Summary
Targeting interleukin‐6 (IL‐6) is a promising strategy to counteract antibody‐mediated rejection (ABMR). In inflammatory states, IL‐6 antagonism was shown to modulate cytochrome P450 (CYP), but its impact on drug metabolism in ABMR treatment was not addressed so far. We report a sub‐study of a phase 2 trial of anti‐IL‐6 antibody clazakizumab in late ABMR (ClinicalTrials.gov, NCT03444103). Twenty kidney transplant recipients were randomized to clazakizumab versus placebo (4‐weekly doses; 12 weeks), followed by a 9‐month extension where all recipients received clazakizumab. To study CYP2C19/CYP3A4 metabolism, we administered pantoprazole (20 mg intravenously) at prespecified time points. Dose‐adjusted C0 levels (C0/D ratio) of tacrolimus (n = 13) and cyclosporin A (CyA, n = 6) were monitored at 4‐weekly intervals. IL‐6 and C‐reactive protein were not elevated at baseline, the latter was then suppressed to undetectable levels under clazakizumab. IL‐6 blockade had no clinically meaningful impact on pantoprazole pharmacokinetics (area under the curve; baseline versus week 52: 3.16 [2.21–7.84] versus 4.22 [1.99–8.18] μg/ml*h, P = 0.36) or calcineurin inhibitor C0/D ratios (tacrolimus: 1.49 [1.17–3.20] versus 1.37 [0.98–2.42] ng/ml/mg, P = 0.21; CyA: 0.69 [0.57–0.85] versus 1.08 [0.52–1.38] ng/ml/mg, P = 0.47). We conclude that IL‐6 blockade in ABMR – in absence of systemic inflammation – may have no meaningful effect on CYP metabolism.
Keywords: antibody‐mediated rejection, clazakizumab, cytochrome P450, drug metabolism, interleukin‐6, kidney transplantation
In inflammatory disease states, interleukin‐6 antagonism was shown to interfere with CYP450 metabolism. In this phase 2 pilot trial of anti‐interleukin‐6 antibody clazakizumab in late antibody‐mediated rejection ‐ in absence of systemic inflammation ‐ we found no significant effect of treatment on the pharmacokinetics of CYP substrate pantoprazole and dose‐adjusted calcineurin inhibitor levels. Abbreviations: ABMR, antibody‐mediated rejection; CYP, cytochrome P450; DSA, donor‐specific antibodies; eGFR, estimated glomerular filtration rate; EP, endpoint; IL‐6, interleukin‐6, PK, pharmacokinetics.

Introduction
Antibody‐mediated rejection (ABMR) is a cardinal cause of renal allograft dysfunction and loss [1]. Its treatment has remained a significant challenge [2], particularly in late ABMR, where randomized controlled trials have failed to demonstrate any effective treatment strategies, despite testing a variety of potential options, including proteasome inhibition or intravenous immunoglobulin plus CD20 antibody rituximab [3, 4, 5]. There is now increasing interest in targeting the interleukin (IL)‐6/IL‐6 receptor (IL‐6R) axis to counteract B‐cell differentiation and plasma cell formation and, as a consequence, donor‐specific antibody (DSA)‐triggered graft injury [6, 7, 8]. Therapeutic efficacy of IL‐6/IL‐6R blockade was first supported by the results of an uncontrolled observational series of 36 renal allograft recipients with refractory chronic active ABMR who received long‐term treatment with anti‐IL‐6R antibody tocilizumab [6]. Our group recently conducted a randomized controlled bi‐center phase 2 pilot trial (20 transplant recipients with late ABMR) and demonstrated that the anti‐IL‐6 monoclonal antibody clazakizumab has a significant impact on DSA levels, morphologic and molecular ABMR activity, and renal function over a period of 12 months [8]. Currently, clazakizumab is being evaluated in a large pivotal long‐duration randomized controlled phase 3 multicenter trial (IMAGINE trial; clinicaltrials.gov identifier: NCT03744910).
When using therapeutic anti‐IL‐6 or anti‐IL‐6R antibodies, one has to take into account potential changes in drug metabolism due to interference with cytochrome P450 (CYP) activity [9, 10, 11]. In cultured hepatocytes, IL‐6 was shown to diminish the expression and activity of CYP enzymes [12, 13], and in various clinical settings, elevated IL‐6 levels were found to modulate CYP‐dependent drug clearance [14, 15]. IL‐6R blockade in vitro was in turn shown to counteract IL‐6‐triggered downregulation of CYP enzymes [16], and in patients with rheumatoid arthritis, tocilizumab restored diminished CYP activity and substantially enhanced the metabolism of simvastatin, a probe substrate of CYP3A4 [17]. Similar effects were reported for anti‐IL‐6 antibody sirukumab or anti‐IL‐6R antibody sarilumab, respectively [18, 19]. These observations may have particular clinical relevance for transplant patients. In these patients, compounds with a narrow therapeutic index (calcineurin inhibitors, mammalian target of rapamycin [mTOR] inhibitors), which are metabolized in a CYP (CYP3A4/CYP3A5)‐dependent manner, are major components of standard baseline immunosuppression. Hence, one may anticipate untoward effects of anti‐IL‐6/IL‐6R antibody treatment in transplant recipients due to changes in the metabolism of immunosuppressive drugs.
The objective of this prespecified sub‐study, which was performed in the context of a recently published randomized controlled trial evaluating clazakizumab in late ABMR [8], was to clarify whether and to what extent IL‐6 antagonism by the antibody interferes with CYP‐based drug metabolism. We systematically studied the impact of clazakizumab on the pharmacokinetics (PK) of intravenous pantoprazole as a probe substrate of CYP2C19 and CYP3A4, administered at baseline, during, and at the end of the trial. Moreover, serial measurements of tacrolimus, cyclosporin A (CyA), and everolimus C0 levels allowed us to dissect the impact of clazakizumab on CYP3A4/CYP3A5‐dependent metabolism of immunosuppressants.
Patients and methods
Study design and patients
This PK sub‐study was performed in the context of a randomized, double‐blind, placebo‐controlled phase 2 pilot trial, which was designed to investigate the safety (primary endpoint), efficacy, and PK (secondary endpoints) of the monoclonal anti‐IL‐6 antibody clazakizumab (Vitaeris Inc., Vancouver, Canada) in late ABMR (ClinicalTrials.gov, NCT03444103) [8, 20]. The trial was conducted at two sites (Medical University of Vienna, Austria; Charité Universitätsmedizin Berlin, Germany) between January 2018 and April 2020. The protocol as well as major safety and efficacy results (levels of DSA, morphologic, and molecular results of two sequential follow‐up biopsies, course of renal function, and proteinuria) have previously been described in detail [8, 20]. In brief, the trial included 20 renal allograft recipients with late ABMR. Key inclusion criteria were biopsy‐proven late active or chronic active ABMR ≥365 days after transplantation, preformed or de novo HLA class I and/or II DSA, and an estimated glomerular filtration rate (eGFR) ≥30 ml/min per 1.73 m2. Major exclusion criteria were pregnancy or breastfeeding, T‐cell‐mediated rejection, acute rejection treatment within >3 months before screening, acute deterioration of graft function, active viral, bacterial, or fungal infections, active malignant disease, and abnormal liver function tests. A detailed description of all inclusion/ exclusion criteria has been provided previously [8, 20]. Main patient characteristics are provided in Table 1. As illustrated in Fig. 1, the study consisted of a 12‐week randomized placebo‐controlled phase (1:1 permuted block randomization) to decipher the short‐term effects of treatment (part A), followed by a 40‐week open‐label extension where all subjects received clazakizumab (part B). All patients provided written informed consent before trial inclusion, and the study was approved by the institutional review boards of the Medical University of Vienna and the Berlin State Ethics Committee and was conducted in accordance with the principles of the Declaration of Helsinki 2008 and the Declaration of Istanbul.
Table 1.
Baseline characteristics.
| Variables | Total (n = 20) | Clazakizumab (n = 10) | Placebo (n = 10) |
|---|---|---|---|
| Variables recorded at transplantation | |||
| Female sex, n (%) | 10 (50) | 3 (30) | 7 (70) |
| Recipient age (years), median (IQR) | 34.2 (24.6–47.6) | 37.4 (27.1–57.9) | 31.4 (22.3–42.3) |
| Deceased donor, n (%) | 14 (70) | 7 (70) | 7 (70) |
| Living donor, n (%) | 6 (30) | 3 (30) | 3 (30) |
| Prior kidney transplant, n (%) | 7 (35) | 4 (40) | 3 (30) |
| Current CDC panel reactivity ≥10%, n (%)* | 6 (33.3) | 3 (33.3) | 3 (33.3) |
| Preformed anti‐HLA DSA, n (%)† | 5 (45.5) | 3 (42.9) | 2 (50) |
| Donor age (years), median (IQR)‡ | 49.0 (21.8–57.3) | 51.0 (21.8–57.3) | 44.0 (23.3–66.0) |
| HLA mismatch (A, B, DR), median (IQR)§ | 3 (2–3) | 3 (3–3) | 3 (2–3) |
| Variables recorded at trial inclusion | |||
| Age of study patients (yr), median (IQR) | 41.5 (36.4–60.1) | 47.2 (38.7–62.1) | 39.6 (30.2–59.6) |
| Year to inclusion in the trial | 10.6 (4.4–16.2) | 9.7 (4.1–16.7) | 11.4 (5.9–16.1) |
| eGFR (ml/min per 1.73 m2 ), median (IQR) | 39.3 (33.6–49.7) | 40.5 (33.3–49.8) | 39.2 (32.9–51.7) |
| Protein/creatinine ratio (mg/g), median (IQR) | 962 (310–1,863) | 727 (197–1,311) | 1,387 (532–3,575) |
| ABMR phenotype (Banff 2017) | |||
| Active ABMR, n (%) | 2 (10) | 2 (20) | 0 |
| Chronic/active ABMR, n (%) | 18 (90) | 8 (80) | 10 (100) |
ABMR, antibody‐mediated rejection; DSA, donor‐specific antibody; CDC, complement‐dependent cytotoxicity; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
CDC panel reactivity was not recorded for 1 recipient in the clazakizumab arm and 1 in the placebo arm.
Pretransplant DSA data were available for 7 recipients in the clazakizumab arm and 4 in the placebo arm (solid‐phase HLA antibody screening on the wait list was implemented at the Vienna transplant unit in July 2009).
Donor age was not recorded for 2 recipients in the placebo arm.
HLA mismatch was not recorded for 1 recipient in the placebo arm.
Figure 1.
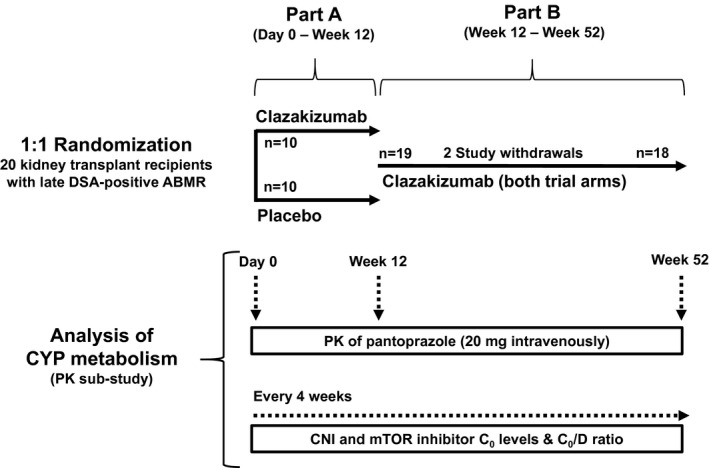
Summary of trial protocol. Twenty renal allograft recipients diagnosed with late antibody‐mediated rejection (ABMR) were randomized to receive clazakizumab or placebo for 12 weeks (part A). After 12 weeks, patients entered part B and they all were scheduled to receive clazakizumab. Two patients were withdrawn from the study because of diverticular disease complications. Both completed part A, one was withdrawn shortly before, the other after the first clazakizumab dose in part B. C0, trough level; CNI, calcineurin inhibitor; CYP, cytochrome P450; D, dose; DSA, donor‐specific antibody; mTOR, mammalian target of rapamycin; PK, pharmacokinetics.
Study medication and baseline immunosuppression
As previously described in detail, the intervention consisted of clazakizumab (25 mg per dose) administered subcutaneously at 4‐weekly intervals. Patients in the placebo arm received injections of 0.9% saline. Table 2 provides details on maintenance immunosuppression. At study inclusion, 13 patients were on tacrolimus, 6 on CyA, and 1 on everolimus. Upon trial inclusion and during the trial, immunosuppressant doses were adjusted to target trough (C0) levels of 5–10 ng/ml (tacrolimus), 80–120 ng/ml (CyA), and 3–8 ng/ml (everolimus). Immunosuppressant blood concentrations were analyzed from ethylenediaminetetraacetic acid (EDTA)‐anticoagulated fresh whole blood samples using high‐performance liquid chromatography‐tandem mass spectrometry. To dissect any potential impact of clazakizumab on calcineurin inhibitor (CNI) or mTOR inhibitor metabolism, dose‐adjusted C0 levels (C0/D ratio) were calculated at 4‐weekly intervals; this was done by dividing C0 levels by the corresponding daily dose. Intrapatient variability (IPV) of CNI or mTOR inhibitor exposure was estimated by calculating the coefficient of variation (CV), according to the following equation: CV (%) = (SD/mean trough concentration) x 100.
Table 2.
Markers of inflammation and immunosuppression at baseline.
| Variables at trial inclusion | Total (n = 20) | Clazakizumab (n = 10) | Placebo (n = 10) |
|---|---|---|---|
| Markers of inflammation | |||
| CRP (mg/dl), median (IQR) | 0.20 (0.05–0.44) | 0.13 (0.04–0.26) | 0.42 (0.08–0.48) |
| IL‐6 (pg/ml), median (IQR) | 1.53 (0.83–2.44) | 1.38 (0.79–2.34) | 1.60 (0.83–2.58) |
| Maintenance immunosuppression | |||
| Triple immunosuppression | 18 (90) | 9 (90) | 9 (90) |
| Dual immunosuppression without steroids | 2 (10) | 1 (10) | 1 (10) |
| Immunosuppressants | |||
| Tacrolimus, n (%) | 13 (65) | 6 (60) | 7(70) |
| C0 level (ng/ml), median (IQR) | 6.0 (5.2–7.1) | 5.6 (4.3–7.4) | 6.0 (5.6–7.0) |
| CyA, n (%) | 6 (30) | 4 (40) | 2 (20) |
| C0 level (ng/ml), median (IQR) | 123 (103–152) | 138 (115–170) | 85, 114 |
| Everolimus, n (%) | 1 (5) | 0 | 1 (10) |
| C0 level (mg/ml) | 5.4 | ‐ | 5.4 |
| MMF, n (%) | 10 (50) | 6 (60) | 4 (40) |
| EC‐MPA, n (%) | 10 (50) | 4 (40) | 6 (60) |
| Steroid, n (%) | 18 (90) | 9 (90) | 9 (90) |
CRP, C‐reactive protein; CyA, cyclosporin A; EC‐MPA, enteric‐coated mycophenolic acid; IL‐6, interleukin‐6; IQR, interquartile range; MMF, mycophenolate mofetil.
Pantoprazole pharmacokinetics
To investigate the impact of IL‐6 blockade on CYP‐dependent drug metabolism, trial subjects received an intravenous bolus of 20 mg pantoprazole, as a probe substrate of CYP drug‐metabolizing enzymes CYP2C19 and CYP3A4. For this dosage, a routine prophylactic dose, the sensitivity of the applied analytical assay was known to be high enough to allow for a complete evaluation of PK. PK sampling was conducted on 3 occasions: on day 0 (before initiation of clazakizumab or placebo treatment), at week 12 (end of part A), and at week 52 (end of part B). In eight patients on continuous proton pump inhibitor therapy (pantoprazole; 20 mg/day: n = 4; 40 mg/day: n = 4; none of the included subjects were on H2 antagonists), oral treatment was paused for three days before PK analysis and restarted one day thereafter. A 3‐day wash‐out period was chosen based on earlier studies that have shown complete absorption of pantoprazole by about 12 hours and a half‐life generally less than two hours [21] and was therefore considered being long enough to cover by far more than 5 half‐lives, even among poor metabolizers with extremely prolonged half‐lives [21] or patients with CYP phenoconversion [22]. Blood samples were drawn in tubes containing EDTA as the anticoagulant before and 1, 2, 3, 4, 5, and 6 hours after dosing. Samples were centrifuged at 2000 g for 10 min at 4°C, and aliquots (500 μL) were stored at −80 °C until the bioanalysis. Blood sampling on day 0 and at week 12 was completed prior to injection of clazakizumab or placebo. Plasma concentrations of pantoprazole were determined using high‐performance liquid chromatography‐tandem mass spectrometry [22]. To estimate PK parameters, noncompartmental methods were used. Maximum plasma concentration (Cmax), area under plasma concentration‐time curve from zero to the last measurable concentration (AUC0‐last) or from zero extrapolated to infinity (AUC0‐inf), and half‐life of drug elimination during the terminal phase (T1/2) were computed using Phoenix WinNonlin version 8.0 (Certara USA, Inc., Princeton, NJ, USA).
Detection of C‐reactive protein (CRP) and IL‐6
High‐sensitive CRP was measured on a Cobas 8000 analyzer by a latex particle enhanced immunoturbidimetric assay (Roche, Mannheim, Germany; functional sensitivity: 0.03 mg/dl; reference range [adults]: <0.5 mg/dl). For IL‐6 detection an ultra‐high sensitive assay based on single molecule array (Simoa™) technology (lower limit of quantitation: 0.14 pg/ml; reference range [adults]: <10 pg/ml) was performed by Myriad RBM (Austin, TX, USA).
Statistics
Continuous data are presented as median and interquartile range (IQR), and categorical variables as absolute and relative frequencies. For inter‐group comparisons, we applied Fisher’s exact, Mann–Whitney U‐test, or Wilcoxon tests, as appropriate. A two‐sided P value <0.05 was considered statistically significant. For statistical analysis, IBM SPSS Statistics version 24 (IBM Corporation, Armonk, NY) was applied.
Results
Patient characteristics and disposition
The trial included 20 patients diagnosed with active (n = 2) or chronic active ABMR (n = 18) a median of 10.6 years post‐transplantation. Baseline variables are provided in Table 1. Ten (50%) subjects were female, and 6 (30%) were recipients of a living donor transplant. The median recipient age at trial inclusion was 41.5 years, and median levels of eGFR and protein/creatinine ratio were 39.3 ml/min per 1.73 m2 and 962 mg/g, respectively. Details on immunosuppressive maintenance therapy are provided in Table 2. At study inclusion, 18 recipients (90%) were on triple baseline immunosuppression and 2 (10%) on dual immunosuppression without steroids. Thirteen (65%) recipients were on tacrolimus‐, 6 (30%) on CyA‐, and one (5%) on everolimus‐based immunosuppression. At baseline, C0 levels were 6.0 (median, IQR: 5.2–7.1) ng/ml, 123 (103–152) ng/ml, and 5.4 ng/ml, respectively. Median levels of CRP were within the normal range (0.20 [0.05–0.44]) mg/dl, as were IL‐6 concentrations, which were consistently below the upper normal limit of 10 pg/ml (median [IQR]: 1.53 [0.83–2.44] pg/ml).
The design of the trial is illustrated in Fig. 1. Recipients were randomized to receive either 4‐weekly injections of clazakizumab or of placebo until week 12 (part A), followed by clazakizumab at 4‐weekly intervals for all included subjects until week 52 (part B). Two patients were withdrawn from the study, one at the end of part A, and one after a single clazakizumab injection in part B. Due to adverse events (n = 10) or personal reasons (n = 1), eleven patients did not receive all 13 scheduled clazakizumab injections. In part A, 17 recipients received all three doses and 3 patients two doses. Ten of the 19 recipients enrolled in part B received less than the 10 scheduled doses (9 doses: n = 4; 8 doses: n = 2, 7 doses: n = 1, 6 doses: n = 1, 5 doses: n = 1, 1 dose: n = 1). Moreover, the clazakizumab dose was reduced to 12.5 mg (last dose in part B) in two active patients with diverticulosis in accordance with a study amendment which was written in response to a second case of complicated diverticular disease.
Figure 2 illustrates the impact of clazakizumab on CRP levels. We observed a virtually complete reduction after the initial clazakizumab injection. This effect was associated with a marked increase in detectable IL‐6 levels, presumably due to the accumulation of large levels of stable IL‐6/anti‐IL‐6 antibody complexes [23]. As shown in Fig. 2, clazakizumab had no meaningful effect on liver parameters.
Figure 2.
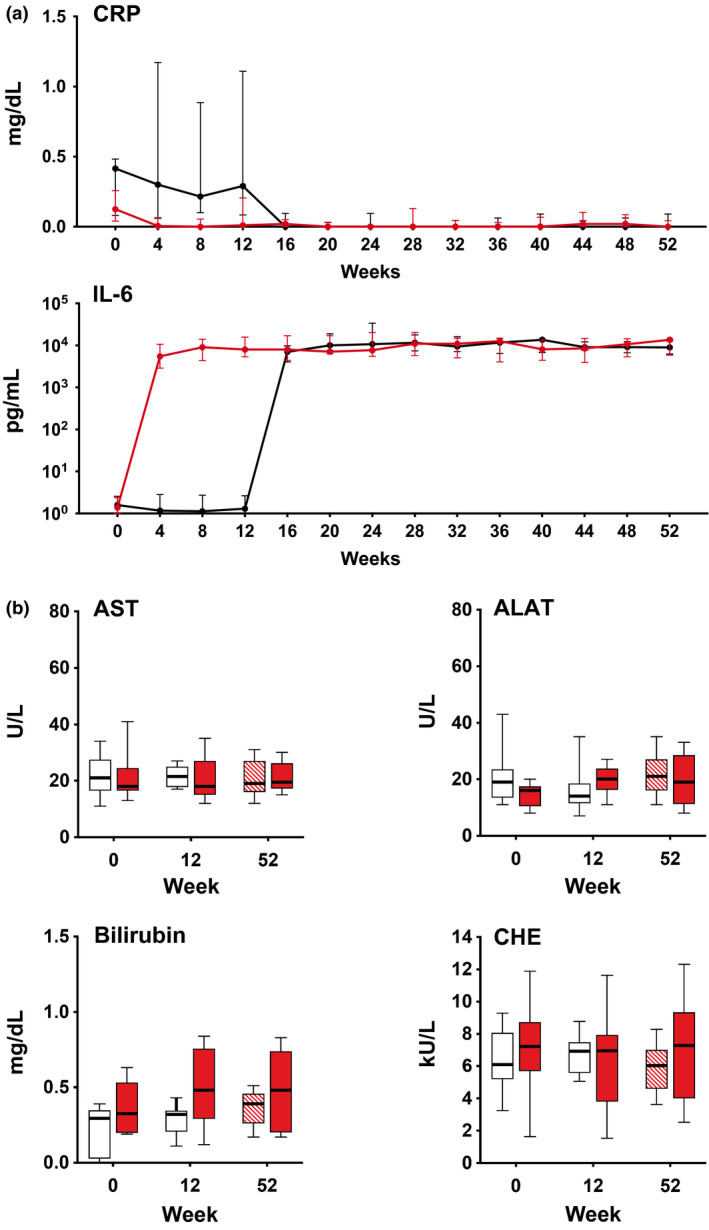
C‐reactive protein (CRP), interleukin‐6 (IL‐6), and liver parameters. Panel a illustrates the course of CRP and IL‐6 levels (median and interquartile range) measured at 4‐weekly intervals in patients randomized to receive clazakizumab (red line) or placebo (black line), respectively. Panel b shows levels (median, interquartile range and range) of liver parameters for patients allocated to clazakizumab (red closed boxplots) versus placebo (part A: open boxplots; part B: red hatched box plots). ALAT, alanine aminotransferase; AST, aspartate aminotransferase; CHE, cholinesterase.
Effect of clazakizumab on pantoprazole pharmacokinetics
PK profiles of intravenous pantoprazole (20 mg) administered on day 0, at week 12, and at week 52 are detailed in Table 3.
Table 3.
Pantoprazole pharmacokinetics.
| PK parameters† | Total (n = 20) | Clazakizumab (n = 10) | Placebo (n = 10) |
|---|---|---|---|
| Day 0 | |||
| T1/2 (h) | 1.08 (0.86–1.72) | 1.55 (0.98–3.09) | 0.94 (0.76–1.33) |
| Cmax (μg/ml) | 1.49 (1.10–2.59) | 1.66 (1.07–3.45) | 1.37 (1.08–2.44) |
| AUC0‐last (μg/ml*h) | 2.88 (1.82–4.02) | 3.67 (1.76–9.18) | 2.71 (1.70–3.32) |
| AUC0‐inf (μg/ml*h) | 3.16 (2.21–7.84) | 4.21 (2.11–13.01) | 3.14 (2.31–3.94) |
| Week 12 | |||
| T1/2 (h) | 1.08 (0.97–1.9) | 1.90 (0.98–3.86) | 1.0 (0.84–1.19) |
| Cmax (μg/ml) | 1.45 (1.09–2.83) | 1.66 (0.98–3.02) | 1.38 (1.26–2.0) |
| AUC0‐last (μg/ml*h) | 2.70 (1.75–5.41) | 3.45 (1.62–11.39) | 2.05 (1.74–3.92) |
| AUC0‐inf (μg/ml*h) | 3.66 (2.03–7.52) | 6.67 (2.47–20.65) | 2.33 (1.91–4.43) |
| Week 52 | |||
| T1/2 (h) | 1.03 (0.89–2.10) | 2.10 (1.41–2.98) | 0.89 (0.80–1.00) |
| Cmax (μg/ml) | 1.97 (0.99–2.89) | 2.75 (1.49–3.82) | 1.57 (0.93–2.32) |
| AUC0‐last (μg/ml*h) | 3.82 (1.78–7.10) | 6.87 (2.79–12.98) | 2.37 (1.72–4.17) |
| AUC0‐inf (μg/ml*h) | 4.22 (1.99–8.18) | 8.09 (2.92–20.60) | 2.75 (1.84–4.66) |
| Percent change from day 0 to week 12 | |||
| T1/2 | 5.9 (−4.5–23.2) | 18.4 (−0.4–23.2) | 0.2 (−14.4‐21.5) |
| Cmax | 2.1 (−12.2‐26.3) | ‐7.6 (−16.2‐17.4) | 5.7 (−10.7‐41.3) |
| AUC0‐last | 10.4 (−8.0‐27.8) | ‐1.8 (−7.9‐27.9) | 15.6 (−18.7‐31.1) |
| AUC0‐inf | 11.6 (−11.7‐31.4) | 4.0 (−6.4‐80.3) | 13.8 (−17.7‐27.8) |
| Percent change from day 0 to week 52 | |||
| T1/2 | ‐0.3 (−19.9‐19.2) | 8.8 (−9.7‐56.8) | ‐9.8 (−34.1‐14.8) |
| Cmax | 5.5 (−22.1‐33.6) | 22.5 (1.6‐37.8) | ‐8.8 (−43.5‐44.5) |
| AUC0‐last | 25.3 (−15.9–51.7) | 41.9 (0.4–84.5) | 13.6 (−47.2–50.5) |
| AUC0‐inf | 31.9 (−22.5–64.0) | 52.3 (−5.6–64.1) | 0.72 (−42.7–64.0) |
Results are provided as median and interquartile range. With the exception of T1/2 at 52 weeks (P = 0.01), inter‐group differences with respect to PK parameters (clazakizumab versus placebo; Mann–Whitney U‐test) were not significant (P > 0.05). Changes from baseline to week 52 (overall study cohort; paired analysis applying Wilcoxon test) were nonsignificant (P > 0.05).
AUC0‐last, area under plasma concentration from zero hours to the last measurable concentration; AUC0‐inf, AUC extrapolated to infinity; Cmax, maximum plasma concentration; PK, pharmacokinetics.
Pantoprazole PK at baseline and at week 12 was available for all patients, at 52 weeks for 18 subjects, following study withdrawal of two study patients.
In the overall cohort, PK parameters did not significantly change from baseline to week 52 (when all patients had received clazakizumab treatment for 9‐12 months): T1/2 (median: 1.08 vs. 1.03 hours; P = 0.98), Cmax (1.49 vs. 1.97 μg/ml; P = 0.78), AUC0‐last (2.88 vs. 3.82 μg/ml*h; P = 0.31), AUC0‐inf, (3.16 vs. 4.22 μg /ml*h; P = 0.36).
At baseline, we observed numerically higher median T1/2, Cmax, and AUC values among patients allocated to clazakizumab than in patients allocated to placebo (bias by chance; Table 3). Inter‐group differences in median levels at the end of randomized controlled part A at week 12 (or differences in percent change from baseline), however, were not significant. PK parameters were as follows: T1/2 (clazakizumab vs. placebo: median [IQR]: 1.90 vs. 1.0 hours; P = 0.059), Cmax (1.66 vs. 1.38 μg/ml; P = 0.68), AUC0‐last (3.45 vs. 2.05 μg/ml*h; P = 0.44), and AUC0‐inf (6.67 vs. 2.33 μg/ml*h; P = 0.11), respectively (Table 3).
The results obtained in a sub‐analysis of nine patients who had received all 13 scheduled doses of clazakizumab are provided in Table S1. Again, there were no significant changes in PK parameters from baseline to week 52. With the exception of a small difference with respect to the percent change in Cmax from day 0 to week 12, inter‐group differences in PK parameters between clazakizumab and placebo were not significant (Table S1).
Effect of clazakizumab on immunosuppressant metabolism
Figure 3 illustrates the course of CNI and everolimus C0/D ratio and C0 levels. In the overall cohort (13 patients on tacrolimus), tacrolimus C0 levels increased within the first weeks after trial inclusion and remained within a narrow range, according to tacrolimus target levels defined in the study protocol (day 0 vs. week 52; median [IQR]: 6.0 [5.2–7.1] vs. 7.5 [6.2–8.2] ng/ml; P = 0.05). The C0/D ratio did not change over time (1.49 [1.17–3.20] vs. 1.37 [0.98–2.42] ng/ml/mg; P = 0.21). At week 12 (end of randomized controlled part A) we did not observe significant differences between the clazakizumab (n = 6) and placebo arms (n = 7) with respect to tacrolimus C0 levels (median [IQR]: 7.0 [4.8–8.7] vs. 6.4 [5.7–8.1] ng/ml; P = 0.84) or C0/D ratio (2.15 [1.13–4.35] vs. 2.0 [0.73–2.03] ng/ml/mg; P = 0.37), respectively. As shown in Table S2, the 13 patients on tacrolimus had a median number of 2.5 (IQR: 1.0–3.5) dose adjustments (part A: 0 [0–1.0]; part B: 1 [1.0–3.5]) and a median tacrolimus CV of 22.0 (19.0–25.5%; part A: 11.9% [10.2–25.2%]; part B: 21.8% [17.2–24.1%]. Differences between clazakizumab and placebo were not significant (Table S2).
Figure 3.
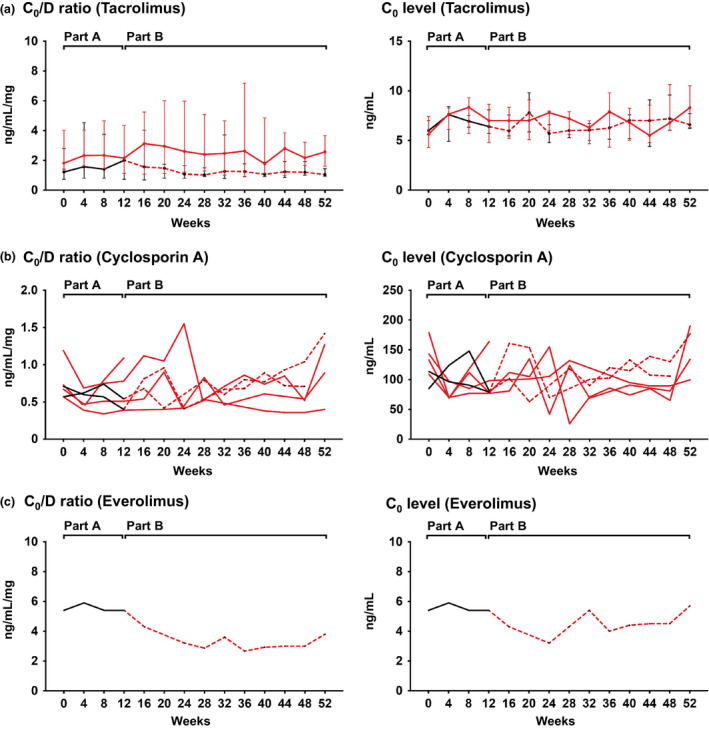
Immunosuppressant C0 level/dose (C0/D) ratio and C0 levels. Results are shown for tacrolimus (a, median and interquartile range), cyclosporin A (b, individual course), and everolimus (c, individual course), in relation to treatment allocation (placebo: black [part A] and hatched red lines [part B]; clazakizumab: red lines [part A and part B]. For part A, data were available for all patients, and for part B, because of study withdrawal of two patients, for 18 subjects.
Analyzing the 6 recipients on CyA, we found that baseline C0 levels and C0/D ratio did not significantly change between the first and last study visit (median [IQR]; C0 levels: 123 [103–152] vs. 156 [108–187] ng/ml; P = 0.47; C0/D ratio: 0.69 [0.57–0.85] vs. 1.08 [0.52–1.38] ng/ml/mg; P = 0.47). A limited number of cases (clazakizumab: n = 4; placebo: n = 2), however, precluded statistical inter‐group comparisons. However, as illustrated in Fig. 3, CyA C0 levels were similar between study arms For the 6 patients on CyA, we recorded in median 2.5 (IQR: 0.75–4.75) dose adjustments and a median CyA CV of 29.8% (23.0–41.6%), respectively.
The individual course of C0 levels and C0/D ratio in the single patient on everolimus is shown in Fig. 3. While C0 levels fluctuated within a range between 3 and 6 ng/ml, we observed a decrease in C0/D ratio of approximately 50%. We recorded a single everolimus dose adjustment and a CV of 16.9%.
Discussion
A major finding of this PK sub‐study of a recently published randomized controlled trial of clazakizumab in late ABMR [8] was that, in contrast to earlier observations made in patients with rheumatic disease being treated with anti‐IL‐6R monoclonal antibodies, prolonged blockade of IL‐6 did not enhance CYP‐dependent drug metabolism. This was supported by the results of systematic analysis of PK profiles of intravenous pantoprazole, a probe drug of CYP2C19 and CYP3A4 metabolism. Most importantly, clazakizumab had no meaningful impact on C0 levels, C0/D ratios, number of dose adjustments, and IPV of CNI, which are predominantly metabolized by CYP3A isozymes (CYP3A4, CYP3A5).
The current interest in targeting IL‐6 in organ transplant patients results from emerging evidence suggesting that this pleiotropic cytokine, which exerts a wide range of effects on components of innate and acquired immunity, may impact the pathogenesis of renal allograft rejection [7]. Previous studies have demonstrated a marked upregulation of intra‐graft IL‐6 gene expression in rejecting allografts, and early rejection was found to associate with an increase in systemic and urinary levels of this cytokine [24, 25]. In the specific context of ABMR, a recent study has revealed a potential role of IL‐6 in the context of HLA‐DQ antibody‐triggered endothelial activation and impaired regulatory T‐cell expansion [26]. Moreover, IL‐6 may be critically involved in B‐cell alloimmunity, given its well‐known role as a mediator of B‐cell activation/differentiation and plasma cell formation [7]. Indeed, in our interventional trial, which was primarily designed to evaluate the safety and tolerability of clazakizumab in patients with late ABMR [20], we were able to demonstrate a significant reduction of DSA already after 3 months and a modulation of ABMR activity after prolonged treatment, as reflected by reduced microcirculation inflammation and molecular ABMR scores. Moreover, treatment significantly halted the progression of graft dysfunction over a period of a year [8].
Our study results suggest that late chronic ABMR, which usually presents silently many years after transplantation, may not, as is believed to be the case in acute rejection, lead to a state of systemic inflammation. This is reinforced by our finding of baseline IL‐6 (and CRP) levels within the normal range. In addition, continuous levels of baseline immunosuppression including steroid treatment may have counteracted systemic inflammatory responses in our patients. Notably, pantoprazole pharmacokinetic parameters, such as the ∼1‐hour plasma half‐life calculated for our transplant patients, were comparable to those earlier reported for healthy volunteers [21]. This may be in contrast to other disease states. For example, in a study of critically ill patients, the half‐life of pantoprazole was approximately 5‐fold longer indicating a substantial downregulation of CYP enzymes [22].
Altogether, this may provide a plausible explanation for our observation that IL‐6 antagonism by clazakizumab did not enhance the metabolism of CNI used as standard immunosuppressants, presumably a result of unaltered CYP isoenzyme expression at baseline. In this respect, ABMR may be different from other disease states, such as infection or autoimmune disease, where systemic inflammation is well known to be associated with markedly elevated IL‐6 levels and other acute‐phase proteins [27, 28]. For example, clinical association studies in patients with heart failure or cancer have shown associations between IL‐6 levels in peripheral blood and CYP‐dependent metabolism [14, 15]. In vitro experiments have demonstrated a substantial impact of pro‐inflammatory cytokines on different isozymes including CYP2C19 and, of high relevance for the metabolism of CNI and mTOR inhibitors, CYP3A4 and CYP3A5 [12, 13]. In such experiments, IL‐6 or IL‐6R antagonism completely prevented CYP downregulation [16], an effect which was also demonstrated for simvastatin as a probe drug for CYP3A4 in patients with rheumatoid arthritis [17, 19]. In a study of 19 patients with active rheumatoid arthritis, simvastatin Cmax and AUC were thereby decreased by about 50%, an effect which was fully reversed by the IL‐6 antibody sarilumab [19]. Notably, in a study by Naito et al. [29] which included 7 patients with rheumatoid arthritis who were treated with tacrolimus on top of tocilizumab demonstrated no effect of IL‐6R blockade on tacrolimus PK, presumably because the included patients were in clinical remission or had only mild disease activity. As in our trial, unaltered CNI PK may be explained by a lack of considerable systemic inflammation and unaffected hepatic oxidative metabolism.
To the best of our knowledge, the impact of IL‐6 antagonism on CNI exposure in allograft recipients has not yet been systematically studied. Given the very narrow therapeutic index of CNI, the observed lack of interference with CNI metabolism may have significant clinical relevance in the transplant setting, where several studies are currently ongoing, including a large pivotal phase 3 randomized controlled trial evaluating clazakizumab over a period of 5 years in chronic ABMR [30].
We are aware of the inherent limitations of our study, in particular, the small sample size (20 recipients) and a heterogeneity regarding immunosuppressive treatment. Moreover, our study design did not include a detailed PK analysis for CNI and everolimus, relying on evaluations of dose‐adjusted C0 levels to estimate drug metabolism and exposure. A limited number of patients on CyA precluded a valid statistical inter‐group comparison between clazakizumab and placebo. Nevertheless, as observed for dose‐adjusted tacrolimus C0 levels, paired analysis failed to reveal meaningful changes in CyA C0/D ratio over a period of 12 months, which may argue against a substantial effect on its metabolism. Our study may provide a valuable basis for an ongoing phase 3 study in chronic ABMR. This trial, which will include more than 300 subjects, can be expected to provide a more in‐depth insight into the pharmacologic effects of clazakizumab. A possible differential effect of IL‐6 blockade on mTOR inhibitor metabolism needs to be established. Our cohort included only one recipient receiving mTOR inhibitor treatment, and we have no meaningful explanations regarding observed fluctuations of everolimus levels, especially with regard to its hepatic metabolism that is very similar to that of CNI. Notably, because of a limited sample size, the results of our present trial may not entirely exclude the possibility of clinically relevant alterations of CYP metabolism in some treated transplant patients, for example, subjects presenting with significant levels of systemic inflammation at baseline. Accordingly, with the use of IL‐6 antagonists in the context of immunosuppressive maintenance therapy, regular and careful drug level monitoring may be of clinical importance.
In conclusion, the results of this study suggest that IL‐6 antagonism in late ABMR, which may not associate with relevant levels of systemic inflammation, may not exert clinically meaningful effects on CYP‐dependent drug metabolism, and, most importantly, may not alter the metabolism of CNI.
Authorship
J.M., M.D., K.B., E.C., B.J and G.A.B. participated in the research design, performance of the research, data analysis, interpretation of results, and writing of the manuscript. C.S., K.D., F.E., K.A.M and S.H.A. participated in performance of research, data analysis and writing of the manuscript. S.S., S.E., B.R. participated in performance of research and data analysis.
Funding
The trial was funded by an investigator‐initiated unrestricted grant from Vitaeris Inc., Vancouver, Canada (a subsidiary of CSL Behring, King of Prussia, PA, USA) (to G.A. Böhmig and B. Jilma).
Conflict of interest
E. Chong was employed by Vitaeris Inc., Vancouver, Canada (a subsidiary of CSL Behring, King of Prussia, PA, USA). S.H. Adler is employed by CSL Behring. G.A. Böhmig is member of the steering committee for an ongoing pivotal phase 3 trial evaluating clazakizumab in chronic active antibody‐mediated rejection (ClinicalTrials.gov number, NCT03744910; sponsored by CSL Behring). All remaining authors have nothing to disclose. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Supporting information
Table S1. Pantoprazole PK in 9 patients who received all 13 scheduled clazakizumab injections.
Table S2. Changes in dosage and intrapatient variation of trough levels among patients on tacrolimus.
Contributor Information
Bernd Jilma, Email: bernd.jilma@meduniwien.ac.at.
Georg A. Böhmig, Email: georg.boehmig@meduniwien.ac.at.
References
- 1.Loupy A, Lefaucheur C. Antibody‐mediated rejection of solid‐organ allografts. N Engl J Med 2018; 379: 1150. [DOI] [PubMed] [Google Scholar]
- 2.Schinstock CA, Mannon RB, Budde K, et al. Recommended Treatment for Antibody‐mediated Rejection After Kidney Transplantation: The 2019 Expert Consensus From the Transplantion Society Working Group. Transplantation 2020; 104: 911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eskandary F, Regele H, Baumann L, et al. A randomized trial of bortezomib in late antibody‐mediated rejection (BORTEJECT). J Am Soc Nephrol 2018; 29: 591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreso F, Crespo M, Ruiz JC, et al. Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: A multicenter, prospective, randomized, double‐blind clinical trial. Am J Transplant 2018; 18: 927. [DOI] [PubMed] [Google Scholar]
- 5.Böhmig GA, Eskandary F, Doberer K, Halloran PF. The therapeutic challenge of late antibody‐mediated kidney allograft rejection. Transpl Int 2019; 32: 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi J, Aubert O, Vo A, et al. Assessment of tocilizumab (anti‐interleukin‐6 receptor monoclonal) as a potential treatment for chronic antibody‐mediated rejection and transplant glomerulopathy in HLA‐sensitized renal allograft recipients. Am J Transplant 2017; 17: 2381. [DOI] [PubMed] [Google Scholar]
- 7.Jordan SC, Ammerman N, Choi J, et al. Interleukin‐6: An Important Mediator of Allograft Injury. Transplantation 2020; 104: 2497. [DOI] [PubMed] [Google Scholar]
- 8.Doberer K, Duerr M, Halloran PF, et al. A randomized clinical trial of anti‐IL‐6 antibody clazakizumab in late antibody‐mediated kidney transplant rejection. J Am Soc Nephrol 2021; 32: 708–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Ostor AJ, Nisar MK. Interleukin‐6 and cytochrome‐P450, reason for concern? Rheumatol Int 2012; 32: 2601. [DOI] [PubMed] [Google Scholar]
- 10.Shah RR, Smith RL. Inflammation‐induced phenoconversion of polymorphic drug metabolizing enzymes: hypothesis with implications for personalized medicine. Drug Metab Dispos 2015; 43: 400. [DOI] [PubMed] [Google Scholar]
- 11.Ferri N, Bellosta S, Baldessin L, Boccia D, Racagni G, Corsini A. Pharmacokinetics interactions of monoclonal antibodies. Pharmacol Res 2016; 111: 592. [DOI] [PubMed] [Google Scholar]
- 12.Abdel‐Razzak Z, Loyer P, Fautrel A, et al. Cytokines down‐regulate expression of major cytochrome P‐450 enzymes in adult human hepatocytes in primary culture. Mol Pharmacol 1993; 44: 707. [PubMed] [Google Scholar]
- 13.Aitken AE, Morgan ET. Gene‐specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos 2007; 35: 1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivory LP, Slaviero KA, Clarke SJ. Hepatic cytochrome P450 3A drug metabolism is reduced in cancer patients who have an acute‐phase response. Br J Cancer 2002; 87: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frye RF, Schneider VM, Frye CS, Feldman AM. Plasma levels of TNF‐alpha and IL‐6 are inversely related to cytochrome P450‐dependent drug metabolism in patients with congestive heart failure. J Card Fail 2002; 8: 315. [DOI] [PubMed] [Google Scholar]
- 16.Mimura H, Kobayashi K, Xu L, et al. Effects of cytokines on CYP3A4 expression and reversal of the effects by anti‐cytokine agents in the three‐dimensionally cultured human hepatoma cell line FLC‐4. Drug Metab Pharmacokinet 2015; 30: 105. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt C, Kuhn B, Zhang X, Kivitz AJ, Grange S. Disease‐drug‐drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther 2011; 89: 735. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang Y, de Vries DE , Xu Z, et al. Evaluation of disease‐mediated therapeutic protein‐drug interactions between an anti‐interleukin‐6 monoclonal antibody (sirukumab) and cytochrome P450 activities in a phase 1 study in patients with rheumatoid arthritis using a cocktail approach. J Clin Pharmacol 2015; 55: 1386. [DOI] [PubMed] [Google Scholar]
- 19.Lee EB, Daskalakis N, Xu C, et al. Disease‐drug interaction of sarilumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacokinet 2017; 56: 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eskandary F, Dürr M, Budde K, et al. Clazakizumab in late antibody‐mediated rejection: study protocol of a randomized controlled pilot trial. Trials 2019; 20: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gawronska‐Szklarz B, Adamiak‐Giera U, Wyska E, et al. CYP2C19 polymorphism affects single‐dose pharmacokinetics of oral pantoprazole in healthy volunteers. Eur J Clin Pharmacol 2012; 68: 1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoergenhofer C, Hobl EL, Schellongowski P, et al. Clopidogrel in critically ill patients. Clin Pharmacol Ther 2018; 103: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu ZY, Brochier J, Wijdenes J, Brailly H, Bataille R, Klein B. High amounts of circulating interleukin (IL)‐6 in the form of monomeric immune complexes during anti‐IL‐6 therapy. Towards a new methodology for measuring overall cytokine production in human in vivo. Eur J Immunol 1992; 22: 2819. [DOI] [PubMed] [Google Scholar]
- 24.Vandenbroecke C, Caillat‐Zucman S, Legendre C, et al. Differential in situ expression of cytokines in renal allograft rejection. Transplantation 1991; 51: 602. [DOI] [PubMed] [Google Scholar]
- 25.Waiser J, Budde K, Katalinic A, Kuerzdorfer M, Riess R, Neumayer HH. Interleukin‐6 expression after renal transplantation. Nephrol Dial Transplant 1997; 12: 753. [DOI] [PubMed] [Google Scholar]
- 26.Cross AR, Lion J, Poussin K, et al. HLA‐DQ alloantibodies directly activate the endothelium and compromise differentiation of FoxP3(high) regulatory T lymphocytes. Kidney Int 2019; 96: 689. [DOI] [PubMed] [Google Scholar]
- 27.Song M, Kellum JA. Interleukin‐6. Crit Care Med 2005; 33: S463. [DOI] [PubMed] [Google Scholar]
- 28.Gottenberg JE, Dayer JM, Lukas C, et al. Serum IL‐6 and IL‐21 are associated with markers of B cell activation and structural progression in early rheumatoid arthritis: results from the ESPOIR cohort. Ann Rheum Dis 2012; 71: 1243. [DOI] [PubMed] [Google Scholar]
- 29.Naito T, Ohshiro J, Sato H, et al. Relationships between concomitant biologic DMARDs and prednisolone administration and blood tacrolimus exposure or serum CYP3A4/5‐related markers in rheumatoid arthritis patients. Clin Biochem 2019; 69: 8. [DOI] [PubMed] [Google Scholar]
- 30.Mayer KA, Doberer K, Eskandary F, Halloran PF, Böhmig GA. New concepts in chronic antibody‐mediated kidney allograft rejection: prevention and treatment. Curr Opin Organ Transplant 2021; 26: 97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Pantoprazole PK in 9 patients who received all 13 scheduled clazakizumab injections.
Table S2. Changes in dosage and intrapatient variation of trough levels among patients on tacrolimus.


