Abstract
Objective
To determine the efficacy and safety of fremanezumab administration in Japanese and Korean patients with chronic migraine (CM).
Background
Available preventive treatments for CM are limited by various efficacy and safety issues. Fremanezumab, a monoclonal antibody that targets the calcitonin gene‐related peptide pathway involved in migraine pathogenesis, has been shown to be effective and well tolerated in large‐scale, international Phase 3 trials.
Methods
Randomized, placebo‐controlled trial of patients with CM who received subcutaneous fremanezumab monthly (675 mg at baseline and 225 mg at weeks 4 and 8), fremanezumab quarterly (675 mg at baseline and placebo at weeks 4 and 8), or matching placebo. Primary endpoint was the mean change from baseline in the monthly (28‐day) average number of headache days of at least moderate severity during the 12 weeks after the first dose.
Results
Among 571 patients randomized (safety set, n = 569; full analysis set, n = 566), the least‐squares mean (±standard error [SE]) reduction in the average number of headache days of at least moderate severity per month during 12 weeks was significantly greater with fremanezumab monthly (–4.1 ± 0.4) and fremanezumab quarterly (–4.1 ± 0.4) than with placebo (–2.4 ± 0.4). The difference from the placebo group in the mean change (95% confidence interval [CI]) was −1.7 days (−2.54, −0.80) for the fremanezumab monthly group and −1.7 days (−2.55, −0.82) for the fremanezumab quarterly group (p < 0.001 vs. placebo for both fremanezumab groups). The percentage of patients with a ≥50% reduction in the average number of headache days of at least moderate severity per month (response rate) was higher with fremanezumab monthly (29.0%) and fremanezumab quarterly (29.1%) than with placebo (13.2%) in addition to other improvements in secondary endpoints, including reduction of acute medication use (mean change from baseline during 12‐week period ± SE: fremanezumab monthly, –3.7 ± 0.4; fremanezumab quarterly, –3.9 ± 0.4; placebo, –2.4 ± 0.4) and improvements in disability scores (mean change from baseline in six‐item Headache Impact Test score at 4 weeks after third injection ± SE: fremanezumab monthly, –8.1 ± 0.7; fremanezumab quarterly, –8.0 ± 0.7; placebo, –6.5 ± 0.7). Fremanezumab was well tolerated with a similar incidence of adverse events including injection‐site reactions as placebo (patients with at least one treatment‐emergent adverse event: fremanezumab total, n = 232 [61.4%]; placebo, n = 118 [61.8%]).
Conclusion
Fremanezumab effectively prevents CM in Japanese and Korean patients and was well tolerated. No safety signal was detected.
Keywords: calcitonin gene‐related peptide, chronic migraine, fremanezumab, Japanese, Korean
Abbreviations
- ANCOVA
analysis of covariance
- CGRP
calcitonin gene‐related peptide
- CM
chronic migraine
- EM
episodic migraine
- HIT‐6
six‐item Headache Impact Test
- LSM
least‐squares mean
- MMRM
mixed‐effects model for repeated measures
- SD
standard deviation
- SE
standard error
- TEAEs
treatment‐emergent adverse events
INTRODUCTION
Chronic migraine (CM), defined as the occurrence of characteristic headaches on at least 15 days per month (at least 8 days of which meet the diagnostic criteria for migraine) for at least 3 months,1 is estimated to affect 1.4%–2.2% of the population.2 Despite the high prevalence and the physical quality of life and functional impairments associated with CM, treatment options are limited. Globally available therapies for migraine prevention include antiseizure drugs, beta‐blockers, antidepressants, and calcium channel blockers. However, these preventive treatments are known to be variously limited by underuse,3 poor adherence,4, 5, 6, 7 short‐ and long‐term adverse reactions,3, 8 and lack of efficacy.3, 7, 8 A real‐world treatment pattern survey in Japan found that patients with CM experience similar challenges with available treatment options.8 Therefore, there is an unmet medical need for efficacious and well‐tolerated migraine‐preventive medications.
More recently, agents that target calcitonin gene‐related peptide (CGRP) have been investigated for their potential as preventive and acute treatments based on preclinical and clinical evidence that CGRP plays a central role in pathogenesis.9, 10 For migraine prevention, monoclonal antibodies act either against the CGRP peptide or its receptor. Fremanezumab is a fully humanized IgG2Δa/kappa monoclonal antibody that has been extensively investigated in clinical trials of chronic or episodic migraine (EM). In particular, the international large‐scale Phase 3 (HALO) trial found that patients with CM randomized to either monthly or quarterly fremanezumab treatment had a significantly greater reduction in average number of headache days of at least moderate severity and greater response rate than patients who received placebo.11 Disability scores, as assessed by the six‐item Headache Impact Test (HIT‐6),12 were also significantly lower in fremanezumab‐treated patients.11 Another placebo‐controlled, randomized trial (FOCUS), which included patients with EM and CM (n = 509), found that fremanezumab was effective and well tolerated in patients with difficult‐to‐treat migraine who had not responded to up to four previous preventive migraine treatments.13
A previous Phase 1 single‐dose trial evaluated the pharmacokinetics, safety, and tolerability of fremanezumab in healthy Japanese and Caucasian participants and demonstrated that the pharmacokinetic profile was comparable between Japanese and Caucasian populations.14 Further, Japanese patients have been included in the international Phase 3 HALO trials that demonstrated fremanezumab was significantly superior to placebo.11, 15, 16 This trial is intended to confirm the efficacy and safety of fremanezumab in Japanese patients with CM. Patients from South Korea were included based on the apparent absence of population differences in CGRP polymorphism based on genomic database searching (dbSNP, https://www.ncbi.nlm.nih.gov/snp/; gnomAD v 2.1.1, https://gnomad.broadinstitute.org/), minimal differences in diagnostic criteria, epidemiology, and therapeutic approach between countries.
In this context of previous trial results, we hypothesized that monthly and quarterly subcutaneous administration of fremanezumab would provide improved efficacy and similar safety compared with placebo for preventive treatment of CM in Japanese and Korean patients.
METHODS
Trial design
This multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group trial in Japanese and Korean patients with CM (ClinicalTrials.gov, NCT03303079) consisted of a 4‐week screening period and a 12‐week double‐blind treatment period. The trial was conducted between November 2017 and November 2019 at 57 institutions in Japan and 10 institutions in Korea (Table S1) with enrolment and informed consent procedures performed at each investigational site by the investigators or their designees. Male or female patients aged 18–70 years were considered initially eligible if they had a history of migraine (age at onset ≤50 years) according to either the International Classification of Headache Disorders, 3rd edition (beta version)17 or clinical judgment suggesting a migraine diagnosis for ≥12 months prior to giving informed consent during the enrolment period. However, to be included in the trial, such patients had to then meet the criteria for CM assessed prospectively during the 28‐day screening period as follows: headache on ≥15 days and headache fulfilling International Classification of Headache Disorders, 3rd edition beta diagnostic criteria, probable migraine (migraine subtype where only one migraine criterion is missing), or the patient used a triptan or ergot derivative to treat an established headache on ≥8 days. Key exclusion criteria were (i) the lack of efficacy of at least two of four clusters of preventive medications despite an adequate treatment, (ii) unremitting headaches with duration more than 80% of waking hours and with less than 4 days without headache per month, and (iii) clinically significant major organ disease. Full inclusion and exclusion criteria are listed in Table S2.
Informed consent was documented on a written informed consent form approved by the same institutional review board or independent ethics committee/ethics committee that approved the trial protocol and which complied with the International Conference on Harmonisation Good Clinical Practice Guideline and local regulatory requirements.
Treatment
After obtaining informed consent and the initial screening for eligibility at Visit 1, eligible patients were randomly assigned at baseline (Visit 2) in a 1:1:1 ratio to receive either monthly fremanezumab, quarterly fremanezumab, or placebo via subcutaneous injection. Randomization was performed by electronic interactive‐response technology, with stratification according to sex, country, and baseline use of preventive medication (yes or no). Both patients and all parties involved in the investigation were blinded to the trial‐group assignments. Assignment of treatment was concealed from investigators and patients by use of a randomization code generated as part of the interactive‐response technology. This was administered by an external contract research organization, which meant that the study sponsor was also blinded to treatment assignment through the use of the randomization codes that were only allowed to be broken in case of a medical emergency.
All treatment groups received either fremanezumab or placebo at 4‐week (“monthly”) intervals for a total of three doses. The monthly fremanezumab group patients received fremanezumab 675 mg as three active injections (225 mg/1.5 ml each) at baseline and then fremanezumab 225 mg as a single active injection (225 mg/1.5 ml) at month 1 (Visit 3) and month 2 (Visit 4). Quarterly fremanezumab group patients received fremanezumab 675 mg as three active injections (225 mg/1.5 ml each) at baseline (Visit 2) and placebo as a single 1.5 ml injection at month 1 (Visit 3) and month 2 (Visit 4). Placebo group patients received three 1.5 ml placebo injections at Visit 2 and a single 1.5 ml placebo injection at Visit 3 and Visit 4. Preventive migraine medications were allowed in no more than 30% of trial patients if the dose had not changed for 2 months prior to screening and was kept consistent throughout the trial; otherwise, they were generally prohibited (Table S3). All randomized patients underwent a final assessment at 12 weeks (Visit 5, Figure S1). Interventions were made essentially identical to each other via use of consistent packaging and identical prefilled syringes each containing 1.5 ml of the investigational product to ensure blinding was not compromised. The number of injections provided at each treatment visit was also identical to avoid study medication assignment being revealed.
Outcomes
Information on headaches was collected via an electronic headache diary provided to individual patients at the screening visit. Patients were instructed to complete diary entries about the previous day from Visit 1 through to Visit 5 or the day of treatment withdrawal.
The primary endpoint was the mean change from baseline in the monthly (28‐day) average number of headache days of at least moderate severity during the 12‐week period after the first dose of trial medication (see Table S4 for definition of headache day of at least moderate severity). This is consistent with the primary endpoint recommended by the Classification Committee of the International Headache Society guidelines for controlled trials of prophylactic treatment of CM in adults.18
Secondary endpoints assessed during the 12‐week treatment period after the first dose of trial medication were the (i) mean change from baseline in the monthly average number of migraine days (see Table S4 for definition of migraine day), (ii) proportion of patients reaching ≥50% reduction in the monthly average number of headache days of at least moderate severity, (iii) mean change from baseline in the monthly average number of days with use of any acute headache medications, and (iv) mean change from baseline in the monthly average number of headache days of at least moderate severity in the subgroup of patients from the full analysis set who did not receive concomitant preventive migraine medications. A further secondary endpoint determined at 4 weeks after the final (third) dose of trial medication was the mean change from baseline in disability score, as measured by the HIT‐6 assessment item (12).
With regard to safety and tolerability evaluation, all adverse events were coded via MedDRA version 22.0 by system organ class and preferred term and summarized as treatment‐emergent adverse events (TEAEs) according to severity, seriousness, and relationship to trial drug and treatment discontinuation. Safety was also assessed by summarizing data related to clinical laboratory tests (chemistry, hematology, coagulation, and urinalysis), 12‐lead ECG, physical examination, vital signs (systolic and diastolic blood pressure, pulse rate, temperature, and respiratory rate), weight, and the electronic Columbia‐Suicide Severity Rating Scale.19 Finally, fremanezumab‐treated patients were assessed for antidrug antibodies.
Statistics
Sample size calculations were based on the assumption that this trial would yield similar results to that of a Phase 2b trial in which the difference in mean change from baseline in the monthly average number of headache days of at least moderate severity between the fremanezumab monthly and the placebo group was 1.7 days when reanalyzed over the same period as this Phase 3 trial.20 The standard deviation (SD) for the change from baseline in the monthly average number of headache days of at least moderate severity during the 12 weeks was 4.9 days. From this result, a sample size of 176 patients per group was considered to provide more than 90% power for the trial to succeed at a significance level of 0.05 (two‐sided) and, taking into account a small percentage of patients who may be excluded, the sample size was determined as 180 patients per group (540 patients in total). Enrolment was stopped when the target sample size was reached. There was no data safety monitoring board in this study and no interim analyses were planned.
Efficacy analyses were conducted on the full analysis set, which included all randomly assigned patients who received at least one dose of a trial regimen and who had baseline and post‐baseline data on monthly average number of headache days of at least moderate severity. Safety analyses were conducted on the safety set, which included all randomly assigned patients who received at least one dose of a trial regimen.
Descriptive statistics related to baseline characteristics and adverse events were evaluated using mean, SD, or absolute frequency count and proportions as appropriate. The primary endpoint was analyzed using an analysis of covariance (ANCOVA) model that included treatment, sex, country, and baseline preventive medication use as fixed effects and baseline number of headache days of at least moderate severity and years since onset of migraine as covariates. Two‐sided 95% confidence intervals and p values were constructed for the least‐squares mean (LSM) differences between each fremanezumab group and the placebo group. Adjustment for multiple comparisons was accomplished using a fixed sequence procedure. If superiority of the fremanezumab monthly group versus placebo was confirmed at a two‐sided significance level of 0.05, then the fremanezumab quarterly group versus placebo was also tested at a two‐sided significance level of 0.05. For the ANCOVA, when the number of evaluation days of the electronic headache diary after administration was 10 days or more, headache diary data were normalized to 28 days of data during the 3‐month period. Therefore, there were no missing values for the primary analysis by ANCOVA. The Wilcoxon rank‐sum test was performed as a sensitivity analysis for normality assumption when comparing each fremanezumab group with placebo. In addition to the primary analysis by ANCOVA, a mixed‐effects model for repeated measures (MMRM) analysis was used to estimate the mean change from baseline in the monthly number of headache days of at least moderate severity by each month. The MMRM included treatment, sex, country, baseline preventive migraine medication use, month and treatment‐by‐month interaction as fixed effects, and baseline value and years since onset of migraine as covariates. For the MMRM analysis, data were also normalized to 28 days of data when the number of evaluation days of the electronic headache diary in each month was 10 days or more. However, data were not available for patients who discontinued, so data for evaluation may be missing for a particular month.
The ANCOVA model was similarly applied to secondary endpoints related to the mean changes from baseline in the monthly average number of migraine days, monthly average number of days with use of any acute headache medications, and the monthly average number of headache days of at least moderate severity in patients not receiving concomitant preventive migraine medications. For the secondary endpoint related to the proportion of patients reaching ≥50% reduction in the monthly average number of headache days of at least moderate severity, each fremanezumab group and the placebo group was compared using the Cochran–Mantel–Haenszel test stratified by baseline preventive medication use. Differences between each fremanezumab group and the placebo group and two‐sided 95% confidence interval (a Mantel–Haenszel estimator of the difference and its two‐sided 95% confidence interval) were computed. For the mean change from baseline in HIT‐6 total score, ANCOVA test was performed in a manner similar to that of the primary endpoint for the mean change from baseline in the HIT‐6 total score.
SAS version 9.4 (SAS Institute, Cary, NC) was used for all statistical calculations.
RESULTS
Subject disposition and baseline characteristics
In total, 571 patients were randomized, and 569 patients received trial treatment (safety set; fremanezumab monthly group, n = 188; fremanezumab quarterly group, n = 190; placebo group, n = 191). Figure 1 shows the flow of patients throughout the phases of the trial. Of the randomized patients, 541 patients (94.7%) completed the trial. Protocol deviation (n = 16) and withdrawal of consent (n = 8) were the most common reasons for discontinuation. The percentage of patients who completed the trial was similar in the fremanezumab monthly (96.3%), fremanezumab quarterly (94.2%), and placebo (93.7%) groups.
FIGURE 1.
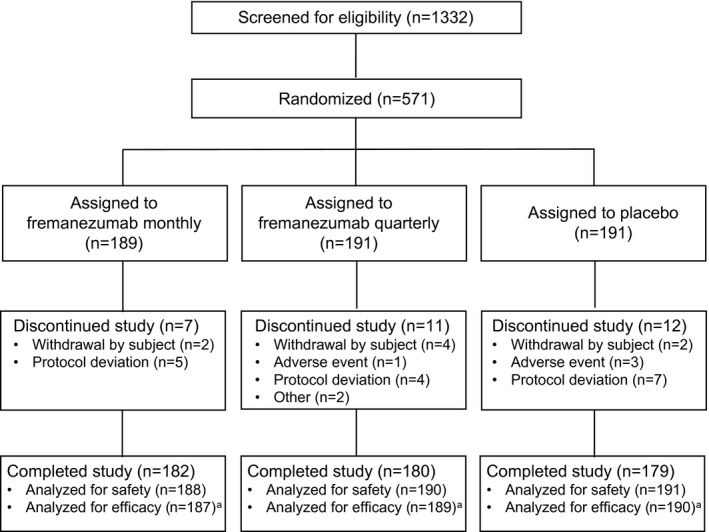
Flow diagram of patient disposition throughout the phases of the trial. aA total of five patients were excluded from the efficacy analysis (full analysis set) as they had less than 10 days of baseline and post‐baseline assessment data on monthly average number of migraine days
In general, demographic and other baseline characteristics were similar among the treatment groups, including in relation to the proportion of female subjects, age, and weight/body mass index (Table 1). In terms of headache characteristics, a similar proportion of patients received preventive migraine medication (range, 20.6%–21.5%), and the mean number of years since onset of migraine ranged from 18.3 (12.4) to 19.0 (11.2) years (Table 1). Disease characteristics were also similar among the treatment groups during the 28‐day preintervention period. During this period, the mean (SD) number of days with headache of any severity and duration ranged from 21.1 (3.9) to 21.6 (4.1) and migraine days per month ranged from 15.2 (5.0) to 16.4 (5.3) days. Further, the proportions of patients reporting use of any acute headache medications or migraine‐specific acute headache medications were highly similar between groups (Table 1).
TABLE 1.
Demographic and baseline clinical characteristics
| Fremanezumab | Placebo (n = 191) | |||
|---|---|---|---|---|
| Monthly (n = 189) | Quarterly (n = 191) | Total (n = 380) | ||
| Age, years, mean (SD) | 42.7 (10.2) | 43.5 (10.2) | 43.1 (10.2) | 42.1 (10.2) |
| Country | ||||
| Japan, n (%) | 159 (84.1) | 159 (83.2) | 318 (83.7) | 161 (84.3) |
| Korea, n (%) | 30 (15.9) | 32 (16.8) | 62 (16.3) | 30 (15.7) |
| Body mass index, mean (SD) | 23.4 (4.1) | 22.4 (3.4) | 22.9 (3.8) | 22.8 (3.4) |
| Female sex, n (%) | 163 (86.2) | 165 (86.4) | 328 (86.3) | 163 (85.3) |
| Disease history | ||||
| Time since onset of migraine, years, mean (SD) | 18.3 (12.4) | 18.7 (12.2) | 18.5 (12.3) | 19.0 (11.2) |
| Use of preventive migraine medication at baseline, yes, n (%) | 39 (20.6) | 40 (20.9) | 79 (20.8) | 41 (21.5) |
| n = 188 | n = 190 | n = 378 | n = 191 | |
|---|---|---|---|---|
| Disease characteristics during 28‐day preintervention period | ||||
| Number of days with headache of any severity and duration, mean (SD) | 21.6 (4.1) | 21.1 (3.9) | 21.4 (4.0) | 21.2 (4.3) |
| Number of headache days of at least moderate severity, mean (SD) | 13.2 (5.4) | 13.4 (5.4) | 13.3 (5.4) | 13.5 (5.0) |
| Number of migraine days, mean (SD) | 16.4 (5.3) | 15.2 (5.0) | 15.8 (5.2) | 15.4 (5.0) |
| Use of any acute headache medications, yes, n (%) | 186 (98.4) | 189 (99.0) | 375 (98.7) | 191 (100.0) |
| Use of migraine‐specific acute headache medicationsa, yes, n (%) | 174 (92.1) | 178 (93.2) | 352 (92.6) | 177 (92.7) |
Triptans and ergot compounds.
Efficacy
Regarding the primary endpoint by ANCOVA for 12‐week analysis, the LSM ± standard error [SE] change from baseline in the monthly average number of headache days of at least moderate severity during the 12‐week period after the first dose of trial medication was −4.1 ± 0.4 days in the fremanezumab monthly group (n = 187), −4.1 ± 0.4 days in the fremanezumab quarterly group (n = 189), and −2.4 ± 0.4 days in the placebo group (n = 190). This corresponded to a difference in the mean change (95% CI) versus placebo of −1.7 (−2.54, −0.80) days in the fremanezumab monthly group and −1.7 (−2.55, −0.82) days in the fremanezumab quarterly group (p < 0.001). Further, in support of the primary analysis by ANCOVA, the LSM ± SE change from baseline in the monthly average number of headache days of at least moderate severity by MMRM analysis for each monthly visit was greater in both fremanezumab treatment groups compared with placebo at all visits (p < 0.05) except for fremanezumab quarterly at month 2 (p = 0.052; Figure 2). Reductions in comparison with the placebo group were observed in both fremanezumab groups from 4 weeks after initial administration (Figure 2).
FIGURE 2.
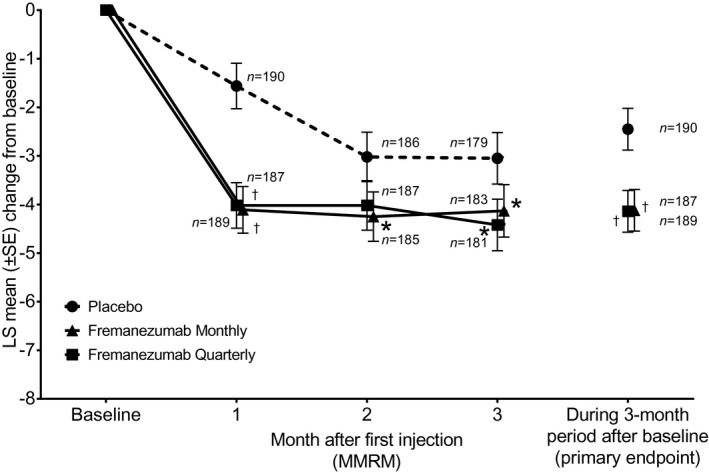
Changes from baseline in the monthly (28‐day) average number of headache days of at least moderate severity (full analysis set population). An asterisk denotes p < 0.05 for the comparison of fremanezumab monthly or quarterly with placebo; mixed‐effects model for repeated measures (MMRM) analysis. A dagger denotes p < 0.001 for the comparison of fremanezumab monthly or quarterly with placebo; MMRM analysis and primary endpoint
Table 2 summarizes the results of the primary and secondary efficacy endpoints. During the 12‐week period after the first dose of fremanezumab, the proportion of patients reaching ≥50% reduction in the monthly average number of headache days of at least moderate severity was greater in patients who received either monthly fremanezumab (29.0%) or quarterly fremanezumab (29.1%), compared with patients who received placebo (13.2%). Similarly, mean changes from baseline in other secondary endpoints during the 12‐week period, including the mean change from baseline in the HIT‐6 disability score assessed at 4 weeks after the final (third) trial medication administration, were also reduced to a greater extent with monthly or quarterly administration of fremanezumab compared with placebo.
TABLE 2.
Summary of primary and secondary endpoints in the full analysis set population
| Fremanezumab | Placebo (n = 190) | ||
|---|---|---|---|
| Monthly (n = 187) | Quarterly (n = 189) | ||
| Primary endpoint | |||
| Average number of headache days of moderate or higher severity per month, mean ± SD | 9.3 ± 5.9 | 9.5 ± 6.0 | 11.2 ± 5.4 |
| Mean change from baseline during 12‐week period ± SE | –4.1 ± 0.4 | –4.1 ± 0.4 | –2.4 ± 0.4 |
| Difference ± SE vs. placebo (95% CI, p)a | –1.7 ± 0.4 (–2.54, –0.80; p < 0.001) | –1.7 ± 0.4 (–2.55, –0.82; p < 0.001) | |
| Secondary endpoints | |||
| Average number of migraine days per month | |||
| Mean change from baseline during 12‐week period ± SE | –4.9 ± 0.5 | –4.1 ± 0.5 | –2.8 ± 0.5 |
| Difference ± SE vs. placebo (95% CI, p)a | –2.1 ± 0.5 (–3.10, –1.12; p < 0.0001) | –1.3 ± 0.5 (–2.27, –0.29; p = 0.011) | |
| ≥50% reduction in the average number of headache days of moderate or higher severity per month during the 12‐week period after the first dose of study medication | |||
| Number of patients evaluated | 186 | 189 | 190 |
| Number of patients with reduction (%) | 54 (29.0) | 55 (29.1) | 25 (13.2) |
| Difference vs. placebo, % (95% CI, p)b | 15.9 (7.8, 24.0; p < 0.001) | 15.9 (7.9, 24.0; p < 0.001) | |
| Average number of days with use of any acute headache medication per month | |||
| Mean change from baseline during 12‐week period ± SE | –3.7 ± 0.4 | –3.9 ± 0.4 | –2.4 ± 0.4 |
| Difference ± SE vs. placebo (95% CI, p)a | –1.3 ± 0.4 (–2.18, –0.43; p = 0.003) | –1.4 ± 0.4 (–2.30, –0.56; p = 0.001) | |
| Average number of headache days of moderate or higher severity in patients not receiving concomitant preventive migraine medication per month | |||
| Number of patients evaluated | 149 | 149 | 149 |
| Mean change from baseline during 12‐week period ± SE | –4.4 ± 0.5 | –4.3 ± 0.5 | –2.7 ± 0.5 |
| Difference ± SE vs. placebo (95% CI, p)a | –1.7 ± 0.5 (–2.63, –0.67; p = 0.001) | –1.6 ± 0.5 (–2.60, –0.63; p = 0.001) | |
| HIT‐6 score | |||
| Number of patients evaluated | 182 | 180 | 179 |
| Mean change from baseline at 4 weeks after third (final) injection ± SE | –8.1 ± 0.7 | –8.0 ± 0.7 | –6.5 ± 0.7 |
| Difference ± SE vs. placebo (95% CI, p)a | –1.6 ± 0.7 (–2.94, –0.19; p = 0.026) | –1.5 ± 0.7 (–2.91, –0.15; p = 0.030) | |
ANCOVA model for change from baseline includes treatment, sex, country, and baseline preventive medication use (yes/no) as fixed effects, and baseline value and years since onset of migraine as covariates.
Comparisons conducted using Mantel–Haenszel test stratified by baseline preventive medication use (yes/no).
Safety
As shown in Table 3, at least one TEAE occurred in a similar proportion of patients in the fremanezumab monthly group (61.7%), fremanezumab quarterly group (61.1%), and the placebo group (61.8%). The proportions of TEAEs potentially related to trial treatment as reported by the investigators were similar in either of the fremanezumab groups (29.3%, 32.1%) compared with the placebo group (28.3%). At least one serious TEAE occurred in only three patients in the fremanezumab monthly group (1.6%; asthma, intestinal hemorrhage, and brain contusion), one patient in the fremanezumab quarterly group (0.5%; influenza) and one patient in the placebo group (0.5%; breast cancer), although no serious TEAEs were deemed as being drug‐related by either the investigators or the sponsor. TEAEs leading to trial discontinuation only occurred in the placebo group (two patients; migraine, eosinophil count, and white blood cell count increased) and no TEAEs leading to treatment discontinuation occurred in any of the groups receiving fremanezumab. Nasopharyngitis and injection‐site reactions were the most common TEAE with injection‐site reactions showing no significant differences between treatment groups.
TABLE 3.
Adverse events
| Fremanezumab | Placebo (n = 191) | |||
|---|---|---|---|---|
| Monthly (n = 188) | Quarterly (n = 190) | Total (n = 378) | ||
| Patients with at least one TEAEa | 116 (61.7) | 116 (61.1) | 232 (61.4) | 118 (61.8) |
| Patients with at least one TEAE potentially related to trial drug | 55 (29.3) | 61 (32.1) | 116 (30.7) | 54 (28.3) |
| Patients with at least one serious adverse event | 3 (1.6) | 1 (0.5) | 4 (1.1) | 1 (0.5) |
| Patients with any adverse event leading to discontinuation of the trial | 0 | 0 | 0 | 2 (1.0) |
| Death | 0 | 0 | 0 | 0 |
| Patients with adverse events reported in >2% of patients in any group | ||||
| Injection‐site reactions | 55 (29.3) | 51 (26.8) | 106 (28.0) | 48 (25.1) |
| Erythema | 29 (15.4) | 23 (12.1) | 52 (13.8) | 21 (11.0) |
| Induration | 33 (17.6) | 23 (12.1) | 56 (14.8) | 24 (12.6) |
| Pain | 14 (7.4) | 24 (12.6) | 38 (10.1) | 17 (8.9) |
| Pruritus | 10 (5.3) | 3 (1.6) | 13 (3.4) | 5 (2.6) |
| Infections and infestations | ||||
| Cystitis | 0 (0.0) | 4 (2.1) | 4 (1.1) | 1 (0.5) |
| Influenza | 4 (2.1) | 2 (1.1) | 6 (1.6) | 3 (1.6) |
| Nasopharyngitis | 30 (16.6) | 40 (21.1) | 70 (18.5) | 36 (18.8) |
| Back pain | 5 (2.7) | 1 (0.5) | 6 (1.6) | 1 (0.5) |
| Nausea | 2 (1.1) | 5 (2.6) | 7 (1.9) | 2 (1.0) |
| Diarrhea | 3 (1.6) | 4 (2.1) | 7 (1.9) | 0 (0.0) |
| Asthma | 2 (1.1) | 4 (2.1) | 6 (1.6) | 0 (0.0) |
| Protocol‐defined adverse events of special interest | ||||
| Cardiovascular events | 7 (3.7) | 5 (2.6) | 12 (3.2) | 4 (2.1) |
| Hepatic enzyme increased | 1 (0.5) | 2 (1.1) | 3 (0.8) | 1 (0.5) |
| Blood bilirubin increased | 0 | 0 | 0 | 1 (0.5) |
| Hy's law eventsb | 0 | 0 | 0 | 0 |
| Ophthalmic events of at least moderate severity | 0 | 0 | 0 | 0 |
| Anaphylaxis | 0 | 0 | 0 | 0 |
| Severe hypersensitivity reactions | 0 | 0 | 0 | 0 |
Adverse events were collected by coding in MedDRA version 22.0.
Treatment‐emergent adverse events, any adverse events that occurred after treatment started.
Defined as aspartate aminotransferase or alanine aminotransferase ≥3 × upper limit of normal (ULN) and total bilirubin ≥2 × ULN or International Normalized Ratio (INR) >1.5.
Regarding adverse events of special interest (Table 3), increases in hepatic enzymes were considered mild in all patients and drug‐induced liver injury was not observed in any group. Anaphylaxis or severe hypersensitivity also did not occur in any group. Cardiovascular events occurred in seven patients (3.7%) in the fremanezumab monthly group, five patients (2.6%) in the fremanezumab quarterly group, and four patients (2.1%) in the placebo group. There were no significant differences in the frequency of these events between fremanezumab‐ and placebo‐treated groups, and none of these events were deemed related to the trial drug.
There were no clinically significant changes in vital signs, weight, or ECG findings in any treatment group. Potentially clinically significant changes in laboratory values tended to occur in similar proportions of patients in each treatment group, and no adverse events associated with these changes were noted. Only one patient in the fremanezumab monthly group and one patient in the placebo group had a positive post‐baseline electronic Columbia‐Suicide Severity Rating Scale measure, and no suicide‐related events were noted in these patients. Antidrug antibodies were observed in 16 of 377 fremanezumab‐treated patients overall but were considered treatment‐related in only four patients with neutralizing antibodies observed in two patients.
DISCUSSION
Efficacy results of this Phase 3 trial in Japanese and Korean patients with CM demonstrated that fremanezumab provided a significant advantage over placebo with respect to the primary efficacy endpoint, namely the average number of headache days of at least moderate severity per month (approximately 1.7 days per month vs. placebo). Benefits were also seen in secondary efficacy endpoints, including the proportion of patients with ≥50% reduction in the monthly average number of headache days of at least moderate severity, the average number of days with use of any acute headache medication per month, the average monthly number of migraine days per month, and headache‐related disability. The onset of effects was rapid with differences between fremanezumab and placebo generally apparent at the first visit (4 weeks). This is important clinically as rapid onset of effect potentially averts early discontinuation by patients due to lack of perceived efficacy.
These efficacy results are highly similar to those of a previous Phase 2 and Phase 3 trial in patients with CM.11, 21 In the Phase 2 trial, an equivalent regimen to that of fremanezumab monthly in the present trial led to changes versus placebo in headache days of at least moderate severity of approximately −1.8 days per month and in migraine days of approximately −1.7 days (compared with approximately −2.1 days for fremanezumab monthly in the present trial).21 Similarly, equivalent fremanezumab monthly and quarterly regimens used in the Phase 3 trial led to comparable results for the primary endpoint with reductions in the average number of headache days of at least moderate severity per month versus placebo of −2.1 days per month for fremanezumab monthly and −1.8 days per month for fremanezumab quarterly.11 Results for corresponding secondary endpoints, including those related to disability, were also generally consistent with the present trial. Overall, this confirms the assumption that Japanese and Korean patients with CM respond similar to Caucasian patients, consistent with results of a Phase 1 trial including both Japanese and Caucasian patients.14
In terms of safety, most adverse events were mild or moderate in all treatment groups, and no patients who received fremanezumab discontinued treatment due to adverse events. The most common adverse events likely related to fremanezumab were injection‐site reactions, which occurred at a slightly higher frequency than in placebo recipients. As a monoclonal antibody, fremanezumab does not go through hepatic metabolism. Although some small‐molecule CGRP antagonists have shown hepatic issues,22 no hepatic safety signal was detected in fremanezumab studies. In the current trial, changes in hepatic laboratory parameters were infrequent, mild, and resolved by trial completion with no overt hepatic impairment observed in any patient. Endogenous CGRP is also a known vasodilator,23 and blocking CGRP could potentially impact the cardiovascular system.24 However, although several cardiovascular events were recorded as adverse events of special interest, almost all were considered mild and not related to trial medication, and no specific hemodynamic abnormalities or cardiac morbidity was observed with fremanezumab during this short‐term trial. These safety findings are also consistent with those from previous Phase 2 and Phase 3 studies in patients with CM,11, 21 as well as in earlier Phase 1 studies in healthy volunteers in which overt safety concerns, including vital signs and laboratory findings, did not emerge.25 These findings are also in line with a long‐term (12‐month) trial of quarterly and monthly fremanezumab in which no safety concerns emerged,26 and with the HALO long‐term trial in which patients with migraine received an additional 12‐month period of fremanezumab treatment.16
The main limitations of this trial are common to those noted previously in Phase 3 studies of fremanezumab in CM and EM.11, 15 These are the lack of inclusion of patients with more refractory disease or coexisting diseases and the short‐term evaluation period. Further studies are needed to determine the efficacy and safety of fremanezumab in a wider range of patients, including those with coexisting diseases, and over longer periods of follow‐up.
CONCLUSION
Fremanezumab is effective and well tolerated during the 3‐month trial treatment period in Japanese and Korean patients with CM to at least a similar extent as noted in previous international trials. Further, no safety concerns were raised in this population as was the case noted in previous international trials.
CONFLICT OF INTEREST
Fumihiko Sakai and Norihiro Suzuki report personal fees from Otsuka Pharmaceutical Co., Ltd. Takao Takeshima reports personal fees from Otsuka Pharmaceutical Co., Ltd. and Eisai Co. Ltd. Byung‐Kun Kim reports personal fees from Otsuka Pharmaceutical Co., Ltd.; consultation fees from Teva Korea and Sanofi Korea; consultation and lecture fees from Lundbeck Korea; and lecture fees from Lilly Korea, Allergan Korea, SK‐Pharma, and YuYu Pharma Inc. Hisaka Igarashi reports personal fees from Otsuka Pharmaceutical Co., Ltd., Pfizer Inc., Eisai Co. Ltd., Eli Lilly Japan K.K., Daiichi Sankyo Co. Ltd., Amgen K.K., and Lundbeck Japan K.K. Koichi Hirata reports personal fees from Otsuka Pharmaceutical Co., Ltd., Eli Lilly Japan K.k., Eisai Co., Pfizer Japan, Daiichi Sankyo Co. Ltd., MSD Co. Ltd., and Amgen Astellas BioPharma K.K. Ltd. Xiaoping Ning is a full‐time employee of Teva Branded Pharmaceutical Products R&D. Tomoko Shima, Miki Ishida, Katsuhiro Iba, Hiroyuki Kondo, and Nobuyuki Koga are full‐time employees of Otsuka Pharmaceutical Co., Ltd.
AUTHOR CONTRIBUTIONS
Study concept and design: Fumihiko Sakai, Norihiro Suzuki, Byung‐Kun Kim, Hisaka Igarashi, Koichi Hirata, Takao Takeshima, Xiaoping Ning, Tomoko Shima, Miki Ishida, Katsuhiro Iba. Acquisition of data: Fumihiko Sakai, Norihiro Suzuki, Byung‐Kun Kim, Hisaka Igarashi, Koichi Hirata, Takao Takeshima, Xiaoping Ning, Tomoko Shima, Miki Ishida, Katsuhiro Iba. Analysis and interpretation of data: Fumihiko Sakai, Norihiro Suzuki, Byung‐Kun Kim, Hisaka Igarashi, Koichi Hirata, Takao Takeshima, Xiaoping Ning, Tomoko Shima, Miki Ishida, Katsuhiro Iba. Drafting of the manuscript: Fumihiko Sakai, Norihiro Suzuki, Byung‐Kun Kim, Hisaka Igarashi, Koichi Hirata, Takao Takeshima, Xiaoping Ning, Tomoko Shima, Miki Ishida, Katsuhiro Iba, Hiroyuki Kondo, Nobuyuki Koga. Revising it for intellectual content: Fumihiko Sakai, Norihiro Suzuki, Byung‐Kun Kim, Hisaka Igarashi, Koichi Hirata, Takao Takeshima, Xiaoping Ning, Tomoko Shima, Miki Ishida, Katsuhiro Iba, Hiroyuki Kondo, Nobuyuki Koga. Final approval of the completed manuscript: Fumihiko Sakai, Norihiro Suzuki, Byung‐Kun Kim, Hisaka Igarashi, Koichi Hirata, Takao Takeshima, Xiaoping Ning, Tomoko Shima, Miki Ishida, Katsuhiro Iba, Hiroyuki Kondo, Nobuyuki Koga.
CLINICAL TRIALS REGISTRATION NUMBER
INSTITUTIONAL REVIEW BOARD APPROVAL
Approval was granted by the relevant institutional review board or independent ethics committee/ethics committee that approved the trial protocol.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank all patients for their participation in the trial, and all trial sites, investigators, and all clinical research staff for their contributions. We also thank Yoshiko Okamoto, PhD, and Mark Snape, MBBS, of inScience Communications, Springer Healthcare, for medical writing assistance, which was funded by Otsuka Pharmaceutical Co., Ltd. All authors contributed to writing the Introduction and Discussion sections. Yasutomo Yutoku, of inScience Communications, Springer Healthcare, provided editorial assistance limited to coordinating revisions from the authors and submitting the final manuscript, which was funded by Otsuka Pharmaceutical Co., Ltd.
Sakai F, Suzuki N, Kim B‐K, et al. Efficacy and safety of fremanezumab for chronic migraine prevention: Multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group trial in Japanese and Korean patients. Headache. 2021;61:1092–1101. 10.1111/head.14169
Funding information
This work was supported by Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan
DATA AVAILABILITY STATEMENT
Anonymized individual participant data that underlie the results of this study will be shared with researchers to achieve aims prespecified in a methodologically sound research proposal.
REFERENCES
- 1.Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 2.Natoli JL, Manack A, Dean B, et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010;30:599‐609. [DOI] [PubMed] [Google Scholar]
- 3.Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second International Burden of Migraine Study (IBMS‐II). Headache. 2013;53:644‐655. [DOI] [PubMed] [Google Scholar]
- 4.Bonafede M, Wilson K, Xue F. Long‐term treatment patterns of prophylactic and acute migraine medications and incidence of opioid‐related adverse events in patients with migraine. Cephalalgia. 2019;39:1086‐1098. [DOI] [PubMed] [Google Scholar]
- 5.Hepp Z, Dodick DW, Varon SF, Gillard P, Hansen RN, Devine EB. Adherence to oral migraine‐preventive medications among patients with chronic migraine. Cephalalgia. 2015;35:478‐488. [DOI] [PubMed] [Google Scholar]
- 6.Woolley JM, Bonafede MM, Maiese BA, Lenz RA. Migraine prophylaxis and acute treatment patterns among commercially insured patients in the United States. Headache. 2017;57:1399‐1408. [DOI] [PubMed] [Google Scholar]
- 7.Loder EW, Rizzoli P. Tolerance and loss of beneficial effect during migraine prophylaxis: clinical considerations. Headache. 2011;51:1336‐1345. [DOI] [PubMed] [Google Scholar]
- 8.Ueda K, Ye W, Lombard L, et al. Real‐world treatment patterns and patient‐reported outcomes in episodic and chronic migraine in Japan: analysis of data from the Adelphi migraine disease specific programme. J Headache Pain. 2019;20:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338‐350. [DOI] [PubMed] [Google Scholar]
- 10.Edvinsson L. Role of CGRP in migraine. In: Brain SD, Geppetti P, eds. Calcitonin Gene‐Related Peptide (CGRP) Mechanisms Handbook of Experimental Pharmacology. Cham: Springer International Publishing; 2019:121‐130. [DOI] [PubMed] [Google Scholar]
- 11.Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377:2113‐2122. [DOI] [PubMed] [Google Scholar]
- 12.Kosinski M, Bayliss MS, Bjorner JB, et al. A six‐item short‐form survey for measuring headache impact: the HIT‐6. Qual Life Res. 2003;12:963‐974. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double‐blind, placebo‐controlled, phase 3b trial. Lancet. 2019;394:1030‐1040. [DOI] [PubMed] [Google Scholar]
- 14.Cohen‐Barak O, Weiss S, Rasamoelisolo M, et al. A phase 1 study to assess the pharmacokinetics, safety, and tolerability of fremanezumab doses (225 mg, 675 mg and 900 mg) in Japanese and Caucasian healthy subjects. Cephalalgia. 2018;38:1960‐1971. [DOI] [PubMed] [Google Scholar]
- 15.Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319:1999‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goadsby PJ, Silberstein SD, Yeung PP, et al. Long‐term safety, tolerability, and efficacy of fremanezumab in migraine: a randomized study. Neurology. 2020;95:e2487‐e2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629‐808. [DOI] [PubMed] [Google Scholar]
- 18.Silberstein S, Tfelt‐Hansen P, Dodick DW, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia. 2008;28:484‐495. [DOI] [PubMed] [Google Scholar]
- 19.Mundt JC, Greist JH, Gelenberg AJ, Katzelnick DJ, Jefferson JW, Modell JG. Feasibility and validation of a computer‐automated Columbia‐Suicide Severity Rating Scale using interactive voice response technology. J Psychiatr Res. 2010;44:1224‐1228. [DOI] [PubMed] [Google Scholar]
- 20.Bigal ME, Edvinsson L, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV‐48125 for preventive treatment of chronic migraine: a multicentre, randomised, double‐blind, placebo‐controlled, phase 2b study. Lancet Neurol. 2015;14:1091‐1100. [DOI] [PubMed] [Google Scholar]
- 21.Bigal ME, Dodick DW, Krymchantowski AV, et al. TEV‐48125 for the preventive treatment of chronic migraine: efficacy at early time points. Neurology. 2016;87:41‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holland PR, Goadsby PJ. Targeted CGRP small molecule antagonists for acute migraine therapy. Neurotherapeutics. 2018;15:304‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Struthers AD, Brown MJ, Macdonald DW, et al. Human calcitonin gene related peptide: a potent endogenous vasodilator in man. Clin Sci (Lond). 1986;70:389‐393. [DOI] [PubMed] [Google Scholar]
- 24.Rubio‐Beltrán E, van den Brink AM . Understanding CGRP and cardiovascular risk. Handb Exp Pharmacol. 2019;255:131‐140. [DOI] [PubMed] [Google Scholar]
- 25.Bigal ME, Escandon R, Bronson M, et al. Safety and tolerability of LBR‐101, a humanized monoclonal antibody that blocks the binding of CGRP to its receptor: results of the Phase 1 program. Cephalalgia. 2014;34:483‐492. [DOI] [PubMed] [Google Scholar]
- 26.Ning X, Cohen J, Bennett N, Yeung P, Yang R. Long‐term safety of fremanezumab: results of a 1‐year study. Neurology. 2019;92:P1.10‐015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Anonymized individual participant data that underlie the results of this study will be shared with researchers to achieve aims prespecified in a methodologically sound research proposal.


