Abstract
Forty-six VanA glycopeptide-resistant enterococci (GRE) from a single patient were investigated for variation in structure and location of VanA resistance elements. Together with identification to species level and pulsed-field gel electrophoresis, these data divided the GRE into 10 groups and subgroups. Combining data in this manner appears helpful when investigating the epidemiology of GRE.
Glycopeptide-resistant enterococci (GRE) with the transmissible VanA phenotype (i.e., resistant to both vancomycin and teicoplanin) were first isolated in 1986 in the United Kingdom (10) and in France (5) and have subsequently been reported widely as a cause of nosocomial infections in the United States and Europe (13). In its prototypic GRE strain, Enterococcus faecium BM4147, the VanA phenotype is conferred by a 10.8-kb transposon, Tn1546 (1), which contains the vanA gene cluster. Other enterococci with the VanA phenotype contain elements that are indistinguishable from or related to Tn1546, but variation arises in some representatives with the insertion of mobile elements into intergenic regions of elements related to Tn1546 (12).
Pulsed-field gel electrophoresis (PFGE) is regarded by many as the “gold standard” for epidemiological comparison of GRE. Nevertheless, epidemiological studies using this method have yielded discordant results. Bonten et al. (2) used PFGE data to suggest that there is little genetic variation among isolates from individual patients who have been colonized for protracted periods with GRE, whereas Schoonmaker et al. (7) recently showed genetic diversity among multiple GRE isolated serially from two patients. Moreover, PFGE does not provide data on the relatedness of the VanA elements themselves with regards to either their structure or location, and it is possible that insertion of transposons or mobile elements into the chromosomes of enterococci may result in significant changes in PFGE profile between related strains (4, 9).
The aim of this study, therefore, was to assess the variability in the structure and location of VanA elements among 46 GRE isolated from multiple fecal screens of one patient and to compare these data with previously determined PFGE types.
Forty-six GRE with the VanA phenotype were isolated from 17 fecal screens of a single patient on the hematology ward at Addenbrooke’s Hospital, Cambridge, United Kingdom, over a 12-week period during which the patient received vancomycin therapy discontinuously. Fecal screens were done as part of a survey to assess the epidemiology of GRE on our hematology unit by plating onto a selective medium containing 4 mg of vancomycin liter−1, and multiple picks of enterococci were selected for identification and sensitivity testing. No screens were negative for enterococci. The 46 GRE isolates had been previously identified to the species level by PCR, their DNA was subjected to PFGE, and the MICs of vancomycin and teicoplanin were determined. All isolates showed high-level resistance to vancomycin and teicoplanin, in accordance with the VanA phenotype, and they comprised 15 Enterococcus faecalis isolates with a single PFGE type and 31 E. faecium isolates distributed among five PFGE types. PFGE profiles were interpreted in accordance with the criteria described by Tenover et al. (8), and details are given in Table 1. Twelve fecal screens contained multiple strains of GRE, and eight screens contained a mixture of E. faecalis and E. faecium.
TABLE 1.
Division of 46 GRE isolates from a single patient into 10 groups and subgroups by combination of identification to species level, PFGE subtype and structure, and location of VanA element
| GRE isolate group | Species | PFGE typea | Date first isolated (mo/day/yr) | Date last isolated (mo/day/yr) | VanA elementb | No. of isolates in group | Subgroups | Hybridization with vanA probe | No. of isolates |
|---|---|---|---|---|---|---|---|---|---|
| 1 | E. faecium | i | 9/28/95 | 11/23/95 | H | 13 | Chromosomal band only | 13 | |
| 2 | E. faecium | ii | 9/28/95 | 10/19/95 | H | 11 | Chromosomal band only | 11 | |
| 3 | E. faecalis | iii | 10/19/95 | 12/18/95 | H | 13 | a | Chromosomal band only | 8 |
| b | Plasmid of ca. 35 MDa and chromosomal band | 1 | |||||||
| c | Plasmid of ca. 70 MDa and chromosomal band | 3 | |||||||
| 4 | E. faecium | iv | 11/3/95 | 12/12/95 | H | 3 | Chromosomal band only | 3 | |
| 5 | E. faecalis | iii | 11/21/95 | 11/23/95 | U | 2 | a | Plasmid of ca. 35 MDa and chromosomal band | 1 |
| b | Plasmid of ca. 70 MDa and chromosomal band | 1 | |||||||
| 6 | E. faecium | v | 12/8/95 | 12/18/95 | H | 3 | Chromosomal band only | 3 | |
| 7 | E. faecium | vi | 12/18/95 | W | 1 | Plasmid of ca. 35 MDa and chromosomal band | 1 |
PFGE profiles were analyzed according to the criteria described by Tenover et al. (8). Profiles of types i and vi differed by four to six bands, suggesting that they were possibly related; the same was true for types ii and iv. All other types differed by more than six bands.
Groups are in accordance with previous designations (12).
Overlapping fragments of VanA elements were amplified by using 10 pairs of PCR primers (1, 12) and scored for the presence or absence of amplicons and the size of each amplicon compared with that obtained from Tn1546. The patterns thereby detected were compared with those of groups A to X as defined in a previous study of VanA enterococci (12). E. faecium BM4147, which contains Tn1546 on plasmid pIP816, and its glycopeptide-sensitive derivative BM4147-1 were used as positive and negative controls, respectively. Plasmids were extracted by an alkaline lysis technique (11), and profiles were compared after electrophoresis on 0.8% agarose gels. The sizes of plasmids were measured by comparing their mobility with those of plasmids of known size from Escherichia coli V517 (NCTC 50193) and 39R861 (NCTC 50192). Plasmid DNA was Southern blotted onto nylon membranes (Hybond N; Amersham Life Sciences, Little Chalfont, United Kingdom) with a VacuGene vacuum blotting apparatus (Pharmacia-LKB, Milton Keynes, United Kingdom), and the blots were hybridized with a digoxigenin-labelled vanA-specific probe under stringent conditions (3).
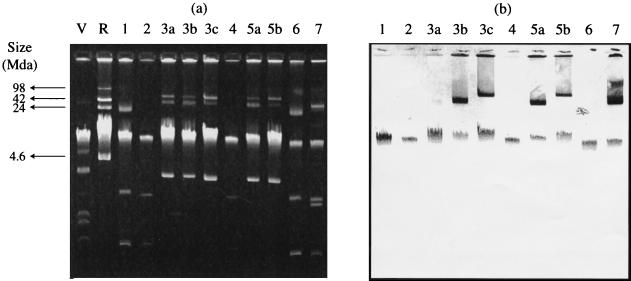
For the 46 enterococci investigated, group H VanA elements were found to be the most common, occurring in 30 E. faecium isolates distributed among four PFGE types and in 13 E. faecalis isolates (Table 1). One E. faecium isolate contained a group W element, and two E. faecalis isolates contained group U elements. The combination of PFGE type and VanA element data sets allowed division of the 46 enterococci into seven groups (Table 1). Hybridization with a vanA probe demonstrated that some isolates had plasmid-borne VanA elements whereas others showed evidence of elements located only on the chromosome. The combination of PCR and hybridization data with PFGE typing allowed the further subdivision of the E. faecalis isolates into five distinct subgroups (Table 1; Fig. 1). One E. faecalis isolate with a type H VanA element failed repeatedly to give any band upon hybridization with the vanA probe. All of the elements identified were highly transferable, including those that were chromosomal (data not shown).
FIG. 1.
(a) Plasmid profiles of the 10 GRE groups and subgroups identified in Table 1; (b) Southern blot corresponding to panel a showing hybridization of these strains with a vanA probe. V, E. coli V517; R, E. coli 39R861.
This study demonstrates that a single patient may be colonized by GRE of distinct species, with several PFGE types, and containing a wide variety of VanA elements. Moreover, isolates with identical PFGE types may contain different VanA elements as determined by the overlapping PCR techniques. It is thought that the variation in VanA elements reflects migration of insertion sequences. These data confirm previous findings with isolates of an epidemic VanA strain (12). It is unclear how significant the variations in structure of VanA elements are for epidemiological investigations or how stable they are. Also, it is uncertain whether and, if so, how the presence of different transposons carrying vanA-mediated resistance may alter the PFGE profile of enterococci.
Forty-three of our isolates carried a group H VanA element, which was the most common type of element among human isolates in a previous analysis of 107 enterococci from hospital patients and nonhuman sources (12). Previous studies suggest that group H elements are carried on the chromosome, while U and W elements reside on plasmids (6). In this study, two groups of E. faecalis isolates that contained H elements also contained plasmids that hybridized with the vanA probe (groups 3b and 3c) (Table 1). These strains may carry more than one VanA element, and a chromosomal H element, giving multiple amplicons with the overlapping PCR primers, might mask another element carried elsewhere and giving fewer amplicons, such as I, J, K, O, R, T, U, V, or X (12). Of these latter types, R, T, U, V, and X elements have previously been shown to be carried on plasmids (6). Conjugation experiments and analysis of transconjugants by PCR and hybridization might demonstrate the carriage of multiple VanA elements in a single donor strain. It is unclear why one isolate of E. faecalis failed to hybridize with the vanA probe. This requires further investigation, since the strain appeared to remain highly resistant to vancomycin by the E test (AB Biodisk, Solna, Sweden) and was shown to contain a group H VanA element by the overlapping PCR method.
These data suggest that analysis of the structure and location of VanA elements may provide useful epidemiological information on the relatedness of GRE, especially as an adjunct to PFGE typing. However, further work must be undertaken to assess the stability and transferability of VanA elements and the possibility that some isolates of GRE may carry multiple VanA elements.
REFERENCES
- 1.Arthur M, Molinas C, Depardieu F, Courvalin P. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1993;175:117–127. doi: 10.1128/jb.175.1.117-127.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonten M J M, Hayden M K, Nathan C, Rice T W, Weinstein R A. Stability of vancomycin-resistant enterococcal genotypes isolated from long-term colonised patients. J Infect Dis. 1998;177:378–382. doi: 10.1086/514196. [DOI] [PubMed] [Google Scholar]
- 3.Garaizar J, Kaufmann M E, Pitt T L. Comparison of ribotyping with conventional methods for type identification of Enterobacter cloacae. J Clin Microbiol. 1991;29:1303–1307. doi: 10.1128/jcm.29.7.1303-1307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Handwerger S, Skoble J. Identification of chromosomal mobile element conferring high-level vancomycin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1995;39:2446–2453. doi: 10.1128/aac.39.11.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leclerq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988;19:157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- 6.Palepou M I, Adebiyi A A, Tremlett C H, Jensen L B, Woodford N. Molecular analysis of diverse elements mediating VanA glycopeptide resistance in enterococci. J Antimicrob Chemother. 1998;42:605–612. doi: 10.1093/jac/42.5.605. [DOI] [PubMed] [Google Scholar]
- 7.Schoonmaker D J, Bopp L H, Baltch A L, Smith R P, Rafferty M E, George M. Genetic analysis of multiple vancomycin-resistant Enterococcus isolates obtained serially from two long-term care patients. J Clin Microbiol. 1998;36:2105–2108. doi: 10.1128/jcm.36.7.2105-2108.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B A, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thal L A, Silverman J, Donabedian S, Zervos M J. The effect of Tn916 insertions on contour-clamped homogeneous electrophoresis patterns of Enterococcus faecalis. J Clin Microbiol. 1997;35:969–972. doi: 10.1128/jcm.35.4.969-972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uttley A H C, Collins C H, Naidoo J, George R C. Vancomycin-resistant enterococci. Lancet. 1988;i:57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- 11.Uttley A H C, George R C, Naidoo J, Woodford N, Johnson A P, Collins C H, Morrison D, Gilfillan A J, Fitch L E, Heptonstall J. High-level vancomycin-resistant enterococci causing hospital infections. Epidemiol Infect. 1989;103:173–181. doi: 10.1017/s0950268800030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodford N, Adebiyi A A, Palepou M I, Cookson B D. Diversity of VanA glycopeptide resistance elements in enterococci from humans and nonhuman sources. Antimicrob Agents Chemother. 1998;42:502–508. doi: 10.1128/aac.42.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodford N, Johnson A P, Morrison D, Speller D C E. Current perspectives on glycopeptide resistance. Clin Microbiol Rev. 1995;8:585–615. doi: 10.1128/cmr.8.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]