Abstract
Background
Specific IgE to Ara h 2 is a diagnostic test for peanut allergy which may reduce the need for double‐blind placebo‐controlled food challenges (DBPCFC); however, guidance for using Ara h 2 in place of DBPCFCs has not been validated.
Objective
To prospectively evaluate 1) diagnostic accuracy of previously published Ara h 2 cut‐off levels to diagnose peanut allergy in children and 2) costs.
Methods
A consecutive series of 150 children age 3.5 to 18 years was evaluated in secondary and tertiary settings in the Netherlands. sIgE to Ara h 2 was the index test, and oral peanut ingestion was the reference test. Oral peanut ingestion was home or supervised introduction for Ara h 2 ≤ 0.1, DBPCFC for 0.1–5.0 and open food challenge for ≥5.0. Costs were calculated using financial healthcare data.
Results
A conclusive reference test was performed in 113 children (75%). Sixty‐four children (57%) had peanut allergy, as confirmed by a DBPCFC (27/47) or an open challenge (37/50). Forty‐nine children (43%) were considered peanut‐tolerant after peanut introduction (19/19), a DBPCFC (20/47) or an open challenge (10/50). Area under the curve for Ara h 2 was 0.94 (95% CI 0.90–0.98). The diagnostic flow chart correctly classified 26/26 (100%; 84–100) of children with Ara h 2 ≤ 0.1 as peanut‐tolerant and 34/35 (97%; 83–100) of children with Ara h 2 ≥ 5.0 as peanut‐allergic. At a cut‐off of ≤0.1 and ≥5.0, a sensitivity of respectively 100% (93–100) and 53% (38–67) was observed and a specificity of 53% (38–67) and 98% (87–100). Mean annual costs of the flow chart were estimated as €320‐€636 per patient lower than following national allergy guidelines.
Conclusions
In this diagnostic accuracy study, which did not take into account pretest probability, we have validated previously published Ara h 2 cut‐off levels which are associated with peanut tolerance and allergy.
Keywords: anxiety, component‐resolved diagnostics, costs, food challenge, peanut allergy
In children with suspected allergy, the sIgE to Ara h 2 cut‐off levels to predict peanut tolerance (cut‐off of ≤0.1) and peanut allergy (cut‐off of ≥5.0) were validated.
Key messages.
In children with suspected peanut allergy, sIgE to Ara h 2 cut‐off levels are validated.
A cut‐off of ≤0.1 and ≥5.0 are associated with respectively peanut tolerance and allergy.
Using these cut‐offs may reduce the burden and costs of double‐blind placebo‐controlled food challenges.
1. INTRODUCTION
Peanut allergy is one of the most common IgE‐mediated food allergies, estimated to affect 0.2–2.5% of children.1, 2 An accurate diagnosis of peanut allergy is important to adequately counsel allergic children and their parents on the elimination of peanut, the prevention and the treatment of accidental allergic reactions. In children with suspected peanut allergy who prove to be peanut‐tolerant, exclusion of peanut allergy is important to guide peanut introduction and to prevent unnecessary elimination diets. In addition, an accurate diagnosis of peanut allergy is important to reduce (parental) anxiety and improve health‐related quality of life.3, 4 The oral food challenge (OFC), particularly the double‐blind placebo‐controlled food challenge (DBPCFC), is currently the gold standard for the diagnosis of peanut allergy according to the EAACI Food Allergy and Anaphylaxis Guidelines.5 However, the DBPCFC is difficult in daily practice as the procedure is time‐consuming, costly to patients and health services and might be difficult to access.5, 6 Thus, there is need for improved diagnostic strategies that could accurately predict the DBPCFC result thereby reducing the number of DBPCFCs.
Various studies, including from our own research group, have reported that sIgE to peanut‐specific component Ara h 2 is useful to distinguish peanut‐allergic from peanut‐tolerant children and is highly superior to sIgE to peanut extract and other peanut components.7, 8, 9, 10, 11 When using the cut‐off levels of sIgE to Ara h 2 with the highest negative and positive predictive value, 62% of children could be classified correctly as peanut‐tolerant or peanut‐allergic. The validation and implementation of scientific research into daily clinical practice are very important to validate previous findings and evaluate costs and adherence. However, until now no studies have investigated validation of cut‐off levels of sIgE to Ara h 2 and the prospective implementation of a diagnostic flow chart based on sIgE to Ara h 2 in daily practice. Thus, it is unknown whether the use of a diagnostic flow chart based on Ara h 2 in children with suspected peanut allergy is accurate and safe, whether it can be used in different clinical settings (secondary and tertiary care) and whether a diagnostic flow chart is beneficial in terms of costs and patients’ welfare.
In the current study, we implemented a diagnostic flow chart based on previously published sIgE to Ara h 2 cut‐off levels in the Netherlands. The primary aim was to validate these cut‐off levels and evaluate the diagnostic accuracy of the flow chart. Secondary aims were to evaluate the direct and indirect costs and the anxiety levels following diagnostic testing.
2. METHODS
2.1. Study population
We performed a prospective cohort study in one tertiary care centre (ie university hospital) and three secondary care centres (ie general hospitals) in the Netherlands between January 2017 and July 2018. All consecutive children aged 3.5 to 18 years with suspected peanut allergy, seen by a paediatrician or an allergist, were eligible for inclusion. Suspected peanut allergy was based on a clinical history of an allergic reaction to peanut or an elimination diet for peanut for more than 1 year with or without peanut sensitization (peanut sIgE ≥0.35 kUA/L or peanut skin prick test mean wheal size ≥3 mm). The study was approved by the ethical committee of the University Medical Center Utrecht (nr. 16‐1456/C). Parents and children aged 12 years and older provided written informed consent before enrolment in the study.
2.2. Study procedures
The index test, specific IgE to Ara h 2, was determined in all children using the ImmunoCAP method (Thermo Fisher Scientific, Uppsala, Sweden) and compared with the following reference tests: home introduction, supervised introduction, a 2‐day double‐blind placebo‐controlled food challenge (DBPCFC) or a 1‐day open oral food challenge (OFC). Physicians were instructed to follow the diagnostic flow chart based on previously found levels of sIgE to Ara h 2 to confirm or exclude peanut allergy as depicted in Figure 1.7 Children with an Ara h 2 level ≤0.1 kUA/L were considered to have a very high probability of being peanut‐tolerant and were instructed to introduce peanut at home. Home introduction consisted of a 7‐day schedule with increasing amounts of peanuts up to 10 g of whole peanuts (i.e 2500 mg peanut protein) on the last day. Supervised introduction consisted of 5 steps of bread with increasing doses of peanut butter, followed by 10 g of whole peanut. Children with an Ara h 2 level ≥5.0 kUA/L were considered to have a very high probability of being peanut‐allergic. The purpose of further diagnostic testing in these children was to gain insight into the severity of peanut allergy and the threshold of the peanut‐allergic reaction. Thus, a 1‐day open OFC instead of a 2‐day DBPCFC was indicated.
FIGURE 1.
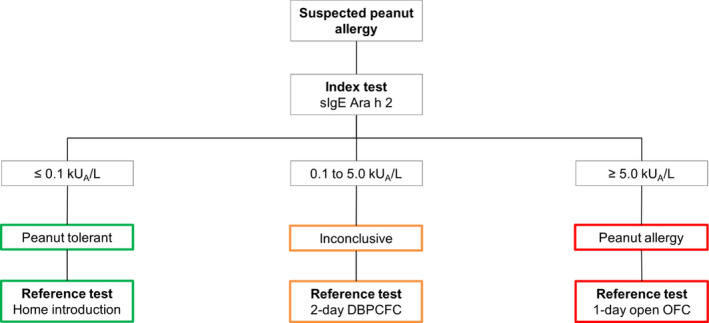
Design of the diagnostic flow chart
DBPCFC, double‐blind placebo‐controlled food challenge; OFC, oral food challenge, n = number
Children with an Ara h 2 level between 0.1 and 5.0 kUA/L were considered inconclusive and were supposed to undergo a 2‐day DBPCFC. The 2‐day DBPCFCs and 1‐day open OFCs were performed in a clinical setting with equipment for resuscitation. The verum day of the DBPCFC and open OFC consisted of 7 increasing doses of gingerbread containing 1 mg to 2500 mg peanut protein.12 Complete methodology of the DBPCFC and open OFC and definitions of the reference test categories are described in the supplemental methods section in the Online Repository.
Demographic and clinical information regarding history of peanut allergy and other atopic diseases was obtained by standardized questionnaires. Asthma, allergic rhinitis, atopic dermatitis and other food allergies were defined as having a doctor's diagnosis.
2.3. Primary aims
2.3.1. Validation of Ara h 2 cut‐off levels
The validity of the sIgE to Ara h 2 cut‐off levels was evaluated by calculating the corresponding sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), negative likelihood ratio (LR‐) and positive likelihood ratio (LR+). Furthermore, the percentage of patients diagnosed correctly using the sIgE to Ara h 2 cut‐off levels was calculated. Children who were not further evaluated after the sIgE to Ara h 2 measurement (with missing data on the reference test) or children with an inconclusive reference test result were excluded from this analysis.
2.4. Diagnostic accuracy of the diagnostic flow chart
The diagnostic accuracy of the diagnostic flow chart was evaluated using the area under the curve of the receiver operating characteristic. These analyses were stratified by secondary and tertiary care. The safety of the diagnostic flow chart was assessed by calculating the number of allergic reactions during home introduction of peanut. The adherence to the diagnostic flow chart was evaluated by calculating the number of children diagnosed according to the diagnostic flow chart and identifying reasons for non‐adherence. By exception and with argumentation, physicians could choose a different diagnostic strategy than the intended reference test indicated in the diagnostic flow chart based on their expert opinion.
2.5. Secondary aims
2.5.1. Cost assessment
The cost assessment was conducted from a societal perspective and included relevant costs borne by the healthcare system (i.e. consultations and diagnostic tests), costs borne by patients and families (i.e. travel expenses) and costs borne by other sectors (i.e. productivity losses) in 12 months after the baseline visit. Costs were based on national guidelines (i.e. Dutch Healthcare Authority and the National Health Care Institute in the Netherlands) and were corrected for inflation using the consumer price index for 2018.13, 14 Complete methodology of the data collection for the cost assessment is presented in the supplemental methods section in the Online Repository.
Mean costs were calculated per patient per year for three scenarios. Scenario A reflected the mean costs of the diagnostic flow chart in daily practice (including children were diagnosed according to the trajectory of the diagnostic flow chart and children who were diagnosed with other strategies). Scenario B reflected the mean costs of the diagnostic flow chart in theory: only the subset of children who followed the trajectory of the diagnostic flow chart were included in this scenario. Scenario C reflected the mean costs of the diagnostic pathway for peanut allergy according to the current Dutch national guideline.15 In scenario C, all children with sensitization to peanut (sIgE peanut extract ≥0.35 kUA/L or skin prick test ≥3 mm) were supposed to undergo a DBPCFC and all children without peanut sensitization were supposed to start eating peanut at home. The costs for scenario C were derived from the costs in scenario B.
2.6. Anxiety levels following diagnostic testing
Anxiety was measured at baseline, after the telephonic consultation communicating the sIgE to Ara h 2 level and discussing the subsequent diagnostic strategy with parents and patients and after 6 months of follow‐up. Anxiety was measured using the Spielberger State‐Trait Anxiety Inventory (STAI) for parents and the State‐Trait Anxiety Inventory for Children (STAIC) for children aged 8 years and older.16, 17 To assess State‐Anxiety (anxiety as an emotional state), parents were instructed to imagine how they would feel if their child was offered peanuts and children to imagine how they would feel if they were offered peanuts.4 The items were scored on a 4‐point scale with a cumulative score ranging between 20 (low anxiety) and 80 points (high anxiety) for the STAI and between 20 and 60 for the STAIC. The difference in scores was considered clinically relevant when the minimal clinically important difference (MCID) of 10 points was exceeded.18
2.7. Statistical analysis
Descriptive statistics were expressed as numbers (percentages) for categorical variables and mean (standard deviation) or median (interquartile range) for continuous variables with a normal or skewed distribution, respectively. Differences in baseline characteristics between children across different sIgE to Ara h 2 levels (≤0.1, 0.1–5.0 and ≥5.0 kUA/L) were statistically evaluated by the chi‐square test for categorical variables and the one‐way analysis of variance (ANOVA) or Kruskal‐Wallis test, as appropriate, with correction for multiple testing using the Benjamini‐Hochberg procedure. The sample size needed to have 95% confidence and 80% power to detect a difference of 5% from a presumed NPV and PPV of 99% were 96 patients.19 Statistical analyses were performed using SPSS for Windows (version 25.0. Armonk, NY: IMB Corp) and R (packages data.table v. 1.12.2 and dplyr v. 0.8.3). A P value of less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Patient characteristics
A total of 227 children with suspected peanut allergy were eligible for inclusion in this study. Seventy‐seven (34%) children or parents were not willing to participate in research. Therefore, 150 children were included in secondary care (65%) and tertiary care (35%) in the Netherlands. Children were grouped based on the level of sIgE to Ara h 2: ≤0.1 kUA/L in 29 (19%) children, 0.1 to 5.0 kUA/L in 68 (45%) children and ≥5.0 kUA/L in 53 (35%) children. Overall, children had a median (interquartile range) age of 7.9 (5.4 to 13.0) years. Children were suspected of peanut allergy because of a previous reaction to peanut (71%) or because of an elimination diet for more than 1 year (29%) (Table 1). Children with a sIgE to Ara h 2 level ≤0.1 kUA/L were significantly older (median age 12.1 years) compared to children with an Ara h 2 level of 0.1 to 5.0 kUA/L or ≥5 kUA/L (median age 7.6 and 7.1 years, respectively) (p00A0= .014). Twenty‐four of 44 (55%) children with an elimination diet for peanut >1 year were known to be sensitized to peanut (peanut sIgE ≥0.35 kUA/L or peanut skin prick test mean wheal size ≥3 mm).
TABLE 1.
Baseline characteristics
|
All children |
sIgE to Ara h 2 category (kUA/L) | ||||
|---|---|---|---|---|---|
| 150 |
≤ 0.1 29 (19) |
0.1–5.0 68 (45) |
≥ 5.0 53 (35) |
P value† | |
| Age in years, median (IQR) | 7.9 (5.4–13) | 12.1 (8–15.7) | 7.6 (5.2–11.5) | 7.1 (5.3–10.5) | 0.014 |
| Gender: male | 88 (59) | 11 (38) | 44 (65) | 33 (62) | 0.090 |
| Setting | 0.860 | ||||
| Secondary care | 97 (65) | 20 (69) | 43 (63) | 34 (64) | |
| Tertiary care | 53 (35) | 9 (31) | 25 (37) | 19 (36) | |
| Suspected peanut allergy | 0.138 | ||||
| Previous reaction | 106 (71) | 17 (59) | 53 (78) | 36 (68) | |
| Elimination diet >1 year | 44 (29) | 12 (41) | 15 (22) | 17 (32) | |
| Previous reaction‡ | 0.453 | ||||
| No reaction | 44 (29) | 12 (41) | 15 (22) | 17 (32) | |
| Grade 1 | 31 (21) | 6 (21) | 15 (22) | 10 (19) | |
| Grade 2 | 32 (21) | 3 (10) | 20 (29) | 9 (17) | |
| Grade 3 | 18 (12) | 2 (7) | 8 (12) | 8 (15) | |
| Grade 4 | 21 (14) | 4 (14) | 9 (13) | 8 (15) | |
| Grade 5 | 0 | 0 | 0 | 0 | |
| Missing data | 4 (3) | 2 (7) | 1 (1) | 1 (2) | |
| Elimination other food allergens | 0.266 | ||||
| No | 42 (28) | 9 (31) | 22 (32) | 11 (21) | |
| 1 or 2 | 43 (29) | 5 (17) | 20 (29) | 18 (34) | |
| 3 or 4 | 30 (20) | 5 (17) | 17 (25) | 8 (15) | |
| 5 or 6 | 27 (18) | 7 (24) | 8 (12) | 12 (23) | |
| 7 or more | 8 (5) | 3 (10) | 1 (1) | 4 (8) | |
| Atopic comorbidities | |||||
| Atopic dermatitis | 118 (79) | 21 (72) | 57 (84) | 40 (75) | 0.453 |
| Allergic rhinitis | 70 (47) | 17 (59) | 37 (54) | 16 (30) | 0.042 |
| Asthma | 48 (32) | 5 (17) | 22 (32) | 21 (40) | 0.207 |
Values are n (%) unless otherwise indicated.
Abbreviations: IQR, interquartile range.
Benjamini‐Hochberg adjusted p‐values for comparison of differences among 3 groups.
Most severe reaction according to the Sampson classification of anaphylaxis.
3.2. Results of the reference tests
The index test was measured in 150 children as previously mentioned. The intended reference test was performed in 92 children (61%), and another reference test was performed in 24 children (16%) and no reference test was performed in 34 children (23%) (Figure 2). Of 116 children who underwent a reference test after Ara h 2 determination, 64 children (55%) had peanut allergy, 49 children (33%) were peanut‐tolerant, and 3 children (3%) were considered inconclusive. Peanut allergy was confirmed after a DBPCFC in 27 children and after an open OFC in 37 children. Peanut tolerance was confirmed after home introduction in 10 children, supervised introduction in 9 children, a DBPCFC in 20 children and an open OFC in 10 children. Three children were considered inconclusive after an open OFC. The number of children diagnosed per intended reference test or another reference tests is presented in Figure 2.
FIGURE 2.
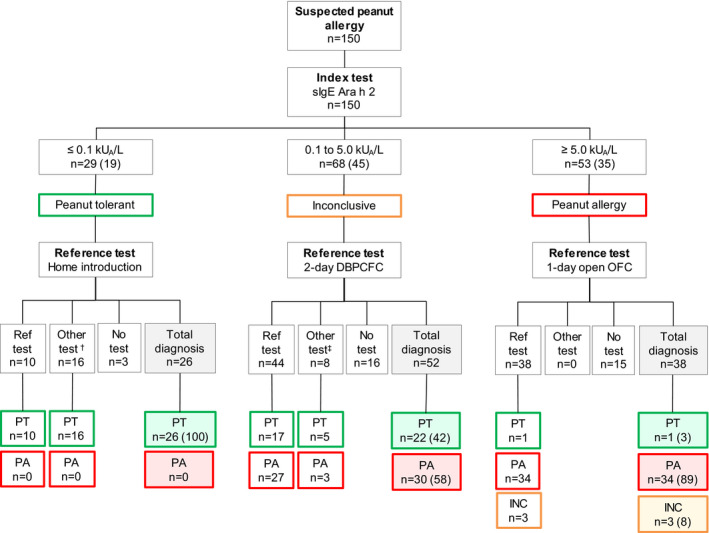
Results of the diagnostic flow chart
Numbers between brackets are percentages.
DBPCFC, double‐blind placebo‐controlled food challenge; INC, inconclusive; OFC, oral food challenge; PA, peanut allergy; PT, peanut‐tolerant; Ref test = reference test; n=number.
† n = 9 supervised introduction, n = 3 DBPCFC and n = 4 open OFC (all n = 16 PT)
‡ n = 8 open OFC
3.3. Validation of Ara h 2 and safety of the flow chart
All children with a sIgE to Ara h 2 level ≤0.1 kUA/L were classified as peanut‐tolerant after the intended reference test (i.e. home introduction) or another reference test (i.e. food challenge or supervised introduction). Fifty‐eight per cent of children with a sIgE to Ara h 2 level between 0.1 and 5.0 kUA/L were classified as peanut‐allergic and 42% as peanut‐tolerant. In this group of children, an increasingly positive diagnostic rate was observed with increasing levels of Ara h 2 ranging from 36% for children with an Ara h 2 level between 0.1 and 1.0 kUA/L and 100% for children with an Ara h 2 level between 3.0 and 5.0 kUA/L. Eighty‐nine per cent of children with a sIgE to Ara h 2 level ≥5 kUA/L were classified as peanut‐allergic, 3% (n = 1) as peanut‐tolerant (Ara h 2 level 7.1 kUA/L) and 8% (n = 3) as inconclusive. Children with an inconclusive outcome did not complete the open OFC due to aversion (n = 1), mild abdominal pain (n = 1) or mild abdominal pain and nausea (n=1). Thus, we could classify 100% of children with a sIgE to Ara h 2 level ≤0.1 kUA/L correctly as peanut‐tolerant (i.e. 100% negative predictive value). Furthermore, we could classify 89% of children with a sIgE to Ara h 2 level ≥5.0 kUA/L correctly as peanut‐allergic if all children with an inconclusive outcome would be peanut‐tolerant. And we could classify 97% of children correctly allergic if all these children with an inconclusive outcome would be peanut‐allergic (i.e. 89–97% positive predictive value). The median (interquartile range) Ara h 2 level of peanut‐tolerant children (n = 49) was 0.10 (0.08–0.49), while the median (interquartile range) Ara h 2 level of peanut‐allergic children (n = 64) was 5.65 (2.00–47.63). Cross‐tabulation of the index test cut‐off results (Ara h 2) and the reference test (home introduction, supervised introduction, DBPCFC or open OFC) are presented in Table E1.
Finally, the diagnostic flow chart was considered safe as none of the children with an Ara h 2 level ≤0.1 kUA/L experienced allergic symptoms during peanut introduction. No adverse events were reported from performing the index or reference test.
3.4. Diagnostic accuracy of Ara h 2
Specific IgE to Ara h 2 was a strong predictor of peanut allergy and showed high discriminative capacity (AUC 0.94; 95% confidence interval [CI] 0.90–0.98) (Figure 3). A stratified analysis by healthcare line showed high discriminative capacity in children in tertiary care (AUC 0.99; 0.98–1.00) and in children in secondary care (AUC 0.92; 0.86–0.98). The sensitivity of Ara h 2 at a cut‐off level ≤0.1 kUA/L was 100% (95% confidence interval 92.8–100) and the specificity 53.1% (38.4–67.2). The sensitivity and specificity of Ara h 2 at a cut‐off level ≥5.0 kUA/L were respectively 53.1% (40.3–65.5) and 98% (87.8–100). All measures of diagnostic accuracy for peanut allergy of these Ara h 2 cut‐off levels are depicted in Table 2. In Table E2, these measures for various cut‐off levels of sIgE to Ara h 2 are listed.
FIGURE 3.
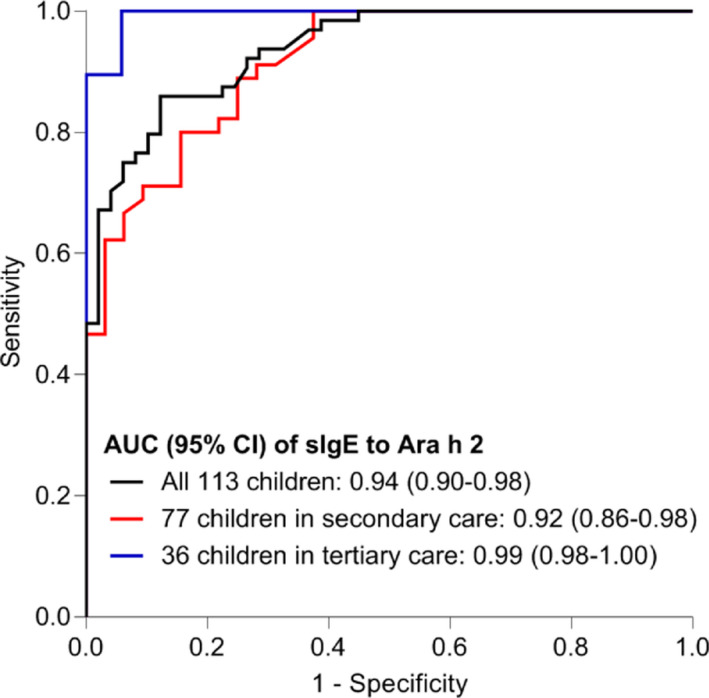
ROC‐curves of sIgE to Ara h 2
Abbreviations: AUC, area under the curve; CI, confidence interval; ROC, receiver operating characteristic
TABLE 2.
Measures of diagnostic accuracy for peanut allergy of specific IgE to Ara h 2 at different cut‐off levels
| Patients | N |
Cut‐off kUA/L |
Sensitivity % (95% CI) |
Specificity (95% CI) |
PPV % (95% CI) |
NPV % (95% CI) |
LR+ Ratio (95% CI) |
LR‐ Ratio (95% CI) |
|---|---|---|---|---|---|---|---|---|
| All children† | 113 | 0.1 | 100 (92.8–100) | 53.1 (38.4–67.2) | 74.3 (62.4–82.0) | 100 (84.0–100) | 2.13 (1.58–2.87) | 0 (NA) |
| 5.0 | 53.1 (40.3–65.5) | 98 (87.8–100) | 97.1 (83.4–100) | 61.5 (49.8–72.1) | 26.03 (3.69–183.60) | 0.48 (0.37–0.62) | ||
| Secondary care | 77 | 0.1 | 100 (90.2–100) | 59.4 (40.8–75.8) | 77.6 (64.4–87.1) | 100 (79.1–100) | 2.46 (1.62–3.74) | 0 (NA) |
| 5.0 | 48.9 (33.9–64.0) | 96.9 (82.0–99.8) | 95.7 (76.0–99.8) | 57.4 (43.3–70.5) | 15.64 (2.22–110.19) | 0.53 (0.40–0.70) | ||
| Tertiary care | 36 | 0.1 | 100 (79.1–100) | 41.2 (19.4–66.5) | 65.5 (45.7–81.4) | 100 (56.1–100) | 1.70 (1.14–2.53) | 0 (NA) |
| 5.0 | 63.2 (38.6–82.8) | 100 (77.1–100) | 100 (69.9–100) | 70.8 (48.8–86.6) | Infinity | 0.37 (0.20–0.66) |
Abbreviations: CI, confidence interval; LR‐, negative likelihood ratio; LR+, positive likelihood ratio; n, number; NA, not applicable; NPV, negative predictive value; PA, peanut allergy; PPV, positive predictive value.
Included children with a reference test with a conclusive outcome n = 113/150 (75%)
3.5. Adherence to the diagnostic flow chart
As previously mentioned, 92 of 150 (61%) children followed the trajectory of the diagnostic flow chart. The mean time interval between the index test (i.e. Ara h 2 determination) and the reference test (i.e. home introduction, supervised introduction, DBPCFC or an open OFC) was respectively 3.4, 6.4, 5.4 and 7.4 months. Thirty‐four (23%) children were not further evaluated after Ara h 2 determination, and in 24 (16%) children, a different diagnostic strategy was chosen. The most frequent reason to choose a different diagnostic strategy in children with an Ara h 2 level ≤0.1 and 0.1 to 5.0 kUA/L was patients’ preference due to anxiety of the child and/or parent(s). However, baseline state anxiety in parents who reported anxiety as reason to choose a different diagnostic strategy was comparable to state anxiety in parents who did not report anxiety (state anxiety 50 and 52, respectively). The most frequent reason to choose a different diagnostic strategy in children with an Ara h 2 level ≥5.0 kUA/L was the physician's preference not to perform an oral food challenge due to a suggestive clinical history of previous reaction to peanut and/or anaphylaxis (Figure E1).
3.6. Cost assessment
Mean (95% CI) cumulative costs of the diagnostic flow chart in daily practice (scenario A) and the diagnostic flow chart in theory (scenario B) were € 2,120 (€ 1,924 ‐ € 2,317) and € 2,437 (€ 2,189 ‐ € 2,685) per patient per year, respectively (Table 3; Figure 4). Mean costs of the diagnostic flow chart in practice were lower because 34 children did not undergo an oral food challenge. Mean cumulative costs of the diagnostic pathway according to the current national guideline were € 2,757 (€ 2,441 ‐ € 3,072) per patient per year. Thus, the use of the diagnostic flow chart may reduce direct and indirect food allergy‐related costs by € 320 (ie flow chart in theory) to €636 (i.e. flow chart in daily practice) per patient per year compared to the current national guideline.
TABLE 3.
Mean costs per patient per year for all children (A), for children diagnosed according to the diagnostic flow chart (B) and a scenario analysis on the costs for children diagnosed according to the guideline (C)
|
A Flow chart in practice n = 150 |
B Flow chart in theory n = 92 |
C Guideline n = 134 |
||||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Total costs | € 2,120 | € 1,924 ‐ € 2,317 | € 2,437 | € 2,189 ‐ € 2,685 | € 2,757 | € 2,441 ‐ € 3,072 |
| Direct costs | ||||||
| Consultations | ||||||
| Outpatient clinic | € 319 | € 270 ‐ € 369 | € 269 | € 227 ‐ € 311 | € 246 | € 179 ‐ € 314 |
| Telephone | € 67 | € 58 ‐ € 77 | € 72 | € 59 ‐ € 84 | € 62 | € 44 ‐ € 80 |
| Diagnostic testing | ||||||
| Food challenges | € 937 | € 809 ‐ € 1,064 | € 1204 | € 1,041 ‐ € 1,367 | € 1,433 | € 1,233 ‐ € 1,634 |
| Laboratory testing | € 112 | € 95 ‐ € 129 | € 103 | € 81 ‐ € 126 | € 116 | € 77 ‐ € 155 |
| Skin prick testing | € 48 | € 34 ‐ € 61 | € 44 | € 28 ‐ € 60 | € 47 | € 20 ‐ € 73 |
| Lung function | € 44 | € 26 ‐ € 61 | € 26 | € 13 ‐ € 40 | € 29 | € 5 ‐ € 53 |
| Travel expenses | € 54 | € 45 ‐ € 63 | € 54 | € 44 ‐ € 64 | € 60 | € 44 ‐ € 75 |
| Indirect costs | ||||||
| Productivity loss | € 540 | € 477 ‐ € 602 | € 666 | € 584 ‐ € 747 | € 763 | € 664 ‐ € 86 |
Abbreviations: CI, confidence interval; n, number.
FIGURE 4.
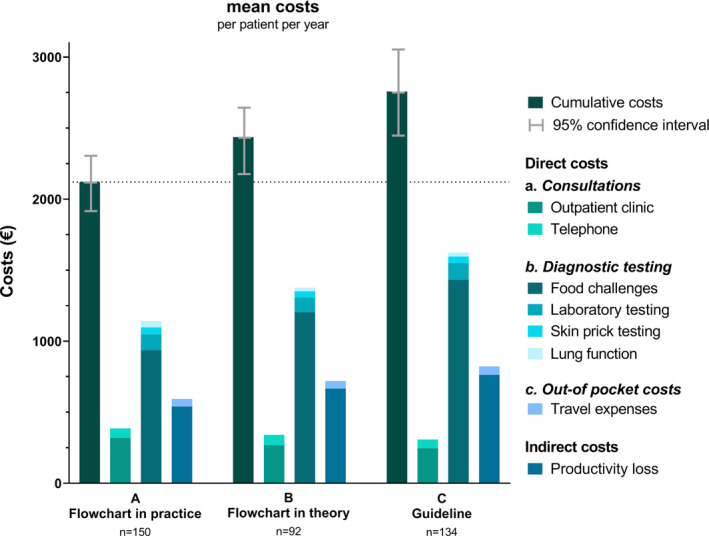
Mean costs per patient per year for all included children (scenario A), for children diagnosed according to the diagnostic flow chart (scenario B) and a scenario analysis on the costs for children diagnosed according to the guideline (scenario C)
3.7. Anxiety following diagnostic testing
Parental state anxiety was high at baseline in all Ara h 2 groups with a mean (standard error [SE]) score of 57.7 (2). Parental state anxiety reduced clinically relevant after the telephonic consultation discussing Ara h 2 if the Ara h 2 level was ≤0.1 kUA/L (mean difference, MD 17; SE 4.1) (Figure E2). In parents of children with an Ara h 2 level >0.1 kUA/L, state anxiety reduced after 6 months of follow‐up if the child underwent an oral food challenge (MD 17.9; SE 3.2) and not if the child did not undergo an oral food challenge (MD 5; SE 2.9). Parental state anxiety reduced clinically relevant both after a positive and negative oral food challenge result with a mean (SE) difference of 16.6 (3.6) and 19.6 (6), respectively. Child‐reported state anxiety did not improve clinically relevant during follow‐up.
4. DISCUSSION
This is the first study investigating the validation of sIgE to Ara h 2 cut‐off levels and the implementation of a diagnostic flow chart based on sIgE to Ara h 2 in children with suspected peanut allergy in the Netherlands. Our previously published sIgE to Ara h 2 cut‐off levels were validated in children in secondary and tertiary care.7 The implemented diagnostic flow chart accurately classified children as peanut‐tolerant and peanut‐allergic with a negative and positive predictive value of 100% and 89–97%, respectively. Furthermore, the diagnostic flow chart could reduce direct and indirect costs of diagnosing peanut allergy by € 320 to € 636 per patient per year compared to diagnosing peanut allergy according to the current national guideline.
All children in our cohort who were not sensitized to Ara h 2 were peanut‐tolerant, even when the child reported a severe allergic reaction to peanut in clinical history. We believe our diagnostic flow chart is safe to use as the probability of an allergic reaction in children with an Ara h 2 level ≤0.1 kUA/L is very low (0% in this and in our previous study) and children are instructed to introduce peanut according to an introduction schedule starting at a very small amount of peanut. However, it should be kept in mind that rare cases of (severe) peanut allergy have been described in literature in children who are not sensitized to Ara h 2.20, 21, 22, 23, 24 These children might be sensitized to other peanut components than Ara h 2, for example Ara h 6 or Ara h 7.25, 26 Furthermore, the adherence to the diagnostic flow chart for children with an Ara h 2 level ≤0.1 kUA/L was low. The majority of children with an Ara h 2 level ≤0.1 kUA/L in our cohort introduced peanut in the hospital and not at home because children, parents or physicians (unfairly afterwards) feared an allergic reaction to peanut. As an alternative, in children with anxiety or a severe allergic reaction in clinical history, physicians may consider introducing peanut at the outpatient clinic instead of at the day care in the hospital. This could further reduce the burden and costs of (delayed) oral food challenges and/or supervised introductions.27 Such a modification to the diagnostic flow chart may increase the feasibility and adherence in daily practice.
We also conclude that total direct and indirect costs seemed to be lower when children were diagnosed according to the diagnostic flow chart compared to the current national guideline. With an estimated 2400 referrals per year in the Netherlands this implicates a reduction in societal costs of € 767,101 per year when all children adhere to the diagnostic flow chart up to € 1,526,688 per year in daily practice.1, 28 A model‐based cost‐effectiveness analysis has been performed in a Norwegian cohort of children with suspected peanut allergy.29 In support of our findings, the authors reported that a diagnostic flow chart based on peanut components (ie Ara h 1, 2, 3, 8 and 9) appeared to be more cost‐effective than current clinical practice based on sIgE to peanut extract, skin prick testing and oral food challenges.
We showed that parental state anxiety is largely reduced after a negative Ara h 2 result and after oral food challenges, irrespective of the oral food challenge result. Parental state anxiety remained high during 6 months of follow‐up if children (were) declined further diagnostic testing after a positive Ara h 2 outcome (ie >0.1 kUA/L). One of the main reasons to decline further diagnostic testing was anxiety. These results further underline the beneficial impact of a clear diagnosis of food allergy on parental state anxiety and quality of life as previously reported.3, 4 Further research is needed to investigate how to handle and/or reduce food‐related anxiety in children, especially when anxiety is the reason to decline further diagnostic testing.
To appreciate the results of our study, several limitations should be taken into account. First, our study lacked a control group limiting the ability to compare the diagnostic flow chart to current daily clinical practice according to the guideline. However, by comparing the flow chart in theory, in practice and a guideline scenario we are able to draw valid conclusions regarding the costs of the diagnostic flow chart. Second, it must be noted that the measures of diagnostic accuracy are dependent on the population (eg peanut allergy prevalence, age, setting and country), limiting generalizability, and we did not take pretest probability into account. Third, we may have underestimated the direct out‐of‐pocket costs borne by patients and families as we included only the costs of travel to the hospital. A recent systematic review assessed the economic burden of food allergy and showed that food allergy is associated with substantial out‐of‐pocket costs, for instance the costs of special allergen‐free foods, costs of safe child care and copayments for medications.30, 31 In addition, there is a risk of registration error as direct costs were based on financial healthcare data and indirect costs were based on the average hourly wage. However, our results on the difference in costs between the diagnostic flow chart and the current guideline are not likely influenced by these limitations as the data collection methods were comparable between the analysed scenarios. Finally, one‐fourth of children were not included in the analysis on the validation of Ara h 2 cut‐off levels and the diagnostic accuracy and safety of the diagnostic flow chart as these children did not undergo further diagnostic testing after the Ara h 2 result. However, the denoted reasons to decline further testing provide meaningful insight into psychosocial barriers and practical implications of the implementation of a new diagnostic strategy in daily practice.
The results of our study are strengthened by the prospective recruitment of all children with a suspected peanut allergy in four centres representing a large area in the Netherlands. In addition, this is the first study that evaluated the validation of Ara h 2 cut‐off levels and the prospective implementation of a diagnostic flow chart based on sensitization to peanut component Ara h 2 in daily practices in secondary and tertiary care. Our study assessed a broad range of relevant outcomes, including validation, diagnostic accuracy, safety, adherence, direct and indirect costs and anxiety levels following diagnostic testing.
In conclusion, previously published cut‐off levels of sIgE to Ara h 2 were validated in children with suspected peanut allergy in secondary and tertiary care. The diagnostic flow chart based on these Ara h 2 cut‐off levels was accurate and beneficial in terms of costs and parental anxiety levels. Our results support the continued use of the diagnostic flow chart and implementation of the diagnostic flow chart in our national guideline. Furthermore, an (adapted) diagnostic flow chart may be used in other countries after international validation studies.
CONFLICTS OF INTEREST
All authors have declared that they have no competing interest in relation to this study. Outside the submitted work, dr. Gorissen reports personal fees from Nutricia and from ALK; dr. van Velzen reports personal fees from Hero and from Mead Johnson; dr. van der Ent reports grants from GSK, grants from Nutricia, grants from TEVA, grants from Gilead, grants from Vertex, grants from ProQR, grants from Proteostasis, grants from Galapagos NV, grants from Eloxx. In addition, dr. van der Ent has a patent 10006904 with royalties paid.
AUTHORS’ CONTRIBUTIONS
HK substantially contributed to design, concept, acquisition of data, analysis and interpretation of data, drafting the article, final approval of the version to be published and agreed to be accountable for all aspects of the work. FE and TL substantially contributed to design, concept, acquisition of data, interpretation of data, drafting the article, final approval of the version to be published and agreed to be accountable for all aspects of the work. DG, MS, MV, AK, CE and YM substantially contributed to design, interpretation of data, revising critically for important intellectual content, final approval of the version to be published and agreed to be accountable for all aspects of the work. WK and GF substantially contributed to design, concept, acquisition of data, interpretation of data, revising critically for important intellectual content, final approval of the version to be published and agreed to be accountable for all aspects of the work.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We thank Ted Klok1, Eva C. Koffeman2 and Jurgen Jansen3 for their help with patient inclusions. We thank Joyce Faber1, Linda Oudenaller3, Lisanne van Berkel2 and Francina de Graaf2 for their help with collecting patient data.
1.Department of Pediatrics, Deventer Hospital, Deventer, the Netherlands
2.Department of Pediatric Pulmonology and Allergology, Wilhelmina Children’s Hospital, University Medical Center Utrecht, University of Utrecht, Utrecht, The Netherlands
3.Department of Pediatrics, Meander Medical Center, Amersfoort, The Netherlands
Kansen HM, van Erp FC, Meijer Y, et al. Diagnostic accuracy of Ara h 2 for detecting peanut allergy in children. Clin Exp Allergy. 2021;51:1069–1079. 10.1111/cea.13987
Funding information
None.
Data Availability Statement
The study protocol and all data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A. Prevalence of common food allergies in Europe: A systematic review and meta‐analysis. Allergy. 2014;69(8):992‐1007. 10.1111/all.12423. [DOI] [PubMed] [Google Scholar]
- 2.Perkin MR, Logan K, Tseng A, et al. Randomized Trial of Introduction of Allergenic Foods in Breast‐Fed Infants. N Engl J Med. 2016;374(18):1733‐1743. 10.1056/NEJMoa1514210. [DOI] [PubMed] [Google Scholar]
- 3.Kansen HM, Le TM, Meijer Y, et al. The impact of oral food challenges for food allergy on quality of life: A systematic review. Pediatr Allergy Immunol. 2018;29(5):527‐537. 10.1111/pai.12905. [DOI] [PubMed] [Google Scholar]
- 4.Zijlstra WT, Flinterman AE, Soeters L, et al. Parental anxiety before and after food challenges in children with suspected peanut and hazelnut allergy. Pediatr Allergy Immunol. 2010;21:e439–e445. 10.1111/j.1399-3038.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- 5.Muraro A, Werfel T, Hoffmann‐Sommergruber K, et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and management of food allergy. Allergy. 2014;69(8):1008‐1025. 10.1111/all.12429. [DOI] [PubMed] [Google Scholar]
- 6.van Erp FC, Knulst AC, Meijer Y, Gabriele C, van der Ent CK. Standardized food challenges are subject to variability in interpretation of clinical symptoms. Clin Transl Allergy. 2014;4(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Erp FC, Knol EF, Pontoppidan B, Meijer Y, van der Ent CK, Knulst AC. The IgE and basophil responses to Ara h 2 and Ara h 6 are good predictors of peanut allergy in children. J Allergy Clin Immunol. 2017;139(1):358‐360.e8. 10.1016/j.jaci.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 8.Klemans RJB, Otte D, Knol M, et al. The diagnostic value of specific IgE to Ara h 2 to predict peanut allergy in children is comparable to a validated and updated diagnostic prediction model. J Allergy Clin Immunol. 2013;131(1):157‐163. 10.1016/j.jaci.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Ebisawa M, Moverare R, Sato S, Borres MP, Ito K. The predictive relationship between peanut‐ and Ara h 2‐specific serum IgE concentrations and peanut allergy. J Allergy Clin Immunol Pr. 2015;3(1):131‐2.e1. 10.1016/j.jaip.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Suratannon N, Ngamphaiboon J, Wongpiyabovorn J, Puripokai P, Chatchatee P. Component‐resolved diagnostics for the evaluation of peanut allergy in a low‐prevalence area. Pediatr Allergy Immunol. 2013;24(7):665‐670. 10.1111/pai.12125. [DOI] [PubMed] [Google Scholar]
- 11.Eller E, Bindslev‐Jensen C. Clinical value of component‐resolved diagnostics in peanut‐allergic patients. Allergy Eur J Allergy Clin Immunol. 2013;68(2):190‐194. 10.1111/all.12075. [DOI] [PubMed] [Google Scholar]
- 12.Vlieg‐Boerstra BJ, Herpertz I, Pasker L, et al. Validation of novel recipes for double‐blind, placebo‐controlled food challenges in children and adults. Allergy Eur J Allergy Clin Immunol. 2011;66(7):948‐954. 10.1111/j.1398-9995.2010.02539.x. [DOI] [PubMed] [Google Scholar]
- 13.Tarieventabel dbc‐zorgproducten en overige zorgproducten per 1 januari. Nederlandse Zorgautoriteit; 2018. https://puc.overheid.nl/nza/doc/PUC_13274_22/1/Accessed December 6, 2019.
- 14.Hakkaart‐van Roijen L, van der Linden N, Bouwmans C, Kanters T, Tan SS. Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg (Bijlage 1). https://www.zorginstituutnederland.nl/over‐ons/publicaties/publicatie/2016/02/29/richtlijn‐voor‐het‐uitvoeren‐van‐economische‐evaluaties‐in‐de‐gezondheidszorg. Published 2015. Accessed December 9, 2019.
- 15.Nederlandse Vereniging voor Allergologie. Richtlijn Voedselprovocatie. https://www.nvk.nl/Portals/0/richtlijnen/Voedselprovocatie/Richtlijn Voedselprovocatie.pdf. Published 2015. Accessed December 9, 2019.
- 16.Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the State‐Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists; 1983. [Google Scholar]
- 17.Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the State‐Trait Anxiety Inventory for Children. Palo Alto (CA): Consulting Psychologists; 1973. [Google Scholar]
- 18.Corsaletti B, Proença M‐D, Bisca G, Leite J, Bellinetti L, Pitta F. Minimal important difference for anxiety and depression surveys after intervention to increase daily physical activity in smokers. Fisioter e Pesqui. 2014;21(4):359‐364. [Google Scholar]
- 19.Dhand NK, Statulator KMS. An online statistical calculator. Sample Size Calculator for Estimating a Single Proportion [Internet]. 2014. [cited 2020 Aug 13]. Available from: http://statulator.com/SampleSize/ss1P.html
- 20.Asarnoj A, Glaumann S, Elfstrom L, et al. Anaphylaxis to peanut in a patient predominantly sensitized to Ara h 6. Int Arch Allergy Immunol. 2012;159(2):209‐212. 10.1159/000336027. [DOI] [PubMed] [Google Scholar]
- 21.Leo SH, Dean JM, Jung B, Kuzeljevic B, Chan ES. Utility of Ara h 2 sIgE levels to predict peanut allergy in Canadian children. J Allergy Clin Immunol Pr. 2015;3(6):968‐969. 10.1016/j.jaip.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Zambrano Ibarra G, Fuentes Aparicio V, Infante Herrero S, Blanca M, Zapatero Remon L. Peanut Allergy in Spanish Children: Comparative Profile of Peanut Allergy versus Tolerance. Int Arch Allergy Immunol. 2019;178(4):370‐376. 10.1159/000495579. [DOI] [PubMed] [Google Scholar]
- 23.Preece K, Bhatia R, Belcher J, et al. The fraction of exhaled nitric oxide improves prediction of clinical allergic reaction to peanut challenge in children. Clin Exp Allergy. 2014;44(3):371‐380. 10.1111/cea.12258. [DOI] [PubMed] [Google Scholar]
- 24.Ballmer‐Weber BK, Lidholm J, Fernandez‐Rivas M, et al. IgE recognition patterns in peanut allergy are age dependent: perspectives of the EuroPrevall study. Allergy. 2015;70(4):391‐407. 10.1111/all.12574. [DOI] [PubMed] [Google Scholar]
- 25.Hazebrouck S, Guillon B, Paty E, Dreskin SC, Adel‐Patient K, Bernard H. Variable IgE cross‐reactivity between peanut 2S‐albumins: The case for measuring IgE to both Ara h 2 and Ara h 6. Clin Exp Allergy. 2019:1107‐1115. 10.1111/cea.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blankestijn MA, Otten HG, Suer W, Weimann A, Knol EF, Knulst AC. Specific IgE to peanut 2S albumin Ara h 7 has a discriminative ability comparable to Ara h 2 and 6. Clin Exp Allergy. 2018;48(1):60‐65. 10.1111/cea.13030. [DOI] [PubMed] [Google Scholar]
- 27.Couch C, Franxman T, Greenhawt M. The economic effect and outcome of delaying oral food challenges. Ann Allergy Asthma Immunol. 2016;116(5):420‐424. 10.1016/j.anai.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Le T‐M, Hoffen E, Kummeling I, et al. Food allergy in the Netherlands: differences in clinical severity, causative foods, sensitization and DBPCFC between community and outpatients. Clin Transl Allergy. 2015;5:8. 10.1186/s13601-015-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunst NR, Lindvik H, Carlsen K‐H, Håland G, Jørgensen E, Lødrup Carlsen KC. Cost‐effectiveness of diagnostic algorithms for peanut allergy in children. J Allergy Clin Immunol. 2019;143(3):1243‐1246. 10.1016/j.jaci.2018.10.050. [DOI] [PubMed] [Google Scholar]
- 30.Protudjer JLP, Jansson SA, Arnlind MH, et al. Household costs associated with objectively diagnosed allergy to staple foods in children and adolescents. J Allergy Clin Immunol Pract. 2015;3(1):68‐75. 10.1016/j.jaip.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Bilaver LA, Chadha AS, Doshi P, Dwyer LO, Gupta RS. Economic burden of food allergy A systematic review. Ann Allergy, Asthma Immunol. 2019;122(4):373‐380.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The study protocol and all data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


