Abstract
Background
Although structured exercise training is strongly recommended in cardiac patients, uncertainties exist about the methods for determining exercise intensity (EI) and their correspondence with effective EI obtained by ventilatory thresholds. We aimed to determine the first (VT1) and second ventilatory thresholds (VT2) in cardiac patients, sedentary subjects, and athletes comparing VT1 and VT2 with EI defined by recommendations.
Methods
We prospectively enrolled 350 subjects (mean age: 50.7±12.9 years; 167 cardiac patients, 150 healthy sedentary subjects, and 33 competitive endurance athletes). Each subject underwent ECG, echocardiography, and cardiopulmonary exercise testing. The percentages of peak VO2, peak heart rate (HR), and HR reserve were obtained at VT1 and VT2 and compared with the EI definition proposed by the recommendations.
Results
VO2 at VT1 corresponded to high rather than moderate EI in 67.1% and 79.6% of cardiac patients, applying the definition of moderate exercise by the previous recommendations and the 2020 guidelines, respectively. Most cardiac patients had VO2 values at VT2 corresponding to very‐high rather than high EI (59.9% and 50.3%, by previous recommendations and 2020 guidelines, respectively). A better correspondence between ventilatory thresholds and recommended EI domains was observed in healthy subjects and athletes (90% and 93.9%, respectively).
Conclusions
EI definition based on percentages of peak HR and peak VO2 may misclassify the effective EI, and the discrepancy between the individually determined and the recommended EI is particularly relevant in cardiac patients. A ventilatory threshold–based rather than a range‐based approach is advisable to define an appropriate level of EI.
Keywords: cardiopulmonary exercise testing, exercise intensity, exercise prescription, lactate, ventilatory threshold
1. INTRODUCTION
Exercise training is a crucial element in the prevention and management of cardiovascular disorders (CVD), as it is associated with proven benefits in terms of quality of life, mortality, disability, and prevention of comorbidities.1, 2, 3 As a consequence, regular exercise training is highly recommended and at least 150 minutes of moderate‐intensity aerobic exercise or at least 75 minutes of high‐intensity exercise training throughout the week is recommended, with additional health benefits with increasing minutes per week.4, 5 Also, strength training is recommended with a frequency of twice a week, at 30–70% of one‐repetition maximum (1RM) for the upper body and 40–80% of 1RM for the lower body, with 12–15 repetitions/set.6 However, particularly in patients with CVD, a tailored exercise prescription is strongly recommended.4, 5, 7, 8, 9 The basic tenets of exercise prescription are usually based on the identification of four main principles: frequency, intensity, time, and type, the so‐called FITT concept. EI is more important than duration to improve the life expectancy and lower the risk for chronic diseases in a primary prevention setting and can be particularly useful not only when exercising with a constant HR but also in specific training programs, such as high‐intensity interval training.10, 11 Although the prescription of frequency, time, and volume per week is intuitive and consolidated in healthy people, the methodology to determine exercise intensity (EI) aimed at prescribing exercise is still debated, particularly in patients with heart failure. Traditionally, the previous recommendations for aerobic exercise prescription identify different EI domains based on the physiological responses to exercise derived by healthy subjects or even competitive athletes: According to this approach, EI is defined based on the corresponding percentages, that is, the percentage of peak oxygen consumption (VO2) and the percentage of peak heart rate (HR).7, 12 Recently, the new 2020 ESC guidelines of sports cardiology proposed a new classification of EI.5 However, this method does not entirely reflect the individual response to exercise and, as a consequence, the effective EI can be misclassified, particularly in patients with aerobic and anaerobic thresholds influenced by clinical, pharmacological (ie, under β‐blockers), or training factors, with a consequent over‐ or under‐estimation of the intensity of exercise training.13, 14, 15, 16
Cardiopulmonary exercise testing (CPET) is the most important tool to assess exercise intensity prescription of a tailored exercise program; however, EI is usually expressed as a percentage of maximal aerobic capacity rather than by CPET‐derived individual ventilatory thresholds (VTs) that more appropriately reflect the variability of personal adaption to exercise and better determine EI, particularly in cardiac patients with left ventricular (LV) dysfunction and β‐blocker therapy.14 Unfortunately, scant data are currently available about the determination of VTs in cardiac patients and their correspondence to EI domains.13, 14 Therefore, this study aimed to determine the first (VT1) and second VTs (VT2) in cardiac patients with those obtained in healthy sedentary subjects and competitive athletes. We also compared the definition of EI by VT1 and VT2 with that recommended by previous and new guidelines, to investigate the correspondence between VTs and EI domains.7, 12 The hypothesis was that recommended EI domains may misclassify the effective EI as assessed by CPET‐derived VTs.
2. METHODS
From January 2018 to June 2020, we prospectively enrolled 390 consecutive subjects referred to three centers qualified in performing CPET: the Siena Centre for Sports Cardiology, the Cardiology Department of the University Hospital of Siena, and the Sports Medicine Unit of “Toscana Centro.” Two hundred and twenty‐four patients with CVD and 150 subjects without known CVD (ie, free from symptoms and evidence of CVD) were enrolled in the study. We also enrolled 33 competitive endurance athletes as a population of supranormal subjects. Patients with CVD were affected by different CVDs, including coronary artery disease, dilated cardiomyopathy, and hypertrophic cardiomyopathy. From the initial population, 26 patients were excluded because they were not in sinus rhythm (ie, atrial fibrillation) and 5 patients because of chronotropic incompetence. The VT1 was not determined in 9 patients because of exercise oscillatory ventilation. Therefore, 350 subjects were included in the final analysis: 167 cardiac patients, 150 healthy sedentary subjects, and 33 competitive non‐professional endurance athletes. Cardiac patients were also divided into 4 categories of LV systolic dysfunction, according to the guideline stratification of LV ejection fraction (EF)17 : severe dysfunction (EF <30%), n=40; moderate dysfunction (30≥EF≤40%), n=82; mild dysfunction (52–54%≤EF>40%), n=32; and preserved LVEF (EF≥52–54% in males and females, respectively), n=13.
All study participants underwent complete clinical and physical examination, 12‐lead resting ECG, transthoracic echocardiographic examination, and CPET.
After the rationale and the study protocol were explained, all patients gave written informed consent. The study protocol was approved by the local Ethical Committee of the University of Siena.
2.1. Physical examination and 12‐lead resting ECG
Information about the presence of a known CVD, cardiovascular risk factors—family history for CVD, hypertension, dyslipidemia, diabetes, and smoking habit—and previous implantation of pacemakers, implantable cardioverters, or cardiac resynchronization therapies was collected. Symptoms suggestive of functional capacity limitation, stratified in categories according to the New York Heart Association (NYHA), were also investigated. Information about drug therapy was also collected. Body height and weight were measured, and body mass index and body surface area were calculated. A standard 12‐lead ECG was performed in all participants in the supine position during quiet respiration using a CARDIOLINE Realclick v.3.4 (Cardioline SpA, Milan, Italy). All ECGs were recorded at a paper speed of 25 mm/s and a standard gain of 1 mV/cm.
2.2. Echocardiographic examination
Echocardiographic examination was performed by expert cardiologists using a high‐quality echocardiograph (Vivid 9, General Electrics, Milwaukee, Wisconsin), equipped with an M4S 1.5‐MHz to 4.0‐MHz transducer, and a one‐lead ECG was continuously displayed. LV end‐diastolic (EDV) and end‐systolic (ESV) volumes were assessed, and LV EF was calculated according to the current guidelines for chamber quantification.17
Right ventricular function was assessed as recommended by current guidelines.18 Valve diseases were reported and quantified as recommended.17
2.3. Cardiopulmonary exercise test
All patients underwent symptom‐limited CPET. All patients were carefully instructed to achieve maximal effort, and all of them were familiar with the 10‐point Borg fatigue scale.19 A standard 12‐lead ECG was recorded at rest, and ECG was continuously monitored during the test. Blood pressure was measured using a manual sphygmomanometer every 2 minutes. All patients were limited by fatigue, except for one patient that experienced a presyncope during the test, 2 patients had angina, and 1 patient was limited by palpitations.
The CPET data were realized on a cycle ergometer (Quark CPET, CosMed USA Inc., Concord, CA, USA), equipped with software OMNIA (CosMed USA Inc., Concord, CA, USA). At the beginning of each test day, a gas and volume calibration was performed according to the manufacturer's instructions. During the test, the environmental temperature was kept stable at 19–21℃. The exercise test (ramp protocol) included a 1‐minute pre‐exercise resting period sitting upright on the bike, a 2‐minute unloaded warm‐up cycling phase, followed by an incremental exercise cycling period with an increasing workload of 5–40W per minute, dependent on the patient's clinical status and aiming to complete the CPET within 8–12 minutes, as recommended.20, 21 VO2, carbon dioxide production (VCO2), and ventilation (VE) during exercise were analyzed breath by breath. The VT1 was determined according to three validated methods to determine VT1 from incremental exercise test data22 : 1) modified V‐slope method; 2) ventilatory equivalent method (VE/VO2 method); and 3) end‐tidal O2 pressure method (PetO2). The VE versus W relationship was also taken into account. The V‐slope was the reference method for VT1 determination, and this point was checked with the point obtained in the other graphs. The VT2 was determined, using the VE/VCO2 plot, on the point where VE increases out of proportion to VCO2, and this threshold was checked by establishing the nadir of the VE versus W relationship and the deflection point of end‐tidal CO2 pressure (PetCO2) versus W (Figure 1).22 These points were measured according to the best agreement between two independent observers (F.A and F.V.). In case of disagreement, a third investigator was asked to assess the thresholds (F.D). In this study, the disagreement between observers 1 and 2 was resolved by a 3rd observed in 1.5% of the cases for VT1 and 2% of the cases for VT2.
FIGURE 1.
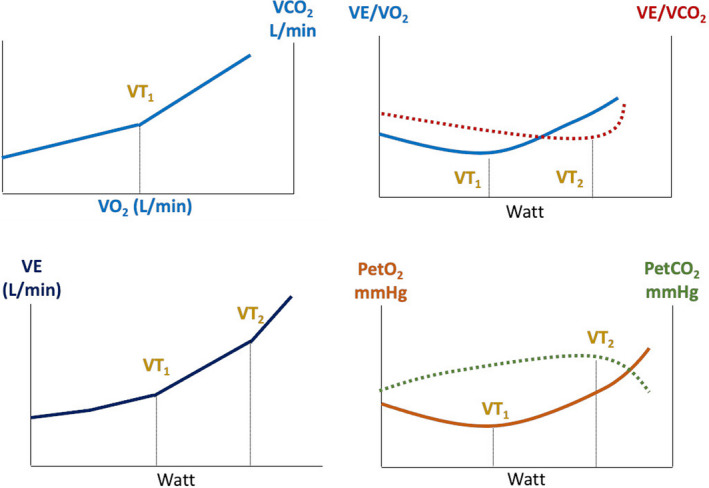
Determination of first and second ventilatory thresholds (VT1 and VT2) by cardiopulmonary exercise test (CPET) in a male: The VT1 and VT2are usually obtained by analyzing all CPET panels, with particular attention to VO2 vs. VCO2, VE/VCO2 and VE/VO2, VE vs. power, and PetO2 and PetCO2 panels
To ensure that peak VO2 was attained, at least two of the following criteria had to be met: 1) maximal HR at a value close to 90% of the theoretical maximal HR, 2) respiratory exchange ratio (RER)≥ 1.10, and 3) pedal rate note maintained at least at 60 rpm at each level of exercise.
VT1 marks the limit between the slight and moderate intensity of exercise and exercising around this threshold allows stimulating aerobic metabolisms while above VT1 blood lactate and pH start to increase and decrease, respectively.7, 14, 23 VT2, that is, respiratory compensation point, has been proposed to be related to the so‐called critical power, that is, the upper‐intensity limit for prolonged aerobic exercise. VT2 marks the limit between moderate and high‐intensity of exercise.7, 23 Therefore, the corresponding HR values obtained at VT1 and VT2 were considered the limit of moderate and high EI domains, respectively, and should be used to prescribe exercise in a different setting.14, 23, 24 At VT1 and VT2, the percentages of peak VO2 (%peak VO2), peak HR (%peak HR), HR reserve (%HRR), and VO2 reserve (%VO2R) were extrapolated for comparison with previous recommendation‐based EI domains7 and with the new 2020 guidelines on sports cardiology and exercise in patients with CVD by European Society of Cardiology (ESC).5 Supplementary Table 1 reports the definition of EI domains according to these documents.
TABLE 1.
Descriptive characteristics of the population
|
Overall population (N=350) |
Cardiac patients (n=167) |
Healthy sedentary (n=150) |
Athletes (n=33) |
|
|---|---|---|---|---|
| Age, yrs | 50.7 ± 12.9 | 54.4 ± 10.9 | 48.0 ± 13.2 | 41.9 ± 13.9 |
| Male, n (%) | 288 (82%) | 147 (88%) | 111 (74%) | 30 (90%) |
| Weight, Kg | 77 ± 14 | 81 ± 14 | 73 ± 14 | 71 ± 10 |
| Height, cm | 173 ± 8 | 173 ± 8 | 173 ± 9 | 176 ± 9 |
| BMI | 25.5 ± 4.1 | 27.0 ± 4.3 | 24.3 ± 3.7 | 22.7 ± 1.8 |
| BSA, m2 | 1.90 ± 0.2 | 1.94 ± 0.2 | 1.85 ± 0.2 | 1,86 ± 0.2 |
| NYHA, n (%) | ||||
| I | 215 (61%) | 44 (26%) | 138 (92%) | 33(100%) |
| II | 118 (34%) | 106 (64%) | 12 (8%) | ‐ |
| III | 17 (5%) | 17 (10%) | ‐ | ‐ |
| IV | 0 (0%) | ‐ | ‐ | ‐ |
| Cardiovascular risk factors, n (%) | ||||
| Hypertension | 43 (12%) | 43 (26%) | ‐ | ‐ |
| Diabetes | 27 (7%) | 27 (16%) | ‐ | ‐ |
| Dyslipidemia | 27 (7%) | 24 (14%) | 3 (2%) | ‐ |
| Current smoker | 21 (6%) | 15 (5%) | 6 (4%) | ‐ |
| Previous smoker | 29 (8%) | 29 (17%) | ‐ | ‐ |
| Medication, n (%) | ||||
| Beta‐blockers | 167 (49%) | 167 (100%) | ||
| ACEi | 89 (25%) | 89 (53%) | ‐ | ‐ |
| ARB | 35 (10%) | 35 (21%) | ‐ | ‐ |
| Sacubitril/valsartan | 38 (11%) | 38 (22%) | ‐ | ‐ |
| MRA | 133 (38%) | 133 (80%) | ‐ | ‐ |
| Diuretics | 142 (41%) | 142 (85%) | ‐ | ‐ |
| PM or ICD, n (%) | 68 (19%) | 68 (40%) | ‐ | ‐ |
| CRT, n (%) | 54 (15%) | 54 (32%) | ‐ | ‐ |
| Resting HR, bpm | 70 ± 12 | 69 ± 11 | 71 ± 12 | 63 ± 12 |
| LBBB, n (%) | 22 (6%) | 22 (13%) | ‐ | ‐ |
| RBBB, n (%) | 8 (2%) | 5 (3%) | 3 (2%) | ‐ |
| Resting SBP, mmHg | 115 ± 15 | 108 ± 14 | 122 ± 10 | 119 ± 9 |
| Resting DBP, mmHg | 74 ± 9 | 71 ± 9 | 78 ± 7 | 74 ± 7 |
Data are presented as mean±standard deviation or as percentages.
Abbreviations: BMI, body mass index; BSA, body surface area; NYHA, New York Heart Association; ACEi, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist; HR, heart rate; LBBB: left bundle branch block; RBBB, right bundle branch block; DBP, diastolic blood pressure; METS, metabolic equivalent of task.
2.4. Statistical analysis
Normal distribution of all continuous variables was examined using the Shapiro‐Wilk test, and data are presented as mean±standard deviation (95th confidence interval). Categorical variables are expressed as percentages. After the descriptive data analysis, the ANOVA test with Bonferroni post‐hoc correction and the Kruskal‐Wallis test were used to assess the significance between the groups of subjects, according to data distribution. A two‐tailed p value <0.05 was considered significant. To identify the independent predictors of VT1 and VT2, expressed as VO2, mL/min, in the study population individual association with clinical and demographic parameters was assessed by univariate and multivariable linear regression analysis. The model included the most important demographic and clinical variables and LVEF, while the CPET‐derived parameters were excluded because of their redundant value. Two different models were set for VT1 and VT2 that were identified as dependent variables. Only the significant predictors identified at univariate regression analysis were included in the final model. Statistics were performed using SPSS, version 21.0 (Statistical Package for the Social Sciences Inc., Chicago, Illinois, USA).
3. RESULTS
3.1. Subjects characteristics
The demographic and clinical characteristics of the study population are reported in Table 1. The majority of patients were male (82%). The mean age was 50.7±12.9 years. Hypertension was the most common cardiovascular risk factor. All cardiac patients were on β‐blocker therapy, although the type of β‐blocker and the dosage differs significantly among the patients. Bisoprolol was the most commonly prescribed β‐blocker, with an average dosage of 5 mg/die, followed by carvedilol and less commonly metoprolol. One hundred and twenty‐two subjects had cardiac stimulation devices (34%), but all of them had sinus rhythm during exercise.
3.2. Cardiopulmonary exercise test
CPET peak parameters in the overall study population and different categories of patients are shown in Table 1 and Table 2, respectively. Mean peak VO2 was reduced in cardiac patients and preserved in healthy sedentary and competitive athletes (overall p<0.0001). All variables showed significant differences among the three different groups of subjects (overall p value <0.0001 for most parameters). The differences between groups are reported in detail in Table 2.
TABLE 2.
Peak cardiopulmonary parameters in different categories of subjects
| Cardiac patients | Healthy sedentary | Athletes | p value | |
|---|---|---|---|---|
| Peak Cycling Power Output, watt |
113.0 ± 36.0 (108–119) |
183.2 ± 63.1* (173–193) |
295.4 ± 54.3* (276–314) |
<0.0001 |
| Peak SBP, mmHg |
137.1 ± 23.9* (133–141) |
179.0 ± 29.4 (174–184) |
187.7 ± 24.9 (179–196) |
<0.0001 |
| Peak DBP, mmHg |
77.6 ± 10.6^ (76–79) |
82.7 ± 8.9 (81–84) |
78.5 ± 8.3 (75–81) |
<0.0001 |
| Peak Borg Scale Dyspnea |
5.9 ± 1.8 (5.6–6.2) |
5.1 ± 1.8° (4.6–5.7) |
8.3 ± 0.8* (7.8–8.8) |
<0.0001 |
| Peak METS |
5.5 ± 1.4 (5.3–5.7) |
8.7 ± 2.2* (8.4–9.1) |
14 ± 2.2* (13.2–14.8) |
<0.0001 |
| Peak RER |
1.11 ± 0.08^ (1.09–1.12) |
1.14 ± 0.08 (1.12–1.15) |
1.11 ± 0.07 (1.08–1.14) |
0.003 |
| Peak HR, bpm |
118 ± 20* (115–121) |
153 ± 19 (150–156) |
162 ± 14§ (157–168) |
<0.0001 |
| Peak HR, % |
70.8 ± 11.3^ (69–75) |
88.9 ± 10.4 (87–91) |
91.2 ± 7.6* (88–94) |
<0.0001 |
| Peak VO2, mL/min |
1533 ± 410 (1471–1596) |
2215 ± 680* (2105–2325) |
3464 ± 562* (3264–3662) |
<0.0001 |
| Peak VO2/Kg, mL/min/Kg |
19.0 ± 4.9 (18.3–19.8) |
30.5 ± 7.9* (29–32) |
49.0 ± 7.7* (46–52) |
<0.0001 |
| Peak VO2, % |
67.9 ± 14.1 (66–70) |
99.0 ± 22.1* (95–103) |
142.7 ± 30.9* (132–154) |
<0.0001 |
| VE/VCO2 slope |
31.8 ± 7.4* (30.6–32.9) |
27.1 ± 3.6 (26.5–27.7) |
25.9 ± 3.1 (24.8–27.1) |
<0.0001 |
| Peak VE, l/min |
58.4 ± 13.9 (56.3–60.5) |
75.5 ± 23.0* (71.8–79.2) |
115.1 ± 26.0* (105.8–124.3) |
<0.0001 |
| Peak VO2/HR |
13.3 ± 3.2 (12.8–13.7) |
14.6 ± 4.1* (13.9–15.3) |
21.5 ± 3.5* (20.3–22.7) |
<0.0001 |
| Peak VO2/HR, % |
98.7 ± 20.6 (96–102) |
112.5 ± 21.6* (109–116) |
152.5 ± 30.6* (142–163) |
<0.0001 |
| Peak VO2/WR |
9.6 ± 1.3 (9.4–9.8) |
9.9 ± 0.8 (9.7–10.0) |
10.6 ± 0.6* (10.3–10.8) |
<0.0001 |
| HRR, bpm |
48 ± 18 (45–51) |
82 ± 19* (79–86) |
100 ± 14* (95–105) |
<0.0001 |
| VO2R, mL/min |
15.5 ± 4.9 (14.8–16.3) |
27.0 ± 7.9* (25.7–28.3) |
45.5 ± 7.7* (42.8–48.2) |
<0.0001 |
Data are presented as mean±standard deviation (95% confidence interval).*p ≤ 0.001 vs. other groups; ^ p≤0.001 vs. healthy sedentary; °p<0.05 vs. cardiac patients; and § p<0.05 vs. healthy sedentary.
Abbreviations: DBP, diastolic blood pressure; HR, heart rate; HRR, heart rate reserve; METS, metabolic equivalent of task; RER, respiratory exchange ratio; SBP, systolic blood pressure; VCO2, exhaled carbon dioxide; VE, ventilation; VO2, oxygen uptake; VO2R, VO2 reserve; WR, work rate.
3.3. Ventilatory thresholds and correspondence with exercise intensity domains
The determination of VT1 and VT2 in different categories of patients and the corresponding values of CPET parameters are reported in Table 3. The VT1 was identified at higher VO2 in athletes as compared to sedentary subjects and cardiac patients (overall p<0.0001). The percentage of peak HR and peak VO2 demonstrated a trend toward a decrease in cardiac patients, sedentary subjects, and competitive athletes (p<0.0001), while the percentage of predicted VO2 demonstrated an opposite trend. The same trend was demonstrated also for VT2 and corresponding peak VO2 and percentage of peak VO2. The difference between HR values corresponding to VT1 and VT2 was the lowest in cardiac patients and the highest in competitive athletes.
TABLE 3.
First and second ventilatory thresholds and their respective parameters determined by cardiopulmonary testing in different categories of subjects
| Cardiac patients | Healthy sedentary | Athletes | p value | ||
|---|---|---|---|---|---|
| VT1 | VO2, mL/min |
955 ± 230 (920–990) |
1286 ± 359* (1228–1344) |
1828±454* (1667–1989) |
<0.0001 |
| VO2/Kg, mL/min/Kg |
11.9 ± 2.8 (11.4–12.3) |
17.8 ± 4.4* (17.1–18.5) |
26.2±7.6* (23.5–28.9) |
<0.0001 | |
| Peak VO2, % |
63.1 ± 7.8 (62–64) |
59.1 ± 8.3* (58–60) |
52.8±10.0* (49–56) |
<0.0001 | |
| Predicted VO2% |
42.6 ± 9.2 (41–44) |
58.0 ± 13.5* (56–60) |
76.8±27.2* (67–86) |
<0.0001 | |
| HR, bpm |
88 ± 13^ (86–90) |
107 ± 17 (104–110) |
111±16* (106–117) |
<0.0001 | |
| Peak HR, % |
75.1 ± 8.1^ (74–76) |
70.0 ± 7.3 (69–71) |
68.8±6.9* (66–71) |
<0.0001 | |
| Work Rate, watt |
53.0 ± 21.9 (50–56) |
87.3 ± 32.9* (82–93) |
139.2±38.6* (125–153) |
<0.0001 | |
| VO2/HR |
11.0 ± 2.5 (10.6–11.4) |
12.2 ± 3.4* (11.6–12.7) |
16.5±3.6* (15.2–17.8) |
<0.0001 | |
| PetCO2, mmHg |
35.8 ± 4.8 (35–37) |
40.6 ± 4.5* (39–41) |
43.8±3.5* (43–45) |
<0.0001 | |
| VT2 | VO2, mL/min |
1301 ± 323 (1252–1350) |
1833 ± 561* (1743–1923) |
2811±505* (2632–2990) |
<0.0001 |
| VO2/Kg, mL/min/Kg |
16.3 ± 3.9 (15.7–16–9) |
25.1 ± 6.4* (24.1–26.2) |
40.4±7.5* (38–43) |
<0.0001 | |
| Peak VO2, % |
85.4 ± 4.9* (84–86) |
83.1 ± 7.7 (82–84) |
81.0±6.2 (79–83) |
<0.0001 | |
| Predicted VO2, % |
57.9 ± 12.0 (56–60) |
82.3 ± 19.5* (79–85) |
116.8±28.6* (107–127) |
<0.0001 | |
| HR, bpm |
104 ± 16 (101–106) |
133 ± 19* (130–136) |
144±15* (139–150) |
<0.0001 | |
| Peak HR, % |
88.2 ± 5.8 (87–89) |
87.1 ± 5.6 (86–88) |
89.4±4.3 (88–91) |
0.053 | |
| Work Rate, watt |
86.8 ± 29.1 (82–91) |
144.0 ± 51.6* (136–152) |
237.1±43.5* (221–252) |
<0.0001 | |
| VO2/HR |
12.7 ± 2.9 (12.2–13.1) |
13.8 ± 3.8° (13.2–14.4) |
19.5±3.6* (18.2–20.8) |
<0.0001 | |
| PetCO2, mmHg |
36.6 ± 5.3* (35–37) |
42.0 ± 4.8 (41–43) |
39.7±5.9§ (39–41) |
<0.0001 | |
| Delta HR (VT2‐VT1), bpm |
16±8 (15–17) |
26 ± 10* (25–28) |
33 ± 11* (30–37) |
<0.0001 | |
Data are presented as mean±standard deviation (95% confidence interval).*p ≤ 0.001 vs. other groups; ^ p≤0.001 vs. healthy sedentary; °p<0.05 vs. cardiac patients; and § p<0.05 vs. healthy sedentary.
Abbreviations: HR, heart rate; PetCO2, CO2 end‐tidal pressure; VCO2, exhaled carbon dioxide; VE, ventilation; VO2, oxygen uptake; VT1, first ventilatory threshold; VT2, second ventilatory threshold; WR, work rate.
Recommendations of exercise intensity in cardiac patients seem to be partly incorrect in the current guideline. Table 4 reports the EI defined by VT1 and VT2 in cardiac patients, healthy sedentary subjects, and competitive athletes and its correspondence to the definition proposed by the previous recommendations and by 2020 ESC guidelines. For the majority of cardiac patients, moderate EI defined by VO2 at VT1 corresponded to high EI domains, applying the cutoffs proposed by the previous recommendations (67.1%), while moderate EI when the 2020 ESC guidelines were considered (79.6%). Similarly, the percentage of sedentary subjects and athletes with moderate EI defined by VO2 at VT1 corresponding to moderate EI cutoffs based on percentages was higher with the ESC 2020 classification than with the previous recommendations. In all groups, the percentage of subjects with VO2R at VT1 corresponding to moderate EI was higher when applying the 2020 ESC guidelines rather than the previous recommendations (90.4% vs. 67.1% for cardiac patients, 92.7% vs. 72% for healthy sedentary subjects, and 72.7% vs 60.6% for competitive athletes).
TABLE 4.
Exercise intensity defined by first and second ventilatory thresholds in cardiac patients, healthy sedentary subjects, and athletes and its correspondence to previous recommendations and 2020 guidelines
| First ventilatory threshold | Second ventilatory threshold | |||||
|---|---|---|---|---|---|---|
| Cardiac patients | Healthy sedentary | Athletes | Cardiac patients | Healthy sedentary | Athletes | |
| Correspondence of exercise intensity according to previous recommendations | ||||||
| % Peak VO2 | ||||||
| Light | 0.6% | 2.7% | 27.3%* | ‐ | ‐ | ‐ |
| Moderate | 31.7% | 53.3%^ | 48.5% | ‐ | ‐ | ‐ |
| High | 67.1% | 43.3% | 24.2% | 40.1%* | 55.3% | 69.7% |
| Very high | 0.6% | 0.7% | ‐ | 59.9%* | 44.7% | 30.3% |
| % Peak HR | ||||||
| Light | 1.2% | 0.7% | ‐ | ‐ | ‐ | ‐ |
| Moderate | 24%* | 47.3% | 45.5% | 1.2% | 0.7% | ‐ |
| High | 73.7% | 50.7%^ | 54.5% | 48.5% | 61.3% | 48.5% |
| Very high | 1.2% | 1.3% | ‐ | 50.3% | 38% | 51.5% |
| % HRR | ||||||
| Light | 57.5%* | 34.7% | 24.2% | 1.8% | ‐ | ‐ |
| Moderate | 37.1%* | 56% | 63.6% | 12.6% | 7.3% | 3% |
| High | 5.4% | 9.3% | 12.2% | 76.6% | 71.3% | 57.6% |
| Very high | ‐ | ‐ | ‐ | 9%* | 21.3% | 42.4% |
| Correspondence of exercise intensity according to 2020 guidelines | ||||||
| % Peak VO2 | ||||||
| Light | ‐ | 0.7% | 3% | ‐ | ‐ | ‐ |
| Moderate | 79.6% | 90% | 93.9%^ | 0.6% | 6% | ‐ |
| High | 19.8% | 8.7%^ | 3% | 49.1% | 56.7% | 75.8%^ |
| Very high | 0.6% | 0.7% | ‐ | 50.3% | 37.3% | 24.2% |
| % Peak HR | ||||||
| Light | 1.2% | 0.7% | ‐ | ‐ | ‐ | ‐ |
| Moderate | 43.1%* | 73.3% | 84.8% | 0.6% | 2.7% | ‐ |
| High | 54.5%* | 26% | 15.2% | 58.1% | 69.3% | 57.6% |
| Very high | 1.2% | ‐ | ‐ | 41.3% | 28% | 42.4% |
| % HRR | ||||||
| Light | 58.7%* | 34.7% | 24.2% | 1.8% | ‐ | ‐ |
| Moderate | 41.3%* | 62.6% | 66.7% | 38.9% | 24.7% | 3% |
| High | ‐ | 2.7% | 9.1% | 51.5% | 56% | 66.7% |
| Very high | ‐ | ‐ | ‐ | 8.4%* | 19.3% | 30.3% |
Data are expressed as percentages of subjects stratified according to exercise intensity for each cardiopulmonary exercise testing parameter. *p<0.05 vs. other groups and ^p<0.05 vs. cardiac patients.
Abbreviations: HR, heart rate; HRR, heart rate reserve; VO2, oxygen uptake.
The VT2 was identified at higher VO2 in athletes as compared to sedentary subjects and cardiac patients (overall p<0.0001). The percentage of peak VO2 demonstrated a trend toward a decrease in cardiac patients, sedentary subjects, and competitive athletes (p<0.0001) (Table 3). For the majority of cardiac patients, VO2 values at VT2 corresponded to very‐high EI when both recommendations were applied. Conversely, a higher percentage of healthy sedentary subjects and competitive athletes showed VO2 at VT2 corresponding to high EI domain according to both previous recommendations and 2020 ESC guidelines.
3.4. Comparison between left ventricular systolic function categories
The EI defined by VT1 and VT2 in subjects stratified according to LV EF and its correspondence to current recommendations and the proposed classification is reported in Table 5. Most of the frequencies did not significantly differ among the groups with different LVEF. Irrespective of the degree of LV systolic dysfunction, the majority of cardiac patients had VO2 values at VT1 corresponding to high EI when the previously recommended classification was applied, while to moderate EI when the definition was based on the 2020 ESC guidelines. Almost all groups of patients had VO2 values at VT2 corresponding mostly to very‐high EI recommendation‐based domain, while the groups with mildly depressed EF and preserved EF had VO2 values at VT2 corresponding mostly to high exercise bases on the 2020 ESC guidelines domains.
TABLE 5.
Exercise intensity defined by first and second ventilatory thresholds in subjects stratified according to left ventricular ejection fraction and its correspondence to previous recommendations and 2020 guidelines
| First ventilatory threshold | Second ventilatory threshold | |||||||
|---|---|---|---|---|---|---|---|---|
| Severely depressed | Moderately depressed | Mildly depressed | Preserved | Severely depressed | Moderately depressed | Mildly depressed | Preserved | |
| Correspondence of exercise intensity according to previous recommendations | ||||||||
| % Peak VO2 | ||||||||
| Light | ‐ | 1.2% | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Moderate | 32.5% | 32.5% | 25.8% | 46.2% | ‐ | ‐ | ‐ | ‐ |
| High | 67.5% | 65.1% | 74.2% | 53.8% | 32.5% | 41% | 41.9% | 53.8% |
| Very high | ‐ | 1.2% | ‐ | ‐ | 67.5% | 79% | 58.1% | 46.2% |
| % Peak HR | ||||||||
| Light | 5% | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Moderate | 27.5% | 26.5% | 19.4% | 15.4% | 2.5% | 1.2% | ‐ | ‐ |
| High | 67.5% | 71.1% | 80.6% | 84.6% | 47.5% | 50.6% | 41.9% | 53.8% |
| Very high | ‐ | 2.4% | ‐ | ‐ | 50% | 48.2% | 58.1% | 46.2% |
| % HRR | ||||||||
| Light | 62.5% | 61.4% | 45.2% | 46.1% | 2.5% | 2.4% | ‐ | ‐ |
| Moderate | 37.5% | 30.1% | 51.6% | 46.2% | 20% | 12% | 6.5% | 7.7% |
| High | ‐ | 8.4% | ‐ | 7.7% | 70% | 77.1% | 83.9% | 76.9% |
| Very high | ‐ | ‐ | ‐ | ‐ | 7.5% | 8.4% | 9.7% | 15.4% |
| Correspondence of exercise intensity according to 2020 guidelines | ||||||||
| % Peak VO2 | ||||||||
| Light | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Moderate | 77.5% | 75.6% | 93.8%* | 76.9% | ‐ | 1.2% | ‐ | ‐ |
| High | 22.5% | 23.2% | 6.2% | 23.1% | 47.5% | 45.1% | 53.1% | 69.2% |
| Very high | ‐ | 1.2% | ‐ | ‐ | 52.5% | 53.7% | 46.9% | 30.8% |
| % Peak HR | ||||||||
| Light | 5% | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Moderate | 40% | 42.7% | 43.8% | 53.8% | 2.5% | ‐ | ‐ | ‐ |
| High | 55% | 54.9% | 56.2% | 46.2% | 55% | 61% | 53.1% | 61.5% |
| Very high | ‐ | 2.4% | ‐ | ‐ | 42.5% | 39% | 46.9% | 38.5% |
| % HRR | ||||||||
| Light | 62.5% | 62.2% | 50% | 46.2% | 2.5% | 2.4% | ‐ | ‐ |
| Moderate | 37.5% | 37.8% | 50% | 53.8% | 50% | 39% | 28.1% | 30.8% |
| High | ‐ | ‐ | ‐ | ‐ | 42.5% | 51.2% | 62.5% | 53.8% |
| Very high | ‐ | ‐ | ‐ | ‐ | 5% | 7.3% | 9.4% | 15.4% |
Data are expressed as percentages of subjects stratified according to left ventricular ejection fraction. *p<0.05 vs. other groups.
Abbreviations: HR, heart rate; HRR, heart rate reserve; VO2, oxygen uptake.
3.5. Regression analysis
To determine the independent predictors of VT1 and VT2, expressed as VO2, mL/min, a multivariate linear regression analysis was performed. The predictors of VT1 were age, gender, height, LVEF, and β‐blocker therapy (R=0.62, adjusted R squared 0.36, p<0.0001, for the entire model). The predictors of VT2 expressed as VO2, mL/min were age, gender, weight, height, LVEF, and β‐blocker therapy (R=0.71, adjusted R squared 0.48, p<0.0001, for the entire model).
4. DISCUSSION
The present study demonstrates that in cardiac patients, the use of EI domains proposed by the previous recommendations determines a misclassification of the effective level of EI assessed by CPET‐derived ventilatory thresholds (ie, VT1 and VT2). In particular, when the percentage of peak VO2 was considered, the majority of cardiac patients had an intensity at VT1 wrongly classified as “high” and an intensity at VT2 wrongly defined as “very high” (67.1% and 59.9%, respectively). Notably, the previously recommended classification defined different levels of EI validated on cohorts of healthy subjects7, 25 but there are concerns about their performance on healthy subjects26 and they may not perform well in patients with CVD, taking medications and having a cardiac or ventilatory deficiency. Indeed, the present study demonstrates that the discrepancy between the individually determined EI and the previously recommended EI levels was less evident in healthy sedentary subjects and competitive athletes. Nevertheless, also in these groups, the same level of effort at VT1 and VT2 corresponded to different levels of EI. The new ESC 2020 ESC guidelines reported a different classification that showed in this study a better correspondence between VT1 parameters and moderate EI domain, especially when the percentage of peak VO2 was considered. However, also applying these guidelines, in cardiac patients HR parameters (ie, % peak HR and %HRR) failed to demonstrate a proper matching between VTs and EI domains. In particular, most cardiac patients had an erroneous classification of the intensity of exercise, when % peak HR and %HRR at VT1 were used.
Previous studies demonstrated that prescribing EI as a fixed percentage of peak VO2 is not a proper method to obtain homogeneous EI grades in different individuals. In particular, the prescription of EI at 70% of peak VO2 resulted in higher concentrations of plasma lactate in untrained rather than in trained subjects.27 Another study reported a large intra‐subject variability in blood lactate observed during an exercise performed at 60 and 75% of peak VO2, highlighting the absence of a lactate steady state at the same presumed level of EI.28 Moreover, a high inter‐subject variability has been observed in highly trained cyclists for RER when cycling at 79% of peak VO2.29 In our population, for the definition of EI based on the percentage of peak HR and of peak VO2, we observed a similar trend: Indeed, the majority of cardiac patients reported a percentage of peak HR at VT1 corresponding to the definition by previous recommendations of high rather than moderate intensity. Moreover, comparing the 2020 ESC guideline–based classification to the confidence interval of % peak VO2 at VTs reported in Table 3, we observed that the range of % peak VO2 at VT1 (ie, 62–64%) and VT2 (84–86%) are consistent with the moderate and high EI domains, respectively. However, these values are near to the upper limits of these ranges, suggesting that prescribing exercise with guidelines‐domain may underestimate the desired intensity, particularly in some categories of individuals. Despite its common use, only one study investigated the validity of the percentage of peak HR to normalize EI and there is no evidence that prescribing EI to fixed percentages of peak HR could be a valid method to achieve homogeneous domains of EI,30, 31 particularly in patients under β‐blocker therapy. Moreover, a loss of linearity of HR versus WR relationship has been reported as peak VO2 is approached in cardiac patients,32 in whom chronotropic incompetence is a frequent finding due to age‐, pathology‐, and drug‐related sinus node dysfunction. Consequently, in cardiac patients, both on‐ and off‐β‐blockers, high uncertainty in predicting %VO2R based on %HRR has been reported.33, 34, 35 In the present study, a population of cardiac patients on β‐blocker therapy was examined and it was observed that a great majority of subjects presented a %HRR at VT1 below the expected level of EI (ie, less than moderate). Thus, our results demonstrated that in cardiac patients, the HR recommendation–based parameters of EI may not correspond to the ventilatory threshold–based intensity of exercise and may misclassify the proper level of EI, leading to the absence of benefit or potential harm of exercise prescription.
Previous recommendations reported also the cutoffs for EI domains for the percentage of VO2R, which has been found to correspond to the same thresholds of %HRR, both in healthy individuals and in cardiac patients.36, 37 Although the 2020 ESC guidelines did not report this parameter in EI classification, when assuming for %VO2R the same cutoffs of %HRR as previously recommended, we observed that a high percentage of subjects in all categories had values of %VO2R at VT1 correctly corresponding to “moderate” EI.
Recently, Hansen et al. observed that, in a population of patients with CVD, at the same level of effort (both at VT1 and VT2) different recommendation‐based EI domains were obtained, suggesting the need for an adjustment of the recommendations.14 Moreover, they demonstrated that patients with lower peak VO2 had a higher level of EI than those with greater peak VO2.14 Only a minority of patients showed VT1 and VT2 parameters corresponding to moderate and high recommendation‐based EI, respectively. Our results are consistent with those reported by this study and provide further information about individual responses and their correspondence to previous recommendations and 2020 ESC guidelines in patients with CVD, in healthy sedentary subjects and athletes.
The lack of correspondence between guideline‐based and ventilatory threshold–based EI domains highlights the need for individualized exercise prescription based on objective parameters derived from quantitative assessment of CPET values. As a consequence, a shift from a “range‐based” to a “ventilatory threshold–based” EI prescription is advisable to prescribe an appropriate level of intensity associated with proven benefits.7, 23 The use of CPET is well established for tailored exercise prescription, as the determination of VTs represents the gold standard for assessment of EI and helps set EI in a highly individualized manner.22 Although CPET gives the unique opportunity to define EI for each specific patient, determining the VTs and identifying the most appropriate target for aerobic exercise38 also in patients with CVD,13 the % of VO2 peak is frequently used to prescribe exercise. However, this study demonstrates the importance of prescribing aerobic exercise according to VT1 and VT2, to obtain the appropriate level of moderate and high EI and to avoid levels of intensity potentially associated with harmful outcomes. Indeed, it has been demonstrated that in patients with hypertrophic cardiomyopathy during incremental effort exercise, concentrations of adrenaline and noradrenaline were stable at a light and moderate EI, whereas above VT2 plasma catecholamine levels rose rapidly.39 Therefore, an EI not exceeding VT2 seems to represent the best and safest exercise option in patients with known CVD and an accurate definition of EI based on VT2 is crucial. This approach should be followed particularly in cardiac patients under β‐blocker therapy, as the misclassification of EI is particularly evident in this group of patients: Indeed, our study demonstrates that, when a certain percentage of VO2 max and HR is used to prescribe moderate EI in a cardiac patient under β‐blocker therapy, in 61% and 74% the EI corresponds to high rather than moderate intensity, failing to identify the appropriate EI suggested by international guidelines and leading to a wrong prescription with a higher than recommended EI. Conversely, if the percentage of HRR is used in a β‐blocked patient, a light EI is prescribed in 58% of the cases, leading to an EI that is below the threshold recommended and that should not allow reaching the established benefits of exercising, given the inappropriateness of EI definition. Accordingly, based on the results of the present study, we strongly encourage physicians prescribing exercise to use an approach based on the determination of ventilatory threshold, that should more appropriately classify EI, particularly in patients under β‐blocker therapy.
In case of impossibility to determine VTs, we suggest the use of the 2020 ESC guideline classification of EI, which at least partially overcomes the limitations of the previously recommended classification and has shown a better correspondence with individual responses, particularly in cardiac patients.
5. LIMITATIONS
This study has some limitations. Firstly, the study population included mostly patients with coronary artery disease, dilated cardiomyopathy, and hypertrophic cardiomyopathy. As a consequence, our results cannot be directly applied to the entire spectrum of CVDs, although the CVDs included in this study represent most of the cardiac patients with an indication to CPET. Secondly, the population of competitive athletes is relatively small. However, these subjects were enrolled to compare VTs obtained in cardiac patients to those identified in competitive athletes, to provide a comprehensive description of the effects of CVDs, sedentary and training on the determination of VTs.
Data on RER and Borg scale values at corresponding first and second VTs were not reported in the present study, and this represents a limitation. However, in Table 3 the corresponding values of the percentages of max HR were reported in the three different groups of subjects to provide informative data also for physicians and centers that cannot use CPET to prescribe exercise.
The population of cardiac patients selected in our study was relatively well compensated and with a less advanced stage of the disease: Accordingly, the VT1 was undeterminable only in 5% of cardiac patients, while some authors found that VT1 was not determinable in a greater proportion of patients (>15%) with more advanced cardiac disease.40 Therefore, the present results cannot be generalized to the entire population of cardiac patients.
Lastly, although we demonstrated that the determination of VTs is crucial to appropriately prescribe exercise, particularly in cardiac patients under β‐blocker therapy, we recognize that the manual analysis is time‐consuming and is affected by a non‐negligible intra‐ and inter‐observer variability.40 However, if VTs are determined by highly experienced clinicians, as in this and other studies by research groups well trained in prescribing exercise by CPET, the CV is rather low (around 2–3.5% for VT1 and 1.9–2.1% for VT2).41, 42, 43, 44 It is therefore essential that physicians are aware of the importance to obtain VTs during CPET and that training is essential to appropriately interpret CPET data. Recently, artificial intelligence has been applied to the determination of VTs demonstrating that neural network achieved expert‐level performances across the tasks (mean absolute error was 9.5% (r=0.79) and 4.2% (r=0.94) for VT1 and VT2, respectively).45 Therefore, to overcome the current limitations, neural networks could potentially be embedded in CPET hardware/software to extend the reach of exercise physiologists beyond their laboratories.
6. PERSPECTIVE
The definition of exercise intensity is crucial to properly prescribe exercise, particularly in cardiac patients under β‐blocker therapy. Exercise intensity defined by percentages of peak HR and peak VO2 may misclassify the effective intensity. Conversely, in this study on 350 patients, we found that in cardiac patients, the use of EI domains proposed by the previous recommendations determines a misclassification of the effective level of EI assessed by CPET‐derived ventilatory thresholds.
The 2020 ESC guideline–based EI domains present a better correspondence with EI derived from VT1 and VT2, as compared to the previous recommendations. However, a shift from a “range‐based” to a “ventilatory threshold–based” EI determination is advisable to prescribe an appropriate level of EI for each individual, according to individual characteristics, personal clinical history, and therapy, particularly in cardiac patients under β‐blocker therapy.
Every approach to prescribe aerobic EI may have some limitations. Indeed, when aerobic EI is prescribed using indices of peak effort (eg, % VO2peak), one of the main limitations is represented by the fact that not all cardiac patients can achieve a near‐maximal effort during CPET and, therefore, this may have a relevant impact on the determination of the appropriate EI. Conversely, VT1 and VT2 are effort‐independent and can be achieved by the vast majority of cardiac patients. Nevertheless, some difficulties may also hamper the reliability of the determination of these VTs. Indeed, VT1 and VT2 cannot be determined in some patients with heart failure.40 However, in this study, we demonstrated that, if cardiac patients enrolled for exercise programs were well compensated, VTs can be determined in the vast majority of the patients with heart failure, even if they have a severe degree of LV dysfunction. Furthermore, a non‐negligible intra‐ and inter‐observer variability have been demonstrated in determining VTs in cardiac patients with heart failure.40 However, in this study the rate of disagreement between the observer 1 and observer 2 was very low, demonstrating that training is essential to appropriately interpret CPET data and, when the same approach was used to prescribe exercise, the intra‐ and inter‐observer variability dramatically decreases. Finally, a ramp cycle protocol may have also positively influenced the results of this study.
7. CONCLUSIONS
A range‐based assessment of EI for prescribing exercise could misclassify the effective EI, as assessed by VTs, particularly in cardiac patients on β‐blocker therapy. The 2020 ESC guideline–based EI domains present a better correspondence with EI derived from VT1 and VT2, as compared to the previous recommendations. However, a shift from a “range‐based” to a “ventilatory threshold–based” EI determination is advisable to prescribe an appropriate level of EI for each individual, according to individual characteristics, personal clinical history, and therapy.
CONFLICTS OF INTEREST
None.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Anselmi F, Cavigli L, Pagliaro A, et al. The importance of ventilatory thresholds to define aerobic exercise intensity in cardiac patients and healthy subjects. Scand J Med Sci Sports. 2021;31:1796–1808. 10.1111/sms.14007
Funding information
None
REFERENCES
- 1.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315‐2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non‐communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kavanagh T, Mertens DJ, Hamm LF, et al. Prediction of long‐term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation. 2002;106:666‐671. [DOI] [PubMed] [Google Scholar]
- 4.Global Recommendations on Physical Activity for Health. Geneva: 2010. [PubMed] [Google Scholar]
- 5.Pelliccia A, Sharma S, Gati S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2020;42(1):17–96. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosetti M, Abreu A, Corra U, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol. 2021;28(5):460–495. 10.1177/2047487320913379 [DOI] [PubMed] [Google Scholar]
- 7.Mezzani A, Hamm LF, Jones AM, et al. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur J Prev Cardiol. 2013;20:442‐467. [DOI] [PubMed] [Google Scholar]
- 8.Vanhees L, De Sutter J, Gelada SN, et al. Importance of characteristics and modalities of physical activity and exercise in defining the benefits to cardiovascular health within the general population: recommendations from the EACPR (Part I). Eur J Prev Cardiol. 2012;19:670‐686. [DOI] [PubMed] [Google Scholar]
- 9.American College of Sports Medicine . ACSM Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 10.Schnohr P, Marott JL, Jensen JS, Jensen GB. Intensity versus duration of cycling, impact on all‐cause and coronary heart disease mortality: the Copenhagen City Heart Study. Eur J Prev Cardiol. 2012;19:73‐80. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell BL, Lock MJ, Davison K, Parfitt G, Buckley JP, Eston RG. What is the effect of aerobic exercise intensity on cardiorespiratory fitness in those undergoing cardiac rehabilitation? A systematic review with meta‐analysis. Br J Sports Med. 2019;53:1341‐1351. [DOI] [PubMed] [Google Scholar]
- 12.Vanhees L, Geladas N, Hansen D, et al. Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular risk factors: recommendations from the EACPR (Part II). Eur J Prev Cardiol. 2012;19:1005‐1033. [DOI] [PubMed] [Google Scholar]
- 13.Guazzi M. Assessment for Exercise Prescription in Heart Failure. Card Fail Rev. 2015;1:46‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen D, Bonne K, Alders T, et al. Exercise training intensity determination in cardiovascular rehabilitation: Should the guidelines be reconsidered? Eur J Prev Cardiol. 2019;26:1921‐1928. [DOI] [PubMed] [Google Scholar]
- 15.Pymer S, Nichols S, Prosser J, Birkett S, Carroll S, Ingle L. Does exercise prescription based on estimated heart rate training zones exceed the ventilatory anaerobic threshold in patients with coronary heart disease undergoing usual‐care cardiovascular rehabilitation? A United Kingdom perspective. Eur J Prev Cardiol. 2020;27:579‐589. [DOI] [PubMed] [Google Scholar]
- 16.Diaz‐Buschmann I, Jaureguizar KV, Calero MJ, Aquino RS. Programming exercise intensity in patients on beta‐blocker treatment: the importance of choosing an appropriate method. Eur J Prev Cardiol. 2014;21:1474‐1480. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233‐270. [DOI] [PubMed] [Google Scholar]
- 18.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713;quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 19.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377‐381. [PubMed] [Google Scholar]
- 20.Mezzani A, Agostoni P, Cohen‐Solal A, et al. Standards for the use of cardiopulmonary exercise testing for the functional evaluation of cardiac patients: a report from the Exercise Physiology Section of the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2009;16:249‐267. [DOI] [PubMed] [Google Scholar]
- 21.Balady GJ, Arena R, Sietsema K, et al. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191‐225. [DOI] [PubMed] [Google Scholar]
- 22.Binder RK, Wonisch M, Corra U, et al. Methodological approach to the first and second lactate threshold in incremental cardiopulmonary exercise testing. Eur J Cardiovasc Prev Rehabil. 2008;15:726‐734. [DOI] [PubMed] [Google Scholar]
- 23.Cavigli L, Olivotto I, Fattirolli F et al. Prescribing, dosing and titrating exercise in patients with hypertrophic cardiomyopathy for prevention of comorbidities: Ready for prime time. Eur J Prev Cardiol. 2020:2047487320928654. [DOI] [PubMed] [Google Scholar]
- 24.D'Ascenzi F, Anselmi F, Fiorentini C, Mannucci R, Bonifazi M, Mondillo S. The benefits of exercise in cancer patients and the criteria for exercise prescription in cardio‐oncology. Eur J Prev Cardiol. 2019. 10.1177/2047487319874900. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine and science in sports and exercise. Med Sci Sports Exerc. 2011;43:1334‐1359. [DOI] [PubMed] [Google Scholar]
- 26.Iannetta D, Inglis EC, Mattu AT, et al. A Critical Evaluation of Current Methods for Exercise Prescription in Women and Men. Med Sci Sports Exerc. 2020;52:466‐473. [DOI] [PubMed] [Google Scholar]
- 27.Baldwin J, Snow RJ, Febbraio MA. Effect of training status and relative exercise intensity on physiological responses in men. Med Sci Sports Exerc. 2000;32:1648‐1654. [DOI] [PubMed] [Google Scholar]
- 28.Scharhag‐Rosenberger F, Meyer T, Gassler N, Faude O, Kindermann W. Exercise at given percentages of VO2max: heterogeneous metabolic responses between individuals. J Sci Med Sport. 2010;13:74‐79. [DOI] [PubMed] [Google Scholar]
- 29.Coyle EF, Coggan AR, Hopper MK, Walters TJ. Determinants of endurance in well‐trained cyclists. J Appl Physiol. 1985;1988(64):2622‐2630. [DOI] [PubMed] [Google Scholar]
- 30.Katch V, Weltman A, Sady S, Freedson P. Validity of the relative percent concept for equating training intensity. Eur J Appl Physiol Occup Physiol. 1978;39:219‐227. [DOI] [PubMed] [Google Scholar]
- 31.Jamnick NA, Pettitt RW, Granata C, Pyne DB, Bishop DJ. An Examination and Critique of Current Methods to Determine Exercise Intensity. Sports Med. 2020;50(10):1729–1756. 10.1007/s40279-020-01322-8 [DOI] [PubMed] [Google Scholar]
- 32.Colucci WS, Ribeiro JP, Rocco MB, et al. Impaired chronotropic response to exercise in patients with congestive heart failure. Role of postsynaptic beta‐adrenergic desensitization. Circulation. 1989;80(2):314–323. 10.1161/01.CIR.80.2.314 [DOI] [PubMed] [Google Scholar]
- 33.Mezzani A, Corra U, Giordano A, Cafagna M, Adriano EP, Giannuzzi P. Unreliability of the %VO2 reserve versus %heart rate reserve relationship for aerobic effort relative intensity assessment in chronic heart failure patients on or off beta‐blocking therapy. Eur J Cardiov Prev Rehabil. 2007;14:92‐98. [DOI] [PubMed] [Google Scholar]
- 34.Witte KK, Cleland JG, Clark AL. Chronic heart failure, chronotropic incompetence, and the effects of beta blockade. Heart (British Cardiac Society). 2006;92:481‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carvalho VO, Guimaraes GV, Bocchi EA. The relationship between heart rate reserve and oxygen uptake reserve in heart failure patients on optimized and non‐optimized beta‐blocker therapy. Clinics (Sao Paulo). 2008;63:725‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swain DP, Leutholtz BC. Heart rate reserve is equivalent to %VO2 reserve, not to %VO2max. Med Sci Sports Exerc. 1997;29:410‐414. [DOI] [PubMed] [Google Scholar]
- 37.Brawner CA, Keteyian SJ, Ehrman JK. The relationship of heart rate reserve to VO2 reserve in patients with heart disease. Med Sci Sports Exerc. 2002;34:418‐422. [DOI] [PubMed] [Google Scholar]
- 38.Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2016;39:1144‐1161. [DOI] [PubMed] [Google Scholar]
- 39.Shah AB, Bechis MZ, Brown M, et al. Catecholamine response to exercise in patients with non‐obstructive hypertrophic cardiomyopathy. J Physiol. 2019;597:1337‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers J, Goldsmith RL, Keteyian SJ, et al. The ventilatory anaerobic threshold in heart failure: a multicenter evaluation of reliability. J Card Fail. 2010;16:76‐83. [DOI] [PubMed] [Google Scholar]
- 41.Jamnick NA, Pettitt RW, Granata C, Pyne DB, Bishop DJ. An Examination and Critique of Current Methods to Determine Exercise Intensity. Sports Med. 2020;50:1729‐1756. [DOI] [PubMed] [Google Scholar]
- 42.Cerezuela‐Espejo V, Courel‐Ibanez J, Moran‐Navarro R, Martinez‐Cava A, Pallares JG. The Relationship Between Lactate and Ventilatory Thresholds in Runners: Validity and Reliability of Exercise Test Performance Parameters. Front Physiol. 2018;9:1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolezal BA, Storer TW, Neufeld EV, Smooke S, Tseng CH, Cooper CB. A Systematic Method to Detect the Metabolic Threshold from Gas Exchange during Incremental Exercise. J Sports Sci Med. 2017;16:396‐406. [PMC free article] [PubMed] [Google Scholar]
- 44.Santos EL, Giannella‐Neto A. Comparison of computerized methods for detecting the ventilatory thresholds. Eur J Appl Physiol. 2004;93:315‐324. [DOI] [PubMed] [Google Scholar]
- 45.Zignoli A, Fornasiero A, Stella F, et al. Expert‐level classification of ventilatory thresholds from cardiopulmonary exercising test data with recurrent neural networks. Eur J Sport Sci. 2019;19:1221‐1229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


