Abstract
The upcycling of waste biomass into valuable materials by resource‐efficient chemical transformations is a prime objective for sustainable chemistry. This approach is demonstrated in a straightforward light‐driven synthesis of polyols and polyurethane foams from the multi‐ton waste products of cashew nut processing. The photo‐oxygenation of cardanol from nutshell oil results in the formation of synthetically versatile hydroperoxides. The choice of the workup method (i. e., reduction, hydrogenation, epoxidation) enables access to a diverse range of alcohols with tunable alkene and OH functions. Condensation with isocyanates to give rigid polyurethane foams provides a resource‐efficient waste‐to‐value chain that benefits from the availability of cardanol and installation of OH groups from aerial O2.
Keywords: biomass valorization, oxidation, photochemistry, polyurethane, polyols
A tough nut to crack: The upcycling of waste biomass into valuable materials is a prime objective for sustainable chemistry. The multi‐ton waste cashew nut shell liquid is used in the light‐driven synthesis of polyols with tunable OH contents and in the subsequent preparation of rigid polyurethane foams.
Introduction
The valorization of renewable feedstocks by resource‐ and energy‐efficient chemical transformations is at the heart of sustainable chemistry (Figure 1, top).[1] Cashew nut shell liquid (CNSL) is a natural material that is produced as waste product of the cashew nut processing on an annual scale of 0.5×106 tons. Unlike carbo‐hydrates or vegetable oils, this phenolic lipid does not compete with food production. The oily liquid consists mainly of three meta‐alkylphenol derivatives: the major component anacardic acid and smaller amounts of cardol and cardanol. Thermal treatment effects decarboxylation of anacardic acid to give a crude CNSL mixture that is very rich in cardanol.[2] Cardanol itself is a mixture of phenolic lipids containing C15 alkyl chains with varying degrees of unsaturation (Scheme 1, top). These trifunctional biomass‐derived chemicals may provide ample opportunities for chemical manipulations of the hydroxy, arene, and alkene motifs toward valorized building blocks. Selected applications of cardanol include the synthesis of surfactants,[3] liquid crystals,[4] plasticizers,[5] antioxidants,[6] and pharmaceuticals[7, 8, 9] were recently reported. Conversions of cardanols to polyalcohol derivatives and further preparation of polyurethanes were also reported (Scheme 1, middle). The introduction of hydroxy functions was achieved by phenol substitution with glycols,[10] and electrophilic aromatic substitution by Mannich bases,[11] alkene epoxidation with peracid,[12a] and thiol‐ene click reactions.[12b] Herein, we report a complementary strategy that involves the direct oxygenation of cardanols with O2 under visible light irradiation, various methods of subsequent polyol formation with high control of OH content, and their addition to polyurethanes.
Figure 1.
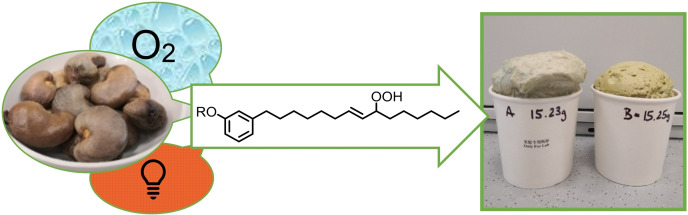
Top: Waste‐to‐value upcycling of biomass waste.[13] Bottom: Photo‐oxygenation of the cashew nut processing waste material cardanol to give polyols and rigid PU oams.
Scheme 1.
Components of CNSL (top), cardanol‐derived polyols (middle), polyol synthesis via photo‐oxygenation (bottom).
Applications of the singlet state of dioxygen (singlet oxygen, 1O2) as reagent in organic synthesis have received significant attention by virtue of its easy availability from ambient air, low price, environmental friendliness, and reactivity with various functional groups. The photosensitized generation of 1O2 in the presence of a catalytic dye and visible light is a highly sustainable method of synthesis. Sufficiently long lifetimes of singlet oxygen in various solvents enable selective oxidations of various alkenes and electron‐rich heteroatom functions.[14, 15, 16]
The CH oxidation of allylic hydrocarbon positions with singlet oxygen is an especially useful reaction that operates under very mild conditions with perfect atom economy by incorporation of both oxygen atoms from 1O2.[17, 18] The resultant allylhydroperoxides are versatile intermediates that can be readily converted to various oxygenates including alcohols, ketones, and epoxy alcohols.[19, 20, 21] High dispersions of the three distinct entities – gas (O2), light, and solution phase – may be challenging so that mass transfer across phase boundaries and light penetration depth may become rate‐determining. Several microreactor setups have been developed to overcome this limitation, such as film,[22] microchannel,[23] or tube reactors.[24, 25] We have recently introduced a home‐built, modular flow reactor that is suited to facilitate light‐driven gas‐liquid reactions and that allows wide variations of flow rate, reaction temperature, light source, and residence time. A biphasic slug flow is generated inside the reactor that ensures constant diffusion of oxygen into the liquid phase. The reactor walls withstand pressures up to 60 bar which highly increases the solubility of gases in the liquid phase. The small diameter of the inner tube results in a high surface‐to‐volume ratio and effective light penetration.[26, 27] The application of such flow reactor to the selective photo‐oxygenation of cardanol oils under visible light irradiation proved highly successful and is reported below.
Results and Discussion
Large amounts of cardanol oil mixtures are available at very low costs from the waste materials of cashew nut processing. The primary technical workup procedure of heating and distillation of the crude waste leads to decarboxylation of the salicylic acid moiety to cashew nut shell liquid (CNSL) in high purity. This mixture, containing the four cardanols as major components and very low amounts of cardol and anarcadic acid, was employed in this study. The individual cardanol olefins (monoene, diene, triene) can be chromatographically separated.[28] Photo‐oxygenations of the cardanol monoene as initial model compound, the mixture of the cardanol diene and triene, and the CNSL mixture containing all four cardanol oils were performed in a capillary flow reactor.[29]
Photo‐oxygenation of cardanol oils
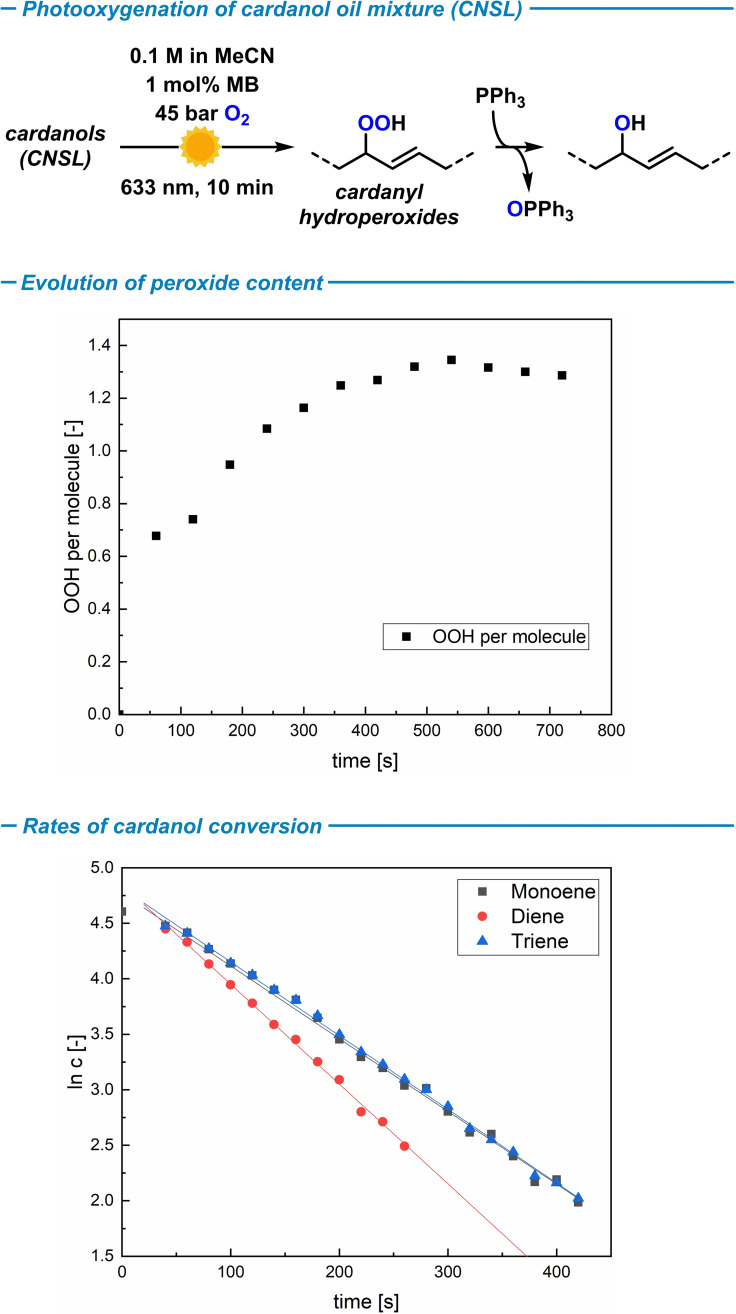
The photo‐oxygenation of cardanols is a straight‐forward method of the introduction of oxygen functions that operates via an insertion of O2 into an allyl‐H motif of hydrocarbons (Scheme 1, bottom). The reactions of the cardanol oils in acetonitrile (0.1 m) were performed in a capillary flow reactor at 20 °C with 45 bar oxygen in the presence of 1 mol% methylene blue (MB) as photocatalyst. The capillary coil was irradiated with red LEDs (633 nm) over 8–10 min (residence time inside the reactor).[29] The solvent acetonitrile enables high solubility of cardanols and the catalyst methylene blue and a reasonably long 1O2 lifetime of roughly 80 μs (longer 1O2 lifetimes can be achieved in toxicologically problematic halogenated solvents, such as CH2Cl2). Photo‐oxygenation of the cardanol monoene under the standard conditions gave full conversion after 8 min irradiation (by 1H NMR spectroscopy). Analysis of the crude product mixture revealed chemoselective formation of two regioisomeric hydroperoxides in equimolar amounts by a Schenck‐ene mechanism (Scheme 3, top).[18] The two regioisomers contained −OOH groups in the allylic C8 and C9 positions, respectively (two sets of 13C NMR signals). The double bonds adopted exclusively E‐configuration (3 J HH=15.5 Hz). Alternative oxidation pathways of the arene, alkene, and alkane moieties and further oxidation of the resultant allyl hydroperoxide in another Schenck ene process were not operative under the reaction conditions. Reactions in the absence of photosensitizer or light gave no conversions, respectively. The cardanol monoene‐derived hydroperoxides were isolated in 96 % yield by SiO2 flash column chromatography (ethyl acetate, pentane). The product mixture is relatively stable in the absence of solvents and can be stored at room temperature for several days (at −30 °C for several months) without signs of decomposition (by 1H NMR spectroscopy).
Scheme 3.
Workup methods for alcohol formation from allyl hydroperoxides.
The photo‐oxygenation of a mixture of cardanol diene and triene was performed under identical reaction conditions to give full conversion after 8 min irradiation time (Scheme 2, center). Again, no conversion was observed in the dark and in the absence of MB, respectively.
Scheme 2.
Reactor set‐up and gas/liquid slug flow of the photo‐oxygenation reactions (top); regioisomeric products of cardanol monoene oxidation (center); product mixture of cardanol diene and triene reactions (bottom).
The use of a mixture of starting materials and the number, sites, and relative orientations of oxygenation events (single/double oxygenation of alkenes; regioisomers; diastereoisomers) and alternative oxidations ([4+2]‐cycloaddition of s‐cis‐dienes with 1O2) make this reaction fairly complex. However, selectivity can be expected from increased steric crowding of higher oxygenation products and the stereoelectronic bias of alkene reactivity (terminal alkene is less reactive than internal). It is important to note that the clean formation of specific alcohol products is not the key criterion of an effective polyol synthesis for further use in polymerizations but rather the facile introduction of various oxygen functions. In full accord with this objective, the standard conditions afforded a complex mixture of oxidation products with a multitude of different OH sites. The crude product mixture was analyzed by NMR spectroscopy. Indeed, no conversion of the terminal alkene in the cardanol triene was observed. Hence, this photooxygenation may be applied as orthogonal method to oxidations that specifically convert terminal double bonds.[30, 31] Then, the CNSL mixture was subjected to the standard flow photo‐oxygenation conditions with a residence time of 10 min (Figure 2, top). The evolution of the peroxy functions was determined by downstream reduction with PPh3 and quantification of the amount of formed triphenylphosphine oxide, OPPh3. (Figure 2, center). A maximum of one peroxy function per internal double bond can be achieved from the standard 1O2‐ene reaction if over‐oxidation is excluded. Based on the composition of the CNSL mixture, (average) maximum yields of 1.58 OOH per molecule can be anticipated. The photo‐oxygenation of CNSL afforded on average 1.38 OOH groups per cardanol molecule after 9 min residence time, i. e. 87 % yield of hydrocarbon oxidation.
Figure 2.
Photo‐oxygenation of the CNSL cardanol mixture (top); oxygenation reaction progress (center); conversion rates of individual cardanol enes within the CNSL mixture (bottom).
The presence of minor amounts of unreactive impurities (such as polymeric material)[2] may give even higher chemical yields. Longer irradiation times resulted in partial decomposition of the peroxy functions, possibly due to oxygen transfer reactions to other CH and C=C sites or solvent molecules.
Further, the kinetic behavior of the photo‐oxygenation of the individual cardanol oil components was analyzed. Consumption of each cardanol ene could be monitored by HPLC analysis (the constant amount of the unreactive saturated cardanol was used as internal reference). Rates of conversions were determined at 10–40 °C with identical flow rates, O2 pressures and residence times. The O2 concentration in solution at 45 bar was 0.3 mol L−1 (experimentally determined).[32] The 1O2 concentration in MeCN is in high excess and can be considered constant over the course of the reaction when assuming uniform illumination and rapid gas diffusion. Therefore, the photo‐oxygenation follows a pseudo‐first order rate behavior, which is in full accordance with the obtained linear relationship between the logarithmic substrate concentration and reaction time (Figure 2, bottom). The individual rate constants k of the three alkene components of CNSL were determined from the ln[c]/t slopes (Table 1): The cardanol diene reacted fastest at all temperatures; the monoene and triene exhibited similar but slower rates. Related kinetic experiments on the structurally related oleic acid, linoleic acid, and linolenic acid documented that the rate of photo‐oxidation was proportional to the number of allyl‐H units (linolenic > linoleic > oleic).[33] The same trend is observed for the cardanol monoene and diene. The cardanol triene does not contain more allyl‐H units than the diene, so no higher reactivity can be expected. However, the lower reaction rate might be due to conformational restraints or physical quenching by the terminal alkene [Eq. 1]:
| (1) |
Table 1.
Rate constants k of the photo‐oxidation of cardanol alkenes.
|
Cardanol |
k at 10 °C [10−3 s−1] |
k at 20 °C [10−3 s−1] |
k at 30 °C [10−3 s−1] |
k at 40 °C [10−3 s−1] |
|---|---|---|---|---|
|
Monoene |
5.7 |
6.5 |
7.2 |
7.6 |
|
Diene |
7.9 |
8.8 |
10.1 |
10.4 |
|
Triene |
5.1 |
6.5 |
7.4 |
7.7 |
where , giving Equation 2:
| (2) |
Solvent properties play a determining role for solubilization and reactivity of reagents, the lifetime of the reactive 1O2 state, the conditions of the gas/liquid slug flow, and the overall environmental sustainability of the process. Acetonitrile (MeCN) is considered more environmentally benign than halogenated solvents, but it still exhibits harmful properties. Short‐chain alcohols display lower environmental impact[34] but result in shorter 1O2 lifetimes due to physical quenching. Consequently, the use of methanol, ethanol, and 2‐propanol as solvents, respectively, in the photo‐oxygenation of CNSL led to lower conversions after 8 min residence time in the flow reactor (Table 2).
Table 2.
Solvent dependence of O2 solubility, 1O2 lifetime, and substrate conversion.
|
Solvent |
O2 solubility at 1 bar [mm L−1] |
O2 solubility at 45 bar [mm L−1][a] |
1O2 lifetime [μs][b] |
Conversion [%] |
Extrapolated reaction time [min] |
|---|---|---|---|---|---|
|
MeCN |
8 |
300 |
80 |
99 |
(8) |
|
iPrOH |
10 |
340 |
22 |
62 |
36 |
|
EtOH |
10 |
320 |
13 |
56 |
41 |
|
MeOH |
10 |
320 |
9.5 |
51 |
50 |
[a] Experimentally determined.[32] [b] Taken from Ref. [35].
A linear correlation between 1O2 lifetime and cardanol conversion was observed (Figure 3, center): Longer lifetimes of the reactive 1O2 species increase the effective concentration of 1O2 and the overall reaction rate. Substrate conversions can be further increased by longer residence times and irradiation times. When assuming the same exponential behavior of substrate conversion as observed above, the required reaction times for full conversion can be estimated (Table 2). For example, the biomass‐derived solvent ethanol has a six‐fold lower lifetime of 1O2 which led to only half of the conversion vs. the standard conditions in MeCN. A fivefold longer reaction time for full conversion can be extrapolated. However, longer reaction times may require lower flow rates and/or longer tubular reactors but may also enhance side reactions (over‐oxidation, oxygen transfer, phenol oxidation). The photo‐oxygenation of CNSL was performed in solvent mixtures of MeCN and EtOH/MeOH. A linear relation between % MeCN solvent composition and CNSL conversion was observed (Figure 3, bottom).
Figure 3.
Solvent dependence of CNSL photo‐oxygenation: 1O2 lifetime and substrate conversion (center); reactions in MeCN/alcohol mixtures (bottom).
Workup of cardanol hydroperoxides
The synthetically versatile cardanol hydroperoxides can be transformed into a variety of functionalized molecules. The inherent phenol, alkene, and hydroperoxide motifs provide many opportunities for the introduction of other substituents, the change of redox states, and the selective removal of functional groups (defunctionalization). The reduction of the hydroperoxide and/or the alkene unit are especially straight‐forward manipulations for which several protocols are available. Alkene epoxidation was accomplished by a metal‐catalyzed self‐epoxidation mechanism, which that utilized the neighboring peroxide function, or by addition of an external oxidant. The high efficacy of such downstream diversifications has been demonstrated with cardanol monoene‐derived product mixtures. A saturated aqueous solution of inexpensive sodium sulfite (Na2SO3)[20] effected clean reduction of the crude product solution of the cardanol monoene hydroperoxides (without any workup) to give full conversion (Scheme 3a). The resultant two regioisomeric allyl alcohol derivatives were isolated in overall 79 % yield (two distinct sets of 13C NMR signals). The presence of double bonds enables various densification reactions in future applications. According to a reported protocol,[36, 37, 38] reduction of hydroperoxides to alcohols by potassium hydroxide was performed by addition of KOH pellets to the crude hydroperoxide/MeCN solution (4 h at 60 °C). The clean allylic alcohols were obtained after acidification, filtration and removal of the solvent in 70 % yield. Hydroperoxides undergo hydrogenation with H2 over Pd/C.[39, 40, 41] Consistently, the saturated alkanols were isolated from the crude photo‐oxygenation mixture in 75 % yield after hydrogenation at 10 bar H2 and 20 °C (Scheme 3b).
A major advantage is the high atom economy of 95 % and the formation of water as the only byproduct. The heterogeneous catalyst was easily removed. Minor amounts of ketone formed as byproduct. Raney nickel catalyzes hydrogenations of hydroperoxides, C=C double bonds, and carbonyl groups,[42, 43] so that such protocol may give high selectivities for the alkanol products. Allylic hydroperoxides can be transformed into epoxy alcohols by Ti(iOPr)4 or VO(acac)2 catalysts with 100 % atom economy in a formal self‐epoxidation that installs both oxygen atoms of the hydroperoxide function into the product structure (Scheme 3c).[21, 44, 45, 46] The cardanol monoene‐derived hydroperoxide solution was treated with catalytic VO(acac)2 downstream of the microreactor, which avoided purification steps. The presence of methylene blue did not impede the reaction. Full conversion was observed with low catalyst loading (1 mol% VO(acac)2) at short reaction times (<2 h) and room temperature. The epoxy alcohols were obtained in up to 68 % yield as a mixture of syn and anti isomers (ca. 1.3 : 1). The allyl alcohol (30 %) and minor amounts (<2 %) of enones were formed as byproducts.[47, 48] VO(acac)2 is also known to catalyze the epoxidation of alkenes with sacrificial inexpensive peroxides such as H2O2 or tert‐butyl hydroperoxide.[49, 50, 51] Accordingly, we adopted an epoxidation protocol by addition of 30 mol% tBuOOH to the cardanyl hydroperoxide mixture which enhanced the yield of the desired epoxy alcohols to 88 % and prevented the formation of allyl alcohol byproducts. The catalyst could be removed by adsorption on silica. The allyl hydroperoxides employed in the epoxidation reactions were mixtures of regioisomers. Thus, all products (allylic alcohols, enones, epoxy alcohols) were obtained as mixtures of regioisomers and stereoisomers. Chromatographic separation of each compound class was achieved, whilst the regioisomers could not be separated. We investigated the ring‐opening reactions of the epoxy alcohols in acidic or basic media, and with catalytic V(TPP)(OTf)2 (Scheme 3d). The resultant triols were mixtures of regioisomers (TPP=meso‐tetraphenylporphyrin, Scheme 3d) and stereoisomers (8,9,10‐ and 9,10,11‐triols, stereoisomers from photo‐oxygenation, epoxidation, hydrolysis; possibly Payne rearrangement). Therefore, no attempt was made at separating the complex alcohol mixtures. As epoxy alcohols are less reactive than alkyl‐substituted epoxides.[52] ring‐opening hydrolysis often relies on strong acids/bases, high temperatures and long reaction times.[53] The cardanol‐based triols were obtained by perchloric acid‐catalyzed hydrolysis in 74 % yield after 48 h. The perchlorate anion is a weak nucleophile that does not give esters as byproducts.[54] Base‐catalyzed hydrolysis in aqueous KOH/dioxane at 85 °C afforded the triols in 72 % overall yield. Hydrolytic ring‐opening can be achieved according to a protocol by Taghavi et al. with catalytic [V(TPP)(OTf)2].[55] The complex was freshly prepared and gave 87 % yield of the triols (2 mol% cat., 3 h). It is important to note that the synthetic versatility of the allyl hydroperoxides and the availability of functional group manipulations (reduction, oxidation, hydrolysis, rearrangement) enable flexible syntheses of diverse alcohol derivatives and polyols with one to three hydroxy functions from the same starting material (Table 3).
Table 3.
Variable oxygen and alkene functions after workup procedures.
|
Method |
OH per OOH |
Alkenes in product |
|---|---|---|
|
Reduction |
1 |
yes |
|
Hydrogenation |
1 |
no |
|
Self‐epoxidation+hydrolysis |
2 |
yes |
|
Epoxidation (tBuOOH)+hydrolysis |
3 |
no |
Application of cardanol‐derived polyols to polyurethane synthesis
Biomass‐derived polyols of low molecular weight (typically<2000 g mol−1) with high OH content can be formulated to rigid polyurethane (PU) foams. Such materials contain special cell structures with superb insulation properties that reduce heat and energy losses of electrical devices, thermal and cryogenic operations, and buildings The growing environmental awareness and rapidly rising energy costs demand new insulating materials. Here, we demonstrate the use of cardanol‐derived polyols to the synthesis of rigid PU foams. A scaled‐up photo‐oxygenation was performed with technical CNSL (Scheme 4). The reactor set‐up was modified to achieve higher productivities: The length of the illuminated capillary tubing (inner diameter 0.79 mm) was increased from 12.8 m to 39.9 m; the flow rate was increased to 2 mL/min; the residence time was 10 min. A 0.3 m solution of CNSL in MeCN with 1 mol% MB as photo‐sensitizer was employed. The average cardanol conversion of repetitive runs under these conditions was about 90 %. About 12 g of CNSL were oxygenated per hour, resulting in an average daily production of 100 g with a single custom‐made flow reactor. The obtained cardanol‐derived hydroperoxide solutions were subsequently subjected to peroxide reductions and alkene epoxidations. The crude allyl hydroperoxide solution was reduced with saturated aqueous Na2SO3 to give allyl alcohols (M w≈330 g mol−1) with OH numbers of 255–270. Epoxidation of the crude allyl hydroperoxide solution with 1 mol% VO(acac)2 followed by HClO4‐catalyzed epoxide‐opening gave polyol mixtures with OH numbers of 350–415. The viscosity of the polyol mixtures increased with higher OH contents, most likely due to enhanced intermolecular hydrogen bonding.[56] For the synthesis of a rigid PU foam, water was added as blowing agent, DABCO (1,4‐diazabicyclo[2.2.2]octane) as basic catalyst, and Niax silicone as surfactant for cell stabilization. Rigid PU foams were prepared upon reaction with the technically used “poly”‐methylene diphenyldiisocyanate (pMDI) in paper cups at room temperature (Scheme 4, bottom).[57] Employment of the cardanol‐derived polyols with OH numbers 255 and 270 gave PU foams with densities of ρ=0.044–0.079 g cm−3 which is in the typical range of rigid foams used for applications as thermal insulation materials.[58] Further performance characteristics of insulation materials, such as heat conductivity or cell structure, were not assessed.[59]
Scheme 4.
Scaled‐up polyol synthesis (top); polyurethane formation (middle); analytics of cardanol‐based PU rigid foams (bottom).
Foam samples (5 cm×5 cm×5 cm) were subjected to a stress‐strain test: The foam specimens were compressed to 60 % of their original height, and a stress‐strain curve was recorded. The Young's moduli (E c=2.81–3.46 MPa) and the compressive strengths (σ=0.240–0.294 MPa) are well comparable with bio‐based rigid foams of similar density.[60, 61] The high viscosity of polyol mixtures with higher OH numbers (350–415) prohibited rapid mixing with pMDI. However, blending with another less viscous polyol may enable effective PU formation in future studies. These results clearly document the successful application of biomass waste to solar‐driven polyol formation and onward PU foam synthesis. As PU formulation is a highly complex operation with several key parameters to be optimized (choice and stoichiometry of catalyst, surfactant, water, isocyanate, and polyol),[62] the fine‐tuning of all PU properties is beyond the scope of this study. We have demonstrated that cashew nut shell liquid is an effective resource for polyol and polyurethane production.
Conclusion
A new example of the waste‐to‐value upcycling of non‐food biomass to synthetic consumer good materials by utilization of sustainable chemical transformations has been successfully demonstrated by the conversion of cashew nut shell liquid (CNSL) to synthetically versatile polyols and polyurethanes. The photo‐oxygenation of the fatty side chains of cardanol, the major component of CNSL, afforded allyl hydroperoxide intermediates upon visible light‐driven singlet oxygen insertion. A custom‐made flow reactor ensured high dispersion of the three entities gas, liquid, light and enabled safe reactions with high product selectivities. Various onward reactions can be performed to convert the allyl hydroperoxide motifs into structurally diverse oxygenates such as allyl alcohols, alkanols, epoxy alcohols, and 1,2,3‐triols. The sequential combination of light‐driven oxygenation and functional group manipulation enabled the flexible introduction of 1–3 additional hydroxy functions into the biomass waste material. Both saturated and unsaturated polyols could be obtained, the latter class provide opportunities for further densification reactions. A larger scale reaction produced around 100 g d−1 of polyol mixtures with OH values of 250–415. The synthesis of rigid polyurethane foams was successfully performed.
The implementation of such sustainable strategy comprising of visible light‐driven oxygenation of waste materials and catalytic transformation to a diverse set of functional building blocks may serve as a general platform that can easily be extended to other substrate classes including non‐food fats, carbohydrates, lignin, but also petrochemical end‐of‐life polymers and waste products.
Experimental Section
Photooxygenation of cardanol monoene
Cardanol monoene (0.423 g, 1.41 mmol) was dissolved in acetonitrile (0.1 m), and methylene blue was added (1 mol%). Ultrasonication assured a homogenous solution which was injected to the flow reactor at 20 °C and irradiated for 8 min with 24 red LEDs in roughly 13 m long 1/16 inch (0.79 mm inner diameter) FEP tubing at 45 bar O2.[19] After photo‐oxidation, the solvent was removed and the crude product purified by column chromatography (ethyl acetate/pentane 1 : 5) to give the regioisomeric mixture of cardanol monoene hydroperoxides (0.449 g, 1.35 mmol) as orange oil in 96 % yield. R f (hexanes/ethyl acetate 4 : 1)=0.44, 1H NMR (300 MHz, CDCl3): δ=7.65 (s, 1H), 7.14 (t, 3 J=7.7 Hz, 1H), 6.74 (d, 3 J=7.7 Hz, 1H), 6.64 (d, 3 J=7.7 Hz, 2H), 5.81–5.73 (m, 1H), 5.40–5.32 (m, 1H), 4.69 (d, 1H), 4.27 (q, 3 J=6.7 Hz, 14.7 Hz, 1H), 2.55 (m, 2H), 2.10–2.04 (m, 2H), 1.71–1.24 (m, 18H), 0.91–0.85 ppm (m, 3H), 13C NMR (75 MHz, CDCl3): δ=155.5 (C), 144.8, 144.7 (C), 137.3, 137.1 (CH), 129.4 (CH), 128.6, 128.4 (CH), 120.9 (CH), 115.3 (CH), 112.6 (CH), 87.2, 87.1 (CH), 35.7 (CH2), 32.4 (CH2), 32.3, 32.2 (CH2), 31.7, 31.4 (CH2), 31.1 (CH2), 29.4, 29.2 (CH2), 29.2, 29.1 (CH2), 28.9 (CH2), 28.9, 28.7 (CH2), 25.3, 25.2 (CH2), 22.6, 22.5 (CH2), 14.1, 14.0 ppm (CH3), GC‐MS (m/z): 316 [M +‐H2O], 300, 281, 253, 207, 147, 133, 120, 108, 95, 91, 81, 77, 67, 55. For full details of reaction setups, procedures, and products, see the Supporting Information.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
This work was generously supported by the Deutsche Forschungsgemeinschaft (DFG: JA 1107/9‐1 and GrK 1626). We thank Cardolite for the provision of cashew nut shell oil. Excellent technical assistance in the PU synthesis and analysis by N. Voigt and M. Zhang (both U Hamburg) is gratefully acknowledged. Open access funding enabled and organized by Projekt DEAL.
R. Stuhr, P. Bayer, C. B. W. Stark, A. Jacobi von Wangelin, ChemSusChem 2021, 14, 3325.
Dedicated to Wittko Francke †.
References
- 1.Anastas P., Eghbali N., Chem. Soc. Rev. 2010, 39, 301–312. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran V. S., Jadhav S. R., Vemula P. K., John G., Chem. Soc. Rev. 2013, 42, 427–438. [DOI] [PubMed] [Google Scholar]
- 3.Tyman J. H. P., Bruce I. E., J. Surfactants Deterg. 2003, 6, 291–297. [Google Scholar]
- 4.John G., Minamikawa H., Masuda M., Shimizu T., Liq. Cryst. 2003, 30, 747–749. [Google Scholar]
- 5.Chen J., Liu Z., Jiang J., Nie X., Zhou Y., Murray R. E., RSC Adv. 2015, 5, 56171–56180. [Google Scholar]
- 6.Feng J., Zhao H., Yue S., Liu S., ACS Sustainable Chem. Eng. 2017, 5, 3399–3408. [Google Scholar]
- 7.Baader S., Podsiadly P. E., Cole-Hamilton D. J., Goossen L. J., Green Chem. 2014, 16, 4885–4890. [Google Scholar]
- 8.Mmongoyo J. A., Mgani Q. A., Mdachi S. J. M., Pogorzelec P. J., Cole-Hamilton D. J., Eur. J. Lipid Sci. Technol. 2012, 114, 1183–1192. [Google Scholar]
- 9.Shi Y., Kamer P. C. J., Cole-Hamilton D. J., Green Chem. 2019, 21, 1043–1053. [Google Scholar]
- 10.Suresh K. I., Kishanprasad V. S., Ind. Eng. Chem. Res. 2005, 44, 4504–4512. [Google Scholar]
- 11.Ionescu M., Wan X., Bilić N., Petrović Z. S., J. Polym. Environ. 2012, 20, 647–658. [Google Scholar]
- 12.
- 12a.Suresh K. I., ACS Sustainable Chem. Eng. 2013, 1, 232–242; [Google Scholar]
- 12b.Wang H., Zhou Q., ACS Sustainable Chem. Eng. 2018, 6, 12088–12095. [Google Scholar]
- 13.Zhou C., Wang Y., Sci. Technol. Adv. Mater. 2020, 21, 787–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clennan E. L., Pace A., Tetrahedron 2005, 61, 6665–6691. [Google Scholar]
- 15.Ogilby P. R., Chem. Soc. Rev. 2010, 39, 3181–3209. [DOI] [PubMed] [Google Scholar]
- 16.DeRosa M., Crutchley R. J., Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar]
- 17.Prein M., Adam W., Angew. Chem. Int. Ed. Engl. 1996, 35, 477–494. [Google Scholar]
- 18.Bayer P., Pérez-Ruiz R., Jacobi von Wangelin A., ChemPhotoChem 2018, 2, 559–570. [Google Scholar]
- 19.Mihelich E. D., Eickhoff D. J., J. Org. Chem. 1983, 48, 4135–4137. [Google Scholar]
- 20.Bayer P., Schachtner J., Májek M., Jacobi von Wangelin A., Org. Chem. Front. 2019, 6, 2877–2883. [Google Scholar]
- 21.Adam W., Griesbeck A., Staab E., Angew. Chem. Int. Ed. 1986, 25, 269–270; [Google Scholar]; Angew. Chem. 1986, 98, 279–280. [Google Scholar]
- 22.Jähnisch K., Dingerdissen U., Chem. Eng. Technol. 2005, 28, 426–427. [Google Scholar]
- 23.Carofiglio T., Donnola P., Maggini M., Rossetto M., Rossi E., Adv. Synth. Catal. 2008, 350, 2815–2822. [Google Scholar]
- 24.Meyer S., Tietze D., Rau S., Schäfer B., Kreisel G., J. Photochem. Photobiol. A 2007, 186, 248–253. [Google Scholar]
- 25.Talla A., Driessen B., Straathof N. J. W., Milroy L. G., Brunsveld L., Hessel V., Noël T., Adv. Synth. Catal. 2015, 357, 2180–2186. [Google Scholar]
- 26.Schachtner J., Bayer P., Jacobi von Wangelin A., Beilstein J. Org. Chem. 2016, 12, 1798–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayer P., Jacobi von Wangelin A., Green Chem. 2020, 22, 2359–2364. [Google Scholar]
- 28.See Supporting Information for cardanol separation procedure.
- 29.See Supporting Information for reactor details.
- 30.Mi C., Meng X. G., Liao X. H., Peng X., RSC Adv. 2015, 5, 69487–69492. [Google Scholar]
- 31.Takacs J. M., Jiang X., Curr. Org. Chem. 2005, 7, 369–396. [Google Scholar]
- 32.See the Supporting Information for the determination of O2 solubility.
- 33.Fukuzumi S., Tanaka T., J. Chem. Soc. Perkin Trans. 2 1989, 2103–2108. [Google Scholar]
- 34.Capello C., Fischer U., Hungerbühler K., Green Chem. 2007, 9, 927–934. [Google Scholar]
- 35.Wilkinson F., Brummer J. G., J. Phys. Chem. Ref. Data 1981, 10, 809–999. [Google Scholar]
- 36.Gardner H. W., Simpson T. D., Hamberg M., Lipids 1996, 31, 1023–1028. [DOI] [PubMed] [Google Scholar]
- 37.Gardner H. W., Simpson T. D., Hamberg M., Lipids 1993, 28, 487–495. [Google Scholar]
- 38.Simpson T. D., Gardner H. W., Lipids 1993, 28, 325–330. [Google Scholar]
- 39.Il'ina I. I., Simakova I. L., Semikolenov V. A., Kinet. Catal. 2002, 43, 652–656. [Google Scholar]
- 40.Zhu Q., Shen B., Ling H., Gu R., J. Hazard. Mater. 2010, 175, 646–650. [DOI] [PubMed] [Google Scholar]
- 41.Coleman J. E., Swern D., J. Am. Oil Chem. Soc. 1955, 32, 221–224. [Google Scholar]
- 42.E. J. Lorand, J. E. Reese, Catalytic hydrogenation of hydroperoxides, US2491926A, 1949.
- 43.Fouilloux P., Appl. Catal. 1983, 8, 1–42. [Google Scholar]
- 44.Adam W., Braun M., Griesbeck A., Staab E., Will B., Lucchini V., J. Am. Chem. Soc. 1989, 111, 203–212. [Google Scholar]
- 45.Hamberg M., Chem. Phys. Lipids 1987, 43, 55–67. [Google Scholar]
- 46.Allison K., Johnson P., Foster G., Sparke M. B., Ind. Eng. Chem. Prod. Res. Dev. 1966, 5, 166–173. [Google Scholar]
- 47.Lacoste J., Carlsson D. J., Falicki S., Wiles D. M., Polym. Degrad. Stab. 1991, 34, 309–323. [Google Scholar]
- 48.Perkel A. L., Voronina S. G., Russ. Chem. Bull. 2019, 68, 480–492. [Google Scholar]
- 49.Aschi M., Crucianelli M., Di Giuseppe A., Di Nicola C., Marchetti F., Catal. Today 2012, 192, 56–62. [Google Scholar]
- 50.Talsi E. P., Chinakov V. D., Babenko V. P., Zamaraev K. I., J. Mol. Catal. 1993, 81, 235–254. [Google Scholar]
- 51.Itoh T., Jitsukawa K., Kaneda K., Teranishi S., J. Am. Chem. Soc. 1979, 101, 159–169. [Google Scholar]
- 52.Pritchard J. G., Long F. A., J. Am. Chem. Soc. 1956, 87, 2667–2670. [Google Scholar]
- 53.Unverferth M., Meier M. A. R., Eur. J. Lipid Sci. Technol. 2018, 120, 1800015. [Google Scholar]
- 54.Eddingsaas N. C., Vandervelde D. G., Wennberg P. O., J. Phys. Chem. A 2010, 114, 8106–8113. [DOI] [PubMed] [Google Scholar]
- 55.Taghavi S. A., Moghadam M., Mohammadpoor-Baltork I., Tangestaninejad S., Mirkhani V., Khosropour A. R., Ahmadi V., Polyhedron 2011, 30, 2244–2252. [Google Scholar]
- 56.Rothfuss N. E., Petters M. D., Environ. Sci. Technol. 2017, 51, 271–279. [DOI] [PubMed] [Google Scholar]
- 57.See Supporting Information for details on polyols and PU foam formation.
- 58.Demharter A., Cryogenics 1998, 38, 113–117. [Google Scholar]
- 59.Mort R., Vorst K., Curtzwiler G., Jiang S., RSC Adv. 2021, 11, 4375–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang M., Zhang J., Chen S., Zhou Y., Polym. Degrad. Stab. 2014, 110, 27–34. [Google Scholar]
- 61.Tu Y.-C., Kiatsimkul P., Suppes G., Hsieh F.-H., J. Appl. Polym. Sci. 2007, 105, 453–459. [Google Scholar]
- 62.Akindoyo J. O., Beg M. D. H., Ghazali S., Islam M. R., Jeyaratnam N., Yuvaraj A. R., RSC Adv. 2016, 6, 114453–114482. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information