Abstract
Chinese herbal medicine (CHM) has long been used for allergic rhinitis (AR). This systematic review aimed to investigate the clinical effects and safety of oral CHM for AR by comparing it to western medications (WM). Nineteen databases were searched up to 27 May 2020. Randomized controlled trials (RCTs) assessing the effects of CHM on the primary or secondary outcomes comparing to WM, in any age of the patients, were included. The pooled results were expressed as mean difference, standardized mean difference or odds ratio with 95% confidence intervals. Eighteen RCTs were included and 17 of them were evaluated in the meta-analysis. CHM may improve total nasal symptom scores, individual symptom scores (rhinorrhea, nasal congestion, sneezing and nasal itching), quality of life and recurrence rate, compared to antihistamines (loratadine and chlorpheniramine). Only mild and transient adverse events of CHM were reported. However, there were no significant differences in some subgroup analyses in total nasal symptom scores, rhinorrhea, nasal obstruction, sneezing, nasal itching and SF-36. Due to the small number of included studies, poor quality of trial design and substantial heterogeneities, the potential of CHM for AR, should be validated in large, multi-center and well-designed RCTs in the future.
Keywords: Allergic diseases, Complementary and alternative medicine, Hay fever, Nasal symptoms, Natural products, Traditional Chinese medicine
1. Introduction
Allergic rhinitis (AR) is a common allergen-induced immunoglobulin E (IgE) mediated inflammatory disease that affects the nasal mucosa membranes (Sin & Togias, 2011). In Australia, the incidence of AR in Australia was approximately 19% (over 4.6 million people) in 2017 to 2018, whereas children seem to be less affected by this condition, accounting for only 10% within all AR patients, compared to other age groups (Australian Institute of Health and Welfare, 2019). There are two different types of AR, including seasonal AR (SAR) and perennial AR (PAR), which are clinically characterized by sneezing, nasal congestion, sneezing, rhinorrhea and itching of the nose and eyes and/or postnasal drips, mainly affects patients’ quality of life (QoL) in allergen exposure, such as plant pollen, dust mites and molds (Seidman et al., 2015). Additionally, a 23-year follow-up survey enrolling 1836 college students claimed that people with AR or positive allergy skin tests are three times higher probability to develop asthma than others (Settipane et al., 1994). Conventional management includes avoidance of exposure to triggers, internasal or oral medication, such as corticosteroids, antihistamines, leukotriene receptor antagonists, decongestants and cromolyn, however, the short-term effects and side effects of them have driven many to seek other solutions (Mahr et al., 2008; Sheth et al., 2008; Wolthers, 2000).
The current pharmacotherapy for AR is prescribed based on a severity spectrum of AR (mild to severe) and the frequency of occurrences of the AR signs and symptoms (intermittent and persistent) (Seidman et al., 2015). In contrary to the conventional interventions, the treatment of AR in Chinese herbal medicine (CHM) are vastly different. Although the terminology “allergic rhinitis” does not exist in Chinese medicine classic texts, the existence of CHM treatment of AR-like signs and symptoms, such as “Bi Qiu”, has long been recorded in classical literature (China Association of Chinese Medicine, 2015). Findings from a retrospective diagnosis research in classic literature revealed that 163 herbs had been used for the management of AR-like signs and symptoms (Kreiner, 2016). Till today, many of these herbs are still used in clinical practice (Kreiner, 2016). Many randomized controlled trials (RCTs) have been carried out to evaluate clinical effects and safety of CHM interventions for AR management with varying results (Yan et al., 2019; Zhao et al., 2019). Some published systematic reviews have indicated that CHM may be beneficial for AR participants, compared to placebo (Wang et al., 2012; Zhang et al., 2018). However, none of the systematic reviews compared the treatment effects and safety between CHM and Western medications (WM) in English Language. Therefore, this review aimed to investigate the clinical effects and safety between oral CHM and WM in the management of AR by systematically evaluating published RCTs.
2. Material and methods
The study design adhered to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins & Green, 2011). The study was conducted according to our published Cochrane protocol (Yang et al., 2009) and reported following the checklist of Preferred Reporting Items for Systematic Reviews and Meta-analyses (Supplementary Table S1) (Moher et al., 2009).
2.1. Search strategies
Nineteen electronic databases were searched from their respective inception to 16 August 2019, updated on 27 May 2020, including the Cochrane Central Register of Controlled Trials; PubMed; EMBASE; AMED; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; ISCTRN; ClinicalTrials.gov; ICTRP; China National Knowledge Infrastructure; Chong Qing VIP; Wanfang Data; and Google. The reference lists of identified publications for RCTs were scanned. Keywords used for the literature search included the following: allergic rhinitis, rhinitis, pollinosis, Chinese herbal medicine, herbal medicine, phytomedicine, ethnobotanical and their related synonyms. Adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0) (Higgins & Green, 2011) were combined with subject strategies, where appropriate. No restrictions on the date of publication nor publication status were imposed. Searching strategy is presented in Supplementary Table S2.
2.2. Selection criteria
RCTs with or without blinding, published in any language, were included. Data from the first phase of randomized cross-over trials were considered for inclusion if cross-over trials were available. Participants of all ages, both genders with SAR or PAR, were included. Allergy must be confirmed using objective tests such as a skin prick test or a serum IgE test. All forms of oral administration of single Chinese herbs or Chinese herbal formulae such as capsule, decoction, tablet, pill or granule used in comparison to different WM were included for analysis. Primary outcome measures included total nasal symptom scores (TNSS) and individual symptom scores (rhinorrhea, nasal congestion, sneezing and nasal itching). Secondary outcome measures included QoL, recurrence rate and adverse events.
Articles were excluded if (1) the participants did not suffer from SAR or PAR that were confirmed by objective tests or (2) the control group applied other interventions such as placebo, or no treatment, or other therapy (such as acupuncture) other than WM or (3) the RCT involved co-intervention in CHM group or both groups or (4) the RCT did not evaluate any of outcome measures specified above. Screening of studies according to the selection criteria was carried out by two independent reviewers (H.L. and J.K.). Any discrepancy between the two reviewers was resolved by the third party (A.Y.).
2.3. Data extraction and quality assessment
Data were extracted based on a self-developed pre-designed Excel form. Data extracted from each included study consisted of characteristics of participants, diagnosis, study setting, sample size, diagnostic criteria, interventions, duration and outcome measures. Primary outcomes include the total and individual nasal symptoms scores (rhinorrhea, nasal congestion, sneezing, nasal itching), and the secondary outcomes consist of quality of life questionnaires (Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ), Visual Analogue Scale (VAS), and 36-Item Short Form Health Survey (SF-36)). Each RCT was evaluated independently by two reviewers (H.L. and A.W.). Any disagreements in the assessment of data were resolved by discussion and consensus was reached in all cases.
The risk of bias of the included RCTs was assessed by two reviewers (M.L. and H.L.) using the risk of bias assessment tool mentioned in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins & Green, 2011). The other reviewer (Y.S.) checked and confirmed the data and assessment process. Any disagreement between the two reviewers was resolved through discussion with the third party (A.Y.). Eight risk of bias items were assessed and listed as followed: (1) random sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants and personnel (performance bias); (4) blinding of outcome assessment (detection bias); (5) incomplete outcome data (attrition bias); (6) selective reporting (reporting bias); (7) baseline (other bias); and (8) funding (other bias). Three judgments including low risk, unclear risk and high risk were utilized to assess each item for individual trials.
2.4. Statistical analysis
Data were synthesized qualitatively and quantitatively using the Cochrane Collaboration software RevMan 5.3 (The Cochrane Collaborations, 2014). For the continuous data (i.e. the total nasal symptom, rhinorrhea, nasal congestion, sneezing and nasal itching scores, and the quality of life measures including visual analogue scale, rhinoconjunctivitis quality of life questionnaire (RQLQ) and SF-36), mean difference (MD) was used when the outcomes were measured with the same scale whereas standardized mean difference (SMD) was used when different scales were used for outcome measures. Odds ratio (OR) was used to analyze dichotomous data (i.e. recurrence rate) utilizing inverse variance. All the results were presented with 95% confidence interval (CI). A ‘worst-case scenario’ method was used as a solution to address the missing data. Heterogeneity was presented with I2 statistics, between 0% and 30% as ‘low’, 30% to 50% as ‘moderate’, and 50% to 100% as ‘substantial’ (Higgins & Green, 2011). Random effects were used to minimize the potential heterogeneity when the I2 value was over 50%. Sensitivity analysis was used where needed. A descriptive summary was used to present all the data in this study.
3. Results
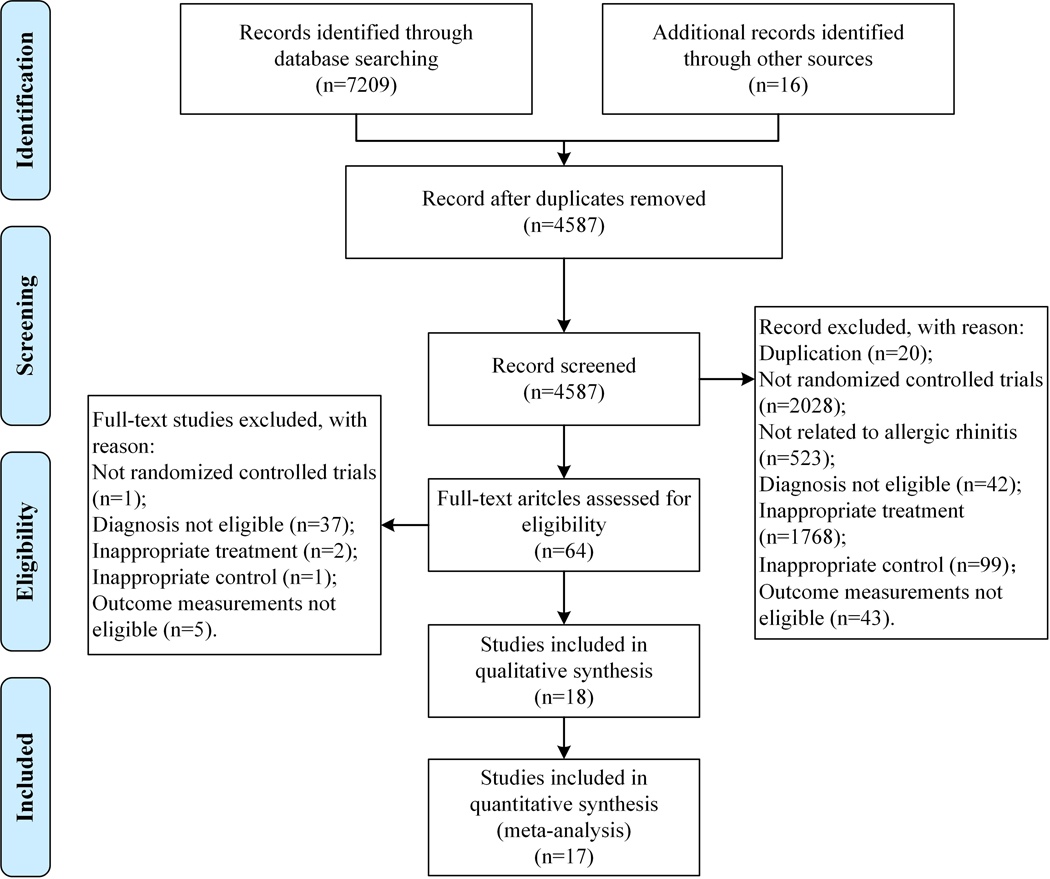
A total of 7225 studies were identified adhering to the search strategies outlined above. Eighteen studies met the inclusion criteria (Duan, 2017; Gao, 2009; Hao, 2017; Hu, 2018; Huang & Teng, 2018; Jia, 2017; Jin, 2010; Liu et al., 2001; Lu et al., 2011; Qiu, 2012; Shi & Liu, 2017; Wang et al., 2018; Wang & Li, 2019; Xuan, 2019; Yan et al., 2019; Zhao et al., 2019; Zhong, 2013; Zou et al., 2012) and 17 of them were included in the meta-analysis (Duan, 2017; Gao, 2009; Hao, 2017; Hu, 2018; Huang & Teng, 2018; Jia, 2017; Jin, 2010; Liu et al., 2001; Lu et al., 2011; Qiu, 2012; Shi & Liu, 2017; Wang et al., 2018; Wang & Li, 2019; Xuan, 2019; Yan et al., 2019; Zhao et al., 2019; Zhong, 2013). The list of the excluded full-text articles has been provided in Supplementary Table S3. Figure 1 details the selection process of this review.
Figure 1.
Flowchart of the selection process of included studies.
3.1. Description of included studies
All 18 included studies are RCTs (17 two-armed trials (Duan, 2017; Gao, 2009; Hao, 2017; Hu, 2018; Huang & Teng, 2018; Jia, 2017; Jin, 2010; Liu et al., 2001; Lu et al., 2011; Qiu, 2012; Shi & Liu, 2017; Wang & Li, 2019; Xuan, 2019; Yan et al., 2019; Zhao et al., 2019; Zhong, 2013; Zou et al., 2012) and one three-armed trial (Wang et al., 2018)). There was no cross-over RCT identified in this review. Seventeen of them were published in Chinese (Duan, 2017; Gao, 2009; Hao, 2017; Hu, 2018; Huang & Teng, 2018; Jia, 2017; Jin, 2010; Liu et al., 2001; Lu et al., 2011; Shi & Liu, 2017; Wang et al., 2018; Wang & Li, 2019; Xuan, 2019; Yan et al., 2019; Zhong, 2013; Zou et al., 2012) and one in English (Zhao et al., 2019). All RCTs were conducted between 2001 to 2019, including 17 trials in mainland China (hospital outpatient departments) (Duan, 2017; Gao, 2009; Hao, 2017; Hu, 2018; Huang & Teng, 2018; Jia, 2017; Jin, 2010; Liu et al., 2001; Lu et al., 2011; Shi & Liu, 2017; Wang et al., 2018; Wang & Li, 2019; Xuan, 2019; Yan et al., 2019; Zhao et al., 2019; Zhong, 2013; Zou et al., 2012) and one in Taiwan (clinical centers) (Qiu, 2012). They recruited 2013 participants totally from two years old to 75 years old, including 1010 participants in the CHM groups, 1003 in the WM groups, 1026/970 male/female participants (two trials did not specify the gender of drop out participants), and 1976 participants were evaluated for one of the outcome measures at the end of the treatment period.
All participants in the included studies were diagnosed by their subjective symptoms, objective signs and objective tests. Participants in seven trials were diagnosed with PAR (Gao, 2009; Hao, 2017; Jin, 2010; Liu et al., 2001; Lu et al., 2011; Zhao et al., 2019; Zhong, 2013), one trial with SAR (Qiu, 2012) and ten trials with non-classified AR (Duan, 2017; Hu, 2018; Huang & Teng, 2018; Jia, 2017; Shi & Liu, 2017; Wang et al., 2018; Wang & Li, 2019; Xuan, 2019; Yan et al., 2019; Zou et al., 2012). Syndrome differentiation for the CHM was not applied in five trials (Huang & Teng, 2018; Liu et al., 2001; Lu et al., 2011; Qiu, 2012; Wang & Li, 2019). Participants in two trials were diagnosed with Kidney Yang deficiency syndrome (Wang et al., 2018; Xuan, 2019), two trials with Lung-Spleen deficiency syndrome (Duan, 2017; Hao, 2017), two trials with Lung Qi deficiency cold syndrome (Jia, 2017; Zhao et al., 2019), and seven trials with other syndromes (Gao, 2009; Hu, 2018; Jin, 2010; Shi & Liu, 2017; Yan et al., 2019; Zhong, 2013; Zou et al., 2012).
Within the 18 included RCTs, 14 of them administrated CHM to participants in decoction form (Duan, 2017; Gao, 2009; Huang & Teng, 2018; Jin, 2010; Liu et al., 2001; Lu et al., 2011; Qiu, 2012; Shi & Liu, 2017; Wang et al., 2018; Wang & Li, 2019; Xuan, 2019; Yan et al., 2019; Zhao et al., 2019; Zhong, 2013), two of them in granule (Hao, 2017; Jia, 2017), one in capsule (Zou et al., 2012), and one in both decoction and granule (Hu, 2018). A total of 18 different CHM formulae were prescribed to the AR participants in the included studies, including 12 newly created formulae (not a classic formula or not a modified formula from a classic one) (Duan, 2017; Hao, 2017; Huang & Teng, 2018; Jia, 2017; Jin, 2010; Liu et al., 2001; Shi & Liu, 2017; Wang et al., 2018; Yan et al., 2019; Zhao et al., 2019; Zhong, 2013; Zou et al., 2012) and six modified from classic formulae (Gao, 2009; Hu, 2018; Lu et al., 2011; Qiu, 2012; Wang & Li, 2019; Xuan, 2019). The treatment period lasted from 14 days to eight weeks. Eleven studies compared CHM with anti-histamines (Gao, 2009; Hao, 2017; Huang & Teng, 2018; Jin, 2010; Liu et al., 2001; Lu et al., 2011; Qiu, 2012; Shi & Liu, 2017; Wang & Li, 2019; Xuan, 2019; Zhong, 2013), five studies with anti-histamines plus corticosteroids (Duan, 2017; Wang et al., 2018; Yan et al., 2019; Zhao et al., 2019; Zou et al., 2012), one study with corticosteroids (Hu, 2018), and one study with anti-histamines plus leukotriene modifiers (Jia, 2017). Characteristics of 18 included RCTs and their herbal ingredients are summarized in Table 1 and detailed in Table 2.
Table 1.
Characteristics of the 18 included randomized controlled trials.
| Study ID | Setting | Sample size | Dropout | Diagnosis | Syndrome differentiation for the CHM | Treatment | Control | Durations | Outcome measurement |
|---|---|---|---|---|---|---|---|---|---|
| Wang & Li, 2019 | HOD, mainland China | T: 58; C: 58 | 0 | Nonclassified AR | N/A | Modified Mahuang Xixin Fuzi Decoction, 1 pack, PO, bid | Chlorpheniramine maleate tablets, 2 mg, PO, tid | 21 days | TNSS; QoL (RQLQ) |
| Xuan, 2019 | HOD, mainland China | T: 80; C: 80 | 0 | Nonclassified AR | Kidney Yang deficiency syndrome | Guizhi Decoction combined with Mahuang Fuzi Xixin Decoction, 100 ml, PO, bid | Loratadine tablets, 10 mg, PO, qd | 4 weeks | Individual symptom scores; Recurrence rate |
| Yan et al., 2019 | HOD, mainland China | T: 50; C: 50 | 0 | Nonclassified AR | Lung-Kidney deficiency cold syndrome | Modified Xi Min Decoction, 200 ml, PO, bid | Fluticasone propionate nasal spray, 2 sprays each nostril, INH, qd; Loratadine tablets, 10 mg, PO, qd | 30 days | TNSS; Individual symptom scores; QoL (RQLQ) |
| Zhao et al., 2019 | HOD, mainland China | T: 54; C: 54 | T: 3; C: 7 | PAR | Lung Qi deficiency cold syndrome | Bi Min Decoction, 200 ml, PO, bid | Fluticasone furoate spray, 2 sprays each nostril, INH, qd; Loratadine tablets, 10 mg, PO, qn | 4 weeks | QoL (RQLQ); AE |
| Hu, 2018 | HOD, mainland China | T: 30; C: 30 | 0 | Nonclassified AR | Lung Qi deficiency and cold attack phenotype syndrome | Huangqi Guizhi Wuwu Decoction plus Cang er zi Granule, 1 pack, PO, bid | Budesonide nasal spray, 2 sprays, INH, bid | 30 days | TNSS |
| Huang & Teng, 2018 | HOD, mainland China | T: 50; C: 50 | 0 | Nonclassified AR | N/A | Yuping Tuomin Decoction, 400 ml, PO, bid | Loratadine tablets, 10 mg, PO, qd | 14 days | TNSS |
| Wang et al., 2018 | HOD, Mainland China | T: 80; C: 80; I: 80 | T: 3; C: 2 | Nonclassified AR | Kidney Yang deficiency syndrome | Bu Yang Liao Ti Decoction, 1 pack, PO, bid | Cetirizine hydrochloride dispersible tablets, 10 mg, PO, qd; Beclomethasone dipropionate aqueous nasal spray, 1–2 sprays, internasal, bid | 8 weeks | Individual symptom score; QoL (VAS, RQLQ); AE |
| Duan, 2017 | HOD, Mainland China | T: 62; C: 66 | T: 2; C: 6 | T: PAR: 34, SAR: 26; C: PAR: 36, SAR: 24; | Dual deficiency of the Lung-Spleen syndrome | Tuo Min Decoction, 100 ml, PO, tid | Ebastine tablets, 10 mg, PO, qn; Fluticasone propionate nasal spray, 2 sprays, INH, qd | 4 weeks | Individual symptom score; Qol (VAS); AE |
| Hao, 2017 | HOD, Mainland China | T: 36; C: 36 | 0 | PAR | Dual deficiency of the Lung-Spleen syndrome | Two Ginseng Xinyi Granule, 150 ml, PO, bid | Loratadine tablets, 10 mg, PO, qd | 4 weeks | TNSS; AE |
| Jia, 2017 | HOD, Mainland China | T: 25; C: 25 | 0 | Nonclassified AR | Lung Qi deficiency cold syndrome | Biyantongqiao Granule, 3–6 yo: 150 ml, PO, qd, 7–12 yo: 200 ml, PO, qd | Loratadine granules, 3–14 yo: body weight > 30 kg, 10 mg, PO, qn, body weight ≤ 30 kg, 5 mg, PO, qn; Montelukast sodium chewable tablets, 3–5 yo: 4 mg, PO, qd, 6–14 yo: 5 mg, PO, qd | 14 days | TNSS; Individual symptom score; AE |
| Shi & Liu, 2017 | HOD, Mainland China | T: 60; C: 60 | 0 | Nonclassified AR | Invasion of cold and Lung deficiency syndrome | Yiqi Tuomin Decoction, 1 pack, PO, bid | Loratadine tablets, 10 mg, PO, qd | 4 weeks | TNSS; Individual symptom score |
| Zhong, 2013 | HOD, Mainland China | T: 35; C: 34 | 0 | PAR | Lung defense Qi deficiency syndrome | Qufeng Tongqiao Decoction, 200 ml, PO, bid | Loratadine tablets, 10 mg, PO, qd | 4 weeks | TNSS; Individual symptom score; Recurrence rate; AE |
| Qiu, 2012 | Clinical center, Taiwan, China | T: 100; C: 100 | 0 | SAR | N/A | Modified Xiao Qing Long Decoction, 1 pack, PO, bid | Loratadine tablets, 10 mg, PO, qd | 30 days | TNSS; AE |
| Zou et al., 2012 | HOD, Mainland China | T: 52; C: 52 | 0 | Nonclassified AR | Qi deficiency syndrome | Tuomin Tongqiao Capsule, 3 capsules, PO, tid | Loratadine tablets, 10 mg, PO, qd; Triamcinolone acetonide nasal spray, 2 sprays, INH, qd | 4 weeks | TNSS; AE |
| Lu et al., 2011 | HOD, Mainland China | T: 60; C: 60 | 0 | PAR | N/A | Modified Guizhi Decoction; 2–6y: 30–80 ml, PO, tid; 7–18y: 150–200 ml, PO, tid | Loratadine Syrup; 2–12 yo: body weight > 30 kg, 10 mg, PO, qd, body weight ≤ 30 kg, 5 mg, PO, qd; Over 12 yo: 10 mg, PO, qd | 14 days | Individual symptom score; Recurrence rate |
| Jin, 2010 | HOD, Mainland China | T: 40; C: 40 | 0 | PAR | Latent heat in Lung channel syndrome | Kemin Decoction, 1 pack, PO, bid | Cetirizine hydrochloride tablets, 10 mg, PO, qd; Diphenhydramine nasal drops, 8 ml, INH, qid | 4 weeks | Recurrence rate |
| Gao, 2009 | HOD, Mainland China | T: 65; C: 65 | T: 6; C: 8 | PAR | Phlegm-rheum attacking upwards syndrome | Modified Lingguizhugan Decoction, 1 pack, PO, tid | Loratadine tablets, 10 mg, PO, qd | 14 days | TNSS; Individual symptom score; QoL (SF-36); AE |
| Liu et al., 2001 | HOD, Mainland China | T: 73; C: 63 | 0 | PAR | N/A | CHM for Nourishing Yin and Calming Liver, 1 pack, PO, qd | Cetirizine hydrochloride tablets, 10 mg, PO, qd | 1 month | Recurrence rate |
Notes: AE: Adverse events; AR: Allergic rhinitis; bid: Twice a day; C: Control group; CHM: Chinese herbal medicine; HOD: Hospital outpatient department; INH: Respiratory (Inhalation); PAR: Perennial allergic rhinitis; PO: Oral administration; qd: Once a day; qn: Once a day before sleep; QoL: Quality of life; RQLQ: Rhinoconjunctivitis Quality of Life Questionnaire; SAR: Seasonal allergic rhinitis; SF-36: 36-Item Short Form Health Survey; T: Treatment group; tid: Three times a day; TNSS: Total nasal symptom scores; yo: Years old.
Table 2.
Ingredients and adverse events of herbal prescriptions in the 18 included studies.
| Study ID | Herbal medicine | Component of prescription | Adverse events |
|---|---|---|---|
| (Wang & Li, 2019) | Modified Mahuang Xixin Fuzi Decoction | Ma huang 5 g, Xi xin 3 g, Zhi fu zi 10 g, Can ger zi 10 g, Bai zhu 10 g, Xin yi hua 10 g, Fang fen 10 g, Huang qi 30 g, Quan xie 3 g, Zhi gan cao 10 g, Wu mei 10 g | Not reported |
| (Xuan, 2019) | Guizhi Decoction combined with Mahuang Fuzi Xixin Decoction | Gui zhi 15 g, Bai shao 15 g, Zhi gan cao 5 g, Sheng jiang 10 g, Da zao 10 g, Ting li zi 12 g, Chan tui 9 g, Ma huang 5 g, Zhi fu zi 10 g, Xi xin 3 g | Not reported |
| (Yan et al., 2019) | Modified Xi Min Decoction | Ma huang5 g, Gui zhi10 g, Bai shao10 g, Pao fu pian 10 g, Xi xin 3 g, Bei chai hu 9 g, Xin yi 10 g, Zhi gan cao 6 g | Not reported |
| (Zhao et al., 2019) | Bi Min Decoction | Fang feng 10 g, Huang qi 15 g, Bai zhu 10 g, Gui zhi 6 g, Bai shao 10 g, Wu mei 6 g, He zi 6 g, Xi xin 3 g, Wu wei zi 6 g, Ma huang 3 g, Gan cao 6 g | Dry nose (2/5); Sore throat (2/5); Sleepiness (1/5) |
| (Hu, 2018) | Huangqi Guizhi Wuwu Decoction plus Cang er zi Granule | Sheng huang qi 30 g, Gui zhi 10 g, Bai shao 10 g, Sheng jiang 10 g, Da zhao 6 g, Cang er zi 10 g, Xin yi 10 g, Bai zhi 10 g, Bo he 9 g | Not reported |
| (Huang & Teng, 2018) | Yuping Tuomin Decoction | Huang qi 30 g, Bai zhu 15 g, Fang feng 10 g, Chai hu 10 g, Fa ban xia 10 g, Bai shao 12 g, Xin yi 10 g, Cang er zi 10 g, Lu feng fang 6 g, Wu mei 10 g, Chan tui 10 g, Gan cao 6 g | Not reported |
| (Wang et al., 2018) | Bu Yang Liao Ti Decoction | Huang qi 20 g, Lu jiao shuang 12 g, Dang shen 20 g, Fang fen 9 g, Bai zhu 15 g, Xin yi 9 g, Gan jiang 15 g, Cang er zi 9 g, Ba ji tian 12 g, Xu chang qing 9 g, Gan cao 6 g | Dry mouth (1/77) |
| (Duan, 2017) | Tuo Min Decoction | Huang qi 15 g, Bai zhu 15 g, Dang shen 10 g, Fang fen 9 g, Chan tui 6 g, Fu ling 10 g, Gan cao 6 g, Wu wei zi 10 g, Gui zhi 9 g, Xi xin 3 g, Ke zi 10 g | Diarrhea (2/60); Nausea (2/60); Vomiting (2/60); Upper limbs rash (1/60) |
| (Hao, 2017) | Two Ginseng Xinyi Granule | Tai zi shen 3 g, Xin yi 1 g, Dang shen 2 g, Gao ben 1 g, Fang fen 1 g, Chuan xiong 1 g, Qiang huo 1 g, Wu wei zi 0.5 g, Huang qin 1 g, Chan tui 0.5 g, Bai zhi 1 g, Sheng ma 1 g | Adverse event was not found in both groups |
| (Jia, 2017) | Biyantongqiao Granule | Huang qi 12 g, Xin yi 6 g, Chao cang er zi 9 g, Bai zhi 9 g, Chuan xiong 9 g, Ke zi rou 9 g, E bu shi cao 6 g, Fang fen 9 g, Chao bai zhu 6 g, Xi xin 3 g, Gan cao 6 g | Adverse event was not found in both groups |
| (Shi & Liu, 2017) | Yiqi Tuomin Tang Decoction | Huang qi 10 g, Bai zhu 10 g, Dang gui 10 g, Wu mei 10 g, Xin yi 10 g Fang fen 6 g, Chai hu 6 g, Gui zhi 6 g, Bai zhi 6 g, Gan cao 3 g | Not reported |
| (Zhong, 2013) | Qufeng Tongqiao Decoction | Sheng huang qi, Yin hua tan, Jing jie tan, Fang feng tan, Dang gui tan (dosage not provided) | Adverse event was not found in both groups |
| (Qiu, 2012) | Modified Xiao Qing Long Decoction | Gui zhi 15 g, Ma huang 5g, Gan jiang 10 g, Bai shao 15 g, Xi xin 5 g, Shu fu zi 15 g, Wu wei zi 10 g, Zhi gan cao 10 g, Cang er zi 15 g, Dan shen 30 g | Adverse event was not found in CHM group |
| (Zou et al., 2012) | TuoMin Tongqiao Capsule | Ginsenoside, Jie geng, Da zao, Cang er zi, Xin yi, Huang qi (dosage not provided) | Adverse event was not found in both groups |
| (Lu et al., 2011) | Modified Guizhi Decoction | Gui zhi 6–10 g, Da zao 6–10 g, Wu mei 6–10 g, Bai shao 6–15 g, Fu ling 6–15 g, Chan tui 3–10 g, Jiang can 3–10 g, Sheng jiang 3–6 g, Gan cao 3–6 g | Not reported |
| (Jin, 2010) | Kemin Decoction | Huang qin 10 g, Zhi zi 10 g, Sang bai pi 10 g, Zhi mu 10 g, Xin yi 10 g, Bai zhi 10 g, Zi cao10 g, Qian cao 10 g, Chan yi 6 g, Gan di long 10 g, Ke zi 10 g, Wu mei 10 g | Not reported |
| (Gao, 2009) | Modified Lingguizhugan Decoction | Fu ling, Gui zhi, Bai zhu, Gan cao (dosage not provided) | Adverse event was not found in CHM group |
| (Liu et al., 2001) | CHM for Nourishing Yin and Calming Liver Decoction | Sheng di 10 g, Shan yu rou 15 g, Wu wei zi 10 g, Gou teng 30 g, Dan pi 10 g, Chai hu 10 g, Bai shao 10 g, Huang qin 10 g | Not reported |
Notes: CHM: Chinese herbal medicine. herb ingredients are listed as Chinese pinyin. Corresponding Latin names refer to the nomenclature list of commonly used Chinese herbal medicines published by the Chinese Medicine Board of Australia. The top 9 of most frequently used herbs were Magnoliae Flos (Xin yi) (n=10), Astragali Radix (Huang qi) (n=9), Saposhnikoviae Radix (Fang feng) (n=9), Glycyrrhizae Radix et Rhizoma (Gan cao) (n=9), Paeoniae Radix Alba (Bai shao) (n=8), Atractylodis Macrocephalae Rhizoma (Cang zu) (n=7), Xanthii Fructus (Can ger zi) (n=7), Cinnamomi Ramulus (Gui zhi) (n=7) and Asari Radix et Rhizoma (Xi xin) (n=7).
3.2. Risk of bias assessment
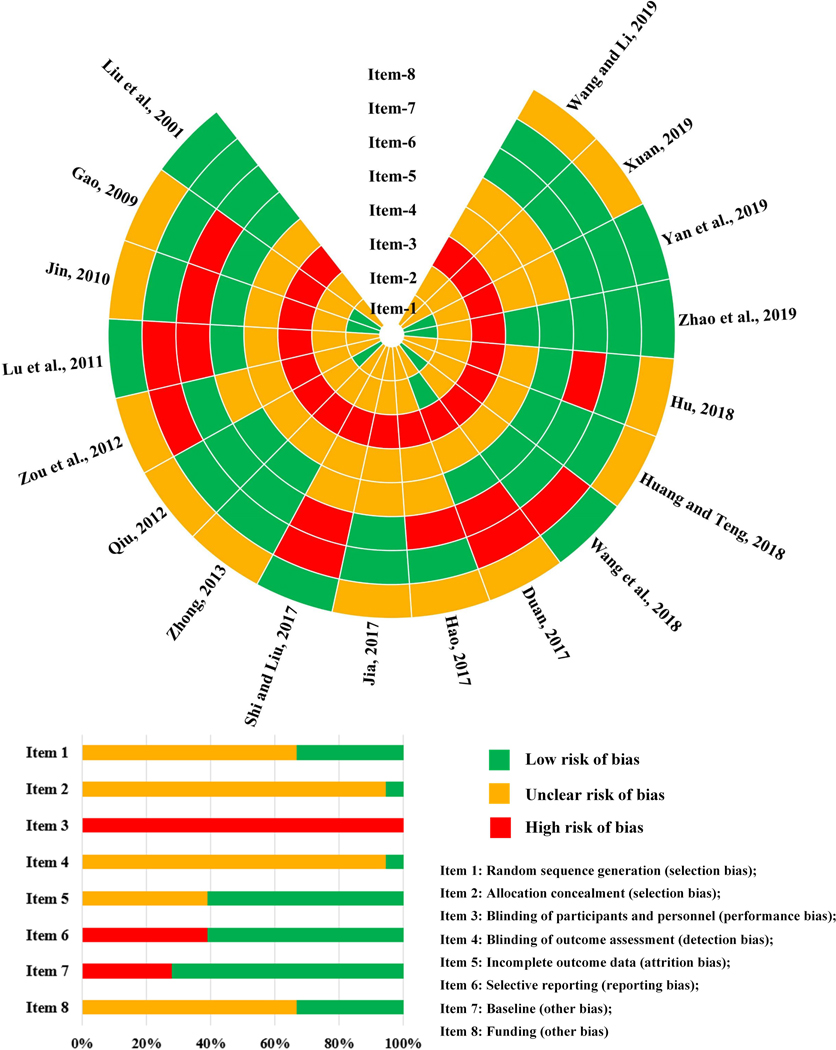
The authors’ assessment of the quality of all included studies is summarized in Figure 2. Although all the included studies claimed as RCTs, only six studies clearly reported the randomization method, including random number table (Jin, 2010; Qiu, 2012; Wang et al., 2018; Yan et al., 2019) or computer-generated numbers (Gao, 2009; Zhao et al., 2019), whilst the other studies did not state randomization generation details. Only one study reported the medications were centrally managed and packed (Duan, 2017), the rest did not provide clear allocation concealment method. None of the included studies were double-blinded. Seventeen studies utilized different forms of interventions in treatment and control groups (Gao, 2009; Hao, 2017; Hu, 2018; Huang & Teng, 2018; Jia, 2017; Jin, 2010; Liu et al., 2001; Lu et al., 2011; Qiu, 2012; Shi & Liu, 2017; Wang et al., 2018; Wang & Li, 2019; Xuan, 2019; Yan et al., 2019; Zhao et al., 2019; Zhong, 2013; Zou et al., 2012). Only one study indicated that all the interventions used the same package; however, the description in the method section stated that CHM decoction was compared to a combination of WM tablets and spray, which implied no blinding was achieved in this study (Duan, 2017). Only one included study stated that blinding of outcome assessors was performed (Zhao et al., 2019). Eleven studies were considered low risk of attrition bias (Duan, 2017; Gao, 2009; Hu, 2018; Huang & Teng, 2018; Jin, 2010; Liu et al., 2001; Lu et al., 2011; Qiu, 2012; Wang et al., 2018; Zhao et al., 2019; Zhong, 2013), while another seven studies used per-protocol analysis based on their vague description and they were assessed to be at unclear risk of bias in reporting complete outcome data (Hao, 2017; Jia, 2017; Shi & Liu, 2017; Wang & Li, 2019; Xuan, 2019; Yan et al., 2019; Zou et al., 2012). Only one study registered their protocol (Zhao et al., 2019). Eleven included RCTs reported consistent outcomes in the method and result sections (low risk) (Huang & Teng, 2018; Jia, 2017; Liu et al., 2001; Qiu, 2012; Wang et al., 2018; Wang & Li, 2019; Xuan, 2019; Yan et al., 2019; Zhao et al., 2019; Zhong, 2013; Zou et al., 2012). Selective reporting was identified in seven included trials, as some of the outcome measures indicated in the method section were not reported in the result section (high risk) (Duan, 2017; Gao, 2009; Hao, 2017; Hu, 2018; Jin, 2010; Lu et al., 2011; Shi & Liu, 2017).
Figure 2.
Risk of bias assessment in the 18 included randomized controlled trials.
All studies claimed that all the participants in treatment and control groups were comparable at baseline. However, five of them had imbalanced baseline data on key nasal symptoms, for example, itchy nose in three studies (MD 0.50, 95% CI 0.18 to 0.82 (Lu et al., 2011); MD −0.25, 95% CI −0.43 to −0.07 (Shi & Liu, 2017); and MD −0.11, 95% CI −0.20 to −0.02 (Wang et al., 2018)); runny nose in one study (MD 0.22, 95% CI 0.05 to 0.39) (Duan, 2017); sneezing in one study (MD 0.72, 95% CI 0.29 to 1.15) (Duan, 2017); nasal obstruction in one study (MD 0.14, 95% CI 0.05 to 0.23) (Wang et al., 2018); and TNSS in one study (MD −0.27, 95% CI −0.35 to −0.19) (Zou et al., 2012). Six studies listed funding details (government or university project) which had a minimum impact on the results (low risk) (Liu et al., 2001; Lu et al., 2011; Shi & Liu, 2017; Wang et al., 2018; Yan et al., 2019; Zhao et al., 2019). The other studies did not provide information on funding (unclear risk) (Duan, 2017; Gao, 2009; Hao, 2017; Hu, 2018; Huang & Teng, 2018; Jia, 2017; Jin, 2010; Qiu, 2012; Wang & Li, 2019; Xuan, 2019; Zhong, 2013; Zou et al., 2012).
3.3. Primary outcomes
TNSS.
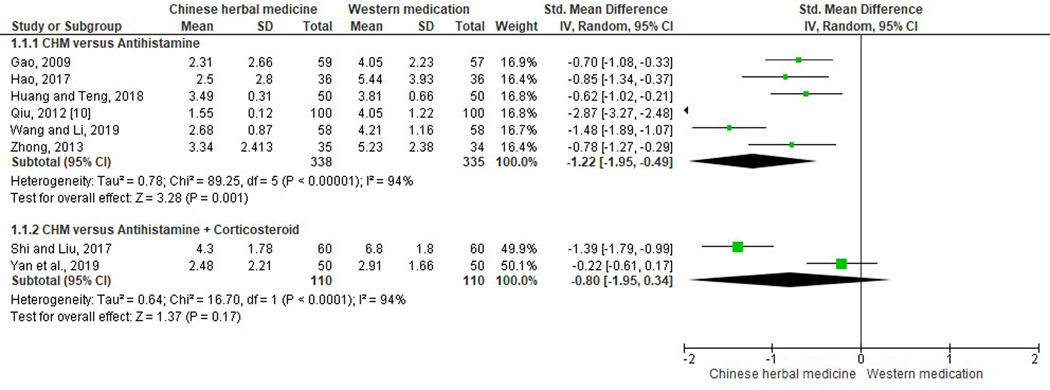
Among included studies, 11 evaluated TNSS (Gao, 2009; Hao, 2017; Hu, 2018; Huang & Teng, 2018; Jia, 2017; Qiu, 2012; Shi & Liu, 2017; Wang & Li, 2019; Yan et al., 2019; Zhong, 2013; Zou et al., 2012). However, one study had a significantly imbalanced baseline and was therefore not included in the meta-analysis (Zou et al., 2012). More specifically, six studies comparing CHM with antihistamines (loratadine n=5; chlorpheniramine n=1) (SMD −1.22, 95% CI −1.95 to −0.49, I2=94%) (Gao, 2009; Hao, 2017; Huang & Teng, 2018; Qiu, 2012; Wang & Li, 2019; Zhong, 2013), and one study comparing CHM with antihistamines plus leukotriene modifiers (SMD −1.63, 95% CI −2.27 to −0.98) (Jia, 2017), revealed significant improvements in TNSS. However, two studies comparing CHM with antihistamines plus corticosteroids (SMD −0.80, 95% CI −1.95 to 0.34, I2=94%) (Shi & Liu, 2017; Yan et al., 2019), and one study comparing CHM with corticosteroids (SMD −0.50, 95% CI −1.01 to 0.02) (Hu, 2018) did not show significant differences between the two groups. The meta-analysis results on the comparison of TNSS between CHM and WM are shown in Figure 3.
Figure 3.
Estimated effects of total nasal symptom scores between Chinese herbal medicine and Western medications.
Rhinorrhea.
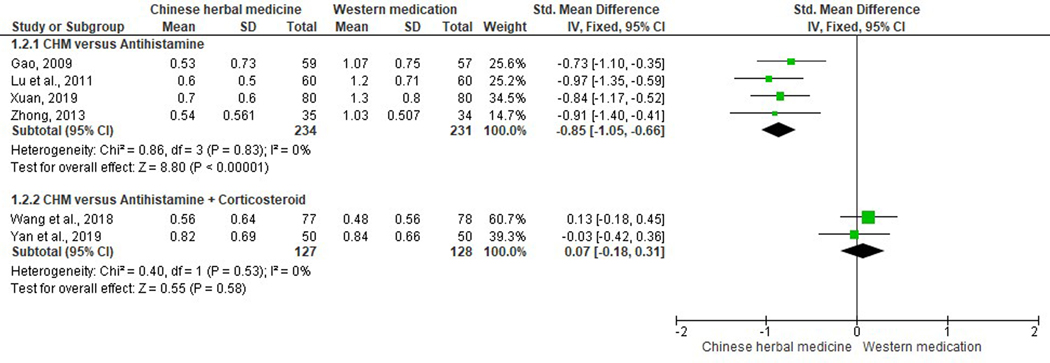
Ten studies reported runny nose scores (Duan, 2017; Gao, 2009; Jia, 2017; Liu et al., 2001; Lu et al., 2011; Shi & Liu, 2017; Wang et al., 2018; Xuan, 2019; Yan et al., 2019; Zhong, 2013). Three studies were excluded from meta-analysis due to data duplication error (Shi & Liu, 2017), incomparable baseline (Duan, 2017), and incomplete data (Liu et al., 2001). Four studies in the CHM versus antihistamines subgroup (SMD −0.85, 95% CI −1.05 to −0.66, I2=0%) (Gao, 2009; Lu et al., 2011; Xuan, 2019; Zhong, 2013) and one study in the CHM versus antihistamines plus leukotriene modifiers subgroup (SMD −0.86, 95% CI −1.44 to −0.28) (Jia, 2017), demonstrated a significant reduction in rhinorrhea scores. There was no statistically significant difference in two studies comparing CHM with antihistamines plus corticosteroids nasal spray (SMD 0.07, 95% CI −0.18 to 0.31, I2=0%) (Figure 4) (Wang et al., 2018; Yan et al., 2019).
Figure 4.
Forest plot on comparison of rhinorrhea scores between Chinese herbal medicine and Western medications.
Nasal congestion.
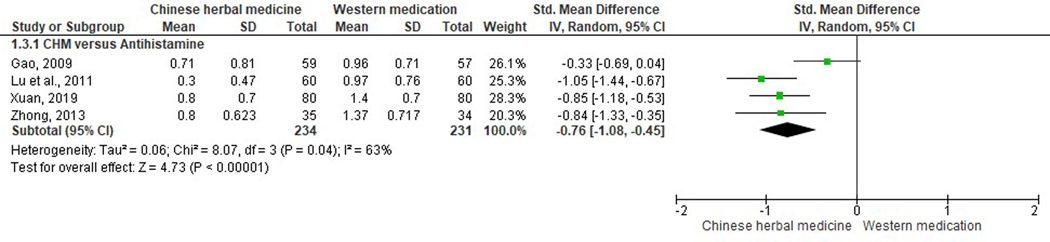
Nine studies reported nasal obstruction scores (Gao, 2009; Jia, 2017; Lu et al., 2011; Qiu, 2012; Shi & Liu, 2017; Wang et al., 2018; Xuan, 2019; Yan et al., 2019; Zhong, 2013). Three studies were excluded from meta-analysis due to data duplication error, incomparable baseline, and incomplete reporting of data respectively (Qiu, 2012; Shi & Liu, 2017; Wang et al., 2018). When comparing studies involving CHM versus antihistamines (Gao, 2009; Lu et al., 2011; Xuan, 2019; Zhong, 2013), all but one (Gao, 2009), indicated significant improvement in unblocking nasal passages (SMD −0.76, 95% CI −1.08 to −0.45, I2=63%). Similarly, one study which compared Biyantongqiao granules with antihistamines plus leukotriene modifiers significantly favored the CHM group (SMD −0.94, 95% CI −1.53 to −0.36) (Jia, 2017). One study in the Modified Xi Min Decoction versus antihistamines plus corticosteroid nasal spray group revealed non-significant results (SMD −0.06, 95% CI −0.45 to 0.33) (Figure 5) (Yan et al., 2019).
Figure 5.
Nasal obstruction scores using Chinese herbal medicine versus Western medications.
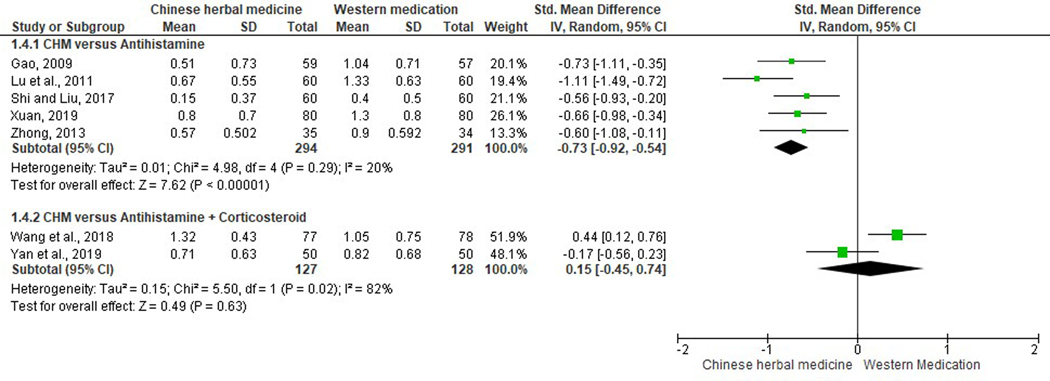
Sneezing.
Ten studies assessed sneezing scores (Duan, 2017; Gao, 2009; Jia, 2017; Liu et al., 2001; Lu et al., 2011; Shi & Liu, 2017; Wang et al., 2018; Xuan, 2019; Yan et al., 2019; Zhong, 2013). Two studies were excluded from meta-analysis due to incomplete reporting of data and incomparable baseline respectively (Duan, 2017; Liu et al., 2001). Five studies comparing CHM with antihistamines (SMD −0.73, 95% CI −0.92 to −0.54, I2=20%) (Gao, 2009; Lu et al., 2011; Shi & Liu, 2017; Xuan, 2019; Zhong, 2013), and one study comparing Biyantongqiao Granules with antihistamines plus leukotriene modifiers (SMD −1.20, 95% CI −1.81 to −0.60) (Jia, 2017), revealed significant reductions in sneezing scores in CHM groups. Pooled data of two studies did not yield significant results when CHM was compared to antihistamines plus corticosteroid nasal spray (SMD 0.15, 95% CI −0.45 to 0.74, I2=82%) (Figure 6) (Wang et al., 2018; Yan et al., 2019).
Figure 6.
Forest plot on comparison of sneezing scores between Chinese herbal medicine and Western medications.
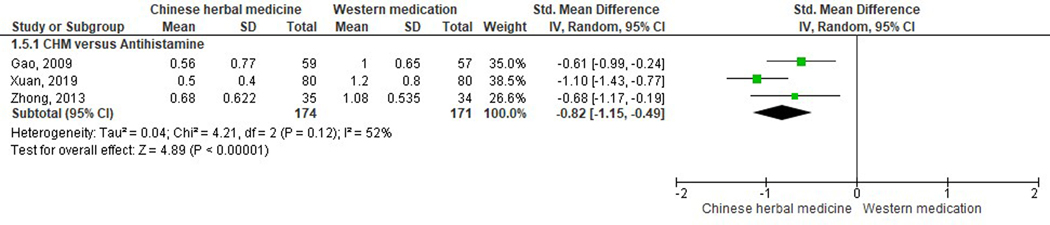
Nasal itching.
Eight studies presented itchy nose scores (Gao, 2009; Jia, 2017; Lu et al., 2011; Shi & Liu, 2017; Wang et al., 2018; Xuan, 2019; Yan et al., 2019; Zhong, 2013). Three of them had incomparable baseline, and thus their symptom scores were hence invalid for further statistical analysis (Lu et al., 2011; Shi & Liu, 2017; Wang et al., 2018). Three studies comparing CHM with antihistamine (loratidine) using Modified Linggui zhugan Decoction (Gao, 2009), Guizhi Decoction combined with Mahuang Fuzi Xixin Decoction (Xuan, 2019), and Qufeng Tongqiao Decoction (Zhong, 2013), had a statistically significant effect favoring CHM (SMD −0.82, 95% CI −1.15 to −0.49, I2=52%). The other two studies did not present significant results when comparing Biyantongqiao Granules versus antihistamine (loratadine granules) plus leukotriene modifiers (montelukast sodium chewable tablets) (SMD −0.40, 95% CI −0.96 to 0.16) (Jia, 2017) and modified Xi Min Decoction versus antihistamine (Loratadine tablets) plus corticosteroid nasal spray (Fluticasone propionate) (SMD −0.10, 95% CI −0.49 to 0.29) (Figure 7) (Yan et al., 2019).
Figure 7.
Estimated effects of nasal itching scores between Chinese herbal medicine and Western medications.
3.4. Secondary outcomes
QoL.
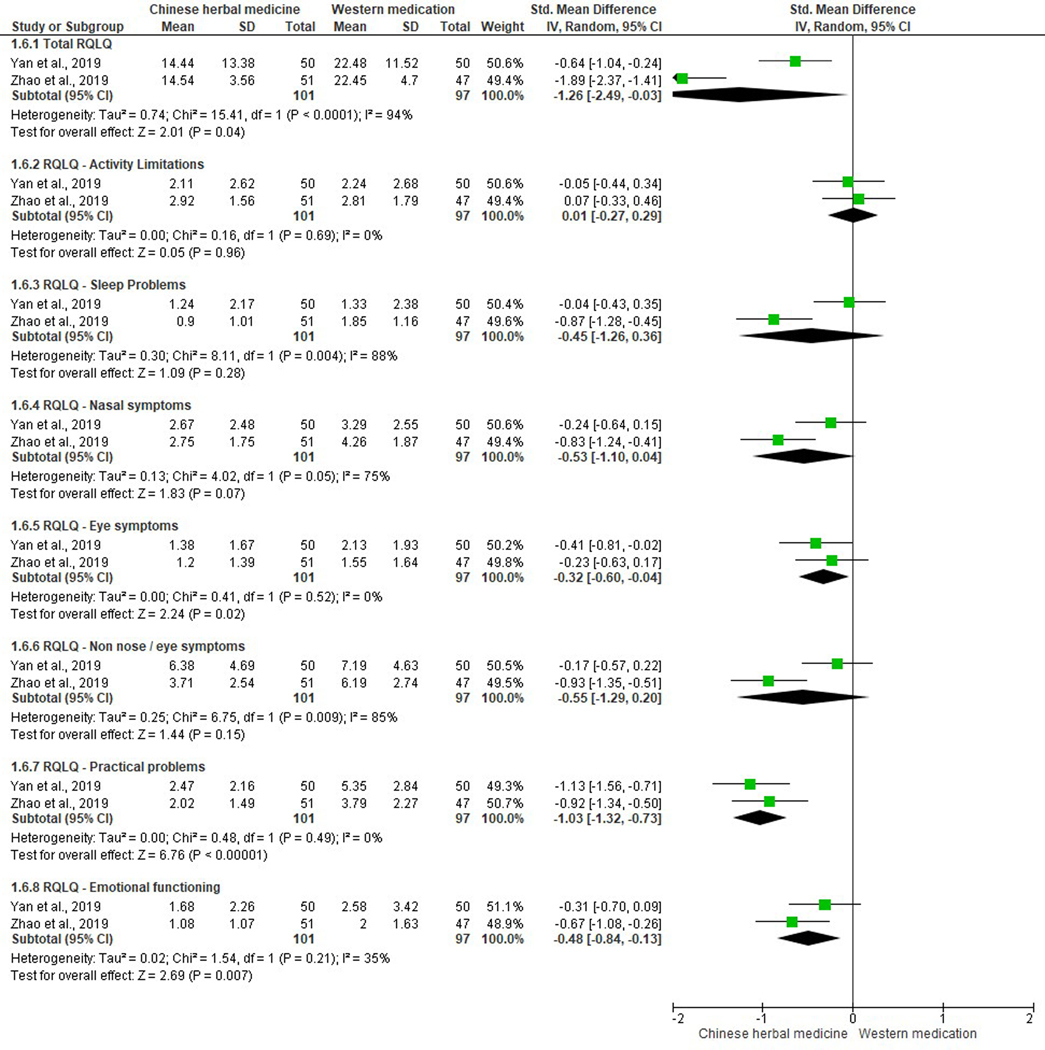
Three different scoring systems were used to assess participants’ QoL among six studies (Duan, 2017; Gao, 2009; Wang et al., 2018; Wang & Li, 2019; Yan et al., 2019; Zhao et al., 2019), including the RQLQ (Wang et al., 2018; Wang & Li, 2019; Yan et al., 2019; Zhao et al., 2019), VAS (Duan, 2017; Wang et al., 2018), and SF-36 (Gao, 2009). One study (Wang et al., 2018) reported both QoL measures, however, the incomparable baseline data limited the validity for further analysis.
For RQLQ, two studies assessed all seven domains, including activity limitations, sleep problems, nasal symptoms, eye symptoms, non-nose / eye symptoms, practical problems, and emotional functioning; and reported a total score for RQLQ (Yan et al., 2019; Zhao et al., 2019). The total RQLQ score in the two studies comparing CHM with loratadine plus a fluticasone propionate nasal spray indicated a significant improvement in QoL favoring CHM (SMD −1.26, 95% CI −2.49 to −0.03, I2=94%) (Yan et al., 2019; Zhao et al., 2019). Data analyses demonstrated a significant improvement in three out of seven domains: eye symptoms (SMD −0.32, 95% CI −0.60 to −0.04, I2=0%), practical problems (SMD −1.03, 95% CI −1.32 to −0.73, I2=0%), and emotional functioning (SMD −0.48, 95% CI −0.84 to −0.13, I2=35%). There were no differences in the rest of the QoL domains revealed between CHM and WM groups: activity limitations (SMD 0.01, 95% CI −0.27 to 0.29, I2=0%), sleep problems (SMD −0.45, 95% CI −1.26 to 0.36, I2=88%), nasal symptoms (SMD −0.53, 95% CI −1.10 to 0.04, I2=75%), and non-nose / eye symptoms (SMD −0.55, 95% CI −1.29 to 0.20, I2=85%) (Figure 8). Another study comparing modified Mahuang Xixin Fuzi Decoction with loratadine assessed the total RQLQ, activity limitation, emotional functioning and nasal symptom scores (Wang & Li, 2019). A significant result was revealed in the total RQLQ score (MD −5.20, 95% CI −7.83 to −2.57). However, due to lack of data reported in each domain, the estimated effects of those three domains were not analyzed.
Figure 8.
Quality of life scores using Chinese herbal medicine versus Western medications (antihistamine plus corticosteroid inhaler).
For VAS, an included study reported Tuo Min Decoction could significantly reduce VAS scores in participants suffering from non-classified AR (MD −1.70, 95% CI −2.40 to −1.00) (Duan, 2017). This study utilized Tuo Min Decoction compared to ebastine and fluticasone propionate inhaler. Another study comparing Guizhi Decoction against loratadine assessed eight domains of SF-36. Opposed to the above studies on QoL, loratadine was reported to improve physical function (MD 2.77, 95% CI 0.49 to 5.05), physical role (MD 6.82, 95% CI 0.90 to 12.74) and general health (MD 6.25, 95% CI 0.28 to 12.22). However, there were no differences between Guizhi Decoction and loratadine in the scores of SF-36 for bodily pain (MD 2.81, 95% CI −2.09 to 7.71), vitality (MD 1.04, 95% CI −5.32 to 7.40), social function (MD 4.38, 95% CI −0.30 to 9.06), emotional role (MD 8.42, 95% CI −2.96 to 19.80) and mental health (MD 1.89, 95% CI −4.10 to 7.88).
Recurrence rate.
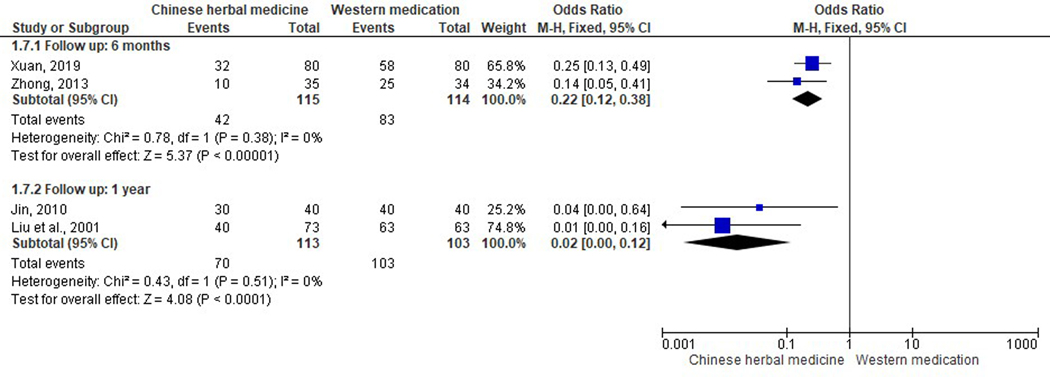
Five trials investigated recurrent rate of AR during six-months to one-year follow-up period (Figure 9) (Jin, 2010; Liu et al., 2001; Lu et al., 2011; Xuan, 2019; Zhong, 2013). According to the worst-case-scenario method for handling missing data, participants lost to follow-up in included studies were assumed to have recurred. All studies, regardless of the length of follow-up, reported that CHM had a significantly lower recurrence rate when compared to western medication (antihistamine). For a six-month follow-up, the recurrence rate of Guizhi Decoction combined with Mahuang Fuzi Xixin Decoction (Xuan, 2019), and Qufeng Tongqiao Decoction (Zhong, 2013) was significantly lower when compared to Loratadine tablets respectively (OR 0.22; 95% CI 0.12 to 0.38, I2=0%). In addition, modest but significant results were observed after an one-year follow-up, when (Kemin Decoction (Jin, 2010) and a CHM formula for nourishing Yin and calming Liver (Liu et al., 2001), was compared to cetirizine tablets plus diphenhydramine spray, and cetirizine tablets respectively (OR 0.02, 95% CI 0.00 to 0.12, I2=0%). Similarly, a study comparing Modified Guizhi Decoction with loratadine syrup indicated a lower recurrence rate when CHM was administered (OR 0.01, 95% CI 0.00 to 0.24, I2=0%) (Lu et al., 2011).
Figure 9.
Forest plot on comparison of the recurrence rate between Chinese herbal medicine and Western medications.
3.5. Adverse events
Four studies reported no adverse events observed in any of the groups (Hao, 2017; Jia, 2017; Zhong, 2013; Zou et al., 2012). Moreover, two studies reported that there were no adverse events in the CHM group whereas adverse events such as fatigue, somnolence, headache, dry mouth and gastrointestinal discomfort were found in the WM group (Gao, 2009; Qiu, 2012). Another three studies reported such minor adverse events in the CHM group as diarrhea (2/60), nausea (2/60), vomiting (2/60), stomach pain (1/50), dry mouth (1/77), and upper limb rashes (1/60) (Supplementary Table S3) (Duan, 2017; Wang et al., 2018; Zhao et al., 2019). However, these studies also indicated that those adverse events were not associated with CHM (Duan, 2017; Wang et al., 2018; Zhao et al., 2019). Half of 18 included studies did not provide the safety data of interventions (Hu, 2018; Huang & Teng, 2018; Jin, 2010; Liu et al., 2001; Lu et al., 2011; Shi & Liu, 2017; Wang & Li, 2019; Xuan, 2019; Yan et al., 2019).
3.6. Sensitivity analysis and publication bias
As all meta-analyses in this review included less than 10 studies, analyses on sensitivity and publication bias could not be conducted.
4. Discussion
CHM has been used to treat AR for thousands of years in China and other Asian countries, and it is still widely used for AR management nowadays (Kreiner, 2016). CHM could be a safe therapy for AR sufferers considering limited adverse events reported. In the meta-analyses of 17 studies comparing CHM with WM, improvements in various primary and secondary outcome measures were revealed. The findings of this review indicate that some herbal formulae may reduce scores in TNSS, individual symptom (rhinorrhea, nasal congestion, sneezing and nasal itching), QoL and recurrence rate, compared to WM, with substantial heterogeneity. In addition, CHM may exert similar effects to WM, in some subgroup analyses, such as corticosteroid or antihistamine plus corticosteroid in TNSS, antihistamine plus corticosteroid in rhinorrhea, nasal obstruction and sneezing, antihistamine plus corticosteroid/leukotriene modifiers in nasal itching and antihistamine in SF-36.
It is interesting to note the favor towards some CHM groups in improving TNSS and individual symptom scores when comparing CHM with antihistamine control (Gao, 2009; Hao, 2017; Huang & Teng, 2018; Jin, 2010; Liu et al., 2001; Lu et al., 2011; Qiu, 2012; Shi & Liu, 2017; Wang & Li, 2019; Xuan, 2019; Zhong, 2013). This may be due to anti-inflammation, anti-allergic and immunoregulatory effects of some high-frequently used Chinese herbs in the included RCTs (Kreiner, 2016). For example, an in vitro study showed that methyleugenol from Asari Radix et Rhizoma (Xi xin) could inhibit the expression of interleukin 4, which plays a significant role in mucus secretion, tumor necrosis factor alpha expression of endothelial molecules adhesion and IgE production at the late stage of AR inflammation (Tang et al., 2015). An animal study reported that aqueous extract of Magnoliae Flos (Xin yi hua) could reduce the level of histamine in plasma which was produced by rat peritoneal mast cells and subsequently suppress IgE-passive cutaneous anaphylactic reaction (World Health Organization, 2009). Furthermore, polysaccharides contained in Astragali Radix (Huang qi) exerted immunoregulatory activities and it could reverse the cyclophosphamide-induced immunosuppressant effect in rats and stimulate the function of interleukin 2 in vitro to activate lymphokine-activated killer cells (World Health Organization, 1999).
Our review has produced consistent findings with another three reviews published in Chinese language on CHM for AR (Li & Liu, 2010; Luo et al., 2017; Wang et al., 2012). A meta-analysis of 10 RCTs published in 2010 revealed a significant increase in the efficacy scores favoring CHM over WM treatment for the management of AR (Li & Liu, 2010). Another meta-analysis of a single CHM formula Yu Ping Feng San, including 22 RCTs, reported a significant decrease in cardinal symptoms (such as itchy nose, sneezing, blocked nose and runny nose), when this formula was used as an adjunct intervention to WM (Luo et al., 2017). Similarly, the recurrence rate of AR at the end of follow-up period in this meta-analysis was reported to be lower in the CHM group (Yu Ping Feng San) compared to antihistamine (cetirizine) (Luo et al., 2017). Furthermore, since only mild and transient adverse events were reported, all three systematic reviews stated that oral administration of CHM for AR was safe (Li & Liu, 2010; Luo et al., 2017; Wang et al., 2012). However, consistent with the aforementioned reviews, the quality of the included studies was low and substantial heterogeneity of meta-analysis was found (Li & Liu, 2010; Luo et al., 2017; Wang et al., 2012).
Despite the attempt to standardize all MD of each outcome measures used across included studies, it is not without risk that results may be over or underestimated due to the different scales utilized by investigators in their trials. Standardization of scales for evaluation of symptoms and QoL are recommended as it will assist the data synthesis and comparison. Furthermore, the standardization of the forms of interventions among RCTs involving CHM and WM should be performed to ensure successful blinding. The different preparation methods ranging from a combination spray, syrup, granule, tablet or decoction administered by participants in the intervention groups in included trials may affect adherence to the treatment regime within the randomly allocated groups and cause exaggerated estimations. The standardization of the intervention routes/regime/administration pathways may improve the methodological quality of RCTs by reducing detection and performance bias among study participants and personnel. Additionally, three-armed design involving double-dummy placebo control is recommended for further studies when comparing CHM with WM for AR.
On the other hand, since only one included paper provided a protocol (Zhao et al., 2019) and some of the included articles provide inconsistent information of outcome measures in their method and result sections (Duan, 2017; Lu et al., 2011; Shi & Liu, 2017; Wang et al., 2018; Zou et al., 2012), in order to avoid the inconsistency, a registered protocol before trial implementation is essential. Prospective registration of protocol aims to prevent duplication of research studies, prevent selective publication and reporting of desired research outcomes, and to inform the potential participants in public about the clinical study. To ensure transparent reporting of trials, the CONSORT statement also indicated the necessity of protocol registration in item 24 (Schulz et al., 2010). Promotion of this requirement in non-English speaking countries is needed.
Various syndromes in the CHM groups may contribute to substantial heterogeneity in the included RCTs. In Chinese medicine, treatment according to syndrome differentiation is a unique concept, which is based on a group of signs and symptoms to determine a coherent picture of pathogenesis of the condition and provide individualized treatment (Wiseman & Ellis, 1996). Even though patients suffering from the same disorder, like AR, syndrome differentiation may be different, demonstrating that they should be treated differently. In this review, 13 out of 18 included studies considering syndrome differentiation in RCT design (Duan, 2017; Gao, 2009; Hao, 2017; Hu, 2018; Jia, 2017; Jin, 2010; Shi & Liu, 2017; Wang et al., 2018; Xuan, 2019; Yan et al., 2019; Zhao et al., 2019; Zhong, 2013; Zou et al., 2012) have involved 10 different patterns which may be associated with high heterogeneity in meta-analyses. Missing syndrome differentiation in another five RCTs published from 2001 to 2019 (Huang & Teng, 2018; Liu et al., 2001; Lu et al., 2011; Qiu, 2012; Wang & Li, 2019) may be a potential cause for the diminished effects. It is recommended that future study design consider syndrome differentiation for AR diagnosis and intervention in RCTs.
The inclusion of CHM into clinical practice guidelines for the management of AR remains a challenge (Seidman et al., 2015). Firstly, it is worth noting that although the ingredients of herbal formulas have been provided in the included RCTs, their mechanisms of action are still unclear. Furthermore, due to lack of rigorous regulation, the need for the manufacturer of the nutraceutical to prove efficacy, safety and quality of a marketed product is less strongly enforced than in the pharmaceutical sector. Therefore, many available products might be ineffective (Colalto, 2018). Additionally, antihistamines have been highly recommended by clinical practice guidelines, however, side effects of antihistamines such as sedation, mucosal dryness, urinary retention and headache, disturb people with AR in their daily life inevitably (Seidman et al., 2015; Wise et al., 2018). With the emerging emphasis of personalized medicine based on the genetic and psycho-social factors for immunological conditions (Licari et al., 2019), clinical management options including evidence-based alternative therapies should be explored. The current review comparing CHM with those accepted WM may provide clinical evidence in effects and safety of CHM. Despite the recent interest in CHM research, the lack of large-scale RCTs and language barriers may have limited the synthesis and interpretation of CHM clinical studies in Western countries (Australian Society of Clinical Immunology and Allergy, 2017). Since systematic reviews and meta-analyses are at the top in the hierarchical levels of the clinical evidence, systematic reviews assimilating high-quality clinical trials are required to provide robust conclusions and convincing evidence to guide future clinical practice (Colalto, 2018). The availability of such evidence may influence the decision-making on clinical practice guidelines development and government rebates on private health insurance for the use of herbal therapies in the future.
5. Conclusions
Compared to WM, CHM may improve scores of total and individual nasal symptoms, QoL and recurrence rate. In addition, CHM may exert similar effects to WM, in some subgroup analyses. However, there is the possibility that most of the studies discussed in the present review have been not performed in accordance to a recent consensus document providing a perspective in best practice in pharmacological research on bioactive preparations from plants (Heinrich et al., 2020). Moreover, due to the small number of included studies, poor quality of trial design and substantial heterogeneities, the results from this review need to be interpreted with caution. The true potential of CHM for AR, compared to WM, should be validated in large-scale, multi-center and well-designed RCTs in the future.
Supplementary Material
Acknowledgement
The authors appreciate the support provided by the Cochrane ENT Disorders Group for the development of the review. We thank Dr Amy Hsiewe Ying Tan for interpretation of the Japanese papers.
Funding
The authors would like to thank the RMIT Emerging Researcher Grant for supporting this review. This work was partially funded by Grant Number R24 AT001293 from the National Center for Complementary and Alternative Medicine (NCCAM). The contents of this systematic review are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or the National Institutes of Health.
Abbreviations
- AR
Allergic rhinitis
- CI
Confidence interval
- CHM
Chinese herbal medicine
- IgE
immunoglobulin E
- MD
Mean difference
- OR
Odds ratio
- PAR
Perennial allergic rhinitis
- QoL
quality of life
- RCTs
Randomized controlled trials
- RQLQ
Rhinoconjunctivitis Quality of Life Questionnaire
- SAR
Seasonal allergic rhinitis
- SF-36
36-Item Short Form Health Survey
- SMD
Standardized mean difference
- TNSS
Total nasal symptom scores
- VAS
Visual Analogue Scale
- WM
Western medications
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Australian Institute of Health and Welfare. (2019). Allergic Rhinitis (‘Hay Fever’). Retrieved Jan 1 from https://www.aihw.gov.au/reports/chronic-respiratory-conditions/allergic-rhinitis-hay-fever/contents/allergic-rhinitis
- Australian Society of Clinical Immunology and Allergy. (2017). Allergic Rhinitis Clinical Update. Australian Society of Clinical Immunology and Allergy. Retrieved Jan 28 from https://www.allergy.org.au/hp/papers/allergic-rhinitis-clinical-update
- China Association of Chinese Medicine. (2015). Zhong Hua Yi Dian (Encyclopaedia of Traditional Chinese Medicine) [CD-ROM]. In (Version 5th) Hunan Electronic and Audio-Visual Publishing House. [Google Scholar]
- Colalto C. (2018). What phytotherapy needs: Evidence-based guidelines for better clinical practice. Phytotherapy Research, 32(3), 413–425. 10.1002/ptr.5977 [DOI] [PubMed] [Google Scholar]
- Duan B. (2017). Clinical research on the treatment of allergic rhinitis with Tuo Mintang [Master’s thesis, Southwest Medical University]. China National Knowledge Infrastructure. [Google Scholar]
- Gao Y. (2009). The effect of Poria, Cinnamon Twig, Ovate Atractylodes, and Liarice decoction on quality of life of patients with perennial allergic rhinitis [Master’s thesis, Chengdu University of Traditional Chinese Medicine]. China National Knowledge Infrastructure. [Google Scholar]
- Hao R. (2017). Two ginseng Xinyi Granule on senile allergic rhinitis (lung and spleen deficiency) clinical study [Master’s thesis, Changchun University of Chinese Medicine]. China National Knowledge Infrastructure. [Google Scholar]
- Heinrich M, Appendino G, Efferth T, Fürst R, Izzo AA, Kayser O, Pezzuto JM, & Viljoen A. (2020). Best practice in research-Overcoming common challenges in phytopharmacological research. Journal of Ethnopharmacology, 246, 112230. 10.1016/j.jep.2019.112230 [DOI] [PubMed] [Google Scholar]
- Higgins JPT, & Green S. (2011). Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0). The Cochrane Collaboration. Retrieved Sep 18 from http://handbook-5-1.cochrane.org/ [Google Scholar]
- Hu S. (2018). Effect of Huangqi Guizhi Wuwu Decoction and Cocklebur powder in the treatment of allergic rhinitis and its effect on immune function [Master’s thesis, Fujian University of Traditional Chinese Medicine]. China National Knowledge Infrastructure. [Google Scholar]
- Huang Y, & Teng F. (2018). Clinical observation of Yuping Tuomin decoction in the treatment of 50 cases of allergic rhinitis. China Practical Medicine, 13(13), 16–18. [Google Scholar]
- Jia Q. (2017). The clinical research of allergic rhinitis among children who were asthenic-cold of the lung qi and were treated by the granule of biyantongqiao [Master’s thesis, Shandong University of Traditional Chinese Medicine]. China National Knowledge Infrastructure. [Google Scholar]
- Jin HM (2010). Clinical observation of Kemin decoction in treating perennial allergic rhinitis. China Journal of Traditional Chinese Medicine and Pharmacy, 25(12), 2192–2193. [Google Scholar]
- Kreiner J. (2016). Clinical effects of Chinese herbal medicine for allergic rhinitis: reviews of classical and modern literature [Master’s thesis, RMIT University]. [Google Scholar]
- Li P, & Liu Y. (2010). Chinese herbal medicine for allergic rhinitis: A meta-analysis Journal of Shaanxi College of Traditional Chinese Medicine, 11(3), 37–39. [Google Scholar]
- Licari A, Castagnoli R, Tosca MA, Marseglia G, & Ciprandi G. (2019). Personalized therapies for the treatment of allergic rhinitis. Expert Review of Precision Medicine and Drug Development, 4(5), 275–281. 10.1080/23808993.2019.1681896 [DOI] [Google Scholar]
- Liu QP, Liu JH, Li YL, & Ge YH (2001). Nourishing Liver Yin in the treatment of allergic rhinitis Journal of Beijing University of Traditional Chinese Medicine, 24(2), 68–69. [Google Scholar]
- Lu B, Chang K, Wang HJ, Guo JJ, & Chen J. (2011). Regulating Ying and Wei in the treatment of 60 allergic rhinitis cases. Shanxi Journal of Traditional Chinese Medicine, 27(3), 10–11. [Google Scholar]
- Luo Q, Zhang CS, Yang L, Zhang AL, Guo X, Xue CC, & Lu C. (2017). Potential effectiveness of Chinese herbal medicine Yu ping feng san for adult allergic rhinitis: a systematic review and meta-analysis of randomized controlled trials. BMC Complementary and Alternative Medicine, 17(1), 485. 10.1186/s12906-017-1988-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahr TA, Sheth KK, & Boyle JM (2008). Lack of efficacy and bothersome effects decrease treatment adherence in children with allergic rhinitis. Annals of Allergy Asthma & Immunology, 100(1), A3. [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine, 151(4), 264–269, w264. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- Qiu W. (2012). The allergic rhinitis TCM dialectical law and clinical research of Xiaoqinglong decoction treatment [Master’s thesis, Guangzhou University of Chinese Medicine]. China National Knowledge Infrastructure. [Google Scholar]
- Schulz KF, Altman DG, & Moher D. (2010). CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Medicine, 8, 18. 10.1186/1741-7015-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman MD, Gurgel RK, Lin SY, Schwartz SR, Baroody FM, Bonner JR, Dawson DE, Dykewicz MS, Hackell JM, Han JK, Ishman SL, Krouse HJ, Malekzadeh S, Mims JW, Omole FS, Reddy WD, Wallace DV, Walsh SA, Warren BE, Wilson MN, & Nnacheta LC (2015). Clinical practice guideline: Allergic rhinitis. Otolaryngology-Head and Neck Surgery, 152(1 Suppl), S1–43. 10.1177/0194599814561600 [DOI] [PubMed] [Google Scholar]
- Settipane RJ, Hagy GW, & Settipane GA (1994). Long-term risk factors for developing asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy and Asthma Proceedings, 15(1), 21–25. 10.2500/108854194778816634 [DOI] [PubMed] [Google Scholar]
- Sheth K, Derebery J, Mahr T, Simmons L, & Boyle J. (2008). Ineffective control of allergic rhinitis symptoms reduces patient satisfaction and adherence With treatment. Journal of Allergy and Clinical Immunology, 121(2, Supplement 1), S106. 10.1016/j.jaci.2007.12.423 [DOI] [Google Scholar]
- Shi J, & Liu Y. (2017). Clinical observation of Yiqi Tuomin Tang for allergic rhinitis of invasion of cold and lung deficiency type. Journal of New Chinese Medicine, 49(12), 110–112. [Google Scholar]
- Sin B, & Togias A. (2011). Pathophysiology of allergic and nonallergic rhinitis. Proceedings of the American Thoracic Society, 8(1), 106–114. 10.1513/pats.201008-057RN [DOI] [PubMed] [Google Scholar]
- Tang F, Chen F, Ling X, Huang Y, Zheng X, Tang Q, & Tan X. (2015). Inhibitory effect of methyleugenol on IgE-mediated allergic inflammation in RBL-2H3 cells. Mediators of Inflammation, 2015, e463530. 10.1155/2015/463530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cochrane Collaborations. (2014). Review Manager (RevMan) [Computer program]. In (Version 5.3.) The Cochrane Collaboration [Google Scholar]
- Wang J, Cai J, Wang H, & Chen W. (2018). Clinical observation of Bu Yang Liao Ti Decoction for allergic rhinitis of kidney yang deficiency type. Journal of Chinese Medicinal Materials, 41(5), 1219–1221. [Google Scholar]
- Wang L, & Li Y. (2019). Effects of Mahuang Xinxin Fuzi decoction on immune function and serum inflammatory factors in children with allergic rhinitis. Journal of Sichuan Traditional Chinese Medicine, 37(6), 172–174. [Google Scholar]
- Wang S, Tang Q, Qian W, & Fan Y. (2012). Meta-analysis of clinical trials on traditional Chinese herbal medicine for treatment of persistent allergic rhinitis. Allergy, 67(5), 583–592. 10.1111/j.1398-9995.2012.02806.x [DOI] [PubMed] [Google Scholar]
- Wise SK, Lin SY, Toskala E, Orlandi RR, Akdis CA, Alt JA, Azar A, Baroody FM, Bachert C, Canonica GW, Chacko T, Cingi C, Ciprandi G, Corey J, Cox LS, Creticos PS, Custovic A, Damask C, Deconde A, Delgaudio JM, Ebert CS, Eloy JA, Flanagan CE, Fokkens WJ, Franzese C, Gosepath J, Halderman A, Hamilton RG, Hoffman HJ, Hohlfeld JM, Houser SM, Hwang PH, Incorvaia C, Jarvis D, Khalid AN, Kilpeläinen M, Kingdom TT, Krouse H, Larenas‐Linnemann D, Laury AM, Lee SE, Levy JM, Luong AU, Marple BF, McCoul ED, McMains KC, Melén E, Mims JW, Moscato G, Mullol J, Nelson HS, Patadia M, Pawankar R, Pfaar O, Platt MP, Reisacher W, Rondón C, Rudmik L, Ryan M, Sastre J, Schlosser RJ, Settipane RA, Sharma HP, Sheikh A, Smith TL, Tantilipikorn P, Tversky JR, Veling MC, Wang DY, Westman M, Wickman M, & Zacharek M. (2018). International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis. International Forum of Allergy & Rhinology, 8(2), 108–352. 10.1002/alr.22073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman N, & Ellis AT (1996). Fundamentals of Chinese medicine [Zhong Yi Xue Ji Chu]. Paradigm Publications. [Google Scholar]
- Wolthers OD (2000). Systemic activity versus systemic adverse effects of nasal glucocorticoids in the treatment of allergic rhinitis. Acta Paediatrica, 89(10), 1158–1161. 10.1111/j.1651-2227.2000.tb00728.x [DOI] [PubMed] [Google Scholar]
- World Health Organization. (1999). WHO Monographs on Selected Medicinal Plants-Volume 1. World Health Organization. Retrieved Sep 18 from https://apps.who.int/medicinedocs/en/m/abstract/Js16713e/ [Google Scholar]
- World Health Organization. (2009). WHO Monographs on Selected Medicinal Plants-Volume 4. World Health Organization. Retrieved Jan 18 from https://apps.who.int/medicinedocs/en/m/abstract/Js16713e/ [Google Scholar]
- Xuan Z. (2019). Clinical observation of Guizhi Decoction combined with Mahuang Fuzi Xixin Decoction for allergic rhinitis. Journal of Practical Traditional Chinese Medicine 35(9), 1052–1053. [Google Scholar]
- Yan X, Wang J, Han J, Zhang H, Zhao J, & Gai J. (2019). Clinical observation on modified Ximin Decoction treating 50 allergic rhinitis patients with deficiency cold of lung and kidney symdrome. Journal of Traditional Chinese Medicine, 60(16), 1384–1388. [Google Scholar]
- Yang AWH, Liu JP, & Xue CCL (2009). Chinese herbal medicine for allergic rhinitis. Cochrane Database of Systematic Reviews(1), CD007643. 10.1002/14651858.CD007643 [DOI] [Google Scholar]
- Zhang X, Lan F, Zhang Y, & Zhang L. (2018). Chinese herbal medicine to treat allergic rhinitis: Evidence from a meta-analysis. Allergy, Asthma & Immunology Research, 10(1), 34–42. 10.4168/aair.2018.10.1.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Yan X, Gai J, Han J, Zhang H, Luo H, Huang S, & Wang J. (2019). Efficacy of Bimin decoction for patients with perennial allergic rhinitis: an open-label non-inferiority randomized controlled trial. Trials, 20(1), 802. 10.1186/s13063-019-3763-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong RQ (2013). Clinical study carminative Tongqiao soup treatment of perennial allergic rhinitis [Master’s thesis, Beijing University of Chinese Medicine]. China National Knowledge Infrastructure. [Google Scholar]
- Zou LQ, Niu QY, Liu XR, & Xing WD (2012). 52 clinically deficient cases of allergic rhinitis utlising desensitisation therapy of Tuomintongqiao capsules. Yunnan Journal of Traditional Chinese Medicine and Materia Medica, 33(6), 36–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.