Abstract
Background
Globally, 3 billion people suffer from either migraine or tension-type headache disorder over their lifetime. Approximately 50% of American adults suffering from headache or migraine have used complementary and alternative medicine (CAM), however, the quality and quantity of recommendations associated with such therapies across clinical practice guidelines (CPGs) for the treatment and/or management of these conditions are unknown. The purpose of this study was to identify the quantity and assess the quality of such CAM recommendations.
Methods
MEDLINE, EMBASE and CINAHL were systematically searched from 2009 to April 2020; the Guidelines International Network and the National Center for Complementary and Integrative Health websites were also searched for eligible CPGs. CPGs were included if they provided any therapy recommendations. Eligible CPGs included those written for adult patients with headache and migraine; CPGs containing CAM recommendations were assessed twice for quality using the AGREE II instrument, once for the overall CPG and once for the CAM sections.
Results
Of 486 unique search results, 21 CPGs were eligible and quality assessed; fifteen CPGs mentioned CAM, of which 13 CPGs made CAM recommendations. The overall CPG assessment yielded higher scaled domain percentages than the CAM section across all domains. The results from highest to lowest were as follows (overall, CAM): clarity of presentation (66.7% vs. 50.0%), scope and purpose (63.9% vs. 61.1%), stakeholder involvement (22.2% vs. 13.9%), rigour of development (13.5% vs. 9.4%), applicability (6.3% vs. 0.0%), and editorial independence (0.0% vs. 0.0%).
Conclusions
Of the eligible CPGs, the CAM sections were of lower quality compared to the overall recommendations across all domains of the AGREE II instrument. CPGs that scored well could serve as a framework for discussion between patients and healthcare professionals regarding use of CAM therapies in the context of headache and migraine.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-021-03401-3.
Keywords: Headache, Migraine, Complementary and alternative medicine, Systematic review, AGREE II, Clinical practice guideline
Background
The prevalence of headache disorders is increasing globally [1]. In 2016, it was estimated that 3 billion people worldwide suffered from either migraine or tension-type headache disorder, with disability adjusted life years approximately 1.9 and 0.3% respectively [1, 2]. Headache, one of the most prevalent conditions in the world, can be associated with more severe primary headache disorders, such as migraine and tension-type disorders [3]. Clinicians regularly consult the International Classification of Headache Disorders (ICHD) to classify and diagnose specific headache disorders, such as migraine, tension-type, and cluster headache [4]. The different types of headache disorders are defined, diagnosed and screened for according to the ICHD, currently in its third edition published in 2018 [4], following the publication of the first two editions [5, 6]. This first version was published in 1988 and mainly based on expert opinions, while the ICHD-II published in 2004 contained a variety of improvements, partly due to new research and partly due to updated expert opinions. Prior to this, the Headache Classification Committee of the International Headache Society released an ICHD-3 beta version in 2013 ahead of the ICHD-3 [7]; at that time, the main reason for this was to synchronize the ICHD-3 with the World Health Organization’s (11th edition) of the International Classification of Diseases (ICD-11) [8], however, based on delays at the WHO, the former was published ahead of the latter [4]. New scientific evidence played a comparatively greater role in the improvements made to the ICHD-3 beta, and all other changes included in the ICHD-3 were based on this evidence. Since this time, it has been found that the ICHD-3 is significantly more specific than the ICHD-3 beta for the diagnosis of migraine with aura and with typical aura [4, 8, 9].
The prevalence of headaches globally has resulted in significant costs and impacts on society as a whole. A review of studies evaluating the quality of life in patients with primary headache disorders indicated that the health-related quality of life for patients suffering from primary headache disorders, such as migraine and cluster headache, was consistently lower than that of the general population [10]. In addition, an American study identified that the average healthcare expenditure of Americans suffering from migraine was significantly higher than those of non-migraine sufferers [11]. Standard treatments for headache disorders include nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen, but there is also an increasing interest among patients in using complementary and alternative medicine (CAM) [12]. According to the National Center for Complementary and Integrative Health (NCCIH), "complementary medicine" is defined as non-mainstream healthcare approaches that are used together with conventional medicine, while "alternative medicine" is defined as non-mainstream healthcare approaches that are used in place of conventional medicine [13, 14].
The main reason that patients use CAM therapies is to avoid the side effects associated with conventional medicine [15]. It was found that the approximate prevalence of CAM use among American adults suffering from migraine was 50%, which is a significantly higher proportion than non-migraine suffering adults who seek CAM therapies [16, 17]. Some common CAM therapies used for headache disorders include acupuncture, massage, chiropractic, and herbal and dietary supplements [15]. To date, some research has found the effectiveness of certain CAM therapies to be promising. In two large clinical trials, it was found that a greater number of individuals exposed to acupuncture experienced at least a 50% reduction in headaches when compared to the control group [18, 19]. Another study discovered that participating in massage therapy, which targets muscular trigger points for chronic tension headaches, reduced the frequency of chronic tension headaches per week when compared to the baseline frequency [20].
Despite the fact that some CAMs are supported by promising evidence, many clinicians lack training on these therapies for the treatment/management of headache and migraine, which may result in them recommending them less frequently [21]. One survey found that only about 25% of American medical students, residents, and clinicians received training in CAM as part of their education [21]. In addition, many physicians do not mention CAM resources in their discussions with patients, and many patients do not report their CAM use [21]. Healthcare professionals routinely consult evidence-based clinical practice guidelines (CPGs) to identify therapy recommendations and their associated risks and benefits. Using the information from CPGs, healthcare professionals can address patient concerns and needs to inform discussions surrounding shared decision-making. Most of the treatments administered for headache and migraine according to CPGs, such as the U.S. Headache Consortium guidelines, include recommendations about NSAIDs and triptans, among other pharmacological therapies [22]. However, CAM recommendations may be included less frequently or inconsistently across CPGs, based on the fact that there is generally a lower quantity and quality of randomized controlled trials and observational studies forming the evidence-base for these types of therapies [23, 24]. The purpose of this study is to conduct a systematic review to determine the mention of CAM therapies in CPGs for the treatment and/or management of headache and migraine, and assess the quality of CAM recommendations using the AGREE II instrument.
Methods
Approach
To identify CPGs for the treatment and/or management of headache and migraine, a systematic review was conducted using standard methods [25] and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [26]. A protocol was registered with PROSPERO, registration number CRD42020182233. The widely-used and validated Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument [27] was used to assess the CPGs containing CAM recommendations; twenty-three items comprise the instrument. These items are grouped into six domains, each designed to assess different aspects of CPGs quality, as follows: scope and purpose, stakeholder involvement, rigor of development, clarity and presentation, applicability, and editorial independence. CPGs containing CAM recommendations were assessed twice with the AGREE II instrument: once for the overall CPG, and once for only the CAM section of the CPG.
Eligibility criteria
The Population, Intervention, Comparison and Outcomes (PICO) framework was used to identify the eligibility criteria for headache and/or migraine CPGs. The population included adults aged 19 years and older with headache and/or migraine. The interventions included evidence-based CPGs that provided treatment and/or management recommendations for headache and/or migraine. From these eligible CPGs, we determined whether they included any mention or recommendations of CAM therapies. Comparisons referred to the assessed overall quality of headache and/or migraine CPGs and the CAM recommendation sections using the AGREE II instrument. The AGREE II scores were the outcomes, reflecting CPG content and format. Additionally, CPGs were restricted to those as follows: developed by non-profit organizations including disease-specific foundations, government agencies, academic institutions or professional associations or societies; published in 2009 to 2020; published in English; and either available publicly or by order through our library system. Ineligible publications included: consensus statements, protocols, abstracts, conference proceedings, letters or editorials; based on primary studies that evaluated headache management or treatment; or focused on headache education, curriculum, research, training, professional certification or performance. The methods used to guide the development of the CPGs were considered when searching and screening for CPGs meeting our eligibility criteria. We specifically included only evidence-based CPGs as they provided recommendations based on a systematic literature search for evidence, as opposed to solely expert opinion or consensus-based CPGs which are reflective of a lower quality of evidence. The only exception for inclusion included the case whereby a CPG was informed by evidence, however, expert opinion was used to formulate a recommendation for certain therapies for which the available evidence-base was lacking.
Searching and screening
The search was conducted on April 17, 2020, from 2009 to April 16, 2020 inclusive, on MEDLINE, EMBASE and CINAHL. The search strategy (Supplementary File 1) included indexed headings and keywords that reflected terms commonly used in the literature to refer to headache and migraine. The Guidelines International Network, a repository of guidelines [https://www.g-i-n.net/], was also searched using keywords, including “headache” and “migraine”. Next, the NCCIH website which contained a single list of CAM guidelines was searched [https://nccih.nih.gov/health/providers/clinicalpractice.htm]. CH and another research assistant screened titles and abstracts from all sources, and they confirmed eligibility by screening full-text items. JYN reviewed the screened titles and abstracts and full-text items to standardize screening, and helped to resolve selection differences between the two screeners (CH and the other research assistant) through discussion.
Data extraction and analysis
CH and the other research assistant data extracted the following items from each eligible CPG: date of publication; country of first author; type of organization that published the CPG (academic institutions, government agencies, disease-specific foundations, or professional associations or societies); and whether any CAMs were mentioned in this CPG. After determining if CAMs were mentioned in a CPG, the types of CAM mentioned, CAM recommendations made, CAM funding sources, and whether any CAM providers were part of the CPG panel were also data extracted. For the purpose of this review, we defined a CAM funding source as that which was provided by a CAM research organization or CAM professional association. Each CPG developer’s website was also searched to identify any associated knowledge-based resources in support of implementation. For eligible CPGs that did not contain CAM therapy recommendations, only demographic information was collected.
Guideline quality assessment
Standardized methods for applying the AGREE II instrument were followed for the extraction and analysis of data from eligible CPGs containing CAM recommendations [27]. The first step involved conducting a pilot test of the AGREE II instrument; JYN, CH and the other research assistant independently assessed three separate CPGs with the AGREE II instrument, then they met to discuss and resolve any discrepancies. Next, CH and the other research assistant independently assessed all eligible CPGs containing CAM therapy recommendations twice (i.e. once for the overall CPG, and once for the CAM sections of the CPG). CPGs were assessed according to the 23 AGREE II items comprised of 6 domains using a seven-point Likert scale from strongly disagree (1) to strongly agree (7). Using the information from these scores, we recommended for or against the use of each CPG. Supplementary File 2 includes the modified AGREE II items that were used to guide the scoring of the CAM sections of each CPG. Any discrepancies in scores between the two assessors were resolved by JYN. To calculate average appraisal scores, we took the average rating for all 23 items of a single appraiser of a single CPG, followed by taking the average of this value for both appraisers. The average of both appraisers’ “overall guideline assessment” scores for each CPG were calculated to provide the average overall assessment score. Calculating the scaled domain percentages required the addition of both appraisers' ratings of items within each domain, and scaling by maximum and minimum possible domain scores before converting this value into a percentage. The scaled domain percentages were generated for inter-domain comparison. Tabulation of the average appraisal scores, average overall assessments, and scaled domain percentages for each CPG was used for comparison.
Results
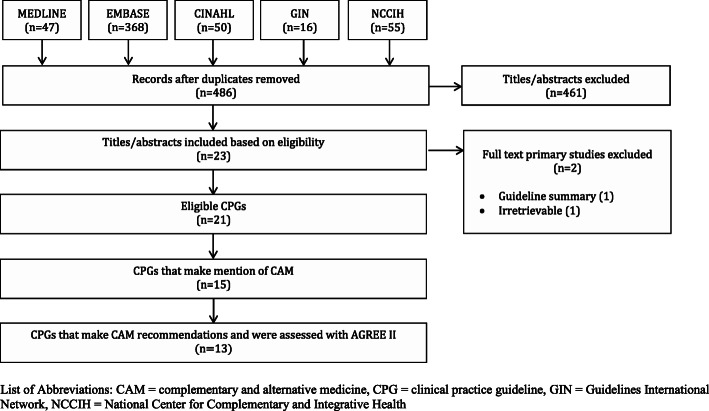
Search results (Fig. 1)
Fig. 1.
PRISMA Diagram
Searches retrieved 536 items, 486 of which were unique. After screening for eligibility, 461 titles and abstracts were eliminated. Of the 23 full-text articles, two were not eligible, because the CPG was irretrievable (n = 1), or the CPG was a summary (n = 1), leaving 21 CPGs eligible for review [28–48]; thirteen CPGs made CAM recommendations [28, 29, 32, 34–36, 38–41, 43–45, 48], two CPGs made mention of CAM but provided no recommendations [33, 48], and the final six CPGs made no mention nor recommendations pertaining to CAM [30, 31, 37, 42, 46, 47]. The citations associated with the excluded full-text items are provided in Supplementary File 3.
Guideline characteristics (Table 1)
Table 1.
Characteristics of eligible guidelines
| Guideline | Country (First Author) | Developer | CAM Category | Guideline Topic |
|---|---|---|---|---|
| Ren 2020 [28] | China | China Association of Chinese Medicine | Acupuncture, herbal therapy, Chinese medicine | Diagnosis and treatment for headache |
| Araki 2019 [29] | Japan | Japanese Society of Neurology and Japanese Headache Society, with collaboration from the Japanese Society of Neurological Therapeutics and the Japan Neurosurgical Society | Acupuncture, herbal, electrotherapy, dietary supplements, oxygen therapy, behavioural therapy | Chronic headache |
| Sacco 2019 [30] | Italy | European Headache Federation | None | Migraine prevention |
| Scottish Intercollegiate Guidelines Network 2018 [31] | Scotland | Scottish Intercollegiate Guidelines Network | None | Pharmacological management of migraine |
| Al Khaled 2017 [32] | Qatar | Ministry of Public Health of Qatar | Acupuncture, herbal therapy, homeopathy, electrotherapy, dietary supplements, oxygen therapy, behavioural therapy | Headache in adults |
| Orr 2016 [33] | USA | American Headache Society | Dietary Supplements | Management of adults with acute migraine in the emergency department |
| Moisset 2016 [34] | France |
French society for the Study of Migraine and Headache Disorders (SFEMC1) and the French Society of Neurology (SFN2) |
Oxygen therapy | Emergency management of headache |
| Robbins 2016 [35] | United States | American Headache Society | Electrotherapy, dietary supplements | Treatment of cluster headache |
| Becker 2015 [36] | Canada | Canadian Family Physician, Alberta College of Family Physicians | Herbal therapy, electrotherapy, dietary supplements, oxygen therapy, behavioural therapy | Primary care management of headache in adults |
| Worthington 2013 [37] | Canada | The Canadian Journal of Neurological Sciences | None | Acute drug therapy for migraine headache |
| Bendtsen 2012 [38] | Denmark | Danish Headache Society | Acupuncture, dietary supplements | Diagnosis and treatment of headache disorders and facial pain |
| Holland 2012 [39] | United States | American Academy of Neurology | Herbal therapy, dietary supplements, oxygen therapy | NSAIDs and complementary treatments for episodic migraine prevention |
| NICE 2012 [40] | United Kingdom | National Institute for Health and Care Excellence | Dietary supplements, oxygen therapy | Diagnosis and management of headache |
| Pringsheim 2012 [41] | Canada | The Canadian Neurological Society | Herbal therapy, dietary supplements | Migraine prophylaxis |
| Silberstein 2012 [42] | USA | American Academy of Neurology | None | Pharmacologic treatment for episodic migraine prevention in adults |
| Sarchielli 2012 [43] | Italy |
Italian Society for the Study of Headaches |
Acupuncture, manual therapy, herbal therapy, electrotherapy, dietary supplements, oxygen therapy behavioural therapy | Guidelines for primary headaches |
| Vukovic 2012 [44] | Croatia | Croatian Society for Neurovascular Disorders, Croatian Medical Association | Acupuncture, manual therapy, herbal therapy, homeopathy, electrotherapy, dietary supplements, oxygen therapy, behavioural therapy | Treatment of primary headache |
| Bryans 2011 [45] | Canada | Canadian Chiropractic Protective Association | Manual therapy (i.e. chiropractic), electrotherapy, behavioural therapy | Chiropractic treatment of adults with headache |
| Evers 2011 [46] | Germany | EFNS Guidelines | None | Treatment of medication overuse headache |
| Saper 2010 [47] | USA | Michigan Head-Pain & Neurological Institute | None | Continuous opioid therapy for refractory daily headache |
| Evers 2009 [48] | Germany | European Federation of Neurological Societies (EFNS) | Herbal therapy, dietary supplements | Drug treatment of migraine |
Eligible CPGs were published from 2009 to 2020 in the USA (n = 5), Canada (n = 4), Germany (n = 2), Italy (n = 2), China (n = 1), Croatia (n = 1), Denmark (n = 1), France (n = 1), Japan (n = 1), Qatar (n = 1), Scotland (only) (n = 1) and the UK (n = 1). The CPGs were funded and/or developed by academic (n = 2) and professional (n = 19) associations or societies. Fifteen CPGs made mention of CAMs, with all 15 CPGs mentioning CAM therapies for different headache disorders, including tension-type, migraine and cluster headaches [28, 29, 32–36, 38–41, 43–45, 48]. These CAMs included dietary supplements (e.g. magnesium, coenzyme Q10, melatonin) (n = 12), herbal medicine (e.g. butterbur, feverfew) (n = 9), oxygen therapy (including hyperbaric, 100%/pure) (n = 9), electrotherapy (e.g. transcutaneous electrical and nerve stimulation (TENS) (n = 7), acupuncture (n = 6), behavioural therapy (e.g. relaxation, cognitive behavioural therapy, hypnosis) (n = 6), manual therapy (e.g. spinal manipulation, massage) (n = 3), homeopathy (n = 2) and Chinese medicine (n = 1). Of the 15 CPGs, recommendations relating to CAM were made in 13 CPGs; only these CPGs were assessed using the AGREE II instrument. CAM funding sources were used in 5 of the CPGs [28, 29, 32, 36, 45], and 3 CPGs included CAM providers as part of the CPG panel [28, 36, 45].
Guidelines mentioning CAM without recommendations
Of 21 eligible CPGs, two CPGs made mention of CAM without making recommendations [33, 48]. The CAMs mentioned included magnesium, butterbur root extract, feverfew (Ternacetum parthenium), riboflavin, and coenzyme Q10. In one CPG, there was a detailed description of various experimental studies about the impact of magnesium on relieving headache, but there was a clear statement that the authors were not making a recommendation for magnesium use [33]. The other CPG had described a variety of herbal treatments, but vaguely [48].
CAM therapies with recommendations across assessed CPGs
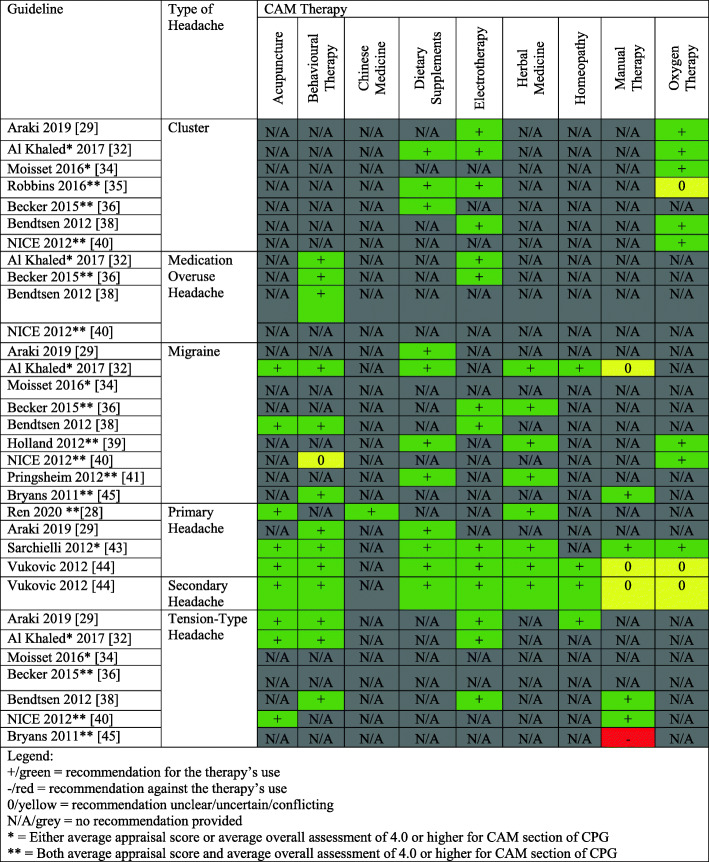
We provide a summary of CAM recommendations made across headache and migraine CPGs for the benefit of clinicians and researchers in Fig. 2. Of the 13 included CPGs, the most commonly recommended CAM therapies were dietary supplements, which were recommended by 10 CPGs, followed by oxygen therapy, herbal medicine, electrotherapy, acupuncture, behavioural therapy, manual therapy, homeopathy and Chinese medicine. This shows that there are similar recommended therapies found across different CPGs, perhaps indicating that the research regarding these CAM therapies in the context of headache/migraine is largely in agreement with one another. Additionally, we provide a legend in Fig. 2 that can support the recommendations that healthcare professionals provide to their patients suffering from headache and/or migraine disorders. This legend indicates that three of the included CPGs either have an average appraisal score or average overall assessment of 4.0 or higher for the CAM section of the CPG, and seven of the included CPGs have both an average appraisal score and average overall assessment of 4.0 or higher for the CAM section of the CPG. Healthcare providers can consult Fig. 2 to identify common CAM therapies recommended for headache and/or migraine, in addition to CAM therapies that are recommended by higher quality CPGs.
Fig. 2.
Summary of CAM Recommendations in Clinical Practice Guidelines
Average appraisal scores, average overall assessments and recommendations regarding use of guidelines: overall guideline (Table 2)
Table 2.
Average appraisal scores and average overall assessments of each guideline
| Guideline | Metric | Appraiser 1 | Appraiser 2 | Average | Standard Deviation |
|---|---|---|---|---|---|
|
Ren 2020 [28] (Overall) |
Appraisal Score | 4.7 | 4.5 | 4.6 | 0.1 |
| Overall Assessment | 5.0 | 4.0 | 4.5 | 0.7 | |
|
Ren 2020 [28] (CAM Section) |
Appraisal Score | 4.7 | 4.5 | 4.6 | 0.1 |
| Overall Assessment | 5.0 | 4.0 | 4.5 | 0.7 | |
|
Araki 2019 [29] (Overall) |
Appraisal Score | 4.0 | 3.4 | 3.7 | 0.4 |
| Overall Assessment | 4.0 | 3.0 | 3.5 | 0.7 | |
|
Araki 2019 [29] (CAM Section) |
Appraisal Score | 3.9 | 3.2 | 3.6 | 0.5 |
| Overall Assessment | 4.0 | 3.0 | 3.5 | 0.7 | |
|
Al Khaled 2017 [32] (Overall) |
Appraisal Score | 4.4 | 4.2 | 4.3 | 0.1 |
| Overall Assessment | 4.0 | 4.0 | 4.0 | 0.0 | |
|
Al Khaled 2017 [32] (CAM Section) |
Appraisal Score | 3.7 | 3.7 | 3.7 | 0.0 |
| Overall Assessment | 3.0 | 4.0 | 3.5 | 0.7 | |
|
Moisset 2016 [34] (Overall) |
Appraisal Score | 4.0 | 3.9 | 4.0 | 0.1 |
| Overall Assessment | 5.0 | 3.0 | 4.0 | 1.4 | |
|
Moisset 2016 [34] (CAM Section) |
Appraisal Score | 3.7 | 3.1 | 3.4 | 0.4 |
| Overall Assessment | 5.0 | 3.0 | 4.0 | 1.4 | |
|
Robbins 2016 [35] (Overall) |
Appraisal Score | 5.0 | 4.9 | 5.0 | 0.1 |
| Overall Assessment | 5.0 | 5.0 | 5.0 | 0.0 | |
|
Robbins 2016 [35] (CAM Section) |
Appraisal Score | 4.7 | 4.3 | 4.5 | 0.3 |
| Overall Assessment | 4.0 | 5.0 | 4.5 | 0.7 | |
|
Becker 2015 [36] (Overall) |
Appraisal Score | 4.9 | 4.6 | 4.8 | 0.2 |
| Overall Assessment | 6.0 | 4.0 | 5.0 | 1.4 | |
|
Becker 2015 [36] (CAM Section) |
Appraisal Score | 4.4 | 3.9 | 4.2 | 0.4 |
| Overall Assessment | 6.0 | 4.0 | 5.0 | 1.4 | |
|
Bendtsen 2012 [38] (Overall) |
Appraisal Score | 3.5 | 3.4 | 3.5 | 0.1 |
| Overall Assessment | 3.0 | 3.0 | 3.0 | 0.0 | |
|
Bendtsen 2012 [38] (CAM Section) |
Appraisal Score | 3.0 | 2.7 | 2.9 | 0.2 |
| Overall Assessment | 3.0 | 3.0 | 3.0 | 0.0 | |
|
Holland 2012 [39] (Overall) |
Appraisal Score | 4.4 | 4.2 | 4.3 | 0.1 |
| Overall Assessment | 6.0 | 4.0 | 5.0 | 1.4 | |
|
Holland 2012 [39] (CAM Section) |
Appraisal Score | 4.3 | 4.0 | 4.2 | 0.2 |
| Overall Assessment | 6.0 | 4.0 | 5.0 | 1.4 | |
|
NICE 2012 [40] (Overall) |
Appraisal Score | 6.3 | 6.2 | 6.3 | 0.1 |
| Overall Assessment | 6.0 | 6.0 | 6.0 | 0.0 | |
|
NICE 2012 [40] (CAM Section) |
Appraisal Score | 6.0 | 5.9 | 6.0 | 0.1 |
| Overall Assessment | 6.0 | 6.0 | 6.0 | 0.0 | |
|
Pringsheim 2012 [41] (Overall) |
Appraisal Score | 6.4 | 6.0 | 6.2 | 0.3 |
| Overall Assessment | 6.0 | 6.0 | 6.0 | 0.0 | |
|
Pringsheim 2012 [41] (CAM Section) |
Appraisal Score | 5.8 | 5.3 | 5.6 | 0.4 |
| Overall Assessment | 6.0 | 6.0 | 6.0 | 0.0 | |
|
Sarchielli 2012 [43] (Overall) |
Appraisal Score | 3.7 | 3.6 | 3.7 | 0.1 |
| Overall Assessment | 5.0 | 4.0 | 4.5 | 0.7 | |
|
Sarchielli 2012 [43] (CAM Section) |
Appraisal Score | 3.3 | 3.2 | 3.3 | 0.1 |
| Overall Assessment | 4.0 | 4.0 | 4.0 | 0.0 | |
|
Vukovic 2012 [44] (Overall) |
Appraisal Score | 3.7 | 3.3 | 3.5 | 0.3 |
| Overall Assessment | 4.0 | 3.0 | 3.5 | 0.7 | |
|
Vukovic 2012 [44] (CAM Section) |
Appraisal Score | 3.3 | 3 | 3.2 | 0.2 |
| Overall Assessment | 3.0 | 3.0 | 3.0 | 0.0 | |
|
Bryans 2011 [45] (Overall) |
Appraisal Score | 4.5 | 4.6 | 4.6 | 0.1 |
| Overall Assessment | 5.0 | 4.0 | 4.5 | 0.7 | |
|
Bryans 2011 [45] (CAM Section) |
Appraisal Score | 4.5 | 4.3 | 4.4 | 0.1 |
| Overall Assessment | 5.0 | 4.0 | 4.5 | 0.7 |
The average appraisal scores for each of the 13 CPGs ranged from 3.5 to 6.3 on the seven-point Likert scale (where 7 equals strongly agree that the item is met); nine CPGs achieved or exceeded an average appraisal score of 4.0, and 3 CPGs achieved or exceeded an average appraisal score of 5.0. Average overall assessments for the 13 CPGs ranged between 3.0 (lowest) and 6.0 (highest), including 10 CPGs equalling or exceeding a score of 4.0, and 5 CPGs equalling or exceeding a score of 5.0.
Average appraisal scores, average overall assessments and recommendations regarding use of guidelines: CAM sections (Table 2)
Average appraisal scores across the 13 CPGs ranged from 2.9 to 6.0 on the seven-point Likert scale (where 7 equals strongly agree that the item is met); seven CPGs achieved or exceeded an average appraisal score of 4.0, and 2 CPGs achieved or exceeded an average appraisal score of 5.0. For the average overall assessments, the 13 CPGs ranged between 3.0 (lowest) and 6.0 (highest), including 9 CPGs with a score of at least 4.0, and 4 CPGs with a score of at least 5.0.
Overall recommendations: overall guideline (Table 3)
Table 3.
Overall recommendations for use of appraised guidelines
| Overall Guideline | CAM Section | |||
|---|---|---|---|---|
| Guideline | Appraiser 1 | Appraiser 2 | Appraiser 1 | Appraiser 2 |
| Ren 2020 [28] | Yes | Yes with Modifications | Yes | Yes with Modifications |
| Araki 2019 [29] | Yes with Modifications | Yes with Modifications | Yes with Modifications | Yes with Modifications |
| Al Khaled 2017 [32] | Yes with Modifications | Yes | No | Yes |
| Moisset 2016 [34] | Yes | No | Yes | No |
| Robbins 2016 [35] | Yes | Yes | Yes with Modifications | Yes |
| Becker 2015 [36] | Yes | Yes with Modifications | Yes | Yes with Modifications |
| Bendtsen 2012 [38] | No | No | No | No |
| Holland 2012 [39] | Yes | Yes with Modifications | Yes | Yes with Modifications |
| NICE 2012 [40] | Yes | Yes | Yes | Yes |
| Pringsheim 2012 [41] | Yes | Yes | Yes | Yes |
| Sarchielli 2012 [43] | Yes | Yes with Modifications | Yes with Modifications | Yes with Modifications |
| Vukovic 2012 [44] | Yes with Modifications | Yes with Modifications | No | Yes with Modifications |
| Bryans 2011 [45] | Yes | Yes | Yes | Yes |
Appraisers agreed in their overall recommendation for 7 of 13 CPGs including 1 “No” [38], 2 “Yes with modifications” [29, 44], and 4 “Yes” [35, 40, 41, 45]. Of the remaining 6 CPGs, 1 was rated by the two appraisers as “No” and “Yes” respectively [34], while 5 CPGs were rated as “Yes” and “Yes with modifications” respectively [28, 32, 36, 39, 43].
Overall recommendations: CAM sections (Table 3)
Appraisers agreed in their overall recommendation for 6 of 13 CPGs including 1 “No” [38], 2 “Yes with modifications” [29, 43], and 3 “Yes” [40, 41, 45]. Of the remaining 7 CPGs, one was rated by the two appraisers as “No” and “Yes with modifications” respectively [44], while 4 CPGs were rated as “Yes” and “Yes with modifications” respectively [28, 35, 36, 39] and 2 were rated as No and Yes [32, 34].
Scaled domain percentage quality assessment (Table 4)
Table 4.
Scaled domain percentages for appraisers of each guideline
| Guideline | Domain score (%) | ||||||
|---|---|---|---|---|---|---|---|
| Scope and Purpose | Stakeholder Involvement | Rigour of Development | Clarity of Presentation | Applicability | Editorial Independence | ||
| Ren 2020 [28] | Overall Guideline | 94.4 | 77.8 | 54.2 | 69.4 | 37.5 | 37.5 |
| CAM Section | 94.4 | 77.8 | 54.2 | 69.4 | 37.5 | 37.5 | |
| Araki 2019 [29] | Overall Guideline | 66.7 | 36.1 | 41.7 | 88.9 | 18.8 | 29.2 |
| CAM Section | 75.0 | 16.7 | 44.8 | 66.7 | 18.8 | 29.2 | |
| Al Khaled 2017 [32] | Overall Guideline | 91.7 | 61.1 | 44.8 | 94.4 | 14.6 | 58.3 |
| CAM Section | 91.7 | 36.1 | 38.5 | 72.2 | 6.3 | 58.3 | |
| Moisset 2016 [34] | Overall Guideline | 97.2 | 61.1 | 32.3 | 80.6 | 12.5 | 45.8 |
| CAM Section | 91.7 | 44.4 | 27.1 | 61.1 | 4.2 | 45.8 | |
| Robbins 2016 [35] | Overall Guideline | 80.6 | 36.1 | 79.2 | 91.7 | 14.6 | 100.0 |
| CAM Section | 80.6 | 27.8 | 68.8 | 75.0 | 10.4 | 100.0 | |
| Becker 2015 [36] | Overall Guideline | 83.3 | 77.8 | 47.9 | 83.3 | 39.6 | 87.5 |
| CAM Section | 80.6 | 52.8 | 40.6 | 63.9 | 31.3 | 87.5 | |
| Bendtsen 2012 [38] | Overall Guideline | 94.4 | 44.4 | 13.5 | 75.0 | 27.1 | 41.7 |
| CAM Section | 80.6 | 33.3 | 9.4 | 50.0 | 16.7 | 41.7 | |
| Holland 2012 [39] | Overall Guideline | 83.3 | 22.2 | 65.6 | 66.7 | 8.3 | 95.8 |
| CAM Section | 83.3 | 13.9 | 60.4 | 66.7 | 6.3 | 95.8 | |
| NICE 2012 [40] | Overall Guideline | 100.0 | 83.3 | 91.7 | 97.2 | 72.9 | 75.0 |
| CAM Section | 100.0 | 66.7 | 85.4 | 97.2 | 68.8 | 75.0 | |
| Pringsheim 2012 [41] | Overall Guideline | 94.4 | 86.1 | 86.5 | 100.0 | 70.8 | 87.5 |
| CAM Section | 94.4 | 47.2 | 79.2 | 100.0 | 54.2 | 87.5 | |
| Sarchielli 2012 [43] | Overall Guideline | 63.9 | 30.6 | 49.0 | 91.7 | 6.3 | 16.7 |
| CAM Section | 61.1 | 19.4 | 42.7 | 77.8 | 2.1 | 16.7 | |
| Vukovic 2012 [44] | Overall Guideline | 91.7 | 44.4 | 25.0 | 86.1 | 25.0 | 0.0 |
| CAM Section | 88.9 | 30.6 | 22.9 | 80.6 | 10.4 | 0.0 | |
| Bryans 2011 [45] | Overall Guideline | 86.1 | 50.0 | 63.5 | 88.9 | 6.3 | 75.0 |
| CAM Section | 86.1 | 41.7 | 62.5 | 91.7 | 0.0 | 75.0 | |
With regards to scaled domain percentages of the overall CPG, scope and purpose scores ranged from 63.9 to 100.0%, stakeholder involvement scores ranged from 22.2 to 86.1%, rigour of development scores ranged from 13.5 to 91.7%, clarity of presentation scores ranged from 66.7 to 100.0%, applicability scores ranged from 6.3 to 72.9%, and editorial independence scores ranged from 0.0 to 100.0%. With regards to scaled domain percentages of the CAM guideline sections, scope and purpose scores ranged from 61.1 to 100.0%, stakeholder involvement scores ranged from 13.9 to 17.8%, rigour of development scores ranged from 9.4 to 85.4%, clarity of presentation scores ranged from 50.0 to 100.0%, applicability scores ranged from 0.0 to 68.8%, and editorial independence scores ranged from 0.0 to 100.0%.
Scope and purpose
For the overall CPG, the overall objectives and health questions were generally well-defined in all CPGs. All CPGs described the goal, the disease or condition that was to be managed by the CAM therapies and the types of CAM therapies that were to be studied. The description of the population for whom the CPG was intended to apply was at times vague, as was the description of the health questions [43]. For the CAM section, the overall objective was generally well-defined in all CPGs. Across the CAM sections of CPGs, descriptions of the population to whom the CPG was meant to apply was less clear [29, 35, 43].
Stakeholder involvement
For the overall CPG, the majority of the CPGs provided detailed characteristics for the members of the CPG development group, including information about the degrees held by each panel member, their institutional affiliations, and geographical location. Most of the CPGs did not elaborate on the views and preferences of the target population, with only a few declaring this [28, 36, 40, 41]. The majority of CPGs thoroughly defined their target users, by providing information about the type of clinician and the specialties. Only three CPGs were vague in their description of target users [29, 39, 43]. For the CAM section, CPGs scored lower across all three sections overall when compared to the overall assessment as there was less involvement of CAM specific specialists. The target users were generally the only detailed subject across all CAM sections. Only two CPGs provided detailed and thorough information to support the three criteria of this domain for both overall and CAM assessments [28, 45].
Rigor of development
For the overall CPG, most of the CPGs used systematic methods to search for evidence according to detailed selection criteria, with the exception of four CPGs [32, 34, 38, 44]. The strengths and limitations of the body of evidence were clearly described in all CPGs except for one [38]. Some CPGs provided detail about the methods for formulating recommendations [28, 34–36, 41, 45], while other CPGs provided minimal detail [29, 32, 38, 39, 43, 44]. All CPGs formulated recommendations with considerations of some health benefits, side effects, and/or risks in formulating their recommendations, with the exception of one [28]. With the exception of one CPG [28], all CPGs provided an explicit link between their recommendations and the supporting evidence. Most of the CPGs did not provide detail or explicitly state that they were externally reviewed by experts prior to publication [29, 32, 34, 36, 38, 43–45], with five CPGs providing explicit statements [28, 35, 39–41]. Most CPGs did not include a procedure for updating the CPG [29, 34, 36, 38, 39, 43–45] and those that did only outlined their methodology vaguely [28, 32, 35, 40, 41]. For the CAM section, the majority of the scores remained the same as the overall assessment, however, many CPGs described health benefits, side effects and/or risks that influenced recommendations [32, 35, 36, 38, 43, 44], as well as the description of external review [32, 34–36, 39–41], more vaguely.
Clarity of presentation
For the overall CPG, the majority of them offered specific and unambiguous recommendations, with the exception of four that lacked details, such as identification of intent/purpose and relevant population [28, 34, 38, 39]. All CPGs presented different options for the management of the condition and were easily identifiable. The generally overall high scores contributed to the high scaled domain percentage [28, 29, 32, 34–36, 38–41, 43, 45]. For the CAM section, the scores were lower for the specificity and unambiguity of the CAM recommendations for several CPGs [29, 32, 35, 36, 38, 43]. Most of the CAM sections provided different management options and were easily identifiable, with the expectation of one [34].
Applicability
For the overall CPG, few CPGs vaguely discussed facilitators and barriers to implementation of the recommendations [28, 35, 40, 41]. Four CPGs included advice and/or tools, such as flowcharts and algorithms, to support implementation of the recommendations [28, 36, 40, 41]. Four CPGs vaguely addressed the resource implications of implementing the recommendations [28, 29, 41, 44], with one addressing it in more detail [40]. Six CPGs [32, 34, 36, 38, 40, 41] provided vague monitoring and auditing criteria, while 7 CPGs contained little to no such information [28, 29, 35, 39, 43–45]. For the CAM section, the scores remained similar to the overall assessment, or the scores were lower across all the applicability criteria, with some related to monitoring and auditing criteria being notably lower than the overall score [28, 44].
Editorial Independence
In the overall CPG, the reporting of the funding source or competing interests of the members of the CPG development panel varied. Several CPGs did not report funding sources or how these sources influenced the CPG development [29, 34, 38, 43, 44]. All the CPGs addressed competing interests, with some varying in the detail regarding how potential competing interests were identified or considered, or how they may have influenced the CPG development process [28, 29, 32, 38, 43, 45]. Some CPGs described in a clear statement that there were no competing interests in the development of the CPGs [28, 29, 45]. One CPG did not address competing interests [44]. For the CAM section, the scores were identical to that of the overall assessment, as the nature of the items in the editorial independence domain are that they pertain to the overall CPG.
Discussion
The purpose of this study was to identify the quantity and assess the quality of CAM recommendations in CPGs for the treatment and/or management of headache and migraine. This research seeks to identify credible, knowledge-based resources which healthcare professionals can use in their discussions and decisions with patients about the use of CAM. We identified 21 eligible CPGs published between 2009 and 2020, of which 13 CPGs made CAM therapy recommendations. The quality of CPGs containing CAM recommendations was assessed by the 23-item AGREE II instrument, which varied widely across CPGs overall and by domain. The overall CPG assessment included three CPGs scoring 5.0 or higher in both average appraisal score and average overall assessment [35, 40, 41]. In assessing the CAM section of each CPG, two CPGs scored 5.0 or higher in both average appraisal score and average overall assessment [40, 41].
To our knowledge, this is the first study to assess the quantity and quality of CAM therapies recommendations in headache and/or migraine CPGs. In this study, the scaled domain percentages for the overall CPGs from highest to lowest were clarity of presentation (66.7%), scope and purpose (63.9%), stakeholder involvement (22.2%), rigour of development (13.5%), applicability (6.3%) and editorial independence (0.0%). The scaled domain percentages for the CAM section of the CPGs from highest to lowest were scope and purpose (61.1%), clarity of presentation (50.0%), stakeholder involvement (13.9%), rigor of development (9.4%), and editorial independence (0.0%) and applicability (0.0%).
Published studies on similar topics relating to CPG quality assessment exist, allowing us to draw comparisons. A study appraising the methodological quality of CPGs for headache, which included some CPGs providing traditional Chinese medicine recommendations, found that, among 23 CPGs published between 1998 and 2014, the scaled domain percentages were similarly ordered from highest (scope and purpose 52.1%) to lowest (editorial independence 24.2%) [49]. One study assessing the quality of CPGs recommending herbal medicines, acupuncture, and spinal manipulation, which are recommended by several CPGs included in the present review, reported quality scores similar to our study, with clarity of presentation being the highest scaled domain percentage (85.3%) and applicability the lowest (20.7%) [50]. Another study examining the quality of CAM recommendations in 15 arthritis CPGs found that the highest scoring domain was clarity of presentation (94.1%), and the lowest was applicability (33.3%) [51]. One study assessed the quality of CAM recommendations across cancer-related pain CPGs, and found that the highest scored domain for both the overall CPG and the CAM section was scope and purpose at 88.1 and 88.1% respectively, while the lowest scored domain was applicability at 21.0 and 8.5% respectively [52]. Finally, two other studies assessing the quality of CAM recommendations across multiple sclerosis and low back pain CPGs respectively found that the highest scoring domains were clarity of presentation and scope of purpose, while the lowest scoring domains were editorial independence and stakeholder involvement [53, 54]. Therefore, the variable quality of headache and migraine CPGs in the context of CAM recommendations is not unique, as the same phenomenon has been observed across CPGs for a variety of diseases and conditions.
This study described the quantity and quality of headache and/or migraine CPGs that included CAM recommendations, revealing that several CPGs can be used to support informed decision-making among healthcare professionals to better inform and support their patients. However, the quality of these recommendations can be improved. Randomized controlled trials used to inform the development of CPGs suffer from several limitations and discrepancies, including insufficient sample sizes, lack of funding and biased grant review processes, making it difficult to formulate conclusions regarding their efficacy [55, 56]. This can be seen in our study, where three CPGs that scored higher in overall assessment also scored higher in their CAM sections than that of CPGs with lower overall assessment scores [28, 40, 41]. Also, there is an increasing interest in and prevalence of CAM use among patients suffering from headache or migraine, but there are discrepancies between patient-reported use and the available evidence-based for CAM therapies [14–16, 56]. Future directions worth exploring given the present review’s findings include the further investigation of how patient preference and experience relating to CAM therapies can better be incorporated into headache/migraine CPGs. Approximately 82% of individuals experiencing headache disorders use CAM therapies [57]. Although healthcare professionals prefer recommending evidence-based therapies, many may be hesitant to recommend even evidence-based CAM therapies due to their lack of knowledge/training, concerns regarding dosing, or personal biases against CAM [21, 58, 59]. Therefore, our finding of lower quality recommendations across most CPGs in combination with the fact that there is increasing interest in CAM therapies among patients further justifies the need to incorporate recommendations for evidence-based and commonly used CAMs in future headache and migraine CPGs.
Strengths and limitations
One strength of this study is the use of a comprehensive systematic review to identify eligible CPGs for the treatment and/or management of headache and/or migraine. Another strength is the use of the validated AGREE II instrument, which is the internationally-accepted gold standard for appraising the quality of CPGs [27]. CPGs were independently assessed by two appraisers instead of four as recommended by the AGREE II instrument to optimize reliability, thus this may limit the interpretation of the findings. In an effort to mitigate this and standardize scoring, JYN, CH and the other research assistant conducted an initial pilot-test during which they each appraised three independent CPGs, then discussed the results to achieve consensus on how to apply the AGREE II instrument. Following appraisal of the 13 CPGs, JYN met with CH and the other research assistant to resolve any uncertainties through discussion without inordinately modifying legitimate discrepancies. Another limitation includes the fact that our inclusion criteria was limited to CPGs that were only published in the English language; this increases the possibility that there could be omissions of other traditional medicine therapy recommendations that originate from different (i.e. non-English speaking) regions of the world, which could also be greatly influenced by the culture of that region. Furthermore, the development of CPGs from other regions of the world could be influenced by the availability of stakeholders and medical resources, indicating that regional and cultural differences can affect the recommendation grade. For example, there could be articles published in Asian countries, which would have more information about traditional Asian medicine, given the higher frequency of use.
Conclusions
This study identified 13 CPGs published between 2009 and 2020 which included CAM therapy recommendations for the treatment and/or management of headache and/or migraine. The CPGs included in this study provided CAM-specific recommendations related to subsets of CAM therapies, including dietary supplements, oxygen therapy, herbal medicine, electrotherapy, and acupuncture. The AGREE II instrument was used for the appraisal of these CPGs, identifying the varied quality within and across them. Some CPGs achieved higher AGREE II scores and favourable overall recommendations, thus patients and healthcare professionals could use them as a basis for discussion about the use of these CAM therapies to treat or manage headache or migraine. CPGs that achieved lower scaled domain percentage and overall recommendations could be improved in future updates according to the criteria as outlined in the AGREE II instrument.
Supplementary Information
Additional file 1. MEDLINE Search Strategy for Headache and Migraine Clinical Practice Guidelines Executed April 17, 2020
Additional file 2. Modified AGREE II Questions Used to Guide Scoring of CAM Sections of Each Guideline
Additional file 3. List of Excluded Full-Text Items
Acknowledgements
We gratefully acknowledge Alisha Sharma for assisting with screening and data extraction. JYN was awarded a Research Scholarship and an Entrance Scholarship from the Department of Health Research Methods, Evidence and Impact, Faculty of Health Sciences at McMaster University.
Abbreviations
- AGREE II
Appraisal of Guidelines for Research & Evaluation II
- CAM
Complementary and Alternative Medicine
- CPG
Clinical Practice Guideline
- ICHD
International Classification of Headache Disorders
- NCCIH
National Center for Complementary and Integrative Health
- PICO
Patients, Intervention, Comparison and Outcomes
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- NSAIDS
Nonsteroidal Anti-Inflammatory Drugs
Authors’ contributions
JYN: designed and conceptualized the study, collected and analysed data, co-drafted the manuscript, and gave final approval of the version to be published. CH: assisted with the collection and analysis of data, co-drafted the manuscript, and gave final approval of the version to be published.
Funding
This study was unfunded.
Availability of data and materials
All relevant data are included in this manuscript.
Declarations
Ethics approval and consent to participate
This study involved a systematic review of peer-reviewed literature only; it did not require ethics approval or consent to participate.
Consent for publication
All authors consent to this manuscript’s publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jeremy Y. Ng, Email: ngjy2@mcmaster.ca
Christina Hanna, Email: hannac6@mcmaster.ca.
References
- 1.Saylor D, Steiner TJ. The global burden of headache. InSeminars Neurol. 2018;38(02):182–190. doi: 10.1055/s-0038-1646946. [DOI] [PubMed] [Google Scholar]
- 2.Stovner LJ, Nichols E, Steiner TJ, Abd-Allah F, Abdelalim A, Al-Raddadi RM, Ansha MG, Barac A, Bensenor IM, Doan LP, Edessa D. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018;17(11):954–976. doi: 10.1016/S1474-4422(18)30322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowson A. The burden of headache: Global and regional prevalence of headache and its impact. Int J Clin Pract Supplement. 2015;1(182):3–7. doi: 10.1111/ijcp.12650. [DOI] [PubMed] [Google Scholar]
- 4.Headache Classification Committee of the International Headache Society The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 5.Headache Classification Subcommittee of the International Headache Society ICHD-II classification: parts 1–3: primary, secondary and other. Cephalalgia. 2004;24(1_suppl):23–136. doi: 10.1111/j.1468-2982.2004.00653.x. [DOI] [Google Scholar]
- 6.Headache Classification Committee of the International Headache Society Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8(7):1–96. [PubMed] [Google Scholar]
- 7.Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders, (beta version) Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. International Classification of Diseases 11th Revision: The global standard for diagnostic health information [Internet].[cited 2021 Jan 31]. Available from: https://icd.who.int/en
- 9.Göbel CH, Karstedt SC, Münte TF, Göbel H, Wolfrum S, Lebedeva ER, Olesen J, Royl G. ICHD-3 is significantly more specific than ICHD-3 beta for diagnosis of migraine with aura and with typical aura. J Headache Pain. 2020;21(1):1–6. doi: 10.1186/s10194-019-1072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu Bakar N, Tanprawate S, Lambru G, Torkamani M, Jahanshahi M, Matharu M. Quality of life in primary headache disorders: a review. Cephalalgia. 2016;36(1):67–91. doi: 10.1177/0333102415580099. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins K, Wang S, Rupnow M. Direct cost burden among insured US employees with migraine. Headache. 2008;48(4):553–563. doi: 10.1111/j.1526-4610.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 12.Verhagen A, Damen L, Berger M, Lenssinck ML, Passchier J, Kroes BW. Treatment of tension type headache: paracetamol and NSAIDs work: a systematic review. Ned Tijdschr Geneeskd 2010;154(27). doi: 10.1111/j.1755-5949.2009.00077.x, 2, 205. [PubMed]
- 13.National Institutes of Health, National Centre for Complementary and Integrative Health (2020). Complementary, alternative, or integrative health: What’s in a name? NCCIH (2018). Accessed July 8, 2020. Available from: https://nccih.nih.gov/health/integrative-health
- 14.Ng JY, Boon HS, Thompson AK, Whitehead CR. Making sense of “alternative”,“complementary”,“unconventional” and “integrative” medicine: exploring the terms and meanings through a textual analysis. BMC Complement Altern Med. 2016;16(1):134. doi: 10.1186/s12906-016-1111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams J, Barbery G, Lui CW. Complementary and alternative medicine use for headache and migraine: a critical review of the literature. Headache. 2013;53(3):459–473. doi: 10.1111/j.1526-4610.2012.02271.x. [DOI] [PubMed] [Google Scholar]
- 16.Rhee TG, Harris IM. Gender differences in the use of complementary and alternative medicine and their association with moderate mental distress in US adults with migraines/severe headaches. Headache. 2017;57(1):97–108. doi: 10.1111/head.12986. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Dennis JA, Leach MJ, Bishop FL, Cramer H, Chung VCH, Moore C, Lauche R, Cook R, Sibbritt D, Adams J. Complementary and alternative medicine use among US adults with headache or migraine: results from the 2012 National Health Interview Survey. Headache. 2017;57(8):1228–1242. doi: 10.1111/head.13148. [DOI] [PubMed] [Google Scholar]
- 18.Jena S, Witt CM, Brinkhaus B, Wegscheider K, Willich SN. Acupuncture in patients with headache. Cephalalgia. 2008;28(9):969–979. doi: 10.1111/j.1468-2982.2008.01640.x. [DOI] [PubMed] [Google Scholar]
- 19.Melchart D, Streng A, Hoppe A, Brinkhaus B, Witt C, Wagenpfeil S, Pfaffenrath V, Hammes M, Hummelsberger J, Irnich D, Weidenhammer W. Acupuncture in patients with tension-type headache: randomised controlled trial. BMJ. 2005;331(7513):376–382. doi: 10.1136/bmj.38512.405440.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn C, Chandler C, Moraska A. Massage therapy and frequency of chronic tension headaches. Am J Public Health. 2002;92(10):1657–1661. doi: 10.2105/AJPH.92.10.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel SJ, Kemper KJ, Kitzmiller JP. Physician perspectives on education, training, and implementation of complementary and alternative medicine. Adv Med Educ Pract. 2017;8:499–503. doi: 10.2147/AMEP.S138572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilmore B, Michael M. Treatment of acute migraine headache. Am Fam Physician. 2011;83(3):271–280. [PubMed] [Google Scholar]
- 23.John PJ, Sharma N, Sharma CM, Kankane A. Effectiveness of yoga therapy in the treatment of migraine without aura: a randomized controlled trial. Headache. 2007;47(5):654–661. doi: 10.1111/j.1526-4610.2007.00789.x. [DOI] [PubMed] [Google Scholar]
- 24.Astin JA, Ernst E. The effectiveness of spinal manipulation for the treatment of headache disorders: a systematic review of randomized clinical trials. Cephalalgia. 2002;22(8):617–623. doi: 10.1046/j.1468-2982.2002.00423.x. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2011. [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 27.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P. AGREE II: advancing guideline development, reporting and evaluation in health care. Can Med Assoc J. 2010;182(18):E839–E842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren Y, Li H, Wang Y, Chen Y. Report of guidelines for diagnosis and treatment of common internal diseases in Chinese medicine: headache. J Evid Based Med. 2020;13(1):70–80. doi: 10.1111/jebm.12378. [DOI] [PubMed] [Google Scholar]
- 29.Araki N, Takeshima T, Ando N, Iizuka T, Igarashi H, Ikeda Y, Ito Y, Inagaki M, Imamura K, Ohkuma H, Ogawa K. Clinical practice guideline for chronic headache 2013. Neurol Clin Neurosci. 2019;7(5):231–259. doi: 10.1111/ncn3.12322. [DOI] [Google Scholar]
- 30.Sacco S, Bendtsen L, Ashina M, Reuter U, Terwindt G, Mitsikostas DD, Martelletti P. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain. 2019;20(1):6. doi: 10.1186/s10194-018-0955-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Network SIG. Pharmacological management of migraine. Edinburgh: SIGN; 2018. [Google Scholar]
- 32.Al-Khaled M. Clinical guidelines for the State of Qatar headache in adults. 2017. [Google Scholar]
- 33.Orr SL, Friedman BW, Christie S, Minen MT, Bamford C, Kelley NE, Tepper D. Management of adults with acute migraine in the emergency department: the American headache society evidence assessment of parenteral pharmacotherapies. Headache. 2016;56(6):911–940. doi: 10.1111/head.12835. [DOI] [PubMed] [Google Scholar]
- 34.Moisset X, Mawet J, Guegan-Massardier E, Bozzolo E, Gilard V, Tollard E, Feraud T, Noëlle B, Rondet C, Donnet A. French guidelines for the emergency management of headaches. Rev Neurol. 2016;172(6-7):350–360. doi: 10.1016/j.neurol.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Robbins MS, Starling AJ, Pringsheim TM, Becker WJ, Schwedt TJ. Treatment of cluster headache: the American headache society evidence-based guidelines. Headache. 2016;56(7):1093–1106. doi: 10.1111/head.12866. [DOI] [PubMed] [Google Scholar]
- 36.Becker WJ, Findlay T, Moga C, Scott NA, Harstall C, Taenzer P. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61(8):670–679. [PMC free article] [PubMed] [Google Scholar]
- 37.Worthington I, Pringsheim T, Gawel MJ, Gladstone J, Cooper P, Dilli E, Aube M, Leroux E, Becker WJ. Canadian headache society guideline: acute drug therapy for migraine headache. Can J Neurol Sci. 2013;40(S3):S1–S3. doi: 10.1017/S0317167100017819. [DOI] [PubMed] [Google Scholar]
- 38.Bendtsen L, Birk S, Kasch H, Aegidius K, Sørensen PS, Thomsen LL, Poulsen L, Rasmussen MJ, Kruuse C, Jensen R. Reference programme: diagnosis and treatment of headache disorders and facial pain. Danish Headache Society, 2012. J Headache Pain. 2012;13(1):1. doi: 10.1007/s10194-011-0402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holland S, Silberstein SD, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults:[RETIRED]: report of the quality standards Subcommittee of the American Academy of neurology and the American headache society. Neurology. 2012;78(17):1346–1353. doi: 10.1212/WNL.0b013e3182535d0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Institute for Health and Care Excellence. Headaches in over 12s: diagnosis and management. 2012 Sep 19. Available from: https://www.nice.org.uk/guidance/cg150/resources/headaches-in-over-12s-diagnosis-and-management-pdf-35109624582853 [PubMed]
- 41.Pringsheim T, Davenport W, Mackie G, Worthington I, Aubé M, Christie SN, Gladstone J, Becker WJ. Canadian headache society guideline for migraine prophylaxis. Can J Neurol Sci. 2012;39(2 Suppl 2):S1–59. [PubMed] [Google Scholar]
- 42.Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the quality standards Subcommittee of the American Academy of neurology and the American headache society. Neurology. 2012;78(17):1337–1345. doi: 10.1212/WNL.0b013e3182535d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarchielli P, Granella F, Prudenzano MP, Pini LA, Guidetti V, Bono G, Pinessi L, Alessandri M, Antonaci F, Fanciullacci M, Ferrari A. Italian guidelines for primary headaches: 2012 revised version. J Headache Pain. 2012;13(2):31–70. doi: 10.1007/s10194-012-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vuković CV, Kes VB, Serić V, Solter VV, Demarin V, Janculjak D, Petravić D, Lakusić DM, Hajnsek S, Lusić I, Bielen I. Report of the Croatian Society for Neurovascular Disorders, Croatian Medical Association. Evidence based guidelines for treatment of primary headaches--2012 update. Acta Clinica Croatica. 2012;51(3):323. [PubMed] [Google Scholar]
- 45.Bryans R, Descarreaux M, Duranleau M, Marcoux H, Potter B, Ruegg R, Shaw L, Watkin R, White E. Evidence-based guidelines for the chiropractic treatment of adults with headache. J Manip Physiol Ther. 2011;34(5):274–289. doi: 10.1016/j.jmpt.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Evers S, Jensen R. Treatment of medication overuse headache–guideline of the EFNS headache panel. Eur J Neurol. 2011;18(9):1115–1121. doi: 10.1111/j.1468-1331.2011.03497.x. [DOI] [PubMed] [Google Scholar]
- 47.Saper JR, Lake AE, Bain PA, Stillman MJ, Rothrock JF, Mathew NT, Hamel RL, Moriarty M, Tietjen GE. A practice guide for continuous opioid therapy for refractory daily headache: patient selection, physician requirements, and treatment monitoring. Headache. 2010;50(7):1175–1193. doi: 10.1111/j.1526-4610.2010.01733.x. [DOI] [PubMed] [Google Scholar]
- 48.Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, Sándor PS. EFNS guideline on the drug treatment of migraine–revised report of an EFNS task force. Eur J Neurol. 2009;16(9):968–981. doi: 10.1111/j.1468-1331.2009.02748.x. [DOI] [PubMed] [Google Scholar]
- 49.Hao L, Hui L, Yangyang W, Sha Y, Wenjie X. Clinical practice guidelines for treating headache with traditional Chinese medicine: quality assessment with the appraisal of guidelines for research and evaluation II instrument. J Tradit Chin Med. 2018;38(3):339–350. doi: 10.1016/S0254-6272(18)30624-1. [DOI] [PubMed] [Google Scholar]
- 50.Ng JY, Liang L, Gagliardi AR. The quantity and quality of complementary and alternative medicine clinical practice guidelines on herbal medicines, acupuncture and spinal manipulation: systematic review and assessment using AGREE II. BMC Complement Altern Med. 2016;16(1):425. doi: 10.1186/s12906-016-1410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng JY, Azizudin AM. Rheumatoid arthritis and osteoarthritis clinical practice guidelines provide few complementary and alternative medicine therapy recommendations: a systematic review. Clin Rheumatol. 2020;39(10):2861–2873. doi: 10.1007/s10067-020-05054-y. [DOI] [PubMed] [Google Scholar]
- 52.Ng JY, Sharma AE. Guidelines for Cancer-related pain: a systematic review of complementary and alternative medicine recommendations. Pain Pract. 2020;21(4):454–467. doi: 10.1111/papr.12964. [DOI] [PubMed] [Google Scholar]
- 53.Ng JY, Kishimoto V. Multiple sclerosis clinical practice guidelines provide few complementary and alternative medicine recommendations: a systematic review. Complementary Therapies Med. 2020;18:102595. doi: 10.1016/j.ctim.2020.102595. [DOI] [PubMed] [Google Scholar]
- 54.Ng JY, Mohiuddin U. Quality of complementary and alternative medicine recommendations in low back pain guidelines: a systematic review. Eur Spine J. 2020;31(8):1–2. doi: 10.1007/s00586-020-06393-9. [DOI] [PubMed] [Google Scholar]
- 55.Veziari Y, Leach MJ, Kumar S. Barriers to the conduct and application of research in complementary and alternative medicine: a systematic review. BMC Complement Altern Med. 2017;17(1):166. doi: 10.1186/s12906-017-1660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wells RE, Beuthin J, Granetzke L. Complementary and integrative medicine for episodic migraine: an update of evidence from the last 3 years. Curr Pain Headache Rep. 2019;23(2):10. doi: 10.1007/s11916-019-0750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaul C, Eismann R, Schmidt T, May A, Leinisch E, Wieser T, Evers S, Henkel K, Franz G, Zierz S. Use of complementary and alternative medicine in patients suffering from primary headache disorders. Cephalalgia. 2009;29(10):1069–1078. doi: 10.1111/j.1468-2982.2009.01841.x. [DOI] [PubMed] [Google Scholar]
- 58.Cowan RP. CAM in the real world: you may practice evidence-based medicine, but your patients don't. Headache. 2014;54(6):1097–1102. doi: 10.1111/head.12364. [DOI] [PubMed] [Google Scholar]
- 59.Wahner-Roedler DL, Vincent A, Elkin PL, Loehrer LL, Cha SS, Bauer BA. Physicians' attitudes toward complementary and alternative medicine and their knowledge of specific therapies: a survey at an academic medical center. Evid Based Complement Alternat Med. 2006;3(4):495–501. doi: 10.1093/ecam/nel036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. MEDLINE Search Strategy for Headache and Migraine Clinical Practice Guidelines Executed April 17, 2020
Additional file 2. Modified AGREE II Questions Used to Guide Scoring of CAM Sections of Each Guideline
Additional file 3. List of Excluded Full-Text Items
Data Availability Statement
All relevant data are included in this manuscript.