Abstract
Background.
Fibrolamellar hepatocellular carcinoma (FLC) is a rare variant of hepatocellular carcinoma (HCC), with most clinical data stemming from single-institution series. The variability in the literature lends support for analysis using a large national dataset. In doing so, we sought to (1) define the characteristics and outcomes of patients with FLC; (2) determine factors associated with survival in patients undergoing resection; and (3) compare the overall survival (OS) of patients with FLC with a matched group of patients with HCC.
Methods.
The National Cancer Database was queried for patients with FLC, and their clinicopathologic features were recorded. Univariate and multivariate analyses were performed to delineate factors associated with survival.
Results.
Between 2004 and 2015, 496 patients were diagnosed with FLC, 229 of whom underwent a curative resection. The median OS for patients with FLC undergoing curative resection was 78.5 months. Factors associated with abbreviated OS in this surgical cohort include multiple tumors [hazard ratio (HR) 3.15, p = 0.025], positive regional lymph nodes (HR 2.83, p = 0.023), and elevated serum α-fetoprotein (AFP; HR 2.81, p = 0.034). When the OS of patients with FLC was compared with a matched group of patients with HCC, no difference was detected (p = 0.748); however, patients with FLC and elevated AFP had abbreviated OS compared with patients with HCC and elevated AFP (43 vs. 82 months, p ≤ 0.001).
Conclusions.
Elevations in serum AFP occur more frequently than previously documented for patients with FLC and are associated with abbreviated OS. AFP levels may help guide the decision for operative intervention in patients with FLC.
INTRODUCTION
Fibrolamellar hepatocellular carcinoma (FLC) is a rare malignant liver tumor known for affecting the non-cirrhotic, young adult population. It was first reported in 1956 in the pediatric population, alongside hepatocellular carcinoma (HCC);1 however, it was not until 1980 that FLC became known for its unique histologic characteristics, making it a distinct variant of HCC.2 The reported incidence of FLC is estimated to be around one in five million people nationally and it carries a 5-year survival rate of 32%.2–6 Currently, FLC is staged and treated similar to HCC, however several studies have reported better survival with FLC compared with HCC, thus demonstrating the need for better disease understanding.6,7
Given the rarity of FLC, most data primarily stem from small, single-institution cohorts, case series, and reports based on the Surveillance, Epidemiology, and End Results (SEER) database, all of which have inherent limitations and resulted in significant ambiguities. For example, in a systematic review of 39 series on FLC, there was a significant amount of variability: female sex was reported anywhere between 32 and 82%, median age ranged from 14 to 33 years, median tumor size was from 8 to 20 cm, and the median overall survival (OS) ranged between 11 and 126 months. That same study reported elevated α-fetoprotein (AFP) in 10% of patients, but fewer than half of the series included in that review reported on the AFP level at all.3 The significant ambiguity reported in the literature prompted us to investigate a multi-institutional cohort of data collected from the National Cancer Database (NCDB) to gain a better insight into this rare liver cancer. We sought to (1) define the characteristics and outcomes of patients with FLC; (2) determine factors associated with survival in patients who underwent curative resection of FLC; and (3) compare the OS of patients with FLC to a matched group of patients with HCC.
METHODS
Data Source
We performed a retrospective cohort study of the NCDB Participant User Files (PUFs) for liver tumors. The NCDB is a joint program of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. NCDB data are collected from over 1500 CoC-accredited facilities and captures more than 70% of newly diagnosed cancer cases in the US. Neither the CoC nor the American Cancer Society have verified or are responsible for the analytic or statistical methods used or the conclusions drawn from these data. This study is exempt for review from our Institutional Review Board.
Selection of the Study Population
The study population included all patients diagnosed with FLC from 2004 to 2015. Patients with FLC were identified using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3), with the primary site code for liver (22.0) and the histology code for FLC (8171). Descriptive data regarding patient and tumor characteristic were collected on all patients with FLC (Table 1).
TABLE 1.
Clinicopathologic data from patients with fibrolamellar hepatocellular carcinoma
| Variables | All patients | Curative resection |
|---|---|---|
| [n (%)]a | [n (%)]a | |
| Patients | 496 | 229 |
| Median age, years (± SD) | 32 (± 21) | 28 (± 18) |
| Sex | ||
| Male | 277 (56) | 106 (46) |
| Female | 219 (44) | 123 (54) |
| Race | ||
| White | 405 (82) | 198 (86) |
| Black | 59 (12) | 20 (9) |
| Asian/other | 32 (6) | 11 (5) |
| Ethnicity | ||
| Non-Hispanic | 422 (85) | 202 (88) |
| Hispanic | 58 (12) | 20 (9) |
| Unknown | 16 (3) | 7 (3) |
| CDCS | ||
| 0 | 383 (77) | 188 (82) |
| 1 | 67 (13) | 33 (14) |
| 2 | 18 (4) | 8 (4) |
| ≥ 3 | 28 (6) | – |
| Median tumor size (cm) | 9.5 (± 5 cm) | 9.2 (± 4.8 cm) |
| Number of tumors | ||
| 1 | 237 (61) | 156 (78) |
| > 1 | 152 (39) | 45 (22) |
| Major vascular involvement | ||
| Absent | 296 (86) | 179 (93) |
| Present | 49 (14) | 13 (7) |
| Lymphovascular invasion | ||
| Absent | 218 (59) | 126 (60) |
| Present | 152 (42) | 85 (40) |
| AFP | ||
| Normal | 196 (57) | 111 (67) |
| Elevated | 146 (44) | 55 (33) |
| Grade | ||
| Well-differentiated | 46 (22) | 25 (17) |
| Moderately differentiated | 127 (60) | 100 (70) |
| Poorly differentiated | 40 (18) | 28 (13) |
| Regional node status | ||
| Negative | – | 71 (55) |
| Positive | – | 57 (45) |
| Resection margin | ||
| R0 | – | 219 (96) |
| R1 | – | 10 (4) |
Percentage does not include missing data
CDCS Charlson–Deyo Comorbidity Score, AFP α-fetoprotein, SD standard deviation
Patient and Tumor Characteristics
Patient and tumor characteristics were examined and recorded. Demographic data included patient age, race, ethnicity, and Charlson–Deyo Comorbidity Score (CDCS), which ranged from 0 to ≥ 3. Tumor characteristics included tumor size, single or multiple tumors, lymphovascular invasion, major vascular involvement, AFP level (normal or elevated), tumor grade, regional lymph node status, and resection margin. AFP was recorded in the NCDB as normal or elevated; an AFP level ≤ 15 ng/mL was recorded as normal and an AFP level > 15 ng/mL was considered elevated. Multiple tumors and major vascular involvement were collected and derived from the Collaborative Stage Data Collection System CS Extension codes included in the NCDB. Multiple tumors included multiple nodules or tumors, including satellitosis, multifocal tumors, and intrahepatic metastases. Major vascular involvement was defined as invasion of the hepatic artery, vena cava, the large branches of the main portal vein (excluding sectoral or segmental branches), or invasion of one or more of the three hepatic veins. Patients were considered to have lymphovascular invasion if they had any vascular invasion, positive lymph nodes, or reported lymphovascular invasion as coded in the NCDB. Cases with missing data were included in the demographic and tumor characteristics but were excluded from univariate and multivariate analyses.
Selection of the Survival Cohort
In order to accurately evaluate the survival of patients with FLC who underwent a potentially curative resection, we chose to exclude patients with a grossly positive margin (R2), a positive margin not otherwise specified, and those with a margin status of not applicable or not assessable. Patients with a CDCS of 3 were also excluded because of the high likelihood of death related to comorbidities, not cancer-related. Based on these criteria, the survival cohort included only those patients who underwent an R0/R1 resection or transplant and who had a CDCS of ≤ 2.
Comparison with a Matched Hepatocellular Carcinoma (HCC) Group
Due to the small amount of data and the limitations associated with single-institution series, we compared our survival cohort with a matched cohort of patients with HCC. It has been thought that patients with FLC have improved survival compared with patients with HCC because of their younger age and the lack of underlying liver disease that is commonly seen in patients with HCC. In order to compare these two hepatic diseases, we created an HCC (histology code 8170) survival cohort using the NCDB and the same selection criteria used for the FLC survival cohort. Kaplan–Meier curves were created to visualize differences in survival.8
Survival Analysis
OS was the primary outcome of the univariate analysis and was completed using a log-rank test. Factors returning statistically significant in the univariate analysis were then placed in a multivariate analysis using the Cox proportional hazard model.9 Age and tumor size were treated as continuous variables. Cases with missing data were excluded from both the univariate and multivariate analyses. Statistical analysis was performed using IBM® SPSS Statistics for Windows version 24 (IBM Corporation, Armonk, NY, USA). All tests were two-sided, and a p value < 0.05 was considered statistically significant.
RESULTS
Patient and Tumor Characteristics
Overall, 496 patients were diagnosed with FLC between 2004 and 2015. Median age was 32 years (range 18–90), nearly half of the patients were female (44%), the majority were Caucasian (82%), and 77% had a CDCS of 0. The median tumor size was 9.50 cm, 39% had multiple tumors, lymphovascular invasion was present in 42%, and major vascular involvement was present in 14%. Elevations in serum AFP were documented in 44% of patients. Clinicopathologic characteristics are displayed in Table 1.
Factors Associated with Survival
Less than half of the patients underwent potentially curative resections (n = 229, 46%), and the median OS for these patients was 78.5 months. Ninety-one percent of patients undergoing a potentially curative resection had complete tumor extirpation (R0) and 45% had positive lymph nodes on final pathology. With univariate log-rank analysis, we found that multiple tumors, major vascular involvement, lymphovascular invasion, elevated serum AFP, regional lymph node involvement, and a microscopically positive resection margin were associated with significantly abbreviated OS (Table 2). When these variables, along with age and tumor size, were included in a multivariate Cox proportional hazards model, we found that multiple tumors [hazard ratio (HR) 3.15, 95% confidence interval (CI) 1.2–8.6], elevated AFP (HR 2.81, 95% CI 1.08–7.33), and positive regional lymph nodes (HR 2.83, 95% CI 1.15–6.96) were independently associated with worse survival.
TABLE 2.
Univariate comparison and multivariate Cox survival model of surgical patients with no underlying liver disease
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Median OS (months) | 95% CI | p value | HR | 95% CI | p value | |
| Age | – | – | – | 0.98 | 0.94–1.02 | 0.321 |
| Sex | 0.15 | |||||
| Male | 67 | 42–92 | ||||
| Female | NR | – | ||||
| Race | 0.69 | |||||
| White | 77 | 46–108 | ||||
| Black | 69 | 21–116 | ||||
| Asian/other | NR | – | ||||
| Ethnicity | 0.23 | |||||
| Non-Hispanic | 76 | 52–100 | ||||
| Hispanic | NR | – | ||||
| Unknown | NR | – | ||||
| CDCS recode | 0.49 | |||||
| 0 | 77 | 58–97 | ||||
| 1 | 87 | 37–137 | ||||
| 2 | 40 | 13–67 | ||||
| Tumor size | – | – | – | 1.012 | 1.00–1.03 | 0.052 |
| Number of tumors | <0.001 | 0.025 | ||||
| 1 | 134 | 82–187 | Reference | |||
| > 1 | 34 | 24–45 | 3.15 | 1.2–8.6 | ||
| Major vascular involvement | 0.011 | 0.19 | ||||
| Absent | 97 | 78–117 | Reference | |||
| Present | 41 | 24–57 | 2.49 | 0.62–9.92 | ||
| Lymphovascular invasion | < 0.001 | 0.29 | ||||
| Absent | 134 | 82–187 | Reference | |||
| Present | 45 | 38–52 | 0.59 | 0.22–1.58 | ||
| AFP | 0.026 | 0.034 | ||||
| Normal | 134 | 60–209 | Reference | |||
| Elevated | 43 | 26–60 | 2.81 | 1.08–7.33 | ||
| Grade | 0.153 | |||||
| Well-differentiated | NR | – | ||||
| Moderately differentiated | 77 | 64–91 | ||||
| Poorly differentiated | 65 | 18–112 | ||||
| Regional node status | <0.001 | 0.023 | ||||
| Negative | 97 | 77–117 | Reference | |||
| Positive | 33 | 24–43 | 2.83 | 1.15–6.96 | ||
| Resection margin | 0.012 | 0.057 | ||||
| R0 | 80 | 62–98 | Reference | |||
| R1 | 29 | 0–66 | ||||
Values in bold represent statistically significant p values
OS overall survival, CI confidence interval, HR hazard ratio, NR not reached, CDCS Charlson–Deyo Comorbidity Score, AFP α-fetoprotein.
Survival Comparison with HCC
A second survival analysis was performed to compare patients with FLC undergoing potentially curative resections with a matched group of patients with HCC (CDCS 0–2, R0 and R1 resections). A log-rank test to compare the OS between the two groups found no significant difference (p = 0.748). When stratified by AFP level, there was a statistically significant difference in OS between the two groups. In patients with FLC and HCC who had normal AFP levels, the median OS was 134 months and 106 months, respectively, whereas those with elevated AFP levels had a median OS of 43 months and 83 months, respectively (p ≤ 0.001) (Fig. 1).
FIG. 1.
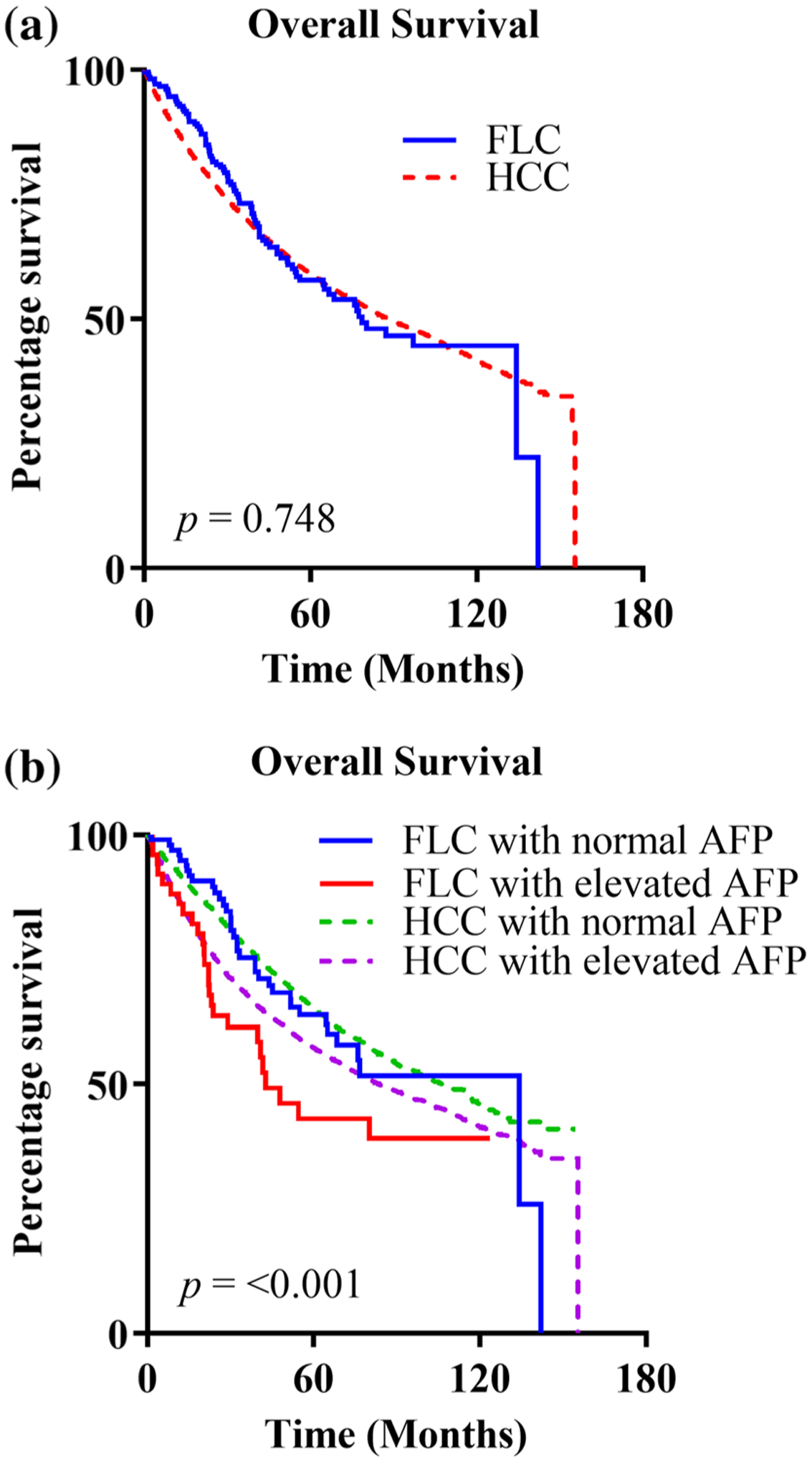
Kaplan–Meier survival curves. a FLC and HCC; b FLC and HCC stratified by AFP. FLC fibrolamellar hepatocellular carcinoma, HCC hepatocellular carcinoma, AFP α-fetoprotein
DISCUSSION
FLC is a rare HCC variant that often affects younger patients with no underlying liver disease. The data that guide current clinical practice are limited to small single- and multi-institutional series, and SEER data. With only small numbers of patients captured in previous studies, there is limited knowledge regarding FLC. Contrary to what has been reported previously, we identified that elevations in serum AFP occur far more often than previously documented, and these elevations are associated with abbreviated OS. Moreover, we found that patients with FLC experience similar OS to that of patients with HCC, with the exception of a subset of patients with FLC who had elevated serum AFP.7
The patient population included in our study is similar to prior studies. The demographic and tumor data of the 496 patients diagnosed with FLC are similar to that reported in other studies with respect to age and sex distribution, as well as rates of vascular invasion, lymph node involvement, and multifocal disease.3,7,10–14 Despite not including patients under the age of 18 years, our findings were also consistent with what has classically been described, with the disease affecting younger patients who do not have underlying liver disease. However, in contrast with the current literature, which reports serum AFP elevations in the range of 0–10%, we identified the percentage of FLC patients with elevated AFP to be at least fourfold higher than the highest reported series. The reasons for this discrepancy are unclear but may also be related to the absence of patients under 18 years of age included in our study, as well as the possibility that the NCDB may capture older patients who are being cared for outside of tertiary referral centers.
AFP levels have a known negative impact on survival for patients with HCC, but this relationship has not previously been demonstrated for patients with FLC.15 We found a significant difference in the median OS for patients with FLC and elevated AFP compared with those with normal AFP levels (43 vs. 134 months). Prior studies suggest there may be an association between poorly differentiated tumors and increasing tumor size with AFP elevation.15,16 Although not an aim of this study, we attempted to identify factors associated with AFP elevations and found moderate and poorly differentiated tumors, increased tumor size, and older age were more likely to be associated with elevated AFP. Perhaps the negative prognostic influence of elevations in serum AFP for patients with FLC had not been previously identified secondary to the small percentage of patients included in prior studies and the inability to capture the older population of patients with FLC. The shorter survival in patients with FLC and elevated AFP likely represents more aggressive tumor biology and a marker for increased risk of recurrence after resection (disease recurs in up to 50% of patients).11
Previous studies have documented improved survival for patients with FLC compared with patients with HCC, with the improved OS attributed to the lack of underlying liver disease and younger patients with FLC.6,7 However, these results must be evaluated with caution as those studies failed to control for underlying liver disease, which can preclude patients from undergoing curative resection. This results in the comparison of a group of patients of whom the majority undergo curative resection with a group of patients of whom the vast majority do not. For example, in the NCDB, 54% of patients diagnosed with FLC underwent a resection, compared with 17% of patients with HCC. When the survival of patients with FLC and HCC who had CDCS scores < 3 and who underwent an R0 and R1 resection was analyzed, we found no difference between groups. We speculate that the tumor biology for adult patients with FLC is similar to patients with HCC, irrespective of the DNAJ-PKAc fusion protein unique to the former.
There are several limitations to this study. The NCDB does not report data on pediatric patients with FLC, which represents an important population affected by this disease as the disease has been reported in patients as young as 12 years of age.13 The NCDB data are collected from different hospitals throughout the US, including both community and academic medical centers. Regional variation and non-standardized management of FLC, inability to standardize the data collection process, and missing/incomplete data may limit the generalizability of the current results. Furthermore, in addition to being retrospective in nature, the data collected in the NCDB did not evaluate for recurrence, details regarding multiple tumors (tumor count, location), or the exact AFP level in the record, but only that it was elevated or not elevated.
CONCLUSION
The number of patients with FLC and elevated serum AFP is much higher than previously thought, and the impact of AFP on the survival of patients with FLC is underreported and underappreciated. More research is needed to determine the relationship of AFP not only to survival but also to the risk of recurrence. This information can be used as a tool by the multidisciplinary team when evaluating the benefits of aggressive surgical resection in suitable patients.
Footnotes
DISCLOSURES
James D. McDonald, Shreya Gupta, Mackenzie L. Shindorf, Lauren A. Gamble, Samantha M. Ruff, Justin Drake, Theo Heller, Jim Y. Wan, Paxton V. Dickson, Evan S. Glazer, Jeremy L. Davis, Jeremiah L. Deneve, Jonathan M. Hernandez declare no conflicts of interest.
REFERENCES
- 1.Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child. 1956;91(2):168–86. [DOI] [PubMed] [Google Scholar]
- 2.Craig JR, Peters RL, Edmondson HA, et al. Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer. 1980;46(2): 372–79. [DOI] [PubMed] [Google Scholar]
- 3.Mavros MN, Mayo SC, Hyder O, et al. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg. 2012;215(6):820–30. [DOI] [PubMed] [Google Scholar]
- 4.Lafaro KJ, Pawlik TM. Fibrolamellar hepatocellular carcinoma: current clinical perspectives. J Hepatocell Carcinoma. 2015;2:151–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groeschl RT, Miura JT, Wong RK, et al. Multi-institutional analysis of recurrence and survival after hepatectomy for fibrolamellar carcinoma. J Surg Oncol. 2014;110(4):412–15. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population-based study. Hepatology. 2004;39(3):798–03. [DOI] [PubMed] [Google Scholar]
- 7.Mayo SC, Mavros MN, Nathan H, et al. Treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma: a national perspective. J Am Coll Surg. 2014;218(2):196–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–81. [Google Scholar]
- 9.Cox DR. Regression models and life-tables. J R Stat Soc Ser B (Methodol). 1972;34(2):187–20. [Google Scholar]
- 10.Ang CS, Kelley RK, Choti MA, et al. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointest Cancer Res. 2013;6(1):3–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita S, Vauthey JN, Kaseb AO, et al. Prognosis of fibrolamellar carcinoma compared to non-cirrhotic conventional hepatocellular carcinoma. J Gastrointest Surg. 2016;20(10):1725–731. [DOI] [PubMed] [Google Scholar]
- 12.Chakrabarti S, Tella SH, Kommalapati A, et al. Clinicopathological features and outcomes of fibrolamellar hepatocellular carcinoma. J Gastrointest Oncol. 2019;10(3):554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darcy DG, Malek MM, Kobos R, et al. Prognostic factors in fibrolamellar hepatocellular carcinoma in young people. J Pediatr Surg. 2015;50(1):153–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaseb AO, Shama M, Sahin IH, et al. Prognostic indicators and treatment outcome in 94 cases of fibrolamellar hepatocellular carcinoma. Oncology. 2013;85(4):197–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva JP, Gorman RA, Berger NG, et al. The prognostic utility of baseline alpha-fetoprotein for hepatocellular carcinoma patients. J Surg Oncol. 2017;116(7):831–40. [DOI] [PubMed] [Google Scholar]
- 16.Blank S, Wang Q, Fiel MI, et al. Assessing prognostic significance of preoperative alpha-fetoprotein in hepatitis B-associated hepatocellular carcinoma: normal is not the new normal. Ann Surg Oncol. 2014;21(3):986–94. [DOI] [PubMed] [Google Scholar]


