Abstract
Background:
Clinically lymph node positive (cLNP) intrahepatic cholangiocarcinoma (ICC) carries a poor prognosis, without clear management guidelines for the practicing clinician. We sought to evaluate current practice patterns for cLNP ICC, including associations with survival.
Methods:
The National Cancer Database was queried for patients with cLNP ICC, without extrahepatic metastases.
Results:
We identified 1023 patients with cLNP ICC, 77%% (n = 784) of whom received chemotherapy alone. Resection was undertaken in 23% (n = 239) of patients and was most commonly utilized in combination with chemotherapy (n = 150). Median survival for all patients was 13.6 months. Patients undergoing resection in combination with chemotherapy were associated with an improved survival (22.5 months) as compared to those patients receiving chemotherapy alone (11.9 months) or resection alone (12.4 months) (p < 0.01). Finally, we compared the survival of patients with cLNP ICC with that of patients with pathologically proved lymph node positive (pLNP) ICC, all of whom were treated with resection with chemotherapy, and found no difference in survival (22.5 months–19.3 months, p = 0.99, respectively).
Conclusions:
While the decision to pursue resection for ICC is multifactorial and patient specific, the presence of clinically positive LNs should not represent a contraindication.
Introduction
Bile duct adenocarcinoma is the second most common primary hepatic malignancy after hepatocellular carcinoma. Intrahepatic cholangiocarcinoma (ICC) represents a subtype of bile duct adenocarcinoma which arises above the second-order bile ducts within the liver, and accounts for approximately 20% of cholangiocarcinomas in the U.S., with an estimated incidence of 0.5–2.0 cases per 100,000.1,2 These tumors are typically very aggressive with complete resection offering the only potential for cure. However, it is estimated that 70% of patients initially present with advanced-stage III or IV disease and are accordingly deemed unresectable.1 Treatment options for advanced-stage or unresectable disease are centered around locoregional and systemic chemotherapy. Despite advances in these modalities, median overall survival for ICC remains less than 20 months.3
Patients presenting with clinically evident lymph node (LN) metastases are considered to have advanced-stage disease and their survival is typically thought to approach that of patients with unresectable disease. The most common sites of lymph node metastases in ICC include the pericholedochal, periportal, and common hepatic artery lymph nodes; all of which are excised during a standard portal lymphadenectomy. Recent studies have reported conflicting findings regarding the survival benefit of resection in patients with pathologically confirmed positive LN.3,4 As such, it remains unclear whether clinically evident LN metastases should be considered a relative contraindication to resection.5,6 While resection alone is associated with poor survival in patients with LN-positive ICC, no consensus exists on the use of surgery combined with other treatment modalities for such cases.7,8
The aim of this study was to examine the use of treatment modalities for patients with clinically lymph node positive (cLNP) ICC. We also sought to assess if any treatment modalities or combinations thereof were associated with a improved survival. Given the relatively rare nature of ICC, single or multi-institutional studies may not provide sufficient insight into our query. Analysis of the National Cancer Database made it possible to examine the cohort on a much larger scale.
Methods
Cohort selection (Fig. 1)
Figure 1.
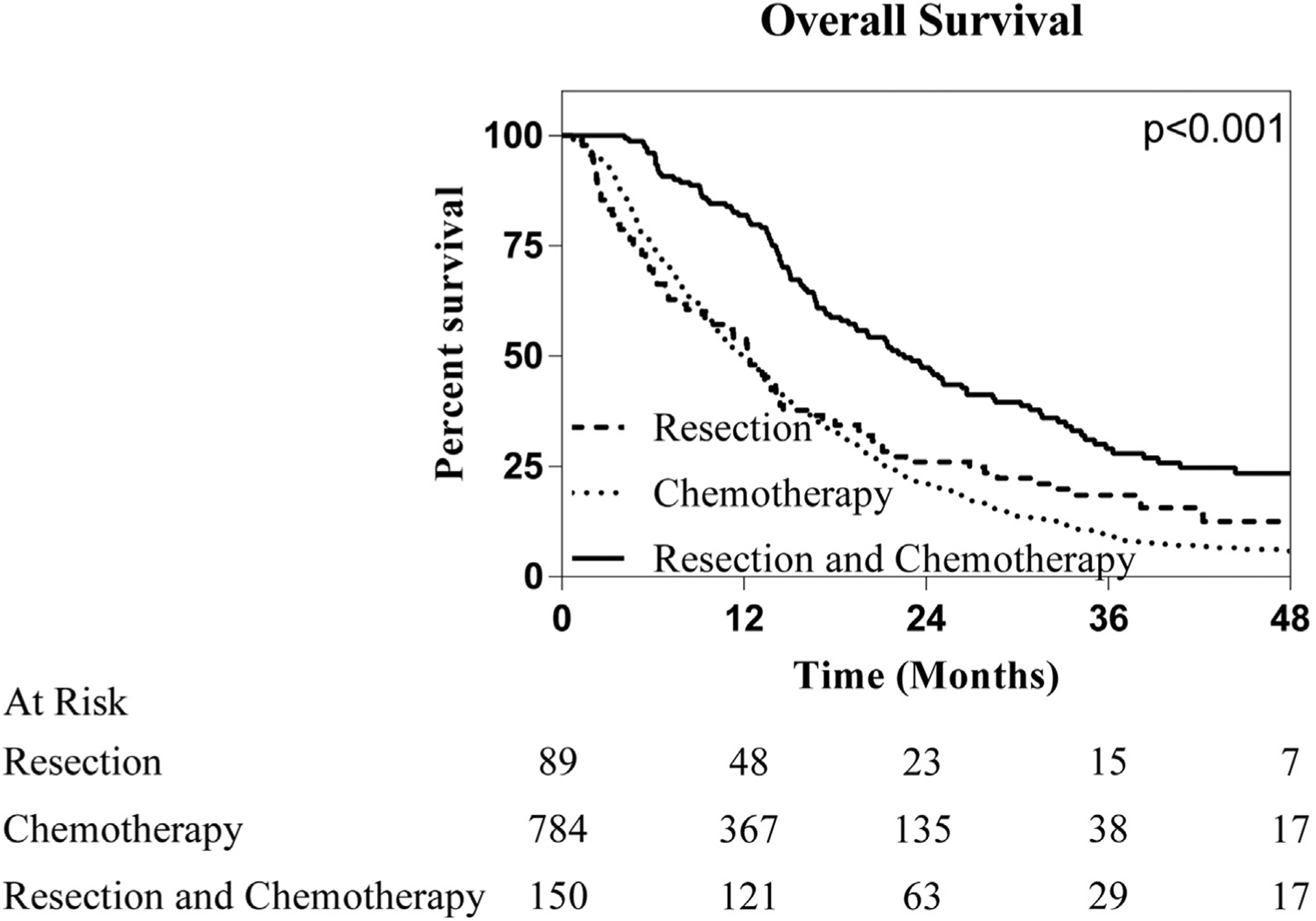
Overall survival stratified by treatment modality in patients with clinically node positive ICC. Patients that underwent a combination of resection and chemotherapy are associated with improved survival compared to those that underwent resection or chemotherapy alone
The National Cancer Database (NCDB) was queried between 2004 and 2014. Patients with ICC were identified using the International Classification of Disease for Oncology 3rd addition (ICD-O-3) with the primary site code specific to intrahepatic bile duct (22.1) as well as histology code for cholangiocarcinoma (8160). Using the NCDB variables for clinical T, N and M staging, patients were restaged in accordance with the most recent staging criteria. The American Joint Committee on Cancer 8th edition defines clinical stage IIIB disease as positive lymph nodes with any T stage and without distant metastatic disease. Only patients with biopsy proven stage IIIB ICC were included. Patients diagnosed on the basis of radiographic or biomarker evidence alone were excluded.
Patients were then stratified into treatment groups based on the NCDB coding for site-specific surgery and chemotherapy. Given the lack of standardized regiments for radiation therapy, patients that received radiation alone were excluded. Patients who did not receive any of the aforementioned treatment options were excluded. The procedures for patients undergoing resection included wedge resections and partial or extended hepatectomy with or without bile duct excision. Patients who underwent transplantation were excluded as were those who received ablative therapy, which we considered to be outside of the scope of this study. In ordered to decrease the degree of selection bias in patients receiving chemotherapy alone, we utilized the “Reason for No Surgery” variable to eliminate patients who were unlikely fit for surgery. Utilizing this code we excluded patients in which surgery was not performed due to patient risk factors and patients who died prior to undergoing resection. With these exclusions, patients included in this study that received chemotherapy alone were those for which surgery was not recommended as part of first line treatment or those who refused surgery. A preliminary analysis demonstrated that the addition of radiation to any of the previously defined regimens did not impact survival and, therefore, a subset of patients in each of the defined treatment categories may have received radiotherapy. Final treatment cohorts included: surgery, chemotherapy alone, or a combination of surgery and chemotherapy.
Basic demographic data was collected on all patients. Patient comorbidity was recorded using a truncated version of the Charlson/Deyo Score ranging from 0 as no comorbidities, to 3 representing the highest possible score. If a patient had missing data on any of the above demographic data, they were excluded from that analysis, but not from the overall survival analysis.
Timing of chemotherapy
To evaluate the effect of the timing of chemotherapy relative to surgery, a subgroup analysis was performed. Patients were included in this subgroup if they had received chemotherapy in conjunction with surgery. Patients were then grouped into neoadjuvant or adjuvant therapy. Patients that received both neoadjuvant and adjuvant therapy were included in the neoadjuvant cohort. If the chemotherapy sequence was unknown, patients were excluded from this analysis, but not the overall survival analysis.
Pathologic lymph node positive patients (pLNP)
In order to better frame the outcomes for patients with cLNP ICC, we queried the entirety of the intrahepatic ducts database utilizing the code for ICC to identify patients who were found to have lymph node metastasis on final pathology. We first identified patients what had undergone a resection for ICC. Patients were then included if they were clinically lymph node negative but later found to have lymph node metastasis on final pathology. Finally, only those patients that went on to receive adjuvant therapy were included in the analysis.
Statistical analysis
Descriptive statistics were calculated for all variables of interest. Continuous variables were summarized using means, whereas categorical variables were summarized using frequency and percentages. Comparisons of categorical variables were performed using the chi-square or Fisher’s exact test, whereas continuous variables were compared with the two-sided Student’s t-test. Overall survival (OS) was calculated using NCDB provided survival times utilizing the Kaplan–Meier method and log-rank test. Following a univariable analysis, a Cox proportional hazards model was constructed using all variables with p < 0.10. Statistical analyses were conducted using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, N.Y., USA).
Results
Patients and treatment modalities employed
From 2004 to 2014, 1023 patients were treated for clinical stage IIIB ICC and met our inclusion criteria. The cohort consisted of slightly more men (n = 520) than women (n = 503), and the average age for all patients was 62 years. Chemotherapy alone was the most commonly utilized treatment modality accounting for 77% (n = 784) of patients. Resection was undertaken in 23% (n = 239) of the patients in this cohort with 15% (n = 150) undergoing resection in combination with chemotherapy, while 8% (n = 89) underwent resection alone. Patient and tumor characteristics, including age, Charleson Deyo score and T-stage, were associated with the type of treatment received (Table 1).
Table 1.
Univariable analysis of demographic factors associated with management of node-positive ICC
| Sex | 0.204 | |||
| Male | 43 (48.3) | 410 (52.3) | 67 (44.7) | |
| Female | 46 (51.7) | 344 (47.7) | 83 (55.33) | |
| Charlson-Deyo Score | 0.039 | |||
| 0 | 63 (70.8) | 545 (69.5) | 115 (76.7) | |
| 1 | 16 (18) | 172 (22.2) | 30 (20.0) | |
| 2 | 4 (4.5) | 45 (5.7) | 1 (0.7) | |
| 3 | 6 (6.7) | 20 (2.5) | 4 (2.7) | |
| Clinical T Stage | 0.007 | |||
| 1 | 29 (18.2) | 131 (36.2) | 35 (22.6) | |
| 2 | 29 (44.7) | 322 (36.2) | 58 (42.3) | |
| 3 | 19 (28.6) | 206 (23.4) | 35 (25.6) | |
| 4 | 2 (8.6) | 62 (3.8) | 9 (6.6) | |
Survival
Median overall survival for the 1023 patients in our analysis was 13.6 months. Log-rank analysis revealed multiple patient and tumor specific factors associated with improved survival, including patient age less than 65 (11.3 mo vs. 14.8 mo, p < 0.001), female sex (12.3 mo vs. 14.4 mo, p = 0.028, Charleson/Deyo Score of 0 (0: 13.9 mo, 1: 12.5 mo, 2: 9.8 mo, 3:8.0 mo; p = 0.012), and clinical T stage of 1 (1: 16.6 mo, 2:13.0 mo, 3: 13.5 mo, 4: 11.1 mo; p < 0.001). In addition to these findings, the treatment modality employed was found to have a strong association with survival. In patient that received a resection or chemotherapy alone, no difference in survival was noted (resection: 12.5 months, chemotherapy: 11.9 months, p = 0.226). Conversely in patients that underwent combination resection and chemotherapy an significant association with survival was noted with a median overall survival of 22.5 months (p < 0.001) (Fig. 1).
To elucidate the impact of treatment modality on survival, a Cox proportional hazards model was created (Table 2). On univariable analysis, treatment modality, age, sex, Charleson/Deyo score, and clinical Tstage were all found to impact survival. These variables were then included in a multivariable Cox regression model to assess whether treatment modality impacted OS independently of these other factors. There was no survival difference between patients undergoing resection or chemotherapy alone (HR 0.85 95%CI: 0.65–1.09, p = 0.201). Conversely, the combination of resection and chemotherapy was found to have a positive impact on survival (HR 0.53, 95%CI 0.43–0.66, p < 0.001). No other factors were associated with improved survival.
Table 2.
Univariable and multivariable cox regression of factors associated with overall survival
| Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | |||
|---|---|---|---|---|
| Chemotherapy | ref | – | ref | ref |
| Resection | 0.18 (0.67–1.08) | 0.187 | 0.85 (0.65–1.09) | 0.201 |
| Resection and Chemotherapy | 0.49 (0.40–0.60) | <0.001 | 0.53 (0.42–0.66) | <0.001 |
| Age | 1.30 (1.13–1.49) | <0.001 | 1.24 (1.07–1.44) | 0.003 |
| Sex | ||||
| Male | ref | – | ref | ref |
| Female | 0.86 (0.75–0.98) | 0.028 | 0.91 (0.79–1.05) | 0.220 |
| Charlson-Deyo Score | ||||
| 0 | ref | – | ref | ref |
| 1 | 1.12 (0.95–1.33) | 0.171 | 1.07 (0.89–1.28) | 0.455 |
| 2 | 1.54 (1.14–2.09) | 0.005 | 1.44 (1.04–1.99) | 0.027 |
| 3 | 1.37 (0.93–2.04) | 0.112 | 1.33 (0.86–2.04) | 0.197 |
| Clinical T Stage | ||||
| 1 | ref | – | ref | – |
| 2 | 1.44 (1.19–1.76) | <0.001 | 1.40 (1.15–1.70) | 0.001 |
| 3 | 1.46 (1.18–1.8) | <0.001 | 1.41 (1.14–1.74) | 0.002 |
| 4 | 1.68 (1.26–2.23) | <0.001 | 1.59 (1.19–2.12) | 0.002 |
Ref = reference variable.
Timing of chemotherapy for patients undergoing resection
Chemotherapy was administered in the neoadjuvant setting for 33% (n = 48) of patients, and in the adjuvant setting for 67% (n = 97) of patients (Table 3). When evaluating patient and tumor characteristics, only sex was found to be associated with the sequence of chemotherapy. Male patients were less likely to undergo neoadjuvant chemotherapy. Overall survival was evaluated via log-rank analysis demonstrating no significant difference in regard to chemotherapy sequence (neoadjuvant 25.1 months vs adjuvant 21.3 months p = 0.572).
Table 3.
Univariable analysis of factors associated with chemotherapy sequence
| Male | 14 (29.2) | 52 (53.6) | |
| Female | 34 (70.8) | 45 (46.4) | |
| Age (mean ± SD) | 57.2 ± 11.8 | 59.9 ± 10.6 | 0.160 |
| Charlson-Deyo Score | 0.855 | ||
| 0 | 38 (79.2) | 73 (75.3) | |
| 1 | 9 (18.7) | 21 (21.6) | |
| 2 | 0 | 0 | |
| 3 | 1 (2.1) | 3 (3.1) | |
| Clinical T Stage | 0.307 | ||
| 1 | 10 (22.2) | 22 (28.7) | |
| 2 | 19 (42.2) | 38 (43.7) | |
| 3 | 15 (33.3) | 18 (20.7) | |
| 4 | 1 (2.2) | 6 (6.9) |
Survival as compared to patients with pLNP ICC
In order to better frame the survival identified for patients undergoing resection and chemotherapy for cLNP ICC, we compared their survival with a similar group of patients with ICC by applying the same search criteria, albeit with pathologically node positive disease and not clinically node positive disease. Based on log rank analysis, no difference in survival was identified for patients that received chemotherapy in conjunction with resection based upon clinical nodal status (cLNP 22.5 mo vs. pLNP 19.3 mo, p = 0.995, Fig. 2).
Figure 2.
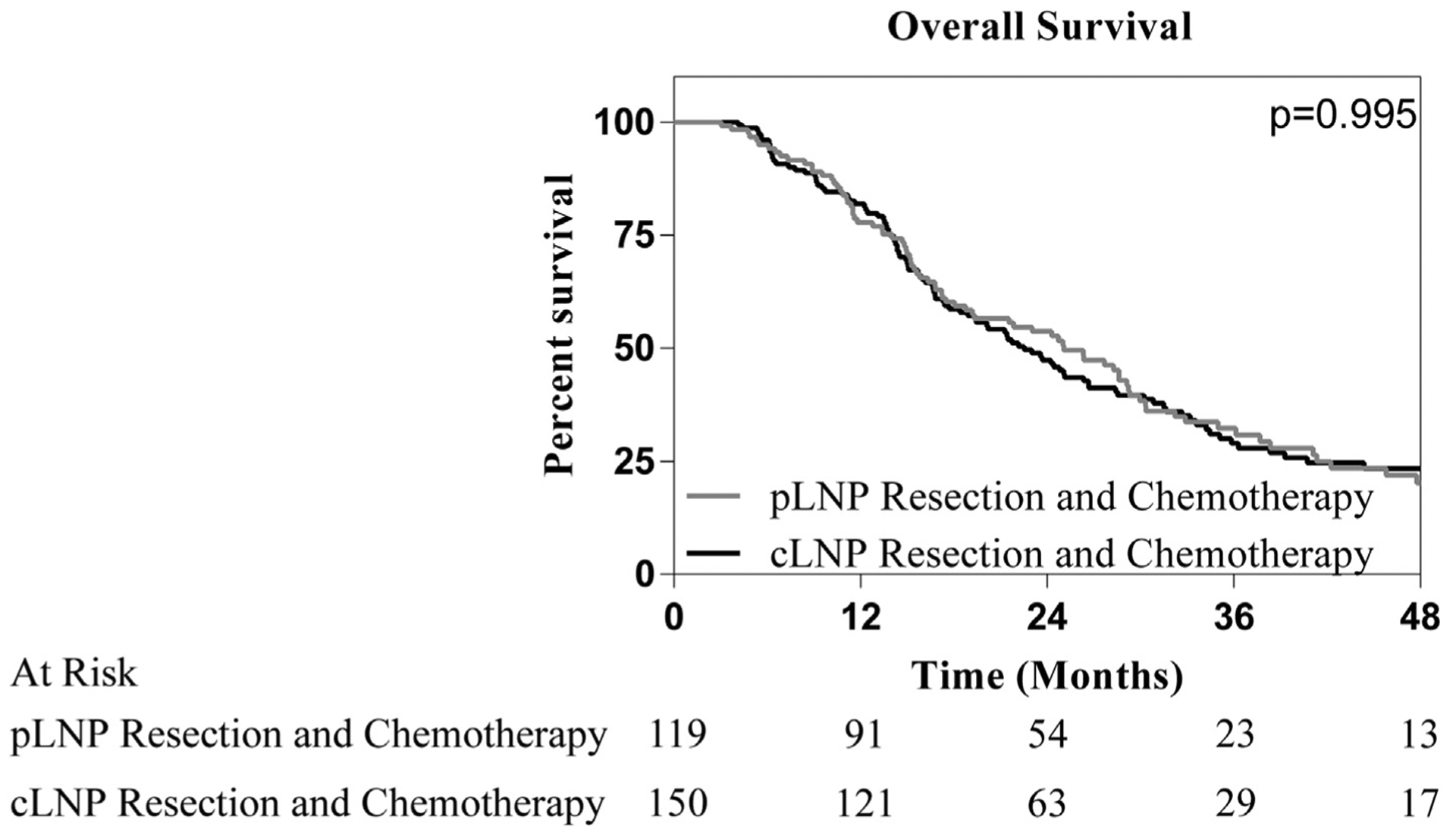
Overall survival stratified by identification of lymph node positive disease in patients that have undergone resection and chemotherapy. No significant difference in survival was noted between patients whose lymph node metastasis were identified clinically or pathologically in those that underwent resection and chemotherapy
Discussion
While the incidence of ICC continues to rise,1 survival remains dismal with reports ranging from 18 to 33 months and fewer than one in four patients alive at 5 years.9 Multiple factors have been shown to have negative prognostic implications, including positive lymph node status, which is among the most devastating. Unfortunately, due to the rarity of the disease, optimal diagnostic and therapeutic treatment algorithms are not well established, especially for patients presenting with clinically detectable LN metastases. Upon examination of the NCDB, we determined national practice patterns for the treatment of this cohort, cLNP ICC. While chemotherapy alone was the most commonly employed treatment approach, we noted an association with improved survival with a combination of resection and chemotherapy as compared to resection or chemotherapy alone. In order to better frame the survival associated with resection and chemotherapy for patients with cLNP ICC, we compared this cohort to a matched cohort with clinically negative, pLNP ICC, and found no difference in outcome.
Lymph node metastasis is among the most important prognostic indicators in ICC. This finding occurs in up to 33% of early stage tumors and would typically preclude surgery.10 We recently reported that for patients who underwent resection, the presence of positive lymph nodes was associated with a 58 month reduction in median OS.10 Poor survival in these patients is directly related to their propensity for recurrence which has been reported to be as high as 93%.11 Moreover, several nomograms aimed at predicting recurrence and survival allocate considerable weight to the negative prognostic value of lymph node positivity.12,13 We hypothesized that preoperative clinical findings of suspicious lymph nodes would dissuade surgeons from offering patients aggressive resections. Accordingly, we noted that the overwhelming majority of such patients were treated with chemotherapy alone and that less than a quarter of these patients underwent resection.
Given the rarity of the disease, formal guidelines for the diagnostic and therapeutic management of ICC are lacking. The current version of the NCCN guidelines has established its treatment algorithm on the basis of resectability without clearly and fully defining it.8 As such, the recommendations do not provide sufficient guidance when managing patients with cLNP ICC. In 2014, the European Association for the Study of the Liver (EASL) published comprehensive guidelines, and recommended resection solely for single tumors without intrahepatic, extrahepatic or nodal extension.7 The decision to classify cLNP patients as unresectable was rooted in previous findings relating to nodal positivity. Firstly, patients with node positive disease who underwent resection have been found to have significantly lower OS than those without nodal disease.10,14 Secondly, data from colorectal cancer metastasis reported that patients with hilar lymphadenopathy had worse outcomes following resection.15 These recommendations are supported by retrospective and anecdotal experiences alone, yet they have shaped the landscape of management for patients with cLNP ICC. Interestingly we have noted that the survival for patients with cLNP ICC undergoing resection and chemotherapy is similar to that of patients with LN metastases on final pathology who are also treated with resection and chemotherapy.
The findings from our study are consistent with previous studies reporting that monotherapy with either chemotherapy or resection for the treatment of lymph node positive ICC is associated with a median survival of approximately 12 months and 13–19 months, respectively.3,10 We examined outcomes following either single or multimodal therapy and observed that there is an association with improved survival with the receipt of combination resection and chemotherapy. When surgical resection was combined with chemotherapy, survival nearly doubled compared to that of patients who received either single modality treatment. Recently, two prospective studies investigated the role of adjuvant therapy following resection for biliary tract cancers, including the BILCAP trial which demonstrated a survival advantage of 17 months, yet neither addressed the role of clinical staging impacting treatment selection.16,17 Rather, each of these studies included patient that were deemed have undergone “curative intent resection” and it is unknowable what factors influenced the decision to offer resection. Conversely, pLNP status was reported in both of these studies (BILCAP: 48%, PRODIGE-12: 40%) and is far lesser than was noted in the cohort of resected patients in our analysis (83%).Interestingly, the sequence in which surgery and chemotherapy were administered did not significantly affect survival in our cohort. This finding suggests that neoadjuvant chemotherapy may offer several potential benefits. Firstly, it allows for a more thorough evaluation of tumor biology and thus a more judicious selection of surgical candidates who stand to benefit from these potentially complex operations. In addition, careful surveillance without adjuvant therapy may be offered to those having received neoadjuvant therapy and a pathologically confirmed complete resection. Finally, administering chemotherapy first allows treatment response to be evaluated early, thereby sparing patients with poor response a burdensome and inefficacious postoperative therapy.
Our analysis of the NCDB allowed for outcomes related to this rare disease to be studied on a much larger scale than had been previously reported. The findings reported in our study suggest that a larger ICC patient population may benefit from resection than had previously been understood. Though these findings have potentially important clinical implications, they must be considered in the setting of the limitations associated with this study. As with any large-scale database that accrues from multiple centers nationwide, errors in reporting are possible. Moreover, given means by which data is reported, adjuvant therapy administered at a non-NCDB reporting facility would not be recorded. The nature of the NCDB precludes our ability to determine why patients did not undergo a resection that may have otherwise been able. Finally, how clinical lymph node positivity was diagnosed is unknowable. Of note, of the 204 patients who underwent surgery with lymph node sampling, 83% were found to have pathologically confirmed lymph node metastases. This lends support to the pre-operative clinical diagnoses established in this study.
Currently, the care of patients with cLNP ICC is centered around chemotherapy. This treatment strategy, while supported by current guidelines, may be suboptimal. While the decision for resection in ICC is multifactorial and patient specific, our findings suggest that the presence of cLNP should not be an absolute contraindication to an operation that would otherwise extirpate the known disease in entirety. In patients who are properly selected, multimodal therapy, which includes resection and chemotherapy, seems optimal.
Acknowledgements
This research was supported in part by the Intramural Research Program of the NIH, NCI ZID BC 011541.
Footnotes
Disclosures
The authors have no disclosures.
Conflicts of interest
None declared.
References
- 1.Saha SK, Zhu AX, Fuchs CS, Brooks GA. (2016) Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist 21:594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. (2018) Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 15:95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kizy S, Altman AM, Marmor S, Wirth K, Ching Hui JY, Tuttle TM et al. (2019) Surgical resection of lymph node positive intrahepatic cholangiocarcinoma may not improve survival. HPB 21:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran Cao HS, Zhang Q, Sada YH, Chai C, Curley SA, Massarweh NN. (2018) The role of surgery and adjuvant therapy in lymph node-positive cancers of the gallbladder and intrahepatic bile ducts. Cancer 124: 74–83. [DOI] [PubMed] [Google Scholar]
- 5.Shimada M, Yamashita Y, Aishima S, Shirabe K, Takenaka K, Sugimachi K. (2001) Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br J Surg 88:1463–1466. [DOI] [PubMed] [Google Scholar]
- 6.Jutric Z, Johnston WC, Hoen HM, Newell PH, Cassera MA, Hammill CW et al. (2016) Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the national cancer database. HPB 18:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T et al. (2014) Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 60:1268–1289. [DOI] [PubMed] [Google Scholar]
- 8.Network NCC. (2018) Hepatobiliary cancers (Version 5.2018) . [Accessed 19 November 2018].
- 9.Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. (2014) Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg 149:565–574. [DOI] [PubMed] [Google Scholar]
- 10.Martin SP, Ruff S, Diggs LP, Drake J, Ayabe RI, Brown ZJ et al. (2019) Tumor grade and sex should influence the utilization of portal lymphadenectomy for early stage intrahepatic cholangiocarcinoma. HPB 21:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D et al. (2008) Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 248:84–96. [DOI] [PubMed] [Google Scholar]
- 12.Hyder O, Marques H, Pulitano C, Marsh JW, Alexandrescu S, Bauer TW et al. (2014) A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg 149:432–438. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z et al. (2013) Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol Offic J Am Soc Clin Oncol 31:1188–1195. [DOI] [PubMed] [Google Scholar]
- 14.Adachi T, Eguchi S. (2014) Lymph node dissection for intrahepatic cholangiocarcinoma: a critical review of the literature to date. J Hepatobiliary Pancreat Sci 21:162–168. [DOI] [PubMed] [Google Scholar]
- 15.Jaeck D (2003) The significance of hepatic pedicle lymph nodes metastases in surgical management of colorectal liver metastases and of other liver malignancies. Ann Surg Oncol 10:1007–1011. [DOI] [PubMed] [Google Scholar]
- 16.Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly J-P et al. (2019) Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. J Clin Oncol 37:658–667. [DOI] [PubMed] [Google Scholar]
- 17.Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D et al. (2019. May) Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 20(5):663–673. [DOI] [PubMed] [Google Scholar]


