Abstract
Objective
We conducted a post hoc analysis of two randomized controlled trials, GWPCARE1 (NCT02091375) and GWPCARE2 (NCT02224703), to estimate the time to onset of cannabidiol (CBD) treatment effects (seizure reduction and adverse events [AEs]) in patients with Dravet syndrome (DS).
Methods
Patients received either plant‐derived highly purified CBD (Epidiolex in the United States; 100 mg/ml oral solution) 10 mg/kg/day (CBD10; GWPCARE2) or 20 mg/kg/day (CBD20; GWPCARE1&2), or matching placebo for 14 weeks. Treatment started at 2.5 mg/kg/day, reached 10 mg/kg/day on Day 7, and went up to 20 mg/kg/day on Day 11 during the 14‐day titration period. Percentage change from baseline in convulsive seizure frequency was calculated by cumulative day (i.e., including all previous days). Time to onset and resolution of AEs were also evaluated.
Results
Overall, 124 patients received placebo and 194 received CBD (CBD10, n = 64; CBD20, n = 130). Mean age was 9.5 years (range = 2.2–18.9). Patients had discontinued a median of four antiepileptic drugs (range = 0–26) and were currently taking a median of three (range = 1–5). Differences in convulsive seizure reduction between placebo and CBD emerged during titration and became nominally significant by Day 12 for CBD20 (p = .02) and Day 13 for CBD10 (p = .03). Additionally, differences in the 50% responder rate between placebo and CBD became apparent during titration. Onset of the first reported AE occurred during the titration period in 48.4% of placebo patients and 54.1% of CBD patients. The three most common AEs of somnolence, decreased appetite, and diarrhea resolved within 4 weeks of onset in the majority of CBD‐treated patients (56.3%–72.9%).
Significance
The therapeutic effect of CBD in DS may start within 2 weeks of treatment in some patients. Although AEs lasted longer for CBD than placebo, most resolved within the 14‐week study period.
Keywords: antiepileptic drug, childhood onset epilepsy, convulsive seizures, efficacy onset
Key Points.
This post hoc analysis evaluated the time to onset of the treatment effects from two trials of CBD versus placebo in patients with DS
Patients were randomized to receive CBD 10 or 20 mg/kg/day or matching placebo; all patients reached the 10 mg/kg/day dose on Day 7
CBD treatment produced greater reduction in seizures versus placebo, and the difference emerged within 2 weeks of starting treatment
Overall, 87% of patients had AEs, with onset during the 2‐week titration period in more than half
The most common AEs of somnolence, decreased appetite, and diarrhea resolved within 4 weeks for the majority of CBD‐treated patients
1. INTRODUCTION
Dravet syndrome (DS) is a rare treatment‐resistant developmental and epileptic encephalopathy1, 2 that affects 1%–6% of children with epilepsy younger than 15 years,3, 4 with an incidence of one in 15 500 live births for SCN1A‐related DS.5 Seizures typically develop in the first year of life in infants who have otherwise been developing normally.6 DS is marked by multiple seizure types, developmental delays, abnormal electroencephalographic results over time, and often, orthopedic problems and hyperactivity1; it is associated with high rates of sudden unexpected death in epilepsy (SUDEP).6 Patients with DS present with a variety of seizure types, including generalized tonic–clonic, generalized clonic, focal clonic (hemiclonic), myoclonic, atypical absence, and focal impaired awareness seizures.6, 7 Complete seizure control is typically not achievable.6, 8 Antiepileptic drugs (AEDs) approved for DS include cannabidiol (CBD; Epidiolex in the United States and Epidyolex in the United Kingdom, European Union, and Australia),9, 10 stiripentol in conjunction with clobazam,11 and fenfluramine.12 Although valproate and clobazam are typically used as first‐line therapies to treat seizures, neither are approved for monotherapy in DS, and there is limited evidence on the efficacy and safety of either AED in DS.13
Add‐on CBD significantly reduced seizures associated with DS, Lennox–Gastaut syndrome (LGS), and tuberous sclerosis complex (TSC) in five randomized placebo‐controlled trials.14, 15, 16, 17, 18 In the two DS trials, both doses of CBD (10 and 20 mg/kg/day) were superior to placebo in reducing convulsive seizure frequency (GWPCARE1, NCT02091375; GWPCARE2, NCT02224703),14, 15 with reductions maintained in the open‐label extension trial (GWPCARE5, NCT02224573).19, 20
Prior publications of the two trials in DS did not include data on the time to onset of the therapeutic effect during the 14 weeks of treatment.14, 15 Here, we present analyses of the combined data from GWPCARE1 and GWPCARE2 to evaluate the precise timing of the CBD treatment effects onset in terms of both efficacy and incidence of adverse events (AEs) in patients with DS.
2. MATERIALS AND METHODS
GWPCARE1 and GWPCARE2 were international, randomized, double‐blind, placebo‐controlled Phase 3 trials. The trial design and patient eligibility criteria were published previously.14, 15 Briefly, the trials consisted of a 4‐week baseline period followed by a 14‐week treatment period, which included a 2‐week titration (dose escalation) and a 12‐week maintenance period. The treatment period was followed by a 10‐day tapering phase and a 4‐week safety follow‐up period. Patients who completed the blinded studies were eligible to transition to an open‐label extension study (GWPCARE5). These trials were conducted with Epidiolex/Epidyolex, and results do not apply to other CBD‐containing products.
Patients with clinically confirmed DS were eligible to enroll if they were 2–18 years of age, were taking at least one AED, and had at least four convulsive seizures during the 4‐week baseline period. Patients were excluded if they had a clinically significant unstable illness (other than epilepsy) during the 4 weeks before screening, a history of alcohol or substance abuse, use of recreational or medicinal cannabis in the previous 3 months, or current use of felbamate for less than 1 year.
Eligible patients were randomized to receive either a pharmaceutical formulation of highly purified CBD derived from the Cannabis sativa L. plant (100 mg/ml oral solution; Epidiolex in the United States and Epidyolex in the United Kingdom, European Union, and Australia; GW Research Ltd) or an equivalent volume of placebo solution (excipients only) in addition to current standard of care treatments. Patients received either 10 or 20 mg/kg/day of add‐on CBD in GWPCARE2 and 20 mg/kg/day of add‐on CBD in GWPCARE1. CBD (or matching placebo) was administered twice daily in two equally divided doses, starting at 2.5 mg/kg/day, and reaching 10 mg/kg/day by Day 7 and 20 mg/kg/day by Day 11 of the titration period (Table 1). Investigators were instructed to maintain doses of concomitant AEDs; however, dose adjustments were permitted for AEs.
TABLE 1.
Titration schedule
| Days | CBD dose received each day | |
|---|---|---|
| Target dose 10 mg/kg/day | Target dose 20 mg/kg/day | |
| 1–2 | 2.5 mg/kg | 2.5 mg/kg |
| 3–4 | 5.0 mg/kg | 5.0 mg/kg |
| 5–6 | 7.5 mg/kg | 7.5 mg/kg |
| 7–8 | 10.0 mg/kga | 10.0 mg/kg |
| 9–10 | 10.0 mg/kg | 15.0 mg/kg |
| 11–14 | 10.0 mg/kg | 20.0 mg/kga |
Study drug or matching placebo was administered daily in two equally divided doses; total daily doses are shown.
Target dose achieved.
After instruction on identification of countable seizure types at the screening visit, caregivers recorded the number and type of convulsive and nonconvulsive seizures each day using an interactive voice response system. Convulsive seizures were defined as tonic–clonic, tonic, clonic, or atonic; nonconvulsive seizures were defined as myoclonic, partial, or absence; and total seizures were all types. All patients had convulsive seizures in addition to other types of seizures; on average, 60.3% of patients’ seizures were convulsive seizures.
Efficacy and safety data for the 10‐mg/kg/day dose were from GWPCARE2, and data for the 20‐mg/kg/day dose and placebo were pooled across GWPCARE1 and GWPCARE2. The antiseizure effect of CBD was assessed by evaluating reduction from baseline in convulsive seizure frequency compared with placebo. Pooled results of the median percentage change from baseline in convulsive and total seizure frequency per 28 days for each CBD dose relative to placebo were summarized for the 2‐week titration period and each 4‐week interval of the maintenance period (Weeks 1–4, 5–8, and 9–12).
To determine a more precise time of onset of antiseizure effect, the percentage reduction in cumulative convulsive seizure count for each day (i.e., including all previous treatment days) of the treatment period was calculated using negative binomial regression. The percentage of patients with a 50% or greater reduction from baseline in convulsive seizure frequency during the treatment period by cumulative day was also calculated. Because evaluation of the antiseizure effect by day was a post hoc analysis, it is subject to multiplicity; therefore, only nominal p values are reported for the primary endpoint of percentage reduction in convulsive seizure frequency. For patients who withdrew from the trial, the seizure frequency was calculated using all available data prior to withdrawal and is included in all subsequent cumulative day summaries.
Incidences of treatment‐emergent AEs by time to onset during the 2‐week titration period and for Weeks 1–4, 5–8, and 9–12 of the maintenance period were also assessed. The time to first onset of an AE was calculated as the start date of an AE minus the date of first dose of study medication plus 1. An additional time to first event analysis by day was conducted for the most common AEs. Time to AE resolution analysis summarized incidence of AEs that resolved within 4 weeks or after 4 weeks by the end of treatment. If any of the AEs did not resolve by the end of treatment, then the AE was categorized as ongoing. The time to AE resolution was calculated as the stop date of an AE minus start date of an AE plus 1.
3. RESULTS
3.1. Patients
A total of 319 patients enrolled in the two trials and were randomized to receive CBD or placebo; however, there was one patient who was randomized in error and did not receive CBD treatment and was subsequently withdrawn. Of the 318 who were treated, 194 patients received CBD at either 10 mg/kg/day (n = 66) or 20 mg/kg/day (n = 128) and 124 patients received matching placebo. Overall, 298 patients (93.4%; CBD, n = 177; placebo, n = 121) completed the studies and 21 patients (6.6%; CBD, n = 18; placebo, n = 3) withdrew, with AEs as the primary reason for withdrawal in 14 patients (CBD, n = 13; placebo, n = 1). Nearly all patients (291/298, 97.7%) who completed the studies entered the open‐label extension trial of CBD (GWPCARE5; Figure S1).
Patients were a mean (minimum, maximum) age of 9.5 years (2.2, 18.9), with the majority (72.0%) less than 12 years of age. There was a near equal proportion of male and female patients. Patients had previously discontinued a median (minimum, maximum) of four AEDs (0, 26) and were currently taking a median (minimum, maximum) of three (1, 5) AEDs at baseline. Valproate (66.0%) and clobazam (64.2%) were the most common concomitant AEDs. Baseline characteristics were mostly consistent between the CBD and placebo groups (Table 2), with a relatively higher number of baseline seizures in the placebo group than the CBD group. The median (minimum, maximum) seizure frequency for the 28‐day baseline period for the placebo group was 16 (3, 771) for convulsive and 46 (4, 3170) for total seizures compared with the CBD group values of 11 (0, 1717) for convulsive and 26 (4, 4141) for total seizures. The most frequently reported convulsive seizure types at baseline were tonic–clonic in 288 patients (90.6%) and tonic seizures in 110 patients (34.6%) across all treatments.
TABLE 2.
Patient demographics and characteristics
| Placebo, n = 124 | CBD 10 and 20 mg/kg/day, n = 194 | |
|---|---|---|
| Age, years | ||
| Mean (minimum, maximum) | 9.7 (2.2, 18.4) | 9.4 (2.2, 18.9) |
| Age group, n (%) | ||
| 2–5 years | 35 (28.2) | 57 (29.4) |
| 6–12 years | 52 (41.9) | 85 (43.8) |
| 13–18 years | 37 (29.8) | 52 (26.8) |
| Sex, n (%) | ||
| Male | 58 (46.8) | 98 (50.5) |
| Weight at baseline, kg | ||
| Mean (minimum, maximum) | 34.6 (12.0, 88.4) | 33.6 (10.8, 133.8) |
| Race, n (%) | ||
| White | 165 (85.1) | 105 (84.7) |
| Region, n (%) | ||
| United States | 69 (55.6) | 96 (49.5) |
| Rest of the world | 55 (44.4) | 98 (50.5) |
| Baseline seizures per 28 days, median (minimum, maximum) | ||
| Convulsive | 15.5 (3, 770.5) | 10.9 (0, 1716.7) |
| Total | 45.8 (4, 3170.0) | 25.6 (3.7, 4141.0) |
| Baseline convulsive seizure types, n (%) | ||
| Tonic | 38 (30.6) | 72 (37.1) |
| Clonic | 25 (20.2) | 49 (25.3) |
| Tonic–clonic | 114 (91.9) | 174 (89.7) |
| Atonic | 21 (16.9) | 25 (12.9) |
| Number of AEDs, median (minimum, maximum) | ||
| Previous | 4 (0, 14) | 4 (0, 26) |
| Current | 3 (1, 5) | 3 (1, 5) |
| Current AEDs [>25%], n (%) | ||
| Valproate | 82 (66.1) | 128 (66.0) |
| Clobazam | 79 (63.7) | 125 (64.4) |
| Stiripentol | 45 (36.3) | 77 (39.7) |
| Levetiracetam | 31 (25.0) | 56 (28.9) |
| Topiramate | 32 (25.8) | 45 (23.2) |
Abbreviations: AED, antiepileptic drug; CBD, cannabidiol.
3.2. Efficacy
The median percentage reduction in convulsive seizure frequency per 28 days during the full treatment period was 41.2% for CBD 10 mg/kg/day (n = 66; reduced from median baseline of 13.5–6.3 per month), 45.4% for CBD 20 mg/kg/day (n = 128; reduced from median baseline of 10.4–5.6 per month), and 21.7% for placebo (n = 124; reduced from median baseline of 15.5–14.1 per month). The median percentage reduction in total seizure frequency per 28 days during the full treatment period was 51.9% for CBD 10 mg/kg/day (reduced from median baseline of 34.5–12.8 per month), 45.0% for CBD 20 mg/kg/day (reduced from median baseline of 24.6–14.9 per month), and 18.1% for placebo (reduced from median baseline of 45.8–33.0 per month).
Greater reduction in the median percentage change from baseline in convulsive and total seizure frequency for CBD treatment compared with placebo was first observed during the titration period; the treatment effect increased over the first 4 weeks of the maintenance period and was sustained over the remaining 4‐week intervals (Figure S2).
Analysis of the percentage reduction in monthly convulsive seizure frequency by cumulative day showed that sustained separation between CBD and placebo emerged as early as Day 7; significant reduction relative to placebo was observed at Day 12 for the CBD 20‐mg/kg/day group (nominal p = .02) and Day 13 for the CBD 10‐mg/kg/day group (nominal p = .03) and was maintained throughout the duration of treatment (Figure 1A). Patients had reached their target maintenance dose by Day 7 for the 10‐mg/kg/day and Day 11 for the 20‐mg/kg/day dose groups.
FIGURE 1.
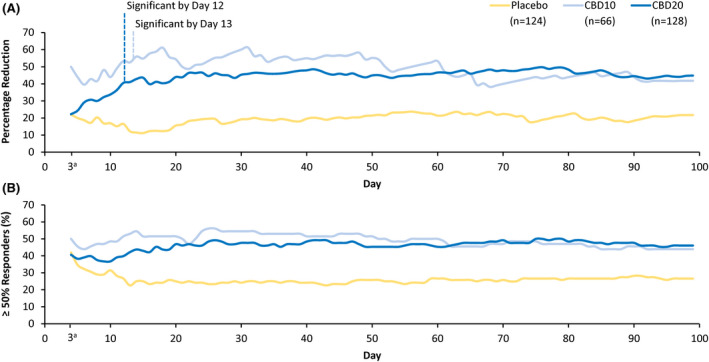
Reduction in convulsive seizure frequency during the treatment period. (A) Percentage reduction from baseline in convulsive seizure frequency by cumulative day; nominally significant by Day 12 for cannabidiol 20 mg/kg/day (CBD20; p = .02) and Day 13 for cannabidiol 10 mg/kg/day (CBD10; p = .03). (B) Percentage of patients reporting ≥50% reduction in convulsive seizure frequency by cumulative day. aData censored until Day 3 because the seizure frequency was ≈1 seizure every 2–3 days. Data are from the efficacy analysis set
A similar pattern was observed for the percentage of patients reporting both 50% or greater and 75% or greater reduction in convulsive seizure frequency by cumulative day, with clear separation from placebo as early as the second week of treatment with either dose of CBD. At least 50% reduction in convulsive seizure frequency during the treatment period was observed in 43.9% of patients (29 of 66) taking CBD 10 mg/kg/day, 46.1% (59 of 128) taking CBD 20 mg/kg/day, and 26.6% (33 of 124) taking placebo (Figure 1B). Additionally, at least 75% reduction in convulsive seizure frequency was observed in 30.3% of patients (20 of 66) taking CBD 10 mg/kg/day, 20.3% (26 of 128) taking CBD 20 mg/kg/day, and 8.9% (11 of 124) taking placebo (Figure S3).
3.3. Safety
Across the two studies, treatment‐emergent AEs were reported in 277 patients (87.1%): 102 (82.3%) taking placebo, 56 (87.5%) CBD 10 mg/kg/day, and 119 (91.5%) CBD 20 mg/kg/day (Table S1). The most commonly reported AEs were somnolence (21.7%), decreased appetite (19.5%), and diarrhea (19.5%). In all three treatment groups, somnolence was reported more frequently by patients taking concomitant clobazam compared with patients who were not (placebo, 13 [16.5%] vs. 2 [4.4%]; CBD 10 mg/kg/day, 15 [34.1%] vs. 1 [5.0%]; CBD 20 mg/kg/day, 31 [37.8%] vs. 7 [14.6%]). Serious AEs were reported in 53 patients (16.7%). There were no deaths.
AEs were listed as one of the reasons for withdrawal in 15 patients and as the primary reason for withdrawal in 14 patients. The most common AEs leading to withdrawal were somnolence (n = 7, five of whom were on clobazam), fatigue (n = 5, all of whom were on clobazam), and decreased appetite (n = 5), all of whom were taking 20 mg/kg/day CBD.
The first postdose measurement of transaminases was performed on Day 15 of treatment (i.e., after the titration period). Increases in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) more than three times the upper limit of normal (ULN) occurred in 9.1% of patients (29 of 318), all of whom were taking concomitant valproate; three were taking CBD 10 mg/kg/day, 25 CBD 20 mg/kg/day, and one placebo. In those who experienced increased ALT/AST levels more than three times ULN, elevations occurred within 30 days of starting treatment in 69.0% of patients (20 of 29) and within 60 days in 79.3% of patients (23 of 29). No patient met the standard criteria for severe drug‐induced liver injury (Hy's law). Elevated ALT/AST levels resolved in all patients either spontaneously (n = 13), following discontinuation from the trial (n = 6), following CBD, valproate, and/or clobazam dose reduction (n = 5), or after enrollment in the open‐label extension trial (n = 5). The decision to discontinue or reduce CBD or other AEDs due to AEs was made by the treating clinician.
Onset of the first AE occurred early in treatment for most patients; during the titration period in 51.9% of patients (165 of 318), and during the first 4 weeks of the maintenance period in 25.2% of patients (80 of 318; Figure 2). Among patients with AEs, onset of the first event occurred during the titration period in 59.6% of patients (165 of 277). In the additional time‐to‐first‐event analysis by day for the most common AEs, somnolence/fatigue/lethargy/sedation were combined into somnolence‐associated AEs. For the three most common AEs of somnolence‐associated, decreased appetite, and diarrhea, most patients reported the first occurrence within 20 days of starting CBD or placebo treatment (Figure 3).
FIGURE 2.
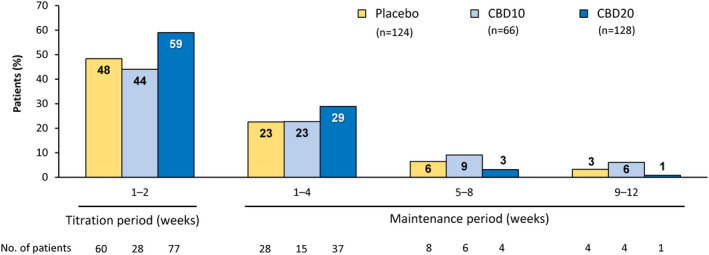
Adverse events (AEs) by time of onset. Onset of AEs occurred early in treatment for most patients: during the titration period in approximately half (165 of 318, 51.9%) and during the first 4 weeks of the maintenance period in approximately a quarter of patients (80 of 318, 25.2%). If a patient had multiple occurrences of an AE, then the AE was counted once for the first occurrence only. The percentage of patients was calculated based on the number of patients in the safety analysis set who had a visit or follow‐up call within each time period. Three patients in the cannabidiol 10 mg/kg/day (CBD10) group and two patients in the placebo group first experienced an AE after the 14‐week treatment period. The titration period includes Days 1–14 of the treatment period (i.e., time taken to reach the target maintenance dose of 10 or 20 mg/kg/day). CBD20, cannabidiol 20 mg/kg/day
FIGURE 3.
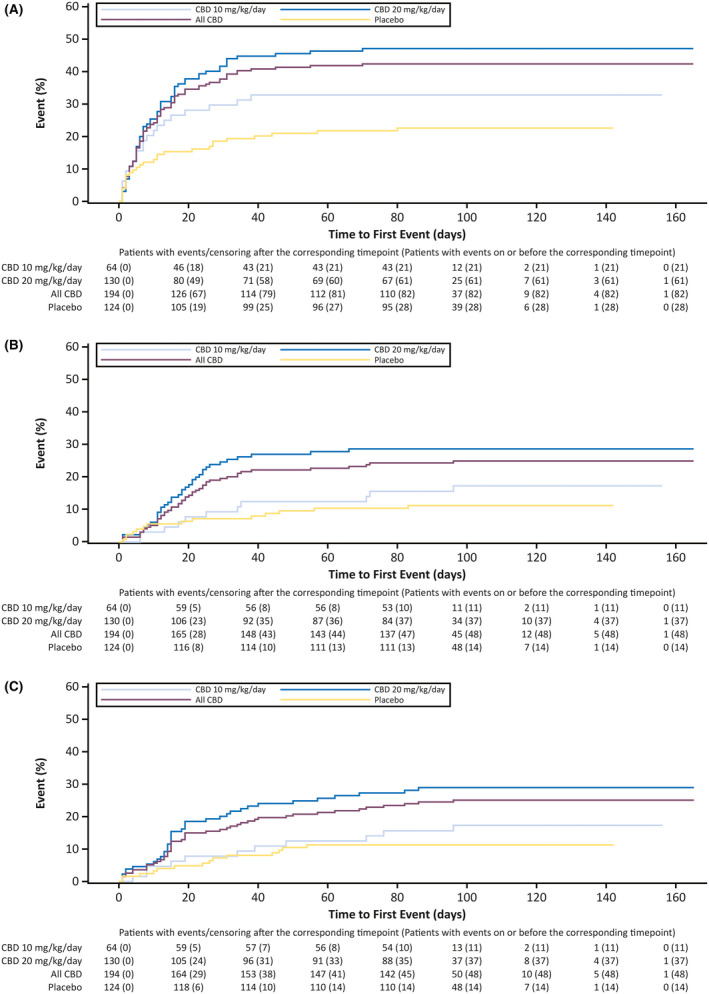
Time to first occurrence of the three most common adverse events by day: (A) somnolence/fatigue/lethargy/sedation, (B) decreased appetite, and (C) diarrhea. CBD, cannabidiol
AEs resolved by the end of the study in nearly two thirds of patients who had events (164 of 277; 59.2%): within 4 weeks of onset in 40.0% of patients (111 of 277) and after 4 weeks of onset (but before the end of the study) in 19.1% of patients (53 of 277; Table 3). AEs resolved within 4 weeks of onset in 47.1% of placebo (48 of 102) and 36.0% of CBD patients (63 of 175) and after 4 weeks of onset (but before the end of study) in 17.6% of placebo (18 of 102) and 20.0% of CBD patients (35 of 175).
TABLE 3.
Time to resolution for all AEs and most common AEs
| Patients, n (%) | |||
|---|---|---|---|
| Placebo, n = 124 | CBD 10 mg/kg/day, n = 64 | CBD 20 mg/kg/day,n = 130 | |
| All AEsa | 102 (82.3) | 56 (87.5) | 119 (91.5) |
| Within 4 weeks | 48/102 (47.1) | 24/56 (42.9) | 39/119 (32.8) |
| After 4 weeks | 18/102 (17.6) | 9/56 (16.1) | 26/119 (21.8) |
| Ongoing | 36/102 (35.3) | 23/56 (41.1) | 54/119 (45.4) |
| Somnolence | 15 (12.1) | 16 (25.0) | 38 (29.2) |
| Within 4 weeks | 9/15 (60.0) | 10/16 (62.5) | 23/38 (60.5) |
| After 4 weeks | 3/15 (20.0) | 3/16 (18.8) | 8/38 (21.1) |
| Ongoing | 3/15 (20.0) | 3/16 (18.8) | 7/38 (18.4) |
| Decreased appetite | 14 (11.3) | 11 (17.2) | 37 (28.5) |
| Within 4 weeks | 7/14 (50.0) | 5/11 (45.5) | 22/37 (59.5) |
| After 4 weeks | 4/14 (28.6) | 4/11 (36.4) | 8/37 (21.6) |
| Ongoing | 3/14 (21.4) | 2/11 (18.2) | 7/37 (18.9) |
| Diarrhea | 14 (11.3) | 11 (17.2) | 37 (28.5) |
| Within 4 weeks | 14/14 (100) | 11/11 (100) | 24/37 (64.9) |
| After 4 weeks | 0 | 0 | 7/37 (18.9) |
| Ongoing | 0 | 0 | 6/37 (16.2) |
Data are from the safety analysis set.
Abbreviations: AE, treatment‐emergent adverse event; CBD, cannabidiol.
For patients with multiple AEs, the longest time to resolution was used; AEs resolved either within the first 4 weeks of treatment or after 4 weeks of treatment by the end of the study, or were ongoing after the study ended. Of the 15 patients who had AEs that led to withdrawal, 12 patients (one placebo, 11 CBD) had AEs that resolved within 4 weeks, one CBD patient had AEs that resolved in >4 weeks, and two CBD patients had ongoing AEs.
In patients reporting the most common AEs of somnolence, decreased appetite, and diarrhea, events resolved by end of treatment in 78.6% or more of patients, ranging from 81.1% to 100% for CBD‐treated patients, and within 4 weeks in the majority of CBD‐treated patients (56.3%–72.9%). Of the three most common AEs, diarrhea had the highest proportion of patients whose AE resolved within 4 weeks and was the only one with a notable difference in time to resolution between treatment groups, with CBD 20 mg/kg/day treatment having the longest time to resolution (Table 3).
4. DISCUSSION
In this post hoc analysis of efficacy and safety data from two randomized, placebo‐controlled trials of add‐on CBD in patients with DS, we observed that the treatment effects of CBD compared with placebo (reduction in seizure frequency and incidence of AEs) occurred as early as the second week of treatment, consistent with our clinical experience. The difference between CBD and placebo for both the percentage reduction in convulsive seizure frequency and the 50% responder rate became apparent during the 2‐week titration period and was maintained throughout the full 14‐week treatment period. Time to onset of AEs showed that over half of patients receiving CBD reported the first occurrence of AEs within the titration period. Early time to AE onset was also observed in the majority of patients for the three most common AEs of somnolence, decreased appetite, and diarrhea.
Pooling data from two similarly designed studies in DS allows for a more precise estimate of the time to onset of CBD efficacy and provides improved estimates of seizure frequency reduction, responder rates, and AE rates in the 20 mg/kg/day CBD versus placebo groups. Analysis of the percentage reduction in monthly convulsive seizure frequency by cumulative day showed that reduction relative to placebo emerged as early as Day 7, reached nominal significance by Days 12 and 13 (CBD 20 mg/kg/day and 10 mg/kg/day groups, respectively), and was maintained throughout the duration of treatment. Given the high seizure burden and risk of status epilepticus and SUDEP in this patient population, early onset of antiseizure effect (during titration period) is particularly important, as a faster onset could reduce the cumulative seizure burden. Although an early treatment effect was observed for the pooled population, the maximum time to effect may be delayed in some individual patients, depending on their daily seizure frequency, underlying epilepsy, and other baseline confounders.
There is a paucity of data for onset of efficacy with other AEDs in patients with DS. However, similar time to efficacy onset analyses of AEDs in other epilepsies have been conducted, suggesting that brivaracetam has an early onset of action during titration in a subset of adult patients with uncontrolled focal onset seizures with an early incidence of AEs21 and adjunctive lamotrigine has an early onset of efficacy during titration in patients 13 years or older with a diagnosis of epilepsy with primary generalized tonic–clonic seizures.22 Additionally, in a study of time to AE onset, perampanel had an early onset of AEs during the titration period in patients 12 years or older with refractory partial seizures.23
AEs occurred within the 2‐week titration period for more than half of CBD‐treated patients. For the AEs of somnolence/fatigue/lethargy/sedation, clear separation in onset between placebo and CBD emerged by 7 days. In the time to onset of the three most common AEs, there was clear separation from placebo for both CBD doses (Figure 3), suggesting that the AEs were related to CBD treatment. Among the ~22% of patients reporting somnolence, more patients were taking concomitant clobazam compared with those who were not. Somnolence/sedation may arise from pharmacokinetic or pharmacodynamic interactions between CBD and clobazam; however, not all patients taking concomitant clobazam experience somnolence/sedation. Thus, it may be advisable to closely monitor patients receiving CBD with concomitant clobazam and consider reducing their clobazam dose if sedation ensues. The majority of AEs (59%) resolved during the study, with 40% resolving within 4 weeks of onset. The three most common AEs (somnolence, decreased appetite, diarrhea) resolved by the end of the study for the vast majority of patients (≥80%).
Increases in ALT/AST more than three times ULN occurred in ~9% of patients (all of whom were taking concomitant valproate), with elevations occurring within 30 days of starting treatment in 69% of those patients. Monitoring transaminase levels in all patients taking CBD, with special caution for those on valproate, is recommended at 1, 3, and 6 months as well as periodically thereafter as clinically indicated.9 Elevated ALT/AST levels resolved in all patients, spontaneously or following discontinuation of CBD or change in AED regimen. This underscores that monitoring is important but is also reassuring given the high rate of resolution and lack of severe liver injury. Longer term data are needed to understand whether ongoing periodic blood testing is necessary.
This study has several limitations. The interpretability and generalizability of the results are limited mostly because it was a post hoc analysis of pooled data from two trials with a rigid titration schedule. Although the observation of an early/clear separation from placebo may not prove to be statistically significant in a prospectively designed trial, the evidence of an early CBD antiseizure effect in two other post hoc analyses of trials in LGS24 and TSC25 robustly supports our results of an early antiseizure effect in DS. We could not evaluate the by‐day onset of efficacy for patients taking versus not taking clobazam due to the small number of patients not taking clobazam combined with the highly variable seizure data from just the first few days of treatment (compared to the full 14‐week treatment period); such an analysis would require a more appropriately powered study with balanced subgroups. However, prior meta‐analysis of the GWPCARE randomized trials in DS and LGS has demonstrated the efficacy of CBD independent of clobazam over the full 14‐week treatment period.26 Another limitation to our study is that the overall and long‐term clinical significance of the time‐to‐treat data is unknown. Further studies would be needed to investigate whether quicker onset of antiseizure effect leads to greater or more sustained seizure control.
The titration schedule used in the pivotal studies does not reflect the prescribing information dosage titration recommendations for a slower rate of dose increases.9 Given the forced titration scheme in the trials, it is possible that slower titration may result in fewer AEs and a longer time to occurrence. The time to onset of efficacy was similar between both dose groups compared to placebo (emerging as early as Day 7 when both groups reached 10 mg/kg/day and reaching significance at Days 12 and 13), consistent with the existing titration guidance described in the product label. It is important to optimize the titration schedule for individual patients, as managing the risks and benefits of treatment is a key clinical decision that must take into account the patient's particular situation: urgency of improving seizure control, concomitant medications, prior experience of side effects, and comorbidities.
In conclusion, this pooled analysis of two individual trials of the safety and efficacy of CBD in DS provides estimates of seizure reduction and AE rates, and new information on the timing of CBD’s treatment effect, which improves the predictability of effect. For many patients, both the onset of reduction in seizure frequency and AEs occurred within 2 weeks of CBD treatment; overall seizure reduction was maintained for the duration of the trials, and the most common AEs tended to resolve, often within 4 weeks.
CONFLICT OF INTEREST
J.M.C. has received speaker fees from Greenwich Biosciences, and has been a principal investigator for GW Research. D.C. is an employee of GW Research, and owns stock in GW Pharmaceuticals. E.D. is an employee of Greenwich Biosciences, and owns stock in GW Pharmaceuticals. B.G. has received consultancy fees from GW Pharmaceuticals, Ovid/Takeda and Zogenix, and has been a principal investigator for GW Research, Zogenix, LivaNova, and Marinus Pharmaceuticals. A.H. has received consultancy and/or speaker fees from Supernus Pharmaceuticals, Eisai, GW Pharmaceuticals, and Aquestive Pharmaceuticals, and has been a principal investigator for GW Research, Sage Therapeutics, Marinus Pharmaceuticals, Neurocrine Biosciences, and UCB Biosciences. D.M. has received speaker fees from Greenwich Biosciences, and has been a been a principal investigator for GW Research. V.V. has participated on advisory boards and in symposia organized by Angelini, Arvelle, Bial, Eisai, Esteve, GSK, GW Pharmaceuticals, Novartis, Sandoz, UCB, and Zogenix, and has been a principal investigator for GW Research. M.Z. has been a principal investigator for GW Research, Zogenix, Ovid/Takeda, Marinus, and Emalex Biosciences. S.M.Z. has received research support from Epilepsy Research UK, Glasgow Children's Hospital Charity, the Tenovus Foundation, and UCB Pharma; has received honoraria for advisory boards, consulting, and educational symposia from GW Pharmaceuticals, Zogenix, Arvelle Therapeutics, Stoke Therapeutics, and Encoded Genomics; and has been a principal investigator for GW Research. His institution has undertaken commercial trials for GW Research and Zogenix. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
AUTHOR CONTRIBUTIONS
All authors provided substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work; drafted the work or revised it critically for important intellectual content; and provided final approval of the version to be published.
Supporting information
Supplementary Material
Fig S1
Fig S2
Fig S3
ACKNOWLEDGMENTS
The authors would like to thank the patients, their families, and the staff at sites that participated in this study. Medical writing support was provided to authors by Keira Kim an employee of Greenwich Biosciences, Inc.
Cohen JM, Checketts D, Dunayevich E, Gunning B, Hyslop A, Madhavan D, et al. Time to onset of cannabidiol treatment effects in Dravet syndrome: Analysis from two randomized controlled trials. Epilepsia. 2021;62:2218–2227. 10.1111/epi.16974
Funding information
This study was sponsored by GW Research Ltd, Cambridge, UK.
REFERENCES
- 1.Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(Suppl 2):3–9. [DOI] [PubMed] [Google Scholar]
- 2.Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durá‐Travé T, Yoldi‐Petri ME, Gallinas‐Victoriano F. Epilepsy in children in Navarre, Spain: epileptic seizure types and epileptic syndromes. J Child Neurol. 2007;22(7):823–8. [DOI] [PubMed] [Google Scholar]
- 4.Wolff M, Cassé‐Perrot C, Dravet C. Severe myoclonic epilepsy of infants (Dravet syndrome): natural history and neuropsychological findings. Epilepsia. 2006;47(Suppl 2):45–8. [DOI] [PubMed] [Google Scholar]
- 5.Symonds JD, Zuberi SM, Stewart K, McLellan A, O'Regan M, MacLeod S, et al. Incidence and phenotypes of childhood‐onset genetic epilepsies: a prospective population‐based national cohort. Brain. 2019;142(8):2303–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirrell EC, Laux L, Donner E, Jette N, Knupp K, Meskis MA, et al. Optimizing the diagnosis and management of Dravet syndrome: recommendations from a North American consensus panel. Pediatr Neurol. 2017;68:18–34.e3. [DOI] [PubMed] [Google Scholar]
- 7.Dam VV, Korff CM. Dravet Syndrome: an update. Swiss Arch Neurol Psychiatry Pyschother. 2013;164(05):153–7. [Google Scholar]
- 8.Chiron C, Dulac O. The pharmacologic treatment of Dravet syndrome. Epilepsia. 2011;52(Suppl 2):72–5. [DOI] [PubMed] [Google Scholar]
- 9.EPIDIOLEX (cannabidiol) oral solution [full prescribing information]. Carlsbad, CA: Greenwich Biosciences; 2020. [Google Scholar]
- 10.Epidyolex (cannabidiol) oral solution [summary of product characteristics]. Amersfoort, the Netherlands: GW Pharma (International); 2019. [Google Scholar]
- 11.DIACOMIT (stiripentol) capsules, powder for oral solution [full prescribing information]. Beauvais, France: Biocodex; 2018. [Google Scholar]
- 12.FINTEPLA (fenfluramine) oral solution, CIV [full prescribing information]. Emeryville, CA: Zogenix; 2020. [Google Scholar]
- 13.Wirrell EC. Treatment of Dravet syndrome. Can J Neurol Sci. 2016;43(Suppl 3):S13–8. [DOI] [PubMed] [Google Scholar]
- 14.Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of cannabidiol for drug‐resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011–20. [DOI] [PubMed] [Google Scholar]
- 15.Miller I, Scheffer IE, Gunning B, Sanchez‐Carpintero R, Gil‐Nagel A, Perry MS, et al. Dose‐ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: a randomized clinical trial. JAMA Neurol. 2020;77(5):613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of cannabidiol on drop seizures in the Lennox‐Gastaut syndrome. N Engl J Med. 2018;378(20):1888–97. [DOI] [PubMed] [Google Scholar]
- 17.Thiele EA, Marsh ED, French JA, Mazurkiewicz‐Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2018;391(10125):1085–96. [DOI] [PubMed] [Google Scholar]
- 18.Thiele EA, Bebin EM, Bhathal H, Jansen FE, Kotulska K, Lawson JA, et al. Add‐on cannabidiol treatment for drug‐resistant seizures in tuberous sclerosis complex: a placebo‐controlled randomized clinical trial. JAMA Neurol. 2021;78(3):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devinsky O, Nabbout R, Miller I, Laux L, Zolnowska M, Wright S, et al. Long‐term cannabidiol treatment in patients with Dravet syndrome: an open‐label extension trial. Epilepsia. 2019;60(2):294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheffer IE, Halford JJ, Nabbout R, Sanchez‐Carpintero R, Shiloh‐Malawksy Y, Wong M, et al. Long‐term safety and efficacy of add‐on cannabidiol in patients with Dravet syndrome: 3‐year results of an open‐label extension trial (GWPCARE5). AES 2019 Annual Meeting Abstract Database. https://cms.aesnet.org/abstractslisting/long‐term‐safety‐and‐efficacy‐of‐add‐on‐cannabidiol‐(cbd)‐treatment‐in‐patients‐with‐dravet‐syndrome‐(ds)‐‐3‐year‐results‐of‐an‐open‐label‐extension‐(ole)‐trial‐(gwpcare5). Accessed 25 Jun 2020.
- 21.Klein P, Johnson ME, Schiemann J, Whitesides J. Time to onset of sustained >/=50% responder status in patients with focal (partial‐onset) seizures in three phase III studies of adjunctive brivaracetam treatment. Epilepsia. 2017;58(2):e21–5. [DOI] [PubMed] [Google Scholar]
- 22.Biton V, Di Memmo J, Shukla R, Lee YY, Poverennova I, Demchenko V, et al. Adjunctive lamotrigine XR for primary generalized tonic‐clonic seizures in a randomized, placebo‐controlled study. Epilepsy Behav. 2010;19(3):352–8. [DOI] [PubMed] [Google Scholar]
- 23.Ko D, Yang H, Williams B, Xing D, Laurenza A. Perampanel in the treatment of partial seizures: time to onset and duration of most common adverse events from pooled phase III and extension studies. Epilepsy Behav. 2015;48:45–52. [DOI] [PubMed] [Google Scholar]
- 24.Privitera M, Bhathal H, Wong M, Cross JH, Wirrell E, Marsh ED, et al. Time to onset of cannabidiol (CBD) treatment effect in Lennox‐Gastaut syndrome: analysis from two randomized controlled trials. Epilepsia. 2021;62:1130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J, Cock H, Devinsky O, Joshi C, Miller I, Roberts C, et al. Time to onset of cannabidiol treatment effect and resolution of adverse events in the tuberous sclerosis complex phase 3 randomized controlled trial (GWPCARE6). Neurology. 2020;94:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devinsky O, Thiele EA, Wright S, Checketts D, Morrison G, Dunayevich E, et al. Cannabidiol efficacy independent of clobazam: meta‐analysis of four randomized controlled trials. Acta Neurol Scand. 2020;142(6):531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Fig S1
Fig S2
Fig S3


