Abstract
Advances in stem cell biology and the understanding of factors that determine lung stem cell self-renewal have enabled long-term in vitro culture of human lung cells derived from airway basal and alveolar type II cells. Improved capability to expand and study primary cells long term, including in clonal cultures that are recently derived from a single cell, will allow experiments that address fundamental questions about lung homeostasis and repair, as well as translational questions in asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and lung cancer research. Here, we provide a brief history of postnatal lung epithelial cell culture and describe recent methodological advances. We further discuss the applications of primary cultures in defining “normal” epithelium, in modeling lung disease, and in future cell therapies.
Keywords: respiratory epithelium, epithelial cells, cell culture, stem cells, cloning
Over the past decade, rapid progress has been made in our understanding of both the composition of human lung epithelia and the stem cell hierarchies that contribute to their maintenance and renewal following injury. In alveoli, alveolar type II (AT2) cells sustain differentiated AT1 cells (1). In the airways, basal cells replenish the pseudostratified epithelium in the trachea and throughout most generations of the intrapulmonary conducting airways (2). Basal cells have the capacity to self-renew as well as differentiate into the major secretory and multiciliated luminal cell types (3) and likely also minor populations such as tuft cells, ionocytes, and pulmonary neuroendocrine cells (4, 5).
The differentiation trajectories of human airway epithelial cells have been refined by single-cell RNA sequencing (scRNAseq) approaches. Both in vivo (6, 7) and in vitro (4, 8) studies converge on a model in which basal cells differentiate through a suprabasal progenitor population to give rise to club-like cells that can contribute to both goblet and multiciliated lineages. The lineage history of minor cell types is more difficult to ascertain in current data sets, but basal cell–descended tuft-like cells might give rise to ionocytes and pulmonary neuroendocrine cells, based on transcriptional similarities (5). Subsets of basal cells, distinguishable by proliferation or differentiation status, have been identified in these studies (9), as have a population of basal cells—“hillock” basal cells—that might uniquely contribute to squamous stratified regions of epithelium (7). Cells residing in airway submucosal glands can be distinguished from surface epithelium based on their distinct gene expression profiles (5, 10). Smoking skews airway epithelial differentiation toward mucosecretory cell production, inhibits ciliogenesis, and globally alters the airway epithelial transcriptome (5, 11). Differentiation is also altered during disease pathogenesis; in asthma, ciliated cells begin to express mucosecretory genes characteristic of goblet cells (12) and chronic obstructive pulmonary disease (COPD) pathogenesis involves epithelial senescence (13) and altered AT2 differentiation (14). Abnormal airway epithelial cell states have also been described (in addition to impaired AT2 cell differentiation and AT2 senescence phenotypes) in pulmonary fibrosis (15–17).
Our understanding of lung cell biology informs in vitro models of the epithelium and vice versa. Cell culture methods have been developed to recapitulate lung epithelial proliferation, differentiation, and responses to environmental stressors, such as cigarette smoke and air pollution. These approaches allow researchers to study airway damage and disease in controlled experimental conditions, including the epithelial response to putative therapies. Here, we review advances in cell culture methodology that allow sustained expansion of lung epithelial cells in culture, with an emphasis on recent advances that allow the study of “clones,” or cell cultures derived from individual cells.
A Brief History of Lung Epithelial Cell Culture
Primary human bronchial epithelial cells (HBECs) have been cultured from various clinical samples, including rejected or excess transplant tissue, lobectomy tissue, induced sputum samples, bronchiolar lavage, and endobronchial forcep or brush biopsies. To optimize HBEC culture conditions, studies were performed to define the calcium and growth factor concentrations in growth media (18). Key factors include epidermal growth factor (EGF), insulin, transferrin, hydrocortisone, phosphoethanolamine, and ethanolamine. Growth media compositions were further modified to increase the initial success and duration of culture; retinoic acid was included as it was found to reduce or reverse the generation of squamous metaplasia in culture (19). Serum-free LHC-9 medium was a further refinement that saw the addition of bovine pituitary extract, epinephrine, and 3,3′,5-triiodo-l-thyronine (T3) to increase proliferation (20). The generation of bronchial epithelial growth medium and several commercially available alternatives built upon these previous media, allowing serial culture of primary HBECs and the initiation of cultures from cryopreserved cells (21, 22). Air–liquid interface (ALI) culture of HBECs in differentiation media allows basal cells to form a confluent, polarized monolayer before generating luminal cell types. In similar medium, three-dimensional (3D) differentiation of basal cells can be achieved either in suspension culture (23) or by embedding cells in extracellular matrices to form cyst-like organoids commonly called “tracheospheres” or “bronchospheres” (24).
Even in optimized cell culture media, few HBECs could be expanded in 2D cultures from small clinical biopsies for functional studies in either ALI or 3D cultures. Serial culture was possible for only a limited number of population doublings during which cells progressively lost their differentiation potential. Three main cell culture strategies have extended primary lung cell culture lifetime and decreased reliance on cancer cell lines to model normal epithelium (Table 1): immortalization, improved 2D cell culture conditions, and improved 3D culture conditions (Figure 1).
Table 1.
A List of Human Pulmonary Epithelial Cell Lines
| Cell Line | Derivation | Characteristics | References |
|---|---|---|---|
| 16HBE14o- | Primary HBECs immortalized with SV40 large T antigen |
|
(28, 131, 132) |
| A549 | Lung adenocarcinoma |
|
(133) |
| BEAS-2B | Primary HBECs transduced with SV40 large T antigen |
|
(27, 29, 134) |
| Calu-3 | Bronchial adenocarcinoma |
|
(131, 135–137) |
| H441 | Lung papillary adenocarcinoma |
|
(138, 139) |
| HBEC-KTs | Primary HBECs transduced with CDK4 and hTERT |
|
(31–33) |
| HPLE | Primary distal lung epithelial transfected with SV40 large T antigen |
|
(140) |
| hSABCi-NS1.1 | Primary small airway basal cells transduced with hTERT |
|
(124) |
| HSAEC1-KT | Primary small airway epithelial cells transduced with CDK4 and hTERT |
|
(31, 141) |
| LIMM-NBE1 | Primary lung cells transduced with hTERT |
|
(142, 143) |
| LL-iPSC-AEC II | iPSCs differentiated to AT2-like cells and transduced with hTERT and BMI-1 |
|
(40) |
| NuLi | Primary airway epithelial cells transduced with hTERT and HPV-16 E6/E7 |
|
(144) |
| TT1 | Primary AT2 cells transduced with hTERT and SV40 large T antigen |
|
(38, 39) |
Definitionof abbreviations: AT1 = alveolar type I; AT2 = alveolar type II; CDK4 = cyclin-dependent kinase 4; CFTR = cystic fibrosis transmembrane conductance regulator; HBEC = human bronchial epithelial cell; HPV = human papillomavirus; hTERT = human telomerase reverse transcriptase; iPSC = induced pluripotent stem cell; p63 = tumour protein p63; SPB = surfactant protein B; SPC = surfactant protein C; SV40 = simian virus 40.
Figure 1.
A timeline of lung epithelial cell culture methodology. Blue boxes indicate advances in 2D primary cell culture, orange boxes indicate strategies involving immortalized lung epithelial cells, and green boxes indicate advances in lung epithelial cell differentiation in cell culture. Created with BioRender.com. 2D = two-dimensional; 3D = three-dimensional.
First, immortalized cell lines have been developed by introducing immortalization factors into primary cells (Table 1) (25, 26). As an example, the partial transformation of primary HBECs with a hybrid adenovirus–simian virus 40 (SV40) virus created the BEAS-2B (27) cell line, which no longer underwent replicative senescence in long-term culture, retained epithelial morphology, but was unable to form tight junctions. On the other hand, 16HBE14o cells—which were immortalized with the SV40 large T antigen—could form tight junctions and also retained a cobblestone appearance in culture, expressed the cystic fibrosis transmembrane conductance regulator (CFTR), and could be differentiated to ciliated epithelium in ALI cultures (28, 29). Unfortunately, because the SV40 large T antigen inhibits the function of multiple tumor suppressor genes including TP53 and Rb, these cells can become tumorigenic with serial passaging (30). However, immortalization can also be achieved without the need for viral oncoproteins. Induction of CDK4 (cyclin-dependent kinase 4) and hTERT (human telomerase reverse transcriptase) expression enabled generation of the HBEC-KT cell lines (31). These cells retain a stable phenotype over serial passaging and are able to differentiate into both ciliated and goblet cells in ALI or in organoid culture (32, 33). Longer-term culture of basal cells with multipotent differentiation potential can also be achieved by overexpression of BMI-1—a polycomb complex protein that acts as an oncogene through regulation of cell cycle inhibitors—either alone, achieving around 20–25 passages before senescence (34, 35), or in combination with hTERT, achieving at least 30 passages (36). Robust protocols exist for obtaining primary AT2 cells from human lung tissue, but alveolar epithelial cell culture results in the transdifferentiation of these cells to an AT1-like phenotype within 2 weeks (37). Immortalization of alveolar epithelial cells led to an AT1-like cell line (38, 39), whereas immortalization of induced pluripotent stem cell (iPSC)-derived AT2 cells maintained an AT2 phenotype for up to 30 passages (40).
Second, coculture of primary HBECs with mitotically inactivated 3T3 fibroblasts allowed the expansion of basal cell cultures derived from single epithelial cells (41, 42), a method first demonstrated and optimized for epidermal keratinocytes (43, 44). The ability to form colonies of different morphologies in these conditions can be used as a surrogate for stem cell potential (42, 45). The introduction of a ROCK (Rho-associated protein kinase) inhibitor, Y-27632, to culture medium improved the plating efficiency and culture duration of HBECs, among other epithelial cell types (46, 47). Nasal, tracheal, bronchial, and small airway epithelial cells have been cultured using this methodology (48, 49). Basal cell differentiation capacity is preserved over a greater number of in vitro population doublings in bulk cultures (48, 49), whereas single cell–derived clonal lines can proliferate for variable timeframes (3–20 passages) while retaining multipotent differentiation potential (50). Culturing alveolar epithelial cells in these conditions enabled brief in vitro proliferation of AT2 cells, but passaging of cells resulted in AT1-like differentiation (51). Further modifications to the 3T3-J2 coculture approach in HBECs have combined bronchial epithelial growth medium, Y-27632, and lower oxygen conditions (52). While lung epithelia are exposed to a partial pressure of oxygen of ∼13–14%, stem cell niches can experience less than 6% in vivo (53). Reducing cell culture oxygen levels to 2% led to improved basal cell culture longevity with multipotent differentiation competency preserved for up to 10 passages in bulk cultures and short term in cultures derived from single cells (52).
Other improvements to 2D culture methodologies have included manipulation of the media composition. Analysis of signaling pathways present in the airway epithelium revealed that SMAD pathway activation was high in luminal epithelial cells but low in basal cells (54). Applying this knowledge to cell culture resulted in a dual SMAD inhibition protocol whereby basal cell culture lifetime was extended with cells retaining the ability to differentiate. Consistent with this, TGF-β pathway inhibition using A83–01 in combination with ROCK inhibition and isoproterenol supplementation supported long-term growth and multiple rounds of single cell cloning in the absence of feeder cells (55). Addition of the mTOR inhibitor rapamycin to TGF-β and ROCK inhibition also extended growth of basal cells from neonatal tracheal aspirates (56).
Third, important instructive signals—including the extracellular matrix and the presence of differentiated cells—are missing from 2D cell cultures. 3D culture of HBECs by encapsulation within extracellular matrix substrates results in “tracheospheres” or “bronchospheres,” organoids containing both basal and luminal differentiated cell types (3, 24). Investigation of clonality in bronchospheres by mixing labeled and unlabeled HBECs identified a clonal seeding threshold of ≤75 cells per well of a 384-well plate, above which density spheres also formed by aggregation (24). Conditions for the long-term culture of airway cells in 3D organoids have been developed whereby a polarized pseudostratified epithelium containing basal, secretory, and ciliated cells can be maintained for over a year of serial culture (57). 3D coculture of human AT2 cells with the human embryonic lung fibroblast cell line MRC5 (1, 58) or adult human lung fibroblasts (59) generates “alveolospheres” containing both AT1 and AT2 cells. Recently, long-term, feeder-free conditions have been described that enable propagation of alveolar epithelial organoids in the absence of fibroblasts (60, 61). Beyond Matrigel-based organoid cultures, lung-on-a-chip devices have also been developed that allow the integration of primary epithelial cells with other lung cell types, mechanical force, and flow (62).
Isolating Single Epithelial Cells for Clonal Expansion
In improved culture conditions, new insights can be gained by isolating single stem cells in culture to ensure that all cells in a culture are derived from a recent common ancestor cell (Figure 2). Single-cell culture is more challenging as stem cells rely on paracrine signals from other cells in bulk cultures that are no longer available in single-cell or sparse culture conditions. To achieve clonal expansion, cells need to be isolated and cultured in a way that prevents anoikis and promotes survival, proliferation and stem cell maintenance, which might include the use of inactivated feeder cells or conditioned medium from bulk epithelial or stromal cultures.
Figure 2.
Methods to expand clonal epithelial cell cultures from lung tissue. Created with BioRender.com. FACS = fluorescence-activated cell sorting.
Most simply, cells can be serially diluted to low density with either one (63) or several (50) cells per well and taking wells in which a single colony forms forward for culture. Alternatively, a mixed population can be cultured at low density and individual colonies picked for further expansion assuming that a single cell initiates colony growth (45, 64, 65). Magnetic bead purification can also be used to enrich for the cell type of interest from primary tissue, for example, positive selection using EPCAM or negative selection for immune (CD45+) and endothelial (CD31+) cells. Similarly, fluorescence-activated cell sorting allows marker-specific cells to be deposited into individual wells. The commonly used basal cell markers KRT5 (keratin 5) and p63 (tumor protein p63) are intracellular and can therefore not be used for live cell sorting, though surface proteins such as ITGA6 (integrin α 6), NGFR (nerve growth factor receptor), and PDPN (podoplanin) are expressed by a majority of basal cells (3, 66). CD66 was recently revealed as a surface marker for basal cells primed for secretory differentiation (9). Anti-HTII-280 antibodies target a surface protein on human alveolar type II cells with high specificity (67) and an antibody targeting TM4SF1 (Transmembrane 4 L Six Family Member 1) has recently been reported to identify a progenitor subset of human AT2 cells that respond to Wnt signals (58). Identification of novel surface marker proteins for lung epithelial stem/progenitor cells would assist in bead or flow cytometric sorting strategies, although this is complicated by donor- and disease-variable expression patterns. Microfluidic technologies can also be used for single cell trapping (68); these may be gentler than flow cytometry protocols, but cell types cannot be isolated based on their surface markers.
Translational Applications of Improved Primary Cell Culture Methods
Defining “Normal” Lung Epithelium
Molecular abnormalities accumulate during lung cancer progression from histologically normal epithelium through preinvasive disease toward invasive cancers (69–71). Tobacco smoking results in distinct mutational signatures in the genomes of both tumours and the histologically normal epithelium surrounding them (72). To reveal information about the genome of the founder cell, a recent study applied whole-genome sequencing to clonal epithelial cell cultures. Colonies were initiated by flow sorting single epithelial cells from the histologically normal airways of smokers, ex-smokers, and never-smokers and cultured for two passages ex vivo. This experiment revealed the predicted higher mutational burden in smokers and ex-smokers but also found that the genomic impact of smoking on the epithelium is heterogeneous (63). In smokers or ex-smokers, some basal cells have a mutational burden comparable to those in never-smokers. Interestingly, the proportion of basal cells with low mutational burden increased in ex-smokers compared with current smokers (63) suggesting that their expansion is actively repressed during smoking or promoted following cessation. Because this clonal culture strategy expanded colonies of basal cells from single cells, it is theoretically possible that other genomic differences, such as copy number alterations, might be present in differentiated cells that could not form colonies in vitro. The accumulation of somatic mutations in physiologically normal tissues during aging has been demonstrated in multiple other organs (73–78), including using cell culture approaches to give single cell resolution within hematopoietic, gastrointestinal, and liver tissues (74, 79, 80).
These data suggest that the lung epithelium is highly dynamic over the life course and in response to injury. Genomic differences might provide a partial explanation for the interindividual variability observed within primary lung cell cultures and suggests that the clonal composition of widely used normal lung cell lines should be more deeply characterized. It will be interesting to explore how somatic evolution varies in smoking-associated chronic lung diseases such as COPD and pulmonary fibrosis, where the epithelium is known to play a key role in pathogenesis. With regard to early lung cancer, questions remain about how oncogenic mutations influence airway epithelial cell behavior, how mutant cells interact with surrounding nonmutant cells, the potential for immune pruning of mutant cells, and the impact of cancer therapies on normal lung epithelial dynamics. Clonal cell culture of normal lung epithelial cells now provides a tractable system in which to investigate these open questions, for example, by looking at direct cell competition between isogenic wild-type and mutant clones.
Modeling Disease Initiation and Pathogenesis
Genetic lung diseases can be caused by mutations in single genes: diverse mutations in CFTR disrupt ion transport and cause cystic fibrosis (CF), those in SERPINA1 cause alpha-1 antitrypsin deficiency, and mutations within a range of genes that affect the structure or function of motile cilia cause primary ciliary dyskinesia (PCD). Steps toward personalized medicine have been achieved in CF by screening primary biopsy-derived intestinal organoids. The fact that forskolin treatment increases intracellular cyclic AMP, activates CFTR, and results in luminal fluid secretion has been exploited in an organoid swelling assay (81, 82). This assay allows the assessment of an individual’s response to currently available treatments and will thus be particularly relevant for patients with rare and poorly characterized mutations. The adaptation of the forskolin-induced swelling assay to airway organoids will likely reduce the requirement for gastrointestinal tract biopsies in these patients (57). Primary cell culture from nasal brush biopsies has allowed individualized cell cultures from patients with PCD as differentiated ALI cultures retain the ciliary defects seen in patients (83, 84). Improved culture methods have allowed miniaturization of ALI cultures to at least 96-well plate format (85, 86), and even higher throughput might be achievable through optimization of plate design. Mechanistic studies of alpha-1 antitrypsin deficiency pathogenesis in the lung might also benefit from new alveolar epithelial cell culture methods because SERPINA1 is prominently expressed by AT2 cells (87).
Beyond monogenic diseases, large genome-wide association studies have revealed germline variants at many loci involved in the susceptibility to and pathogenesis of chronic lung diseases, whereas acquired somatic mutations have been associated with chronic diseases in other organs (88, 89). In both cases, primary cell culture methods offer a valuable opportunity to test the functional impact of variants in epithelial cells, for example, by using CRISPR-based genome editing approaches. Knockout of MUC18 (90), which is upregulated during lung inflammation, or of transcription factors involved in epithelial fate decisions (5) has been achieved in primary cells, demonstrating proof of principle for this approach. Although the strategies used so far have resulted in heterogeneous bulk populations of edited cells, recent advances in clonal expansion will allow the selection of a single genotype and facilitate characterization of possible off-target effects. Alternatively, gene editing can be performed once airway cells have differentiated in ALI cultures (91).
Epithelial cell culture can also be used to study disease initiation in several contexts, providing a window into a disease stage that is impossible to study in patients. For example, ALI and organoid cultures have provided a system to study epithelial cell infection with respiratory viruses such as influenza (92, 93), respiratory syncytial virus (57, 94) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (60, 95–97). Clonal primary human lung epithelial cell expansion also offers a unique opportunity to study early carcinogenesis as multiple cancer-associated genetic events can be experimentally introduced into lung epithelium in a stepwise fashion. Clonal derivation of lung cancer organoids is also feasible using similar methods to primary airway organoids (57, 98, 99). Such cultures offer opportunities to study intratumor heterogeneity, monitor ongoing mutational processes in vitro, and study tumor–immune cell interactions in cocultures.
The abnormal differentiation phenotypes, such as goblet cell hyperplasia, that are associated with asthma and COPD can be studied either by exposing cells from healthy donors to relevant growth factors, cytokines, or small molecules or by studying primary cells derived from patients with those diseases. A human recombinant protein screen using bronchospheres identified that EGF promoted basal cell proliferation while IL-13 and IL-17a caused goblet cell metaplasia in a Notch2-dependent manner (24). Although individual organoids in these assays are likely to have been clonally derived, analyses were performed at the well level and thereby represent mixed lineage cultures. Future experiments might expand this approach using clonally derived cell cultures of known genotypes. Alternatively, cell culture methods can also be applied to epithelium from patients with respiratory diseases. Evidence from bulk cultures suggests that some phenotypes associated with asthma, COPD, and pulmonary fibrosis are maintained in epithelial cell cultures. For example, differences in epithelial barrier formation (100), delayed wound repair (101), and reduced innate defense against bacterial infection (102) persist in COPD cultures. Recently, RNA sequencing of clonal airway epithelial cell cultures expanded from normal and COPD lung tissue revealed four clusters of basal cell variants. These four variant clone types had distinct differentiation phenotypes, resulting in normal epithelium, goblet cell metaplasia, squamous cell metaplasia, and inflammatory squamous cell metaplasia, respectively (65). The metaplastic basal cell phenotypes were present at low levels in cultures from normal adult and fetal lungs but abnormally expanded in those from COPD airways and the phenotypes persisted after as many as 25 passages (65), suggesting at least partial epigenetic stability in culture. It will be interesting to unravel the extent to which the predominance of these four clone subtypes determines the interindividual variability in bulk patient cultures, how variable transcriptomically defined clone types are in other respiratory diseases, and whether specific clone types respond differently to cigarette smoke or infection.
Cell Therapy
Cell therapies aiming to directly replace epithelial cells have been proposed for lung diseases including bronchopulmonary dysplasia, bronchiolitis obliterans, COPD, pulmonary fibrosis, and CF (103). There is precedent for cell therapy using cultured epithelial cells in the context of epidermal and limbal burns injuries (44, 104). In the airways, epithelium from split-skin grafts has been used to provide “biological inhibition” in the context of laryngotracheal stenosis (105, 106), where chronically damaged epithelium might contribute to fibrotic reactions and recurrence of stenoses. Clinical applications of cultured autologous cells have been limited. In tissue-engineered grafts used in airway transplantation cases (107, 108), the epithelial components are not thought to have engrafted, with any epithelialization instead attributed to in-growth from surrounding host cells (109); this view is supported by difficulties in engrafting airway epithelium in preclinical models (110). While two patients with bronchiectasis also received cultured basal cells via bronchoscopy in a pilot clinical study (111), the fate of these cells is unknown and additional preclinical work is required before further human trials.
Nevertheless, existing preclinical data are promising. Human bronchial basal cells (112) and developmental precursors derived from iPSCs (113) transplanted into the injured airways of immunocompromised mice can engraft and differentiate into both secretory and ciliated cell types, restoring the airway epithelium at least in the short term.
In the future, epithelial cell therapies might be used to treat genetic lung diseases through the replacement of impaired cells with functional gene-corrected cells. Primary epithelial cells from CF (50) and PCD (85) expand and differentiate similarly in culture to those from healthy control donors, making this a plausible approach, and iPSC-based alternatives are also under development (114). Advances have been made toward functional gene correction in both CF and PCD. Using CRISPR-Cas9 approaches, CFTR function has been restored in intestinal organoids (115, 116) and in CF iPSCs that could be guided toward a lung epithelial lineage (117). Promisingly, the most common CF CFTR mutation—ΔF508—has been corrected using CRISPR-Cas9 delivery in airway basal cells that retained the ability to differentiate into pseudostratified epithelium in ALI culture (118). The protocol did not use cell selection or cloning to preferentially expand the gene-corrected cells, resulting in a heterogeneous cell population in terms of CFTR function. In PCD, TALEN (transcription activator–like effector nuclease)-mediated gene editing allowed correction of DNAH11 mutations with the wild-type sequence in bulk cell cultures, restoring ciliary function (119).
However, a number of hurdles remain. First, estimates for the number of cells required for therapeutic airway epithelial cell engraftment converge in the range of 50–100 million cells (49, 50). Given the small number of basal cells that are recovered from BAL (<2,000 [54]), induced sputum (<2,000 [54]), bronchial brush biopsy (10,000–200,000 [121]), or bronchial forcep biopsy (5,000–200,000 [121]) samples, and the difficulty to manipulate freshly isolated cells, it is likely that such a therapy will require cell expansion in culture. It remains to be seen whether clonal, gene-corrected cells from patients with CF can be expanded to this extent while retaining the capacity to regenerate the airway epithelium long term. Second, available gene-editing technologies can cause unintended off-target effects. The use of clonal cell cultures might reduce the heterogeneity of on-target phenotype modification and simultaneously limit the extent of off-target safety concerns associated with these approaches (120). A further consideration is that preconditioning of the patient is likely to be required (122) but the methods used to generate injury experimentally in mice are unlikely to be acceptable clinically.
Future Directions
Historically, airway epithelial cells have been more readily cultured than their alveolar counterparts. Nevertheless, recent advances make clonal cell culture of alveolar epithelium possible (97), albeit in organoids that lack many of the morphological aspects of alveoli. This breakthrough makes a number of experiments—such as determining the genomic landscape of individual AT2 cells in normal distal lung epithelium during aging and following tobacco exposure, recapitulating human AT2 to AT1 differentiation in vitro, and determining the engraftment potential of cultured human alveolar cells in mouse injury models—achievable in the near term. A future application of alveolar organoids might be in cellular therapy, but substantial challenges exist in addition to those outlined above for basal cells; the alveolus has a complex 3D structure including precise cellular architecture that will prove difficult to recapitulate using exogenous cells. However, cell-matrix scaffolds in combination with more extensive partial tissue replacement should be prioritized for investigation.
Several aspects of lung epithelial biology discovered in the mouse remain uncertain in human lungs. Basal cells are increasingly sparse in the distal-most human airways as the epithelium becomes simple columnar rather than pseudostratified. Mouse studies suggest the involvement of club cells as stem cells in simple columnar airway epithelium, but human cell culture studies suggest that human club cells arise from basal cells (123). Epithelial cell culture can shed light on the cell biology of this region in humans because multiple lines of evidence suggest that region-specific phenotypic differences persist in vitro: transcriptomic differences are found between cultured basal cells from nasal, tracheal, or small airway epithelium (41); immortalized cell lines can retain small airway–like differentiation capacity (124); and ALI cultures from nasal, large, and small airways retain differential sensitivity to viral infection (96). Cell culture might also help to ascertain whether airway epithelial cells can acquire greater plasticity following severe injury than is seen during normal cell turnover, as is seen when club cells contribute to the basal stem cell pool in mice (125, 126). Similarly, future studies might address whether human AT2 and club cells pass through a transition state characterized by KRT8 expression and TP53 pathway activation before reaching full AT1 differentiation, as has been observed in mice and has been suggested by analyses of human disease states (127–129).
Gene editing technology advances applied to model organisms allow in vivo clonal analyses under experimental control. In one example that might be applicable to cultured lung epithelial cells—Lineage and RNA recovery (LARRY)—cells are transduced with a lentivirus containing an eGFP construct with a DNA barcode in the 3′ untranslated region. After brief culture to allow cell division, a proportion of these cells are analyzed by scRNAseq with the remaining cells either replated in vitro or transplanted to assess in vivo differentiation. Analysis of scRNAseq data from before or after plating/transplantation allows mapping of progenitor transcriptomes to their cell fates through barcode matching, potentially determining cell states associated with self-renewal, differentiation, and/or engraftment. Entirely in vivo systems might also be deployed. For example, the CARLIN (CRISPR array repair lineage tracing) mouse model barcodes cells to track their progeny (130). A doxycycline-inducible Cas9, guide RNAs, and an array of their target sites sit within the Col1a1 locus and allow temporally controlled and/or sequential editing of the array to generate diverse barcodes trackable using scRNAseq. Although initially used to reconstruct lineages and investigate functional heterogeneity in the hematopoietic system (130), CARLIN-edited gene transcripts are observed in lung tissue. Improvements to this mouse line that allow greater barcode diversity would provide an opportunity to trace cell lineages through recovery after lung injury or infection in adult mice.
Conclusions
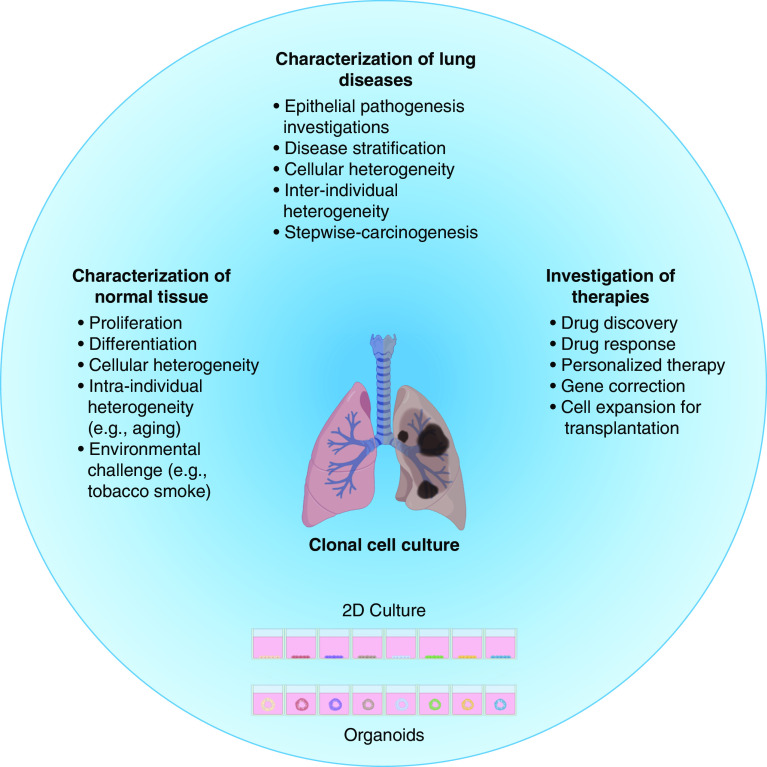
Primary human lung epithelial cell cultures represent powerful tools for understanding lung health and disease. Improvements to cell culture methodologies allow prolonged culture of both airway and alveolar epithelial cells, including as clonal subcultures. In combination with other technological advances—particularly in gene editing and next-generation sequencing—these will help to further dissect the molecular heterogeneity of the lung epithelium in patients and preclinical models (Figure 3). Future cell therapies might also benefit through the capacity to expand cells of a single known genotype to therapeutic quantities.
Figure 3.
Translational applications of clonal lung epithelial cell culture. Created with BioRender.com.
Acknowledgments
Acknowledgment
The authors apologize to authors whose work it was not possible to cite here owing to space constraints. They also thank Dr. Eva Grönroos (The Francis Crick Institute, London, UK) for proofreading a draft manuscript and colleagues at the Great Ormond Street Institute of Child Health (University College London, UK) and UCL Respiratory (University College London, UK) for feedback on the revised manuscript.
Footnotes
J.C.O. is funded by a Ph.D. studentship from the Longfonds BREATH lung regeneration consortium. R.E.H. is a Wellcome Trust Sir Henry Wellcome Fellow (WT209199/Z/17/Z) and is supported by the Roy Castle Lung Cancer Foundation, the James Tudor Foundation, and the Cancer Research UK Lung Cancer Centre of Excellence. R.E.H. is a member of the UK Regenerative Medicine Platform (UKRMP2) Engineered Cell Environment Hub (MRC; MR/R015635/1).
Author Contributions: J.C.O. wrote and edited a draft manuscript and prepared figures. R.E.H. edited a draft manuscript and approved the final manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0440TR on January 11, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basil MC, Katzen J, Engler AE, Guo M, Herriges MJ, Kathiriya JJ, et al. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell. 2020;26:482–502. doi: 10.1016/j.stem.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Plasschaert LW, Žilionis R, Choo-Wing R, Savova V, Knehr J, Roma G, et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560:377–381. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldfarbmuren KC, Jackson ND, Sajuthi SP, Dyjack N, Li KS, Rios CL, et al. Dissecting the cellular specificity of smoking effects and reconstructing lineages in the human airway epithelium. Nat Commun. 2020;11:2485. doi: 10.1038/s41467-020-16239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560:319–324. doi: 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deprez M, Zaragosi L-E, Truchi M, Becavin C, Ruiz García S, Arguel M-J, et al. A single-cell atlas of the human healthy airways. Am J Respir Crit Care Med. 2020;202:1636–1645. doi: 10.1164/rccm.201911-2199OC. [DOI] [PubMed] [Google Scholar]
- 8. Ruiz García S, Deprez M, Lebrigand K, Cavard A, Paquet A, Arguel MJ, et al. Novel dynamics of human mucociliary differentiation revealed by single-cell RNA sequencing of nasal epithelial cultures. Development. 2019;146:dev177428. doi: 10.1242/dev.177428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carraro G, Mulay A, Yao C, Mizuno T, Konda B, Petrov M, et al. Single-cell reconstruction of human basal cell diversity in normal and idiopathic pulmonary fibrosis lungs. Am J Respir Crit Care Med. 2020;202:1540–1550. doi: 10.1164/rccm.201904-0792OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fischer AJ, Goss KL, Scheetz TE, Wohlford-Lenane CL, Snyder JM, McCray PB., Jr Differential gene expression in human conducting airway surface epithelia and submucosal glands. Am J Respir Cell Mol Biol. 2009;40:189–199. doi: 10.1165/rcmb.2008-0240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duclos GE, Teixeira VH, Autissier P, Gesthalter YB, Reinders-Luinge MA, Terrano R, et al. Characterizing smoking-induced transcriptional heterogeneity in the human bronchial epithelium at single-cell resolution. Sci Adv. 2019;5:eaaw3413. doi: 10.1126/sciadv.aaw3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vieira Braga FA, Kar G, Berg M, Carpaij OA, Polanski K, Simon LM, et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat Med. 2019;25:1153–1163. doi: 10.1038/s41591-019-0468-5. [DOI] [PubMed] [Google Scholar]
- 13. Barnes PJ, Baker J, Donnelly LE. Cellular senescence as a mechanism and target in chronic lung diseases. Am J Respir Crit Care Med. 2019;200:556–564. doi: 10.1164/rccm.201810-1975TR. [DOI] [PubMed] [Google Scholar]
- 14. Sauler M, McDonough JE, Adams TS, Kothapalli N, Schupp JS, Nouws J, et al. Single-cell RNA sequencing identifies aberrant transcriptional profiles of cellular populations and altered alveolar niche signalling networks in chronic obstructive pulmonary disease (COPD) [preprint] medRxiv. 2020 [accessed 2020 Sep 14]. Available from: https://www.medrxiv.org/content/10.1101/2020.09.13.20193417v1.
- 15. Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, et al. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight. 2016;1:e90558. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adams TS, Schupp JC, Poli S, Ayaub EA, Neumark N, Ahangari F, et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv. 2020;6:eaba1983. doi: 10.1126/sciadv.aba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Habermann AC, Gutierrez AJ, Bui LT, Yahn SL, Winters NI, Calvi CL, et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci Adv. 2020;6:eaba1972. doi: 10.1126/sciadv.aba1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lechner JF, Haugen A, McClendon IA, Pettis EW. Clonal growth of normal adult human bronchial epithelial cells in a serum-free medium. In Vitro. 1982;18:633–642. doi: 10.1007/BF02796396. [DOI] [PubMed] [Google Scholar]
- 19. Yoon J-H, Koo JS, Norford D, Guzman K, Gray T, Nettesheim P. Lysozyme expression during metaplastic squamous differentiation of retinoic acid-deficient human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1999;20:573–581. doi: 10.1165/ajrcmb.20.4.3127. [DOI] [PubMed] [Google Scholar]
- 20. Lechner JF, LaVeck MA. A serum-free method for culturing normal human bronchial epithelial cells at clonal density. J Tissue Cult Methods. 1985;9:43–48. [Google Scholar]
- 21. Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 22. Fulcher ML, Randell SH. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol. 2013;945:109–121. doi: 10.1007/978-1-62703-125-7_8. [DOI] [PubMed] [Google Scholar]
- 23. Jorissen M, Van der Schueren B, Tyberghein J, Van der Berghe H, Cassiman JJ. Ciliogenesis and coordinated ciliary beating in human nasal epithelial cells cultured in vitro. Acta Otorhinolaryngol Belg. 1989;43:67–73. [PubMed] [Google Scholar]
- 24. Danahay H, Pessotti AD, Coote J, Montgomery BE, Xia D, Wilson A, et al. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep. 2015;10:239–252. doi: 10.1016/j.celrep.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 25. Sato M, Shay JW, Minna JD. Immortalized normal human lung epithelial cell models for studying lung cancer biology. Respir Investig. 2020;58:344–354. doi: 10.1016/j.resinv.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 26. Gruenert DC, Finkbeiner WE, Widdicombe JH. Culture and transformation of human airway epithelial cells. Am J Physiol. 1995;268:L347–L360. doi: 10.1152/ajplung.1995.268.3.L347. [DOI] [PubMed] [Google Scholar]
- 27. Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, et al. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988;48:1904–1909. [PubMed] [Google Scholar]
- 28. Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, et al. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 29. Bérubé K, Prytherch Z, Job C, Hughes T. Human primary bronchial lung cell constructs: the new respiratory models. Toxicology. 2010;278:311–318. doi: 10.1016/j.tox.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 30. Reddel RR, Salghetti SE, Willey JC, Ohnuki Y, Ke Y, Gerwin BI, et al. Development of tumorigenicity in simian virus 40-immortalized human bronchial epithelial cell lines. Cancer Res. 1993;53:985–991. [PubMed] [Google Scholar]
- 31. Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 32. Delgado O, Kaisani AA, Spinola M, Xie X-J, Batten KG, Minna JD, et al. Multipotent capacity of immortalized human bronchial epithelial cells. PLoS One. 2011;6:e22023. doi: 10.1371/journal.pone.0022023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vaughan MB, Ramirez RD, Wright WE, Minna JD, Shay JW. A three-dimensional model of differentiation of immortalized human bronchial epithelial cells. Differentiation. 2006;74:141–148. doi: 10.1111/j.1432-0436.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 34. Torr E, Heath M, Mee M, Shaw D, Sharp TV, Sayers I. Expression of polycomb protein BMI-1 maintains the plasticity of basal bronchial epithelial cells. Physiol Rep. 2016;4:e12847. doi: 10.14814/phy2.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Munye MM, Shoemark A, Hirst RA, Delhove JM, Sharp TV, McKay TR, et al. BMI-1 extends proliferative potential of human bronchial epithelial cells while retaining their mucociliary differentiation capacity. Am J Physiol Lung Cell Mol Physiol. 2017;312:L258–L267. doi: 10.1152/ajplung.00471.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fulcher ML, Gabriel SE, Olsen JC, Tatreau JR, Gentzsch M, Livanos E, et al. Novel human bronchial epithelial cell lines for cystic fibrosis research. Am J Physiol Lung Cell Mol Physiol. 2009;296:L82–L91. doi: 10.1152/ajplung.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bove PF, Grubb BR, Okada SF, Ribeiro CM, Rogers TD, Randell SH, et al. Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling. J Biol Chem. 2010;285:34939–34949. doi: 10.1074/jbc.M110.162933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van den Bogaard EH, Dailey LA, Thorley AJ, Tetley TD, Forbes B. Inflammatory response and barrier properties of a new alveolar type 1-like cell line (TT1) Pharm Res. 2009;26:1172–1180. doi: 10.1007/s11095-009-9838-x. [DOI] [PubMed] [Google Scholar]
- 39. Kemp SJ, Thorley AJ, Gorelik J, Seckl MJ, O’Hare MJ, Arcaro A, et al. Immortalization of human alveolar epithelial cells to investigate nanoparticle uptake. Am J Respir Cell Mol Biol. 2008;39:591–597. doi: 10.1165/rcmb.2007-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tamò L, Hibaoui Y, Kallol S, Alves MP, Albrecht C, Hostettler KE, et al. Generation of an alveolar epithelial type II cell line from induced pluripotent stem cells. Am J Physiol Lung Cell Mol Physiol. 2018;315:L921–L932. doi: 10.1152/ajplung.00357.2017. [DOI] [PubMed] [Google Scholar]
- 41. Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghosh M, Ahmad S, Jian A, Li B, Smith RW, Helm KM, et al. Human tracheobronchial basal cells: normal versus remodeling/repairing phenotypes in vivo and in vitro. Am J Respir Cell Mol Biol. 2013;49:1127–1134. doi: 10.1165/rcmb.2013-0049OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 44. Hynds RE, Bonfanti P, Janes SM. Regenerating human epithelia with cultured stem cells: feeder cells, organoids and beyond. EMBO Mol Med. 2018;10:139–150. doi: 10.15252/emmm.201708213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suprynowicz FA, Upadhyay G, Krawczyk E, Kramer SC, Hebert JD, Liu X, et al. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc Natl Acad Sci USA. 2012;109:20035–20040. doi: 10.1073/pnas.1213241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reynolds SD, Rios C, Wesolowska-Andersen A, Zhuang Y, Pinter M, Happoldt C, et al. Airway progenitor clone formation is enhanced by y-27632-dependent changes in the transcriptome. Am J Respir Cell Mol Biol. 2016;55:323–336. doi: 10.1165/rcmb.2015-0274MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Butler CR, Hynds RE, Gowers KHC, Lee DdoH, Brown JM, Crowley C, et al. Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am J Respir Crit Care Med. 2016;194:156–168. doi: 10.1164/rccm.201507-1414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hayes D, Jr, Kopp BT, Hill CL, Lallier SW, Schwartz CM, Tadesse M, et al. Cell therapy for cystic fibrosis lung disease: regenerative basal cell amplification. Stem Cells Transl Med. 2019;8:225–235. doi: 10.1002/sctm.18-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bove PF, Dang H, Cheluvaraju C, Jones LC, Liu X, O’Neal WK, et al. Breaking the in vitro alveolar type II cell proliferation barrier while retaining ion transport properties. Am J Respir Cell Mol Biol. 2014;50:767–776. doi: 10.1165/rcmb.2013-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peters-Hall JR, Coquelin ML, Torres MJ, LaRanger R, Alabi BR, Sho S, et al. Long-term culture and cloning of primary human bronchial basal cells that maintain multipotent differentiation capacity and CFTR channel function. Am J Physiol Lung Cell Mol Physiol. 2018;315:L313–L327. doi: 10.1152/ajplung.00355.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mas-Bargues C, Sanz-Ros J, Román-Domínguez A, Inglés M, Gimeno-Mallench L, El Alami M, et al. Relevance of oxygen concentration in stem cell culture for regenerative medicine. Int J Mol Sci. 2019;20:1195. doi: 10.3390/ijms20051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mou H, Vinarsky V, Tata PR, Brazauskas K, Choi SH, Crooke AK, et al. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell. 2016;19:217–231. doi: 10.1016/j.stem.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang C, Lee HJ, Shrivastava A, Wang R, McQuiston TJ, Challberg SS, et al. Long-term in vitro expansion of epithelial stem cells enabled by pharmacological inhibition of PAK1-ROCK-myosin II and TGF-β signaling. Cell Rep. 2018;25:598–610.e5. doi: 10.1016/j.celrep.2018.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lu J, Zhu X, Shui JE, Xiong L, Gierahn T, Zhang C, et al. Rho/SMAD/mTOR triple inhibition enables long-term expansion of human neonatal tracheal aspirate-derived airway basal cell-like cells. Pediatr Res. doi: 10.1038/s41390-020-0925-3. [online ahead of print] 4 May 2020; DOI: 10.1038/s41390-020-0925-3. [DOI] [PubMed] [Google Scholar]
- 57. Sachs N, Papaspyropoulos A, Zomer-van Ommen DD, Heo I, Böttinger L, Klay D, et al. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019;38:e100300. doi: 10.15252/embj.2018100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ng-Blichfeldt JP, de Jong T, Kortekaas RK, Wu X, Lindner M, Guryev V, et al. TGF-β activation impairs fibroblast ability to support adult lung epithelial progenitor cell organoid formation. Am J Physiol Lung Cell Mol Physiol. 2019;317:L14–L28. doi: 10.1152/ajplung.00400.2018. [DOI] [PubMed] [Google Scholar]
- 60. Youk J, Kim T, Evans KV, Jeong YI, Hur Y, Hong SP, et al. Three-dimensional human alveolar stem cell culture models reveal infection response to sars-cov-2. Cell Stem Cell. 2020;27:905–919.e10. doi: 10.1016/j.stem.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Katsura H, Sontake V, Tata A, Kobayashi Y, Edwards CE, Heaton BE, et al. Human lung stem cell-based alveolospheres provide insights into sars-cov-2-mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell. 2020;27:890–904.e8. doi: 10.1016/j.stem.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Benam KH, Villenave R, Lucchesi C, Varone A, Hubeau C, Lee HH, et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods. 2016;13:151–157. doi: 10.1038/nmeth.3697. [DOI] [PubMed] [Google Scholar]
- 63. Yoshida K, Gowers KHC, Lee-Six H, Chandrasekharan DP, Coorens T, Maughan EF, et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature. 2020;578:266–272. doi: 10.1038/s41586-020-1961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang X, Yamamoto Y, Wilson LH, Zhang T, Howitt BE, Farrow MA, et al. Cloning and variation of ground state intestinal stem cells. Nature. 2015;522:173–178. doi: 10.1038/nature14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rao W, Wang S, Duleba M, Niroula S, Goller K, Xie J, et al. Regenerative metaplastic clones in copd lung drive inflammation and fibrosis. Cell. 2020;181:848–864.e18. doi: 10.1016/j.cell.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weeden CE, Chen Y, Ma SB, Hu Y, Ramm G, Sutherland KD, et al. Lung basal stem cells rapidly repair DNA damage using the error-prone nonhomologous end-joining pathway. PLoS Biol. 2017;15:e2000731. doi: 10.1371/journal.pbio.2000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gonzalez RF, Allen L, Gonzales L, Ballard PL, Dobbs LG. HTII-280, a biomarker specific to the apical plasma membrane of human lung alveolar type II cells. J Histochem Cytochem. 2010;58:891–901. doi: 10.1369/jhc.2010.956433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen H, Sun J, Wolvetang E, Cooper-White J. High-throughput, deterministic single cell trapping and long-term clonal cell culture in microfluidic devices. Lab Chip. 2015;15:1072–1083. doi: 10.1039/c4lc01176g. [DOI] [PubMed] [Google Scholar]
- 69. Ooi AT, Gower AC, Zhang KX, Vick JL, Hong L, Nagao B, et al. Molecular profiling of premalignant lesions in lung squamous cell carcinomas identifies mechanisms involved in stepwise carcinogenesis. Cancer Prev Res (Phila) 2014;7:487–495. doi: 10.1158/1940-6207.CAPR-13-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen H, Carrot-Zhang J, Zhao Y, Hu H, Freeman SS, Yu S, et al. Genomic and immune profiling of pre-invasive lung adenocarcinoma. Nat Commun. 2019;10:5472. doi: 10.1038/s41467-019-13460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Teixeira VH, Pipinikas CP, Pennycuick A, Lee-Six H, Chandrasekharan D, Beane J, et al. Deciphering the genomic, epigenomic, and transcriptomic landscapes of pre-invasive lung cancer lesions. Nat Med. 2019;25:517–525. doi: 10.1038/s41591-018-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tang X, Shigematsu H, Bekele BN, Roth JA, Minna JD, Hong WK, et al. EGFR tyrosine kinase domain mutations are detected in histologically normal respiratory epithelium in lung cancer patients. Cancer Res. 2005;65:7568–7572. doi: 10.1158/0008-5472.CAN-05-1705. [DOI] [PubMed] [Google Scholar]
- 73. Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, et al. Tumor evolution: high burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yokoyama A, Kakiuchi N, Yoshizato T, Nannya Y, Suzuki H, Takeuchi Y, et al. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature. 2019;565:312–317. doi: 10.1038/s41586-018-0811-x. [DOI] [PubMed] [Google Scholar]
- 75. Martincorena I, Fowler JC, Wabik A, Lawson ARJ, Abascal F, Hall MWJ, et al. Somatic mutant clones colonize the human esophagus with age. Science. 2018;362:911–917. doi: 10.1126/science.aau3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Suda K, Nakaoka H, Yoshihara K, Ishiguro T, Tamura R, Mori Y, et al. Clonal expansion and diversification of cancer-associated mutations in endometriosis and normal endometrium. Cell Rep. 2018;24:1777–1789. doi: 10.1016/j.celrep.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 77. Lee-Six H, Olafsson S, Ellis P, Osborne RJ, Sanders MA, Moore L, et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature. 2019;574:532–537. doi: 10.1038/s41586-019-1672-7. [DOI] [PubMed] [Google Scholar]
- 78. Brunner SF, Roberts ND, Wylie LA, Moore L, Aitken SJ, Davies SE, et al. Somatic mutations and clonal dynamics in healthy and cirrhotic human liver. Nature. 2019;574:538–542. doi: 10.1038/s41586-019-1670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Blokzijl F, de Ligt J, Jager M, Sasselli V, Roerink S, Sasaki N, et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature. 2016;538:260–264. doi: 10.1038/nature19768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lee-Six H, Øbro NF, Shepherd MS, Grossmann S, Dawson K, Belmonte M, et al. Population dynamics of normal human blood inferred from somatic mutations. Nature. 2018;561:473–478. doi: 10.1038/s41586-018-0497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19:939–945. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 82. de Winter-de Groot KM, Janssens HM, van Uum RT, Dekkers JF, Berkers G, Vonk A, et al. Stratifying infants with cystic fibrosis for disease severity using intestinal organoid swelling as a biomarker of CFTR function. Eur Respir J. 2018;52:1702529. doi: 10.1183/13993003.02529-2017. [DOI] [PubMed] [Google Scholar]
- 83. Jorissen M, Willems T, Van der Schueren B. Ciliary function analysis for the diagnosis of primary ciliary dyskinesia: advantages of ciliogenesis in culture. Acta Otolaryngol. 2000;120:291–295. doi: 10.1080/000164800750001116. [DOI] [PubMed] [Google Scholar]
- 84. Hirst RA, Rutman A, Williams G, O’Callaghan C. Ciliated air-liquid cultures as an aid to diagnostic testing of primary ciliary dyskinesia. Chest. 2010;138:1441–1447. doi: 10.1378/chest.10-0175. [DOI] [PubMed] [Google Scholar]
- 85. Lee DDH, Cardinale D, Nigro E, Butler CR, Rutman A, Fassad MR, et al. High-content screening for rare respiratory diseases: readthrough therapy in primary ciliary dyskinesia [preprint] bioRxiv. 2020 doi: 10.1183/13993003.00455-2020. [accessed 2020 Feb 28]. Available from: https://www.biorxiv.org/content/10.1101/2020.02.28.959189v1. [DOI] [PMC free article] [PubMed]
- 86. Bluhmki T, Bitzer S, Gindele JA, Schruf E, Kiechle T, Webster M, et al. Development of a miniaturized 96-Transwell air-liquid interface human small airway epithelial model. Sci Rep. 2020;10:13022. doi: 10.1038/s41598-020-69948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Venembre P, Boutten A, Seta N, Dehoux MS, Crestani B, Aubier M, et al. Secretion of alpha 1-antitrypsin by alveolar epithelial cells. FEBS Lett. 1994;346:171–174. doi: 10.1016/0014-5793(94)80695-0. [DOI] [PubMed] [Google Scholar]
- 88. Zhu M, Lu T, Jia Y, Luo X, Gopal P, Li L, et al. Somatic mutations increase hepatic clonal fitness and regeneration in chronic liver disease. Cell. 2019;177:608–621.e12. doi: 10.1016/j.cell.2019.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Olafsson S, McIntyre RE, Coorens T, Butler T, Jung H, Robinson PS, et al. Somatic evolution in non-neoplastic IBD-affected colon. Cell. 2020;182:672–684.e11. doi: 10.1016/j.cell.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chu HW, Rios C, Huang C, Wesolowska-Andersen A, Burchard EG, O’Connor BP, et al. CRISPR-Cas9-mediated gene knockout in primary human airway epithelial cells reveals a proinflammatory role for MUC18. Gene Ther. 2015;22:822–829. doi: 10.1038/gt.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rapiteanu R, Karagyozova T, Zimmermann N, Singh K, Wayne G, Martufi M, et al. Highly efficient genome editing in primary human bronchial epithelial cells differentiated at air-liquid interface. Eur Respir J. 2020;55:1900950. doi: 10.1183/13993003.00950-2019. [DOI] [PubMed] [Google Scholar]
- 92. Slepushkin VA, Staber PD, Wang G, McCray PB, Jr, Davidson BL. Infection of human airway epithelia with H1N1, H2N2, and H3N2 influenza A virus strains. Mol Ther. 2001;3:395–402. doi: 10.1006/mthe.2001.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhou J, Li C, Sachs N, Chiu MC, Wong BH, Chu H, et al. Differentiated human airway organoids to assess infectivity of emerging influenza virus. Proc Natl Acad Sci USA. 2018;115:6822–6827. doi: 10.1073/pnas.1806308115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Smith CM, Kulkarni H, Radhakrishnan P, Rutman A, Bankart MJ, Williams G, et al. Ciliary dyskinesia is an early feature of respiratory syncytial virus infection. Eur Respir J. 2014;43:485–496. doi: 10.1183/09031936.00205312. [DOI] [PubMed] [Google Scholar]
- 95. Mulay A, Konda B, Garcia G, Yao C, Beil S, Sen C, et al. SARS-CoV-2 infection of primary human lung epithelium for COVID-19 modeling and drug discovery [preprint] bioRxiv. 2020 doi: 10.1016/j.celrep.2021.109055. [accessed 2020 Jun 29]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7337376/ [DOI] [PMC free article] [PubMed]
- 96. Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH, III, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–446.e14. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Salahudeen AA, Choi SS, Rustagi A, Zhu J, de la O SM, Flynn RA, et al. Progenitor identification and SARS-CoV-2 infection in long-term human distal lung organoid cultures [preprint] bioRxiv. 2020 doi: 10.1038/s41586-020-3014-1. [accessed 2020 Jul 27]. Available from: https://www.biorxiv.org/content/10.1101/2020.07.27.212076v1. [DOI] [PMC free article] [PubMed]
- 98. Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ, Chun SM, et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 2019;10:3991. doi: 10.1038/s41467-019-11867-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dijkstra KK, Monkhorst K, Schipper LJ, Hartemink KJ, Smit EF, Kaing S, et al. Challenges in establishing pure lung cancer organoids limit their utility for personalized medicine. Cell Rep. 2020;31:107588. doi: 10.1016/j.celrep.2020.107588. [DOI] [PubMed] [Google Scholar]
- 100. Heijink IH, Noordhoek JA, Timens W, van Oosterhout AJ, Postma DS. Abnormalities in airway epithelial junction formation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:1439–1442. doi: 10.1164/rccm.201311-1982LE. [DOI] [PubMed] [Google Scholar]
- 101. Perotin JM, Adam D, Vella-Boucaud J, Delepine G, Sandu S, Jonvel AC, et al. Delay of airway epithelial wound repair in COPD is associated with airflow obstruction severity. Respir Res. 2014;15:151. doi: 10.1186/s12931-014-0151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Amatngalim GD, Schrumpf JA, Henic A, Dronkers E, Verhoosel RM, Ordonez SR, et al. Antibacterial defense of human airway epithelial cells from chronic obstructive pulmonary disease patients induced by acute exposure to nontypeable haemophilus influenzae: modulation by cigarette smoke. J Innate Immun. 2017;9:359–374. doi: 10.1159/000455193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ikonomou L, Wagner DE, Turner L, Weiss DJ. Translating basic research into safe and effective cell-based treatments for respiratory diseases. Ann Am Thorac Soc. 2019;16:657–668. doi: 10.1513/AnnalsATS.201812-890CME. [DOI] [PubMed] [Google Scholar]
- 104. Pellegrini G, Rama P, Di Rocco A, Panaras A, De Luca M. Concise review: hurdles in a successful example of limbal stem cell-based regenerative medicine. Stem Cells. 2014;32:26–34. doi: 10.1002/stem.1517. [DOI] [PubMed] [Google Scholar]
- 105. Bowe SN, Wentland CJ, Sandhu GS, Hartnick CJ. Management of complex pediatric laryngotracheal stenosis with skin graft reconstruction. Int J Pediatr Otorhinolaryngol. 2018;108:46–48. doi: 10.1016/j.ijporl.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 106. Nouraei SA, Sandhu GS. Outcome of a multimodality approach to the management of idiopathic subglottic stenosis. Laryngoscope. 2013;123:2474–2484. doi: 10.1002/lary.23949. [DOI] [PubMed] [Google Scholar]
- 107. Elliott MJ, De Coppi P, Speggiorin S, Roebuck D, Butler CR, Samuel E, et al. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet. 2012;380:994–1000. doi: 10.1016/S0140-6736(12)60737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Elliott MJ, Butler CR, Varanou-Jenkins A, Partington L, Carvalho C, Samuel E, et al. Tracheal replacement therapy with a stem cell-seeded graft: lessons from compassionate use application of a gmp-compliant tissue-engineered medicine. Stem Cells Transl Med. 2017;6:1458–1464. doi: 10.1002/sctm.16-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hamilton NJ, Kanani M, Roebuck DJ, Hewitt RJ, Cetto R, Culme-Seymour EJ, et al. Tissue-engineered tracheal replacement in a child: a 4-year follow-up study. Am J Transplant. 2015;15:2750–2757. doi: 10.1111/ajt.13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hamilton NJI, Lee DDH, Gowers KHC, Butler CR, Maughan EF, Jevans B, et al. Bioengineered airway epithelial grafts with mucociliary function based on collagen IV- and laminin-containing extracellular matrix scaffolds. Eur Respir J. 2020;55:1901200. doi: 10.1183/13993003.01200-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ma Q, Ma Y, Dai X, Ren T, Fu Y, Liu W, et al. Regeneration of functional alveoli by adult human SOX9+ airway basal cell transplantation. Protein Cell. 2018;9:267–282. doi: 10.1007/s13238-018-0506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ghosh M, Ahmad S, White CW, Reynolds SD. Transplantation of airway epithelial stem/progenitor cells: a future for cell-based therapy. Am J Respir Cell Mol Biol. 2017;56:1–10. doi: 10.1165/rcmb.2016-0181MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Miller AJ, Hill DR, Nagy MS, Aoki Y, Dye BR, Chin AM, et al. In vitro induction and in vivo engraftment of lung bud tip progenitor cells derived from human pluripotent stem cells. Stem Cell Reports. 2018;10:101–119. doi: 10.1016/j.stemcr.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Berical A, Lee RE, Randell SH, Hawkins F. Challenges facing airway epithelial cell-based therapy for cystic fibrosis. Front Pharmacol. 2019;10:74. doi: 10.3389/fphar.2019.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Schwank G, Koo B-K, Sasselli V, Dekkers JF, Heo I, Demircan T, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 116. Geurts MH, de Poel E, Amatngalim GD, Oka R, Meijers FM, Kruisselbrink E, et al. Crispr-based adenine editors correct nonsense mutations in a cystic fibrosis organoid biobank. Cell Stem Cell. 2020;26:503–510.e7. doi: 10.1016/j.stem.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 117. Firth AL, Menon T, Parker GS, Qualls SJ, Lewis BM, Ke E, et al. Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient ipscs. Cell Rep. 2015;12:1385–1390. doi: 10.1016/j.celrep.2015.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Suzuki S, Crane AM, Anirudhan V, Barillà C, Matthias N, Randell SH, et al. Highly efficient gene editing of cystic fibrosis patient-derived airway basal cells results in functional cftr correction. Mol Ther. 2020;28:1684–1695. doi: 10.1016/j.ymthe.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lai M, Pifferi M, Bush A, Piras M, Michelucci A, Di Cicco M, et al. Gene editing of DNAH11 restores normal cilia motility in primary ciliary dyskinesia. J Med Genet. 2016;53:242–249. doi: 10.1136/jmedgenet-2015-103539. [DOI] [PubMed] [Google Scholar]
- 120. Droz-Georget Lathion S, Rochat A, Knott G, Recchia A, Martinet D, Benmohammed S, et al. A single epidermal stem cell strategy for safe ex vivo gene therapy. EMBO Mol Med. 2015;7:380–393. doi: 10.15252/emmm.201404353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gowers KHC, Hynds RE, Thakrar RM, Carroll B, Birchall MA, Janes SM. Optimized isolation and expansion of human airway epithelial basal cells from endobronchial biopsy samples. J Tissue Eng Regen Med. 2018;12:e313–e317. doi: 10.1002/term.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Rosen C, Shezen E, Aronovich A, Klionsky YZ, Yaakov Y, Assayag M, et al. Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice. Nat Med. 2015;21:869–879. doi: 10.1038/nm.3889. [DOI] [PubMed] [Google Scholar]
- 123. Zuo WL, Shenoy SA, Li S, O’Beirne SL, Strulovici-Barel Y, Leopold PL, et al. Ontogeny and biology of human small airway epithelial club cells. Am J Respir Crit Care Med. 2018;198:1375–1388. doi: 10.1164/rccm.201710-2107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang G, Lou HH, Salit J, Leopold PL, Driscoll S, Schymeinsky J, et al. Characterization of an immortalized human small airway basal stem/progenitor cell line with airway region-specific differentiation capacity. Respir Res. 2019;20:196. doi: 10.1186/s12931-019-1140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zheng D, Yin L, Chen J. Evidence for Scgb1a1(+) cells in the generation of p63(+) cells in the damaged lung parenchyma. Am J Respir Cell Mol Biol. 2014;50:595–604. doi: 10.1165/rcmb.2013-0327OC. [DOI] [PubMed] [Google Scholar]
- 126. Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Strunz M, Simon LM, Ansari M, Kathiriya JJ, Angelidis I, Mayr CH, et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat Commun. 2020;11:3559. doi: 10.1038/s41467-020-17358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kobayashi Y, Tata A, Konkimalla A, Katsura H, Lee RF, Ou J, et al. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat Cell Biol. 2020;22:934–946. doi: 10.1038/s41556-020-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Choi J, Park JE, Tsagkogeorga G, Yanagita M, Koo BK, Han N, et al. Inflammatory signals induce at2 cell-derived damage-associated transient progenitors that mediate alveolar regeneration. Cell Stem Cell. 2020;27:366–382.e7. doi: 10.1016/j.stem.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bowling S, Sritharan D, Osorio FG, Nguyen M, Cheung P, Rodriguez-Fraticelli A, et al. An engineered crispr-cas9 mouse line for simultaneous readout of lineage histories and gene expression profiles in single cells. Cell. 2020;181:1693–1694. doi: 10.1016/j.cell.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wan H, Winton HL, Soeller C, Stewart GA, Thompson PJ, Gruenert DC, et al. Tight junction properties of the immortalized human bronchial epithelial cell lines Calu-3 and 16HBE14o- Eur Respir J. 2000;15:1058–1068. doi: 10.1034/j.1399-3003.2000.01514.x. [DOI] [PubMed] [Google Scholar]
- 132. Ehrhardt C, Kneuer C, Fiegel J, Hanes J, Schaefer UF, Kim K-J, et al. Influence of apical fluid volume on the development of functional intercellular junctions in the human epithelial cell line 16HBE14o-: implications for the use of this cell line as an in vitro model for bronchial drug absorption studies. Cell Tissue Res. 2002;308:391–400. doi: 10.1007/s00441-002-0548-5. [DOI] [PubMed] [Google Scholar]
- 133. Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17:62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- 134. Ke Y, Reddel RR, Gerwin BI, Miyashita M, McMenamin M, Lechner JF, et al. Human bronchial epithelial cells with integrated SV40 virus T antigen genes retain the ability to undergo squamous differentiation. Differentiation. 1988;38:60–66. doi: 10.1111/j.1432-0436.1988.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 135. Shen BQ, Finkbeiner WE, Wine JJ, Mrsny RJ, Widdicombe JH. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl- secretion. Am J Physiol. 1994;266:L493–L501. doi: 10.1152/ajplung.1994.266.5.L493. [DOI] [PubMed] [Google Scholar]
- 136. Foster KA, Avery ML, Yazdanian M, Audus KL. Characterization of the Calu-3 cell line as a tool to screen pulmonary drug delivery. Int J Pharm. 2000;208:1–11. doi: 10.1016/s0378-5173(00)00452-x. [DOI] [PubMed] [Google Scholar]
- 137. Grainger CI, Greenwell LL, Lockley DJ, Martin GP, Forbes B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm Res. 2006;23:1482–1490. doi: 10.1007/s11095-006-0255-0. [DOI] [PubMed] [Google Scholar]
- 138. Salomon JJ, Muchitsch VE, Gausterer JC, Schwagerus E, Huwer H, Daum N, et al. The cell line NCl-H441 is a useful in vitro model for transport studies of human distal lung epithelial barrier. Mol Pharm. 2014;11:995–1006. doi: 10.1021/mp4006535. [DOI] [PubMed] [Google Scholar]
- 139. O’Reilly MA, Weaver TE, Pilot-Matias TJ, Sarin VK, Gazdar AF, Whitsett JA. In vitro translation, post-translational processing and secretion of pulmonary surfactant protein B precursors. Biochim Biophys Acta. 1989;1011:140–148. doi: 10.1016/0167-4889(89)90201-2. [DOI] [PubMed] [Google Scholar]
- 140. Masuda A, Kondo M, Saito T, Yatabe Y, Kobayashi T, Okamoto M, et al. Establishment of human peripheral lung epithelial cell lines (HPL1) retaining differentiated characteristics and responsiveness to epidermal growth factor, hepatocyte growth factor, and transforming growth factor beta1. Cancer Res. 1997;57:4898–4904. [PubMed] [Google Scholar]
- 141. Kalita M, Tian B, Gao B, Choudhary S, Wood TG, Carmical JR, et al. Systems approaches to modeling chronic mucosal inflammation. Biomed Res Int. 2013;2013:505864. doi: 10.1155/2013/505864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Stead LF, Egan P, Devery A, Conway C, Daly C, Berri S, et al. An integrated inspection of the somatic mutations in a lung squamous cell carcinoma using next-generation sequencing. PLoS One. 2013;8:e78823. doi: 10.1371/journal.pone.0078823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Stead LF, Berri S, Wood HM, Egan P, Conway C, Daly C, et al. The transcriptional consequences of somatic amplifications, deletions, and rearrangements in a human lung squamous cell carcinoma. Neoplasia. 2012;14:1075–1086. doi: 10.1593/neo.121380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zabner J, Karp P, Seiler M, Phillips SL, Mitchell CJ, Saavedra M, et al. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am J Physiol Lung Cell Mol Physiol. 2003;284:L844–L854. doi: 10.1152/ajplung.00355.2002. [DOI] [PubMed] [Google Scholar]