Abstract
Introduction:
The aim of this study was to perform a quantitative assessment of the prostate anatomy with a focus on the relation of prostatic urethral anatomic variation to urinary symptoms.
Methods:
This retrospective study involved patients undergoing magnetic resonance imaging for prostate cancer who were also assessed for lower urinary tract symptoms. Volumetric segmentations were utilized to derive the in vivo prostatic urethral length and urethral trajectory in coronal and sagittal planes using a piece-wise cubic spline function to derive the angle of the urethra within the prostate. Association of anatomical factors with urinary symptoms was evaluated using ordinal univariable and multivariable logistic regression with IPSS score cutoffs of ≤7, 8–19, and >20 to define mild, moderate, and severe symptoms, respectively.
Results:
A total of 423 patients were included. On univariable analysis, whole prostate volume, transition zone volume, prostatic urethral length, urethral angle, and retrourethral volume were all significantly associated with worse urinary symptoms. On multivariable analysis prostatic urethral length was associated with urinary symptoms with a normalized odds ratio of 1.5 (95% confidence interval 1.0–2.2, p = 0.04). In a subset analysis of patients on alpha blockers, maximal urethral angle, transition zone volume as well as urethral length were all associated with worse urinary symptoms.
Conclusion:
Multiple parameters were associated with worse urinary symptoms on univariable analysis, but only prostatic urethral length was associated with worse urinary symptoms on multivariable analysis. This study demonstrates the ability of quantitative assessment of prostatic urethral anatomy to predict lower urinary tract symptoms.
Keywords: Prostate, Prostatic urethra, Benign prostatic hyperplasia, MRI, Lower urinary tract symptoms
INTRODUCTION
Prostatic tissue surrounding the proximal portion of the male urethra commonly undergoes age-related hyperplasia resulting in enlargement of the prostate (1). This benign growth can impede the flow of urine and is a common source of lower urinary tract symptoms (LUTS), which include symptoms of obstruction and bladder irritation (2). LUTS are observed in over 50% of men over the age of 50 (3) with 24% of men in in 60–69 age group reporting moderate to severe symptoms (4).
The impact of benign prostatic enlargement on urinary symptoms is most often assessed by evaluating the volume of the entire prostate (5). However, factors other than overall prostate size have also been shown to correlate with LUTS including the size of the transition zone and the presence of tissue posterior to the urethra commonly referred to as the median lobe (5). Magnetic resonance imaging (MRI) provides excellent depiction of prostatic anatomy and is most frequently used for the detection of prostate cancer. MRI has also been utilized to further characterize the enlargement of specific zones of the prostate although this is usually reserved for the research setting (6). Such characterization is usually subjective but is amenable to more quantitative approaches (7). The aim of this project was to provide a quantitative methodology to comprehensively evaluate the intraprostatic urethral anatomy in relation to prostate zonal volumes, total prostatic urethral length, and deviation from a direct trajectory (angular path) using 3D urethral volumes derived from MRI. Additionally, we assess the relationship between these quantitative attributes of the prostatic urethra and urinary symptoms.
PATIENTS AND METHODS
Patient Selection and MRI Protocol
The patient population utilized in this study was derived from a cohort of patients undergoing prostate MRI on an IRB-approved protocol evaluating men with prostate cancer (18-C-0017) from 2/2018 to 11/2018. This protocol specifically allows for computational analysis of the prostate images, and it began accrual in 2/2018. Exclusion criteria included men who had previously undergone treatment of their prostate cancer (including androgen deprivation therapy, radiation therapy, etc.) or presented for MRI with an indwelling foley catheter. All MRI studies were performed with a 3T MRI with a 16-channel cardiac coil utilizing parallel imaging (Philips Medical Systems, Best, the Netherlands). Patients undergoing their initial MRI were scanned with an endorectal coil tuned to 127.8 MHz and filled with 45 mL of galden perfluorinated fluid (Solvay Specialty Polymers, Milan, Italy). The following series were obtained: T1-weighted, triplanar T2-weighted turbo-spin-echo, apparent diffusion coefficient maps based on b-value sweeps of 0–750, high b-value weighted images with b-values of 2000 s/mm2 for patients with an endorectal coil and 1500 s/mm2 for patients without an endorectal coil. Axial contrast-enhanced sequences were obtained before, during, and after a single dose of gadoterate dimeglumine (Dotarem; Guerbet-US Princeton, NJ) administered via peripheral vein at a dose of 0.1 mmol/kg using a mechanical injector (Spectris MR Injection System Medrad). Only axial T2-weighted sequences were considered for analysis in this study.
Chart review was performed to obtain clinical information including age, prostate-specific antigen, prior benign prostatic hyperplasia (BPH) treatment, and use of alpha blockers and 5-alpha reductase inhibitors. Urinary symptoms were scored utilizing the American Urological Association Symptom Index (2) provided they were within 2 years of the MRI. Symptom scores were grouped into three categories: mild (≤7), moderate (8–19), and severe (≥20). Lesions concerning for prostate cancer, scored according to PI-RADS v2.0 were also noted.
Urethral Segmentation
All segmentations were performed using the DynaCAD (Invivo Corp) software on the axial T2 series by a radiologist with >10 years of experience in the field or a urologist with 1 year of training in prostate MRI segmentation and specific supervised training in urethral segmentation. Both were blinded to urinary symptoms scores during the segmentation process. Prior to urethral segmentation, the boundaries of the entire prostate and transition zone were delineated and saved as separate files. Coronal and sagittal reconstructions from the axial sequence were also utilized to assist in defining the boundaries of the prostate. Urethral segmentation was completed in 4 steps: (1) The proximal verumontanum was located and a segmentation sphere was placed in this location (Fig 1a). (2) The view was converted to the sagittal reconstruction and the segmentation sphere was enlarged in a straight line between distal prostatic urethra and the bladder neck (Fig 1b). (3) The angle was then contoured on the sagittal reconstruction, using the dedicated sagittal T2-weighted series for assistance in another window (Fig 1c). (4) The contour was then adjusted on the coronal and axial planes using the dynamic contrast enhanced series to localize the urethra in cases where the course was not clear, particularly in the proximal urethra (Fig 1d). All segmentations generated in DynaCAD were saved to MIPAV VOI polygon point files.
Figure 1.
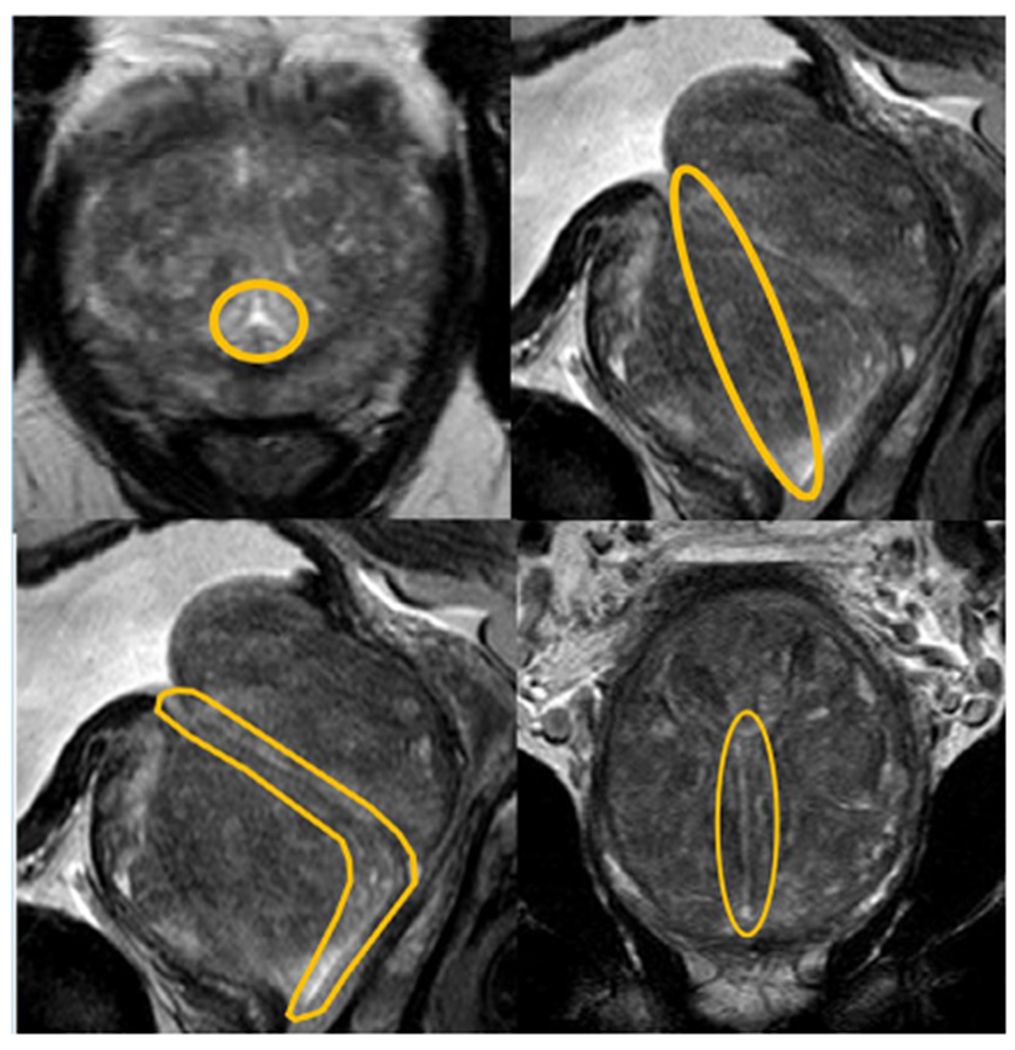
Prostatic urethra segmentation technique.
A systematic approach was taken to the segmentation of the prostatic urethra. Segmentation began by inserting a segmentation circle on the axial cut at the level where the verumontanum was clearly visible (A). This segmentation circle was then extended to the bladder neck in a straight line (b). The contour of the urethra was estimated on the sagittal section (c) and adjusted in the coronal section (d). The final contour was then adjusted in the axial section (a), using the dynamic contrast enhanced series to elucidate the location of the urethra when not obvious on the T2 series. (Color version of figure is available online.)
Calculation of Volumes, Angles, and Lengths
To calculate the volume of the prostate and transition zone, DICOM files were converted to NIFTI format using the open source python library dicom2nifti (https://github.com/icometrix/dicom2nifti) and binary NIFTI masks of whole prostate, TZ, and urethra were created using custom python scripts. The resolution of each scan in the x, y, and z planes was obtained from DICOM headers to obtain the voxel volume, and the volume of each zone was calculated by summing the points of the NIFTI mask and multiplying by the voxel volume.
The prostatic urethra was characterized by its length and trajectory in both sagittal and coronal planes. To model this, urethra polygon segmentation files were imported as an inclusive binary mask to MATLAB. The center-of-mass volume of urethra track length was derived from the center of mass points within each slice and a radius determined by the ratio of x-y resolution to z resolution. Using the center-of-mass volume, a piece-wise cubic spline function was fit to map the path of urethra across all visible slices using splinefit function (8). To identify deviations from anatomical expectation (a straight line traversing the entire volume of the prostate), points of inflection (curvature) were defined as the point where the urethral trajectory maximally deviated from a straight-line plane between the apical and basal most points of the prostate. The angle between the maximal inflection point and apical/base points were used to define angular deviation in both sagittal and coronal planes. Finally, the total piecewise length from apex to base in three dimensions was also reported. The original urethra segmentation and center-of-mass volume of urethra track were visualized using surface mesh derived from boundary points of each object (Fig 2).
Figure 2.
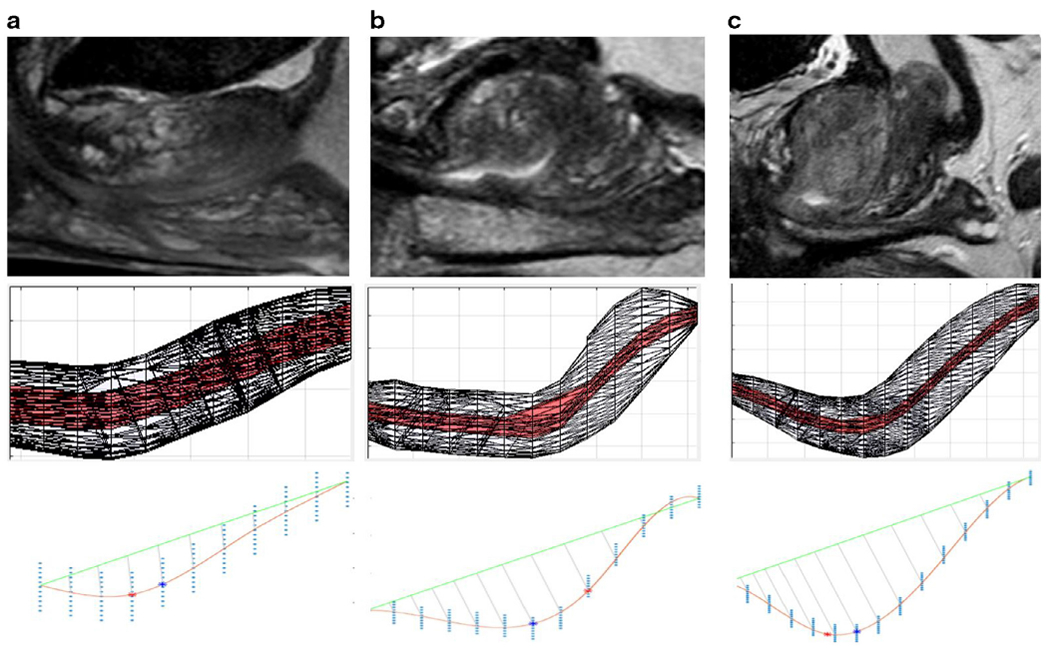
Anatomic variation impact on surface mesh and curvature measurement.
This is a substantial amount of variability in the course of the prostatic urethra, with Figure 2a demonstrating a straight urethra, Figure 2b demonstrating a moderate curve, and Figure 2c demonstrating a severe curve with lengthening of the urethra. The second row of this figure demonstrates a 3D mesh visualization of the urethra with the central portion of the urethra demonstrated in red. The third row demonstrates the maximal curvature with the lower red line compared with the straight-line distance demonstrated by the green line, with the point of maximal curvature demonstrated as the red dot (inflection-based calculation) and blue dot (distance-based calculation). (Color version of figure is available online.)
To characterize the impact of prostatic volume on the displacement and trajectory of the prostatic urethra, a 2D sagittal plane from the apical-most point of the prostatic urethra and the maximal point of inflection was created to measure the burden of both transition zone and whole prostate volume anterior and posterior to the observed urethral deviation (Fig 3a). The volume posterior to this plane was termed the “retrourethral burden” of both the transition and whole prostate volumes, which is a method for quantification of the entity commonly known as the “median lobe” (Fig 3b).
Figure 3.
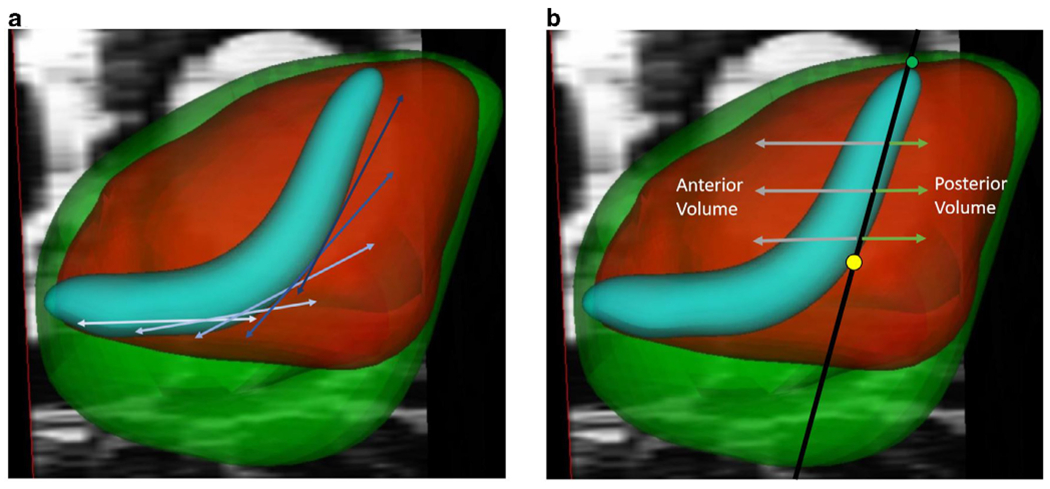
Measurement of prostatic urethral length and rectourethral volume.
A piecewise spline function was utilized to determine the length of the entire urethra taking deviations in the urethra into account (a). A delineation to determine the tissue posterior to the urethra is seen in Figure 2b. This median lobe correlate was made by creating a plane between the superior point of urethra (green dot) and the maximum point of inflection (yellow dot), with the volume of the prostate posterior to this considered as part of the retrourethral volume. (Color version of figure is available online.)
Statistical Analysis
Statistical analysis was performed using R version 3.5.1 (http://www.R-project.org). Univariable ordinal regression analysis was performed by evaluating each variable independently against IPSS score severity category. Multivariable ordinal regression analysis was performed including the statistically significant variables from the univariable analysis. All data were scaled as part of the regression to ensure relative odds ratios were consistent across variables.
Code Availability
All python, MATLAB, and R scripts used in the analysis of this paper can be found in the following Gitub repository (https://github.com/NIH-MIP/prostatic_urethra).
RESULTS
Demographics and Clinical Data
A total of 423 patients met study inclusion criteria and all intraprostatic structures could be segmented for analysis. An IPSS score could be found for 380 patients. The average age of the patients was 66 years (range 16–87). The median prostate-specific antigen value was 6.71 ng/mL (range of 0.8–429.5 ng/mL). Of the patients with IPSS scores, 109 (29%) were listed as taking alpha blockers. The most common alpha blocker was Tamsulosin (60%) followed by Alfuzosin (12%), Silodosin (11%), Unspecified (11%), Terazosin (3%), and Doxazosin (3%). Fourteen patients (3%) had undergone a prior transurethral resection for BPH. A total of 41 patients with IPSS scores (11%) were noted to be on 5-alpha reductase inhibitors with 28 patients taking finasteride (68%), 9 patients (22%) taking dutasteride, 3 patients (<1%) taking an unspecified 5-alpha reductase inhibitor, and 1 patient (7%) taking saw palmetto. Twenty-two patients (6%) were on combination alpha blocker/5-alpha reductase therapy.
Quantitative Measurements
The quantitative metrics from all 423 patients are summarized in Table 1. The median whole prostate size was 62 cc (range 14–271 cc). The median transition zone size was 35 (range 3–222 cc). The median 3D length was 4.8 cm with a range 2.2–10.2 cm. On average the proportion of prostatic volume anterior to the urethral segment from apex to the point of maximal angulation relative to the remaining volume posterior was 0.4 for both the whole prostate as well as the transition zone. A total of 155 of the patients (37%) were imaged using an endorectal coil. There were a total of 1247 lesions detected (182 PIRAS 2 (15%), 468 PIRADS 3 (38%), 404 PIRADS 4 (32%), and 173 PIRADS 5 (14%). A total of 387 lesions (31%) were in the transition zone, 832 (67%) were in the peripheral zone, and 28 (2%) were in the central zone.
TABLE 1.
Summary of Measurements
| Median | Range | IQR | |
|---|---|---|---|
| Prostate volumes (cc) | |||
| Whole prostate | 62.6 | (14.8–271.2) | (44.2–90.8) |
| Transition zone | 36.2 | (2.9–222.4) | (19.9–59.0) |
| Prostatic urethral length (mm) | |||
| Sagittal length | 47.9 | (21.6–101.3) | (40.0–55.6) |
| Coronal length | 42.0 | (18.0–81.0) | (36.1–48.0) |
| 3D length | 48.0 | (21.5–102.0) | (40.0–55.9) |
| Urethral angle (degrees) | |||
| Sagittal angle | 38 | (9–77) | (29–47) |
| Coronal angle | 8 | (0–52) | (4–14) |
| Whole prostate delineation (cc) | |||
| Volume anterior | 40.8 | (0.0–248) | (25.3–60.7) |
| Volume posterior | 21.9 | (4.5–147.9) | (15.5–30.7) |
| Anterior/posterior ratio | 0.4 | (0.1–1.0) | (0.3–0.4) |
| Transition zone delineation (cc) | |||
| Volume anterior | 22.1 | (0.0–206.4) | (11.3–38.5) |
| Volume posterior | 11.7 | (0.6–59.5) | (7.2–19.1) |
| Anterior/posterior ratio | 0.4 | (0.1–1.0) | (0.3–0.5) |
IQR, interquartile range.
Symptoms
Of the 380 patients with IPSS scores, 130 (34%) had mild symptom, 196 (52%) had moderate symptoms, and 54 (14%) had severe urinary symptoms. Univariable and multivariable ordinal linear regression analysis results relating quantitative measures of the prostatic urethra and urinary symptoms are shown in Table 2. On univariable analysis, nearly all quantitative variables demonstrated a statistically significant relationship with urinary symptoms including whole prostate and transition zone volume, urethra length, and volume of prostate posterior to the urethra (aka retrourethral prostate, which is a median lobe corollary). However, on multivariable analysis of the entire cohort, only prostatic urethral length maintained a statistically significant relationship with urinary symptoms with a normalized odds ratio of 1.5 (95% confidence interval 1.0–2.2, p = 0.04).
TABLE 2.
Univariable and Multivariable Analysis
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| OR (95%) | p Value | OR (95%) | p Value | |
| Prostate volumes (cc) | ||||
| Whole prostate | 1.5 (1.3–1.9) | <0.001 | 1.5 (0.5–4.3) | 0.443 |
| Transition zone | 1.4 (1.2–1.8) | <0.001 | 0.5 (0.2–1.0) | 0.067 |
| Prostatic urethral length (mm) | ||||
| Sagittal length | 1.6 (1.3–2.0) | <0.001 | ||
| Coronal length | 1.6 (1.3–1.9) | <0.001 | ||
| 3D length | 1.6 (1.3–2.0) | <0.001 | 1.5 (1.0–2.2) | 0.038 |
| Urethral angle (degrees) | ||||
| Sagittal angle | 1.2 (1.0–1.5) | 0.018 | 0.9 (0.7–1.1) | 0.391 |
| Coronal angle | 1.2 (1.0–1.5) | 0.027 | ||
| Median lobe corollary (cc) | ||||
| Volume posterior urethra | 1.4 (1.1–1.7) | 0.003 | 1.5 (0.8–2.9) | 0.184 |
| Anterior/posterior ratio | 0.8 (0.6–0.9) | 0.022 | 0.7 (0.5–1.1) | 0.101 |
Bold numbers indicate statistical significance.
Patients on alpha blockers had a higher median IPSS score than patients not on alpha blockers (14 vs 9, respectively, p < 0.001). Separate statistical analysis for patients on and off alpha blockers is demonstrated in Table 3. For patients on alpha blockers, prostatic urethral length was the factor with the strongest association with urinary symptoms with a normalized odds ratio of 3.5 (confidence interval 1.5–8.8, p = 0.004). Urethral angle was also positively associated with urinary symptoms whereas transition zone volume was inversely associated with urinary symptoms when controlling for all anatomical characteristics in multivariable model. For patients not on alpha blockers, there was no statistically significant relationship between any variables and urinary symptoms.
TABLE 3.
Multivariable Analysis for Patients on and off Alpha Blockers
| On Alpha-Blockers (N = 109) |
No Alpha-Blockers (N = 283) |
|||
|---|---|---|---|---|
| OR (95%) | p Value | OR (95%) | p Value | |
| Whole prostate volume | 1.7 (0.2–19.0) | 0.649 | 1.4 (0.4–4.9) | 0.560 |
| Transition zone volume | 0.2 (0.1–0.8) | 0.030 | 0.7 (0.3–1.8) | 0.506 |
| Prostatic urethral length | 3.5 (1.5–8.5) | 0.004 | 1.0 (0.3–1.8) | 0.891 |
| Maximum urethral angle | 1.7 (1.1–2.6) | 0.022 | 1.0 (0.8–1.3) | 0.863 |
| Volume posterior prostate | 1.8 (0.5–7.6) | 0.392 | 1.3 (0.7–2.8) | 0.444 |
| Anterior/posterior TZ ratio | 0.6 (0.3–1.5) | 0.294 | 0.7 (0.4–1.3) | 0.265 |
Bold numbers indicate statistical significance.
DISCUSSION
The majority of men will experience age-related proliferation of stromal and epithelial elements of prostatic tissue surrounding the urethra at some point in their life (9). Population-based studies demonstrate a concomitant increase in LUTS with age (10) and commensurate reductions in urinary flow (11). However, the relationship between prostate volume and LUTS is unpredictable. For instance, it is widely held that LUTS is caused by obstruction of the urethra by an enlarged prostate (12). This is supported by the higher prevalence of urinary retention in men with larger prostates and the correlation of lower peak flow rates with increased risk of urinary retention (13). However, multiple subsequent studies demonstrate only a weak association between the size of the prostate and urinary symptoms in men (11,14), and some studies found no association between prostate size and urinary symptoms (15).
There have been multiple studies evaluating anatomical characteristics of intra-prostate structures to explain the co-occurrence of LUTS and prostatic hyperplasia beyond total prostate volume. Attention has been paid to the volume of the transition zone however the correlation with LUTS is also weak (16,17). Prostatic urethral length has been correlated with symptoms (18). The angle of the urethra has also been measured via ultrasound and found to have a correlation with urinary flow (19) and symptoms (20). Most studies thus far have been limited to ultrasound assessment, which has a high degree of inter-observer and intraobserver variation (21). Additionally, calculation of volumes by ultrasound relies on an approximation of prostatic shape to an ellipsoid which may differ significantly from direct volumetric assessment by true anatomic segmentation (22).
In this study, we sought to utilize volumetric segmentation of the prostate, transition zone, and urethra on MRI to provide quantitative metrics to correlate with LUTS. Benign growth of prostatic tissues is known to occur predominately in the transition zone of the prostate with some patients having more growth laterally (known as the lateral lobes) while other patients having more growth in retrourethral tissue, (known as the “median lobe”) (23). Growth of the retrourethral tissue can protrude into the bladder (intravesicle prostatic protrusion) and be a source of obstruction (1), and therefore, it is important to quantify the amount of tissue in this space. To our knowledge, this is the first description of a methodology defined to quantify the volume of the retrourethral tissue in an objective manner. Quantification of the nonlinear prostatic urethral length as well as a quantitative measurement of the maximum urethral angle allowed for an objective assessment of multiple aspects of the prostatic urethra derived from the anatomic segmentation.
Consistent with prior studies (16,17,19,20), there was a statistically significant positive relationship between urinary symptoms and each of the following variables on univariable analysis: whole prostate volume, transition zone volume, prostatic urethral length, urethral angle, and retrourethral volume. However, in a multivariable model with all patients, only prostatic urethral length had a statistically significant relationship with symptoms with a normalized hazard ratio of 1.5. This is consistent with the known principles of fluid dynamics where resistance increases directly with length and flow varies inversely with resistance (24). This finding also explains the weak relationship between whole prostate volume and transition zone volume and urinary symptoms on a population basis: larger prostates may have more BPH, but it is really the length of the urethra that is most strongly associated with urinary symptoms when controlling for other variables.
Alpha blockers have become a mainstay of therapy for men with mild to moderate LUTS (25). Alpha blockers improve symptoms by an average of 30–40% (26), but some patients fail medical treatment (27). For patients on alpha blockers, prostatic urethral length had a stronger association with worse urinary symptoms with an odds ratio of 3.5. Two other factors were also statistically significant in the multivariable model: maximum urethral angle (OR 1.7) and TZ volume (0.2). The odds ratio of the TZ volume must be interpreted in the context of the multivariable model given that TZ volume odds ratio is greater than 1 on univariable analysis and less than 1 on multivariable analysis. Specifically, this model describes a situation where the size of the whole prostate constant as well as the volume of the retrourethral tissue is held constant. In this setting, an individual who has a relatively small transition zone with a steep angle creating a long urethra is more likely to have severe urinary symptoms despite alpha blockade use. This is a phenomenon that has been described as a “high bladder neck,” which has been postulated to be a source of obstruction in men with LUTS (19). Identification of these anatomical factors related to treatment failure allows for improved monitoring and consideration for earlier intervention.
There are important limitations to this study. This is a single institution retrospective evaluation, and IPSS scores for 11% of the data were not able to be located. Furthermore, there was no systematic measurement of urinary flow, post-void residuals, and no urodynamic evaluation was performed to assess the effect of quantitative measurements included in this study on the presence of anatomical obstruction. Finally, we did not include information regarding the radius of the urethra given the urethra was in a collapsed state during the MRI and therefore the true luminal volume during urination could not be assessed.
It should be noted that all analysis performed in this study required only one source of input: segmentation of MRI data. While MRI is not recommended for BPH per se, prostate MRI data are increasingly available as it is used to diagnose cancer, and for those patients with concomitant urinary symptoms, a segmentation-based methodology could provide a substantial amount of additional information, particularly for those patients who are not responding to alpha blockade. Furthermore, prostatic urethral length can also be measured during a routine cystoscopy or transrectal ultrasound.
In summary, this study demonstrates a quantitative method to evaluate the prostate on MRI. There is substantial variation in prostate and transition zone size and location of enlargement, leading to considerable variation in the prostatic urethral length and maximal angle in the sagittal plane. While multiple anatomic features were correlated with outcome on univariable analysis, on multivariable analysis prostatic urethral length emerged as the single variable most associated with more severe urinary symptoms. This study demonstrates the value of quantitative analysis of anatomic features for evaluation of BPH.
FUNDING
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Abbreviations:
- BPH
benign prostatic hyperplasia
- MRI
magnetic resonance imaging
- LUTS
lower urinary tract symptoms
- TZ
transition zone
- IPSS
International Prostate Symptom Score
- PSA
prostate-specific antigen
- IRB
institutional review board
Footnotes
DECLARATION OF COMPETING INTEREST
No author has any conflict of interest.
REFERENCES
- 1.Kuo HC. Clinical prostate score for diagnosis of bladder outlet obstruction by prostate measurements and uroflowmetry. Urology 1999; 54:90–96. [DOI] [PubMed] [Google Scholar]
- 2.Barry MJ, Fowler FJ Jr, O’leary MP, et al. The American Urological Association Symptom Index for benign prostatic hyperplasia. J Urol 2017; 197:S189–S197. [DOI] [PubMed] [Google Scholar]
- 3.Irwin DE, Milsom I, Kopp Z, et al. Prevalence, severity, and symptom bother of lower urinary tract symptoms among men in the EPIC study: impact of overactive bladder. Eur Urol 2009; 56:14–20. [DOI] [PubMed] [Google Scholar]
- 4.Boyle P, Robertson C, Mazzetta C, et al. The prevalence of lower urinary tract symptoms in men and women in four centres. The UrEpik study. BJU Int 2003; 92:409–414. [DOI] [PubMed] [Google Scholar]
- 5.Simon RM, Howard LE, Moreira DM, et al. Does prostate size predict the development of incident lower urinary tract symptoms in men with mild to no current symptoms? Results from the REDUCE trial. Eur Urol 2016; 69:885–891. [DOI] [PubMed] [Google Scholar]
- 6.Wasserman NF, Spilseth B, Golzarian J, et al. Use of MRI for lobar classification of benign prostatic hyperplasia: potential phenotypic biomarkers for research on treatment strategies. AJR Am J Roentgenol 2015; 205:564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wasserman NF, Niendorf E, Spilseth B. Precision and accuracy of magnetic resonance imaging for lobar classification of benign prostatic hyperplasia. Abdom Radiol 2019; 44:2535–2544. [DOI] [PubMed] [Google Scholar]
- 8.Lundgren J: SPLINEFIT. Available at: https://www.mathworks.com/matlabcentral/fileexchange/71225-splinefit, Accessed June 30, 2019.
- 9.Lepor H Pathophysiology of benign prostatic hyperplasia in the aging male population. Rev Urol 2005; 7(Suppl 4):S3–S12. [PMC free article] [PubMed] [Google Scholar]
- 10.Maserejian NN, Chen S, Chiu GR, et al. Incidence of lower urinary tract symptoms in a population-based study of men and women. Urology 2013; 82:560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barry MJ, Cockett A, Holtgrewe HL, et al. Relationship of symptoms of prostatism to commonly used physiological and anatomical measures of the severity of benign prostatic hyperplasia. J Urol 1993; 150:351–358. [DOI] [PubMed] [Google Scholar]
- 12.Lepor H, Roehrborn CG. Historical overview of medical therapy for benign prostatic hyperplasia. Rev Urol 2005; 7(Suppl 4):S1–S2. [Google Scholar]
- 13.Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol 1997; 158:481–487. [DOI] [PubMed] [Google Scholar]
- 14.Girman CJ, Jacobsen SJ, Guess HA, et al. Natural history of prostatism: relationship among symptoms, prostate volume and peak urinary flow rate. J Urol 1995; 153:1510–1515. [DOI] [PubMed] [Google Scholar]
- 15.Deebajah M, Bazzi M, Walton E, et al. Prostate volume measured by magnetic resonance imaging is not a predictor of lower urinary tract symptoms. J Family Med Prim Care 2019; 8:1370–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corica FA, Jacobsen SJ, King BF, et al. Prostatic central zone volume, lower urinary tract symptom severity and peak urinary flow rates in community dwelling men. J Urol 1999; 161:831–834. [PubMed] [Google Scholar]
- 17.Kaplan SA, Te AE, Pressler LB, et al. Transition zone index as a method of assessing benign prostatic hyperplasia: correlation with symptoms, urine flow and detrusor pressure. J Urol 1995; 154:1764–1769. [PubMed] [Google Scholar]
- 18.Kim BS, Ko YH, Song PH, et al. Prostatic urethral length as a predictive factor for surgical treatment of benign prostatic hyperplasia: a prospective, multiinstitutional study. Prostate Int 2019; 7:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho KS, Kim JH, Kim DJ, et al. Relationship between prostatic urethral angle and urinary flow rate: its implication in benign prostatic hyperplasia pathogenesis. Urology 2008; 71:858–862. [DOI] [PubMed] [Google Scholar]
- 20.Hou C-P, Chen C-L, Lin Y-H, et al. Prostatic urethral angle might be a predictor of treatment efficacy of α-blockers in men with lower urinary tract symptoms. Drug Des Devel Ther 2014; 8:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews GJ, Motta J, Fracehia JA. The accuracy of transrectal ultrasound prostate volume estimation: clinical correlations. J Clin Ultrasound 1996; 24:501–505. [DOI] [PubMed] [Google Scholar]
- 22.Garvey B, Turkbey B, Truong H, et al. Clinical value of prostate segmentation and volume determination on MRI in benign prostatic hyperplasia. Diagn Interv Radiol 2014; 20:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasserman NF. Benign prostatic hyperplasia: a review and ultrasound classification. Radiol Clin North Am 2006; 44(5):689–710. [DOI] [PubMed] [Google Scholar]
- 24.Sutera SP, Skalak R. The history of Poiseuille’s law. Annu Rev Fluid Mech 1993; 25:1–20. [Google Scholar]
- 25.Blankstein U, Asseldonk BV, Elterman DS. BPH update: medical versus interventional management. Can J Urol 2016; 23:10–15. [PubMed] [Google Scholar]
- 26.Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of α1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol 1999; 36:1–13. [DOI] [PubMed] [Google Scholar]
- 27.Hong SJ, Ko WJ, Kim SI, et al. Identification of baseline clinical factors which predict medical treatment failure of benign prostatic hyperplasia: an observational cohort study. Eur Urol 2003; 44:94–100. [DOI] [PubMed] [Google Scholar]


