Abstract
Background:
Concerns about ketamine for treating depression include abuse potential and the occurrence of psychotomimetic effects. This study sought to comprehensively assess side effects (SEs) associated with a single subanesthetic-dose intravenous ketamine infusion. A secondary aim was to examine the relationship between Clinician-Administered Dissociative States Scale (CADSS) scores and dissociative symptoms reported on a comprehensive, clinician-administered SE questionnaire.
Methods:
Data from 188 participants were pooled from four placebo-controlled, crossover ketamine trials and one open-label study (n=163 with either treatment-resistant major depressive disorder or bipolar disorder and 25 healthy controls). SEs were actively solicited in a standardized fashion and monitored over the time-course of each study. Statistical analyses assessed the effect of drug (ketamine, placebo) on SEs and measured the relationship between CADSS total score and SEs contemporaneously endorsed during structured interviews.
Results:
Forty-four of 120 SEs occurred in at least 5% of participants over all trials. Thirty-three of these 44 SEs were significantly associated with active drug administration (versus placebo). The most common SE was feeling strange/weird/loopy. Most SEs peaked within an hour of ketamine administration and resolved completely by two hours post-infusion. No serious drug-related adverse events or increased ketamine craving/abuse post-administration were observed. A positive correlation was found between dissociative SEs and total CADSS score.
Limitations:
The post-hoc nature of the analysis; the limited generalizability of a single subanesthetic-dose ketamine infusion; and the lack of formal measures to assess ketamine’s cognitive, urological, or addictive potential.
Conclusions:
No long-lasting significant SEs occurred over the approximately three-month follow-up period.
Keywords: ketamine, major depressive disorder, side effects, adverse events, dissociative
Introduction
On March 5, 2019, the U.S. Food and Drug Administration (FDA) approved the glutamatergic modulator esketamine for treatment-resistant depression (TRD) (U.S. Food & Drug Administration, 2019a). Esketamine, the S-enantiomer of ketamine, is delivered intranasally and represents the first rapid-acting antidepressant drug treatment based on this novel mechanism of action. In its New Drug Application (NDA) review (U.S. Food & Drug Administration, 2019d), the FDA identified sedation, dissociation, and increased blood pressure, as well as the potential for misuse and abuse, as the primary safety concerns associated with esketamine. In order to mitigate risk, the FDA required that a Risk Evaluation and Mitigation Strategy (REMS) accompany esketamine administration. The REMS consists of prescriber training; esketamine administration only in certain health care settings; monitoring by a healthcare provider for two hours after administration; the certification of dispensing pharmacies, practitioners, or healthcare settings; and the creation of a registry for all esketamine patients to monitor risk.
Ketamine was originally approved in 1970 by the FDA for use as an anesthetic agent. Originally developed as a safer alternative and analog to phencyclidine (PCP), ketamine shares its mechanism of action as a glutamatergic N-methyl-D-aspartate (NMDA) receptor antagonist. In many decades of use as an anesthetic and analgesic agent, as well as in unsupervised illicit use, ketamine has been associated with dissociative symptoms, perceptual disturbances, cognitive and memory impairment, ulcerative cystitis (gross hematuria, urgency frequency, severe dysuria) (Shahani et al., 2007), emergence delirium, and abuse potential (Sassano-Higgins et al., 2016; Strayer and Nelson, 2008). Given its pro-glutamatergic activity, excessive and/or prolonged use of ketamine (particularly in developing brains) (Brambrink et al., 2012) has raised potential concerns about excitotoxic neuronal injury (Scallet et al., 2004; Zou et al., 2009). Pathomorphological vacuolization of neurons, known as “Olney lesions”, has been observed in adult rats after administration of high doses of ketamine (40mg/kg) but not at lower doses (20mg/kg) (Olney et al., 1989). At anesthetic intravenous doses (i.e., 1-4.5 mg/kg), the primary safety concerns associated with ketamine include emergence phenomena (e.g., dissociative/psychotomimetic symptoms, confusion, excitement, and irrational behavior), which have been reported in up to 12% of patients (U.S. Food & Drug Administration, 2019c). Ketamine’s psychotomimetic SEs are thought to be related to its effects on the glutamate system, namely increased synaptic glutamate levels following NMDA receptor inhibition (Javitt et al., 2011; Lodge and Mercier, 2015). Its ability to exert cognitive changes is thought to stem from disrupted functioning of the frontal, cingulate, and temporal cortices, as well as interactions between nicotinic receptors, gamma aminobutyric acid (GABA)-ergic neurons in the hippocampus and medial prefrontal cortex, and dopamine and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in the hippocampus (Liu et al., 2016). Other adverse events, such as hypertension, tachycardia, respiratory depression, diplopia, nystagmus, tonic/clonic motor activity, anorexia, nausea, vomiting, cystitis, anaphylaxis, and abuse have also been reported (U.S. Food & Drug Administration, 2019c).
While ketamine has routinely been used as a dissociative anesthetic and analgesic agent for several decades, particularly in pediatric populations, research into its potential for treating depression has grown substantially in the past two decades, culminating in the aforementioned approval of intranasal esketamine for TRD in March 2019. Even before FDA approval, the past few years have witnessed the proliferation of outpatient clinics in the US providing off-label intravenous racemic ketamine (Wilkinson et al., 2017). When administered intravenously, antidepressant doses are lower than anesthetic doses (0.5 mg/kg compared to 1-4.5 mg/kg) and have a slower rate of delivery. Nevertheless, relatively little is known about the overall risks of using ketamine as an antidepressant. Likewise, little is known about the intranasal route of administration that esketamine uses (U.S. Food & Drug Administration, 2019b), though initial reports suggest a similar side effect (SE) profile.
In the existing literature, the most common concerns regarding subanesthetic-dose ketamine include dissociation, abuse potential (Bonnet, 2015), and potential excitotoxic neuronal injury, as has been demonstrated in animal models (Scallet et al., 2004; Zou et al., 2009). One study of 205 intravenous subanesthetic-dose (0.5 mg/kg) ketamine infusions in 97 participants found that ketamine was well tolerated and not associated with increased abuse potential or neuronal injury (Wan et al., 2015). The most commonly reported SEs were drowsiness, dizziness, poor coordination, blurred vision, and feeling strange or unreal. All SEs resolved quickly post-infusion, and no significant medical effects or increased substance use was observed (Schak et al., 2016; Wan et al., 2015). A recent systematic review of 60 studies examined SEs associated with subanesthetic-dose ketamine to treat depression in 902 individuals who received a single dose of ketamine and 356 who received repeated doses (Short et al., 2018). The most common SEs were headache, dizziness, dissociation, elevated blood pressure, and blurred vision. The most common psychiatric SE was anxiety. Most SEs occurred immediately after initiation of the dose and resolved shortly thereafter. However, the authors noted that a major limitation of the literature was that most SE assessments were conducted via passive monitoring rather than via active and structured surveillance of emerging SEs by study personnel (Short et al., 2018); moreover, only a handful of studies included a placebo control and, to our knowledge, no formal comparison of SEs exists that examined differences between intravenous administration of subanesthetic-dose ketamine and placebo control (Short et al., 2018).
Notably, it has been especially difficult to quantify the dissociative experience during subanesthetic-dose ketamine administration (Neehoff and Glue, 2019; van Schalkwyk et al., 2018). The Clinician-Administered Dissociative States Scale (CADSS), which was originally developed and validated to measure present-state dissociative symptoms, has routinely been used to assess dissociative SEs associated with subanesthetic-dose ketamine. However, it should be noted that this tool was validated in individuals with posttraumatic stress disorder (Bremner et al., 1998), and recent work suggests that the current version of the CADSS may not suffice to capture every dissociative SE associated with ketamine (van Schalkwyk et al., 2018).
An additional concern is that the use of multiple ketamine infusions as a treatment for TRD has increased in recent years (Wilkinson et al., 2017). While most of the literature has examined the effects of single-dose (or limited repeat-dose) intravenous ketamine, one study of 10 TRD participants who received six infusions of intravenous ketamine (0.5 mg/kg) over 12 days reported that three participants had significant but transient dissociative symptoms, two participants had transient hypertension and tachycardia, and one participant had transient bradycardia and hypotension (aan het Rot et al., 2010). In all cases, participants received all six treatments and did not need to withdraw prior to the completion of infusions. Throughout the study, no evidence of cognitive impairment was observed.
Another complicating factor is that ketamine has a long history as a drug of abuse. Its psychotomimetic SEs (e.g., dissociative symptoms, derealization, depersonalization, hallucinations) have made this drug attractive in the “rave” culture of the last several decades. Ketamine also has potentially unpleasant physiologic (cardiovascular, gastrointestinal, and urologic) and psychological SEs that resemble a psychotic state; symptoms include severe dissociation or depersonalization (sometimes referred to as a ‘K-hole’) that can include out-of-body experiences that can be quite distressing, especially for inexperienced users (Curran and Monaghan, 2001; Liu et al., 2016; Muetzelfeldt et al., 2008). These tend to be transient in recreational users but may be chronic in long-term users (Liu et al., 2016; Sassano-Higgins et al., 2016). Those who engage in long-term, daily ketamine abuse have also reported memory and learning impairments, persistent symptoms of dissociation, delusional thinking, and depressed mood (Sassano-Higgins et al., 2016).
Given the recent approval of intranasal esketamine and the proliferation of off-label intravenous ketamine use in the community, it is imperative for health care providers, as well as patients, to have as much information as possible about ketamine’s safety. The primary aim of this study was to comprehensively report SEs associated with ketamine by reviewing 120 symptoms associated with a single intravenous infusion of subanesthetic-dose ketamine across studies conducted over 13 years at the National Institutes of Health (NIH) Intramural Program. In the extant literature, most SEs have been measured via passive assessment (Short et al., 2018); in contrast, the NIH studies used active and structured solicitation at various time points to assess both dissociative and non-dissociative SEs. We hypothesized that each SE would occur more frequently during ketamine infusion than during placebo, particularly for dissociative SEs. Because of the related importance of examining whether the CADSS effectively measures the dissociative symptoms associated with subanesthetic-dose ketamine, a secondary aim of this study was to examine the relationship between the dissociative symptoms reported on an internal SE questionnaire and CADSS total score. We expected to observe a positive relationship between the two and to subsequently quantify the amount of CADSS variation accounted for by commonly reported dissociative SEs
Methods
Study design
Data were pooled from five ketamine substudies that were part of a larger protocol, entitled “Investigation of the rapid (next day) antidepressant effects of an NMDA antagonist” ( NCT00088699). All studies involved the intravenous administration of ketamine (either placebo-controlled or open-label). Participants had a primary diagnosis of treatment-resistant major depressive disorder (MDD) or bipolar disorder (BD) (defined as having failed to respond to at least one previous adequate antidepressant trial) or were healthy controls (HCs) enrolled in one of the substudies. All studies examined SEs systematically over time.
One substudy was a randomized, placebo-controlled, crossover trial of a single infusion of subanesthetic-dose ketamine (0.5 mg/kg) versus placebo that sought to examine ketamine’s antidepressant effects in individuals with MDD (KET-MDD; (Zarate et al., 2006)). Two substudies were randomized, placebo-controlled, crossover trials of a single infusion of subanesthetic-dose ketamine (0.5 mg/kg) versus placebo to examine ketamine’s antidepressant effects in BD (KET-BD; (Diazgranados et al., 2010; Zarate et al., 2012)). Unlike the other studies, participants were maintained on a therapeutic and stable dose of lithium or valproate at the time of ketamine infusion. Given the nearly identical methodology of the two BD studies, they were grouped in analyses here (i.e., treated as one substudy). Another substudy was a randomized, placebo-controlled, crossover trial of a single infusion of subanesthetic-dose ketamine versus placebo to examine ketamine’s mechanism of action in MDD, BD, and HC participants (KET-MOA; (Nugent et al., 2019)). The placebo control for all of these trials was an intravenous infusion of an identical volume of normal saline infused over 40 minutes. The final substudy was conducted to examine the use of riluzole to extend ketamine’s antidepressant effects in individuals with MDD (KET-RIL; (Ibrahim et al., 2012)). In this substudy, an intravenous infusion of subanesthetic-dose ketamine was administered in an open-label fashion. Post-infusion, participants received oral riluzole or placebo tablets. For this analysis, SEs were assessed after ketamine infusion but before the administration of riluzole or placebo. Specific methodologies, including eligibility criteria, are described in the prior publications.
Demographic and clinical characteristics of the substudy populations can be found in Table 1. Treatment conditions and data collection procedures for each substudy are detailed in Table 2.
Table 1.
Demographics
| Variable | KET-MDD | KET-BD* | KET-RIL | KET-MOA |
|---|---|---|---|---|
| Subjects (N) | 21 | 44 | 57 | 66 |
| DSM IV Diagnosis | MDD | BD | MDD | MDD, BD, & HC |
| Age (yrs) | 48 (11) | 47 (10.6) | 48 (12.9) | 36.4 (10.1) |
| Male | 8 (38%) | 21 (47.7%) | 36 (63.2%) | 15 (39%) |
| Race – White | 13 (61.9%) | 37 (84.1%) | 53 (93%) | 31 (81.5%) |
| BMI | 32.7 (8.8) | 29.96 (5.8) | 30.1 (6.6) | 27.5 (6.9) |
| Age of Onset (yrs) | 24 (13.8) | 18 (7.1) | 20 (10.7) | 15 (8.6) |
| Length of illness (yrs) | 25 (13) | 28 (10.3) | 27 (13.2) | 21 (11.5) |
KET-BD represents two studies whose methods were nearly identical, combined here for analysis.
BMI: body mass index; MDD: major depressive disorder; BD: bipolar disorder; HC: healthy control
Table 2.
Substudies included in this report
| Substudy | References | Subjects receiving ketamine (N) |
DSM IV Diagnosis |
Dose (mg/kg) and duration of infusion |
Route | Assessment Time Points Day of Infusion |
Design |
|---|---|---|---|---|---|---|---|
| KET-MDD | Zarate et al, 2006 | 21 | MDD | 0.5 over 40 min | IV | −60,230 | Double-blind, placebo-controlled, crossover RCT |
| KET-BD * | Diazgranados et al, 2010; Zarate et al, 2012 |
44 | BD | 0.5 over 40 min | IV | −60,40,80,120,230 | Double-blind, placebo-controlled crossover RCT |
| KET-RIL | Ibrahim et al, 2012 | 57 | MDD | 0.5 over 40 min | IV | −60,40,80,120,230 | Open-label ketamine followed by riluzole in RCT |
| KET-MOA | Nugent et al, 2019 | 66 | MDD, BD, and HC | 0.5 over 40 min | IV | −60,40,80,120,230 | Double-blind, placebo-controlled crossover RCT |
KET-BD represents two studies whose methods were nearly identical, combined here for analysis.
RCT: randomized controlled trial; MDD: major depressive disorder; BD: bipolar disorder; HC: healthy control.
Instruments
SEs were assessed via active solicitation by trained clinicians using an Adverse Side Effects Form questionnaire developed in-house for our studies (hereafter referred to as “the questionnaire”). The questionnaire assesses up to 120 symptoms organized by organ system, and includes both dissociative and non-dissociative symptoms (see Supplemental Table S1). Over the 13 years of data collection, the questionnaire was slightly expanded (particularly in earlier phases of the research) to include additional SEs that had been spontaneously reported to clinical staff. These minor changes to the questionnaire over time were accounted for when reporting percentages for SEs in the present study. For each substudy, the questionnaire was administered at least 60 minutes prior to each infusion, at 40, 80, 120, and/or 230 minutes post-infusion (for some patients in the KET-RIL substudy, SEs were assessed at 230 minutes only), and on several subsequent days, depending on the relevant substudy (see Table 2). Symptoms were recorded on a Likert scale where 0 = none, 1 = mild, 2 = moderate, and 3 = severe.
The CADSS (Bremner et al., 1998) was also administered in each substudy at the same time intervals as the questionnaire: at least 60 minutes prior to each infusion, at 40, 80, 120, and 230 minutes post-infusion, and on Days 1, 2, 3, 7, 10, and 14 post-infusion. As noted above, the CADSS is commonly used to assess dissociative SEs associated with subanesthetic-dose ketamine and was validated in individuals with post-traumatic stress disorder (Bremner et al., 1998).
Data collection and preparation
The present study retroactively investigated acute SEs in response to a single infusion of subanesthetic-dose ketamine and included participants’ SE data from the day of infusion (ketamine or placebo) and Day 1 post-infusion. For each participant, an SE was identified as occurring if it increased in severity by two points from baseline, where baseline was defined as study day zero, up to one hour prior to the first infusion. For each instance of a symptom, its peak severity, study day/timepoint reported, and study day/timepoint resolved (i.e., returned to baseline) were also recorded. This was done for the duration of study participation. If a symptom resolved and then significantly reappeared, it was recorded as a separate occurrence. Data from all substudies were merged by time of assessment, condition (ketamine or placebo), and the SE, so that each person had observed or missing data for each of the possible condition x time x SE combinations. For quality assurance purposes, 10% of SE records (one record per person) were randomly selected to be reviewed for accuracy. Only symptoms reported within a day of infusion were included in the statistical analysis, as the focus of the study was on acute SEs post-ketamine infusion.
Discharge summaries for the research participants who had completed their inpatient stay at our research facility were systematically reviewed by conducting a word search for the terms “craving” and “addiction” in order to assess for possible intent to abuse or misuse ketamine following trial completion.
Statistical analyses
In order to estimate the rate of occurrence for all SEs post-ketamine and post-placebo infusion, the number of people who reported a symptom within a day of infusion was divided by the total number of people who were asked about that symptom. SEs that occurred in fewer than 5% of all participants (see Supplemental Table S1) were then excluded to maintain consistency with FDA reporting rules, and also because estimates of low-occurring events tend to have poor reliability. Rates were based on data from all participants as there were only minor differences when rates were calculated using only data from the double-blind, placebo-controlled studies (average absolute difference in rates= 0.8%).
Prescott’s tests were used to explore the effect of treatment (ketamine vs. placebo) on each SE; these are essentially exact conditional tests for a linear trend on a contingency table. Prescott’s tests are valid in the presence of period effects and are more sensitive than McNemar tests because they maximize information from participants who did not experience an SE, or who experienced an SE following both ketamine and placebo (Jones and Kenward, 2014). Because Prescott’s tests were calculated using data from three of the four substudies (KET-RIL participants were not included as they did not have a placebo condition), substudy was included as a fixed effect in the linear trend model. The false discovery rate was controlled at q=0.05 using the Benjamini- Yekutieli (2001) method (Benjamini and Yekutieli, 2001), which controls false discovery rate when there is dependency among the p-values.
To examine the relationship between CADSS total score and dissociative SEs from the questionnaire, per-person sums were calculated at each post-infusion time point (40, 80, 120, and 230 minutes) for the 18 SEs that were categorized as dissociative following DSM-5 criteria/descriptions of dissociative disorders (American Psychiatric Association., 2013) (see Supplemental Table S1). To account for the possible confounding effects of substudy-to-substudy variation, the same models were run with substudy included as a main effect, as well as models that also allowed substudy to interact with SE predictors (dissociative and non-dissociative SEs). All analyses were conducted in R.
Results
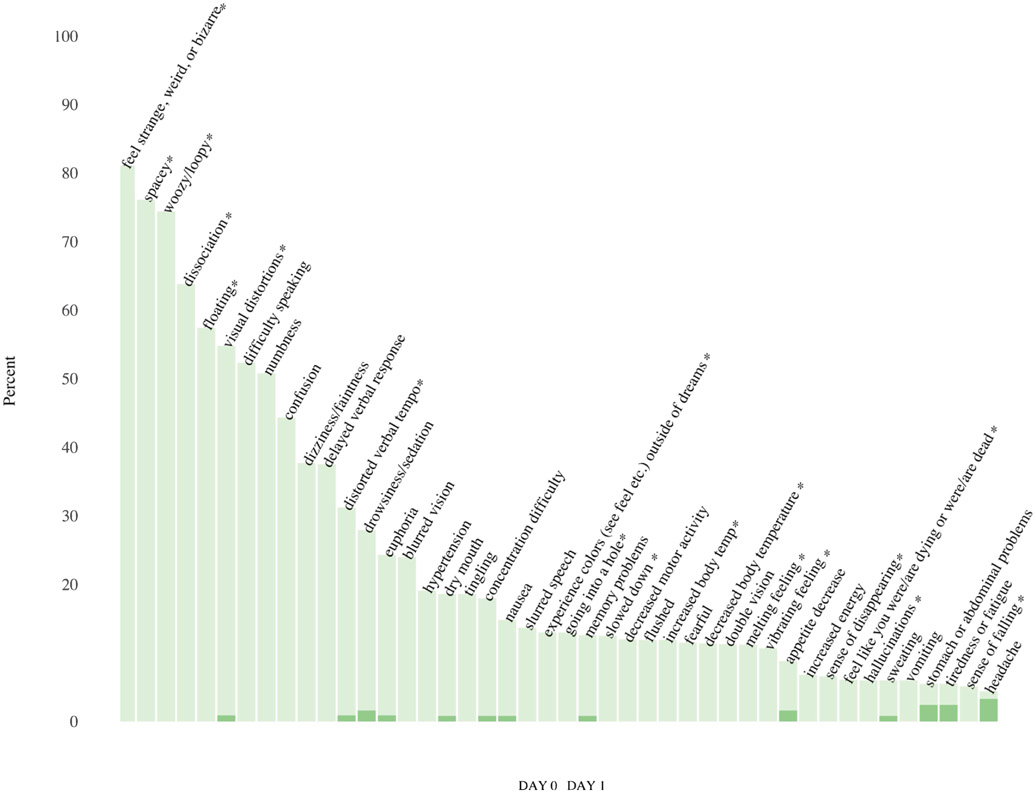
Forty-four of 120 SEs (36.7%) met the aforementioned criterion of having ≥ 5% rate of occurrence within a day following ketamine infusion. Of these 44 SEs, eight occurred in at least 50% of participants: feeling strange, weird, or bizarre; feeling spacey; feeling woozy/loopy; dissociation; floating; visual distortions; difficulty speaking; and numbness. Conversely, half of the 44 SEs occurred less than 13% of the time. Each of the 44 SEs that occurred in at least 5% of the sample rarely persisted or appeared the day following ketamine infusion (see Figure 1).
Figure 1:
Frequency of side effects (SEs) on day of intravenous ketamine infusion and Day 1 post-infusion. The figure illustrates the frequency with which SEs were reported after a single ketamine infusion for up to four hours post-infusion on Day 0 as well as on Day 1 post-infusion. (*) SEs identified as dissociative for comparison analysis with Clinician-Administered Dissociative States Scale (CADSS) total score.
Prescott tests indicated that 33 of the 44 most commonly occurring SEs (75%) were significantly associated with treatment after controlling for substudy (FDR q threshold=0.05), and each of these 33 SEs occurred at a higher rate post-ketamine versus post-placebo (see Table 3). The same pattern of results was also evident when substudy was not included as a covariate. For simplicity, only results from models that included substudy are reported here.
Table 3.
Prescott Tests: Effects of treatment on side effect
| Side Effect | Ket and PBO |
KET only |
PBO only |
Neither | N for Prescott test (BD/MOA/MDD) |
Ketamine | Placebo | p-values (controlling for study) |
|---|---|---|---|---|---|---|---|---|
| Feel strange, weird, or bizarre | 2 | 74 | 0 | 22 | 98(34/59/5) | 78% | 2% | p< 0.00001 |
| Spacey | 1 | 75 | 1 | 26 | 103(34/59/10) | 74% | 2% | p< 0.00001 |
| Woozy/loopy | 2 | 72 | 1 | 28 | 103(34/59/10) | 72% | 3% | p< 0.00001 |
| Dissociation | 1 | 63 | 1 | 39 | 104(34/59/11) | 62% | 2% | p< 0.00001 |
| Visual distortions | 0 | 59 | 1 | 44 | 104(34/59/11) | 57% | 1% | p< 0.00001 |
| Floating | 2 | 55 | 0 | 46 | 103(34/59/10) | 55% | 2% | p< 0.00001 |
| Numbness | 2 | 53 | 1 | 48 | 104(34/59/11) | 53% | 3% | p< 0.00001 |
| Difficulty speaking | 1 | 49 | 0 | 53 | 103(34/59/10) | 49% | 1% | p< 0.00001 |
| Delayed verbal response | 0 | 41 | 0 | 62 | 103(34/59/10) | 40% | 0% | p< 0.00001 |
| Confusion | 0 | 41 | 0 | 67 | 108(34/59/15) | 38% | 0% | p< 0.00001 |
| Dizziness/faintness | 0 | 40 | 1 | 67 | 108(34/59/15) | 37% | 1% | p< 0.00001 |
| Distorted verbal tempo | 0 | 30 | 0 | 73 | 103(34/59/10) | 29% | 0% | p< 0.00001 |
| Euphoria | 0 | 28 | 1 | 75 | 104(34/59/11) | 27% | 1% | p< 0.00001 |
| Blurred vision | 0 | 26 | 0 | 82 | 108(34/59/15) | 24% | 0% | p< 0.00001 |
| Drowsiness/sedation | 0 | 25 | 3 | 80 | 108(34/59/15) | 23% | 3% | 0.00002 |
| Concentration difficulty | 0 | 21 | 0 | 87 | 108(34/59/15) | 19% | 0% | p< 0.00001 |
| Dry mouth | 0 | 20 | 0 | 88 | 108(34/59/15) | 19% | 0% | p< 0.00001 |
| Hypertension | 0 | 20 | 0 | 88 | 108(34/59/15) | 19% | 0% | p< 0.00001 |
| Tingling | 0 | 20 | 3 | 81 | 104(34/59/11) | 19% | 3% | 0.0004 |
| Nausea | 1 | 16 | 0 | 91 | 108(34/59/15) | 16% | 1% | 0.00003 |
| Vibrating feeling | 0 | 16 | 0 | 82 | 98(34/59/5) | 16% | 0% | 0.00003 |
| Decreased body temperature | 0 | 15 | 1 | 87 | 103(34/59/10) | 15% | 1% | 0.00057 |
| Increased body temperature | 0 | 13 | 0 | 90 | 103(34/59/10) | 13% | 0% | 0.00027 |
| Flushed | 0 | 12 | 0 | 92 | 104(34/59/11) | 12% | 0% | 0.00042 |
| Slurred speech | 0 | 13 | 0 | 95 | 108(34/59/15) | 12% | 0% | 0.00023 |
| Double vision | 0 | 12 | 0 | 92 | 104(34/59/11) | 12% | 0% | 0.00045 |
| Melting feeling | 0 | 12 | 0 | 86 | 98(34/59/5) | 12% | 0% | 0.00051 |
| Going into a hole | 0 | 10 | 0 | 88 | 98(34/59/5) | 10% | 0% | 0.0009 |
| Experience colors (see feel etc.) outside of dreams | 0 | 10 | 0 | 88 | 98(34/59/5) | 10% | 0% | 0.00206 |
| Fearful | 0 | 8 | 0 | 83 | 91(32/59/0) | 9% | 0% | 0.00713 |
| Memory problems | 0 | 10 | 1 | 97 | 108(34/59/15) | 9% | 1% | 0.01153 |
| Sense of disappearing | 0 | 8 | 0 | 79 | 87(28/59/0) | 9% | 0% | 0.00832 |
| Appetite decrease | 0 | 10 | 2 | 96 | 108(34/59/15) | 9% | 2% | 0.03863 |
| Decreased motor activity | 0 | 10 | 2 | 96 | 108(34/59/15) | 9% | 2% | 0.03647 |
| Sweating | 1 | 8 | 0 | 99 | 108(34/59/15) | 8% | 1% | 0.00765 |
| Increased energy | 0 | 8 | 0 | 95 | 103(34/59/10) | 8% | 0% | 0.00252 |
| Headache | 0 | 9 | 6 | 93 | 108(34/59/15) | 8% | 6% | 0.44386 |
| Stomach or abdominal problems | 0 | 8 | 1 | 99 | 108(34/59/15) | 7% | 1% | 0.01876 |
| Tiredness or fatigue | 0 | 7 | 2 | 99 | 108(34/59/15) | 7% | 2% | 0.09408 |
| Sense of falling | 0 | 5 | 0 | 82 | 87(28/59/0) | 6% | 0% | 0.06292 |
| Hallucinations | 0 | 6 | 0 | 102 | 108(34/59/15) | 6% | 0% | 0.01256 |
| Vomiting | 0 | 6 | 0 | 102 | 108(34/59/15) | 6% | 0% | 0.03228 |
| Slowed down | 0 | 3 | 0 | 100 | 103(34/59/10) | 3% | 0% | 0.06083 |
| Feel like you were/are dying or were/are dead | 0 | 3 | 0 | 88 | 91(32/59/0) | 3% | 0% | 0.11123 |
Abbreviations: PBO: placebo; BD: KET-BD substudy MDD: KET-MDD substudy; MOA: KET-MOA substudy
Dissociative SEs and CADSS scores
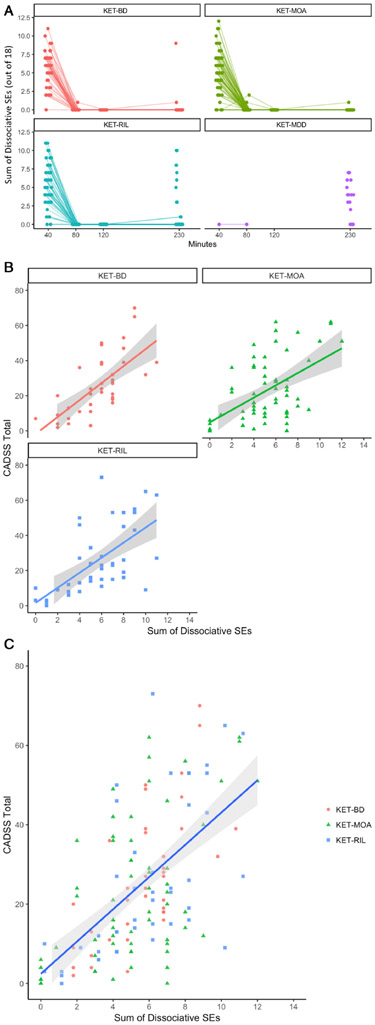
Variation in both CADSS score and dissociative SE sum was dramatically reduced after 40 minutes post-infusion, and the near absence of variation in dissociative SE sums not due to study design factors after 40 minutes meant that there was little to no useful information in the post-40-minute time points with which to explain CADSS variation (see Figure 2A). Thus, the relationship between CADSS score and dissociative SEs was explored using measurements taken at 40 minutes post-infusion.
Figure 2:
Dissociative and non-dissociative side effects (SEs). A) The sum of dissociative SEs per participant per substudy post-ketamine infusion across time. B) The sum of dissociative SEs versus Clinician-Administered Dissociative States Scale (CADSS) total score at 40 minutes post-ketamine infusion. C) The sum of dissociative SEs versus CADSS total score at 40 minutes post-ketamine infusion. Panel A illustrates the sum of dissociative SEs per substudy, as reported per participant across time. Time points varied according to study design evolution per substudy. Panel B illustrates the relationship between CADSS total score and the sum of dissociative SEs per substudy, as reported per participant 40 minutes post-ketamine infusion. Panel C illustrates the relationship across all studies between CADSS total score and the sum of dissociative SEs per participant at 40 minutes post-ketamine infusion. Because there were no data points at 40 minutes post-ketamine infusion in the KET-MDD study per protocol design, that study was excluded from panels B and C.
CADSS total scores were positively related to the sum of 18 dissociative SEs at 40 minutes post-infusion; this relationship was evident in the KET-MOA, KET-BD, and KET-RIL studies (the KET-MDD study did not include a 40-minute assessment) (see Figures 2B and 2C). At 40 minutes post-infusion, a linear regression analysis indicated that the sum of 18 dissociative SEs accounted for 36% (adjusted R2) of the variance in CADSS total score, and the estimated slope indicated that for every dissociative SE, there was a corresponding increase of 4.06 (t=9.1 p<0.0001) in CADSS total score. The estimated CADSS score corresponding to zero dissociative SEs was not statistically different from zero (intercept=2.4, t=.90, p=0.37). When substudy was included in the model, the effect of substudy was not significant (F(2, 142)=0.04, p=0.96), and the results did not meaningfully change. Similarly, allowing the relationship of dissociative SEs and CADSS total score to vary by substudy did not meaningfully change the results or contribute to the model (F(2, 140)=0.36, p=0.84).
Cravings/Addiction behaviors
A chart review of clinical assessments revealed no cravings or addiction behavior towards ketamine during participants’ inpatient stay in our research facility, which could last up to three months after study participation ended; this observation period of up to three months included passes into the community, random room checks, and urine drug screens.
Discussion
This study was an exploratory meta-analysis of secondary measures drawn from five substudies examining the antidepressant effects of ketamine conducted at the NIH over a period of 13 years. The results indicate that a single intravenous subanesthetic-dose ketamine infusion was relatively safe for the treatment of TRD. While the few previous studies examining this issue largely assessed SEs via passive monitoring, the present study used active and structured surveillance of emerging SEs by study personnel. Furthermore, unlike past studies that focused largely on assessing dissociative symptoms, the present study inquired about all body systems and comprehensively assessed both dissociative and non-dissociative SEs. In general, the SEs associated with intravenous ketamine use were mainly dissociative, although some general/somatic SEs were also commonly experienced (i.e., numbness, dizziness/faintness, blurred vision, and drowsiness/sedation were reported for >20% of ketamine infusions). These SEs were largely transient, starting immediately after ketamine infusion and returning to baseline within four hours post-infusion. These findings are consistent with previous reports of SEs associated with intravenous ketamine and with the SE profile of intranasal esketamine (Popova et al., 2019). In this sample, no serious long-lasting SEs were associated with a single infusion of 0.5mg/kg of ketamine, including cystitis, emergence delirium, or anaphylaxis. There was also no evidence of any significant cognitive or memory deficits, or of any increased propensity for recreational ketamine use or abuse as observed over an approximately three-month follow-up in our research inpatient unit.
The analysis also found that CADSS total scores at 40 minutes post-infusion were positively related to dissociative SEs within and across studies. Dissociative SEs had a linear relationship that was estimated to start at CADSS total score=0 with a slope of approximately one. This suggests that the CADSS and active solicitation of dissociative symptoms may measure different aspects of the dissociative experience, but that one is not necessarily superior to the other.
Strengths and limitations
Strengths of this study include its unique methodology, which used a structured assessment tool to actively solicit data about adverse SEs associated with subanesthetic-dose ketamine at several time points pre- and post-infusion across a number of similarly conducted substudies. Given that most of the data in the present study were derived from placebo-controlled, crossover trials, we were able to conduct a formal statistical analysis demonstrating that the reported SEs were associated with active treatment with ketamine compared to placebo. Because of this methodology, the present results provide further details regarding the characteristics and resolution over time of specific SEs experienced as a result of subanesthetic-dose ketamine infusion. Another major strength of the study was its comprehensive assessment of SEs across all body systems, which included both dissociative and non-dissociative symptoms. These findings are especially relevant after the recent FDA approval of esketamine. Finally, the relatively large sample size, and the fact that data were drawn across 13 years of single-site studies in an inpatient setting, are also significant strengths.
Nevertheless, the study is also associated with several limitations. First, the secondary, exploratory nature of the study limits the conclusions that can be derived from these data. Second, the secondary nature of this study also precluded our ability to examine diagnostic differences in SEs associated with ketamine versus placebo due to the confounding of diagnosis and substudy. Specifically, all participants diagnosed with major depressive disorder were enrolled in one of three substudies (KET-MDD, KET-MOA, and KET-RIL); conversely, all but three of the participants with bipolar depression were enrolled in another substudy (KET-BD). Because we treated our dataset as meta-analytic and controlled for study, we are essentially unable to tease diagnosis apart in our models. Third, because SEs were only assessed for a single infusion of subanesthetic-dose intravenous ketamine, data regarding other doses, repeated dosing, or other routes of administration are lacking. Fourth, results regarding cognitive, urological, or addictive SEs associated with subanesthetic-dose ketamine are also limited because we did not complete formal neuropsychological, cognitive, or memory testing, nor did we formally measure cravings or other indicators of abuse or dependence during the participants’ inpatient stay or following discharge from our unit. However, it is important to mention that, while formal instrument-based assessment of these potential SEs was not conducted, participants received an extended period of clinical follow-up and observation—up to three months post-infusion. Finally, a potential limitation is our method of classifying dissociative and non-dissociative SEs. While we were confident in the 18 items we classified as dissociative, some non-dissociative symptoms were more challenging to classify (e.g., confusion, anxiety, memory problems) as they could potentially be seen as, or related to, dissociative symptoms. To address this issue, we attempted a data-driven decision rule by exploring the relationship between each of these items and the 18 dissociative items as well as clearly non-dissociative items (e.g., headache, nausea); that analysis did not yield conclusive information. Therefore, we relied on the consensus opinion of four psychiatrists (EEAD, LP, CK, and BK) and one psychologist (DG) to classify the SEs, ultimately opting for a less inclusive list of dissociative symptoms.
Future studies designed to prospectively assess the effect of repeat ketamine doses may help to better substantiate our findings as well as weigh the usefulness of the CADSS for assessing subanesthetic-dose ketamine as pharmacotherapy for TRD. Another key area of research is to improve our understanding of ketamine’s underlying mechanism of antidepressant action and its relationship, if any, to the SEs associated with its use.
Supplementary Material
Highlights.
Ketamine treatment is associated with transient dissociative side effects (SEs)
Some somatic transient SEs were also commonly experienced
CADSS scores post-ketamine were related to dissociative SEs
There was no evidence of long-lasting delirium, abuse liability, or cystitis
Acknowledgments
The authors thank the 7SE Inpatient Mood and Anxiety Disorders Research Unit for their clinical support during the writing of this manuscript. Ioline Henter, MA (NIMH) provided invaluable editorial assistance.
Funding and Role of Funding Source
The authors gratefully acknowledge the support of the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA-MH002857; NCT00088699). The NIMH had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Declaration of Interest
Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. Dr. Kraus received travel support from Roche and AOP Orphan. All other authors have no conflict of interest to disclose, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ, 2010. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67,139–145. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association., 2013. Diagnostic and statistical manual for mental disorders, Fifth edition ed. American Psychiatric Publishing, Washington, DC, pp. 291 – 307. [Google Scholar]
- Benjamini Y, Yekutieli D, 2001. The control of the false discovery rate in multiple testing under dependency. Ann Statist 29, 1165–1188. [Google Scholar]
- Bonnet U, 2015. Long-term ketamine self-injections in major depressive disorder: focus on tolerance in ketamine's antidepressant response and the development of ketamine addiction. J Psychoactive Drugs 47, 276–285. [DOI] [PubMed] [Google Scholar]
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Martin LD, Dissen GA, Creeley CE, Olney JW, 2012. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology 116, 372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV, Monaghan L, 2001. In and out of the K-hole: a comparison of the acute and residual effects of ketamine in frequent and infrequent ketamine users. Addiction 96, 749–760. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Schoepp D, Kalivas PW, Volkow ND, Zarate CA Jr., Merchang K, Bear MF, Umbricht D, Hajos M, Potter WZ, Lee C-M, 2011. Translating glutamate: from pathophysiology to treatment. Science Transl Med 3, 102mr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lin D, Wu B, Zhou W, 2016. Ketamine abuse potential and use disorder. Brain Res Bull 126, 68–73. [DOI] [PubMed] [Google Scholar]
- Lodge D, Mercier MS, 2015. Ketamine and phencyclidine: the good, the bad and the unexpected. Br J Pharmacol 172, 4254–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muetzelfeldt L, Kamboj SK, Rees H, Taylor J, Morgan CJA, Curran HV, 2008. Journey through the K-hole: Phenomenological aspects of ketamine use. Drug Alcohol Depend 95, 219–229. [DOI] [PubMed] [Google Scholar]
- Neehoff S, Glue P, 2019. Dissociation after ketamine dosing: Is the CADSS fit for purpose? J Affect Disord 244, 239–240. [DOI] [PubMed] [Google Scholar]
- Olney JW, Labruyere J, Price MT, 1989. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science 244, 1360–1362. [DOI] [PubMed] [Google Scholar]
- Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, Mazzucco C, Hough D, Thase ME, Shelton RC, Molero P, Vieta E, Bajbouj M, Manji H, Drevets WC, Singh JB, 2019. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry 176, 428–438. [DOI] [PubMed] [Google Scholar]
- Sassano-Higgins S, Baron D, Juarez G, Esmaili N, Gold M, 2016. A review of ketamine abuse and diversion. Depress Anxiety 33, 718–727. [DOI] [PubMed] [Google Scholar]
- Scallet AC, Schmued LC, Slikker W Jr., Grunberg N, Faustino PJ, Davis H, Lester D, Pine PS, Sistare F, Hanig JP, 2004. Developmental neurotoxicity of ketamine: morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci 81, 364–370. [DOI] [PubMed] [Google Scholar]
- Schak KM, Vande Voort JL, Johnson EK, Kung S, Leung JG, Rasmussen KG, Palmer BA, Frye MA, 2016. Potential risks of poorly monitored ketamine use in depression treatment. Am J Psychiatry 173, 215–218. [DOI] [PubMed] [Google Scholar]
- Shahani R, Streutker C, Dickson B, Stewart RJ, 2007. Ketamine-associated ulcerative cystitis: a new clinical entity. Urology 69, 810–812. [DOI] [PubMed] [Google Scholar]
- Short B, Fong J, Galvez V, Shelker W, Loo CK, 2018. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry 5, 65–78. [DOI] [PubMed] [Google Scholar]
- Strayer RJ, Nelson LS, 2008. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med 26, 985–1028. [DOI] [PubMed] [Google Scholar]
- U.S. Food & Drug Administration, 2019a. FDA approves new nasal spray medication for treatment-resistant depression. [Google Scholar]
- U.S. Food & Drug Administration, 2019b. FDA Briefing Document: Psychopharmacologic Drugs Advisory Committee (PDAC) and Drug Safety and Risk Management (DSaRM) Advisory Committee Meeting. February12, 2019 FDA, Washington, DC. [Google Scholar]
- U.S. Food & Drug Administration, 2019c. Ketalar (ketamine hydrochloride) injection. FDA. [Google Scholar]
- U.S. Food & Drug Administration, 2019d. Risk evaluation and mitigation strategy (REMS) document: SPRAVATO (esketamine hydrochloride) REMS program. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/rems/Spravato_2019_03_05_REMS_Document.pdf.
- van Schalkwyk GI, Wilkinson ST, Davidson L, Silverman WK, Sanacora G, 2018. Acute psychoactive effects of intravenous ketamine during treatment of mood disorders: Analysis of the Clinician Administered Dissociative State Scale. J Affect Disord 227, 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan LB, Levitch CF, Perez AM, Brallier JW, Iosifescu DV, Chang LC, Foulkes A, Mathew SJ, Charney DS, Murrough JW, 2015. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry 76, 247–252. [DOI] [PubMed] [Google Scholar]
- Wilkinson ST, Toprak M, Turner MS, Levine SP, Katz RB, Sanacora G, 2017. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry 174, 695–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Patterson TA, Divine RL, Sadovova N, Zhang X, Hanig JP, Paule MG, Slikker W Jr., Wang C, 2009. Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J Dev Neurosci 27, 727–731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.