Summary
Objective
This study was undertaken to examine long‐term (up to 7.8 years) retention rate, safety, and tolerability of the antiseizure medication (ASM) cenobamate as adjunctive treatment in the open‐label extension (OLE) of study YKP3089C013 (C013; ClinicalTrials.gov: NCT01397968).
Methods
Patients who completed the 12‐week, multicenter, multinational, double‐blind, randomized, placebo‐controlled C013 study, which examined adjunctive cenobamate treatment of adults with uncontrolled focal seizures, were eligible to enroll in the OLE. During the OLE, dose adjustments of cenobamate and concomitant ASMs were allowed. Safety assessments included frequency of treatment‐emergent adverse events (TEAEs) and serious TEAEs, TEAE severity, and TEAEs leading to discontinuation. Probability of patient continuation in the OLE was examined using a Kaplan–Meier analysis.
Results
One hundred forty‐nine patients entered the OLE (median duration of cenobamate treatment = 6.25 years). As of the data cutoff, 57% of patients (85/149) remained in the OLE (median treatment duration = 6.8 years, range = 6.4–7.8 years). The median modal daily cenobamate dose was 200 mg (range = 50–400 mg). The probability of treatment continuation at 1–6 years of cenobamate treatment was 73%, 67%, 63%, 61%, 60%, and 59%, respectively. Among patients who continued at 1 year (n = 107), the probability of continuing at Years 2–5 was 92%, 87%, 83%, and 82%. The most common discontinuation reasons were patient withdrawal (19.5%, 29/149), adverse event (10.1%, 15/149), and lack of efficacy (5.4%, 8/149). TEAEs leading to discontinuation in 1% or more of patients were fatigue (1.3%, 2/149), ataxia (1.3%, 2/149), and memory impairment or amnesia (1.3%, 2/149). Dizziness (32.9%, 49/149), headache (26.8%, 40/149), and somnolence (21.5%, 32/149) were the most frequently reported TEAEs and were primarily mild or moderate in severity.
Significance
Long‐term retention in the C013 OLE study demonstrated sustained safety and tolerability of adjunctive cenobamate treatment up to 7.8 years in adults with treatment‐resistant focal seizures taking one to three ASMs.
Keywords: epilepsy, focal seizures, long‐term, retention, safety
Key Points.
High long‐term treatment retention is a clinically meaningful outcome because epilepsy is a chronic disease requiring long‐term treatment
This open‐label extension of the cenobamate RCT YKP3089C013 (C013; Chung et al.) examined long‐term treatment retention and tolerability
Sustained safety and tolerability of cenobamate up to 7.8 years was demonstrated in adults with treatment‐resistant focal seizures
Cenobamate treatment retention ranged from 73% of patients at 1 year to 59% of patients at 6 years
1. INTRODUCTION
Cenobamate is an oral tetrazole carbamate derivative antiseizure medication (ASM) that is distinct from other carbamate‐containing ASMs.1 Although the specific mechanism of action of cenobamate is not fully known, it reduces repetitive neuronal firing by inhibiting voltage‐gated sodium currents (preferentially inhibiting the persistent Na+ current) and is also a positive allosteric modulator of the γ‐aminobutyric acid type A (GABAA) receptor.2, 3 Cenobamate's efficacy through its novel mechanism of action of combined actions on persistent sodium currents and tonic GABA currents is supported by a comparative analysis of its unique preclinical profile versus other ASMs.4 Cenobamate was approved by the US Food and Drug Administration for the treatment of adults with focal (partial onset) epilepsy based on the results from two adequate and well‐controlled clinical studies that demonstrated substantial and statistically significant reductions in seizure frequency and high responder rates, including 100% seizure reduction (zero seizures), in patients with uncontrolled focal seizures treated with adjunctive cenobamate.5, 6
Although randomized controlled trials are essential to demonstrate efficacy and safety of a new ASM, they are limited in their application to real‐life clinical practice treating patients with epilepsy due to short trial duration and nonflexible dosing regimens.7 Longer duration assessment is needed to evaluate potential late onset adverse events (AEs) and long‐term tolerability of the ASM.7 An open‐label extension (OLE) study allows examination of treatment continuation (i.e., retention) over a longer treatment duration and with flexible dosing, complementing the evidence provided by a randomized controlled trial. Retention is often used as an indicator of overall treatment satisfaction, incorporating efficacy, safety, and tolerability into the patient decision to continue or discontinue treatment.8, 9 Because epilepsy is a chronic disorder with the need for long‐term ASM treatment, knowledge of the long‐term safety and tolerability of an ASM is needed for clinical practice treatment decisions.
In the 12‐week (6‐week titration and 6‐week maintenance phases), double‐blind, placebo‐controlled, Phase 2 study (YKP3089C013 [C013]; NCT01397968), cenobamate significantly improved seizure control in adults with uncontrolled focal seizures taking one to three concomitant ASMs and was well‐tolerated.5 Patients who completed the 12‐week double‐blind treatment period of study C013 were eligible to participate in an OLE and receive cenobamate treatment. Because seizure diaries were not collected during the OLE, we report here the long‐term safety and retention outcomes from the C013 OLE study. This OLE provides the longest duration evaluation of the safety and tolerability of cenobamate, over a median treatment duration of 6.25 years among all patients who entered the OLE, and more closely reflects clinical practice by allowing flexible dosing of cenobamate and concomitant ASMs.
2. MATERIALS AND METHODS
2.1. Study design and participants
This multicenter, multinational, single‐arm, long‐term OLE enrolled patients who had completed the 12‐week, multicenter, multinational, double‐blind, randomized, placebo‐controlled C013 study, which examined adjunctive cenobamate treatment of adults with uncontrolled focal seizures.5 The double‐blind and OLE studies were performed in accordance with the International Conference on Harmonization Good Clinical Practice Guidelines and country‐specific regulations. The study protocols were approved by independent ethics committees or institutional review boards according to site local regulations. Patients provided written informed consent prior to participation.
Details of the C013 study and patient inclusion and exclusion criteria have been previously published.5 Briefly, patients were randomized to receive placebo or cenobamate at a target dose of 200 mg once daily for 12 weeks (6‐week titration phase, 6‐week maintenance phase). Key inclusion criteria included adult patients 18–65 years old with a diagnosis of treatment‐resistant focal epilepsy,10, 11 history of epilepsy for at least 2 years, and taking one, two, or three ASMs at stable doses for at least 12 weeks before randomization. Within the 8‐week baseline period, patients had to have three or more focal aware seizures with motor component, including aphasia and other observable symptoms; focal impaired awareness; and/or focal to bilateral tonic–clonic (secondarily generalized) seizures per month with no consecutive 21‐day seizure‐free period. Patients receiving phenytoin, phenobarbital, or metabolites of these drugs were excluded due to potential for drug–drug interactions, and patients who received vigabatrin within the past year, felbamate for less than 18 continuous months, or intermittent rescue benzodiazepines more than once per month within the past month were excluded from participation.5 Patients who completed the double‐blind study and continued to meet inclusion/exclusion criteria (except for seizure frequency) were eligible for the OLE.
2.2. Procedures
The OLE was added to the C013 study in a protocol amendment. Patients who completed the double‐blind period before the OLE amendment was approved could re‐enter the study and continue in the OLE. India did not approve the OLE, and thus the C013 study participants in India were excluded from the OLE. Following the protocol amendment that established the OLE, patients entered the OLE after the study drug (cenobamate or placebo) was tapered and discontinued at up to 3–4 weeks following the end of the double‐blind period. Following a later protocol amendment, patients could undergo a blinded conversion to open‐label cenobamate without being tapered off study drug, if in the opinion of the investigator the patient had a clinically meaningful response and it was medically warranted to skip the taper period.
All patients entering the OLE received cenobamate at 100 mg/day, with subsequent dose increases of 50 mg/day every 2 weeks as tolerated. As was done in the double‐blind study, if a patient could not tolerate the next higher dose, they were continued on the current dose or had their dose reduced. The initial maximum cenobamate dose during the OLE was 200 mg/day. Following an amendment approximately 2 years after the initiation of the OLE, the maximum allowable dose during the OLE was increased to 400 mg/day. Whereas no dose changes were allowed in concomitant ASMs during the double‐blind study, investigators could adjust the dosage of, remove, or add concomitant ASMs as clinically indicated during the OLE, with the exception that monotherapy with cenobamate was not allowed.
OLE study visits began at 2‐week intervals, then transitioned to monthly visits, followed by 3 months between visits. After the first year within the OLE, patients had four visits per study year.
2.3. Study outcomes
Study outcomes in the OLE included demographic and safety information only. Safety assessments included frequency of treatment‐emergent AEs (TEAEs) and serious TEAEs, severity of TEAEs, and TEAEs leading to discontinuation. TEAEs were defined as AEs with onset after the start of the OLE, or onset before OLE start with worsening during OLE. TEAEs were assessed up to the last dose of cenobamate during the OLE plus 30 days (or analysis cutoff date if earlier). TEAEs were coded using the MedDRA Dictionary version 20.0.
2.4. Data analysis
OLE safety outcomes and study discontinuations were summarized with descriptive statistics. Probability of study continuation from the first cenobamate dose in the OLE was examined using a Kaplan–Meier analysis, with patients who completed the study or were ongoing at the data cutoff considered censored. The OLE safety population was defined as all patients who entered the OLE and took at least one dose of cenobamate. The date of data cutoff for the current analysis of the OLE was July 1, 2019.
3. RESULTS
3.1. Patients
Of the 201 patients who completed the C013 double‐blind study, 43 were from study sites that did not participate in the OLE (i.e., India did not approve the OLE amendment). Of the remaining 158 patients, 149 entered the OLE, including 76 patients originally randomized to cenobamate and 73 patients originally randomized to placebo during the double‐blind period. Among the patients who received cenobamate during the double‐blind period, 25.0% (19/76) transitioned to the OLE without cenobamate discontinuation. In this group of patients, 68.4% (13/19) of patients were taking cenobamate 200 mg/day at the end of the double‐blind period and two patients each were taking cenobamate 50, 100, and 150 mg/day. One patient had no cenobamate treatment for 66 days between the end of the double‐blind period and the start of the OLE. The remaining 73.7% of patients (56/76) who received cenobamate during the double‐blind period had no cenobamate for up to 32 days between the end of the double‐blind period and start of the OLE. Among these patients, 3.6% (2/56) had no cenobamate for ≤7 days, 8.9% (5/56) had no cenobamate for >7 to ≤14 days, 7.1% (4/56) had no cenobamate for >14 days to ≤21 days, and most patients (80.4%, 45/56) had no cenobamate for >21 days (up to 32 days) between the end of the double‐blind period and the start of the OLE. The demographic and clinical characteristics of the patients who entered the OLE are shown in Table 1.
TABLE 1.
Demographics and baseline characteristics (OLE population)
| All patients receiving cenobamate in the OLE, n = 149 | |
|---|---|
| Age, years, mean (SD) | 37.6 (10.9) |
| Sex, n (%) | |
| Female | 77 (51.7) |
| Male | 72 (48.3) |
| Race, n (%) | |
| Caucasian/White | 99 (66.4) |
| Asian | 37 (24.8) |
| African American/Black | 5 (3.4) |
| Other/unknown | 8 (5.4) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 6 (4.0) |
| Not Hispanic or Latino | 137 (91.9) |
| Not reported | 6 (4.0) |
| Seizure types by history, n (%)a | |
| Focal aware nonmotor | 34 (22.8) |
| Focal aware motor | 35 (23.5) |
| Focal impaired awareness | 124 (83.2) |
| Focal to bilateral tonic–clonic | 56 (37.6) |
| Baseline ASMs, n (%)b | |
| 1 | 13 (8.7) |
| 2 | 70 (47.0) |
| 3 | 62 (41.6) |
| >3 | 4 (2.7) |
| Concomitant ASMs in OLE in ≥10% of patients, n (%) | |
| Levetiracetam | 67 (45.0) |
| Valproatec | 57 (38.3) |
| Lamotrigine | 52 (34.9) |
| Carbamazepine | 39 (26.2) |
| Lacosamide | 39 (26.2) |
| Topiramate | 37 (24.8) |
| Oxcarbazepine | 27 (18.1) |
| Clobazam | 17 (11.4) |
Abbreviations: ASM, antiseizure medication; OLE, open‐label extension.
Patients may be reported in >1 category.
Baseline ASMs defined as ASMs starting prior to and ongoing at the time of first study drug dose in the double‐blind period.
Including valproate semisodium, valproate sodium, valproic acid, and Ergenyl chrono.
3.2. Exposure to cenobamate and retention rate
As of the July 2019 data cutoff, the total duration of cenobamate treatment was 652.8 patient‐years, and 85 patients (57.0%) remained in the OLE. These 85 patients received cenobamate in the OLE for a median of 6.8 years (mean ± SD = 6.9 ± 0.3, range = 6.4–7.8 years). The median duration of cenobamate treatment among all 149 patients who entered the OLE was 6.25 years (median weeks = 325, range in weeks = 1–407). The probability of continuing cenobamate treatment was 73% after 1 year, 67% after 2 years, 63% after 3 years, 61% after 4 years, 60% after 5 years, and 59% after 6 years (Figure 1). Among the patients who continued treatment at 12 months (n = 107), the probability of continuing treatment was 92% after 2 years, 87% after 3 years, 83% after 4 years, and 82% after 5 years (Figure 2). In these patients who continued cenobamate at 12 months, 79.4% (85/107) were still receiving cenobamate after a median duration of 6.8 years. Reasons for OLE discontinuation are shown in Figure 3. The most common reasons were withdrawal by patient (19.5%, 29/149), AE (10.1%, 15/149), and lack of efficacy (5.4%, 8/149). No specific reasons were recorded for the “withdrawal by patient” category.
FIGURE 1.
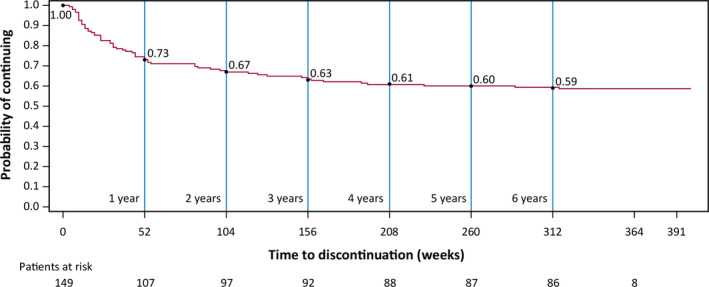
Kaplan–Meier plot of continuation during the open‐label extension (OLE). Event = early discontinuation from OLE. Patients who completed the study and patients ongoing at the date of data cutoff are considered censored
FIGURE 2.
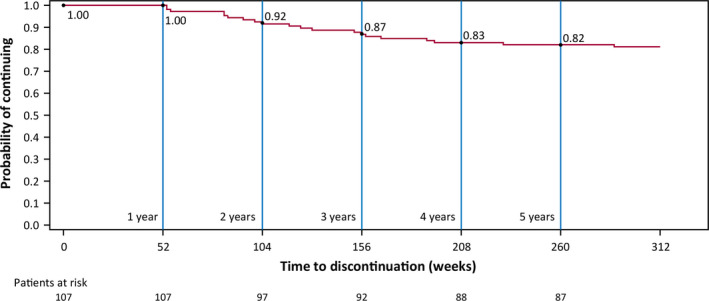
Kaplan–Meier plot of continuation during the open‐label extension (OLE) in the patients who remained in the OLE at 12 months. Event = early discontinuation from OLE. Patients who completed the study and patients ongoing at the date of data cutoff are considered censored
FIGURE 3.
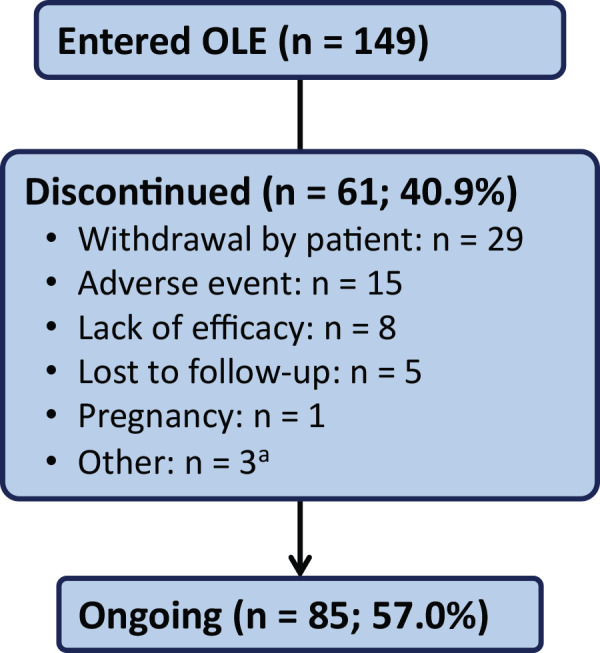
Patient disposition and reason for discontinuation (open‐label extension [OLE] population). aAn additional three patients completed the OLE as reported on the end of study case report form. The patients completed 9.0 months, 11.0 months, and 12.3 months of treatment in the OLE
3.3. Cenobamate dose
The median modal daily cenobamate dose was 200 mg (range = 50–400 mg). The last recorded dose received by patients at or before the OLE data cutoff, including patients who received cenobamate taper prior to discontinuation, was 400 mg/day (8.1%, 12/149), 350 mg/day (5.4%, 8/149), 300 mg/day (6.7%, 10/149), 250 mg/day (3.4%, 5/149), 200 mg/day (26.2%, 39/149), 150 mg/day (8.1%, 12/149), 100 mg/day (23.5%, 35/149), 50 mg/day (16.1%, 24/149), and 0 mg/day (2.7%, 4/149) in patients who had discontinued treatment. The last recorded modal dose before the OLE data cutoff in ongoing patients in the OLE was 200 mg/day (mean ± SD = 230.6 ± 100.6 mg/day, median = 200 mg/day, range = 50–400 mg/day), whereas in patients who discontinued the OLE the modal last recorded dose was 100 mg/day (mean ± SD = 103.3 ± 71.8 mg/day, median = 100 mg/day, range = 0–400 mg/day).
3.4. Concomitant ASMs
In the OLE, 53.7% (80/149) of patients discontinued and remained off one or more concomitant ASMs. One or more concomitant ASMs were added by 26.2% (39/149) of patients. Among the patients who discontinued and remained off one or more concomitant ASMs, 76.3% (61/80) remained in the OLE at the data cutoff.
3.5. Safety
Over the 7.8‐year duration of the OLE, TEAEs occurred in 89.3% (133/149) of patients (Table 2). TEAEs were primarily mild (28.2%, 42/133) or moderate (47.0%, 70/133) in severity. Dizziness (32.9%, 49/149), headache (26.8%, 40/149), and somnolence (21.5%, 32/149) were the most frequently reported TEAEs. Dizziness was mild (44.9%) or moderate (51.0%) in most patients. In all patients, headache was mild (80.0%) or moderate (20.0%) and somnolence was mild (56.3%) or moderate (43.8%). The timing for the onset of the most common TEAEs showed the median onset for dizziness was 36 days, for somnolence was 41 days, and for fatigue was 57.5 days, whereas median onset of headache was 354 days.
TABLE 2.
Summary of TEAEs
| All patients receiving cenobamate in the OLE, N = 149, n (%) | |
|---|---|
| Any TEAE | 133 (89.3) |
| TEAE severity | |
| Mild | 42 (28.2) |
| Moderate | 70 (47.0) |
| Severe | 21 (14.1) |
| Serious TEAEs | 38 (25.5) |
| TEAEs in ≥10% of patients | |
| Dizziness | 49 (32.9) |
| Headache | 40 (26.8) |
| Somnolence | 32 (21.5) |
| Viral upper respiratory tract infection | 30 (20.1) |
| Upper respiratory tract infection | 24 (16.1) |
| Nausea | 16 (10.7) |
| Fatigue | 16 (10.7) |
| Urinary tract infection | 16 (10.7) |
Abbreviations: OLE, open‐label extension; TEAE, treatment‐emergent adverse event.
Serious TEAEs occurred in 25.5% (38/149) of patients and those occurring in >1% of patients were seizure (n = 6) and vomiting, pneumonia, sepsis, and osteoarthritis (n = 2 each). There were three deaths during the OLE: one sudden unexplained death in epilepsy (SUDEP) rated as unlikely to be related to study treatment; one cardiac arrest resulting in death rated as not related to study treatment; and one completed suicide rated as unlikely to be related to study treatment.
TEAEs leading to discontinuation were reported in 10.1% (15/149) of patients, and those leading to discontinuation in >1% of patients were fatigue (1.3%, 2/149), ataxia (1.3%, 2/149), and memory impairment or amnesia (1.3%, 2/149). Of the two patients who discontinued the OLE due to fatigue, one patient discontinued at 8 days following onset of fatigue and one patient discontinued at 63 days after onset of fatigue. Of the two patients who discontinued the OLE due to ataxia, one patient discontinued at 8 days after ataxia onset and one patient discontinued at 652 days after ataxia onset. The patient reporting memory impairment discontinued the OLE at 4 days after onset, and the patient reporting amnesia discontinued at 46 days after onset. Among TEAEs leading to discontinuation in one patient, discontinuation occurred at 4 days following aphasia onset; 23 days after asthenia, diplopia, and dizziness onset; and 101 days after headache onset.
4. DISCUSSION
Long‐term examination of retention rate is an accepted measure of effectiveness encompassing efficacy, safety, and tolerability, and one that may provide useful information for clinical practice.12, 13 Unlike the rigid dosing protocols for study drug and concomitant ASMs that occur in the double‐blind phase of pivotal clinical trials, OLE studies allow the evaluation of long‐term safety and tolerability within the clinically meaningful context of more individualized dosing of both the study drug and concomitant ASMs. OLE studies likely better represent clinical practice and patient experiences than controlled clinical trials. In this OLE of the C013 Phase 2 study, adjunctive cenobamate was generally well tolerated by those patients treated over the longer term (up to 7.8 years). Among all patients who entered the OLE, the median duration of treatment with cenobamate was 6.25 years. Notably, among the group of patients who continued cenobamate at 1 year, 79% were still receiving cenobamate after a median duration of 6.8 years. During treatment with cenobamate in the OLE, approximately half of the patients discontinued and remained off one or more concomitant ASMs. These patients had a high retention rate, with 76% continuing in the OLE at the data cutoff, suggesting these patients were benefiting from cenobamate treatment.
A high long‐term retention rate is a clinically meaningful outcome because epilepsy is a chronic disease that requires continuing treatment. In the absence of methodologically rigorous real‐world comparative effectiveness and safety studies for ASMs,14 indirect comparisons of long‐term OLE retention outcomes may provide important information for clinical practice treatment decisions. The 1‐year (73%) and 2‐year (67%) retention rates with cenobamate are similar to the retention rates reported for other recently approved adjunctive ASMs for focal seizures.15, 16 In an analysis of retention rates in adjunctive treatment of focal seizures using pooled extension studies, which primarily included patients who had completed double‐blind, randomized, controlled trials and to a lesser extent open‐label safety studies, 1‐ and 2‐year rates were 75.4% and 61.7% for lacosamide, 75.3% and 35.9% for perampanel, 72.5% (no 2‐year OLE available) for eslicarbazepine acetate, and 79.8% and 68.1% for brivaracetam.15 Retention rates reported in other 1‐year OLE studies have included 71% with topiramate,17 and 84% with extended‐release oxcarbazepine.18 One‐ and 2‐year treatment continuation rates for clinic patients of tertiary referral epilepsy centers have been reported as 75.2% and 69.2% for lamotrigine, 65.6% and 45.8% for levetiracetam, and 51.7% and 38.3% for topiramate.19 An analysis of ASM treatment continuation rates at 2 years, identified using medical records and patient interviews, showed 53.6% of patients continued levetiracetam, 74.1% continued lamotrigine, 58.8% continued oxcarbazepine, 44.2% continued topiramate, and 60.2% continued zonisamide.8
Cenobamate longer term retention rates were stable across Years 3–6 and are higher than the rates that have been reported for other ASMs in OLE studies in patients with focal seizures (Figure 4).20, 21, 22, 23, 24, 25, 26, 27 The higher long‐term retention rates with cenobamate are notable, because some comparator OLE studies included only patients whom investigators had a reasonable expectation would benefit from long‐term treatment, rather than all patients who completed the previous study; or, in their analysis of retention rates, the discontinuations not due to AEs or lack of efficacy were censored.21, 22, 23, 27 Cenobamate retention rates also exceeded those of clinic patients of tertiary referral epilepsy centers, including the 3‐year retention rates of lacosamide (37%),28 perampanel (42.7%),29 and levetiracetam (58%).30 The high rate of long‐term retention in the C013 OLE study, along with 79% retention at 1 year in the open‐label safety study,31 supports patient satisfaction with cenobamate treatment, serving as an indirect measure of combined efficacy and safety and the overall benefits of cenobamate as experienced by patients.
FIGURE 4.
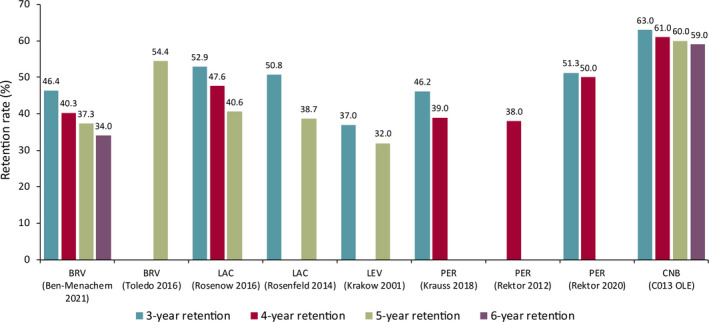
Long‐term retention rates during open‐label extension (OLE) studies. BRV, brivaracetam; CNB, cenobamate; LAC, lacosamide; LEV, levetiracetam; PER, perampanel
Most of the TEAEs in the C013 OLE were central nervous system‐related and consistent with the Phase 2 double‐blind studies5, 6 and the Phase 3 open‐label safety study.31 They also are similar to the most common TEAEs experienced by patients receiving lacosamide, perampanel, eslicarbazepine acetate, and brivaracetam in OLE studies, including dizziness, headache, somnolence, and fatigue.15 The most common serious TEAE, reported in six patients, was seizures, which is not unexpected for patients with treatment‐resistant focal epilepsy. Safety assessments did not find any unanticipated AEs over the 7.8‐year duration of the OLE or any late drug reactions to long‐term treatment with cenobamate.
Limitations of the C013 OLE study are similar to other OLE studies, including no control comparison group, reduced sample size over time, and potential confounders such as changes in concomitant ASM therapy. There was no collection of seizure data to directly assess efficacy during the C013 OLE. The analysis of retention began at the start of cenobamate dosing in the OLE rather than in the double‐blind phase, which could lead to an overestimate of the retention rate because patients who discontinued during the double‐blind study are not included in the analysis.16 The decision to analyze retention from the start of the OLE was due to most patients ceasing cenobamate for approximately 3 weeks from the end of the double‐blind period to the beginning of the OLE, and to the loss of patients from sites in India who participated in the double‐blind study but were not eligible for the OLE. If analysis started from dosing in the double‐blind period, the loss of these patients would have artificially lowered the retention rate. Additionally, discontinuation from cenobamate in the C013 double‐blind period was relatively low (11/113, 9.7% of patients vs. 10/109, 9.2% of placebo patients, including four patients from each group discontinuing due to AEs during the double‐blind period).
Alternatively, analysis of OLE treatment discontinuation did not exclude patients who discontinued for reasons other than efficacy and tolerability, such as lost to follow‐up, which may underestimate retention.32 Importantly, the patient population of the C013 study is characteristic of patients experiencing treatment‐resistant focal seizures, and the long‐term OLE adds to the understanding of the long‐term safety and tolerability of cenobamate. The outcomes of the second Phase 2 randomized, double‐blind, placebo‐controlled dose–response study (YKP3089C017; NCT0186611)6 OLE will include examination of retention rate, percentage seizure reduction, ≥50% responder rates, safety, and tolerability during cenobamate treatment over a median of 4 years.
In conclusion, long‐term retention findings from the C013 OLE study demonstrated sustained safety and tolerability of adjunctive cenobamate up to 7.8 years in adults with treatment‐resistant focal seizures taking one to three ASMs. High long‐term retention was seen in the patients who continued cenobamate at 1 year, in that 79% were still receiving cenobamate after a median duration of 6.8 years.
PREVIOUS PRESENTATION
French JA, et al. Long‐term safety of adjunctive cenobamate in patients with uncontrolled focal seizures: open‐label extension of a randomized clinical study. Presented at the American Epilepsy Society Annual Meeting, December 6–10, 2019, Baltimore, MD (Abstract 3.297) and encored at the American Academy of Neurology Annual Meeting, April 25–May 1, 2020.
CONFLICT OF INTEREST
J.A.F.: Salary support to New York University: Epilepsy Foundation; consultant/advisor (on behalf of the Epilepsy Study Consortium): Adamas, Aeonian/Aeovian, Anavex, Arkin Holdings, Arvelle, Athenen Therapeutics/Carnot Pharma, Baergic, Biogen, BioXcel, Cavion, Cerebral, Cerevel, Crossject, CuroNZ, Eisai, Eliem, Encoded, Engage, Engrail, Epiminder, Equilibre, Fortress, Greenwich, GW Pharma, Janssen, Knopp, Lundbeck, Marinus, Mend Neuroscience, Merck, NeuCyte, Neurocrine, Otsuka, Ovid, Passage, Praxis, Redpin, Sage, SK Life Science, Sofinnova, Stoke, Supernus, Synergia Medical, Takeda, UCB Pharma, West Therapeutic Development, Xenon, Xeris, Zogenix, Zynerba; research support: Epilepsy Research Foundation, Epilepsy Study Consortium (funded by Andrews Foundation, Eisai, Engage, Lundbeck, Pfizer, SK Life Science, Sunovion, UCB Pharma, Vogelstein Foundation), Epilepsy Study Consortium/Epilepsy Foundation (funded by Engage, Neurelis, SK Life Science, UCB Pharma), GW/One8 Foundation/FACES, National Institute of Neurological Disorders and Stroke; editorial board: Lancet Neurology, Neurology Today; travel reimbursement: Arvelle, Biogen, Cerevel, Engage, Epilepsy Study Consortium, Epilepsy Foundation, Lundbeck, NeuCyte, Otsuka, Sage, UCB Pharma, Xenon, Zogenix. S.S.C.: Consultant/advisor: Adamas, Eisai, SK Life Science, UCB Pharma; speaker: Eisai, Greenwich Biosciences, Sunovion, UCB Pharma; research support: Engage, SK Life Science, UCB Pharma. G.L.K.: Consultant/advisor: Adamas, Eisai, Otsuka, Shire; research support: Biogen, SK Life Science, UCB Pharma, Upsher‐Smith. S.K.L.: Consultant/advisor: Eisai, SK Life Science, UCB Pharma. M.M.: Speaker: Biogen, Merck, Novartis, Roche; research support: Roche. W.E.R.: Consultant/advisor: SK Life Science; speaker: Eisai, Greenwich Biosciences (GW Pharmaceuticals), SK Life Science, Sunovion, UCB Pharma; research support: Greenwich Biosciences, Marinus, Medtronic, Neurelis, Ovid, SK Life Science, Takeda, UCB Pharma, Upsher‐Smith. M.R.S.: Consultant/advisor: Medtronic; speaker: Eisai, International Medical Press, Medscape, NeurologyLive, Projects in Knowledge; research support: Cavion, Cerevel, Eisai, Engage, Medtronic, Neurelis, SK Life Science, Takeda, UCB Pharma, Xenon. M.K.: Employee, SK Life Science. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
This study was funded by SK Life Science. The authors thank Lynanne McGuire, PhD, and Don Fallon, ELS, of MedVal Scientific Information Services for medical writing and editorial assistance, which were funded by SK Life Science. This article was prepared according to the International Society for Medical Publication Professionals’ Good Publication Practice for Communicating Company‐Sponsored Medical Research: GPP3.
French JA, Chung SS, Krauss GL, Lee SK, Maciejowski M, Rosenfeld WE, et al. Long‐term safety of adjunctive cenobamate in patients with uncontrolled focal seizures: Open‐label extension of a randomized clinical study. Epilepsia. 2021;62:2142–2150. 10.1111/epi.17007
[Correction added on 27 July, 2021, after first online publication: The copyright line has been updated].
DATA AVAILABILITY STATEMENT
The data for the analyses described in this article are available by request from the corresponding author or SK Life Science, the company sponsoring the clinical development of cenobamate for the treatment of focal epilepsy.
REFERENCES
- 1.Keam SJ. Cenobamate: first approval. Drugs. 2020;80(1):73–8. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura M, Cho JH, Shin HS, Jang IS. Effects of cenobamate (YKP3089), a newly developed anti‐epileptic drug, on voltage‐gated sodium channels in rat hippocampal CA3 neurons. Eur J Pharmacol. 2019;855:175–82. [DOI] [PubMed] [Google Scholar]
- 3.Sharma R, Nakamura M, Neupane C, Jeon BH, Shin H, Melnick SM, et al. Positive allosteric modulation of GABAA receptors by a novel antiepileptic drug cenobamate. Eur J Pharmacol. 2020;879:173117. [DOI] [PubMed] [Google Scholar]
- 4.Guignet M, Campbell A, White HS. Cenobamate (XCOPRI): can preclinical and clinical evidence provide insight into its mechanism of action? Epilepsia. 2020;61(11):2329–39. [DOI] [PubMed] [Google Scholar]
- 5.Chung SS, French JA, Kowalski J, Krauss GL, Lee SK, Maciejowski M, et al. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. 2020;94(22):e2311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krauss GL, Klein P, Brandt C, Kun Lee S, Milanov I, Milovanovic M, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double‐blind, randomised, placebo‐controlled, dose‐response trial. Lancet Neurol. 2020;19(1):38–48. [DOI] [PubMed] [Google Scholar]
- 7.Perucca E, Wiebe S. Not all that glitters is gold: a guide to the critical interpretation of drug trials in epilepsy. Epilepsia Open. 2016;1(1–2):9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung S, Wang N, Hank N. Comparative retention rates and long‐term tolerability of new antiepileptic drugs. Seizure. 2007;16(4):296–304. [DOI] [PubMed] [Google Scholar]
- 9.Ben‐Menachem E, Sander JW, Privitera M, Gilliam F. Measuring outcomes of treatment with antiepileptic drugs in clinical trials. Epilepsy Behav. 2010;18(1–2):24–30. [DOI] [PubMed] [Google Scholar]
- 10.Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–30. [DOI] [PubMed] [Google Scholar]
- 11.Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben‐Menachem E, Gabbai AA, Hufnagel A, Maia J, Almeida L, Soares‐da‐Silva P. Eslicarbazepine acetate as adjunctive therapy in adult patients with partial epilepsy. Epilepsy Res. 2010;89(2–3):278–85. [DOI] [PubMed] [Google Scholar]
- 13.Mohanraj R, Brodie MJ. Early predictors of outcome in newly diagnosed epilepsy. Seizure. 2013;22(5):333–44. [DOI] [PubMed] [Google Scholar]
- 14.Thieffry S, Klein P, Baulac M, Plumb J, Pelgrims B, Steeves S, et al. Understanding the challenge of comparative effectiveness research in focal epilepsy: a review of network meta‐analyses and real‐world evidence on antiepileptic drugs. Epilepsia. 2020;61(4):595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwok CS, Johnson EL, Krauss GL. Comparing safety and efficacy of "third‐generation" antiepileptic drugs: long‐term extension and post‐marketing treatment. CNS Drugs. 2017;31(11):959–74. [DOI] [PubMed] [Google Scholar]
- 16.Toledo M, Beale R, Evans JS, Steeves S, Elmoufti S, Townsend R, et al. Long‐term retention rates for antiepileptic drugs: a review of long‐term extension studies and comparison with brivaracetam. Epilepsy Res. 2017;138:53–61. [DOI] [PubMed] [Google Scholar]
- 17.Chung SS, Hogan RE, Blatt I, Lawson PB, Nguyen H, Clark AM, et al. Long‐term safety and sustained efficacy of USL255 (topiramate extended‐release capsules) in patients with refractory partial‐onset seizures. Epilepsy Behav. 2016;59:13–20. [DOI] [PubMed] [Google Scholar]
- 18.Chung SS, Johnson JK, Brittain ST, Baroldi P. Long‐term efficacy and safety of adjunctive extended‐release oxcarbazepine (Oxtellar XR®) in adults with partial‐onset seizures. Acta Neurol Scand. 2016;133(2):124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bootsma HP, Ricker L, Hekster YA, Hulsman J, Lambrechts D, Majoie M, et al. The impact of side effects on long‐term retention in three new antiepileptic drugs. Seizure. 2009;18(5):327–31. [DOI] [PubMed] [Google Scholar]
- 20.Ben‐Menachem E, Baulac M, Hong SB, Cleveland JM, Reichel C, Schulz AL, et al. Safety, tolerability, and efficacy of brivaracetam as adjunctive therapy in patients with focal seizures, generalized onset seizures, or Unverricht‐Lundborg disease: an open‐label, long‐term follow‐up trial. Epilepsy Res. 2021;170:106526. [DOI] [PubMed] [Google Scholar]
- 21.Toledo M, Whitesides J, Schiemann J, Johnson ME, Eckhardt K, McDonough B, et al. Safety, tolerability, and seizure control during long‐term treatment with adjunctive brivaracetam for partial‐onset seizures. Epilepsia. 2016;57(7):1139–51. [DOI] [PubMed] [Google Scholar]
- 22.Rosenow F, Kelemen A, Ben‐Menachem E, McShea C, Isojarvi J, Doty P. Long‐term adjunctive lacosamide treatment in patients with partial‐onset seizures. Acta Neurol Scand. 2016;133(2):136–44. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld W, Fountain NB, Kaubrys G, Ben‐Menachem E, McShea C, Isojarvi J, et al. Safety and efficacy of adjunctive lacosamide among patients with partial‐onset seizures in a long‐term open‐label extension trial of up to 8 years. Epilepsy Behav. 2014;41:164–70. [DOI] [PubMed] [Google Scholar]
- 24.Krakow K, Walker M, Otoul C, Sander JW. Long‐term continuation of levetiracetam in patients with refractory epilepsy. Neurology. 2001;56(12):1772–4. [DOI] [PubMed] [Google Scholar]
- 25.Krauss GL, Perucca E, Kwan P, Ben‐Menachem E, Wang XF, Shih JJ, et al. Final safety, tolerability, and seizure outcomes in patients with focal epilepsy treated with adjunctive perampanel for up to 4 years in an open‐label extension of phase III randomized trials: study 307. Epilepsia. 2018;59(4):866–76. [DOI] [PubMed] [Google Scholar]
- 26.Rektor I, Krauss GL, Bar M, Biton V, Klapper JA, Vaiciene‐Magistris N, et al. Perampanel Study 207: long‐term open‐label evaluation in patients with epilepsy. Acta Neurol Scand. 2012;126(4):263–9. [DOI] [PubMed] [Google Scholar]
- 27.Rektor I, Krauss GL, Inoue Y, Kaneko S, Williams B, Patten A, et al. Assessment of the long‐term efficacy and safety of adjunctive perampanel in tonic‐clonic seizures: analysis of four open‐label extension studies. Epilepsia. 2020;61(7):1491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novy J, Bartolini E, Bell GS, Duncan JS, Sander JW. Long‐term retention of lacosamide in a large cohort of people with medically refractory epilepsy: a single centre evaluation. Epilepsy Res. 2013;106(1–2):250–6. [DOI] [PubMed] [Google Scholar]
- 29.Wehner T, Mannan S, Turaga S, Vallabhaneni K, Yip HM, Wiggans C, et al. Retention of perampanel in adults with pharmacoresistant epilepsy at a single tertiary care center. Epilepsy Behav. 2017;73:106–10. [DOI] [PubMed] [Google Scholar]
- 30.Depondt C, Yuen AW, Bell GS, Mitchell T, Koepp MJ, Duncan JS, et al. The long term retention of levetiracetam in a large cohort of patients with epilepsy. J Neurol Neurosurg Psychiatry. 2006;77(1):101–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperling MR, Klein P, Aboumatar S, Gelfand M, Halford JJ, Krauss GL, et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open‐label safety study. Epilepsia. 2020;61(6):1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohanraj R, Brodie MJ. Measuring the efficacy of antiepileptic drugs. Seizure. 2003;12(7):413–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for the analyses described in this article are available by request from the corresponding author or SK Life Science, the company sponsoring the clinical development of cenobamate for the treatment of focal epilepsy.


