Abstract
Chronic graft-versus-host disease (cGVHD) is an immune-mediated disorder characterized by chronic inflammation and fibrosis. Rho-associated coiled-coil–containing protein kinases (ROCKs) are key coordinators of tissue response to injury, regulating multiple functions, such as gene expression and cell migration, proliferation and survival. Relevant to cGVHD and autoimmunity, only the ROCK2 isoform drives a pro-inflammatory type 17 helper T (Th17) cell response. Moreover, ROCK2 inhibition shifts the Th17/regulatory T (Treg) cell balance toward Treg cells and restores immune homeostasis in animal models of autoimmunity and cGVHD. Furthermore, the selective inhibition of ROCK2 by belumosudil reduces fibrosis by downregulating both transforming growth factor-β signaling and profibrotic gene expression, thereby impeding the creation of focal adhesions. Consistent with its anti-inflammatory and antifibrotic activities, belumosudil has demonstrated efficacy in clinical studies, resulting in an overall response rate of >70% in patients with cGVHD who failed 2 to 5 prior lines of systemic therapy. In summary, selective ROCK2 inhibition has emerged as a promising novel therapeutic approach for treating cGVHD and other immunologic diseases with unique mechanisms of action, targeting both immune imbalance and detrimental fibrotic responses.
Keywords: ROCK2, chronic graft-versus-host disease, autoimmunity, fibrosis, immunomodulation, belumosudil
1. Introduction
Since the discovery of the ROCK pathway more than 20 years ago [1], an increasing body of research has continued to unravel the myriad effects of this pathway in controlling tissue response to stress and injury [1,2]. Dysregulated ROCK activity has been associated with a wide spectrum of diseases, ranging from cardiovascular [3] and neurologic [4] disorders to immune-mediated [5] and fibrotic [6] diseases. Although the 2 ROCK isoforms, ROCK1 and ROCK2, share >90% homology within their kinase domains [7], the function of these proteins is not redundant and depends on the cellular system where they are expressed and activated [8–11]. More recently, extensive experimental data highlight the specific contribution of the ROCK1 and ROCK2 proteins in multiple disease states [3,12–15], providing essential knowledge for the development of isoform-specific ROCK-targeted therapies [12,15–17]. In this review, we discuss the current understanding of the role of the ROCK2 signaling pathway in the regulation of immune responses and fibrotic processes. Here, we focus on the therapeutic potential of selective ROCK2 inhibition in cGVHD, an immune-mediated, life-threatening complication of alloHCT.
2. ROCK2, but not ROCK1, controls the balance between pro-inflammatory and Treg cell subsets
ROCK signaling is critical in the coordination of T-cell–mediated immune responses, including cellular movement, cell proliferation and the acquisition of the appropriate T-cell effector phenotype [18–20]. ROCK signaling has been found to be upregulated in patients with autoimmune disorders [21–25], including rheumatoid arthritis [21], SLE [22,23], giant cell arteritis [24] and multiple sclerosis [25], as well as in animal models of type 1 diabetes [26]. Although dysregulated ROCK activity has been associated with the development of autoimmune responses through its broad effects on cytoskeletal dynamics in immune cells [12,20,22,23], only the ROCK2 isoform has been shown to be integral in controlling the balance between pro-inflammatory and Treg cell subsets [12]. Under Th17 cell–polarizing conditions, ROCK2, but not ROCK1, phosphorylates IRF4, a transcription factor required for IL-17 and IL-21 cytokine production [27–29]. Given the recognized contribution of these cytokines to the development of autoimmunity, ROCK2 targeting effectively reduced clinical scores and pathology in a range of preclinical animal models [30–32], including collagen-induced arthritis [30], MRL-lpr SLE [31] and trinitrobenzene sulfonic acid–induced colitis [32].
In studies with human cells, it was demonstrated that ROCK2, but not ROCK1, interacts with pSTAT3 in T cells stimulated by Th17 cell–skewing conditions [33]. Furthermore, ROCK2 activity controls the formation of the ROCK2/STAT3/JAK2 complex [33], allowing optimal STAT3 activation and Th17 cell–specific gene transcription [30,33,34]. In addition, both ROCK2 signaling and STAT3 phosphorylation are required for the development of the Th17 cell–induced subset of Tfh cells [31], a specialized T-cell subset that promotes the production of self-reactive mature B cells and autoantibodies implicated in the pathogenesis of autoimmune disorders [35]. Indeed, pharmacologic inhibition of ROCK2 decreased the number and function of murine Tfh cells in vitro and in vivo, as well as decreased the number and function of Tfh cells in cultures of normal human T cells or PBMCs from patients with active SLE stimulated ex vivo [28,31].
In nonhematopoietic cells, ROCK proteins have been implicated in TGF-β signaling, which plays an instrumental role in controlling the generation of both pro-inflammatory Th17 cells and immunosuppressive Treg cells [36,37]. Specifically, ROCK2 targeting increases STAT5 phosphorylation, the percentage of Treg cells and IL-10 secretion while decreasing Th17 and Tfh cells [30,31]. The immunomodulatory potential of selective ROCK2 inhibition was demonstrated in cell cultures, animal models and patients [30–32,38]. In the clinic, selective ROCK2 inhibition improved clinical symptoms in patients with psoriasis vulgaris that were associated with attenuating Th17 cell–driven autoimmune responses while significantly increasing IL-10 levels and the percentage of Treg cells [38]. These data suggest that targeting of ROCK2 may therefore restore disrupted immune homeostasis through the concurrent regulation of pro-inflammatory and Treg cell subsets (Fig. 1A) [12].
Fig 1. ROCK2 controls the balance between pro-inflammatory and Treg cell subsets, regulates cytoskeletal dynamics and profibrotic gene expression and drives both chronic inflammation and fibrosis in cGVHD.
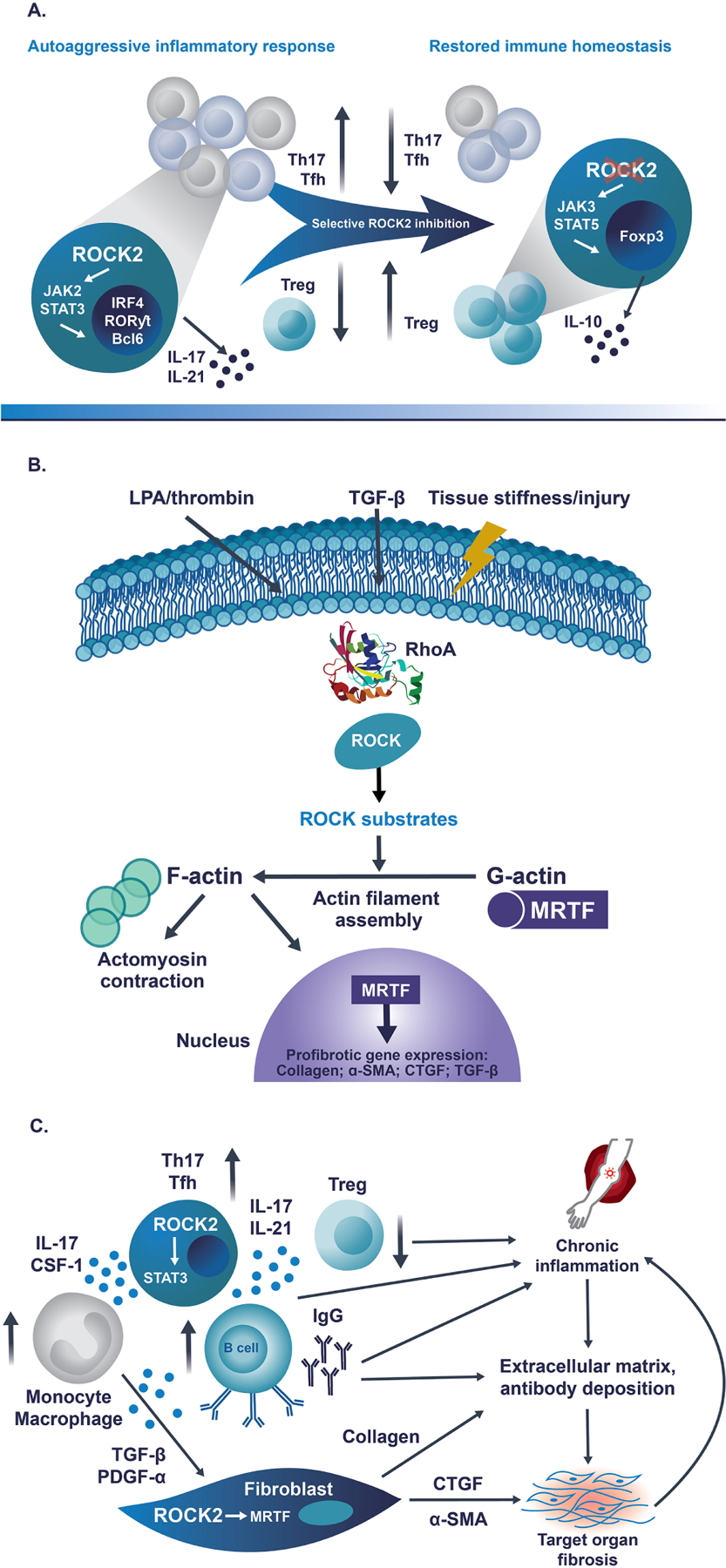
A) During pro-inflammatory immune response, ROCK2 specifically interacts with phosphorylated STAT3. ROCK2 activity is required for the formation of the JAK2/STAT3 complex in Th17 and Tfh cells. Therefore, ROCK2 inhibition results in decreased activation of STAT3 and other Th17/Tfh transcription factors, including IRF4, RORγT and Bcl6, triggering the significant downregulation of both Th17 and Tfh cells. In addition, selective ROCK2 inhibition promotes interaction of ROCK2 with JAK3, leading to the increased phosphorylation of STAT5, the upregulation of Treg cells and the restoration of immune homeostasis. B) Extracellular mediators, such as LPA, thrombin and TGF-β, as well as mechanotransduction forces, activate the RhoA/ROCK signaling pathway and its numerous downstream substrates. This results in the polymerization of G-actin to F-actin and the formation of contractile fibers. ROCK-induced actin polymerization frees the transcription factor MRTF, which is normally sequestered in cytoplasm, to translocate to the nucleus and initiate transcription of several profibrotic genes, including collagen, α-SMA, CTGF and TGF-β, resulting in changes to the cellular structure and an increase in tissue stiffness. C) ROCK2 promotes the secretion of pro-inflammatory cytokines IL-17 and IL-21 via STAT3 phosphorylation, consequently leading to the imbalanced activation of B cells and monocytes/macrophages, which results in the excessive production of autoantibodies and the secretion of profibrotic factors, respectively. The pro-inflammatory milieu downregulates the number and function of immunosuppressive Treg cells to sustain the chronic inflammation. In addition, ROCK2 facilitates MRTF-mediated transcription and increased expression of smooth muscle actin and CTGF, stimulating the differentiation of fibroblasts into myofibroblasts and the increased production of collagen, which promote the development of fibrosis in target organs. Thus, ROCK2 is integral in the cross-talk between the inflammatory and the fibrotic processes driving the pathology of cGVHD.
α-SMA, alpha smooth muscle actin; Bcl6, B-cell lymphoma 6; cGVHD, chronic graft-versus-host disease; CSF-1, colony-stimulating factor-1; CTGF, connective tissue growth factor; Foxp3, forkhead box P3; IgG, immunoglobulin G; IL-10, interleukin 10; IL-17, interleukin 17; IL-21, interleukin 21; IRF4, interferon regulatory factor 4; JAK2, Janus-associated kinase 2; JAK3, Janus-associated kinase 3; LPA, lysophosphatidic acid; MRTF, myocardin-related transcription factor; PDGF-α, platelet-derived growth factor α; ROCK2, rho-associated coiled-coil-containing protein kinase-2; RORγt, retinoic-acid-receptor-related orphan nuclear receptor γ; STAT3, signal transducer and activator of transcription 3; STAT5, signal transducer and activator of transcription 5; Tfh, follicular helper T [cell]; TGF-β, transforming growth factor β; Th17, type 17 helper T [cell]; Treg, regulatory T [cell].
3. ROCKs as profibrotic mediators
ROCK activation is driven by a variety of profibrotic signals, including mechanical forces (increased tissue stiffness) and biochemical mediators (LPA, thrombin, TGF-β) [13]. Although the role of ROCKs as master regulators of cytoskeletal dynamics has been well established, the activity of the ROCK1 and ROCK2 isoforms is nonredundant with distinct properties [39]. For example, evidence points to isoform-specific roles in the regulation of actomyosin organization, cell polarity and cell migration via activation of different intracellular targets [39]. ROCK1 initiates polarity through the formation of stable actomyosin filament bundles, whereas ROCK2 generates contractile forces, locally attenuates the small GTPase Rac1 that inhibits actin polymerization and mediates the formation of the leading edge in migratory cells [40]. By using the small interfering RNA-mediated ROCK isoform knockdown approach, ROCK2, but not ROCK1, was shown to control LPA-induced monocytic migration and adhesion [41]. While ROCK1 is critical for the formation of stress fibers and focal adhesions, ROCK2 controls cortical contractility and phagocytosis of fibronectin by fibroblasts [8].
Changes in cytoskeletal dynamics by ROCKs also lead to increased transcription of key profibrotic genes (Fig. 1B) [13,42]. In particular, recent studies indicate that ROCK2 facilitates MRTF-mediated transcription and increased expression of smooth muscle actin and CTGF [42–44]. Activation of these genes promotes the differentiation of fibroblasts into myofibroblasts and increases the production of collagen, both of which are key features of fibrotic diseases [42].
Pharmacologic inhibition of ROCK activity using nonselective ROCK inhibitors has been demonstrated by several groups to prevent fibrosis in animal models; however, the specific contribution of each isoform to antifibrotic effects in vivo was unclear [42,45]. Knipe et al reported that reduced expression of either ROCK1 or ROCK2 was sufficient to protect mice from bleomycin-induced vascular leak and pulmonary fibrosis [46]. In addition, ROCK2, but not ROCK1, regulates TGF-β–induced fibrotic gene expression in mesangial cells, urinary albumin excretion and glomerulosclerosis in diabetic animals [44]. Deletion of ROCK2 in cardiac fibroblasts diminished cardiac hypertrophy and fibrosis induced by angiotensin II in mice, suggesting that selective ROCK2 targeting may have therapeutic benefits in patients with fibrotic diseases [43].
4. ROCK2 is an emerging therapeutic target in cGVHD
Chronic GVHD remains a frequent complication of alloHCT [47–49] that is associated with considerable morbidity [49], impaired quality of life [50] and late nonrelapse mortality [49,51]. Chronic GVHD is an immune-mediated multiorgan disorder that is characterized by persistent inflammation due to a dysregulated immune response and aberrant tissue repair with fibrosis [52]. The initiation phase of the disease is characterized by the excessive expansion and differentiation of naive alloreactive T-cell subsets, including Th1, Th17 and Tfh cells, driven by high levels of IL-1, IL-6 and TNF-α cytokines secreted in response to tissue injury [53]. Th17 cells contribute to the pathogenesis of cGVHD by trafficking to target organs and secreting IL-17, IL-21, GM-CSF and CSF-1 [54]. These cytokines subsequently recruit innate immune cells and promote their differentiation into pathogenic phenotypes, leading to extensive tissue fibrosis through myofibroblast activation via TGF-β signaling and collagen production in target organs [55–57]. Indeed, the disruption of CSF-1 signaling has been shown to deplete tissue macrophages and reduce cGVHD-associated cutaneous and pulmonary fibrosis [54].
In addition, the B-cell arm of the adaptive immune response is also markedly dysregulated in cGVHD owing to increased secretion of IL-21 and activation of Tfh cells [58]. This can lead to abnormal germinal center or extrafollicular B-cell expansion, followed by secretion and deposition of auto- and alloreactive antibodies in target organs [59,60]. These antibodies bind to the Fc receptor on macrophages, which further stimulates TGF-β secretion and tissue fibrosis [53].
Another key event occurring during this phase of the disease is the development of a relative deficiency of thymus-derived Treg cells [61]. Conditioning regimens used prior to transplant, immunosuppressant-based prophylaxis post transplant and other factors cause thymic injury that leads to failure of negative selection and reduced Treg cell production, as well as increased Treg cell susceptibility to apoptosis that cannot be compensated by increased Treg cell proliferation [52,61]. Several studies have reported that Treg cell–boosting therapies, such as Treg cell infusion or administration of low-dose IL-2, demonstrated clinical benefits in patients [54]. Thus, the pathogenesis of cGVHD is characterized by an imbalance in effector Th17/Tfh cells and Treg cells, resulting in the inability to rebound the pro-inflammatory environment and a loss of immune tolerance, which subsequently lead to the development of irreversible fibrosis and damage in target organs [52,64].
Many of the cellular processes regulated by the ROCK2 signaling pathway, particularly the control of immune cell abnormalities and fibrosis, are relevant to the pathophysiology of cGVHD (Fig. 1C)[13,31,35,44,54]. Belumosudil, also known as KD025, is an investigational novel oral selective ROCK2 inhibitor that has 100-fold more selectivity for the ROCK2 isoform than the ROCK1 isoform [16,30]. Although the unique functions of the ROCK isoforms continue to be defined, selective ROCK2 inhibition offers the potential for specific therapeutic effects, such as restoring Th17/Treg cell balance and reducing fibrosis, without the profound effect of dual ROCK1/2 inhibition that could be harmful to patients [12,62]. Indeed, a placebo-controlled, randomized, phase1 clinical study showed that the oral administration of belumosudil was well tolerated, without significant AEs, in healthy individuals. Moreover, an analysis of PBMCs revealed that belumosudil effectively downregulated the secretion of pro-inflammatory cytokines IL-21 and IL-17 during stimulation ex vivo, which is consistent with the exclusive role of the ROCK2 isoform in the regulation of the Th17 pathway [30]. ROCK2 inhibition with belumosudil significantly diminished disease severity in association with a decrease in Th17 cells and the simultaneous upregulation of Treg cells in therapeutic animal models of cGVHD, such as a multiorgan system cGVHD model with BOS and a sclerodermatous mouse GVHD model. The improvememt in the clinical and immunologic symptoms was associated with a significant reduction in targeted organ fibrosis in both models. Furthermore, a study using human PBMCs from patients with active cGVHD demonstrated that belumosudil inhibited the production of IL-21, IL-17 and IFNƔ, as well as decreased Tfh cells, confirming the therapeutic potential of ROCK2 inhibition in cGVHD [62].
The use of belumosudil has been investigated in a phase 2a study (KD025–208) that included adult patients with steroid-dependent or steroid-refractory cGVHD who had received 1 to 3 prior lines of systemic treatment. Patients were treated with belumosudil at dosages of 200 mg once daily (n=17), 200 mg twice daily (n=16) or 400 mg once daily (n=21) in 28-day cycles until disease progression [63]. The primary end point was ORR, defined as PR or CR by the 2014 NIH cGVHD Consensus Criteria [63]. Data from 54 patients demonstrated an ORR of 65%, with >75% of responders achieving a response by week 8 [63]. Responses were observed in all affected organ systems, including organs with fibrotic disease. The median DOR was 35 weeks, with 1- and 2-year FFS rates of 47% and 33%, respectively [63]. The most common AEs included upper respiratory tract infection (46%), diarrhea (33%), nausea (33%), increased liver function tests (33%), fatigue (33%) and dyspnea (30%). However, AEs were overall consistent with those expected in patients with cGVHD receiving corticosteroids [63].
A pivotal phase 2, open-label, randomized study (KD025–213, ROCKstar) of belumosudil in patients with cGVHD who received at least 2 prior lines of systemic therapy is being conducted; results have since been published. Patients were randomized to belumosudil at dosages of 200 mg once daily (n=66) or 200 mg twice daily (n=66) and were treated to clinically significant progression. Analysis 12 months after completion of enrollment indicated that both cohorts achieved the primary end point, with an ORR of 74% and 77% in patients receiving belumosudil 200 mg once daily and 200 mg twice daily, respectively. CR was observed in all affected organ systems. Similar to the KD025–208 study, AEs were overall consistent with those expected in patients with cGVHD receiving corticosteroids. The most commonly reported AEs included fatigue (38%), diarrhea (33%), nausea (31%), cough (28%), upper respiratory tract infection (27%), dyspnea (25%), headache (24%), liver-related AEs (24%), peripheral edema (23%), vomiting (21%) and muscle spasms (20%) [64].
5. Future direction for clinical development of belumosudil
The critical role of ROCK2 in regulating immune and fibrotic processes underscores its potential as a therapeutic target for a number of autoimmune and fibrotic disorders [12,35,42,43]. This potential led to preclinical studies in systemic lupus erythematosus and multiorgan system cGVHD with BOS and scleroderma, and subsequently progressed into clinical trials, including two phase 2 trials in patients with cGVHD treated with prior lines of systemic treatment [31, 62–64]. Accordingly, the role of ROCK2 inhibition with belumosudil is being explored in systemic sclerosis (scleroderma) [65], a chronic autoimmune disease characterized by small vessel vasculopathy, dysregulated innate and adaptive immunity and extensive fibrosis of the skin and visceral organs [66,67]. Currently considered incurable, systemic sclerosis is associated with the highest mortality of all rheumatic diseases [66,67]. Although the pathogenesis of this condition remains poorly understood, ROCK signaling pathways have been implicated in facilitating the disease through their broad effects in controlling vascular smooth muscle, differentiating fibroblasts into myofibroblasts [67,68] and driving fibrosis through TGF-β–mediated mechanisms and transcription of profibrotic genes [62,68,69]. Further, significant associations between ROCK1, ROCK2, RhoA and RhoC gene polymorphisms and systemic sclerosis have been demonstrated [68].
Based on these findings, a phase 2 investigation of belumosudil is ongoing in patients with diffuse cutaneous systemic sclerosis. Patients are being randomized to double-blinded treatment with belumosudil 200 mg once daily, belumosudil 200 mg twice daily or matched placebo for 28 weeks, followed by open-label treatment of 24 weeks. The primary end point is CRISS score at 24 weeks, and secondary end points include CRISS response at 52 weeks, lung fibrosis change via high-resolution computed tomography and AEs [65].
6. Conclusions
An extensive body of evidence has highlighted the crucial roles of ROCK2 in regulating pro-inflammatory immune responses and fibrotic processes involved in the pathogenesis of multiple diseases. Importantly, ROCK2-mediated effects on cytoskeletal dynamics and intracellular inflammatory cascades provide a promising therapeutic target for multiple immune-mediated and fibrotic disorders. Belumosudil, a selective ROCK2 inhibitor, has demonstrated important immunomodulatory properties, ameliorating the symptoms of cGVHD in both animal models and patients in clinical trials with this devastating disorder. The 200-mg QD belumosudil dose has been approved by the FDA for adult and pediatric patients aged ≥12 years with cGVHD after failure of ≥2 prior lines of systemic therapy [70]. Additional clinical research is expected to further characterize the therapeutic potential of ROCK2 inhibition in cGVHD, as well as other autoimmune and fibrotic disorders.
Highlights.
ROCK2 balances pro-inflammatory/Treg cell subsets via STAT3/STAT5 phosphorylation
ROCK2 facilitates cell contractility and transcription of profibrotic genes
Belumosudil, a selective ROCK2 inhibitor, has shown clinical benefit in cGVHD
ROCK2 inhibition has therapeutic potential for other autoimmune/fibrotic disorders
ACKNOWLEDGMENTS:
The authors thank all the patients, caregivers, health care professionals, investigators and Kadmon employees who were and are involved in the clinical studies of belumosudil. This work was supported by Kadmon Corporation, LLC, who funded medical writing assistance provided by RevHealth, and the National Institutes of Health (grants P01 CA142106, P01 AI056299 and R01 HL147324).
ABBREVIATIONS:
- AE
adverse event
- alloHCT
allogeneic hematopoietic cell transplant
- BOS
bronchiolitis obliterans syndrome
- cGVHD
chronic graft-versus-host disease
- CRISS
Combined Response Index for Systemic Sclerosis Score
- CSF-1
colony-stimulating factor 1
- CTGF
connective tissue growth factor
- FFS
failure-free survival
- GTPase Rac1
guanosine triphosphatase Ras-related C3 botulinum toxin substrate 1
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IFNƔ
interferon Ɣ
- IL-1
interleukin 1
- IL-2
interleukin 2
- IL-6
interleukin 6
- IL-10
interleukin 10
- IL-17
interleukin 17
- IL-21
interleukin 21
- IRF4
interferon regulator factor 4
- JAK2
Janus-associated kinase 2
- LPA
lysophosphatidic acid
- MRL-lpr
lymphoproliferation mutant
- MRTF
myocardin-related transcription factor
- NIH
National Institutes of Health
- ORR
overall response rate
- PBMCs
peripheral blood mononuclear cells
- pSTAT3
phosphorylated signal transducer and activator of transcription 3
- RNA
ribonucleic acid
- ROCK
rho-associated coiled-coil–containing protein kinase
- ROCK1
rho-associated coiled-coil–containing protein kinase-1
- ROCK2
rho-associated coiled-coil–containing protein kinase-2
- SLE
systemic lupus erythematosus
- STAT3
signal transducer and activator of transcription 3
- STAT5
signal transducer and activator of transcription 5
- Tfh
follicular helper T [cell]
- TGF-β
transforming growth factor-β
- Th17
type 17 helper T [cell]
- TNF-α
tumor necrosis factor α
- Treg
regulatory T [cell]
Footnotes
Disclosures: Bruce Blazar receives remuneration as an advisor to Magenta Therapeutics and BlueRock Therapeutics and receives research funding from BlueRock Therapeutics, Rheos Medicines, Equilibre Biopharmaceuticals, Carisma Therapeutics, Inc., Children’s Cancer Research Fund and Kids First Fund. He is a cofounder of Tmunity Therapeutics and serves as a member of a steering committee for Kadmon Corporation (and was previously a paid consultant for Kadmon but has not received any consulting compensation of any kind in the past year). He is also an ad hoc consultant to Incyte Corporation.
Disclosures: Alexandra Zanin-Zhorov was a full-time employee of Kadmon Corporation, LLC from January 2012 to December 14, 2020. She is now Senior Vice President, Translational Medicine at Equilibre Biopharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Riento K, Ridley AJ, ROCKS: multifunctional kinases in cell behaviour, Nat. Rev. Mol. Cell. Biol 4 (2003) 446–456. doi: 10.1038/nrm1128 [DOI] [PubMed] [Google Scholar]
- [2].Leung T, Chien X-Q, Manser E, Lim L, The p160 rhoA-binding kinase ROKα is a member of a kinase family and is involved in the reorganization of the cytoskeleton, Mol. Cell. Biol 16 (1996) 5313–5327. doi: 10.1128/mcb.16.10.5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hartmann S, Ridley AJ, Lutz S, The function of rho-associated kinases ROCK1 and ROCK2 in the pathogenesis of cardiovascular disease, Front. Pharmacol 6 (2015) 276. doi: 10.3389/fphar.2015.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Koch JC, Tatenhorst L, Roser A-E, Saal K-A, Tönges L, Lingor P, ROCK inhibition in models of neurodegeneration and its potential for clinical translation, Pharmacol. Ther 189 (2018) 1–21. doi: 10.1016/j.pharmthera.2018.03.008 [DOI] [PubMed] [Google Scholar]
- [5].Pernis AB, Ricker E, Weng C-H, Rozo C, Yi W, Rho kinases in autoimmune diseases, Annu. Rev. Med 67 (2016) 355–374. doi: 10.1146/annurev-med-051914-022120 [DOI] [PubMed] [Google Scholar]
- [6].Riches DWH, Backos DS, Redente EF, ROCK and rho: promising therapeutic targets to ameliorate pulmonary fibrosis, Am. J. Pathol 185 (2015) 909–912. doi: 10.1016/j.ajpath.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S, ROCK-I and ROCK-II, two isoforms of rho-associated coiled-coil forming protein serine/threonine kinase in mice, FEBS. Lett 392 (1996) 189–193. doi: 10.1016/0014-5793(96)00811-3 [DOI] [PubMed] [Google Scholar]
- [8].Yoneda A, Multhaupt HAB, Couchman JR, The rho kinases I and II regulate different aspects of myosin II activity, J. Cell. Biol 170 (2005) 443–453. doi: 10.1083/jcb.200412043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shi J, Wu X, Survma M, et al. , Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment, Cell Death and Disease 4 (2013) e483. doi: 10.1038/cddis.2013.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shi J, Surma M, Zhang L, Wei L, Dissecting the roles of ROCK isoforms in stress-induced cell detachment, Cell. Cycle 12 (2013) 1492–1500. doi: 10.4161/cc.24699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huang L, Dai F, Tang L, et al. , Distinct roles for ROCK1 and ROCK2 in the regulation of Oxldl-mediated endothelial dysfunction, Cell. Physiol. Biochem 49 (2018) 565–577. doi: 10.1159/000492994 [DOI] [PubMed] [Google Scholar]
- [12].Zanin-Zhorov A, Flynn R, Waksal SD, Blazar BR, Isoform-specific targeting of ROCK proteins in immune cells, Small. GTPases 7 (2016) 173–177. doi: 10.1080/21541248.2016.1181698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Knipe RS, Tager AM, Liao JK, The rho kinases: critical mediators of multiple profibrotic processes and rational targets for new therapies for pulmonary fibrosis, Pharmacol. Rev 67 (2015) 103–117. doi: 10.1124/pr.114.009381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee JH, Zheng Y, von Bomstadt D, et al. , Selective ROCK2 inhibition in focal cerebral ischemia, Ann. Clin. Transl. Neurol 1 (2014) 2–14. doi: 10.1002/acn3.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pan P, Shen M, Yu H, Li Y, Li D, Hou T, Advances in the development of rho-associated protein kinase (ROCK) inhibitors, Drug. Discov. Today 18 (2013) 1323–1333. doi: 10.1016/j.drudis.2013.09.010 [DOI] [PubMed] [Google Scholar]
- [16].Boerma M, Fu Q, Wang J, et al. , Comparative gene expression profiling in three primary human cell lines after treatment with a novel inhibitor of rho kinase or atorvastatin, Blood. Coagul. Fibrinolysis 19 (2008) 709–718. doi: 10.1097/MBC.0b013e32830b2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shah S, Savjani J, A review on ROCK-II inhibitors: from molecular modelling to synthesis, Bioorg. Med. Chem. Lett 26 (2016) 2383–2391. doi: 10.1016/j.bmcl.2016.03.113 [DOI] [PubMed] [Google Scholar]
- [18].Tybulewicz VLJ, Henderson RB, Rho family GTPases and their regulators in lymphocytes, Nat. Rev. Immunol 9 (2009) 630–644. doi: 10.1038/nri2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tharaux P-L, Bukoski RC, Rocha PN, et al. , Rho kinase promotes alloimmune responses by regulating the proliferation and structure of T cells, J. Immunol 171 (2003) 96–105. doi: 10.4049/jimmunol.171.1.96 [DOI] [PubMed] [Google Scholar]
- [20].Apostolidis SA, Rauen T, Hedrich CM, Tsokos GC, Crispin JC, Protein phosphatase 2A enables expression of interleukin 17 (IL-17) through chromatin remodeling, J. Biol. Chem 288 (2013) 26775–26784. doi: 10.1074/jbc.M113.483743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].He Y, Xu H, Liang L, et al. , Antiinflammatory effect of rho kinase blockade via inhibition of NF-ΚB activation in rheumatoid arthritis, Arthritis. Rheum 58 (2008) 3366–3376. doi: 10.1002/art.23986 [DOI] [PubMed] [Google Scholar]
- [22].Li Y, Harada T, Juang Y-T, et al. , Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus, J. Immunol 178 (2007) 1938–1947. doi: 10.4049/jimmunol.178.3.1938 [DOI] [PubMed] [Google Scholar]
- [23].Isgro J, Gupta S, Jacek E, et al. , Enhanced rho-associated protein kinase activation in patients with systemic lupus erythematosus, Arthritis. Rheum 65 (2013) 1592–1602. doi: 10.1002/art.37934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lally L, Pernis A, Narula N, Huang W-T, Spiera R, Increased rho kinase activity in temporal artery biopsies from patients with giant cell arteritis, Rheumatology (Oxford) 54 (2015) 554–558. doi: 10.1093/rheumatology/keu364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang X, Tao Y, Troiani L, Markovic-Plese S, Simvastatin inhibits IFN regulatory factor 4 expression and Th17 cell differentiation in CD4+ T cells derived from patients with multiple sclerosis, J. Immunol 187 (2011) 3431–3437. doi: 10.4049/jimmunol.1100580 [DOI] [PubMed] [Google Scholar]
- [26].Biswas PS, Gupta S, Chang E, Bhagat G, Pernis AB, Aberrant ROCK activation promotes the development of type I diabetes in NOD mice, Cell. Immunol 266 (2011) 111–115. doi: 10.1016/j.cellimm.2010.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Biswas PS, Gupta S, Chang E, et al. , Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice, J. Clin. Invest 120 (2010) 3280–3295. doi: 10.1172/JCI42856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rozo C, Chinenov Y, Maharaj RK, et al. , Targeting the rhoA-ROCK pathway to reverse T-cell dysfunction in SLE. Ann. Rheum. Dis 76 (2017) 740–747. doi: 10.1136/annrheumdis-2016-209850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kasahara DI, Matthews JA, Ninin FMC, Wurmbrand AP, Liao JK, Shore SA, Role of ROCK2 in CD4+ cells in allergic airways responses in mice, Clin. Exp. Allergy 47 (2017) 224–235. doi: 10.1111/cea.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zanin-Zhorov A, Weiss JM, Nyuydzefe MS, et al. , Selective oral ROCK2 inhibitor downregulates IL-21 and IL-17 secretion in human T cells via STAT3-dependent mechanism, Proc. Natl. Acad. Sci. USA 111 (2014) 16814–16819. doi: 10.1073/pnas.1414189111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Weiss JM, Chen W, Nyuydzefe MS, et al. , ROCK2 signaling is required to induce a subset of T follicular helper cells through opposing effects on STATs in autoimmune settings, Sci. Signal 9 (2016) ra73. doi: 10.1126/scisignal.aad8953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang W, Zhou G, Yu T, et al. , Critical role of ROCK2 activity in facilitating mucosal CD4+ T cell activation in inflammatory bowel disease, J. Autoimmun 89 (2018) 125–138. doi: 10.1016/j.jaut.2017.12.009 [DOI] [PubMed] [Google Scholar]
- [33].Chen W, Nyuydzefe MS, Weiss JM, Zhang J, Waksal SD, Zanin-Zhorov A, ROCK2, but not ROCK1 interacts with phosphorylated STAT3 and co-occupies TH17/TFH gene promoters in TH17-activated human T cells, Sci. Rep 8 (2018) 16636. doi: 10.1038/s41598-018-35109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tengesdal IW, Kitzenberg D, Li S, et al. , The selective ROCK2 inhibitor KD025 reduces IL-17 secretion in human peripheral blood mononuclear cells independent of IL-1 and IL-6, Eur. J. Immunol 48 (2018) 1679–1686. doi: 10.1002/eji.201847652 [DOI] [PubMed] [Google Scholar]
- [35].Gensous N, Charrier M, Duluc D, et al. , T follicular helper cells in autoimmune disorders, Front. Immunol 9 (2018) 1637. doi: 10.3389/fimmu.2018.01637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Edlund S, Landström M, Heldin C-H, Aspenström P, Transforming growth factor-β–induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA, Mol. Biol. Cell 13 (2002) 902–914. doi: 10.1091/mbc.01-08-0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bettelli E, Carrier Y, Gao W, et al. , Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells, Nature 441 (2006) 235–238. doi: 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- [38].Zanin-Zhorov A, Weiss JM, Trzeciak A, et al. , Cutting edge: selective oral ROCK2 inhibitor reduces clinical scores in patients with psoriasis vulgaris and normalizes skin pathology via concurrent regulation of IL-17 and IL-10, J. Immunol 198 (2017) 3809–3814. doi: 10.4049/jimmunol.1602142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Amano M, Nakayama M, Kaibuchi K, Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity, Cytoskeleton (Hoboken) 67 (2010) 545–554. doi: 10.1002/cm.20472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Newell-Litwa KA, Badoual M, Asmussen H, Patel H, Whitmore L, Horwitz AR, ROCK1 and 2 differentially regulate actomyosin organization to drive cell and synaptic polarity, J. Cell. Biol 210 (2015) 225–242. doi: 10.1083/jcb.201504046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Takeda Y, Matoba K, Kawanami D, et al. , ROCK2 regulates monocyte migration and cell to cell adhesion in vascular endothelial cells, Int. J. Mol. Sci 20 (2019) 1331. doi: 10.3390/ijms20061331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shimizu T, Liao JK, Rho kinases and cardiac remodeling, Circ. J 80 (2016) 1491–1498. doi: 10.1253/circj.CJ-16-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shimizu T, Narang N, Chen P, et al. , Fibroblast deletion of ROCK2 attenuates cardiac hypertrophy, fibrosis, and diastolic dysfunction, JCI. Insight 2 (2017) e93187. doi: 10.1172/jci.insight.93187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nagai Y, Matoba K, Kawanami D, et al. , ROCK2 regulates TGF-β–induced expression of CTGF and profibrotic genes via NF-ΚB and cytoskeleton dynamics in mesangial cells, Am. J. Physiol. Renal. Physiol 317 (2019) F839–F851. doi: 10.1152/ajprenal.00596.2018 [DOI] [PubMed] [Google Scholar]
- [45].Zhou Y, Huang X, Hecker L, et al. , Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis, J. Clin. Invest 123 (2013) 1096–1108. doi: 10.1172/JCI66700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Knipe RS, Probst CK, Lagares D, et al. , The rho kinase isoforms ROCK1 and ROCK2 each contribute to the development of experimental pulmonary fibrosis, Am. J. Respir. Cell. Mol. Biol 58 (2018) 471–481. doi: 10.1165/rcmb.2017-0075OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Damlaj M, Snnallah M, Alhejazi A, et al. , Graft vs host disease impacts overall survival post allogeneic hematopoietic stem cell transplantation for acute lymphoblastic leukemia/lymphoma, World. J. Transplant 8 (2018) 252–261. doi: 10.5500/wjt.v8.i7.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Arai S, Arora M, Wang T, et al. ; for the Graft-vs-Host Disease Working Committee of the CIBMTR, Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research, Biol. Blood. Marrow. Transplant 21 (2015) 266–274. doi: 10.1016/j.bbmt.2014.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Arora M, Cutler CS, Jagasia MH, et al. , Late acute and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation, Biol. Blood. Marrow. Transplant 22 (2016) 449–455. doi: 10.1016/j.bbmt.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wong FL, Francisco L, Togawa K, et al. , Long-term recovery after hematopoietic cell transplantation: predictors of quality-of-life concerns, Blood 115 (2010) 2508–2519. doi: 10.1182/blood-2009-06-225631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wingard JR, Majhail NS, Brazauskas R, et al. , Long-term survival and late deaths after allogeneic hematopoietic cell transplantation, J. Clin. Oncol 29 (2011) 2230–2239. doi: 10.1200/JCO.2010.33.7212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zeiser R, Blazar BR, Pathophysiology of chronic graft-versus-host disease and therapeutic targets, New. Engl. J. Med 337 (2017) 2565–2579. doi: 10.1056/NEJMra1703472 [DOI] [PubMed] [Google Scholar]
- [53].Cooke KR, Luznik L, Sarantopoulos S, et al. , The biology of chronic graft-versus-host disease: a task force report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease, Biol. Blood. Marrow. Transplant 23 (2017) 211–234. doi: 10.1016/j.bbmt.2016.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].MacDonald KPA, Hill GR, Blazar BR, Chronic graft-versus-host disease: biological insights from preclinical and clinical studies, Blood 129 (2017) 12–21. doi: 10.1182/blood-2016-06-686618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Alexander KA, Flynn R, Lineburg KE, et al. , CSF-1–dependant donor-derived macrophages mediate chronic graft-versus-host disease, J. Clin. Invest 124 (2014) 4266–4280. doi: 10.1172/JCI75935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hill GR, Olver SD, Kuns RD, et al. , Stem cell mobilization with G-CSF induces type 17 differentiation and promotes scleroderma, Blood 116 (2010) 819–828. doi: 10.1182/blood-2009-11-256495 [DOI] [PubMed] [Google Scholar]
- [57].Du J, Paz K, Flynn R, et al. , Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-β production, Blood 129 (2017) 2570–2580. doi: 10.1182/blood-2017-01-758854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Flynn R, Du J, Veenstra RG, et al. , Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans, Blood 123 (2014) 3988–3998. doi: 10.1182/blood-2014-03-562231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Srinivasan M, Flynn R, Price A, et al. , Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans, Blood 119 (2012) 1570–1580. doi: 10.1182/blood-2011-07-364414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Deng R, Hurtz C, Song Q, et al. , Extrafollicular CD4+ T-B interactions are sufficient for inducing autoimmune-like chronic graft-versus-host disease, Nat. Commun 8 (2017) 978. doi: 10.1038/s41467-017-00880-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Matsuoka K-I, Kim HT, McDonough S, et al. , Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation, J. Clin. Invest 120 (2010) 1479–1493. doi: 10.1172/JCI41072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Flynn R, Paz K, Du J, et al. , Targeted rho-associated kinase 2 inhibition suppresses murine and human chronic GVHD through a STAT3-dependent mechanism, Blood 127 (2016) 2144–2154. doi: 10.1182/blood-2015-10-678706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jagasia M, Lazaryan A, Bachier CR, et al. , ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graft-versus-host disease, J. Clin. Oncol 39(2021) 1888–1898. doi: 10.1200/JCO.20.02754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cutler CS, Lee SJ, Arai S, et al. , Belumosudil for chronic graft-versus-host disease (cGVHD) after 2 or more prior lines of therapy: the ROCKstar study, Blood 2021. doi: 10.1182/blood.2021012021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].National Institutes of Health, KD025 in subjects with diffuse cutaneous systemic sclerosis https://clinicaltrials.gov/ct2/show/NCT03919799, 2019. (accessed 29 October 2020).
- [66].Volkmann ER, Varga J, Emerging targets of disease-modifying therapy for systemic sclerosis, Nat.Rev. Rheumatol 15 (2019) 208–224. doi: 10.1038/s41584-019-0184-z [DOI] [PubMed] [Google Scholar]
- [67].Fuschiotti P, Current perspectives on the immunopathogenesis of systemic sclerosis, Immunotargets. Ther 5 (2016) 21–35. doi: 10.2147/ITT.S82037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pehlivan Y, Yolbas S, Cetin GY, et al. , Investigation of the association between rho/rho-kinase gene polymorphisms and systemic sclerosis, Rheumatol. Int 36 (2016) 421–427. doi: 10.1007/s00296-015-3400-4 [DOI] [PubMed] [Google Scholar]
- [69].Akhmetshina A, Dees C, Pileckyte M, et al. , Rho-associated kinases are crucial for myofibroblast differentiation and production of extracellular matrix in scleroderma fibroblasts, Arthritis. Rheum 58 (2008) 2553–2564. doi: 10.1002/art.23677 [DOI] [PubMed] [Google Scholar]
- [70].REZUROCK. Package insert. Kadmon Pharmaceuticals, LLC; 2021. [Google Scholar]


