Idiopathic pulmonary fibrosis (IPF) is the most common and deadly of the idiopathic interstitial pneumonias (1). Through uncontrolled fibroproliferation and excess deposition of extracellular matrices, IPF destroys the lung architecture, resulting in progressive decline in lung function. No intervention has been shown to halt or reverse this disease, and only two drugs have been reported to delay decline in lung function (2). The limited understanding we have about how and which lung cells mediate fibrogenesis represents an important challenge to overcome.
Excess inflammation and activated immune mechanisms are known to contribute to the development and/or progression of pulmonary fibrosis in conditions such as connective tissue–related interstitial lung diseases (3). Data in support of a role for aberrant immunity in IPF emerged as early as 1983, when investigators reported an increased frequency of the B-lymphocyte alloantigen HLA-DR2 in patients with IPF, which correlated with radiographic abnormalities (4). However, today, inflammation is considered less relevant in IPF, as the lungs of affected individuals show a paucity of inflammation, and antiinflammatory agents have not shown beneficial therapeutic effect. Instead, the prevailing concept is that IPF results from repeated injury to the lung epithelium, which in turn elicits an aggressive repair response that drives fibrogenesis (5). Nevertheless, the recent identification of genes coding for components of the immune system (e.g., TOLLIP and TLR3) as contributors of IPF pathogenesis has reignited interest in this system (6).
In this issue of the Journal, Ali and colleagues (pp. 722–733) further our understanding of the role of adaptive immunity in IPF while focusing on how activated B lymphocytes or B cells affect fibroblast functions (7). Their work builds on prior observations showing a decrease in regulator B cells in the peripheral blood of patients with IPF, with the proportion of regulatory B cells correlating with the annual relative change in diffusing capacity of the lungs for carbon monoxide (8). In other work, increased B-cell activation and an increased number of IgA+ memory B cells and plasmablasts were found in the blood and lungs of patients with IPF when compared with control subjects (9). Moreover, B cells in the circulation of patients with IPF were more antigen differentiated and had greater plasmablast proportions than in control subjects, with the extent of B-cell differentiation correlating with patient lung volumes (10).
Together, these observations suggest that loss of self-tolerance to lung-specific proteins might promote or exacerbate IPF and could represent a target for intervention (11). Consistent with this, others have reported increased concentrations of anti–collagen V antibodies in some patients with IPF and have shown that collagen type V tolerance inhibits bleomycin-induced lung fibrosis (12). More recently, a pilot study was conducted in which 11 critically ill patients with acute exacerbations of IPF were treated with therapeutic plasma exchanges and rituximab, which was in some cases supplemented with intravenous immunoglobulin therapy (13); nine patients showed improvements compared with one in the historical control.
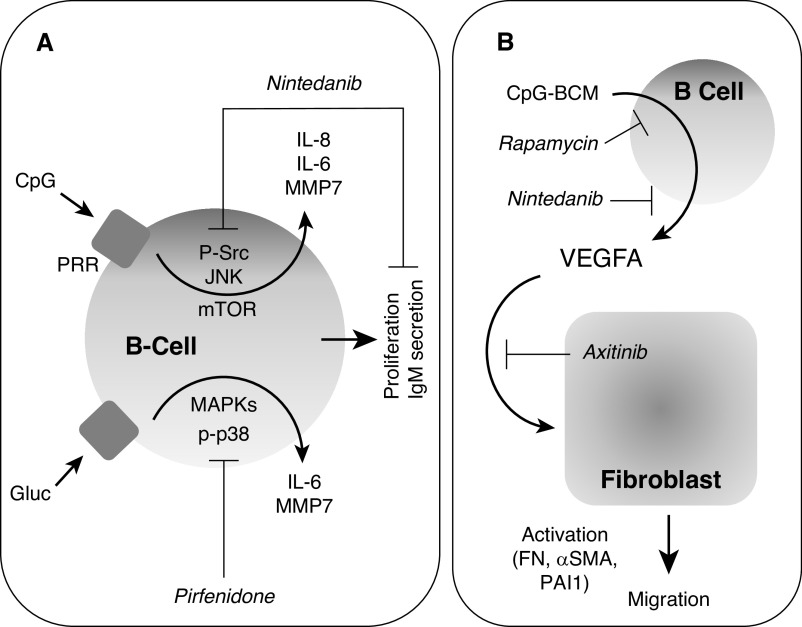
Ali and colleagues previously reported that activation of B cells obtained from normal donors with microbial antigens stimulates their release of proinflammatory mediators (14, 15). This earlier work led the group to speculate that B-cell activation with microbial antigens might contribute to the inflammatory and profibrotic milieu observed in IPF. To test this idea, blood samples and B cells were collected from patients with IPF and normal volunteers who were not undergoing an exacerbation and who were not on antifibrotic or steroid treatment for at least 3 months prior (7). The cells were exposed to CpG, an unmethylated synthetic oligodeoxynucleotide similar to those found in bacterial and fungal DNA, or β-glucans, carbohydrates present in the cell walls of many fungi. They found that B cells from patients with IPF produced higher amounts of IL-8, IL-6, and MMP7 in response to CpG or β-glucan and that the supernatants of cultured stimulated B cells promoted lung fibroblast migration and proliferation via mTOR-dependent and independent pathways. Next, they tested the effects of the antifibrotic agents nintedanib and pirfenidone by incubating B cells from patients with IPF with the drugs before stimulation with either CpG or β-glucan. Both antifibrotic agents impaired cytokine secretion but not in an identical fashion. Of note, the mTOR inhibitor rapamycin inhibited IL-6 secretion in B cells stimulated with CpG but not in cells stimulated with β-glucan. Nintedanib decreased Src phosphorylation and mTOR activation, whereas pirfenidone decreased p38 phosphorylation. Further work pointed to B cell–derived VEGFA (vascular endothelial growth factor A) as being responsible for many of the effects observed on lung fibroblast activation and migration; this effect was inhibited by nintedanib. The findings were considered highly relevant to the human condition, as activated CD20-positive B cells were found to form aggregates in the fibrotic areas adjacent to fibroblastic foci in IPF lungs.
A few intriguing observations described by Ali and coworkers should be highlighted. First, they observed that both microbial antigens stimulate B-cell production of soluble mediators that affect the functions of lung fibroblasts, thereby pointing to a potential role for B cell–lung fibroblast interactions in IPF pathogenesis. As acknowledged by the authors, this might have important implications for infection-mediated IPF exacerbation as well as for understanding the role of the lung microbiome in IPF pathogenesis (16). Second, even though both stimulants affected B cells, their effect differed, which is not surprising considering the intrinsic differences between CpG and β-glucan. Third, both nintedanib and pirfenidone reduced the impact of CpG and β-glucan stimulation on lung fibroblasts, suggesting that these agents are capable of affecting immune responses in IPF. However, the effect of the antifibrotic agents differed with respect to their capacity to affect B-cell proliferation and activation as well as the actions of B-cell activation on lung fibroblasts, with nintedanib being more effective. These differences might be due to the effect nintedanib has on tyrosine kinases versus the effects of pirfenidone. Either way, it is interesting that these agents have such divergent effects in vitro (Figure 1) while having very similar effects in patients, perhaps suggesting more important antifibrotic effects on other cells.
Figure 1.
Effects of microbial antigens on B cells and their interaction with lung fibroblasts. (A) Microbial antigens promote B-cell proliferation. CpG and Gluc act via PRR on B cells to stimulate mTOR-dependent and mTOR-independent signaling pathways leading to the expression of cytokines and matrix metalloproteinase-7 (MMP7) as well as B-cell proliferation and IgM secretion. Nintedanib inhibits the mTOR-dependent signals, whereas pirfenidone inhibits mTOR-independent pathways. (B) Role of VEGFA (vascular endothelial growth factor A) in B cell–mediated activation of lung fibroblasts. Microbial antigens stimulate B cells to produce VEGFA via mTOR-dependent pathways inhibited by rapamycin and nintedanib. VEGFA stimulates the activation and migration of fibroblasts, which is inhibited by axitinib. αSMA = α-smooth muscle actin; FN = fibronectin; Gluc = B-glucan; JNK = c-Jun N-terminal kinase; MAPK = mitogen-activated protein kinases; PAI-1 = plasminogen activator inhibitor-1; PRR = pattern recognition receptors.
In short, the work by Ali and colleagues enhances our understanding of the role adaptive immunity may play in IPF and how antifibrotics might work to modulate these processes. Studies testing these pathways in patients will be required to determine the importance of these mechanisms of action. Emmil von Behring and Shibasaburo Kitasato noted the importance of circulating antitoxins in immunity to diphtheria and tetanus in 1890, which represented the first clear indication of the existence of cells that we now know as B cells (17). Little did they know that these cells would be implicated in the pathogenesis of IPF more than a century later. Such new knowledge is likely to drive further research in the field using more sophisticated techniques capable of identifying new targets for intervention (18).
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2021-0101ED on March 16, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378:1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 2. Somogyi V, Chaudhuri N, Torrisi SE, Kahn N, Müller V, Kreuter M. The therapy of idiopathic pulmonary fibrosis: what is next? Eur Respir Rev. 2019;28:190021. doi: 10.1183/16000617.0021-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mathai SC, Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ. 2016;352:h6819. doi: 10.1136/bmj.h6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Libby DM, Gibofsky A, Fotino M, Waters SJ, Smith JP. Immunogenetic and clinical findings in idiopathic pulmonary fibrosis: association with the B-cell alloantigen HLA-DR2. Am Rev Respir Dis. 1983;127:618–622. doi: 10.1164/arrd.1983.127.5.618. [DOI] [PubMed] [Google Scholar]
- 5. Hewlett JC, Kropski JA, Blackwell TS. Idiopathic pulmonary fibrosis: epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol. 2018;71–72:112–127. doi: 10.1016/j.matbio.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noth I, Zhang Y, Ma SF, Flores C, Barber M, Huang Y, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1:309–317. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali MF, Egan AM, Shaughenessy GF, Anderson DK, Kottm TJ, Dasri H, et al. Antifibrotics modify B-cell–induced fibroblast migration and activation in patients with idiopathic pulmonary fibrosis Am J Respir Cell Mol Biol 202164722–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asai Y, Chiba H, Nishikiori H, Kamekura R, Yabe H, Kondo S, et al. Aberrant populations of circulating T follicular helper cells and regulatory B cells underlying idiopathic pulmonary fibrosis. Respir Res. 2019;20:244. doi: 10.1186/s12931-019-1216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heukels P, van Hulst JAC, van Nimwegen M, Boorsma CE, Melgert BN, von der Thusen JH, et al. Enhanced Bruton’s tyrosine kinase in B-cells and autoreactive IgA in patients with idiopathic pulmonary fibrosis. Respir Res. 2019;20:232. doi: 10.1186/s12931-019-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xue J, Kass DJ, Bon J, Vuga L, Tan J, Csizmadia E, et al. Plasma B lymphocyte stimulator and B cell differentiation in idiopathic pulmonary fibrosis patients. J Immunol. 2013;191:2089–2095. doi: 10.4049/jimmunol.1203476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoyne GF, Elliott H, Mutsaers SE, Prêle CM. Idiopathic pulmonary fibrosis and a role for autoimmunity. Immunol Cell Biol. 2017;95:577–583. doi: 10.1038/icb.2017.22. [DOI] [PubMed] [Google Scholar]
- 12. Vittal R, Mickler EA, Fisher AJ, Zhang C, Rothhaar K, Gu H, et al. Type V collagen induced tolerance suppresses collagen deposition, TGF-β and associated transcripts in pulmonary fibrosis. PLoS One. 2013;8:e76451. doi: 10.1371/journal.pone.0076451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donahoe M, Valentine VG, Chien N, Gibson KF, Raval JS, Saul M, et al. Autoantibody-targeted treatments for acute exacerbations of idiopathic pulmonary fibrosis. PLoS One. 2015;10:e0127771. doi: 10.1371/journal.pone.0127771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ali MF, Dasari H, Van Keulen VP, Cornec D, Vasmatzis G, Peikert T, et al. Microbial antigens stimulate metalloprotease-7 secretion in human B-lymphocytes using mTOR-dependent and independent pathways. Sci Rep. 2017;7:3869. doi: 10.1038/s41598-017-04199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ali MF, Dasari H, Van Keulen VP, Carmona EM. Canonical stimulation of the NLRP3 inflammasome by fungal antigens links innate and adaptive B-lymphocyte responses by modulating IL-1β and IgM production. Front Immunol. 2017;8:1504. doi: 10.3389/fimmu.2017.01504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salisbury ML, Han MK, Dickson RP, Molyneaux PL. Microbiome in interstitial lung disease: from pathogenesis to treatment target. Curr Opin Pulm Med. 2017;23:404–410. doi: 10.1097/MCP.0000000000000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooper MD. The early history of B cells. Nat Rev Immunol. 2015;15:191–197. doi: 10.1038/nri3801. [DOI] [PubMed] [Google Scholar]
- 18. McDonough JE, Ahangari F, Li Q, Jain S, Verleden SE, Herazo-Maya J, et al. Transcriptional regulatory model of fibrosis progression in the human lung. JCI Insight. 2019;4:e131597. doi: 10.1172/jci.insight.131597. [DOI] [PMC free article] [PubMed] [Google Scholar]