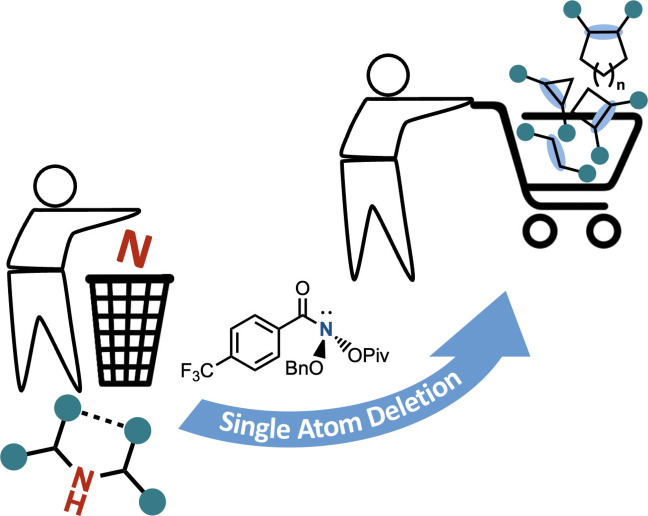
Abstract
Late‐stage modification is highly desirable for the diversification and modification of biologically active compounds. Peripheral editing (e.g., C−H activation) has been the predominant methodology, whereas skeletal editing is in its infancy. The single‐atom N‐deletion using anomeric amide reagents constitutes a powerful tool to modify the underlying molecular skeletons of secondary amines. N‐pivaloyloxy‐N‐alkoxyamide is easily prepared on a large scale and promotes C−C bond formation in good yields under the extrusion of N2 for a variety of (cyclic) aliphatic amines. The exploitation of widely available amines allows the use of existing amine synthesis protocols to translate into the construction of new C−C bonds, enabling ring contraction and the potential for structure optimization of biologically active compounds.
Keywords: amines, anomeric amide, isodiazene, late-stage, skeletal editing
A novel method for structural modification by skeletal editing of secondary amines employs N‐pivaloyloxy‐N‐alkoxyamide as a reagent. Single‐atom N‐deletion is a novel approach for constructing C−C bonds under N2 extrusion and uses widely available secondary amines in late‐stage skeletal modification enabling ring contraction and structure optimization of biologically active compounds.
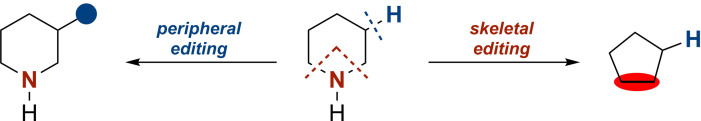
Organic synthesis relies heavily on the selective manipulation of functional groups to build molecular complexity. Aiming for operational efficiency, high atom economy, and reducing the general amount of waste produced has led to the advancement of direct C−H functionalization in organic synthesis.[1] Using the ubiquitously present C−H bonds in organic molecules to form new C−C or C−X bonds not only offers the possibility to diversify the periphery of the molecular skeleton at a late stage but is also a conceptually new retrosynthetic approach. Peripheral editing, that is C−H functionalization, is being extensively studied and refined, whereas modification of the underlying skeleton itself is yet to achieve the same level of refinement (Scheme 1).[1, 2]
Scheme 1.
Concept of peripheral and skeletal editing.
Skeletal editing by single‐atom insertion or deletion is best understood from carbonyl chemistry by which C, N, or O atoms can easily be inserted into or removed from a molecular skeleton by, e.g., Wolff, Favorskii, and Baeyer–Villiger rearrangement. The retrosynthetic simplicity of this type of skeletal editing entails an appealing strategy for late‐stage modification to build complexity.[3]
In particular, nitrogen deletion reactions, in which widely available C−N bonds (accessible via, e.g., hydroamination and iminium‐based chemistry) can serve as a surrogate for the formation of C−C bonds, are highly desirable.[4] This type of single‐atom deletion can be achieved by the intermediary formation of isodiazenes, which form diradical species that can undergo intramolecular C−C bond formation under the extrusion of N2.[5]
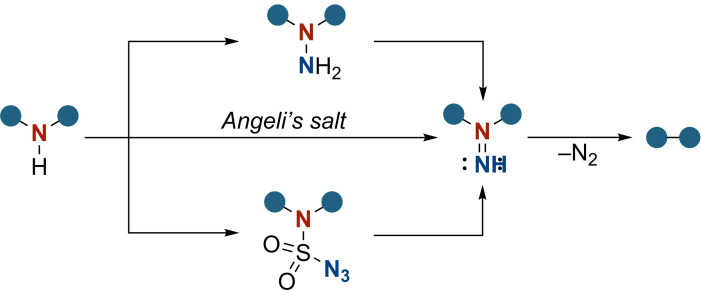
Common transformations of secondary amines into isodiazenes include their reaction with Angeli's salt (Na2[N2O3]),[6] the rearrangement of sulfamoyl azides which was recently applied by Lu and coworkers for N‐heterocyclic compounds,[7] or the oxidation of 1,1‐hydrazines with lead‐ or mercury‐based oxidants (Scheme 2).[5c] Each approach involves either hazardous reagents or laborious multi‐step procedures.
Scheme 2.
Selection of existing protocols for the synthesis of isodiazenes.
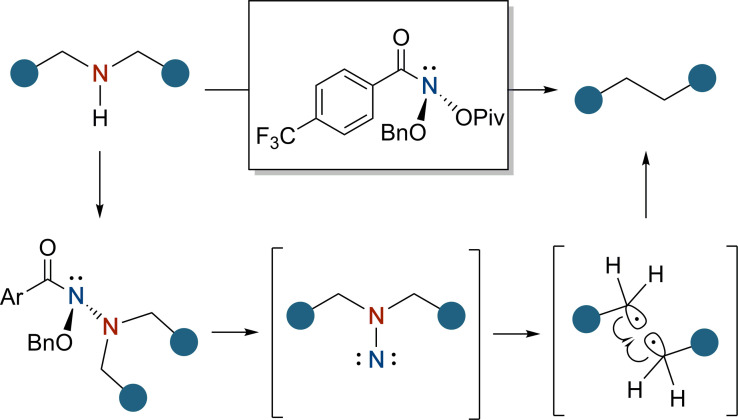
The recently reported procedure by Levin and co‐workers[8] makes use of anomeric amide reagents to achieve the deletion of nitrogen atoms from secondary amines in a single‐step procedure (Scheme 3). Anomeric amides, bearing two oxygen atoms at the amide nitrogen, display high electrophilicity, enabling the nucleophilic substitution by secondary amines. The formed ONN‐system then undergoes a concerted rearrangement (Heteroatom Rearrangement on Nitrogen, also known as HERON reaction), where the oxygen migrates from the nitrogen to the carbonyl group, forming an isodiazene intermediate.[9] The extrusion of N2 forms a radical species in stabilized or conjugated systems and enables the formation of C−C bonds.
Scheme 3.
Proposed mechanism of the nitrogen deletion initiated by anomeric amides.
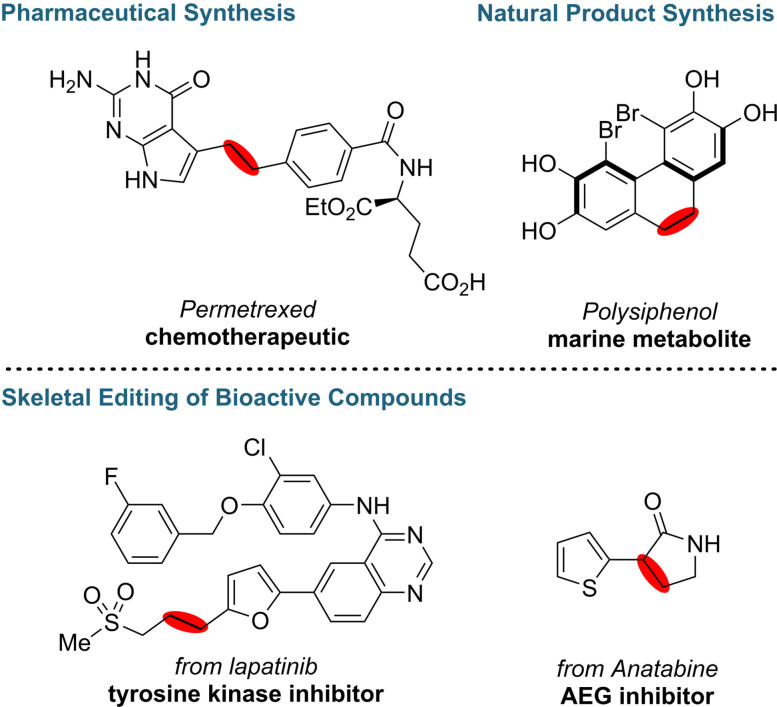
The anomeric amide reagent can be synthesized on a multi‐gram scale from commercially available reagents in excellent yield over three steps. Applying this reagent, high chemoselectivity and functional group tolerance towards basic N‐containing heterocycles, protic, reducing, and Lewis basic functionalities were observed. Additionally, cyclic amines undergo ring contraction, yielding (n−1) carbon frameworks (n=4–8). The late‐stage functionalization of highly functionalized molecules was demonstrated in various biologically active compounds that were either synthesized or modified by this methodology (Scheme 4).
Scheme 4.
N‐Deletion in the synthesis of skeletal editing of selected biologically active compounds.
So far, bulky α‐branched amines were found to impede the reaction, while hydrazone formation or radical fragmentation were competing for reactions in less stabilized or highly strained systems.
The use of such anomeric amides forgoes toxic mercury‐ or lead‐based oxidants that are used in previous protocols and are undesirable in the synthesis of pharmaceuticals. Anomeric amide reagents are more compatible with the synthesis of bioactive molecules, whereas the handling itself is of concern due to its potentially cancerogenic properties. A series of similar N‐(benzoyloxy)‐N‐(benzyloxy)benzamides have been identified via Ames assay to be mutagenic in Salmonella TA100.[10] Bearing in mind that the biological activity of such compounds is heavily dependent on their substitution, the specific compound has to be examined to rule out potential health concerns and should therefore be handled with special care.
The direct deletion of one nitrogen atom not only offers a new retrosynthetic perspective on the exploitation of amine bonds in general but may also streamline the discovery of drugs and their modification. It is a convenient and straightforward method for the formation of C−C bonds and can enable the construction of complex carbon skeletons from widely available amine precursors. The current methodology precludes the formation of C−C bonds from sterically hindered α‐substituted amines and is a factor that limits its general application. The opportunity to address aspects of scope and limitations will arise through its application in natural products or drug synthesis.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
The authors acknowledge support provided by Deutsche Forschungsgemeinschaft (DFG) under SFB/TRR 88 “3MET” and 3DMM2O, “3D Matter Made to Order” Germany's Excellence Strategy 2082/1‐390761711 as well as the Landesgraduiertenförderung (LGF) and Manchot fellowship. Open access funding enabled and organized by Projekt DEAL.
C. Zippel, J. Seibert, S. Bräse, Angew. Chem. Int. Ed. 2021, 60, 19522.
References
- 1.Roudesly F., Oble J., Poli G., J. Mol. Cat. A 2017, 426, 275–296. [Google Scholar]
- 2.
- 2a.Davies H. M., Beckwith R. E., Chem. Rev. 2003, 103, 2861–2904; [DOI] [PubMed] [Google Scholar]
- 2b.Giri R., Shi B. F., Engle K. M., Maugel N., Yu J. Q., Chem. Soc. Rev. 2009, 38, 3242–3272; [DOI] [PubMed] [Google Scholar]
- 2c.Crabtree R. H., Lei A., Chem. Rev. 2017, 117, 8481–8482; [DOI] [PubMed] [Google Scholar]
- 2d.Lam N. Y. S., Wu K., Yu J. Q., Angew. Chem. Int. Ed. 2020, 60, 15767–15790; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 15901–15924; [Google Scholar]
- 2e.Cernak T., Dykstra K. D., Tyagarajan S., Vachal P., Krska S. W., Chem. Soc. Rev. 2016, 45, 546–576. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a.Donald J. R., Unsworth W. P., Chem. Eur. J. 2017, 23, 8780–8799; [DOI] [PubMed] [Google Scholar]
- 3b.Roque J. B., Kuroda Y., Gottemann L. T., Sarpong R., Nature 2018, 564, 244–248; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3c.Hu Y., Stumpfe D., Bajorath J., J. Med. Chem. 2017, 60, 1238–1246; [DOI] [PubMed] [Google Scholar]
- 3d.Szpilman A. M., Carreira E. M., Angew. Chem. Int. Ed. 2010, 49, 9592–9628; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 9786–9823; [Google Scholar]
- 3e.Cao Z. C., Shi Z. J., J. Am. Chem. Soc. 2017, 139, 6546–6549. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a.Hartwig J. F., Pure Appl. Chem. 2004, 76, 507–516; [Google Scholar]
- 4b.Togni G., Grützmacher H., Catalytic Heterofunctionalisation, VCH, Weinheim, 2001, p. 91; [Google Scholar]
- 4c.Pohlki F., Doye S., Chem. Soc. Rev. 2003, 32, 104–114; [DOI] [PubMed] [Google Scholar]
- 4d.Afanasyev O. I., Kuchuk E., Usanov D. L., Chusov D., Chem. Rev. 2019, 119, 11857–11911; [DOI] [PubMed] [Google Scholar]
- 4e.Roughley S. D., Jordan A. M., J. Med. Chem. 2011, 54, 3451–3479; [DOI] [PubMed] [Google Scholar]
- 4f.Brown D. G., Bostrom J., J. Med. Chem. 2016, 59, 4443–4458. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a.Glover S. A., Rauk A., Buccigross J. M., Campbell J. J., Hammond G. P., Mo G., Andrews L. E., Gillson A.-M. E., Can. J. Chem. 2005, 83, 1492–1509; [Google Scholar]
- 5b.Glover S. A., Mo G., J. Chem. Soc. Perkin Trans. 2 2002, 1728–1739; [Google Scholar]
- 5c.Hinman R. L., Hamm K. L., J. Am. Chem. Soc. 1959, 81, 3294–3297. [Google Scholar]
- 6.Lemal D. M., Rave T. W., J. Am. Chem. Soc. 1965, 87, 393–394. [Google Scholar]
- 7.
- 7a.Zou X., Zou J., Yang L., Li G., Lu H., J. Org. Chem. 2017, 82, 4677–4688; [DOI] [PubMed] [Google Scholar]
- 7b.H. Qin, W. Cai, S. Wang, T. Guo, G. Li, H. Lu, Angew. Chem. Int. Ed., 10.1002/ange.202107356; Angew. Chem. Int. Ed., . [DOI]
- 8.Kennedy S. H., Dherange B. D., Berger K. J., Levin M. D., Nature 2021, 593, 223–227. [DOI] [PubMed] [Google Scholar]
- 9.Glover S. A., Tetrahedron 1998, 54, 7229–7271. [Google Scholar]
- 10.Glover S. A., Hammond G. P., Bonin A. M., J. Org. Chem. 1998, 63, 9684–9689. [Google Scholar]