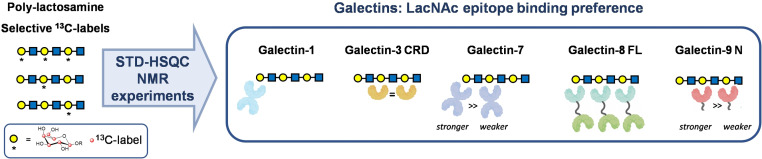
Abstract
A combined chemo‐enzymatic synthesis/NMR‐based methodology is presented to identify, in unambiguous manner, the distinctive binding epitope within repeating sugar oligomers when binding to protein receptors. The concept is based on the incorporation of 13C‐labels at specific monosaccharide units, selected within a repeating glycan oligomeric structure. No new chemical tags are added, and thus the chemical entity remains the same, while the presence of the 13C‐labeled monosaccharide breaks the NMR chemical shift degeneracy that occurs in the non‐labeled compound and allows the unique identification of the different components of the oligomer. The approach is demonstrated by a proof‐of‐concept study dealing with the interaction of a polylactosamine hexasaccharide with five different galectins that display distinct preferences for these entities.
Keywords: galectins, molecular recognition, NMR, polylactosamine, selective 13C-labels
Repeating carbohydrate patterns are commonly found in Nature in extended oligosaccharides and polysaccharides. When recognized by protein receptors, dissecting the contribution of each repeating unit to the binding event is not easy. Here, the introduction of 13C‐labels at specific positions of a tri‐LacNAc repeating hexasaccharide has been used in STD‐HSQC NMR experiments to reveal the preferred binding epitopes for a panel of human galectins.
Introduction
Repeating‐sugar sequences to form extended oligosaccharides and polysaccharides are ubiquitous among living organisms. These elongated structures can interact with carbohydrate‐specific antibodies[1] or glycan binding proteins[2] to mediate diverse biological events.[3] From a supramolecular chemistry perspective, the oligo‐ or polymeric glycan pattern is thought to increase the avidity (and thus apparent affinity) of these binding processes that are otherwise weak.[4] Understanding the mechanisms that regulate such multivalent interactions at the molecular level, and the resulting consequences in terms of binding affinity and selectivity, represents a challenging task, usually hampered by the difficulty in deconvoluting the single contributions of each of the repeating units to the global binding event.
Poly‐N‐acetyllactosamine (poly‐LacNAc), which is a chemical structure present on many mammalian glycans, is composed of repeating units of the disaccharide N‐acetyllactosamine (LacNAc, Galβ1‐4GlcNAcβ). These extensions are commonly found on glycolipids and glycoproteins,[5] where their presence has been associated to key biological processes, especially related to immune response regulation.[6] Poly‐LacNAc extensions serve as elongated scaffolds for the presentation of specific epitopes (such as ABO, Lewis or HNK‐1 antigens, sialic acid or keratan sulfate)[7] for their interaction with specific receptors, but they are also binding epitopes themselves, acting as ligands for a family of endogenous lectins,[8] the galectins.
This lectin family, widely distributed in animals, comprises 15 different members in humans that are associated with a remarkable number of pathological phenomena, mostly related to immunity,[9] cancer[10] and autoimmune disorders.[11] All galectins share the ability to bind LacNAc. Structural modifications of this common epitope, including its sequential repetition into poly‐LacNAc chains, modulate the affinity and selectivity for the different galectins.[12, 13] In fact, different binding mechanisms and preferences are expected for each galectin towards poly‐LacNAc chains.[16] These associations become particularly significant on cell surfaces, where they give rise to the so‐called galectin lattice,[14] which impacts cell surface dynamics and regulates receptor localization and signaling.[15] Studies of the differential binding preferences of galectins for poly‐LacNAc have yielded somehow conflicting results. The discrepancies have been interpreted[16] in terms of the intrinsic differences of the various techniques employed in each study, which ultimately reflect the unique binding mode of each galectin. Indeed, an additional level of regulation operating in galectin‐mediated binding events is provided by the multivalent nature of galectins, which leads to different binding outcomes when surface‐bound ligands versus in solution are compared.[16]
Galectins are classified into three different groups depending on the organization of their Carbohydrate Recognition Domains (CRDs). Prototype galectins, which include Galectins (Gal) ‐1, ‐2, ‐7, ‐10, ‐11, ‐13, ‐14 and ‐15, are non‐covalent homodimers with two identical CRDs; tandem repeat galectins, including Gal‐4, ‐6, ‐8, ‐9 and ‐12, are heterodimers containing two different CRDs covalently linked through a short peptide; while Gal‐3, the only member of the chimera type, features a single CRD with a long peptide chain through which it forms oligomers of different size. It is nowadays assumed that Gal‐3, the C‐terminal domain of Gal‐8 (Gal‐8C), and both the C‐ and N‐terminal domains of Gal‐9 (Gal‐9C and Gal‐9N) preferentially bind internal LacNAc units, thus acting as endo‐type lectins, while Gal‐1, Gal‐2, Gal‐7 and the N‐terminal domain of Gal‐8 (Gal‐8N) prefer terminal positions, thus acting as exo‐type lectins.[2] These conclusions have been based on macroscopic‐type biological/biochemical assays and, to the best of our knowledge, no unambiguous experimental evidence has been yet presented for some of these systems and the structural basis for selectivity remains elusive. When multiple poly‐LacNAc chains are installed into glycoconjugates, as part of branched N‐ and O‐glycans, understanding how these extensions affect galectin binding becomes a challenging endeavor.[17, 18]
Herein, an NMR‐based approach to easily identify the preferred binding epitope within repeating oligomeric glycans when binding to protein receptors is presented. It is based on the incorporation of 13C‐labels in selected monosaccharide units within a repeating glycan oligomer. Thus, the chemical entity remains the same, but the 13C‐label breaks the NMR chemical shift degeneracy that occurs in the non‐labeled compound. Therefore, ligand‐based NMR strategies can be employed to report on the specific binding epitopes. The introduction of selective 13C‐labels into carbohydrate oligomers has been reported for a linear hexamer of β1‐6 linked glucose,[19] providing structural and conformational information otherwise not accessible.
Herein, 13C‐labels have been introduced at specific positions of a poly‐LacNAc fragment composed of three repeating LacNAc units (tri‐LaNAc). The study of their specific interaction with a panel of galectins was carried out by 2D STD‐1H,13C‐HSQC NMR experiments,[20] which revealed the precise preference of each galectin for a specific LacNAc unit. Previously, the enzymatic synthesis of di‐ and tri‐LacNAc oligosaccharides with 13C‐galactose enrichment has been reported[21] to monitor Gal‐1 binding through 1H‐line broadening effects. Herein, we describe the enzymatic synthesis of hexasaccharides composed of three LacNAc units (tri‐LacNAc) with uniformly 13C‐labeled‐galactose (UL‐13C6‐galactose) units introduced at selected positions. The complementary UL‐13C6‐galactose labeling Scheme (Scheme 1) permitted to distinguish the individual contribution of each LacNAc moiety to tri‐LacNAc binding by different galectins.
Scheme 1.
Left. The typical N‐glycan structure containing one poly‐LacNAc chain at the α6 branch. Right. The tri‐LacNAc oligomers containing selective 13C‐C6‐galactose labeling, synthesized and employed in this study.
Results and Discussion
Three tri‐LacNAc hexasaccharide were obtained with different labeling: 5 a with all the three‐galactose residues 13C‐labeled, and two tri‐LacNAc hexasaccharides, with a single‐galactose 13C‐labeled at either the middle (5 b) or the reducing‐end (5 c). Tetrasaccharides 3 (3 a, 3 b and 3 c) were straightforward prepared by the subsequent treatment of commercial N‐acetyllactosamine 1 b and 1 a with β1,3‐N‐acetylglucosaminyltransferase (HP‐39) in the presence of uridine‐5′‐diphospho (UDP)‐N‐acetyl‐glucosamine (GlcNAc) and then with β1,4‐galactosyltransferase (LgtB) and UDP‐galactose or UDP‐UL‐13C6‐galactose, respectively. Repeated action with HP‐39 and LgtB installed a third modified LacNAc motif to furnish the target hexasaccharides 5 (5 a, 5 b and 5 c) (Figure 1).[22, 23]
Figure 1.
Synthesis of tri‐LacNAc compounds 5 a, 5 b and 5 c with different 13C‐labeling schemes. Reagents and conditions: a) HP‐39, UDP‐GlcNAc; b) LgtB, UDP‐UL‐13C6‐galactose; c) LgtB, UDP‐galactose.
In this study, five galectins (or galectin domains) from all three families were selected to be interrogated about their binding preferences toward poly‐LacNAc structures: (i) two homodimeric prototype galectins, Gal‐1 and Gal‐7, (ii) the CRD of the chimera‐type Gal‐3 (Gal‐3 CRD), and (iii) the full length tandem‐repeat Gal‐8 (Gal‐8 FL) were used, along with the N‐terminal domain of Gal‐9 (Gal‐9N), for which X‐ray crystallographic structures have been obtained in complex with a tri‐LacNAc fragment.[24] All galectins were obtained through E. coli overexpression and purified as described in the Supporting Information.
Binding of the differently labeled tri‐LacNAc compounds (5 a, 5 b, 5 c) to the selected galectins was monitored through 2D STD‐1H,13C‐HSQC NMR experiments. Conventional 1D 1H‐STD NMR experiments have been extensively used to study the recognition of β‐galactose containing glycans by galectins,[25, 26] whereby the strongest STD effect systematically corresponds to protons H6, H5 and H4 of the β‐galactose residue, central element in these binding events, providing most of the intermolecular interactions, including a key CH‐π stacking with a conserved tryptophan (Trp) residue at the galectin binding site.[27, 28]
In the regular HSQC spectrum of the tri‐labeled compound 5 a (Figure 2 and SI), the cross‐peaks for C2, C3, C4 and C5 of the B and C β‐galactose residues resonate at identical positions, while those for residue A display clearly differentiated cross‐peaks. On the contrary, the signals for C1 (anomeric) and C6 were indistinguishable for all the three β‐galactose A, B and C residues. Thus, this compound permits a straightforward discrimination of the binding preference of the different galectins for the terminal (A) versus non‐terminal β‐galactose residues (B and C), just by observing the relative STDs of the different signals (C2‐H2, C3‐H3, C4‐H4 and C5‐H5) in the 2D STD‐1H,13C ‐HSQC spectra. This is shown in Figure 2, where the 2D STD‐1H,13C ‐HSQC spectra for the interaction of tri‐LacNAc 5 a with the 5 galectins are shown in panel A. The analysis of the individual STD spectra and the epitope mapping for each galectin is shown in Figure S14. Remarkably, for all spectra, within every β‐galactose residue, the signal showing the strongest STD effect is consistently that of the C5‐H5, followed by that of C4‐H4, and finally C3‐H3 and C2‐H2, in agreement with the expected binding mode described above. To facilitate the comparison among all systems, the STD values (in %) of all the signals corresponding to the A residue were added and compared to the sum of the STDs corresponding to residues B and C, which are indistinguishable (Figure 2 B). The conclusions about the terminal versus non‐terminal epitope binding preferences of the five galectins can be easily drawn from Figure 2. Gal‐1 is the only galectin that shows specificity for the terminal LacNAc, while the binding of the other prototype galectin, Gal‐7 is not exclusive for the terminal epitope. In contrast, chimera type Gal‐3 CRD and the N‐terminal domain of tandem repeat type Gal‐9 preferentially recognize non‐terminal LacNAc epitopes, while the likewise tandem repeat Gal‐8 does not discriminate between terminal and non‐terminal LacNAc moieties.
Figure 2.
(A) 2D STD‐1H,13C‐HSQC NMR spectra of tri‐LacNAc 5a (with 13C6‐labeled‐galactose at residues A, B and C) in the presence of Gal‐1, Gal‐3 CRD, Gal‐7, Gal‐8 FL, and Gal‐9N (ligand:galectin molar ratios are 30:1). Left, 2D 1H,13C‐HSQC spectrum with signal assignment and right, 2D STD‐1H,13C‐HSQC NMR spectra. (B) Corresponding sum of STD effects (in percent) for all signals of the terminal galactose A (blue) and the two spectrally indistinguishable residues B and C (gray). The STD sum was computed from the net signal integrals in the 2D 1H,13C‐HSQC spectrum with on‐resonant protein saturation (2 s at 0.84 ppm using a train of 33 ms PC9_4 90° pulses) vs. off‐resonant irradiation (at −25 ppm), * with exclusion of the indistinguishable C1‐H1 and C6‐H6 signals, and C5‐H5 in Gal‐8 FL. See the Supplementary Material for further details.
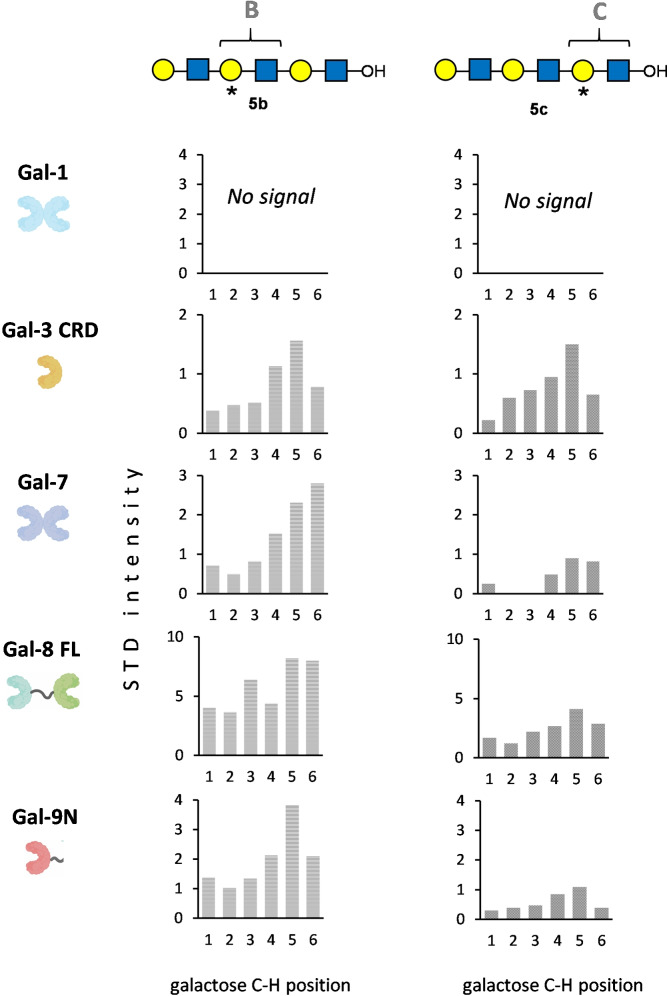
Next, to further distinguish binding preferences for the reducing‐end (residue C) versus internal (residue B) LacNAc units, compounds 5 c and 5 b, with a single 13C6‐labeled‐galactose moiety in the corresponding positions, were employed. The 2D STD‐1H,13C‐HSQC spectra are shown in the SI. In this case, all the signals in each spectrum correspond unambiguously to the only labeled β‐galactose residue in each case: B in compound 5 b and C in compound 5 c. The analysis of the STD values determined for all the cross‐peaks in the 2D STD‐1H,13C‐HSQC spectra of 5 b and 5 c with the different galectins is shown in Figure 3. Remarkably, prototype Gal‐1 showed absence of any STD effects, evidencing no recognition of either internal or reducing‐end β‐galactose residues. The fact that the 2D STD‐1H,13C‐HSQC spectrum with the tri‐labeled analogue (5 a) did showed detectable, although very weak, STD signal for these non‐terminal positions (Figure 2 A) could be explained by a very weak interaction, below the detection limit in the experiments with compounds 5 b and 5 c, but observable with 5 a where the cross‐peaks display double intensity with respect to those in the mono‐labeled compounds.
Figure 3.
Analysis of 2D STD‐1H,13C‐HSQC NMR experiments for 5b and 5c with the different galectins. The plots represent the STD intensity in % ((cross‐peak integral in the STD spectrum/cross‐peak intensity in the reference spectrum) ×100) of every CH cross‐peak of the galactose residue (represented in the X axis). Pertaining 2D STD‐1H,13C‐HSQC NMR spectra were recorded as detailed in Figure 2.
For all the other galectins, the 2D STD‐1H,13C‐HSQC spectra for 5 b and 5 c showed STD signals, and for all of them except for Gal‐3 CRD, the STD intensities were always stronger for 5 b than for 5 c. This fact demonstrates that, although both β‐galactose residues at positions B and C are binding epitopes, the binding through the reducing‐end LacNAc (C) is much weaker than through the middle LacNAc (B) epitope. This difference is less pronounced for tandem‐repeat Gal‐8 for which the STD for residue B is only 2‐fold stronger than that for residue C, and not the case for Gal‐3 CRD, which binds B and C epitopes equally.
Besides yielding residue‐specific information, the strategy described herein also affords atomic‐specific information, providing molecular details of the complexes. For 5 b and 5 c, the STD values for all the CH correlations of the bound galactose residue were available. The corresponding information was not available for 5 a, given the overlapping for the galactose H6s for all the three LacNAc units. The obtained STD profiles for each β‐galactose with the different galectins (Figure 3) were very similar, showing a general trend where the strongest STD effect is experienced by position 5 of the galactose residue, which basically agrees with the known binding modes for the reported complexes between galectins and Lac/LacNAc‐containing molecules complexes. Interestingly, minor differences were found for the Gal‐7/5 b complex, where the strongest STD corresponded instead to position 6. This minor difference could be indicative of subtle differences in the binding pose. Thus, the deposited X‐ray crystallographic structures of the complexes formed between LacNAc and Gal‐3, Gal‐9N and Gal‐7 were compared, showing that the tilt of the galactopyranose ring with respect to the conserved Trp residue at the binding site is slightly different in the complex with Gal‐7 to those of Gal‐3 and Gal‐9N (Figure S16), thus providing a possible explanation of the STD NMR experimental observations
For the interaction of Gal‐3 with 5 a, the HSQC (Figure S15) and 2D STD‐1H,13C‐HSQC spectrum (Figure S10) showed a new set of weak cross‐peaks. A 13C‐decoupled‐ROESY spectrum (Figure S15) confirmed that they correspond to the bound ligand form, as deduced from their chemical exchange cross‐peaks with the signals of B+C Gal residues in the free form. Gal‐3 has the strongest binding affinity for a single LacNAc unit (K D=53 μM)[25] among the galectins studied herein. We have previously reported the detection of the galectin‐bound ligand NMR signals;[25, 29] however, this is generally difficult to achieve given the typical low affinity interactions. Herein, the 13C‐labeling provides the impetus to deduce exquisite structural information of the ligand in the bound form.[30] In this case, all CH signals of the β‐galactose were shifted up‐field due to the sugar‐aromatic CH‐π stacking interaction, being the extent of the shift proportional to the distance of the CH to the conserved galectin Trp (W168 in Gal‐3 CRD) residue in the binding site.[25]
Conclusion
Poly‐ and oligo‐meric glycans, composed of repeating (mono‐ or oligo‐) saccharide units, such as polysialic acid, polylactosamine, glucan, chitin, glycosaminoglycans, capsular polysaccharides, etc…, are ubiquitous structures in Nature. Although NMR is one of the best suited techniques to provide atomic detailed information on their recognition by protein receptors, the NMR chemical shift degeneracy occurring in homo‐oligomers significantly limits the profundity of the analysis. Herein, the introduction, of 13C‐labels at selected positions permits to pinpoint the specific glycan binding epitopes in a repeating glycan oligomer sequence.
As proof‐of‐concept, the proposed approach has been applied to examine the binding preferences of various galectins for poly‐LacNAc sequences. As expected,[12, 13, 31, 32] Gal‐1 showed specificity for the terminal LacNAc epitope, while Gal‐3 CRD exhibited the full opposite preference. Gal‐7 also displayed preference for the terminal position, although with less selectivity than Gal‐1. In fact, this lectin also binds the internal LacNAc (B) epitope, with only slightly lower affinity than the terminal one, while the affinity was drastically reduced (Figure 2 & Figure 3) for the reducing‐end LacNAc (C). This is an interesting result considering that previous studies on Gal‐7 binding to poly‐LacNAc had shown little or even no effect on the affinity depending on the number of LacNAc units.[12, 32] Gal‐9N showed similar preferences to Gal‐3 CRD, with almost no interaction with the terminal (A) epitope. While this was expected for Gal‐3, this is an interesting result for Gal‐9N, since crystals of this domain only bound to the terminal β‐galactose unit in di‐LacNAc sequences have been obtained and solved by X‐ray crystallography (pdb 2ZHK and 2ZHL).[24] Our data shows however, that in solution, binding to the internal epitope is clearly preferred. Finally, full‐length Gal‐8 showed no discrimination for the different LacNAc positions.[12] Therefore, the methodology presented herein allows answering precise molecular recognition questions in a direct manner, pushing the limits of applications of NMR to the analysis of complex glycans with repetitive or multivalent presentations. Morever, this combined specific‐labeling/NMR‐based approach should also be useful beyond its application for disentangling the role of specific residues in complex glycans. The concept can be also adapted to study and dissect multivalent interactions in diverse supramolecular systems that contain repetitions of the same building block.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
This research was funded by European Research Council for financial support (ERC‐2017‐AdG, project number 788143‐RECGLYCANMR). We also thank Agencia Estatal de Investigación (Spain) for project RTI2018‐094751‐B‐C21 and the Severo Ochoa Excellence Accreditation (SEV‐2016‐0644). We also thank Marcos Gómez‐Redondo and Sara Bertuzzi for their kelp with the purification of Gal‐8 and Gal‐1, respectively.
M. J. Moure, A. Gimeno, S. Delgado, T. Diercks, G.-J. Boons, J. Jiménez-Barbero, A. Ardá, Angew. Chem. Int. Ed. 2021, 60, 18777.
Dedicated to Professor Manuel Martin‐Lomas on the occasion of his 80th anniversary
Contributor Information
Prof. Jesús Jiménez‐Barbero, Email: jjbarbero@cicbiogune.es.
Dr. Ana Ardá, Email: aarda@cicbiogune.es.
References
- 1.
- 1a.Zhang Y., Gómez-Redondo M., Jiménez-Osés G., Ardá A., Overkleeft H. S., Marel G. A., Jiménez-Barbero J., Codée J. D. C., Angew. Chem. Int. Ed. 2020, 59, 12746–12750; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 12846–12850; [Google Scholar]
- 1b.Gómez-Redondo M., Ardá A., Gimeno A., Jiménez-Barbero J., Drug Discovery Today Technol. 2020, 35–36, 1–11; [DOI] [PubMed] [Google Scholar]
- 1c.Zhang Q., Gimeno A., Santana D., Wang Z., Valdés-Balbin Y., Rodríguez-Noda L. M., Hansen T., Kong L., Shen M., Overkleeft H. S., Vérez-Bencomo V., van der Marel G. A., Jiménez-Barbero J., Chiodo F., Codée J. D. C., ACS Cent. Sci. 2019, 5, 1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagae M., Yamaguchi Y., Int. J. Mol. Sci. 2014, 15, 3768–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.
- 3a.Lenman A., Liaci A. M., Liu Y., Frängsmyr L., Frank M., Blaum B. S., Chai W., Podgorski I. I., Harrach B., Benkő M., Feizi T., Stehle T., Arnberg N., Proc. Natl. Acad. Sci. USA 2018, 115, E4264–E4273; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3b.Briard B., Fontaine T., Samir P., Place D. E., Muszkieta L., Malireddi R. K. S., Karki R., Christgen S., Bomme P., Vogel P., Beau R., Mellado E., Ibrahim-Granet O., Henrissat B., Kalathur R. C., Robinson C., Latgé J.-P., Kanneganti T.-D., Nature 2020, 588, 688–692; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3c.Tamura K., Dejean G., Van Petegem F., Brumer H., J. Biol. Chem. 2021, 296, 100415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiessling L. L., Grim J. C., Chem. Soc. Rev. 2013, 42, 4476–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.
- 5a.Benicky J., Sanda M., Brnakova Kennedy Z., Grant O. C., Woods R. J., Zwart A., Goldman R., J. Proteome Res. 2021, 20, 485–497; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5b.Liu Y., Huang P., Jiang B., Tan M., Morrow A. L., Jiang X., PLoS One 2013, 8, e78113; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5c.Nimrichter L., Burdick M. M., Aoki K., Laroy W., Fierro M. A., Hudson S. A., Von Seggern C. E., Cotter R. J., Bochner B. S., Tiemeyer M., Konstantopoulos K., Schnaar R. L., Blood 2008, 112, 3744–3752; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5d.Handa K., Stroud M. R., Hakomori S., Biochemistry 1997, 36, 12412–12420. [DOI] [PubMed] [Google Scholar]
- 6.Togayachi A., in Glycosci. Biol. Med. (Eds.: Taniguchi N., Endo T., Hart G. W., Seeberger P. H., Wong C.-H.), Springer Japan, Tokyo, 2015, pp. 691–698. [Google Scholar]
- 7.
- 7a.Yaji S., Manya H., Nakagawa N., Takematsu H., Endo T., Kannagi R., Yoshihara T., Asano M., Oka S., Glycobiology 2015, 25, 376–385; [DOI] [PubMed] [Google Scholar]
- 7b.Caterson B., Melrose J., Glycobiology 2018, 28, 182–206; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7c.P. Stanley, R. D. Cummings, “Structures Common to Different Glycans,” can be found under http://www.ncbi.nlm.nih.gov/pubmed/28876849, 2015.
- 8.
- 8a.Johannes L., Jacob R., Leffler H., J. Cell Sci. 2018, 131, jcs208884; [DOI] [PubMed] [Google Scholar]
- 8b.Bertuzzi S., Quintana J. I., Ardá A., Gimeno A., Jiménez-Barbero J., Front. Chem. 2020, 8, 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.
- 9a.Giovannone N., Smith L. K., Treanor B., Dimitroff C. J., Front. Immunol. 2018, 9, 2839; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9b.Robinson B. S., Arthur C. M., Evavold B., Roback E., Kamili N. A., Stowell C. S., Vallecillo-Zúniga M. L., Van Ry P. M., Dias-Baruffi M., Cummings R. D., Stowell S. R., Front. Immunol. 2019, 10, 1762; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9c.Thiemann S., Baum L. G., Annu. Rev. Immunol. 2016, 34, 243–264. [DOI] [PubMed] [Google Scholar]
- 10.Dimitroff C. J., Cancer Res. 2015, 75, 3195–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabinovich G. A., Croci D. O., Immunity 2012, 36, 322–335. [DOI] [PubMed] [Google Scholar]
- 12.Hirabayashi J., Biochim. Biophys. Acta Gen. Subj. 2002, 1572, 232–254. [DOI] [PubMed] [Google Scholar]
- 13.Stowell S. R., Arthur C. M., Mehta P., Slanina K. A., Blixt O., Leffler H., Smith D. F., Cummings R. D., J. Biol. Chem. 2008, 283, 10109–10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nabi I. R., Shankar J., Dennis J. W., J. Cell Sci. 2015, 128, 2213–2219. [DOI] [PubMed] [Google Scholar]
- 15.Mkhikian H., Mortales C. L., Zhou R. W., Khachikyan K., Wu G., Haslam S. M., Kavarian P., Dell A., Demetriou M., eLife 2016, 5, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamili N. A., Arthur C. M., Gerner-Smidt C., Tafesse E., Blenda A., Dias-Baruffi M., Stowell S. R., Proteomics 2016, 16, 3111–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patnaik S. K., Potvin B., Carlsson S., Sturm D., Leffler H., Stanley P., Glycobiology 2006, 16, 305–317. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen M. I., Stegmayr J., Grant O. C., Yang Z., Nilsson U. J., Boos I., Carlsson M. C., Woods R. J., Unverzagt C., Leffler H., Wandall H. H., J. Biol. Chem. 2018, 293, 20249–20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delbianco M., Kononov A., Poveda A., Yu Y., Diercks T., Jiménez-Barbero J., Seeberger P. H., J. Am. Chem. Soc. 2018, 140, 5421–5426. [DOI] [PubMed] [Google Scholar]
- 20.
- 20a.Mayer M., Meyer B., Angew. Chem. Int. Ed. 1999, 38, 1784–1788; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 1999, 111, 1902–1906; [Google Scholar]
- 20b.Meyer B., Peters T., Angew. Chem. Int. Ed. 2003, 42, 864–890; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2003, 115, 890–918. [Google Scholar]
- 21.Di Virgilio S., Glushka J., Moremen K., Pierce M., Glycobiology 1999, 9, 353–364. [DOI] [PubMed] [Google Scholar]
- 22.Peng W., Pranskevich J., Nycholat C., Gilbert M., Wakarchuk W., Paulson J. C., Razi N., Glycobiology 2012, 22, 1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park J.-E., Lee K.-Y., Do S.-I., Lee S.-S., BMB Rep. 2002, 35, 330–336. [Google Scholar]
- 24.Nagae M., Nishi N., Murata T., Usui T., Nakamura T., Wakatsuki S., Kato R., Glycobiology 2008, 19, 112–117. [DOI] [PubMed] [Google Scholar]
- 25.Gimeno A., Delgado S., Valverde P., Bertuzzi S., Berbís M. A., Echavarren J., Lacetera A., Martín-Santamaría S., Surolia A., Cañada F. J., Jiménez-Barbero J., Ardá A., Angew. Chem. Int. Ed. 2019, 58, 7268–7272; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 7346–7350. [Google Scholar]
- 26.
- 26a.Bertuzzi S., Gimeno A., Núñez-Franco R., Bernardo-Seisdedos G., Delgado S., Jiménez-Osés G., Millet O., Jiménez-Barbero J., Ardá A., Chem. Eur. J. 2020, 26, 15643–15653; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26b.Gómez-Redondo M., Delgado S., Nuñez-Franco R., Jiménez-Osés G., Ardá A., Jimenez-Barbero J., Gimeno A., RSC Chem. Biol. 2021, 2, 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasta G. R., in Adv. Exp. Med. Biol., Springer, Singapore, 2020, pp. 169–196. [Google Scholar]
- 28.Spiwok V., Molecules 2017, 22, 1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintana J. I., Delgado S., Nuñez-Franco R., Cañada F. J., Jimenez-Osés G., Jimenez-Barbero J., Ardá A., Front. Chem. 2021, 10.3389/fchem.2021.664097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nestor G., Anderson T., Oscarson S., Gronenborn A. M., J. Am. Chem. Soc. 2017, 139, 6210–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leppänen A., Stowell S., Blixt O., Cummings R. D., J. Biol. Chem. 2005, 280, 5549–5562. [DOI] [PubMed] [Google Scholar]
- 32.Brewer C. F., Glycoconjugate J. 2004, 19, 459–465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information