Abstract
Annual variation in phenology can have profound effects on the behavior of animals. As climate change advances spring phenology in ecosystems around the globe, it is becoming increasingly important to understand how animals respond to variation in the timing of seasonal events and how their responses may shift in the future. We investigated the influence of spring phenology on the behavior of migratory, barren‐ground caribou (Rangifer tarandus), a species that has evolved to cope with short Arctic summers. Specifically, we examined the effect of spring snow melt and vegetation growth on the current and potential future space‐use patterns of the Porcupine Caribou Herd (PCH), which exhibits large, inter‐annual shifts in their calving and post‐calving distributions across the U.S.–Canadian border. We quantified PCH selection for snow melt and vegetation phenology using machine learning models, determined how selection resulted in annual shifts in space‐use, and then projected future distributions based on climate‐driven phenology models. Caribou exhibited strong, scale‐dependent selection for both snow melt and vegetation growth. During the calving season, caribou selected areas at finer scales where the snow had melted and vegetation was greening, but within broader landscapes that were still brown or snow covered. During the post‐calving season, they selected vegetation with intermediate biomass expected to have high forage quality. Annual variation in spring phenology predicted major shifts in PCH space‐use. In years with early spring phenology, PCH predominately used habitat in Alaska, while in years with late phenology, they spent more time in Yukon. Future climate conditions were projected to advance spring phenology, shifting PCH calving and post‐calving distributions further west into Alaska. Our results demonstrate that caribou selection for habitat in specific phenological stages drive dramatic shifts in annual space‐use patterns, and will likely affect future distributions, underscoring the importance of maintaining sufficient suitable habitat to allow for behavioral plasticity.
Keywords: Arctic, caribou, climate change, large herbivore, migration, phenology, Porcupine Caribou Herd, range shift, Rangifer tarandus, resource selection
The Porcupine Caribou Herd spends their summer calving and post‐calving seasons on the Arctic coastal plain of Alaska and Yukon but can exhibit substantial annual shifts in their precise distributions. Our models demonstrated that annual variation in spring snow melt and vegetation phenology drove variation in summer range locations. Based on climate projections, the future summer ranges were predicted to shift further west and north into the Alaskan coastal plain due to advancing spring phenology, underscoring the need for sufficient habitat to allow for behavioral adaptation in the changing environment.

1. INTRODUCTION
As changing climate conditions alter phenology of ecosystems across the globe (Myers‐Smith et al., 2011; Parmesan & Yohe, 2003; Root et al., 2003), there is growing interest in understanding how animals respond to variation in the timing of seasonal events (Cohen et al., 2018). Phenology often elicits strong behavioral responses in animals, altering their movements, habitat use patterns, and spatial distributions as they react to annual shifts in resource availability (Beever et al., 2017; Helm et al., 2013; Resano‐Mayor et al., 2019; Scharf et al., 2019). These behavioral responses often enable animals to accrue fitness benefits by accessing key resources during critical times of the year, most notably, moving to areas of food abundance during the reproductive season (Dingle & Drake, 2007). As climate change advances spring phenology, animals exhibit a variety of novel behaviors including shifting their seasonal ranges, changing the timing of their movements, and altering their foraging patterns (Beever et al., 2017; Root et al., 2003; Samplonius et al., 2016). Such behavioral plasticity has buffered some species from mismatches in the timing of peak resource availability and their consumer needs but may be unable to keep pace with rapidly changing conditions (Beever et al., 2017) having the potential for demographic consequences (Visser & Gienapp, 2019). As a result, it is becoming increasingly important to understand the behavioral mechanisms animals use to respond to annual phenological variation and predict how they may respond to future environmental conditions (Beever et al., 2017; Buchholz et al., 2019; Cohen et al., 2018).
In seasonal environments, the timing and rate of vegetation growth during spring is particularly important in driving the behavior of large, migratory herbivores. During spring, these species often track the “green wave” of newly emergent vegetation as it advances along elevational or latitudinal gradients to extend their access to high quality forage (Aikens et al., 2020; Merkle et al., 2016). Forage quality (i.e., digestible energy and protein) tends to be high in new plant growth at the start of the growing season, and then declines as plants mature and their defensive tissues and compounds increase (Hebblewhite et al., 2008; Van Soest, 1982). By selecting vegetation in earlier phenological stages, large herbivores optimize trade‐offs between forage quality and quantity to maximize consumption of digestible nutrients (Fryxell, 1991; Hebblewhite et al., 2008). While investigators are increasingly recognizing the influence of phenology on behavior of large herbivores, studies have primarily focused on its role during spring migration (Aikens et al., 2020; Bischof et al., 2012; Merkle et al., 2016), even though forage conditions continue to change throughout the growing season (Felton et al., 2018). Selection by herbivores for specific plant stages, combined with the spatial arrangement of preferred plants, suggests that changes in phenology can result in temporally dynamic space‐use patterns (Paolini et al., 2018) with key implications for how seasonal ranges may shift under changing climate conditions.
As the Arctic warms at more than twice the global rate (Intergovernmental Panel on Climate Change Climate Change [IPCC], 2007), advances in growing season phenology are likely to strongly influence the behavior of migratory caribou (Rangifer tarandus). Caribou are the dominant large herbivore in the Arctic and are ecologically important due to their effects on vegetation dynamics (Newton et al., 2014; Zamin & Grogan, 2013) and socially important for providing subsistence food and cultural resources to rural and indigenous communities (Fall, 2016; Kenny et al., 2018). Arctic caribou exhibit the longest migrations of terrestrial mammals on the planet (Joly et al., 2020) as they travel to summer ranges to birth their calves and access high‐quality forage (Griffith et al., 2002; White et al., 1975). Unlike many other large herbivores that migrate in synchrony with the green wave of vegetation growth in spring, Arctic caribou migrate through the snow and often arrive on their calving grounds prior to the onset of green‐up (Gurarie et al., 2019; Gustine et al., 2017). While Arctic caribou typically exhibit strong fidelity to their calving grounds and summer ranges (Skoog, 1968), some herds have been observed to shift these ranges over time, sometimes by hundreds of kilometers (Gunn et al., 2008; Newton et al., 2015; Taillon et al., 2012). The cause of these range shifts is unknown, however, investigators have speculated that it may be, in part, to access habitat with earlier plant phenology and improved foraging conditions (Gunn et al., 2008; Newton et al., 2015). As the summer ranges of many Arctic caribou herds are being impacted by climate change and industrial development (Festa‐Bianchet et al., 2011; Mallory & Boyce, 2018), there is a critical need to identify preferred habitat conditions, and understand how suitable habitat may be distributed in the future (Taillon et al., 2012).
To examine the influence of spring phenology on resource selection and space‐use patterns of Arctic caribou, we investigated the behavior of the Porcupine Caribou Herd (PCH). The PCH is currently one of the largest Arctic herds in North America (ca. 218,000; Caikoski, 2020) with an annual distribution extending across the United States−Canada border. The locations of the PCH calving and post‐calving ranges vary annually but tend to be further west (in Alaska) during years with earlier snow melt and further east (in Yukon) during years of late snow melt (Griffith et al., 2002; Figure 1). Under changing climate conditions, this pattern could shift PCH distributions predominately into Alaska, which might exacerbate the potential effects of proposed oil development within the Alaskan calving and post‐calving ranges (Bureau of Land Management [BLM], 2019). To better understand the role of phenology in governing current and future patterns of PCH space‐use, we (1) examined caribou selection of multiple phenology and habitat characteristics at several spatial scales; (2) determined how annual variation in phenology affected annual calving and post‐calving distributions; and (3) projected future calving and post‐calving distributions based on predicted climate‐driven shifts in annual phenology.
FIGURE 1.
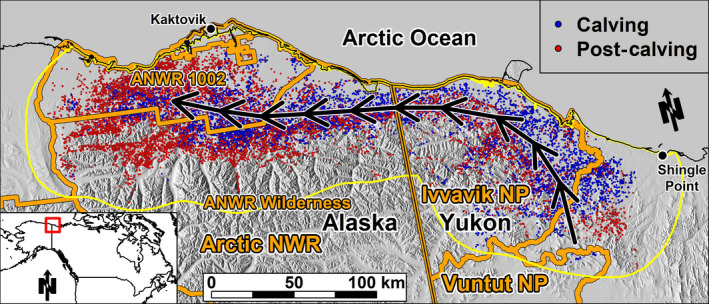
Study area boundary (yellow polygon) for assessing resource selection associated with calving (blue dots) and post‐calving (red dots) locations of the Porcupine Caribou Herd along the Arctic coast of Alaska and Yukon (black lines) during 2012–2018. Black arrows indicate the typical movement path during these seasons. The boundaries of the Arctic National Wildlife Refuge (ANWR), sub‐units of ANWR (1002 Area and Wilderness Area), Ivvavik National Park (NP), and Vuntut NP are shown in orange. Locations represent a random subsample of 20% of the total data for clarity
2. METHODS
2.1. Study area
During the calving and post‐calving seasons, the PCH typically inhabits the Arctic coastal plain and foothills of the northwestern Yukon Territory and northeastern Alaska (Figure 1; Griffith et al., 2002; Russell et al., 1993). In April and May, caribou migrate to the Yukon's coastal plain by wrapping east around the Brooks Range or by travelling north from winter ranges in northern Yukon and Northwest Territories (Russell et al., 1993). Once on Yukon's coastal plain, caribou may remain there for calving or continue westward into Alaska (Figure 1). As a result, the annual calving distribution can be located predominately in the Yukon, in Alaska, or split across the international border (Porcupine Caribou Technical Committee [PCTC], 1993). After caribou birth their calves (early June), caribou that calved in Yukon continue to move west to join those already in Alaska for the post‐calving season (Figure 1).
Spring snow melt and vegetation growth on the Arctic coastal plain typically follow a longitudinal gradient, occurring earlier in the eastern portion of the study area and progressing westward. Latitudinally, the pattern is more complex with spring conditions occurring earlier in the foothills south of the coastal plain (further from the Arctic Ocean) and later at high elevations in the mountains (Griffith et al., 2002; Russell et al., 1993). During 2001–2017 (the years of our phenology analysis; see below) in our study area, the average February temperature (coldest month) was −21.2℃ (range: −27.8 to 16.1℃), the average July temperature (warmest month) was 10.6℃ (range: 7.2–12.9℃), and average total annual precipitation was 388 mm (range: 261–479 mm; http://ckan.snap.uaf.edu/dataset/historical‐and‐projected‐dynamically‐downscaled‐climate‐data‐for‐the‐state‐of‐alaska‐and‐surrou). Vegetation is dominated by herbaceous tundra, tussock tundra, and low shrubs, and a large portion of the balance was sparsely vegetated alluvial and montane habitat (Supporting Information S1; https://daac.ornl.gov/; Eastland et al., 1989; Russell et al., 1993). Lands are managed primarily by the U.S. federal government in Alaska (Arctic National Wildlife Refuge [ANWR]) and the Canadian federal government in Yukon (Ivvavik National Park; Figure 1). Within ANWR, there are two management units, the Wilderness Area and the 1002 Area, the latter of which allows for potential energy development (BLM, 2019).
2.2. Caribou location data
During 2012–2018, primarily in February–March, the Yukon Government and Alaska Department of Fish and Game (ADFG) captured adult female (≥3 year old) PCH caribou from a helicopter using a net gun (ADFG Protocol #0018). Caribou were fitted with global positioning system (GPS) collars (ATS Iridium G2210E and Lotek Iridium Track M) programmed to collect locations every 2–5 h. These data were archived by the Yukon Government and approved for our use by the PCTC. We used locations from all collared adult females (parturient and non‐parturient) and divided locations into those occurring during the calving season (26 May–10 June; ordinal day 146–161) and the post‐calving season (11–30 June; ordinal day 162–181) following previous definitions for the behavior of the herd (PCTC, 1993). Median calving dates are generally consistent within a few days ranging between May 30 and June 6 (Griffith et al., 2002; Hepler, 2019). Data from animals with <50 locations/season/year were excluded from our analyses.
2.3. Phenology and habitat variables
To quantify annual variation in phenology during the calving and post‐calving seasons, we used metrics derived from Moderate Resolution Imaging Spectroradiometer (MODIS) imagery. We used MODIS‐derived snow‐free date (Lindsay et al., 2015; 500‐m resolution) from the Geographic Information Network of Alaska (GINA; https://gina.alaska.edu/), which represented the ordinal day each pixel was estimated to be free of snow. We also acquired MODIS‐derived onset of greenness (250‐m resolution) from GINA, which represented the estimated ordinal day the vegetation in a pixel became green based on a modeled normalized difference vegetation index (NDVI) threshold (Zhu et al., 2013). Finally, using the NDVI metrics from GINA (Zhu et al., 2013) for each pixel, we also estimated the date when NDVI reached 50% of its maximum value for the year, approximating the time when biomass levels are intermediate and forage quality is high, ideal foraging conditions for ungulates early in the growing season (Fryxell, 1991; Hebblewhite et al., 2008). Intermediate NDVI values coincide with maximum concentrations of forage protein for Arctic caribou on the coastal plain (Johnson et al., 2018) which is hypothesized to be nutritionally limiting (Barboza et al., 2018). We interpolated the date when NDVI was 50% of its maximum value (50% max NDVI) by using the dates of greenness onset and maximum NDVI, assuming a constant rate of change.
In addition to phenology, we included terrain and landcover variables previously shown to be important to Arctic caribou resource selection (Fullman et al., 2017; Nelleman & Thomsen, 1994). We acquired a digital elevation model (DEM; 30‐m resolution) from the U.S. Geological Survey (https://www.usgs.gov/core‐science‐systems/ngp/tnm‐delivery/) and calculated terrain ruggedness, topographic position, slope, and aspect across the study area using the Geomorphometry and Gradient Metrics 2.0 toolbox (Evans et al., 2014) in ArcGIS 10.6.1 (ESRI). We used the Global Self‐consistent, Hierarchical, High‐resolution Geography coastline shapefile from the National Oceanic and Atmospheric Administration (https://www.ngdc.noaa.gov/mgg/shorelines/; Wessel & Smith, 1996) to define the northern boundary of our study area and to measure distance to the coastline.
To characterize landcover across the study area, we used Landsat‐derived data from the Arctic Boreal Vulnerability Experiment (ABoVE; Wang et al., 2019; 30 m). Landcover classes in our analysis included water, tussock tundra, tall shrub, low shrub, barren and sparsely vegetated (Supporting Information S1). Upon comparison with aerial photographs, barren and sparsely vegetated classes appeared similar and were interspersed, so we combined these classes and recategorized them as being either “montane barren,” in rugged, high elevation habitat, or “riverine barren”, in gravel bars, deltas, and other alluvial habitat associated with rivers based on topographic criteria (Table S1.1). The ABoVE data included annual landcover classifications from 1984 to 2014, so we used 2014 data in our analyses to coincide with the period we collected caribou locations.
2.4. Resource selection analysis
We assessed population‐level selection (second order selection by individuals within the population range; Meyer & Thuiller, 2006) of phenology and habitat variables by adult female caribou during the calving and post‐calving seasons by comparing the characteristics of used and available locations. We delineated the habitat available to caribou with a kernel density of calving and post‐calving locations using the “density.ppp” function in the “spatstat” package (Baddeley et al., 2016) in the R 3.6.3 computing environment (R Core Team, 2020). We used the ad hoc method of defining kernel smoothing bandwidth (Worton, 1989), and because sample size varied by year, weighted our density estimate by the annual number of caribou locations collected (Supporting Information S2). We used all locations from both calving and post‐calving seasons to define availability because caribou distributions overlapped extensively between seasons (Figure 1) and this allowed a direct comparison of seasonal and annual shifts across a large area. We then extracted the contour defining the 95% volume of the kernel density and buffered the contour by 13 km, the mean daily movement rate during our study period, which together, approximated habitat that was generally available to the herd (Pop et al., 2018). Areas within the Arctic Ocean were excluded (Figure 1). Within the study area boundary, we randomly generated five available locations for every used caribou location and attributed phenology, terrain, and landcover values to all locations. We did not use elevation or distance to coast in resource selection models because these variables often serve as proxies for phenology characteristics (Haugen & Brown, 1980; Macander et al., 2015), the primary variables of interest. To evaluate and interpret the influence of spatial scale on caribou resource selection, we averaged values for all habitat variables within buffers around locations with radii of 500, 1000, 2000, 5000, and 10,000 m. The largest buffer represented approximate average daily movement distances of caribou throughout the calving and post‐calving seasons.
To discriminate between the characteristics of used and available locations we employed a machine learning, random forest classification model (Breiman, 2001). Random forest models are resistant to collinearity of variables, do not assume independence of samples, can model non‐linear relationships, inherently consider interactions among variables, and often perform better (i.e., lower predictive error rates) than parametric models (e.g., logistic regression) of ecological data (Cutler et al., 2007; Evans et al., 2011). A random forest model is an ensemble of tree models that use bootstrap aggregation (i.e., bagging) in which a unique sample of the data is drawn to grow each tree, and a splitting variable (e.g., phenology or habitat variable) is chosen from a random sample of variables drawn for each node (Breiman, 2001). In the “ranger” package (Wright & Ziegler, 2017) in R, we constructed probability forests (required by “ranger” to calculate classification probabilities) using sampling with replacement and used the Gini index for node splitting (Hastie et al., 2009; see Supporting Information S3 for details). Forests comprised 500 trees during the variable selection phase to reduce computation time, but final forests comprised 1000 trees. We applied weights to the bootstrap sampling to account for the imbalanced number of available and used locations (i.e., 5:1) and unequal sample sizes among years and individuals. We used the square root of the number of variables in the model as the number of randomly selected variables to consider at each node (Breiman, 2001). Trees in random forest classification models are often grown to maximum depth (Breiman, 2001), but to limit overfitting (Hastie et al., 2009; Segal, 2004) and to improve generalization across years, we limited the trees to a maximum depth of 13 node splits and minimum node size of 10 samples (Supporting Information S3; Figure S.3.1).
To select the best resource selection model for each season, we conducted a recursive variable removal procedure (Guyon et al., 2002). We started with a global model with all variables at all buffers, removed the least important variable (as calculated by improvement in Gini impurity; Liaw & Wiener, 2002), and then calculated the area under the receiver operating characteristic curve (AUC), which is resistant to class‐imbalanced data (Evans et al., 2011). We continued this process until only one variable remained. In preliminary analyses, the AUC often plateaued before declining, and a clear “best” model was not evident. To further regularize the model, we bootstrapped the AUC to produce 95% confidence intervals (Hastie et al., 2009) and selected the simplest model whose intervals overlapped those of the model with the highest AUC (Supporting Information S3 and S4; Figure S4.1). We then compared the global model with the best recursive selection model and chose the one with the highest AUC. To facilitate model interpretation, we generated partial dependence plots for main effects, compared relative variable importance, and mapped spatial predictions of the top models.
2.5. Projected resource selection under future climate conditions
To project future PCH calving and post‐calving distributions, we first needed projections of snow melt and vegetation conditions under future climate scenarios that could be used as inputs into our resource selection models. Because those phenology projections were not available, we derived our own by developing phenology prediction models from observed past weather data, and then used those models to project phenology under future climate conditions (described below).
To model each phenology metric (i.e., dates of snow melt, onset of greenness, and 50% max NDVI), we used 20‐km resolution weather data produced by the European Centre for Medium‐Range Weather Forecasts interim reanalysis project (ERA‐Interim) during 2001 (earliest year of phenology data) to 2017 (the most recent year of weather data) which had been dynamically downscaled by the Scenarios Network for Alaska and Arctic Planning (SNAP) using the Weather Research and Forecasting model Version 3.5 (Bieniek et al., 2016). The weather variables we used included snow water equivalent (SWE) on 1 May, representing the accumulated snow present shortly before the calving season. We also calculated an average “spring” temperature during May and June representing the heat available to melt snow and initiate the growing season. Additionally, we calculated “summer” total precipitation and average temperature during June and July, which we hypothesized would affect the rate of green up and maximum NDVI values and, therefore, were only used in the model predicting the date of 50% max NDVI. We also used terrain variables in the models, including elevation, ruggedness, topographic position, slope, aspect, and distance to coast, using the definitions and scales described above.
The random forest model specification and selection for the phenology metrics were similar to the resource selection models, but because the phenology models were regression rather than classification, we used variance for node splitting and for variable importance (Hastie et al., 2009). Weighting was not necessary because each year had the same sample size (i.e., number of pixels in the study area). Also, due to large sample sizes and computation time, model selection was completed with forests composed of 100 trees, and final models comprised 500 trees. The best models were selected using the predictive (OOB) R 2 values (Liaw & Weiner, 2002; Hastie et al., 2009).
To estimate future phenology values, we used projected weather from the 20‐km dynamically downscaled climate projection data from SNAP (Lader et al., 2017; Walsh et al., 2018). Values were averaged from two global climate models (GCM; GFDL‐CM3 and NCAR‐CCSM4) from the Coupled Model Inter‐Comparison Project 5 (CMIP5) which have been found to be representative for Alaska (Lader et al., 2017; Walsh et al., 2018). Projection values were only available for representative concentration pathway (RCP) 8.5, the highest CMIP5 concentration pathway. This pathway may overestimate emissions from fossil fuels (Hausfather & Peters, 2020) but tracks recent climate trends in the Arctic (Lader et al., 2017). Its estimates of warming are increasingly plausible, particularly through the mid‐21st century (Schwalm et al., 2020a) before most of the divergence occurs between RCP 8.5 and more moderate scenarios (Hawkins & Sutton, 2009; Knutti & Sedlácek, 2013). To account for climate variability and limit bias, we averaged climate projections across three decades: 2030–2039, 2040–2049, and 2050–2059 (Terando et al., 2020). We bias‐corrected the data by adjusting the climate projections by the offset between the ERA‐Interim average and the 2010–2019 climate projection average (Lader et al., 2016, 2017). The adjustment was proportional for SWE and precipitation, because these data were constrained on the lower limit by “0”, and was absolute for temperature because, for practical purposes, the values were unconstrained. The median proportional pixel bias between the ERA‐Interim observed data and the climate projection data (ERA‐Interim divided by projection) was 0.63 for SWE and 1.20 for spring precipitation, and the absolute median pixel bias (ERA‐Interim minus projection) was 1.16℃ for spring temperature and 0.88℃ for summer temperature.
We input the adjusted decadal averages of the climate projections into the phenology prediction models to project future phenology characteristics throughout the study area. The projected phenology metrics were then used as inputs in the resource selection models to estimate the future probability of calving and post‐calving habitat use within the study area under the climate conditions predicted for each decade. To assess how the average distribution of habitat may change through time, we determined a classification probability cutoff value in the resource selection models to identify “suitable” habitat. We calculated the mean classification success of the used and available locations (i.e., the average of the used location classification success and the available location classification success) across all probability values and identified the probability cutoff where the classification success was maximized (Guisan et al., 2017). To interpret temporal shifts in resource use from current conditions into the future, we plotted spatial predictions and calculated the amount of average suitable habitat in Alaska and Yukon. While the suitable habitat delineation averaged across multiple years does not encompass the full range of annual variation, it represented a consistent metric for comparing distribution shifts through time.
3. RESULTS
3.1. Resource selection
The number of collared caribou increased across the study period, starting with seven collared females in 2012 and ending with 52 in 2018 (Supporting Information S2). Across those years, during the calving season, we collected data from 89 individuals, totaling 236 animal‐years, and comprising 40,880 locations. During the post‐calving season, we collected data from 88 individuals, totaling 238 animal‐years, and comprising 52,305 locations.
The best calving season model (AUC = 0.942; Supporting Information S4; Tables S4.1–S4.3) included 16 phenology and habitat variables and, except for snow melt at the pixel scale, all variables were included in the model at the 5‐ and 10‐km scales (Figure 2; Supporting Information S4; Tables S4.2 and S4.3). The most important variable was the proportion of low shrub cover at the 10‐km scale followed by snow melt and onset of greenness dates at the 10‐km scale. Snow melt and onset of greenness at the 5‐km scale were also included with moderate importance, and snow melt at the pixel scale was the least important of the included variables. At large spatial scales (5 and 10 km) caribou selected habitat where the snow had melted approximately 0–9 days before the start of the calving season (Figure 3a). At the pixel scale, however, selection peaked sharply with snow melt dates ~9 days prior to the start of the calving season. This suggests that at finer scales, caribou selected habitat that had been snow‐free for several days, but which were within broader landscapes that may still have snow or more recently melted. Similarly, within the 5‐km buffer, caribou selected habitat where the vegetation turned green at the beginning of the calving season, while within the 10‐km buffer, they selected vegetation which turned green toward the end of the calving season (Figure 3b). This suggests that caribou selected patches of green vegetation within a mostly brown, or potentially snow‐covered, landscape, seeking out areas with greater foraging opportunities. Partial dependence plots for the other variables are included in Supporting Information S4 (Figure S4.2). During the calving season, caribou generally selected habitat comprising moderate to high proportions of low shrub, tussock tundra, and herbaceous habitats and low proportions of tall shrub and riverine barren habitats. They also selected low to moderately rugged terrain and gentle slopes.
FIGURE 2.
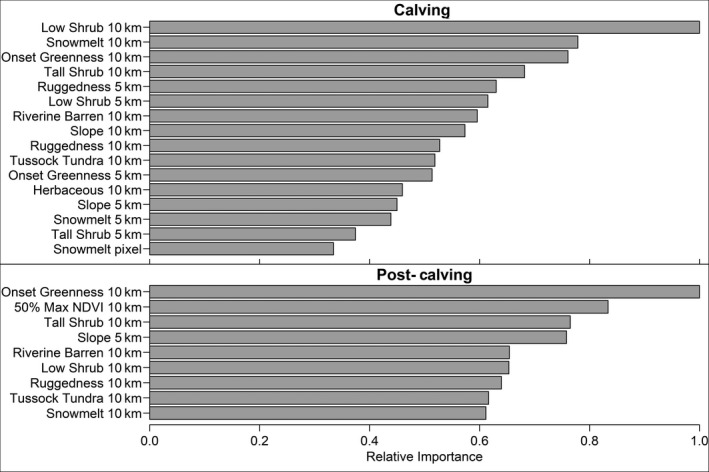
Variable importance plots for models of caribou resource selection during the calving (top) and post‐calving (bottom) seasons by the Porcupine Caribou Herd, 2012–2018
FIGURE 3.
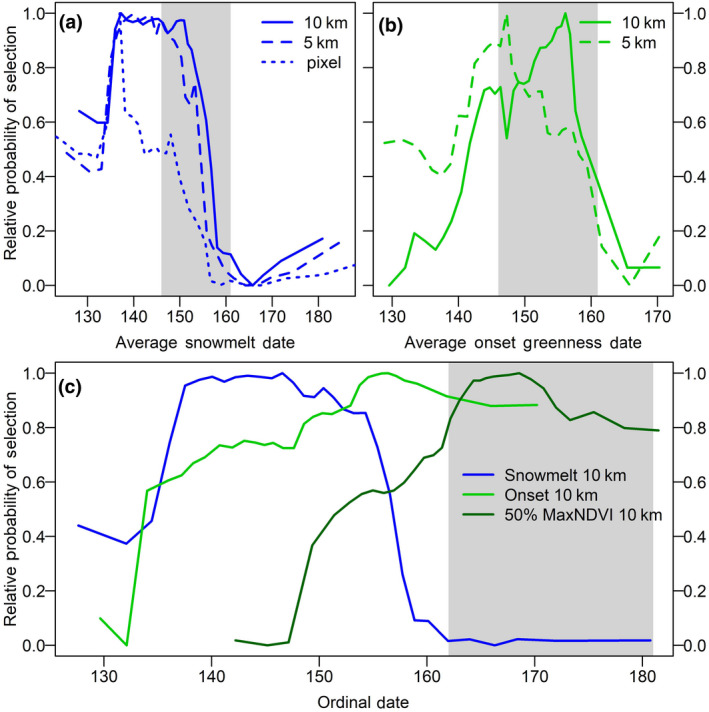
Partial dependence plots for caribou selection of phenology variables during the calving (a, b) and post‐calving (c) seasons by the Porcupine Caribou Herd, 2012–2018. Gray polygons depict the timing of either the calving or post‐calving season, respectively. During the calving season, snow melt date (a) was included in the model at three spatial scales and the onset of greenness was included at two scales (b). During the post‐calving season, the phenology variables were included in the model at only one spatial scale (c)
During the post‐calving season, nine phenology and habitat variables were retained in the top model (AUC = 0.919; Supporting Information S4; Table S4.1, S4.2, and S4.4), each at only one spatial scale. All the variables retained in the model were measured at the 10‐km scale except for slope, which was measured at 5 km (Figure 2). Onset of greenness and 50% max NDVI dates were the most important variables (Figure 2). Caribou selected habitat where the snow had melted ~20–40 days earlier, and the vegetation had turned green ~10–20 days earlier (Figure 3c). Additionally, they selected habitat where the 50% max NDVI date occurred within the post‐calving season (i.e., while they were using the habitat; Figure 3c). Partial dependence plots for other variables are included in Supporting Information S4 (Figure S4.3). During the post‐calving season, caribou selected habitat comprising moderate to high proportions of tussock tundra and low shrub and low proportions of tall shrub and riverine barren habitats. They also tended to select slightly steeper and more rugged terrain than during the calving season.
The optimal probability cutoff value to delineate “suitable” habitat (i.e., the maximum separation between used and available locations) was 0.53 for the calving season and 0.52 for the post‐calving season (Supporting Information S5). The study area predictions during 2012–2018 exhibited large annual variation in PCH calving and post‐calving space‐use (Figures 4 and 5). Specifically, in years when spring phenology was early (e.g., 2015), calving habitat shifted westward and was located primarily within Alaska, whereas in years when phenology was later (e.g., 2018), calving habitat shifted eastward and was primarily within Yukon (Figure 6). During the post‐calving season, years of early phenology were associated with caribou use of the Alaskan coastal plain, whereas years with later phenology were associated with caribou being widely distributed longitudinally across the study area (in both Yukon and Alaska) and primarily in the foothills (Figure 7).
FIGURE 4.
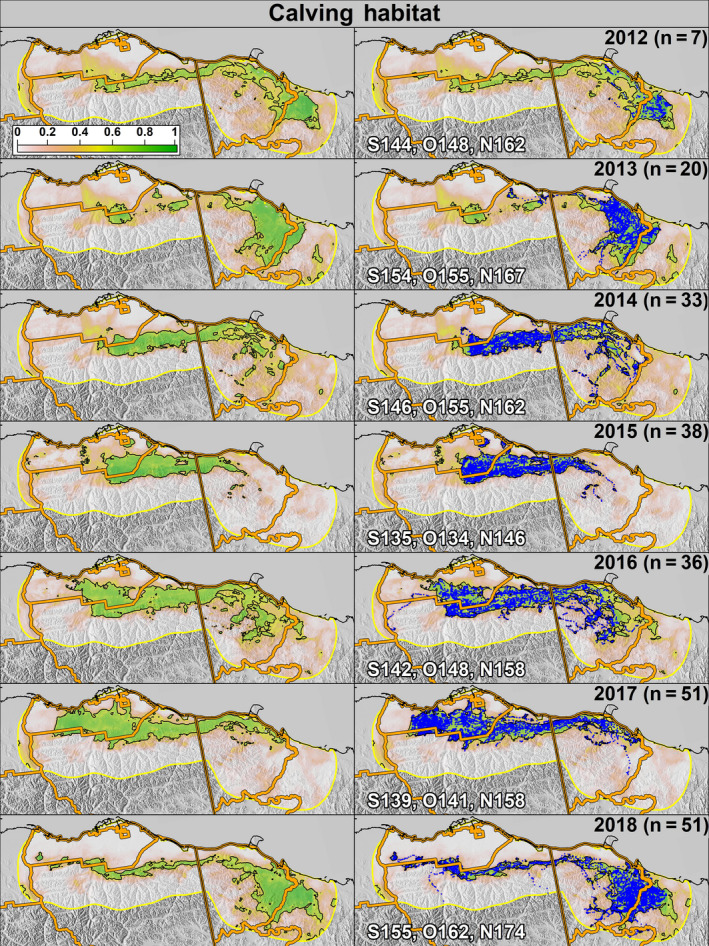
Predicted probability of calving habitat use for the Porcupine Caribou Herd from 2012 (top) to 2018 (bottom) as a function of annual variation in snow melt and vegetation greening. The left column shows only the predicted probabilities and the right column includes caribou locations (blue dots). The number of individuals tracked each year is represented as “n.” The black outline depicts areas classified as suitable habitat. White lettering represents the median ordinal date of snow melt (S), onset of greenness (O), and 50% max NDVI (N) in the study area each year
FIGURE 5.
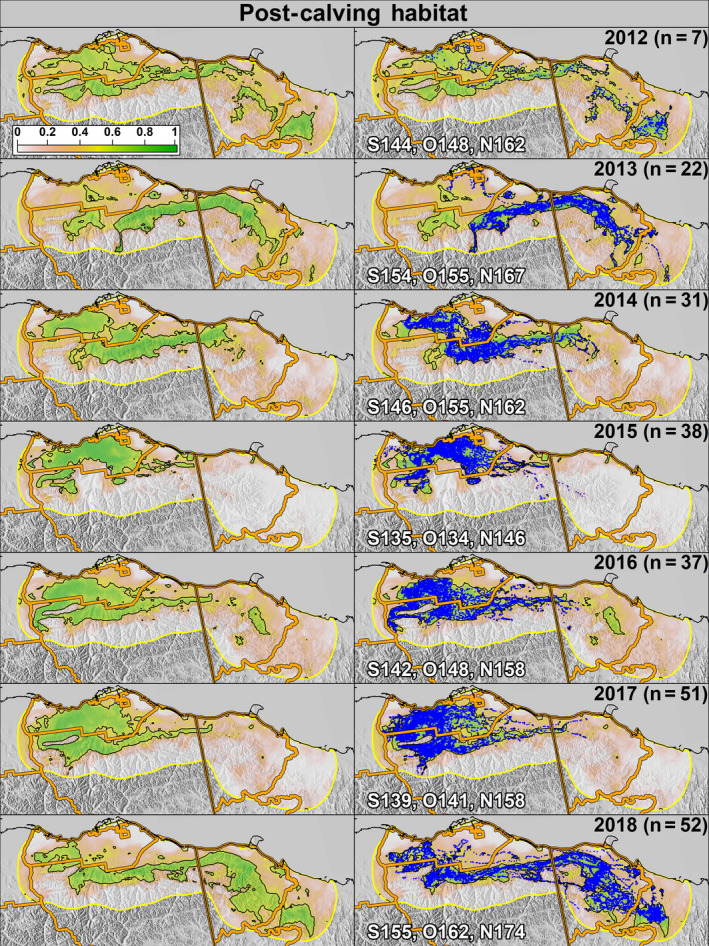
Predicted probability of post‐calving habitat use for the Porcupine Caribou Herd from 2012 (top) 2018 (bottom) as a function of annual variation in snow melt and vegetation greening. The left column shows only the predicted probabilities and the right column includes caribou locations (blue dots). The number of individuals tracked each year is represented as “n.” The black outline depicts areas classified as a suitable habitat. White lettering represents the median ordinal date of snow melt (S), onset of greenness (O), and 50% max NDVI (N) in the study area each year
FIGURE 6.
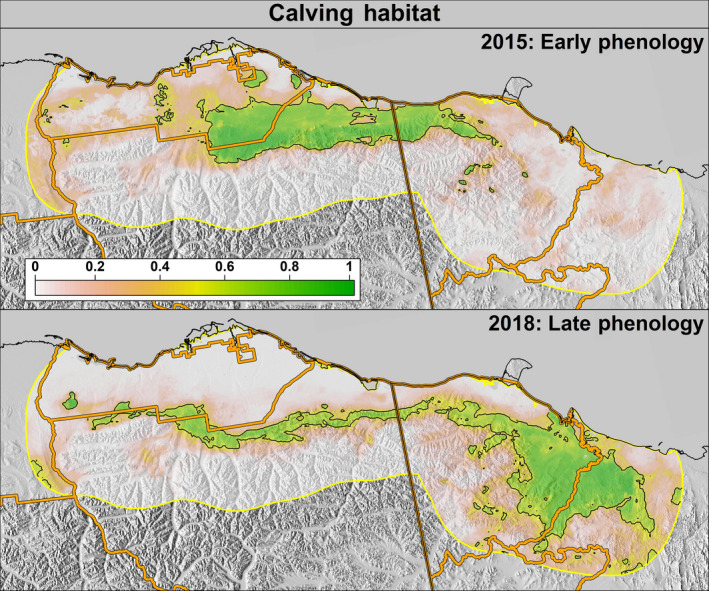
Examples of predicted probabilities of Porcupine Caribou Herd calving habitat use during years when phenology was early (2015; top) and late (2018; bottom). The black outline depicts areas classified as suitable habitat. The median phenology ordinal dates in 2015 and 2018, respectively, were snow melt: 135 and 155; onset of greenness: 134 and 162; 50% max NDVI: 146 and 174
FIGURE 7.
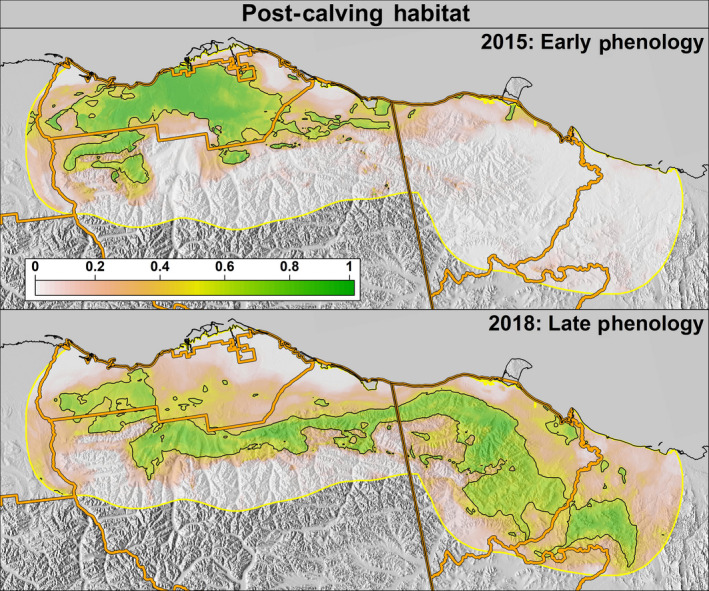
Examples of predicted probabilities of Porcupine Caribou Herd post‐calving habitat use during years when phenology was early (2015; top) and late (2018; bottom). The black outline depicts areas classified as a suitable habitat. The median phenology ordinal dates in 2015 and 2018, respectively, were snow melt: 135 and 155; onset of greenness: 134 and 162; 50% max NDVI: 146 and 174
3.2. Projected space‐use under future climate conditions
The recursive selection models for all three phenology metrics performed better than the global models (Supporting Information S6; Table S6.1). The snow melt model included 10 variables with a predictive R 2 of 0.93, the onset of greenness model included 10 variables with a predictive R 2 of 0.82, and the 50% max NDVI date model included 17 variables with a predictive R 2 of 0.68. The top two variables for all phenology models were spring temperature and May SWE (Supporting Information S6; Figure S6.1), whereas the terrain variables had less influence. Summer temperature and precipitation were also important in predicting the 50% max NDVI date.
Our models predicted that spring phenology would advance in the study area in the future. For our study period (2012–2018) and for the future decades of the 2030s, 2040s, and 2050s, the median snow melt dates across the study area were 148, 148, 140, and 140, respectively. The median onset of greenness dates were 149, 147, 138, and 134, and the median 50% max NDVI dates were 161, 162, 154, and 152. Thus, between the study period and the 2050s, the median snow melt date, onset of greenness date, and 50% max NDVI dates advanced by 8, 15, and 9 days, respectively. During the study period, median onset of greenness dates occasionally preceded snow melt dates due to differences in the spatial resolution of the original data (e.g., onset of greenness was estimated at 250‐m pixel resolution, while snow melt was the 500‐m pixel resolution).
In the future, projected average calving and post‐calving ranges generally depicted increased use of western and northern portions of the range (i.e., Alaska and further north onto the coastal plain) and decreased use of the eastern portion of the range (i.e., Yukon; Figures 8 and 9). Comparing the present average distribution to the 2050s projected average distribution, the area delineated as suitable calving habitat was projected to increase by 115% in Alaska, decrease by 31% in Yukon, and increase by 46% overall (Table 1). The area delineated as suitable post‐calving habitat was projected to decrease by 4% in Alaska, decrease by 88% in Yukon, and decrease by 13% overall (Table 1). The average delineated habitat in the 1002 Area, which allows for potential energy development, was projected to increase by 429% and 35% during the calving and post‐calving seasons, respectively.
FIGURE 8.

Average predicted calving resource use for the Porcupine Caribou Herd during 2012–2018 (top) and average projected use during the 2030s, 2040s, and 2050s. The black outline depicts areas considered suitable habitat. Predictions are based on projected climate data from representative concentration pathway 8.5
FIGURE 9.
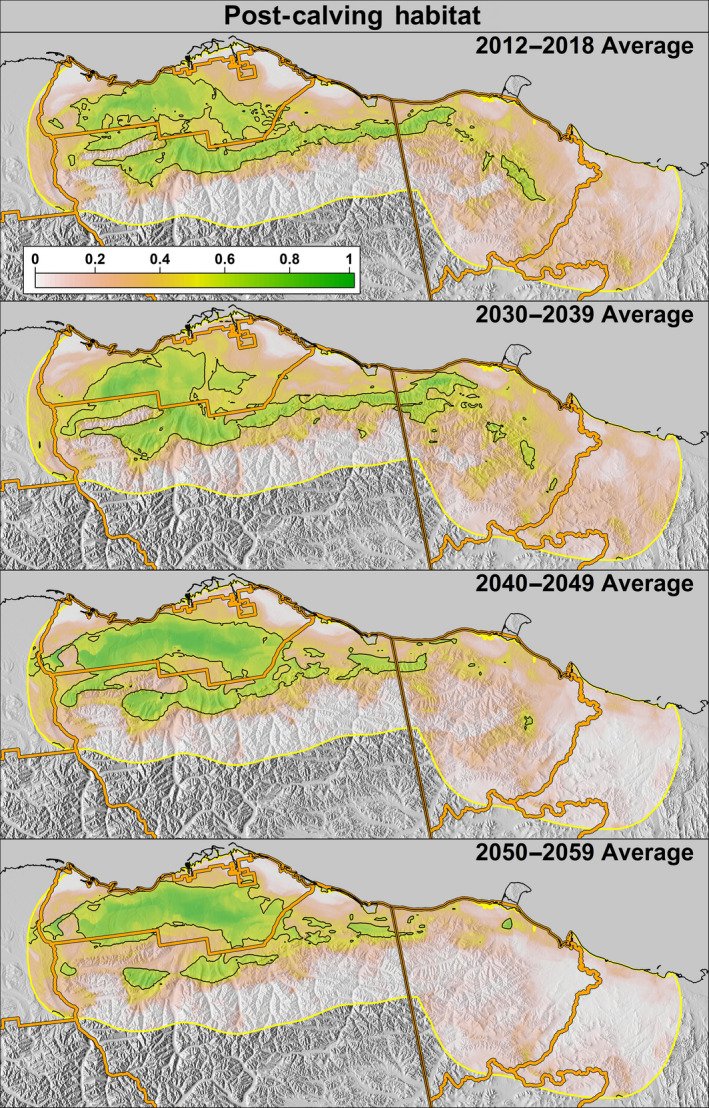
Average predicted post‐calving resource use for the Porcupine Caribou Herd during 2012–2018 (top) and average projected use during the 2030s, 2040s, and 2050s. The black outline depicts areas considered a suitable habitat. Predictions are based on projected climate data from representative concentration pathway 8.5
TABLE 1.
Present (2012–2018 average) and projected (decadal averages) future suitable habitat during the calving and post‐calving seasons for the Porcupine Caribou Herd. The percent change was calculated between present delineated habitat and the 2050s projected habitat. The area was calculated from average suitability values and does not represent the total suitable area among years. Suitable habitat is shown for different jurisdictional boundaries including that within Alaska, Yukon, the Arctic National Wildlife Refuge (ANWR), the management units within ANWR (1002 Area and Wilderness Area), and Ivvavik National Park
| Season | Boundary | Average annual habitat area (km2) | ||||
|---|---|---|---|---|---|---|
| Present | 2030s | 2040s | 2050s | % Change | ||
| Calving | Alaska | 1829.6 | 2542.3 | 3541.3 | 3935.4 | 115.1 |
| ANWR | 1824.4 | 2486.9 | 3541.3 | 3934.5 | 115.7 | |
| 1002 area | 316.3 | 922.4 | 1522.1 | 1673.5 | 429.2 | |
| Wilderness area | 1507.9 | 1564.3 | 2014.5 | 2255.3 | 49.6 | |
| Yukon | 1647.4 | 1760.4 | 1497.7 | 1128.2 | −31.5 | |
| Ivvavik National Park | 1511.8 | 1672.1 | 1438.3 | 1103.9 | −27.0 | |
| Total | 3477.0 | 4302.7 | 5038.9 | 5063.6 | 45.6 | |
| Post‐calving | Alaska | 4902.1 | 4769.8 | 5516.5 | 4699.9 | −4.1 |
| ANWR | 4898.8 | 4746.6 | 5440.7 | 4645.0 | −5.2 | |
| 1002 Area | 2388.8 | 1968.9 | 3189.1 | 3232.6 | 35.3 | |
| Wilderness area | 2507.6 | 2769.5 | 2248.9 | 1388.6 | −44.6 | |
| Yukon | 584.3 | 881.9 | 177.7 | 72.3 | −87.6 | |
| Ivvavik National Park | 580.2 | 877.4 | 177.7 | 68.1 | −88.3 | |
| Total | 5486.4 | 5651.7 | 5694.2 | 4772.3 | −13.0 | |
4. DISCUSSION
As changing climate conditions alter seasonal phenology in habitats across the globe (Cleland et al., 2007; Parmesan, 2007), there is a critical need to understand the mechanisms by which animals adapt to phenological variation and how those mechanisms may influence animal populations in the future (Cohen et al., 2018). Our results indicate that spring phenology plays a dominant role in driving major inter‐annual shifts in calving and post‐calving distributions of the PCH, as they exhibit strong selection for specific phenological windows of snow melt and vegetation growth. While studies often examine the influence of a single phenology metric on animal space‐use or movements (e.g., the onset of greenness), our results show that caribou respond to multiple phenological processes at multiple spatial and temporal scales. In years with accelerated phenology, these patterns generally resulted in the PCH predominately using habitat in Alaska, while in years with delayed phenology, they spent more time in the Yukon (Figures 6 and 7). In the future, as Arctic temperatures increase and the snowpack declines (Lader et al., 2017; Littell et al., 2018), suitable calving and post‐calving habitat for PCH is projected to occur more frequently in Alaska as caribou move to areas in preferred phenological stages. Such changes in space‐use patterns of the PCH across the international border have significant implications for conservation planning given bilateral commitments to protect habitat and provide subsistence opportunities for indigenous communities (McMillan & Hodel, 1987).
During the calving season, snow melt and the onset of vegetation growth were strongly associated with caribou resource selection (Figure 2). Early studies of the PCH reported that calving grounds were associated with landscapes still largely covered by snow, yet noted that caribou birthed their calves in bare patches of ground where the snow had melted and new plant growth was accessible (Eastland et al., 1989; Fancy & Whitten, 1991). Our multiscale analysis was clearly able to quantify both these patterns, demonstrating that caribou selection for snow melt and green‐up are indeed scale‐dependent. Caribou selected habitat at coarse scales (5 and 10 km) that were still mostly snow covered until early in the calving season, but within those landscapes caribou used areas at the pixel scale that had been snow‐free for several days and were starting to green (Figure 3a,b). Caribou use of habitat with mottled snow has been hypothesized to reduce predation rates on calves, while providing adult females access to high‐quality, immature cottongrass (Eriophorum) flowers which emerge shortly after snow melt (Fancy & Whitten, 1991; Griffith et al., 2002; Johnstone et al., 2002). In response to inter‐annual differences in phenology, female caribou appear to move as far west along the coastal plain as possible into the receding snow edge during the calving season, occupying patches of snow‐free habitat with greening vegetation. In some years, our calving model identified “islands” of suitable habitat that appeared to be unoccupied by caribou (Figures 4 and 5). We hypothesize that such areas were likely inaccessible to caribou due to barriers created by large expanses of persistent snow cover, and additional analyses specifically assessing connectivity may be beneficial.
During the post‐calving season, caribou selected habitat most strongly based on forage conditions, targeting areas where green‐up had commenced approximately a week prior to the start of the post‐calving season, and where vegetation reached 50% max NDVI early in the post‐calving season (Figure 3c). These results support the forage maturation hypothesis where herbivores are predicted to prefer earlier vegetation growth stages where the tradeoff between biomass and forage quality is optimized (Fryxell, 1991; Hebblewhite et al., 2008). On the coastal plain, digestible energy for caribou is relatively high throughout the growing season, generally remaining above the threshold needed to store body reserves (Barboza et al., 2018). Digestible protein, however, only remains above the threshold for body storage during early summer (Barboza et al., 2018), providing a short window for caribou to amass protein to reproduce the following year, while also meeting the increased demands of lactation (Barboza & Parker, 2008; Taillon et al., 2013). In the Central Arctic Caribou Herd, located directly to the west of PCH, Johnson et al. (2018) found that forage protein peaked when NDVI was approximately half of its maximum value, suggesting that selection for 50% max NDVI may also serve as a proxy for protein acquisition. By shifting the location of their annual post‐calving range to use earlier phenological stages of vegetation, caribou ensure access to high protein forage during the short window it is available. This behavior is analogous to large herbivores in other systems “surfing the green wave” as they track new vegetation growth along latitudinal or elevation gradients (Aikens et al., 2020; Merkle et al., 2016). In our study system, however, the PCH follows the phenological wave from the Yukon (east side of the study area) where snow melt and green‐up first occurs, to Alaska (west side of the study area) where snow melt and green‐up commence later. We did not account for the influence of insect harassment in our analyses, as this typically begins in late June, toward the end of the post‐calving season (Russell et al., 1993). It is important to note, however, that warmer weather could result in earlier emergence of insects and increased harassment of caribou during the post‐calving season (Culler et al., 2015), which could subsequently alter caribou space‐use and foraging efficiency (Witter et al., 2012).
Because caribou selected for specific phenological stages during the calving and post‐calving seasons, annual variation in phenology was responsible for dramatic shifts in PCH space‐use (Figures 6 and 7). Indeed, only phenology variables were temporally dynamic in our resource selection models (i.e., landcover and terrain variables were static), but these variables successfully predicted extreme shifts in space‐use (Figures 4 and 5). This demonstrates the importance of preferred phenological windows in defining early summer habitat for the PCH, and how the locations of important habitat areas can shift based on environmental conditions (Ito et al., 2013; Singh et al., 2010). In years of early phenology, our models predicted the PCH to predominately use Alaska during both the calving and post‐calving seasons, while in years of late phenology, they predicted greater use of the Yukon during the calving season and a distribution spanning the international border during the post‐calving season. While other recent studies of resource selection have considered temporal variation in habitat conditions (e.g., Dupke et al., 2017; Resano‐Mayor et al., 2019), our work underscores the importance of phenology in driving early summer PCH behavior and may help explain dramatic range shifts for other populations of caribou (Taillon et al., 2012) and other large herbivores. It is important to note that our study period (2012–2018; based on available GPS collar data) included wide variation in early summer PCH distributions, yet did not include the full extent of spatial variation exhibited since the herd has been monitored (BLM, 2019; PCTC, 1993).
Future climate projections are often constrained to temperature and precipitation metrics, limiting their utility in studies of animals that may not directly respond to these parameters (Berteaux et al., 2006; Seavy et al., 2008). By using temperature and precipitation variables to model variation in spring snow melt and vegetation phenology, however, we were able to link climate projections to our models of caribou resource selection. In doing so, we found that earlier springs were likely to increase the PCH use of the western portion of their early summer range in Alaska (Figures 8 and 9). While the Yukon is likely to remain an important spring migratory path for caribou, by 2050, our models project that the average amount of suitable calving habitat in the Yukon will decline by 32% and post‐calving habitat by 88%. Conversely, in Alaska, the average amount of suitable calving habitat is projected to substantially increase (115%) as areas further west and near the coast experience earlier snow melt and vegetation growth, while the amount of post‐calving habitat will remain relatively static (Table 1). It is important to recognize that these averages serve only as a point of comparison for habitat predicted to be most frequently used, and do not encompass the extent of suitable habitat in all years; collectively, however, they suggest that the Alaskan coastal plain will become increasingly important for the PCH.
Recent plans to produce oil in the 1002 Area of ANWR (BLM, 2019) have raised concerns about the loss of habitat and connectivity within the PCH early summer range, given that caribou tend to avoid industrial development, particularly during the calving season (Cameron et al., 2005; Johnson et al., 2020). We found that most of the projected future increases in suitable habitat in Alaska occurred within the 1002 Area (Table 1; Figures 8 and 9), particularly during the calving season, emphasizing the increased importance of this habitat under future climate conditions. Such projected shifts in habitat use highlight the potential limitations of using past locations for future land‐use planning (e.g., BLM, 2019), as changes in PCH distributions may significantly alter the anticipated impacts of proposed development. Whereas, the neighboring Central Arctic Caribou Herd shifted their calving range within coastal plain habitat when the Kuparuk oil field was developed (Cameron et al., 2005), coastal plain habitat for the PCH is already constrained to a narrow band between the Arctic Ocean and the Brooks Range (Figure 1). As a result, there are limited options for displacement, which are likely to be further constrained in the future by the availability of preferred habitat under advancing phenology.
Climate‐driven temporal shifts in phenology can create “mismatches” between resource availability and animal life‐history requirements (Visser & Gienapp, 2019), with potential demographic consequences (Ross et al., 2017). Although investigators have raised concerns that accelerated spring phenology may cause the timing of peak nutrient availability to be misaligned with the timing of parturition and lactation in caribou (when nutritional requirements are maximized; Parker et al., 2009), the evidence for such an effect remains unclear. For example, studies have found that earlier springs were associated with negative effects (Post & Forchhammer, 2008), positive effects (Tveraa et al., 2013), and no effects (Vieberg et al., 2017) on reproductive success in caribou and reindeer. Recently, in the Qamanirjuaq caribou herd in Canada, Mallory et al. (2020) found that the dates of vegetation green‐up, migration, and parturition all advanced during the study period (2004–2016), with no evidence of a mismatch. Their study suggests that caribou can make temporal adjustments when they access resources and even the timing of their own biology, to adapt to changing phenology. Our results demonstrate that caribou can also make spatial adjustments in response to phenological variation, moving to where available resources are consistent with their needs.
In our resource selection analyses, we found that scale was an important consideration for improving model fit, with habitat attributes evaluated at larger buffers (5 and 10 km) yielding greater predictive power than smaller ones. Given that we investigated landscape‐scale, second‐order selection (Meyer & Thuiller, 2006) for a species with high movement rates (Joly et al., 2020), it is unsurprising that phenology and habitat variables were most strongly selected at such coarse scales. That said, the practice of explicitly testing different spatial scales of selection within resource selection models (e.g., Anderson et al., 2005; Meyer et al., 1998) has not been commonly employed for caribou, as analyses typically use a single spatial scale that is determined a priori (e.g., 1 km). Caribou have been found to respond to human disturbance at landscape scales (several km; Boulanger et al., 2012; Johnson et al., 2020), and we found that they similarly respond to natural environmental features at such scales, an analysis detail which should be considered in future studies. It is important to note, however, that evaluations of finer‐scale selection or movement analyses (i.e., the scale of the foraging patch) would likely detect selection at appropriately smaller buffers.
While our analyses elucidate the role of spring phenology on caribou resource selection and space‐use, there were several limitations that are important to recognize. For example, the climate projection dataset we used provided the best available localized downscaling for Alaska (Lader et al., 2017; Walsh et al., 2018), but only included the RCP 8.5 scenario. Recent studies suggest that warming approximating RCP 8.5 has become increasingly likely (Peters et al., 2013; Schwalm et al., 2020a, 2020b), but it would have been preferable to explore additional scenarios. Additional GCMs may also have been helpful in producing more accurate future mean trends in climate variables, but only two were available in the climate dataset we used, which had demonstrated proficiency in Alaska (Bieniek et al., 2018; Lader et al., 2017; Walsh et al., 2018). Additionally, our projections assumed that landcover was static, as future predictions of landcover change were not available for our study area. While we know that the composition of Arctic vegetation is changing over time (Myers‐Smith et al., 2011), such changes are often heterogeneous and subtle (Bjorkman et al., 2020; Pattison et al., 2015), and complicated by the additional influence of declining sea ice (Buchwal et al., 2020), thereby limiting our ability to speculate about their effects on future caribou distributions. Lastly, we investigated PCH resource selection during years (2012–2018) when the herd was large and relatively stable in size (ca. 197,000–218,000), but population estimates have ranged from a low of approximately 100,000 in the early 1970s to the present highs (Caikoski, 2020). Because early summer resource selection could be density dependent (van Beest et al., 2016), habitat relationships could vary in the future as a function of population size.
Our results demonstrate the importance of spring phenology in driving large‐scale shifts in space‐use patterns of the PCH, but the influence of these shifts on caribou demographic trends remains unclear. Animals have evolved to select habitat that maximizes their fitness (Fretwell & Lucas, 1970); however, habitat that is available in the appropriate phenological stage (e.g., relative to snow melt and green up) may vary in quality due to underlying differences in plant composition, predator densities, or other factors (Griffith et al., 2002). For example, Jorgenson et al. (2002) found that the abundance of preferred forage plants was higher in western portions of the coastal plain in ANWR than sampling locations further to the south and east, suggesting that forage composition for caribou varies spatially across their early summer range. An important next step will be to determine how phenology‐driven changes in space‐use influence the ability of the PCH to obtain nutrients, survive, and reproduce, and how such relationships could be altered in the future. Given our results, and uncertainty in how different habitat areas confer fitness benefits for caribou, it will be critical to maintain habitat that enables behavioral flexibility as animals respond to changing environmental conditions (Ito et al., 2013; Singh & Milner‐Gulland, 2011; Taillon et al., 2012).
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We appreciate the comments and guidance that improved this analysis and manuscript, including those from J. Caikoski, J. Pearce, V. Patil, L. Adams, J. Littell, S. McAfee, P. Bieniek, and T. Kurkowski. We thank the Porcupine Caribou Technical Committee for providing location data, and particularly M. Kienzler for technical assistance. This project was funded by the U.S. Fish and Wildlife Service and the USGS Changing Arctic Ecosystems Initiative which is supported by the Wildlife Program of the USGS Ecosystem Mission Area. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Severson, J. P., Johnson, H. E., Arthur, S. M., Leacock, W. B., & Suitor, M. J. (2021). Spring phenology drives range shifts in a migratory Arctic ungulate with key implications for the future. Global Change Biology, 27, 4546–4563. 10.1111/gcb.15682
DATA AVAILABILITY STATEMENT
Porcupine Caribou Herd location data are archived by the Yukon Government and were approved for our use by the Porcupine Caribou Technical Committee. All other data used in the analysis are available from the sources cited in the manuscript. Spatial predictions from resource selection models and habitat suitability polygons are available in Severson et al. (2021).
REFERENCES
- Aikens, E. O., Mysterud, A., Merkle, J. A., Cagnacci, F., Rivrud, I. M., Hebblewhite, M., Hurley, M. A., Peters, W., Bergen, S., De Groeve, J., Dwinnell, S. P. H., Gehr, B., Heurich, M., Hewison, A. J. M., Jarnemo, A., Kjellander, P., Kröschel, M., Licoppe, A., Linnell, J. D. C., … Kauffman, M. J. (2020). Wave‐like patterns of plant phenology determine ungulate movement tactics. Current Biology, 30(17), 3444–3449.e4. 10.1016/j.cub.2020.06.032 [DOI] [PubMed] [Google Scholar]
- Anderson, D. P., Turner, M. G., Forester, J. D., Zhu, J., Boyce, M. S., Beyer, H., & Stowell, L. (2005). Scale‐dependent summer resource selection by reintroduced elk in Wisconsin, USA. Journal of Wildlife Management, 69, 298–310. [DOI] [Google Scholar]
- Baddeley, A., Rubak, E., & Turner, R. (2016). Spatial point patterns: Methodology and applications with R. Chapman & Hall/CRC Interdisciplinary Statistics Series. CRC Press, Taylor & Francis Group. [Google Scholar]
- Barboza, P. S., & Parker, K. L. (2008). Allocating protein to reproduction in Arctic reindeer and caribou. Physiological and Biochemical Zoology, 81, 835–855. 10.1086/590414 [DOI] [PubMed] [Google Scholar]
- Barboza, P. S., Van Someren, L. L., Gustine, D. D., & Bret‐Harte, M. S. (2018). The nitrogen window for arctic herbivores: Plant phenology and protein gain of migratory caribou (Rangifer tarandus). Ecosphere, 9, e02073. 10.1002/ecs2.2073 [DOI] [Google Scholar]
- Beever, E. A., Hall, L. E., Varner, J., Loosen, A. E., Dunham, J. B., Gahl, M. K., Smith, F. A., & Lawler, J. J. (2017). Behavioral flexibility as a mechanism for coping with climate change. Frontiers in Ecology and the Environment, 15, 299–308. 10.1002/fee.1502 [DOI] [Google Scholar]
- Berteaux, D., Humphries, M. M., Krebs, C. J., Lima, M., McAdam, A. G., Pettorelli, N., Réale, D., Saitoh, T., Tkadlec, E., Weladji, R. B., & Stenseth, N. C. (2006). Constraints to projecting the effects of climate change on mammals. Climate Research, 32, 151–158. 10.3354/cr032151 [DOI] [Google Scholar]
- Bieniek, P. A., Bhatt, U. S., Walsh, J. E., Lader, R., Griffith, B., Roach, J. K., & Thoman, R. L. (2018). Assessment of Alaska rain‐on‐snow events using dynamical downscaling. Journal of Applied Meteorology and Climatology, 57, 1847–1863. 10.1175/JAMC-D-17-0276.1 [DOI] [Google Scholar]
- Bieniek, P. A., Bhatt, U. S., Walsh, J. E., Rupp, T. S., Zhang, J., Krieger, J. R., & Lader, R. (2016). Dynamical downscaling of ERA‐interim temperature and precipitation for Alaska. Journal of Applied Meteorology and Climatology, 55, 635–654. 10.1175/JAMC-D-15-0153.1 [DOI] [Google Scholar]
- Bischof, R., Loe, L. E., Meisingset, E. L., Zimmermann, B., Van Moorter, B., & Mysterud, A. (2012). A migratory northern ungulate in the pursuit of spring: Jumping or surfing the green wave? The American Naturalist, 180, 407–424. 10.1086/667590 [DOI] [PubMed] [Google Scholar]
- Bjorkman, A. D., Criado, M. G., Myers‐Smith, I. H., Ravolainen, V., Jónsdóttir, I. S., Westergaard, K. B., Lawler, J. P., Aronsson, M., Bennett, B., Gardfjell, H., Heiðmarsson, S., Stewart, L., & Normand, S. (2020). Status and trends in Arctic vegetation: Evidence from experimental warming and long‐term monitoring. Ambio, 49, 678–692. 10.1007/s13280-019-01161-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger, J., Poole, K. G., Gunn, A., & Wierzchowski, J. (2012). Estimating the zone of influence of industrial developments on wildlife: A migratory caribou Rangifer tarandus groenlandicus and diamond mine case study. Wildlife Biology, 18, 164–179. 10.2981/11-045 [DOI] [Google Scholar]
- Breiman, L. (2001). Random forests. Machine Learning, 45, 5–32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- Buchholz, R., Banusiewicz, J. D., Burgess, S., Crocker‐Buta, S., Eveland, L., & Fuller, L. (2019). Behavioural research priorities for the study of animal response to climate change. Animal Behaviour, 150, 127–137. 10.1016/j.anbehav.2019.02.005 [DOI] [Google Scholar]
- Buchwal, A., Sullivan, P. F., Macias‐Fauria, M., Post, E., Meyers‐Smith, I. H., Stroeve, J. C., Blok, D., Tape, K. D., Forbes, B. C., Ropars, P., Levesque, E., Elberling, B., Angers‐Blondin, S., Boyle, J. S., Boudreau, S., Boulanger‐Lapointe, N., Gamm, C., Hallinger, M., Rachlewicz, G., … Welker, J. M. (2020). Divergence of Arctic shrub growth associated with sea ice decline. Proceedings of the National Academy of Sciences of the United States of America, 117, 33334–33344. 10.1073/pnas.2013311117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Land Management (BLM) . (2019). Coastal plain oil and gas leasing program, final environmental impact statement. U.S. Department of Interior, Bureau of Land Management. DOI/BLM/AK 0000‐2018‐0002‐EIS. [Google Scholar]
- Caikoski, J. R. (2020). Porcupine caribou herd management report and plan, Game Management Unit 25A, 25B, 25D, and 26C: Report period 1 July 2012–30 June 2017, and plan period 1 July 2017–30 June 2022. Alaska Department of Fish and Game, Species Management Report and Plan ADF&G/DWC/SMR&P‐2020‐22. [Google Scholar]
- Cameron, R. D., Smith, W. T., White, R. G., & Griffith, B. (2005). Central arctic caribou and petroleum development: Distributional, nutritional, and reproductive implications. Arctic, 58, 1–9. 10.14430/ARCTIC382 [DOI] [Google Scholar]
- Cleland, E. E., Chuine, I., Menzel, A., Mooney, H. A., & Schwartz, M. D. (2007). Shifting plant phenology in response to global change. Trends in Ecology & Evolution, 22, 357–365. 10.1016/j.tree.2007.04.003 [DOI] [PubMed] [Google Scholar]
- Cohen, J. M., Lajeunesse, M. J., & Rohr, J. R. (2018). A global synthesis of animal phenological responses to climate change. Nature Climate Change, 8, 224–228. 10.1038/s41558-018-0067-3 [DOI] [Google Scholar]
- Culler, L. E., Ayres, M. P., & Virginia, R. A. (2015). In a warmer Arctic, mosquitos avoid increased mortality from predators by growing faster. Proceedings of the Royal Society Series B: Biological Sciences, 282, 20151549. 10.1098/rspb.2015.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, D. R., Edwards, T. C., Beard, K. H., Cutler, A., Hess, K. T., Gibson, J. C., & Lawler, J. J. (2007). Random forests for classification in ecology. Ecology, 88, 2783–2793. 10.1890/07-0539.1 [DOI] [PubMed] [Google Scholar]
- Dingle, H., & Drake, V. A. (2007). What is migration? BioScience, 57, 113–121. 10.1641/B570206 [DOI] [Google Scholar]
- Dupke, C., Bonefant, C., Reineking, B., Hable, R., Zeppenfeld, T., Ewald, M., & Heurich, M. (2017). Habitat selection by a large herbivore at multiple spatial and temporal scales is primarily governed by food resources. Ecography, 40, 1014–1027. 10.1111/ecog.02152 [DOI] [Google Scholar]
- Eastland, W. G., Bowyer, R. T., & Fancy, S. G. (1989). Effects of snow cover on selection of calving sites by caribou. Journal of Mammalogy, 70, 824–828. 10.2307/1381720 [DOI] [Google Scholar]
- Evans, J. S., Murphy, M. A., Holden, Z. A., & Cushman, S. A. (2011). Modeling species distribution and change using random forest. In Drew C. A., Wiersma Y. F., & Huettmann F. (Eds.), Predictive species and habitat modeling in landscape ecology (pp. 139–159). Springer. [Google Scholar]
- Evans, J. S., Oakleaf, J., Cushman, S. A., & Theobald, D. (2014). An ArcGIS toolbox for surface gradient and geomorphometric modeling, version 2.0‐0. https://evansmurphy.wixsite.com/evansspatial/arcgis‐gradient‐metrics‐toolbox
- Fall, J. A. (2016). Regional patterns of fish and wildlife harvests in contemporary Alaska. Arctic, 69, 47–64. 10.14430/arctic4547 [DOI] [Google Scholar]
- Fancy, S. G., & Whitten, K. R. (1991). Selection of calving sites by Porcupine herd caribou. Canadian Journal of Zoology, 69, 1736–1743. 10.1139/z91-242 [DOI] [Google Scholar]
- Felton, A. M., Wam, H. K., Stolter, C., Mathisen, K. M., & Wallgren, M. (2018). The complexity of interacting nutritional drivers behind food selection, a review of northern cervids. Ecosphere, 9, e02230. 10.1002/ecs2.2230 [DOI] [Google Scholar]
- Festa‐Bianchet, M., Ray, J. C., Boutin, S., Côté, S. D., & Gunn, A. (2011). Conservation of caribou (Rangifer tarandus) in Canada: An uncertain future. Canadian Journal of Zoology, 89, 419–434. 10.1139/z11-025 [DOI] [Google Scholar]
- Fretwell, S. D., & Lucas, H. L. (1970). On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheoretica, 19, 136–156. 10.1007/bf01601953 [DOI] [Google Scholar]
- Fryxell, J. M. (1991). Forage quality and aggregation by large herbivores. The American Naturalist, 138, 478–498. 10.1086/285227 [DOI] [Google Scholar]
- Fullman, T. J., Joly, K., & Ackerman, A. (2017). Effects of environmental features and sport hunting on caribou migration in northwestern Alaska. Movement Ecology, 5, 4. 10.1186/s40462-017-0095-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith, B. G., Douglas, D. C., Walsh, N. E., Young, D. D., McCabe, T. R., Russell, D. E., White, R. G., Cameron, R. D., & Whitten, K. R. (2002). Section 3: The Porcupine caribou herd. In Douglas D. C., Reynolds P. E., & Rhode E. B. (Eds.), Arctic Refuge coastal plain terrestrial wildlife research summaries: USGS Biological Science Report 2002–0001 (pp. 8–37). USGS. [Google Scholar]
- Guisan, A., Thuiller, W., & Zimmerman, N. E. (2017). Habitat suitability and distribution models with applications in R. Cambridge University Press. [Google Scholar]
- Gunn, A., Poole, K. G., & Wierzchowski, J. (2008). A geostatistical analysis for the patterns of caribou occupancy on the Bathurst calving grounds 1966–2007. Indian and Northern Affairs. [Google Scholar]
- Gurarie, E., Hebblewhite, M., Joly, K., Kelly, A. P., Adamczewski, J., Davidson, S. C., Davison, T., Gunn, A., Suitor, M. J., Fagan, W. F., & Boelman, N. (2019). Tactical departures and strategic arrivals: Divergent effects of climate and weather on caribou spring migrations. Ecosphere, 10, e02971. 10.1002/ecs2.2971 [DOI] [Google Scholar]
- Gustine, D., Barboza, P., Adams, L., Griffith, B., Cameron, R., & Whitten, K. (2017). Advancing the match‐mis‐match framework for large herbivores in the Arctic: Evaluating the evidence for trophic mismatch in caribou. PLoS One, 12, e0171807. 10.1371/journal.pone.0171807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon, I., Weston, J., Barnhill, S., & Vapnik, V. (2002). Gene selection for cancer classification using support vector machines. Machine Learning, 46, 389–422. 10.1023/A:1012487302797 [DOI] [Google Scholar]
- Hastie, T., Tibshirani, R., & Friedman, J. H. (2009). The elements of statistical learning: Data mining, inference, and prediction (2nd ed.). Springer series in statistics. Springer. [Google Scholar]
- Haugen, R. K., & Brown, J. (1980). Coastal‐inland distributions of summer air temperature and precipitation in northern Alaska. Arctic and Alpine Research, 12, 403–412. 10.2307/1550491 [DOI] [Google Scholar]
- Hausfather, Z., & Peters, G. P. (2020). Emissions – The ‘business as usual’ story is misleading. Nature, 577, 618–620. 10.1038/d41586-020-00177-3 [DOI] [PubMed] [Google Scholar]
- Hawkins, E., & Sutton, R. (2009). The potential to narrow uncertainty in regional climate predictions. Bulletin of the American Meteorological Society, 90, 1095–1108. 10.1175/2009BAMS2607.1 [DOI] [Google Scholar]
- Hebblewhite, M., Merrill, E., & McDermid, G. (2008). A multi‐scale test of the forage maturation hypothesis in a partially migratory ungulate population. Ecological Monographs, 78, 141–166. 10.1890/06-1708.1 [DOI] [Google Scholar]
- Helm, B., Ben‐Shlomo, R., Sheriff, M. J., Hut, R. A., Foster, R., Barnes, B. M., & Dominoni, D. (2013). Annual rhythms that underlie phenology: Biological time‐keeping meets environmental change. Proceedings of the Royal Society B: Biological Sciences, 280(1765), 20130016. 10.1098/rspb.2013.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler, J. D. (2019). Validating a GPS collar‐based method to estimate parturition events and calving locations for two barren‐ground caribou herds. Thesis, University of Alaska Fairbanks. [Google Scholar]
- Intergovernmental Panel on Climate Change Climate Change (IPCC) . (2007). The physical science basis 2007. Cambridge University Press. [Google Scholar]
- Ito, T. Y., Tsuge, M., Lhagvasuren, B., Buuveibaatar, B., Chimeddorj, B., Takatsuki, S., Tsunekawa, A., & Shinoda, M. (2013). Effects of interannual variations in environmental conditions on seasonal range selection by Mongolian gazelles. Journal of Arid Environments, 91, 61–68. 10.1016/j.jaridenv.2012.12.008 [DOI] [Google Scholar]
- Johnson, H. E., Golden, T. S., Adams, L. G., Gustine, D. D., & Lenart, E. A. (2020). Caribou use of habitat near energy development in Arctic Alaska. The Journal of Wildlife Management, 84, 401–412. 10.1002/jwmg.21809 [DOI] [Google Scholar]
- Johnson, H. E., Gustine, D. D., Golden, T. S., Adams, L. G., Parrett, L. S., Lenart, E. A., & Barboza, P. S. (2018). NDVI exhibits mixed success in predicting spatiotemporal variation in caribou summer forage quality and quantity. Ecosphere, 9, e02461. 10.1002/ecs2.2461 [DOI] [Google Scholar]
- Johnstone, J., Russell, D. E., & Griffith, B. (2002). Variations in plant forage quality in the range of the Porcupine caribou herd. Rangifer, 22, 83–91. 10.7557/2.22.1.693 [DOI] [Google Scholar]
- Joly, K., Gurarie, E., Sorum, M. S., Kaczensky, P., Cameron, M. D., Jakes, A. F., Borg, B. L., Nandintsetseg, D., Hopcraft, J. G. C., Buuveibaatar, B., Jones, P. F., Mueller, T., Walzer, C., Olson, K. A., Payne, J. C., Yadamsuren, A., & Hebblewhite, M. (2020). Longest terrestrial migrations and movements around the world. Scientific Reports, 9, 15333. 10.1038/s41598-019-51884-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgenson, J. C., Udevitz, M. S., & Felix, N. A. (2002). Section 5: Forage quantity and quality. In Douglas D. C., Reynolds P. E., & Rhode E. B. (Eds.), Arctic Refuge coastal plain terrestrial wildlife research summaries: USGS Biological Science Report 2002–0001 (pp. 46–50). USGS. [Google Scholar]
- Kenny, T. A., Fillion, M., Simpkin, S., Wesche, S. D., & Chan, H. M. (2018). Caribou (Rangifer tarandus) and Inuit nutrition security in Canada. EcoHealth, 15, 590–607. 10.1007/s10393-018-1348-z [DOI] [PubMed] [Google Scholar]
- Knutti, R., & Sedlácek, J. (2013). Robustness and uncertainties in the new CMIP5 climate model projections. Nature Climate Change, 3, 369–373. 10.1038/nclimate1716 [DOI] [Google Scholar]
- Lader, R., Bhatt, U. S., Walsh, J. E., Rupp, T. S., & Bieniek, P. A. (2016). Two‐meter temperature and precipitation from atmospheric reanalysis evaluated for Alaska. Journal of Applied Meteorology and Climatology, 55, 901–922. 10.1175/JAMC-D-15-0162.1 [DOI] [Google Scholar]
- Lader, R., Walsh, J. E., Bhatt, U. S., & Bieniek, P. A. (2017). Projections of twenty‐first‐century climate extremes for Alaska via dynamical downscaling and quantile mapping. Journal of Applied Meteorology and Climatology, 56, 2393–2409. 10.1175/JAMC-D-16-0415.1 [DOI] [Google Scholar]
- Liaw, A., & Wiener, M. (2002). Classification and regression by randomForest. R News, 2(3), 18–22. 10.1023/A:1012487302797 [DOI] [Google Scholar]
- Lindsay, C., Zhu, J., Miller, A., Kirchner, P., & Wilson, T. (2015). Deriving snow cover metrics for Alaska from MODIS. Remote Sensing, 7, 12961–12985. 10.3390/rs71012961 [DOI] [Google Scholar]
- Littell, J. S., McAfee, S. A., & Hayward, G. D. (2018). Alaska snowpack response to climate change: Statewide snowfall equivalent and snowpack water scenarios. Water, 10, 668. 10.3390/w10050668 [DOI] [Google Scholar]
- Macander, M. J., Swingley, C. S., Joly, K., & Raynolds, M. K. (2015). Landsat‐based snow persistence map for northwest Alaska. Remote Sensing of Environment, 163, 23–31. 10.1016/j.rse.2015.02.028 [DOI] [Google Scholar]
- Mallory, C. D., & Boyce, M. S. (2018). Observed and predicted effects of climate change on Arctic caribou and reindeer. Environmental Reviews, 26, 13–25. 10.1139/er-2017-0032 [DOI] [Google Scholar]
- Mallory, C. D., Williamson, S. N., Campbell, M. W., & Boyce, M. S. (2020). Responses of barren‐ground caribou to advancing spring phenology. Oecologia, 192, 837–852. 10.1007/s00442-020-04604-0 [DOI] [PubMed] [Google Scholar]
- McMillan, T. M., & Hodel, D. P. (1987). Agreement between the government of Canada and the government of the United States of America on the conservation of the Porcupine Caribou Herd. https://www.treaty‐accord.gc.ca/text‐texte.aspx?id=100687 [Google Scholar]
- Merkle, J. A., Monteith, K. L., Aikens, E. O., Hayes, M. M., Hersey, K. R., Middleton, A. D., Oates, B. A., Sawyer, H., Scurlock, B. M., & Kauffman, M. J. (2016). Large herbivores surf waves of green‐up during spring. Proceedings of the Royal Society B: Biological Sciences, 283(1833), 20160456. 10.1098/rspb.2016.0456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, C. B., & Thuiller, W. (2006). Accuracy of resource selection functions across spatial scales. Diversity and Distributions, 12, 288–297. 10.1111/j.1366-9516.2006.00241.x [DOI] [Google Scholar]
- Meyer, J. S., Irwin, L. L., & Boyce, M. S. (1998). Influence of habitat adundance and fragmentation on northern spotted owls in western Oregon. Wildlife Monographs, 139, 3–51. [Google Scholar]
- Myers‐Smith, I. H., Forbes, B. C., Wilmking, M., Hallinger, M., Lantz, T., Blok, D., Tape, K. D., Marcias‐Fauria, M., Sass‐Klassen, U., & Levesque, E. (2011). Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environmental Research Letters, 6, 045509. 10.1088/1748-9326/6/4/045509 [DOI] [Google Scholar]
- Nelleman, C., & Thomsen, M. G. (1994). Terrain ruggedness and caribou forage availability during snowmelt on the Arctic coastal plain, Alaska. Arctic, 47, 361–367. 10.14430/arctic1309 [DOI] [Google Scholar]
- Newton, E. J., Abraham, K. F., Schaefer, J. A., Pond, B. A., Brown, G. S., & Thompson, J. E. (2015). Causes and consequences of broad‐scale changes in the distribution of migratory caribou (Rangifer tarandus) of Southern Hudson Bay. Arctic, 68, 472–485. https://doi.org/10.14430/arctic4524 [Google Scholar]
- Newton, E. J., Pond, B. A., Brown, G. S., Abraham, K. F., & Schaefer, J. A. (2014). Remote sensing reveals long‐term effects of caribou on tundra vegetation. Polar Biology, 37(5), 715–725. 10.1007/s00300-014-1472-3 [DOI] [Google Scholar]
- Paolini, K. E., Strickland, B. K., Teget, J. L., VerCauteren, K. C., & Street, G. M. (2018). Seasonal variation in preference dictates space use in an invasive generalist. PLoS One, 13, e0199078. 10.1371/journal.pone.0199078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, K. L., Barboza, P. S., & Gillingham, M. P. (2009). Nutrition integrates environmental responses of ungulates. Functional Ecology, 23, 57–69. 10.1111/j.1365-2435.2009.01528.x [DOI] [Google Scholar]
- Parmesan, C. (2007). Influence of species, latitudes and methodologies on estimates of phenological response to global warming. Global Change Biology, 13, 1860–1872. 10.1111/j.1365-2486.2007.01404.x [DOI] [Google Scholar]
- Parmesan, C., & Yohe, G. (2003). A globally coherent fingerprint of climate change impacts across natural systems. Nature, 421, 37–42. 10.1038/nature01286 [DOI] [PubMed] [Google Scholar]
- Pattison, R. R., Jorgenson, J. C., Raynolds, M. K., & Welker, J. M. (2015). Trends in NDVI and tundra community composition in the Arctic of NE Alaska between 1984 and 2009. Ecosystems, 18, 707–719. 10.1007/s10021-015-9858-9 [DOI] [Google Scholar]
- Peters, G. P., Andrew, R. M., Boden, T., Canadell, J. G., Ciais, P., Le Quéré, C., Marland, G., Raupach, M. R., & Wilson, C. (2013). The challenge to keep global warming below 2°C. Nature Climate Change, 3, 4–6. 10.1038/nclimate1783 [DOI] [Google Scholar]
- Pop, M. I., Iosif, R., Miu, I. V., Rozylowicz, L., & Popescu, V. D. (2018). Combining resource selection functions and home‐range data to identify habitat conservation priorities for brown bears. Animal Conservation, 21, 352–362. 10.1111/acv.12399 [DOI] [Google Scholar]
- Porcupine Caribou Technical Committee . (1993). Sensitive habitats of the Porcupine Caribou Herd. International Porcupine Caribou Board. [Google Scholar]
- Post, E., & Forchhammer, M. C. (2008). Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Philosophical Transactions of the Royal Society B: Biological Sciences, 363, 2369–2375. 10.1098/rstb.2007.2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R‐project.org/ [Google Scholar]
- Resano‐Mayor, J., Korner‐Nievergelt, F., Vignali, S., Horrenberger, N., Barras, A. G., Braunisch, V., Pernollet, C. A., & Arlettaz, R. (2019). Snow cover phenology is the main driver of foraging habitat selection for a high‐alpine passerine during breeding: Implications for species persistence in the face of climate change. Biodiversity and Conservation, 28, 2669–2685. 10.1007/s10531-019-01786-9 [DOI] [Google Scholar]
- Root, T. L., Price, J. T., Hall, K. R., Schneider, S. H., Rosenzweig, C., & Pounds, J. A. (2003). Fingerprints of global warming on wild animals and plants. Nature, 421, 57–60. 10.1038/nature01333 [DOI] [PubMed] [Google Scholar]
- Ross, M. V., Alisauskas, R. T., Douglas, D. C., & Kellett, D. K. (2017). Decadal declines in avian herbivore reproduction: Density‐dependent nutrition and phenological mismatch in the Arctic. Ecology, 98, 1869–1883. 10.1002/ecy.1856 [DOI] [PubMed] [Google Scholar]
- Russell, D. E., Martell, A. M., & Nixon, W. A. C. (1993). Range ecology of the Porcupine Caribou Herd in Canada. Rangifer, 8, 1–167. 10.7557/2.13.5.1057 [DOI] [Google Scholar]
- Samplonius, J. M., Kappers, E. F., Brands, S., & Both, C. (2016). Phenological mismatch and ontogenetic diet shifts interactively affect offspring condition in a passerine. Journal of Animal Ecology, 85, 1255–1264. 10.1111/1365-2656.12554 [DOI] [PubMed] [Google Scholar]
- Scharf, H. R., Hooten, M. B., Wilson, R. R., Durner, G. M., & Atwood, T. C. (2019). Accounting for phenology in the analysis of animal movement. Biometrics, 75, 810–820. 10.1111/biom.13052 [DOI] [PubMed] [Google Scholar]
- Schwalm, C. R., Glendon, S., & Duffy, P. B. (2020a). RCP8.5 tracks cumulative CO2 emissions. Proceedings of the National Academy of Sciences of United States of America, 117, 19656–19657. 10.1073/pnas.2007117117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalm, C. R., Glendon, S., & Duffy, P. B. (2020b). Reply to Hausfather and Peters: RCP8.5 is neither problematic nor misleading. Proceedings of the National Academy of Sciences of the United States of America, 117, 27793–27794. 10.1073/pnas.2018008117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seavy, N. E., Dybala, K. E., & Snyder, M. A. (2008). Climate models and ornithology. The Auk, 125, 1–10. 10.1525/auk.2008.125.1.1 [DOI] [Google Scholar]
- Segal, M. R. (2004). Machine learning benchmarks and random forest regression. eScholarship repository technical report. University of California. [Google Scholar]
- Severson, J. P., Johnson, H. E., Arthur, S. M., Leacock, W. B., & Suitor, M. J. (2021). Predicted calving and post‐calving season resource use of the Porcupine Caribou Herd during 2012–2018 with future projections for the 2030s, 2040s, and 2050s. USGS Data Release. 10.5066/P9TTRPAC [DOI]
- Singh, N. J., Grachev, I. A., Bekenov, A. B., & Milner‐Gulland, E. J. (2010). Tracking greenery across a latitudinal gradient in central Asia – The migration of the saiga antelope. Diversity and Distributions, 16, 663–675. 10.1111/j.1472-4642.2010.00671.x [DOI] [Google Scholar]
- Singh, N. J., & Milner‐Gulland, E. J. (2011). Conserving a moving target: Planning protection for a migratory species as its distribution changes. Journal of Applied Ecology, 48, 35–46. 10.1111/j.1365-2664.2010.01905.x [DOI] [Google Scholar]
- Skoog, R. O. (1968). Ecology of caribou (Rangifer tarandus granti) in Alaska. Dissertation, University of California, Berkley, USA. [Google Scholar]
- Taillon, J., Barboza, P. S., & Côté, S. D. (2013). Nitrogen allocation to offspring and milk production in a capital breeder. Ecology, 94, 1815–1827. 10.1890/12-1424.1 [DOI] [PubMed] [Google Scholar]
- Taillon, J., Festa‐Bianchet, M., & Côté, S. D. (2012). Shifting targets in the tundra: Protection of migratory caribou calving grounds must account for spatial changes over time. Biological Conservation, 147, 163–173. 10.1016/j.biocon.2011.12.027 [DOI] [Google Scholar]
- Terando, A., Reidmiller, D., Hostetler, S. W., Littell, J. S., BeardJr., T. D., Weiskopf, S. R., Belnap, J., & Plumlee, G. S. (2020). Using information from global climate models to inform policymaking – The role of the U.S. Geological Survey. Open‐file report 2020‐1058. U.S. Geological Survey. [Google Scholar]
- Tveraa, T., Stein, A., Bårdsen, B., & Fauchald, P. (2013). Population densities, vegetation green‐up, and plant productivity: Impacts on reproductive success and juvenile body mass in reindeer. PLoS One, 8, e56450. 10.1371/journal.pone.0056450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beest, F. M., McLoughlin, P. D., Mysterud, A., & Brook, R. K. (2016). Functional responses in habitat selection are density dependent in a large herbivore. Ecography, 39, 515–523. 10.1111/ecog.01339 [DOI] [Google Scholar]
- Van Soest, P. J. (1982). Nutritional ecology of the ruminant. O&B Books. [Google Scholar]
- Vieberg, V., Loe, L. E., Albon, S. D., Irvine, R. J., Tveraa, T., Ropstad, E., & Stein, A. (2017). Maternal winter body mass and not spring phenology determine annual calf production in an Arctic herbivore. Oikos, 126, 980–987. 10.1111/oik.03815 [DOI] [Google Scholar]
- Visser, M. E., & Gienapp, P. (2019). Evolutionary and demographic consequences of phenological mismatches. Nature Ecology & Evolution, 3, 879–885. 10.1038/s41559-019-0880-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, J. E., Bhatt, U. S., Littell, J. S., Leonawicz, M., Lindgren, M., Kurkowski, T. A., Bieniek, P. A., Thoman, R., Gray, S., & Rupp, T. S. (2018). Downscaling of climate model output for Alaskan stakeholders. Environmental Modelling & Software, 110, 38–51. 10.1016/j.envsoft.2018.03.021 [DOI] [Google Scholar]
- Wang, J. A., Sulla‐Menashe, D., Woodcock, C. E., Sonnentag, O., Keeling, R. F., & Friedl, M. A. (2019). ABoVE: Landsat‐derived annual dominant land cover across above core domain, 1984‐2014. 15817.400714 MB. ORNL Distributed Active Archive Center. 10.3334/ORNLDAAC/1691 [DOI] [Google Scholar]
- Wessel, P., & Smith, W. H. F. (1996). A global, self‐consistent, hierarchical, high‐resolution shoreline database. Journal of Geophysical Research: Solid Earth, 101, 8741–8743. 10.1029/96JB00104 [DOI] [Google Scholar]
- White, R. G., Thomsen, B. R., Skogland, T., Person, S. J., Russell, D. E., Holleman, D. F., & Luick, J. R. (1975). Ecology of caribou at Prudhoe Bay, Alaska. In Brown J. (Ed.), Ecological investigations of the tundra biome in the Prudhoe Bay Region (pp. 151–201). University of Alaska. [Google Scholar]
- Witter, L. A., Johnson, C. J., Croft, B., Gunne, A., & Gillingham, M. P. (2012). Behavioural trade‐offs in response to external stimuli: Time allocation of an Arctic ungulate during varying intensities of harassment by parasitic flies. Journal of Animal Ecology, 81, 284–295. 10.1111/j.1365-2656.2011.01905.x [DOI] [PubMed] [Google Scholar]
- Worton, B. J. (1989). Kernel methods for estimating the utilization distribution in home‐range studies. Ecology, 70, 164–168. 10.2307/1938423 [DOI] [Google Scholar]
- Wright, M. N., & Ziegler, A. (2017). ranger: A fast implementation of random forests for high dimensional data in C++ and R. Journal of Statistical Software, 77, 1–17. 10.18637/jss.v077.i01 [DOI] [Google Scholar]
- Zamin, T. J., & Grogan, P. (2013). Caribou exclusion during a population low increases deciduous and evergreen shrub species biomass and nitrogen pools in low Arctic tundra. Journal of Ecology, 101, 671–683. 10.1111/1365-2745.12082 [DOI] [Google Scholar]
- Zhu, J., Miller, A. E., Lindsay, C., Broderson, D., Heinrichs, T., & Martyn, P. (2013). MODIS NDVI products and metrics user manual, version 1.0. Geographic Information Network of Alaska, University of Alaska Fairbanks. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Porcupine Caribou Herd location data are archived by the Yukon Government and were approved for our use by the Porcupine Caribou Technical Committee. All other data used in the analysis are available from the sources cited in the manuscript. Spatial predictions from resource selection models and habitat suitability polygons are available in Severson et al. (2021).


