Eosinophilic gastrointestinal diseases (EGIDs) are single-organ hypereosinophilic syndromes (HESs) characterized by eosinophilic inflammation involving any segment of the gastrointestinal (GI) tract and include eosinophilic esophagitis, eosinophilic gastritis, eosinophilic gastroenteritis, and eosinophilic colitis. Peripheral eosinophilia (≥500/mm3) is common in patients with EGID, and some patients, especially those with multisegment or serosal involvement, present with peripheral hypereosinophilia (absolute eosinophil count [AEC], ≥1500/mm3).1 It is important to exclude other subtypes of HESs that may progress to multisystem involvement or require alternative therapies in the evaluation of such cases.
HESs encompass a heterogeneous group of rare disorders characterized by hypereosinophilia on 2 examinations separated by at least 1 month and/or organ damage attributed to the eosinophilia. Multiorgan involvement is common, with dermatologic (69%), pulmonary (44%), and GI (38%) accounting for most clinical manifestations. The GI tract is the third most common organ system involved at initial presentation,2 and predominate GI involvement as the presenting symptom in HESs has been reported.3–5 We describe a patient with platelet-derived growth factor alpha (PDGFRA)-positive HES who presented with isolated, biopsy-proven EGID and describe the clinical course and response to therapy.
A 32-year-old man presented in 2012 to an outside institution complaining of abdominal pain and diarrhea. Initial AEC was 3000/mm3 (Figure 1). Esophagogastroduodenoscopy (EGD) and colonoscopy were performed, with biopsies revealing eosinophilic infiltration of the esophagus, terminal ileum, and colon (Figure 2). He was treated for presumed EGID with oral budesonide capsules and cromolyn, with persistent symptoms in the setting of increasing peripheral eosinophilia (AEC, 4800/mm3). Additional evaluation revealed elevated serum tryptase (56.1 μg/L) and B12 (1367 pg/mL) levels. Peripheral blood flow cytometry did not show any evidence of a clonal process, and testing for parasitic infection, including Strongyloides stercoralis IgG, was unrevealing. A bone marrow biopsy was performed. The specimen contained scant marrow but showed increased eosinophils with a focal aggregate of CD25-positive mast cells suggestive (but not diagnostic) of systemic mastocytosis. Fluorescence in situ hybridization (FISH) assay was negative for FIP1L1-PDGFRA, and peripheral blood testing was reportedly negative for D816V KIT. He was treated with prednisone (20–50 mg daily) beginning in early 2017 with persistent symptoms and AEC ranging from 5800 to 10,700/mm3.
FIGURE 1.
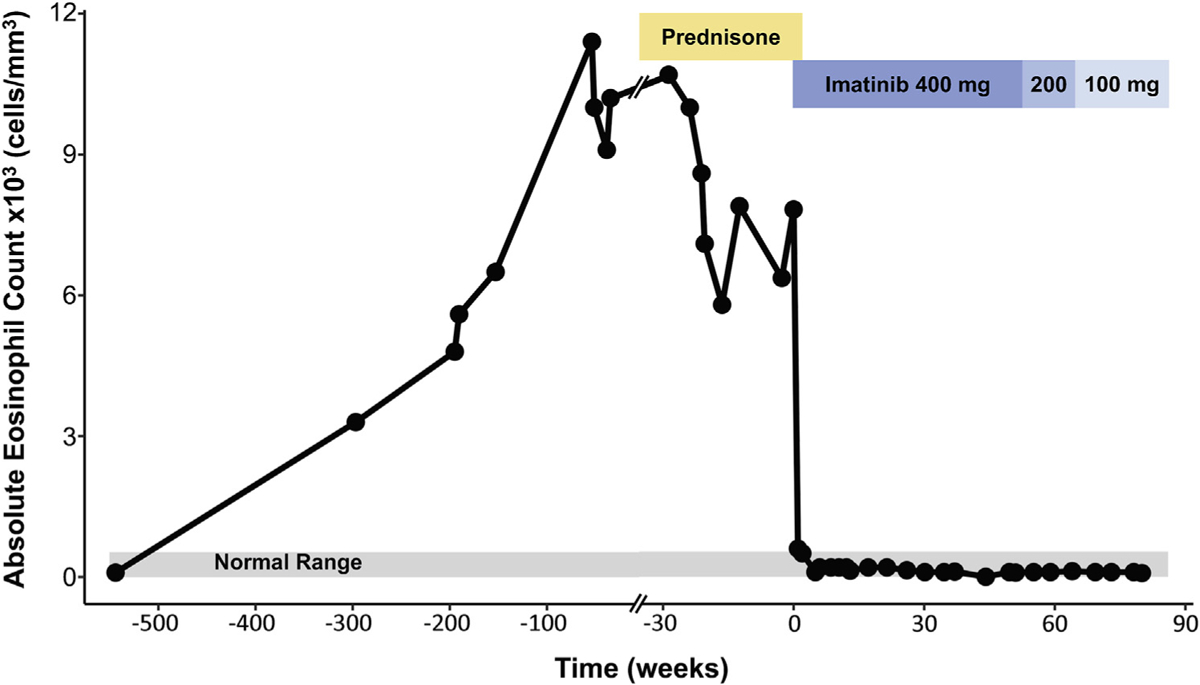
Clinical course and AEC and treatment over time.
FIGURE 2.
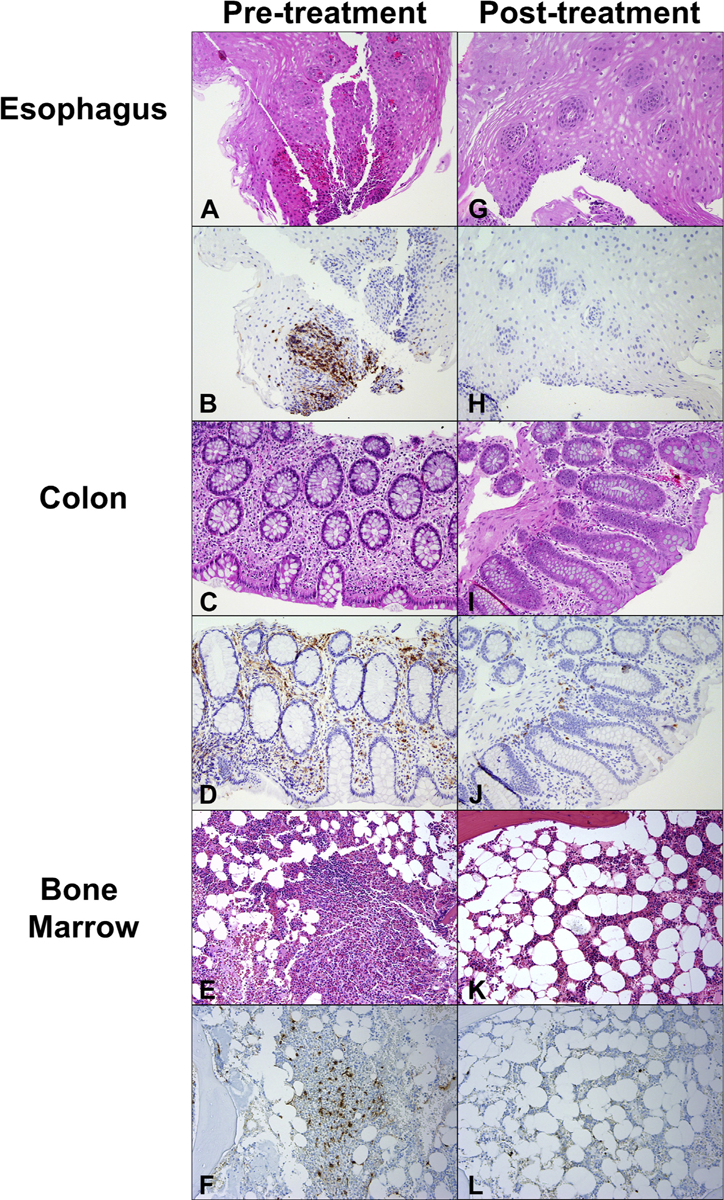
(A) Intraepithelial eosinophilia of the esophagus before imatinib stained with hematoxylin and eosin (H&E). (B) Esophageal eosinophilia stained with eosinophil peroxidase (EPX). (C) Colonic eosinophilic infiltration before treatment demonstrated on H&E staining and EPX staining (D). (E) Bone marrow biopsy stained with H&E. (F) Tryptase stain showing aggregates of mast cells with spindle morphology, lymphocytes, and increased eosinophils before imatinib therapy. (G-J) Resolution of GI tract eosinophilia on imatinib therapy. (K and L) Resolution of bone marrow findings on imatinib therapy. Magnification is 20× for all sections.
He was referred to the National Institutes of Health and enrolled on protocols NCT00001406 and NCT00044304 after giving written informed consent. At his initial visit on October 4, 2017, he complained of persistent abdominal pain and diarrhea. Physical examination was notable only for splenomegaly, which was subsequently confirmed by computed tomography scan (16 cm). Laboratory assessment revealed persistent eosinophilia (AEC, 6300/mm3) and elevated serum tryptase level (19.7 ng/mL). Despite a 5-year interval from the time of initial presentation, the patient’s clinical symptoms were stable, and additional laboratory and diagnostic testing, including routine chemistries, serum troponin, EKG, echocardiography, pulmonary function tests, and computed tomography scan, showed no evidence of new end-organ involvement. Repeat bone marrow biopsy confirmed normal cellularity with increased eosinophils and atypical mast cell aggregates (Figure 2, E and F). RT-PCR detected the FIP1L1-PDGFRA fusion rearrangement in both peripheral blood and bone marrow. Allele-specific PCR testing for D816V KIT was negative. Imatinib therapy (400 mg daily) resulted in clinical improvement and resolution of peripheral eosinophilia (AEC, 200/mm3) within 1 week of initiation of therapy (NCT00044304), and prednisone was tapered and discontinued. Bone marrow examination performed after 6 weeks on imatinib monotherapy was normo-cellular without eosinophilia or mast cell aggregates (Figure 2, K and L). Repeat EGD and colonoscopy with biopsies at the same visit demonstrated normal architecture of the esophagus, stomach, and small and large intestine, with the notable absence of eosinophils (Figure 2, G–J). Molecular testing for FIP1L1-PDGFRA in blood and bone marrow became negative by 13 weeks after initiation of imatinib therapy, and the imatinib dose was decreased to 100 mg daily in February 2019 (Figure 1). The patient has continued to do well, maintaining clinical, hematologic, and molecular remission since that time.
Although GI symptoms (abdominal pain and diarrhea) were described in as many as 16% (7 of 44) of patients with PDGFRA-positive HES in one series,6 biopsy-proven eosinophilic infiltration of the GI tract has only rarely been documented. The present case provides evidence that isolated eosinophilic GI involvement can occur in patients with PDGFRA-positive HES and may mimic the presentation of EGID with peripheral eosinophilia. Clues to the diagnosis include the lack of response to corticosteroid therapy and other features of myeloid neoplasms, including splenomegaly and elevated serum tryptase and B12 levels.7 Laboratory evaluation should include testing for the FIP1L1-PDGFRA gene fusion product via FISH or RT-PCR, although false-negative results can occur, particularly with FISH assays (as in our patient).8 Although PDGFRA-positive HES typically follows an aggressive course with high mortality in the absence of treatment, the FIP1L1-PDGFRA fusion is very sensitive to imatinib mesylate, with nearly all patients achieving durable clinical, hematological, and molecular remission.2,6,7,9 Thus, imatinib therapy should be instituted promptly even in asymptomatic patients once the diagnosis is confirmed. In situations in which confirmatory testing is unavailable, treatment should be initiated on the basis of clinical suspicion.
Clinical Implications.
An underlying eosinophilic myeloid neoplasm should be considered in patients with eosinophilic gastrointestinal disease and peripheral eosinophilia refractory to corticosteroid therapy.
Acknowledgments
This work was supported in part by the Divisions of Intramural Research at the National Institute of Allergy and Infectious Diseases and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health (under contract no. HHSN261200800001E). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Footnotes
Conflicts of interest: We confirm that there are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome. Informed consent was obtained from all individual participants included in this report.
REFERENCES
- 1.Lee J, Dierkhising R, Wu T-T, Alexander J, Weiler C. Eosinophilic gastrointestinal disorders (EGID) with peripheral eosinophilia: a retrospective review at Mayo Clinic. Dig Dis Sci 2011;56:3254–61. [DOI] [PubMed] [Google Scholar]
- 2.Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol 2009;124: 1319–1325.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeon YW, Hong SJ, Kim HJ, Han JP, Kim HK, Ko BM, et al. A hypereosinophilic syndrome presenting as eosinophilic colitis. Clin Endosc 2012;45:444–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitini V, Sturniolo G, Cavallari V, Arrigo C. Rapid reversion of eosinophilic gastroenteritis associated with FIP1L1-PDGFRA fusion after targeted therapy with imatinib. Br J Haematol 2006;132:123. [DOI] [PubMed] [Google Scholar]
- 5.Nanagas VC, Kovalszki A. Gastrointestinal manifestations of hypereosinophilic syndromes and mast cell disorders: a comprehensive review. Clin Rev Allergy Immunol 2019;57:194–212. [DOI] [PubMed] [Google Scholar]
- 6.Legrand F, Renneville A, Macintyre E, Mastrilli S, Ackermann F, Cayuela JM, et al. The spectrum of FIP1L1-PDGFRA-associated chronic eosinophilic leukemia: new insights based on a survey of 44 cases. Medicine 2013;92:e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klion AD, Noel P, Akin C, Law MA, Gilliland DG, Cools J, et al. Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness. Blood 2003;101: 4660–6. [DOI] [PubMed] [Google Scholar]
- 8.Olsson-Arvidsson L, Norberg A, Sjogren H, Johansson B. Frequent false-negative FIP1L1-PDGFRA FISH analyses of bone marrow samples from clonal eosinophilia at diagnosis. Br J Haem 2020;188:e76–9. [DOI] [PubMed] [Google Scholar]
- 9.Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med 2003;348:1201–14. [DOI] [PubMed] [Google Scholar]


