Summary
Due to an increasing scarcity of pancreases with optimal donor characteristics, islet isolation centers utilize pancreases from extended criteria donors, such as from donation after circulatory death (DCD) donors, which are particularly susceptible to prolonged cold ischemia time (CIT). We hypothesized that hypothermic machine perfusion (HMP) can safely increase CIT. Five human DCD pancreases were subjected to 6 h of oxygenated HMP. Perfusion parameters, apoptosis, and edema were measured prior to islet isolation. Five human DBD pancreases were evaluated after static cold storage (SCS). Islet viability, and in vitro and in vivo functionality in diabetic mice were analyzed. Islets were isolated from HMP pancreases after 13.4 h [12.9–14.5] CIT and after 9.2 h [6.5–12.5] CIT from SCS pancreases. Histological analysis of the pancreatic tissue showed that HMP did not induce edema nor apoptosis. Islets maintained >90% viable during culture, and an appropriate in vitro and in vivo function in mice was demonstrated after HMP. The current study design does not permit to demonstrate that oxygenated HMP allows for cold ischemia extension; however, the successful isolation of functional islets from discarded human DCD pancreases after performing 6 h of oxygenated HMP indicates that oxygenated HMP may be a useful technology for better preservation of pancreases.
Keywords: donation after circulatory death, hypothermic machine perfusion, islet isolation
Doppenberg and Leemkuil et al. demonstrate clinically relevant human pancreatic islet isolation results, in vitro and in vivo, after six hours oxygenated hypothermic machine perfusion of discarded DCD pancreases during transport.

Introduction
Allogeneic transplantation of pancreatic islets is an effective treatment for a selected group of patients with long‐standing type 1 diabetes mellitus. Using modern immunosuppression regimes, improved glycemic control can be achieved in most transplanted patients [1, 2]. In the Eurotransplant region, pancreases are preferentially offered for vascularized pancreas transplantation, leaving pancreases with less favorable donor characteristics for islet isolation [3]. This has led to greater numbers of pancreases accepted for isolation from extended criteria donors (ECDs) such as donation after circulatory death donors (DCDs) [4]. In fact, in the Netherlands DCD procedures accounted for 34% of all multi‐organ donation procedures in 2010, which increased to 59% in 2019 [5]. There are perceived added risks for the utilization of DCD organs as DCD kidneys and livers have been shown to be more susceptible to the harmful effects of warm and cold ischemia [6, 7, 8, 9]. Islet isolation from DCD donors has shown to result in lower yields compared with DBD pancreases (87 000–100 000 IEQ less) [10, 11]. Therefore, donor characteristics are often more favorable for DCD pancreases: younger age, more often male donors, without a recent history of cardiac arrest, leading to comparable viability, and function after islet isolation [10]. Prolonged cold ischemia time (CIT) has been demonstrated to be an independent risk factor for technical failure after pancreas transplantation [12, 13, 14]. It is hypothesized that these harmful effects are even more pronounced in DCD pancreases [14, 15]. Coupled with cold ischemia, during islet isolation from a donor pancreas, islets are lost because of exposure to multiple physiochemical stress factors [16, 17]. One possibility to extend the CIT without sacrificing quality is to decrease injury during preservation [18]. Hypothermic machine perfusion (HMP) has been proposed as an alternative strategy to fulfill this role. HMP is shown to decrease delayed graft function (DGF) after kidney transplantation and to improve the quality of higher risk livers prior to transplantation [19, 20, 21, 22, 23]. Recently, it was reported that adequate preservation of pancreatic tissue could be achieved by oxygenated HMP [24, 25], but an extensive examination of the effects of oxygenated HMP in human islet isolation and during the subsequent days in culture is still lacking. We hypothesized that we can safely extend CIT of human DCD pancreases by performing 6 h of oxygenated HMP.
Methods
Donor selection
Ten human donor pancreases procured from multi‐organ donors in the Netherlands were included in this study. For this study, a statement of no objection was given by the Medical Ethical Committee of the University Medical Center Groningen (METc2012‐438). The clinical and research activities were in line with the principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.” To study the effect of oxygenated HMP on the islets of marginal donor pancreases, five discarded DCD organs were included. After 6 h of oxygenated HMP, islets were isolated, and after 3–4 days of culture, islets were tested for viability and functionality, both in vitro and in vivo. Also, five discarded DBD pancreases preserved by static cold storage (SCS) were studied. Table 1 summarizes donor demographics.
Table 1.
Donor characteristics of pancreases from the SCS and HMP groups
| Donor variable | HMP | SCS |
|---|---|---|
| DCD/DBD | 5/0 | 0/5 |
| Cause of death |
Postanoxia 3/5 iCVA 1/5 Euthanasia 1/5 |
SAB 4/5 Trauma capitis 1/5 |
| Cardiac arrest | 4/5 | 1/5 |
| Duration cardiac arrest | 18 min [14–30] | 20 min |
| Age (years) | 50 [33.5–54.5] | 64 [47–68] |
| Sex (M/F) | 4/1 | 2/3 |
| Weight (kg) | 83 [67–95] | 82 [57.5–89.5] |
| Height (cm) | 185 [180–187.5] | 169 [162.5–176] |
| BMI (kg/m2) | 23 [19.5–29] | 28 [20.5–30.5] |
| Donor amylase (U/l) | 46.5[27.3–89] | 77 [44.8–128] |
| fWIT (min) | 26 [24.5–30.5] | ‐ |
| CIT (hours) | 13.4 [12.9–14.5] | 9.2 [6.5–12.5] |
| Pancreas mass (grams) | 96 [76–121.5] | 103 [81.5–127] |
Donor demographics for islet isolations after HMP group (n = 5) and after SCS group (n = 5). Variables are described as median and interquartile range.
BMI, body mass index; CIT, cold ischemia time (start of aortic preservation solution flush until start enzymatic perfusion); DBD, donation after brain death; DCD, donation after circulatory death; fWIT, functional warm ischemia time (O2 saturation <80% or MAP <50 mmHg until cardiac arrest, minutes); HMP, hypothermic machine perfusion; iCVA, ischemic cerebrovascular accident; SAB, subarachnoid bleeding; SCS, static cold storage.
Organ procurement and transport
The pancreases were procured by the regional multi‐organ recovery teams according to the no‐touch technique as described earlier [26]. Initially, all pancreases were flushed and stored by SCS in the University of Wisconsin Cold Storage Solution (UWS; Bridge to Life, London, UK). The DCD pancreases were transported under hypothermic conditions in UWS to the machine perfusion facility at the University Medical Center Groningen (UMCG) to prepare for perfusion. Oxygenated HMP was performed during transport to the islet isolation facility at the Leiden University Medical Center (LUMC). DBD pancreases were directly transported (SCS) to the islet isolation facility at the LUMC. CIT was calculated as time from the start of aortic cold flush in the donor to the initiation of ductal enzymatic perfusion in the pancreas, therefore including oxygenated HMP.
Oxygenated hypothermic machine perfusion
Upon arrival at the UMCG, the pancreases were prepared to be connected to the transportable pressure‐controlled dual perfusion device (Fig. 1) as described earlier [24]. In short, a splenectomy was performed, the gastroduodenal artery was ligated and the superior mesenteric artery and the splenic artery were cannulated for separate perfusion at 25 mmHg. The portal vein was left open to ensure passive drainage of perfusion fluid back into the reservoir. One liter of University of Wisconsin Machine Perfusion Solution (UW‐MPS; Bridge to Life, London, UK) was used, which was oxygenated by delivery of 100% O2 through the hollow fiber oxygenators with a fixed flow of 100 ml/min. The pancreas was placed in a plastic netted organ holder inside an insulated box surrounded by melting ice. Ambient temperature was maintained between 4° and 7°C during the entire transport.
Figure 1.

Transportable dual arterial oxygenated hypothermic machine perfusion device. The perfusion system consists of two separate centrifugal pumps (1), two hollow fiber oxygenators (2), perfusion pressure, flow and temperature sensors (3), an organ holder (4) in an insulated container filled with melting ice (5).
Markers of edema and apoptosis
Upon arrival at the isolation facility, independent investigators examined HMP and SCS pancreases for visible macroscopic edema. Tissue samples (ventral head and tail) were taken from the HMP pancreases just before the start of HMP and from both HMP and SCS group shortly before islet isolation, using a 12G needle biopsy (Bio‐feather; Medax, San Possidonio, Italy). The samples were fixed in 4% paraformaldehyde, subsequently embedded in paraffin, and cut into sections of 4 µm. Light microscopy of hematoxylin and eosin (H&E)‐stained sections were performed to evaluate changes in morphology. Edema was quantified using a custom‐made logarithm in Image‐Pro Premier 9.1 (Media Cybernetics, Silver Springs, MD), segmenting interstitial tissue area compared with total area. Immunofluorescent staining of insulin (Santa Cruz, Heidelberg, Germany; Rabbit, 1:100) and caspase‐3 (Cell Signaling, Leiden, the Netherlands; Asp175, Rabbit, 1:100) was performed to identify islets and evaluate the extent of apoptosis in the islets. For each slide, the percentage of cells in an islet staining positive was graded in in a semi‐quantitative scale: grade 0 (no caspase‐3 staining), grade 1 (<50% of an islet stained positive for caspase‐3), and grade 2 (≥50% of the islet stained positive for caspase‐3). The H‐score of each slide was calculated using the following formula: [1x (% cells grade 0) + 2x (% cells grade 1) + 3x (% cells grade 3) ].
Islet isolations
Pancreatic islet isolations were performed at the LUMC using an adapted version of the semi‐automated method as described earlier [10]. Isolation protocols for both HMP‐ and SCS‐preserved pancreases were identical. The percentage of digested tissue was calculated by the mass of the pancreas after peri‐pancreas tissue dissection (pancreas mass) minus the tissue mass remaining in the Ricordi chamber after digestion, divided by the pancreas mass. Embedded and nonembedded islets were counted from images taken from samples (>20 islets) of each fraction after purification. When ≥50% of the perimeter of an islet was surrounded by exocrine tissue, it was considered embedded. The fraction of embedded islets was then multiplied by the IEQ of that fraction and divided by the total IEQ of the isolation and was added to all other fractions to achieve the percentage of embedded islets. A second, blinded investigator confirmed the results.
Islet culture
Isolated islets were cultured in fractions based on purity and amount of embedding, using CMRL 1066 (Mediatech, Herndon, VA, USA), supplemented with 10% human serum, 10 mM HEPES, 2 mM l‐glutamine, 50 µg/ml gentamycin, 0.25 µg/ml fungizone (GIBCO BRL), and 20 µg/ml ciprofloxacin (Bayer Healthcare AG, Leverkusen, Germany) in platelet culture bags (Terumo BCT Accessory Platelet Bag 70300; Lakewood, CO, USA) at 37⁰C in 5% CO2. Islet yield (in islet equivalents, IEQ) [27] was determined after isolation (day 0), after the first medium change one day after isolation (day 1), and after the second medium change three days after isolation (day 3).
Glucose‐stimulated insulin secretion test
In vitro functionality of isolated islets (in duplo of ±20 islets) was tested after one day of culture via a dynamic glucose‐stimulated insulin secretion (GSIS) test using a Brandel Suprafusion 1000 System (Brandel, Gaithersburg, USA), as previously described [27]. The insulin concentration at each time point was then divided by the average insulin concentration (Human Insulin ELISA Kit 10‐1113‐01; Mercodia AB, Uppsala, Sweden) of the last three insulin concentrations during the second low‐glucose phase. This stimulation index per time point was averaged for HMP and SCS pancreases. To calculate the area under the curve of the stimulation indices, the stimulation index curves were integrated over time.
Islet viability
Islets preparations were assessed for viability on day 0, day 1, and day 3 using a fluorescein diacetate–propidium iodide (FDA‐PI) staining. Briefly, an aliquot of approximately 50 islets was obtained from the purest fraction and washed in 15 ml Ringer’s acetate solution (B. Braun, Melsungen, Germany). FDA (0.67 µg/l) and PI (4 µg/l) were added simultaneously and incubated at room temperature for 30 min under dark conditions. Using a fluorescent microscope, the percentage of living cells (FDA‐positive) to dead cells (PI‐positive) in each islet was determined. Using a grading system, the viability of the preparation was quantified [28].
Islet transplantation in diabetic mice
Diabetes was induced by intraperitoneal injection of streptozotocin (220 mg/kg) in 6‐ to 8‐week‐old male immunodeficient (NOD.CB17‐prkdc<scid>/J, NOD‐SCID) mice (Charles River, L'arbresle, France). Weight and blood glucose measurements (from tail vein blood) were taken three times a week, and mice were considered diabetic after two consecutive measurements >20 mmol/l glucose. On day 3 after islet isolation, a pellet of 3000 IEQ of the purest fraction was obtained and washed in CMRL 1066 without additives. One to four mice per isolation were anesthetized with 5% isoflurane (maintenance 2% isoflurane). Buprenorphine (0.1 mg/kg) was given as analgesia. A small incision was made in the renal capsule where the islet pellet was implanted. At day 28 post‐transplantation, a nephrectomy of the kidney containing the transplant was performed and two additional blood glucose measurements were taken in the following three days to monitor a return to hyperglycemia.
In vivo functionality
At 28 days post‐transplant, all mice underwent an intraperitoneal glucose tolerance test. After 4 h of fasting, two grams of glucose (dissolved in phosphate‐buffered saline) per kilogram body weight was injected into the peritoneum. Blood samples were taken from the tail vein at 0, 15, 30, 60, and 120 min after glucose administration for the measurement of glucose and insulin concentrations. Plasma insulin concentration was determined analogously to the GSIS measurements (Human Insulin ELISA Kit 10‐1113‐01; Mercodia AB, Uppsala, Sweden).
Statistical analysis
Continuous variables are presented as median and interquartile range (IQR), unless otherwise specified. Wilcoxon signed‐rank tests were performed to compare related samples. IBM SPSS Statistics version 23 for Windows was used in analyses regarding perfusion flow and apoptosis, and GraphPad Prism 8.1.1 (La Jolla, CA, USA) was used in all analyses regarding donor characteristics, pancreas graft edema, and islet isolation.
Results
Donor characteristics
Three out of five DCD donors suffered from postanoxic encephalopathy, one from an ischemic cerebrovascular accident, and one died after euthanasia. Four out of five DCD donors experienced cardiac arrest with resuscitation with a median time of 18 min [14–30]. Donors in the HMP group were 185 cm [180–187.5] and that in the SCS group were 169 cm [162.5–176]. Pancreases in the HMP group were preserved for 13.4 h [12.9–14.5] (including 6 h of oxygenated HMP), and in the SCS group, pancreases were stored for 9.2 h [6.5–12.5] prior to isolation. Donor characteristics are shown in Table 1.
Machine perfusion
Pancreases were connected to the machine perfusion device by two investigators directly after arrival at the UMCG. Median SCS time before the start of oxygenated HMP was 356 min [296–437]. In the first 30 min, flow in the SA and in the SMA increased, after which it stabilized to 31 ml/min [26–38] and 37 ml/min [20–44] in the SA and SMA, respectively, P < 0.05 (Fig. 2).
Figure 2.
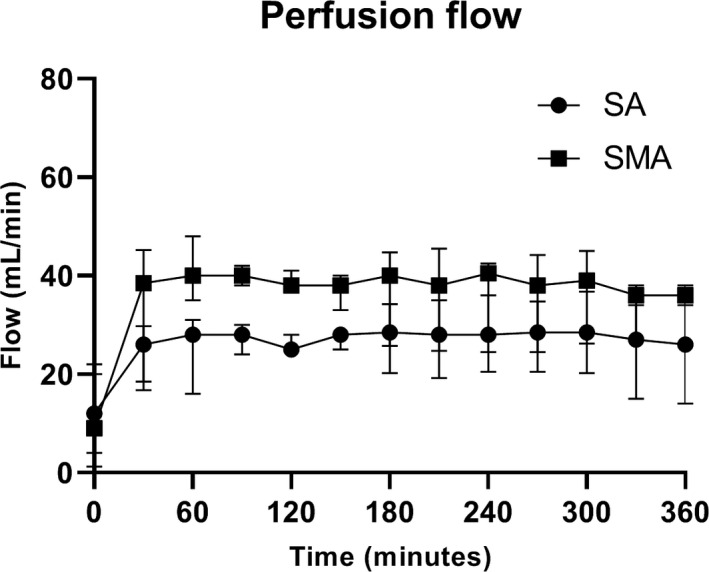
Average flow rates through the splenic artery and superior mesenteric artery during HMP. Perfusion flow in both arteries stabilized after 30 min of perfusion and remained so during 6 h of machine perfusion. Flow in the SMA was higher than in the SA in all DCD pancreases. SA, splenic artery; SMA, superior mesenteric artery.
Histology
Macroscopically, none of the pancreases were considered to be edematous, postprocurement, and during and after HMP or SCS. Figure 3 shows a representative example of an oxygenated HMP pancreas prior to isolation. The change in percentage of interstitial space (either post‐HMP—postprocurement or post‐SCS—postprocurement) reflecting microscopic edema did not change significantly (an increase of 0.45 ± 3.49% after oxygenated HMP and a decrease of 2.4 ± 4.93% after SCS, Fig. 4). The percentage of caspase‐3‐positive islets was described using H‐scores, as a measure of amount and intensity of caspase‐3 staining per slide. In the HMP group, no significant difference was observed before and after 6 h of oxygenated HMP. The H‐scores were 19 (11–85) and 32 (12–40) before and after HMP, respectively (P = 0.128). The H‐score in the SCS group before islet isolation was 26 (33–69).
Figure 3.

Macroscopic aspect of the pancreas directly after hypothermic machine perfusion. No visible indication of edema formation after machine perfusion was seen.
Figure 4.
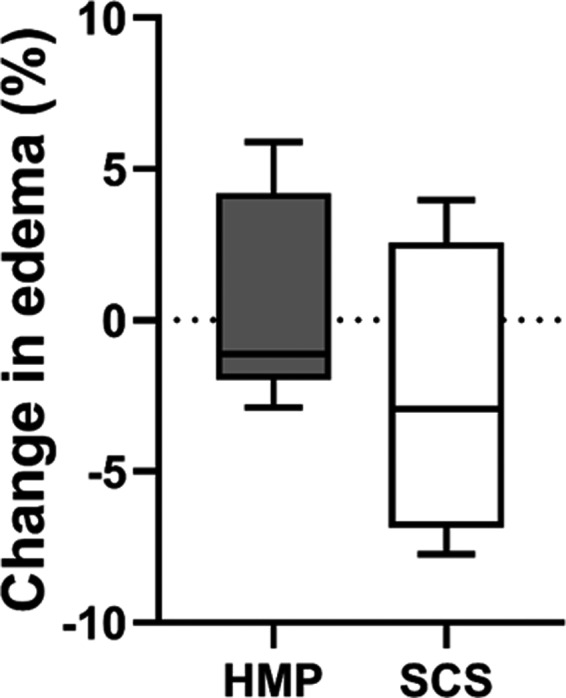
Change in percentage of edema in HMP and SCS pancreases. Edema was measured as a percentage of interstitial space divided by the total area of tissue measured after H&E staining. The difference in percentage after and before either HMP or SCS is shown. HMP, hypothermic machine perfusion; SCS, static cold storage. Results are shown as box‐and‐whisker median ± interquartile range.
Islet isolation
Pancreatic islets were isolated from HMP pancreases without any necessity to adjust the isolation in technique. 91% [85.5–99%] of the initial pancreas mass was digested for HMP pancreases and 82% [74–92.5%] for SCS pancreases (Fig. 5a). After density gradient separation, the highest purity (of the 12 fractions) in the HMP was 85% [77.5–92.8%] and 85% [80–92.5%] for SCS pancreases (Fig. 5b). The average purity of the fractions that were considered suitable for culture (≥25% purity) was 52% [45–59%] for HMP pancreases and 57% [48–59.5%] for SCS pancreases (Fig. 5c). The proportion of embedded islets that were cultured from HMP pancreases was 27.3% [19.5–58.3%] and 36.6% [13.5–49.9%] from SCS pancreases (Fig. 5d).
Figure 5.
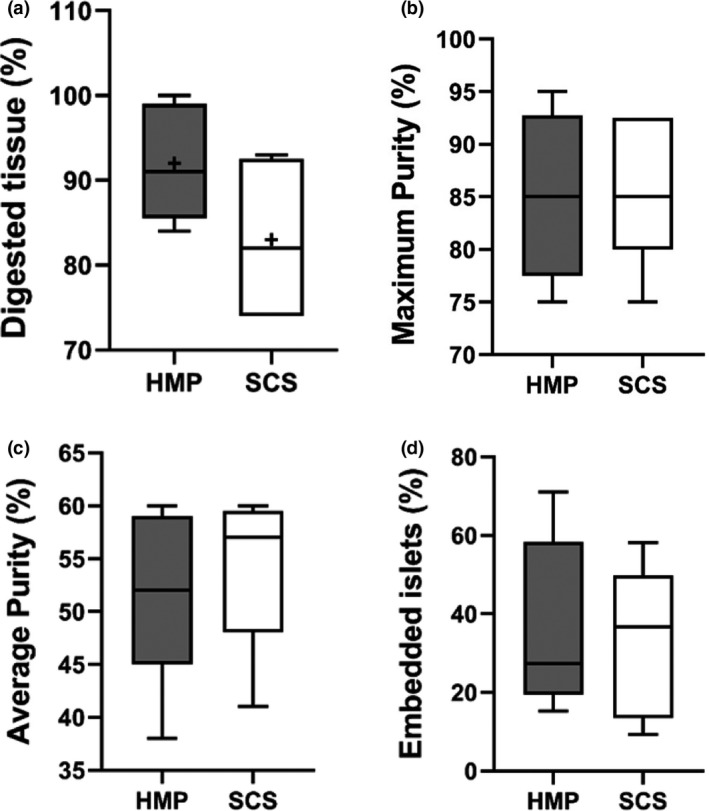
Islet isolation parameters. (a) Percentage of digested pancreas tissue [g/g]. (b) Maximum islet purity after density separation. (c) Average purity of combined islet fractions (≥25% purity). (d) Percentage of embedded islets as part of all isolated islets. HMP, hypothermic machine perfusion; SCS, static cold storage. Results are shown as box‐and‐whisker median ± interquartile range.
After isolation, HMP pancreases yielded 378 261 [217 392–526 359] IEQ. The observed IEQ decrease during culture for HMP islets was 36 ± 52% from day 0 to day 3. The SCS pancreases yielded 254 891 [146 739–595 652] IEQ. This yield declined by 40 ± 63% after 3 days of culture (Fig. 6).
Figure 6.
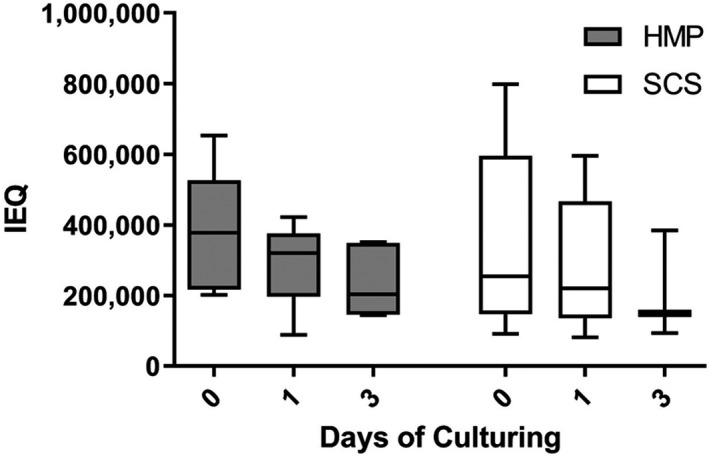
Changes in total IEQ after islet isolation from SCS and HMP pancreases. IEQ after islet isolation (day 0), after one day of culturing (day 1), and after three days of culturing (day 3). IEQ, islet equivalent; HMP, hypothermic machine perfusion; SCS, static cold storage. Results are shown as box‐and‐whisker median ± interquartile range.
Viability of islets
Islet viability was analyzed directly after isolation, after the first culture medium change (one day of culture later, day 1), and after the second medium change (three days after isolation, day 3). On day 0, HMP islets were 94% [79.7–97.5%] viable at day 0, 97.9% [93.7–98.6%] at day 1, and 93.9% [82–98.4%] at day 3. SCS islets were 92.8% [90.8–93%] viable at day 0, 93.4% [86.9–97.3%] at day 1, and 92% [80.2–99.6%] at day 3 (Fig. 7).
Figure 7.
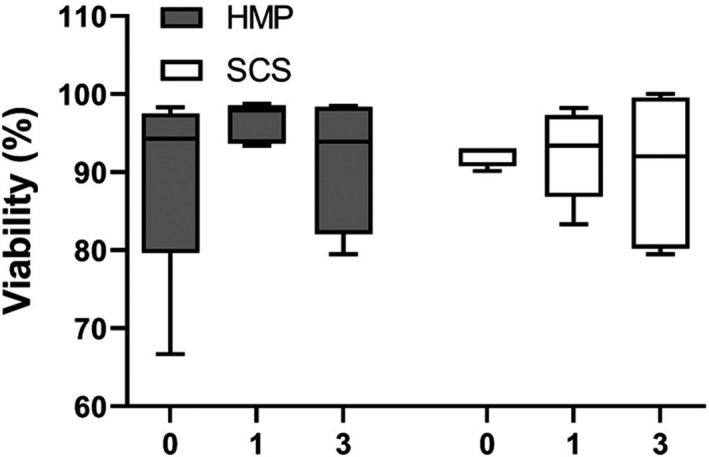
Postisolation viability of SCS and HMP pancreases. Islet viability, as assessed by FDA‐PI staining, was calculated directly after isolation (day 0), after the first medium change (day 1), and after the second medium change (day 3). HMP, hypothermic machine perfusion; SCS, static cold storage. Results are shown as box‐and‐whisker median ± interquartile range.
In vitro functionality
In vitro functionality of isolated islets from pancreases after 6 h of oxygenated HMP‐ and SCS‐preserved pancreases was assessed by a dynamic glucose‐stimulated insulin secretion (dGSIS) test after the first medium change, one day after isolation (Fig. 8). There was a biphasic insulin release response to glucose in both groups. The peak stimulation index for HMP islets was 3.8 with an AUC fold increase in insulin of 288 ± 44.3. For SCS islets, the peak stimulation index was 3.3 with an AUC fold increase in insulin of 259 ± 34.3.
Figure 8.
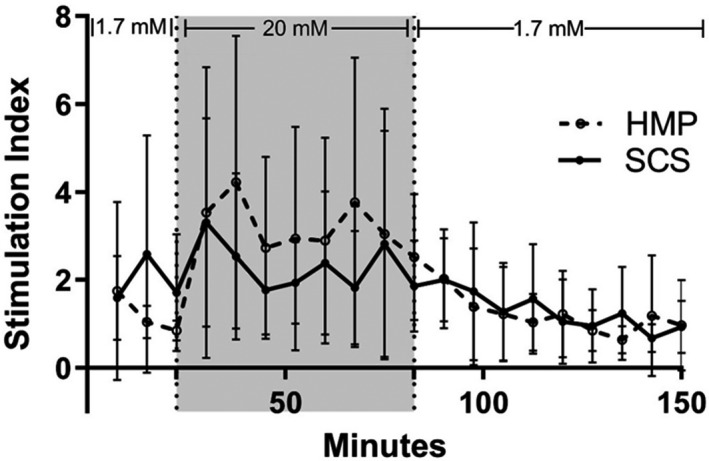
Average glucose‐stimulated insulin response in islets from SCS and HMP pancreases. After day 1 medium change, cultured islets from every isolation were perfused in duplo with a low concentration glucose solution (1.7 mM), followed by a high concentration glucose solution (20 mM, gray area), and again by the low concentration glucose solution. Each time point is averaged per group, shown as mean ± SD.
In vivo functionality
In vivo functionality of the HMP islets was assessed by transplantation of isolated islets under the kidney capsule of diabetic mice. At day 28 post‐transplantation, the AUC fold increase in insulin during the intraperitoneal glucose tolerance test (IPGTT) in mice receiving HMP islets was 167 ± 28 and SCS islets was 198 ± 45 (Fig. 9a). There was also a concomitant rise in glucose concentration (Fig. 9b, AUC blood glucose of HMP islets: 1118 ± 337; AUC blood glucose of SCS islets: 1276 ± 149).
Figure 9.
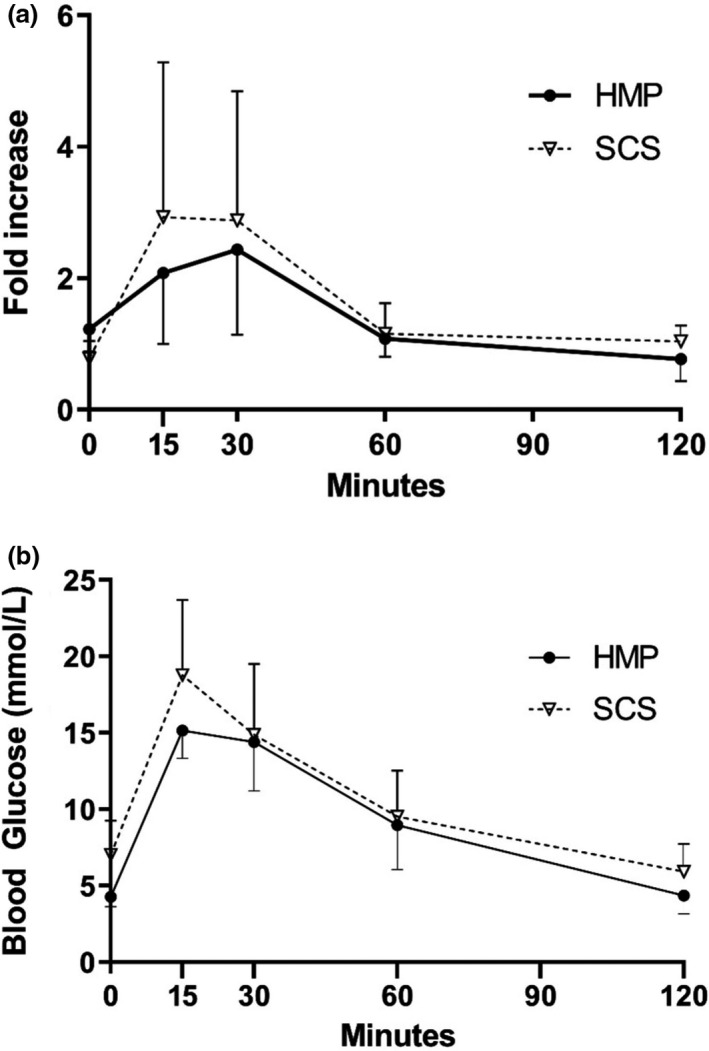
In vivo functionality of islets isolated from SCS and HMP pancreases. In vivo functionality of the isolated islets from HMP‐ and SCS‐preserved pancreases as assessed via transplantation of islets under the kidney capsule of diabetic mice three days after islet isolation. (a) Fold increase in insulin concentrations during the IPGTT at day 28. Insulin concentrations at each time point were divided by insulin concentrations at t = 120 min, averaged over all mice. (b) Glucose concentrations during the intraperitoneal glucose tolerance test (IPGTT) at day 28 after transplantation.
Discussion
This study describes the successful preservation of human DCD pancreases using hypothermic machine perfusion. This report shows the feasibility to isolate a clinically relevant number of viable islets from discarded human DCD pancreases after oxygenated HMP, thereby prolonging the cold ischemia time. Based on the donor characteristics, these pancreases would generally be rejected for clinical islet isolation. However, after HMP, we were able to isolate an adequate number of viable, functional islets and demonstrate proper in vitro and in vivo functionality.
There are many factors contributing to outcome after isolation and graft function after transplantation: donor characteristics, procurement, preservation, islet isolation protocol, culture, transplantation, and engraftment [17, 29]. Pancreatic islet β‐cells are known to be highly susceptible to oxidative stress, because of their reduced levels of endogenous antioxidants [16], which can eventually, after a period of extreme stress, lead to the production of reactive oxygen species. Evidence suggests that the oxidative stress during pancreas procurement and the isolation procedure can lead to chronic impaired islet function after transplantation [16]. The current standard preservation method for donor pancreases after multi‐organ donation is SCS, whether intended for whole organ transplantation or islet isolation [30]. Although simple and affordable, extended SCS time inevitably leads to the accumulation of intracellular toxins and ATP depletion, because of residual metabolic activity in the absence of oxygen [31]. The CIT a pancreas is subjected to is generally a consequence of the location of the donor hospital in relation to the recipient hospital combined with logistic hurdles of an islet isolation center. Data from several studies show CIT to be an independent variable that decreases islet yield and/or function (as a continuous variable, or in arbitrary cutoff values of 7, 8, 12, or 16 h CIT) [32, 33, 34, 35, 36, 37, 38, 39]. In our center, we have found a correlation between prolonged CIT and a lower islet yield in 126 DCD islet isolations [10]. In the current study, CIT was extended by 6 h in the oxygenated HMP‐preserved pancreases.
In the Netherlands, an increased number of organ donors are derived from more marginal donors including DCD donors [5]. Research has indicated that organs procured from DCD donors are especially susceptible to detrimental cold ischemia effects [40]. This cold ischemic insult combined with the inherent injury resulting from the agonal phase after withdrawal of life support and a period of warm ischemia prevalent in DCD procedures results in a reduced capacity to deal with this accumulation of stress factors compared with DBD organs [41, 42]. In three small studies concerning islet isolation from DCD donors, no significant differences in islet yield obtained from DCD compared with DBD pancreases were reported [43, 44, 45]. However, two more recent studies reported a reduction of up to 100 000 IEQ from DCD pancreases compared with DBD pancreases [10, 11], and another study reporting a 30% lower yield for DCD pancreases when calculated as beta‐cell number [46].
Recent studies reported that hypothermic machine perfusion (HMP) is beneficial for the preservation of DCD kidneys and livers [19, 20, 21, 22]. In an earlier study performed by our group, 6 h of oxygenated HMP using a custom‐made, transportable, dual arterial perfusion system with perfusion pressure of 25 mmHg was technically feasible and safe [24]. The usage of a dual perfusion system with two centrifugal pumps enables the separate perfusion of both the splenic and superior mesenteric arteries. In this way, flow in both systems was continuously monitored and potential perfusion problems could be traced and treated if needed, which earlier ensured uniform perfusion of the pancreatic tissue. The addition of oxygen to the perfusion system led to significantly increased ATP concentrations in the tissue; we reported a 6.8‐fold ATP increase in DCD pancreases and a 2.6‐fold increase in DBD pancreases after 6 h of oxygenated HMP [24].
In this report, we studied whether the effects of oxygenated HMP persist in pancreatic islets several days after isolation, during culturing, and after xenotransplantation in diabetic mice. Perfusion flow was stable during the 6‐h period of oxygenated HMP without visible macroscopic or microscopic edema formation. Furthermore, oxygenated HMP did not lead to increased apoptosis levels as indicated by caspase‐3 staining. Other systems using low perfusion pressures have documented similar findings of low edema and injury markers in experimental HMP of pancreases that were not transplanted or used for islet isolation [47, 48]. A recent study on HMP of porcine pancreases showed moderate and severe edema after 12 and 24 h of HMP, respectively. Histological examination showed similar results up to 12 h of preservation when compared to SCS [25]. In a study regarding the effect of 24 h of HMP on isolated porcine islets, it was speculated that edema induced by HMP aided the enzymatic digestion during isolation [49]. In our hands, enzymatic digestion of perfused pancreatic tissue was as efficient as static‐preserved organs. Also, HMP did not appear to improve the separation of islets from surrounding exocrine tissue, as evidenced by a similar prevalence of embedded islets. Other factors, such as donor age and enzyme perfusion technique, may play a larger role in that respect. It has long been a goal of organ preservation, in all its forms, to inhibit edema formation because of its deleterious effects [50], so the ability to isolate islets in a normal fashion without the inherent risks associated with edema should be seen as advantageous. We observed similar yield, maximum purity, and average purity between HMP and SCS islets. The similar results in islet yield between our groups, despite extended CIT, indicate that HMP can inhibit the deleterious effects of CIT once perfusion has started. In vitro functionality tests showed a biphasic insulin response to glucose by the HMP islets. Graft function after transplantation in diabetic mice remained adequate (blood glucose levels <10 mmol/L) for 55% of the mice during the study. IPGTT at day 28 showed a normal glucose peak at 15 min and an insulin peak at 30 min. After transplantectomy, all mice reverted to hyperglycemia. These xenotransplantation results support the data from in vitro analyses and suggest that HMP‐preserved DCD islets function properly after recovery in culture.
The present study does have potential limitations. Islet isolations were performed at a single center. Also, because of limited access to discarded donor pancreases, only five HMP (DCD) and five SCS (DBD) pancreases could be included in this study. The inclusion of a second SCS group consisting of DCD pancreases could have controlled for differences in cold ischemic injury between DCD and DBD pancreases. Despite this shortcoming, we postulate that referencing our HMP results to theoretically superior DBD donors can provide insight into the effectiveness of HMP. In order to definitively demonstrate the superiority of oxygenated HMP, a randomized controlled trial comparing clinical SCS DCD pancreases to HMP DCD pancreases should be performed.
Besides studying the biological benefit of HMP to islet isolations, this study was also initiated to investigate the feasibility of postprocurement pancreas perfusion in a clinical setting. While results presented indicate that HMP can safely extend CIT without sacrificing islet yield, function, or viability, the methodology in using the transportable dual arterial oxygenated hypothermic machine perfusion system used in this study is challenging (a period of SCS during transportation to the HMP center, followed by a period of HMP during transportation to the islet isolation facility). This complex undertaking should be optimized and ideally be centralized in dedicated perfusion centers before it is more widely applied. Different approaches are hypothetically possible, direct start of HMP after procurement at the donor center (which is currently performed for all kidneys in the Netherlands) [51]: short (2–6 h) end‐ischemic HMP after SCS in the recipient hospital, or prolonged (6–12 h) end‐ischemic HMP in order to postpone the isolation procedure for logistical reasons. The protocol used in this study could theoretically be implemented in all three approaches, but is most likely best applicable in the latter two, because of the surgical complexity and time‐consuming nature of connecting the pancreas to the device. Furthermore, HMP may be combined with other machine perfusion techniques during which viability or functionality of ECD pancreases can be tested such as normothermic regional perfusion or end‐ischemic normothermic machine perfusion. Normothermic regional perfusion may be performed for the sake of other abdominal organs, and islets have been isolated from pancreases after these procedures although the success of which has not been well‐documented so far [52]. Normothermic machine perfusion (NMP) could be performed after HMP for (islet) viability and functionality testing prior to isolation. However, only a single report of NMP of the pancreas has been documented. Unfortunately, although the endocrine function was maintained during a short period of NMP, the perfusion itself may have conferred additional necrotic damage to the pancreases [53].
Conclusion
This study demonstrates that we were able to successfully isolate functional islets from discarded human DCD pancreases after performing 6 h of oxygenated HMP that increased the cold ischemia time.
Authorship
JD, ML, and ME participated in research design, performance of the research, data analysis, and writing of the paper. CK, EdK, and HL participated in research design, data analysis, and writing of the paper.
Funding
This project was financially supported by the Diabetes Cell Therapy Initiative (DCTI) FES 2009 Program LSH‐DCTI including the Dutch Diabetes Research Foundation.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol 2017; 13: 268. [DOI] [PubMed] [Google Scholar]
- 2.Nijhoff MF, Engelse MA, Dubbeld J, et al. Glycemic stability through islet‐after‐kidney transplantation using an alemtuzumab‐based induction regimen and long‐term triple‐maintenance immunosuppression. Am J Transplant 2016; 16: 246. [DOI] [PubMed] [Google Scholar]
- 3.Eurotransplant chapter 7: ET pancreas allocation system (EPAS). Updated 2019.
- 4.Proneth A, Schnitzbauer AA, Schenker P, et al. Extended pancreas donor program‐the EXPAND study: a prospective multicenter trial testing the use of pancreas donors older than 50 years. Transplantation 2018; 102: 1330. [DOI] [PubMed] [Google Scholar]
- 5.Www.eurotransplant.org/statistics. Accessed May 6, 2020.
- 6.Jochmans I, Akhtar MZ, Nasralla D, et al. Past, present, and future of dynamic kidney and liver preservation and resuscitation. Am J Transplant 2016; 16: 2545. [DOI] [PubMed] [Google Scholar]
- 7.Snoeijs MG, Winkens B, Heemskerk MB, et al. Kidney transplantation from donors after cardiac death: a 25‐year experience. Transplantation 2010; 90: 1106. [DOI] [PubMed] [Google Scholar]
- 8.Bellingham JM, Santhanakrishnan C, Neidlinger N, et al. Donation after cardiac death: a 29‐year experience. Surgery 2011; 150: 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jay C, Ladner D, Wang E, et al. A comprehensive risk assessment of mortality following donation after cardiac death liver transplant – an analysis of the national registry. J Hepatol 2011; 55: 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doppenberg JB, Nijhoff MF, Engelse MA, de Koning EJP . Clinical use of donation after circulatory death pancreas for islet transplantation. Am J Transplant 2021. 10.1111/ajt.16533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito T, Kenmochi T, Kurihara K, et al. The effects of using pancreases obtained from brain‐dead donors for clinical islet transplantation in japan. J Clin Med 2019; 8: 1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humar A, Ramcharan T, Kandaswamy R, Gruessner RW, Gruessner AC, Sutherland DE. Technical failures after pancreas transplants: Why grafts fail and the risk factors–a multivariate analysis. Transplantation 2004; 78: 1188. [DOI] [PubMed] [Google Scholar]
- 13.Keck T, Werner J, Schneider L, Gebhard MM, Klar E. Characterization of ischemia/reperfusion injury after pancreas transplantation and reduction by application of monoclonal antibodies against ICAM‐1 in the rat. Surgery 2003; 134: 63. [DOI] [PubMed] [Google Scholar]
- 14.Barlow AD, Hosgood SA, Nicholson ML. Current state of pancreas preservation and implications for DCD pancreas transplantation. Transplantation 2013; 95: 1419. [DOI] [PubMed] [Google Scholar]
- 15.Miñambres E, Suberviola B, Dominguez‐Gil B, et al. Improving the outcomes of organs obtained from controlled donation after circulatory death donors using abdominal normothermic regional perfusion. Am J Transplant 2017; 17: 2165. [DOI] [PubMed] [Google Scholar]
- 16.Bottino R, Balamurugan AN, Tse H, et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes 2004; 53: 2559. [DOI] [PubMed] [Google Scholar]
- 17.Iwanaga Y, Sutherland DE, Harmon JV, Papas KK. Pancreas preservation for pancreas and islet transplantation. Curr Opin Organ Transplant 2008; 13: 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Neill S, Srinivasa S, Callaghan CJ, et al. Novel organ perfusion and preservation strategies in transplantation – where are we going in the UK? Transplantation 2020; 104: 1813. [DOI] [PubMed] [Google Scholar]
- 19.Moers C, Smits JM, Maathuis MH, et al. Machine perfusion or cold storage in deceased‐donor kidney transplantation. N Engl J Med 2009; 360: 7. [DOI] [PubMed] [Google Scholar]
- 20.Tingle SJ, Figueiredo RS, Moir JA, Goodfellow M, Talbot D, Wilson CH. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst Rev 2019;3:CD011671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Rijn R , Karimian N, Matton APM, et al. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg 2017; 104: 907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlegel A, Kron P, Dutkowski P. Hypothermic machine perfusion in liver transplantation. Curr Opin Organ Transplant 2016; 21: 308. [DOI] [PubMed] [Google Scholar]
- 23.Jochmans I, Brat A, Davies L, et al. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): a randomised, double‐blind, paired, phase 3 trial. Lancet 2020; 396: 1653. [DOI] [PubMed] [Google Scholar]
- 24.Leemkuil M, Lier G, Engelse MA, et al. Hypothermic oxygenated machine perfusion of the human donor pancreas. Transplant Direct 2018; 4: e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prudhomme T, Kervella D, Ogbemudia AE, et al. Successful pancreas allotransplantations after hypothermic machine perfusion in a novel diabetic porcine model: a controlled study. Transpl Int 2021; 34: 353. [DOI] [PubMed] [Google Scholar]
- 26.Baranski A. Pancreas procurement. In: Surgical Technique of Abdominal Organ Procurement. First ed. London: Springer‐Verlag, 2009, 149–155. 10.1007/978-1-84800-251-7 [DOI] [Google Scholar]
- 27.Goto M, Eich TM, Felldin M, et al. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation 2004; 78: 1367. [DOI] [PubMed] [Google Scholar]
- 28.London NJ, Contractor H, Lake SP, Aucott GC, Bell PR, James RF. A microfluorometric viability assay for isolated human and rat islets of langerhans. Diabetes Res 1989; 12: 141. [PubMed] [Google Scholar]
- 29.Shintaku H, Okitsu T, Kawano S, et al. Effects of fluid dynamic stress on fracturing of cell‐aggregated tissue during purification for islets of langerhans transplantation. J Phys D Appl Phys 2008; 41: 15507. [Google Scholar]
- 30.Dholakia S, Royston E, Sharples EJ, Sankaran V, Ploeg RJ, Friend PJ. Preserving and perfusing the allograft pancreas: past, present, and future. Transplant Rev (Orlando). 2018; 32: 127. [DOI] [PubMed] [Google Scholar]
- 31.Kuan KG, Wee MN, Chung WY, et al. Extracorporeal machine perfusion of the pancreas: technical aspects and its clinical implications–a systematic review of experimental models. Transplant Rev (Orlando). 2016; 30: 31. [DOI] [PubMed] [Google Scholar]
- 32.Balamurugan AN, Naziruddin B, Lockridge A, et al. Islet product characteristics and factors related to successful human islet transplantation from the collaborative islet transplant registry (CITR) 1999–2010. Am J Transplant 2014; 14: 2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaddis JS, Danobeitia JS, Niland JC, Stiller T, Fernandez LA. Multicenter analysis of novel and established variables associated with successful human islet isolation outcomes. Am J Transplant 2010; 10: 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hilling DE, Bouwman E, Terpstra OT, Marang‐van de Mheen PJ. Effects of donor‐, pancreas‐, and isolation‐related variables on human islet isolation outcome: A systematic review. Cell Transplant 2014; 23: 921. [DOI] [PubMed] [Google Scholar]
- 35.Lakey JR, Burridge PW, Shapiro AM. Technical aspects of islet preparation and transplantation. Transpl Int 2003; 16: 613. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann R, Zuellig RA, Kugelmeier P, et al. Superiority of small islets in human islet transplantation. Diabetes 2007; 56: 594. [DOI] [PubMed] [Google Scholar]
- 37.Warnock GL, Meloche RM, Thompson D, et al. Improved human pancreatic islet isolation for a prospective cohort study of islet transplantation vs best medical therapy in type 1 diabetes mellitus. Arch Surg 2005; 140: 735. [DOI] [PubMed] [Google Scholar]
- 38.Wassmer CH, Perrier Q, Combescure C, et al. Impact of ischemia time on islet isolation success and posttransplantation outcomes: A retrospective study of 452 pancreas isolations. Am J Transplant 2021; 21: 1493. [DOI] [PubMed] [Google Scholar]
- 39.Caballero‐Corbalán J, Brandhorst H, Malm H, et al. Using HTK for prolonged pancreas preservation prior to human islet isolation. J Surg Res 2012; 175: 163. [DOI] [PubMed] [Google Scholar]
- 40.Summers DM, Watson CJ, Pettigrew GJ, et al. Kidney donation after circulatory death (DCD): state of the art. Kidney Int 2015; 88: 241. [DOI] [PubMed] [Google Scholar]
- 41.Peters‐Sengers H, Houtzager JHE, Heemskerk MBA, et al. DCD donor hemodynamics as predictor of outcome after kidney transplantation. Am J Transplant 2018; 18: 1966. [DOI] [PubMed] [Google Scholar]
- 42.Berney T, Boffa C, Augustine T, et al. Utilization of organs from donors after circulatory death for vascularized pancreas and islet of langerhans transplantation: recommendations from an expert group. Transpl Int 2016; 29: 798. [DOI] [PubMed] [Google Scholar]
- 43.Andres A, Kin T, O'Gorman D, et al. Clinical islet isolation and transplantation outcomes with deceased cardiac death donors are similar to neurological determination of death donors. Transpl Int 2016; 29: 34. [DOI] [PubMed] [Google Scholar]
- 44.Clayton HA, Swift SM, Turner JM, James RF, Bell PR. Non‐heart‐beating organ donors: a potential source of islets for transplantation? Transplantation 2000; 69: 2094. [DOI] [PubMed] [Google Scholar]
- 45.Markmann JF, Deng S, Desai NM, et al. The use of non‐heart‐beating donors for isolated pancreatic islet transplantation. Transplantation 2003; 75: 1423. [DOI] [PubMed] [Google Scholar]
- 46.De Paep DL, Van Hulle F, Ling Z, et al. Lower beta cell yield from donor pancreases after controlled circulatory death prevented by shortening acirculatory warm ischemia time and by using IGL‐1 cold preservation solution. PLoS One 2021; 16: e0251055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Branchereau J, Renaudin K, Kervella D, et al. Hypothermic pulsatile perfusion of human pancreas: preliminary technical feasibility study based on histology. Cryobiology 2018; 85: 56. [DOI] [PubMed] [Google Scholar]
- 48.Hamaoui K, Gowers S, Sandhu B, et al. Development of pancreatic machine perfusion: translational steps from porcine to human models. J Surg Res 2018; 223: 263. [DOI] [PubMed] [Google Scholar]
- 49.Taylor MJ, Baicu S, Leman B, Greene E, Vazquez A, Brassil J. Twenty‐four hour hypothermic machine perfusion preservation of porcine pancreas facilitates processing for islet isolation. Transplant Proc 2008; 40: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ridgway D, Manas D, Shaw J, White S. Preservation of the donor pancreas for whole pancreas and islet transplantation. Clin Transplant 2010; 24: 1. [DOI] [PubMed] [Google Scholar]
- 51.Rijkse E, IJzermans JNM, Minnee RC. Machine perfusion in abdominal organ transplantation: current use in the Netherlands. World J Transplant 2020; 10: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oniscu GC, Randle LV, Muiesan P, et al. In situ normothermic regional perfusion for controlled donation after circulatory death–the united kingdom experience. Am J Transplant 2014; 14: 2846. [DOI] [PubMed] [Google Scholar]
- 53.Barlow AD, Hamed MO, Mallon DH, et al. Use of ex vivo normothermic perfusion for quality assessment of discarded human donor pancreases. Am J Transplant 2015; 15: 2475. [DOI] [PMC free article] [PubMed] [Google Scholar]


