Abstract
Objective
Discontinuation of antiepileptic drugs (AEDs) in seizure‐free patients is an important goal because of possible long‐term side effects and the social stigma burden of epilepsy. The purpose of this work was to assess seizure recurrence risk after suspension of AEDs, to evaluate predictors for recurrence, and to investigate the recovery of seizure control after relapse. In addition, the accuracy of a previously published prediction model of seizure recurrence risk was estimated.
Methods
Seizure‐free patients with epilepsy who had discontinued AEDs were retrospectively enrolled. The frequency of seizure relapses after AED withdrawal as well as prognosis after recurrence were assessed and the predictive role of baseline clinical‐demographic variables was evaluated. The aforementioned prediction model was also validated and its accuracy assessed at different seizure‐relapse probability levels.
Results
The enrolled patients (n = 133) had been followed for a median of 3 years (range 0.8–33 years) after AED discontinuation; 60 (45%) of them relapsed. Previous febrile seizures in childhood (hazard ratio [HR] 3.927; 95% confidence interval [CI] 1.403–10.988), a seizure‐free period on therapy of less than 2 years (HR 2.313; 95% CI 1.193–4.486), and persistent motor deficits (HR 4.568; 95% CI 1.412–14.772) were the clinical features associated with relapse risk in univariate analysis. Among these variables, only a seizure‐free period on therapy of less than 2 years was associated with seizure recurrence in multivariate analysis (HR 2.365; 95% CI 1.178–4.7444). Pharmacological control of epilepsy was restored in 82.4% of the patients who relapsed. In this population, the aforementioned prediction model showed an unsatisfactory accuracy.
Significance
A period of freedom from seizure on therapy of less than 2 years was the main predictor of seizure recurrence. The accuracy of the previously described prediction tool was low in this cohort, thus suggesting its cautious use in real‐world clinical practice.
Keywords: AED withdrawal, anti‐seizure medications, epilepsy
Key Points.
Discontinuation of antiepileptic drugs (AEDs) in seizure‐free patients is an important goal because of side effects and the social stigma burden of epilepsy.
Seizure relapses and possible predictors of recurrence were evaluated in 133 patients followed for a median of 3 years after AED withdrawal.
Regain of control after seizure relapse and the accuracy of a prediction model of seizure recurrence risk published by Lamberink et al. were also estimated.
A period of freedom from seizure on therapy of less than 2 years was the main predictor of seizure recurrence.
About 82% of patients regained seizure control after relapse but no predictor of the recovery could be identified.
The accuracy of the Lamberink prediction model was low in this cohort suggesting its cautious use in real‐world clinical practice
1. INTRODUCTION
Antiepileptic drugs (AEDs) are effective in about 65%–85% of patients with epilepsy.1, 2 One of the main open questions in this field is treatment duration after achieving seizure remission. In fact, the discontinuation of AEDs in seizure‐free patients remains a desirable clinical achievement, since at least one side effect of AEDs is observed in up to 88% of cases (eg, behavioral and cognitive effects, teratogenic risk, and drug interactions).1, 2, 3, 4, 5, 6 In addition, interrupting AEDs can lead to a significant improvement in the quality of life of patients with epilepsy, with important psychosocial implications, since patients perceive themselves not only as free from drugs and seizures but also without any social stigma.3, 7, 8, 9, 10, 11
However, AED discontinuation is a demanding clinical task due to its possible detrimental consequences in the case of seizure recurrence (eg, injuries, loss of self‐esteem, unemployment, loss of the driving license, and so on).12 To properly tailor this decision, many potential risk factors of recurrence have been reported in the literature, but evidence supporting them is still weak.13 In particular, only two randomized controlled trials (RCTs) comparing seizure recurrence risk between AED discontinuation and maintenance have been published, showing opposite results. The first one, a British Medical Research Council‐based multicenter RCT, showed a seizure recurrence risk in the AED discontinuation group higher than in continuers, whereas the second one, a Norway‐based multicenter double‐blinded RCT, did not.14, 15
Several multivariate prediction models have also been proposed in the literature but these tools have shown limitations that make them poorly applicable as they are based on too selected populations.13, 16, 17, 18, 19 In addition, the available models are hardly comparable, in terms of inclusion criteria.13, 16, 17, 18, 19 In 2017, starting from an individual participant data meta‐analysis based on the raw data of 10 studies (including a total of 1769 patients), Lamberink et al. developed a predictive model estimating seizure recurrence risk at 2 and 5 years after AED withdrawal (from now on called Lamberink's prediction model, LPM).2 This model is believed to be well applicable in daily clinical practice as it is based on a heterogeneous cohort, close to real‐life experience.2, 20, 21 The accuracy of LPM was internally cross‐validated but also externally by only two studies, both in Chinese populations.2, 20, 21
In the present study, seizure recurrence after AED discontinuation was retrospectively evaluated in an Italian patient cohort evaluating several risk factors correlated with seizure relapse. Moreover, clinical course after seizure recurrence was also estimated by assessing the number of patients recovering pharmacological control and the clinical variable associated with this achievement. Finally in this cohort, a retrospective validation of the accuracy of LPM was performed.
The LPM for risk of seizure recurrence at 2 and 5 years provides an individual recurrence probability, without defining a threshold value dividing patients as high or low risk of recurrence, making it difficult to apply in clinical practice.2 For this reason, the purpose of this work was also to identify a practical threshold probability.
2. METHODS
2.1. Study design
This work is an observational retro‐prospective cohort study conducted at the Epilepsy Regional Referral Center (ERRC) of the Neurology 2 Department of Careggi University Hospital (Florence, Italy), a third‐level medical center. The study respects the Declaration of Helsinki and has been approved by the hospital ethics committee (code:14385_oss). Written informed consent for data gathering was obtained from each patient.
2.2. Patients
Patients who fulfilled the diagnostic criteria for epilepsy,22 followed between January, 1983 and November, 2018 at the ERRC, were included according to the following criteria: seizure‐free subjects who interrupted AEDs from the age of at least 16 and were thereafter followed for a minimum of 2 years or until seizure recurrence. Exclusion criteria were incomplete clinical records, a follow‐up shorter than 2 years without a recurrence, AED discontinuation without being seizure‐free, seizures not fulfilling the diagnostic criteria for epilepsy (eg, acute symptomatic seizures occurring within 1 week of a brain insult23), interruption of prophylactic AEDs (eg, patients undergoing neurosurgery who had never experienced seizures), and AED withdrawal performed after surgery for epilepsy. Clinical records were used to gather all the necessary clinical demographic variables. The data were collected retrospectively if patients had discontinued AEDs before 2010 and partially prospectively until January 1, 2020, if they had interrupted AEDs thereafter. In order to collect follow‐up data also of the patients who missed on‐site visits, the clinical status of these patients (ie, seizure freedom in the years after the beginning of AEDs withdrawal) was investigated by phone calls. To analyze seizure‐free intervals on therapy before AED withdrawal and duration of AED tapering as possible risk factors of seizure recurrence, these parameters were not part of the inclusion criteria.
2.3. Clinical‐demographic characteristics analyzed
The following clinical‐demographic characteristics were recorded: sex, age at seizure onset (stratified according to Lamberink et al.2), duration of epilepsy (from the first to the last seizure), family history of epilepsy, neonatal seizures (until the first month of life), febrile seizures in childhood, number of seizures before AED discontinuation (<10 or ≥10),2 duration of the seizure‐free period on therapy (from the last seizure attack to AED withdrawal; variable was recorded both categorized according to Wang et al.24 and continuous), number of discontinued drugs, seizure type (focal/generalized), etiology of epilepsy (structural, assessed by magnetic resonance imaging [MRI]; genetic; unknown), diagnosis of a self‐limiting epilepsy syndrome (eg, absence epilepsy, benign epilepsy with centrotemporal spikes, Panayiotopoulos syndrome), developmental delay (assessed only by clinical judgment and history of need for specialized schooling, as in 3/10 of the papers included in the meta‐analysis of Lamberink et al.2), electroencephalography (EEG) epileptiform abnormalities before discontinuation, age at last seizure, age at AED withdrawal, plasma levels of AEDs at the beginning of withdrawal (within, below, or above therapeutic range), persistent motor deficits, psychiatric disorders for which the patient assumed a specific drug, failure of previous AED discontinuations, duration of AED tapering (0–3 months; 4–12 months; more than 1 year), epileptiform abnormalities on EEG during or at the end of AED withdrawal, epileptic encephalopathy, and presence of juvenile myoclonic epilepsy.
For LPM validation in this patient cohort, the 2‐ and 5‐year seizure recurrence probability for each patient was estimated using the web‐based tool developed by the authors (http://epilepsypredictiontools.info).2
As for the time to seizure recurrence, this variable was evaluated starting from the beginning of AED tapering, since seizure recurrence risk can increase starting from this time.25 Therefore, as in previous studies, the relapses that occurred during the tapering period were also included in the analyis.14, 25, 26, 27, 28
Seizures were accepted when validated by an EEG recorded during the event, or when they were reported to the neurologist with suggestive features by a witness (clinician/relative/friend of the patient), or self‐reported by the patients themselves.
In the patients who experienced seizure recurrence, the recovery of pharmacological control of epilepsy after the recurrence and time to new seizure control, defined as the presence at the last follow‐up of a seizure‐free interval of at least 1 year,29, 30 were also evaluated. Finally, the type of the new therapy started again after recurrence was also assessed (same AEDs as before withdrawal at same dose/same AEDs at a lower dose/same AEDs at a higher dose/different monotherapy/polytherapy).
2.4. Statistics
Statistical analyses were carried out using SPSS version 12 and R (The R Project for Statistical Computing, Vienna, Austria) version 4.0.3 packages. All statistical tests were two‐tailed and p < 0.05 was considered statistically significant.
Frequencies were used for categorical variables, whereas average/standard deviation (SD) or median/range were used for continuous variables according to their distribution (normal or not, respectively, according to the Shapiro‐Wilk test).
Statistical analysis of the selected outcomes (seizure recurrence and recovery of pharmacological control after recurrence) was based on the evaluation of the time to the first event by the Kaplan‐Meier method.
To evaluate the baseline clinical‐demographic variables associated with increased risk of seizure recurrence, univariate and multivariate analyses with the Cox regression model were performed, calculating the hazard ratios (HRs) with their 95% confidence intervals (CIs). For multivariate analysis, only the variables that resulted significant at the univariate were included. Univariate and multivariate analyses with the Cox regression model were also performed to evaluate the variables associated with the chance to recover a new pharmacological seizure control in the patients who experienced seizure relapse.
Considering that the authors of LPM did not define a threshold value that divides patients as high or low risk of seizure recurrence, a specifically devised algorithm was developed to assess the accuracy of LPM in this patient cohort that permits its evaluation at each possible recurrence risk threshold probability value. Each possible threshold probability value was identified in the sequence of increase of 2% between 0 and 100% (51 recurrence risk threshold probability values, extremes included). The extreme values of LPM are <10% and >90%. No patient in this study showed a recurrence risk probability value >90% but for those with a probability <10%, the central value of that interval was assigned (ie, 5%). This algorithm was implemented with the R software and also produced the CIs at 95% both for sensitivity and specificity curves through the bootstrap technique. The bootstrap is a statistical resampling method used to obtain an estimation of the sampling distribution of almost any statistics. In this analysis, empirical CIs were generated by extracting 10,000 subsamples of 80 patients for each possible threshold value and by calculating the 2.5 and 97.5 percentiles from the sensitivity and specificity subsample distribution (with a cohort of 133 patients, the universe of all the possible subsamples of 80 patients has a cardinality of 4.861139e+37).
3. RESULTS
3.1. Seizure recurrence risk
Of the 4154 patients diagnosed with epilepsy between January 1, 1983 and November 30, 2018, a total of 205 had discontinued AEDs. After applying the selection criteria, a final cohort of 133 patients was extracted. For 12 of the 72 excluded patients, exclusion occurred because they were lost at follow‐up before the end of the second year after AED withdrawal, or before any eventual seizure recurrence, although in each case phone contacts were repeatedly attempted (Figure 1).
FIGURE 1.
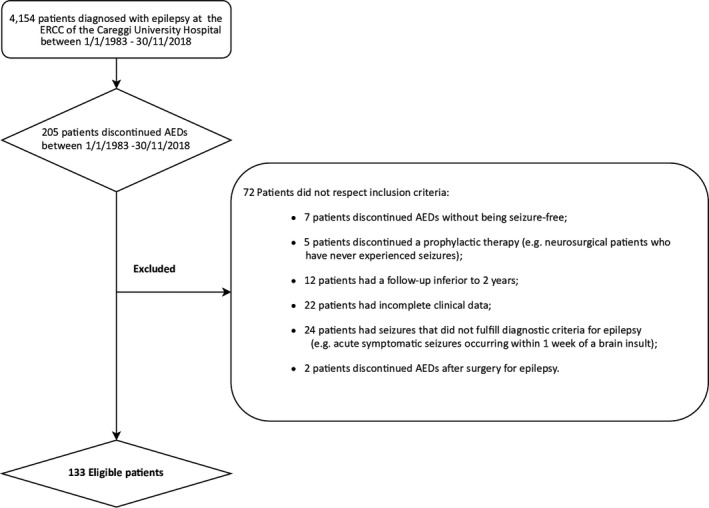
Patients enrolled in the study. Two hundred five patients discontinued antiepileptic drugs (AEDs) of the 4154 patients diagnosed with epilepsy between January 1, 1983 and November 30, 2018, at Epilepsy Regional Referral Center (ERCC) of the Neurology 2 Department of Careggi University Hospital (Florence, Italy). After applying the inclusion criteria, a final cohort of 133 patients was selected.
The 133 patients included were followed for at least 2 years or until any recurrence, for a median of 3 years (0.8–33 years) after the beginning of AED withdrawal. The main baseline characteristics of this cohort are reported in Table 1. The AEDs most used were levetiracetam (30.1% of patients), carbamazepine (24.8%), valproic acid (13.5%), lamotrigine (11.3%), and phenobarbital (11.3%).
TABLE 1.
Clinical‐demographic features and potential risk factors of seizure recurrence after AED discontinuation evaluated by Cox regression model
| Variables | N = 133 | Univariate for seizure recurrence | |
|---|---|---|---|
| HR (95% CI) | p‐value | ||
| Sex (%) | |||
| F | 63 (47.4%) | 1.110 (0.665–1.852) | 0.689 |
| M | 70 (52.6%) | Reference value | |
| Family history of epilepsy (%) | 11 (8.3%) | 1.012 (0.404–2.536) | 0.980 |
| History of neonatal seizures (%) | 0 | ‐ | ‐ |
| History of febrile seizures in childhood (%) | 4 (3%) | 3.927 (1.403–10.988) | 0.009 * |
| Age at seizure onset (%)a | |||
| Childhood (0–10 years) | 12 (9%) | Reference value | |
| Adolescent (11–17 years) | 20 (15%) | 1.088 (0.361–3.280) | 0.881 |
| Adult age (≥18 years) | 101 (75.9%) | 1.179 (0.464–2.996) | 0.729 |
| No. of seizures before AED withdrawal | |||
| 0–9 | 110 (82.7%) | Reference value | |
| 10 or more | 23 (17.3%) | 0.485 (0.209–1.130) | 0.093 |
| Median duration of epilepsy in months (range) | 6 (0–480) | 0.998 (0.995–1.002) | 0.378 |
| Median age at last seizure (range) | 34 (9–80) | 0.997 (0.984–1.010) | 0.647 |
| Seizure type | |||
| Generalized | 19 (14.3%) | Reference value | |
| Focal | 114 (85.7%) | 0.864 (0.434–1.722) | 0.678 |
| Etiology of epilepsy | |||
| Structural | 75 (56.4%) | 1.023 (0.613–1.708) | 0.930 |
| Genetic | 0 | – | |
| Unknown | 58 (43.6%) | Reference value | |
| Self‐limiting epilepsy syndrome | 2 (1.5%) | 21.230 (0.010–43698.239) | 0.433 |
| Juvenile myoclonic epilepsy | 6 (4.5%) | 0.720 (0.175–2.953) | 0.648 |
| Epileptic encephalopathy | 1 (0.8%) | 3.936 (0.537–28.864) | 0.178 |
| Development delay | 5 (3.8%) | 1.976 (0.616–6.337) | 0.252 |
| Persistent motor deficits | 3 (2.3%) | 4.568 (1.412–14.772) | 0.011 ** |
| Psychiatric abnormalities | 26 (19.5%) | 0.786 (0.398–1.553) | 0.489 |
| Seizure‐free period on therapy (months)b | |||
| 0–23 | 20 (15%) | 2.313 (1.193–4.486) | 0.013 *** |
| 24–35 | 16 (12%) | 1.479 (0.665–3.291) | 0.338 |
| 36–47 | 21 (15.8%) | 0.834 (0.375–1.855) | 0.656 |
| 48–59 | 15 (11.3%) | 0.913 (0.349–2.389) | 0.853 |
| 60 or more | 61 (45.9%) | Reference value | |
| Median age at withdrawal (range) | 43 (16–84) | 0.993 (0.980–1.007) | 0.313 |
| EEG before AEDs withdrawal | |||
| Normal | 72 (54.1%) | Reference value | |
| Epileptiform abnormality | 2 (1.5%) | 1.302 (0.173–9.789) | 0.797 |
| Failure of previous AEDs discontinuations | 12 (9%) | 0.843 (0.337–2.109) | 0.714 |
| Number of AEDs discontinued | |||
| One | 130 (97.7%) | Reference value | |
| Two | 3 (2.3%) | 2.346 (0.571–9.641) | 0.237 |
| Plasma levels of AEDs at the beginning of withdrawal | |||
| Below range | 21 (15.8%) | 1.226 (0.534–2.815) | 0.631 |
| Within range | 66 (49.6%) | Reference value | |
| Above range | 0 | – | |
| Duration of AEDs tapering | |||
| 0–3 months | 41 (30.8%) | 5.912 (0.794–44.044) | 0.083 |
| 4–12 months | 69 (51.9%) | 3.678 (0.498–27.144) | 0.202 |
| More than 1 year | 1 (0.8%) | Reference value | |
| EEG during/at the end of AEDs withdrawal | |||
| Normal | 94 (70.7%) | Reference value | |
| Epileptiform abnormality | 9 (6.8%) | 2.059 (0.869–4.879) | 0.101 |
Missing data for the variable “EEG before AED withdrawal”: 59 (44.4%).
Missing data for the variable “Plasma levels of AEDs at the beginning of withdrawal”: 46 (34.6%).
Missing data for the variable “Duration of AED tapering”: 14 (10.5%).
Missing data for the variable “EEG during/at the end of AED withdrawal”: 30 (22.6%).
AED, antiepileptic drug; CI, confidence interval; EEG, electroencephalography; HR, hazard ratio.
The subdivision of age groups is the same as in Lamberink et al2
The subdivision of seizure‐free period on therapy is the same as in Wang et al21
Multivariate analysis: HR 2.865 (95% CI 0.709–11.567) p = 0.139.
Multivariate analysis: HR 2.842 (95% CI 0.566–14.265) p = 0.204.
Multivariate analysis: 0–23 months: HR 2.600 (95% CI 1.318–5.129) p = 0.006; 24–35 months: HR 1.694 (95% CI 0.750–3.827) p = 0.205; 36–47 months: HR 0.905 (95% CI 0.401–2.043) p = 0.811; 48–59 months: HR 1.041 (95% CI 0.393–2.758) p = 0.935.
Sixty patients (45%) relapsed after AED discontinuation; 73 (55%) did not. Nineteen of the 73 patients (26%) who did not relapse were followed for at least 5 years and, including the duration of their seizure‐free interval on therapy, 13 of them (68.4%) had been seizure‐free for 10 years, without AEDs for at least the last 5 years. Thus according to International League Against Epilepsy (ILAE) criteria, these patients can be considered to have resolved epilepsy.22
For the 60 patients (45%) who relapsed after AED discontinuation, the cumulative risk of seizure recurrence at 6 months was 13.5%, at 1 year 30.8%, at 2 years 39.1%, at 3 years 41.4%, and at 5 years 44.7% (Figure 2A). The median time to seizure recurrence from the beginning of AED tapering was 304 days (30–5840 days). Of the 60 patients who had a seizure recurrence, it occurred within the first year from the beginning of tapering in 68.3% of them, and within 2 years in 86.7% of them. Only 13.3% of these 60 patients had recurrence after 2 years and only 6.7% after 5 years.
FIGURE 2.
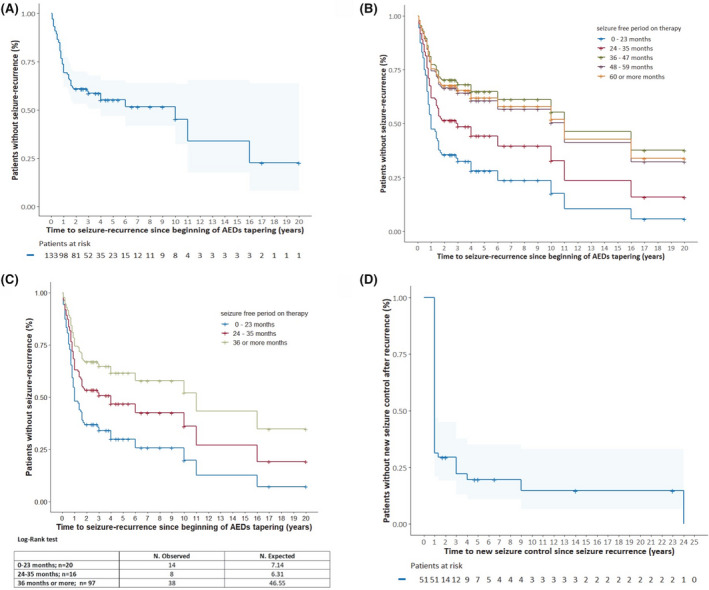
A, Time to first seizure recurrence from the beginning of antiepileptic drug (AED) tapering is displayed according to the Kaplan‐Meier method. On the x‐axis, time 0 represents the beginning of AED tapering up to the discontinuation. The number of patients at risk at each time is reported under the x‐axis. B, Multivariate analysis with the Cox regression model is presented, stratified according to the duration of the seizure‐free period on therapy. Each curve represents the whole population and it is weighted in the same way for the other two factors implemented in the multivariate analysis (febrile seizures in childhood and persistent motor deficit; both are fixed to their average values). The only element differentiating the curves are the five different values that were assigned to the variable “seizure‐free period on therapy.” A seizure‐free period on therapy shorter than 2 years was significantly associated with recurrence risk (hazard ratio [HR] 2.365; 95% confidence interval [CI] 1.178–4.7444). It is notable that the strata encompassing the longest seizure‐free periods (36–47 months, 48–59 months, and 60 or more months) substantially overlap: Having a seizure‐free period on therapy longer than 3 years does not seem to lead to any further reduction in seizure recurrence risk (not significant). C, Post hoc analysis of the results represented in 2B. The curves displayed are the graphical representation of multivariate analysis with the Cox regression model where the possible values assigned to the variable “duration of the seizure‐free period on therapy” are 0–23 months, 24–35, and 36 or more months. A period shorter than 2 years is still the only significant seizure recurrence risk factor but, as shown in the table below the graph, also a period of 2 years or longer but shorter than 3 years leads to an increased seizure recurrence risk with more observed recurrences than expected (not significant). D, Time to restore seizure pharmacological control after relapse, displayed using the Kaplan‐Meier method. On the x‐axis, 0 represents the time of seizure relapse after AED discontinuation. The patients at risk at each time are reported under the curve.
Univariate analysis showed that significant risk factors associated with seizure relapse were: the presence of febrile seizures in childhood (HR 3.927; 95% CI 1.403−10.988), duration of the seizure‐free period on therapy shorter than 2 years (HR 2.313; 95% CI 1.193–4.486), and presence of persistent motor deficits (HR 4.568; 95% CI 1.412 −14.772) (Table 1). Multivariate analysis revealed that among these factors, only the duration of the seizure‐free period on therapy shorter than 2 years holds a statistically significant association with seizure relapse (HR 2.365; 95% CI 1.178–4.744) (Figure 2B). Subsequently, a post hoc analysis was carried out to better define the effect on seizure recurrence risk of a seizure‐free period on therapy of 2 years or longer but shorter than 3 years. Thus only three possible values were assigned to the variable “duration of the seizure‐free period on therapy” (36–47 months, 48–59 months, and 60 or more months) and a log‐rank test was performed among the three curves to evaluate the difference between the expected and observed recurrences. As shown in Figure 2C, also a seizure‐free period on therapy of 2 years or longer but shorter than 3 years led to an increased seizure recurrence risk with more observed recurrences than expected (not significant). Instead, having a seizure‐free period on therapy of 3 years or longer did not seem to further reduce seizure recurrence risk (Figure 2C).
3.2. Probability of gaining a new seizure control after recurrence
Among the 60 patients who relapsed, 59 agreed to start AEDs again. Of these, 51 patients were followed for at least 1 year after the relapse (median 5 years; range 1–33). The main clinical features of this cohort are reported in Table 2. During this period, of these 51 patients, 42 regained seizure control (82.4%), 5 did not (10%), and 4 (7.8%) developed drug‐resistant epilepsy. Out the 42 patients who regained seizure control, 35 (83.3%) did it within the first year from recurrence, 36 (85.7%) within the first 2 years, 39 (92.9%) within the first 3 years, and 40 (95.2%) within the first 4 years. The last two patients gained a new control after 9 and 24 years, respectively (Figure 2D). The majority of patients gained new seizure control with monotherapy (41 of 42 patients, 97.6%); in particular, 26 with the same AEDs as before the discontinuation at a higher dose (6 patients ), at the same dose (13 patients), or a lower dose (7 patients). From univariate analysis, no clinical‐demographic features resulted as associated with the gain of new seizure control (Table 2).
TABLE 2.
Clinical‐demographic features of the 51 patients who restarted AEDs after seizure recurrence and were followed for at least one year. Factors associated with the achievement of new pharmacological control evaluated by the Cox regression model are also reported.
| Variables | N = 51 | Univariate for new seizure control | |
|---|---|---|---|
| HR (95% CI) | p‐value | ||
| Sex (%) | |||
| F | 27 (52.9%) | 1.048 (0.567–1.937) | 0.881 |
| M | 24 (47.1%) | Reference value | |
| Family history of epilepsy (%) | 4 (7.8%) | 0.405 (0.95–1.733) | 0.223 |
| History of neonatal seizures (%) | 0 | ‐ | |
| History of febrile seizures in childhood (%) | 3 (5.9%) | 1.5 (0.459–4.898) | 0.502 |
| Age at seizure onset (%)a | |||
| Childhood (0–10 years) | 5 (9.8%) | Reference value | |
| Adolescent (11–17 years) | 7 (13.7%) | 0.991 (0.279–3.522) | 0.989 |
| Adult age (≥18 years) | 39 (76.5%) | 1.037 (0.365–2.951) | 0.945 |
| No. of seizures before AED withdrawal | |||
| 0–9 | 45 (88.2%) | Reference value | |
| 10 or more | 6 (11.8%) | 0.736 (0.260–2.080) | 0.563 |
| Median duration of epilepsy in months (range) | 6 (0–480) | 0.999 (0.996–1.003) | 0.651 |
| Median age at last seizure (range) | 31 (12–80) | 0.999 (0.983–1.015) | 0.905 |
| Seizure type | |||
| Generalized | 10 (19.6%) | Reference value | |
| Focal | 41 (80.4%) | 0.807 (0.384–1.697) | 0.573 |
| Etiology of epilepsy | |||
| Structural | 29 (56.9%) | 0.767 (0.412–1.427) | 0.402 |
| Genetic | 0 | – | |
| Unknown | 22 (43.1%) | Reference value | |
| Self‐limiting epilepsy syndrome | 0 | – | |
| Juvenile myoclonic epilepsy | 2 (3.9%) | 1.198 (0.288–4.985) | 0.804 |
| Epileptic encephalopathy | 1 (2%) | 1.471 (0.201–10.743) | 0.704 |
| Development delay | 3 (5.9%) | 0.715 (0.171–2.987) | 0.645 |
| Persistent motor deficits | 2 (3.9%) | 0.044 (0–21.567) | 0.323 |
| Psychiatric abnormalities | 8 (15.7%) | 0.717 (0.281–1.829) | 0.486 |
| Seizure‐free period on therapy (months)b | |||
| 0–23 | 13 (25.5%) | 1.042 (0.472–2.299) | 0.918 |
| 24–35 | 7 (13.7%) | 1.074 (0.421–2.735) | 0.882 |
| 36–47 | 6 (11.8%) | 1.036 (0.382–2.813) | 0.944 |
| 48–59 | 5 (9.8%) | 0.693 (0.202–2.377) | 0.560 |
| 60 or more | 20 (39.2%) | Reference value | |
| Median age at withdrawal (range) | 39 (16–81) | 0.999 (0.982–1.016) | 0.885 |
| EEG before AED withdrawal | |||
| Normal | 25 (49%) | Reference value | |
| Epileptiform abnormality | 1 (2%) | 1.471 (0.196–11.050) | 0.708 |
| Failure of previous AED discontinuations | 4 (7.8%) | 0.867 (0.266–2.822) | 0.812 |
| Number of AEDs discontinued | |||
| One | 49 (96%) | Reference value | |
| Two | 2 (4%) | 0.688 (0.094–5.024) | 0.712 |
| Plasma levels of AEDs at the beginning of withdrawal | |||
| Below range | 7 (13.7%) | 1.021 (0.383–2.723) | 0.967 |
| Within range | 14 (27.5%) | Reference value | |
| Above range | 0 | ||
| Duration of AED tapering | |||
| 0–3 months | 18 (35.3%) | 1.051 (0.519–2.131) | 0.890 |
| 4–12 months | 21 (41.2%) | Reference value | |
| More than 1 year | 0 | ||
| EEG during/at the end of AED withdrawal | |||
| Normal | 34 (66.7%) | Reference value | |
| Epileptiform abnormality | 5 (9.8%) | 1.381 (0.529–3.610) | 0.510 |
| Seizure recurrence | |||
| During AED tapering | 9 (17.6%) | Reference value | |
| At the end of AED withdrawal | 42 (82.4%) | 1.039 (0.460–2.346) | 0.926 |
| Median time to seizure recurrence (days, range) | 304 (30–5840) | 1 (1–1) | 0.762 |
| Median duration of the seizure‐free interval after restarting a treatment (years, range) | 4 (1–33) | ||
| Therapeutic modifications after recurrence | |||
| Same AEDs lower dose | 7 (13.7%) | Reference value | |
| Same AEDs same dose | 13 (25.5%) | 1.064 | 0.895 |
| Same AEDs higher dose | 8 (15.7%) | 0.596 | 0.358 |
| Different monotherapy | 17 (33.3%) | 0.737 | 0.513 |
| Polytherapy | 5 (9.8%) | 0.149 | 0.077 |
| No therapy | 1 (2%) | 0 (0–0) | 0.980 |
Missing data for the variable “EEG before AED withdrawal”: 25 (49%).
Missing data for the variable “Plasma levels of AEDs at the beginning of withdrawal”: 30 (58.9%).
Missing data for the variable “Duration of AED tapering”: 12 (23.5%).
Missing data for the variable “EEG during/at the end of AED withdrawal”: 12 (23.5%).
AED, antiepileptic drug; CI, confidence interval; EEG, electroencephalography; HR, hazard ratio.
The subdivision of age groups is the same as the paper of Lamberink et al2
The subdivision of seizure‐free period on therapy is the same as the paper of Wang et al21
3.3. Retrospective validation of LPM in our cohort
The accuracy of the LPM in our cohort was analyzed via our algorithm at each possible identified threshold probability value, showing that the model has an unsatisfactory accuracy for the risk both at 2 and 5 years. As shown in Figure 3, applying, for instance, a threshold probability value of 0.5 at 2 years, sensitivity would be 40% and specificity 60%, and at 5 years sensitivity would be 60% and specificity 40%. For the prediction risk at 2 years, using a sensitivity level of 75%, at the corresponding threshold value we would have a specificity level of 40%: in 60% of patients, we would predict a false seizure recurrence. If instead, we set a higher specificity, for instance at 80%, the sensitivity would be 20% at the corresponding threshold value, meaning that in 80% of patients a seizure recurrence would not be predicted. With a sensitivity of 90%, a specificity of 10% would have been observed, meaning that a false seizure recurrence would have been predicted in 90% of patients (Figure 3A). The same unsatisfactory risk predictions could be made with reference to 5 years (Figure 3B).
FIGURE 3.
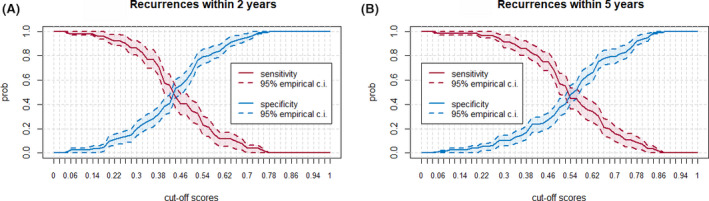
A, Sensitivity and specificity (y‐axis) plotted at different probability threshold values generated by the Lamberink prediction model (LPM) for seizure recurrence risk at year 2. Dashed curves identify the confidence intervals (CIs). At year 2, under these experimental conditions, LPM shows low accuracy. B, Same parameters at year 5. Under these experimental conditions, LPM shows low accuracy also at year 5.
4. DISCUSSION
The current work presents long‐term follow‐up data of an Italian cohort of adult patients with epilepsy who discontinued their AEDs. The frequency of relapses is consistent with previous studies where the range of seizure recurrence was 12%‐67%.2, 12, 13, 20, 21, 30, 31 In particular, in two other external validation papers on LPM, 59%20 and 44%,21 respectively, of patients relapsed.
The risk factors evaluated were the same reported in the previous papers, and definitions of these variables are consistent with them.2, 13, 17, 30 However, to broadly evaluate the potential effect on seizure recurrence risk, patients with different tapering times and different seizure‐free periods on therapy were included.
In this work, a seizure‐free period on therapy of less than 2 years is an independent predictor of increased recurrence risk. This result is coherent with some previous reports,1, 14, 27 whereas others showed either different cut‐offs (5 or 3 years) or progressive reduction of risk without giving any definite cut‐off value.2, 24, 31 Therefore, until more evidence is gathered, it seems important that future studies include patients with different duration of seizure‐free period on therapy.
The results about the disease's natural history after seizure recurrence are encouraging and they should reassure clinicians facing decisions regarding AED withdrawal. The percentage of patients who obtained a new control of epilepsy after recurrence (82.4%) is consistent with other published series (64–91%).2, 12 Ultimately, only 7.8% of our patients developed drug‐resistant epilepsy (other groups have shown results ranging from 7 to 23%).2, 12 Unfortunately, no predictor of the recovery of seizure control could be identified, and there was not sufficient statistical power to analyze the drug‐resistant cohort from the sample size of the study. Very few studies have addressed the issue of epilepsy prognosis after recurrence following AED discontinuation, and there is no evidence that either discontinuation itself or pharmacological treatment of a recurrence are predictors of seizure outcome.12, 29, 32, 33, 34, 35 Other works identified the following negative predictive factors regarding new control: a structural etiology with or without mental delay and a long duration of epilepsy in children, the diagnosis of juvenile myoclonic epilepsy in adolescence, and epilepsy with focal motor seizure with impaired awareness in adults.12, 33
The unsatisfactory accuracy of the LPM in the present cohort is not consistent with the internal validation performed by Lamberink et al.,2 and only in part consistent with the two other external validations of the method published in the past, by Lin et al. and Chu et al.20, 21 This could be due to the different populations included in the analysis or to the sample size. In the present study, the proportion of adults is higher than in the LPM2 as for age both at AED withdrawal (a median of 43 years in this study vs 15 years in LPM work2) and at seizure onset (75.9% of the patients in the present work developed epilepsy at adult age vs 17% of patients in the LPM paper2). The different ages at seizure onset may have determined different frequencies of specific epilepsy syndromes, for example, self‐limiting syndromes (just 1.5% in the present study vs 19% in Lamberink et al.2), which are usually associated with a favorable prognosis. Despite that, the frequency of relapses in this work is consistent with that of the LPM study2 (45% and 46%, respectively). Moreover, in a population similar to that of the original meta‐analysis, the accuracy of LPM was confirmed only as for the risk of seizure recurrence at 5 years.21 Then, the size of the present cohort is similar to the above‐mentioned external validation papers and it should be remarked that the LPM is supposed to be widely applicable in clinical practice.2, 20, 21
A major obstacle to the practical implementation of LPM is represented by the lack of a single threshold probability value that separates high‐risk from low‐risk patients. Lin et al. tried to overcome this issue by a decision curve analysis: This method, unfortunately, did not offer a specific cut‐off but it revealed that the model's usefulness resides in a specific probability range.20 On the contrary, Chu et al. proposed a cut‐off by calculating the largest Youden index on receiver‐operating characteristic (ROC) curves: at 2 years this value was 47% (with a sensitivity of 0.758 and a specificity of 0.410), whereas at 5 years it was 77% (sensitivity of 0.358 and a specificity of 0.979).21 These findings do not seem clinically satisfactory (eg, with a cut‐off value of 77% at 5 years, true seizure recurrence would have not been predicted in 64.2% of patients).21 Moreover, the Youden index is a mathematical way of summarizing the performance of a dichotomous diagnostic test: Its maximum value is often used as a detecting criterion of the optimal cut‐off point.
Instead, in the present paper, a full evaluation of the accuracy of LPM was performed by assessing its sensitivity and specificity for each possible threshold value. In addition, thanks to the bootstrap method, we were able to show the reliability of our measures with their confidence intervals. We aimed to provide clinicians with a complete appraisal of LPM, by allowing them to tailor decisions on a single patient. However, no threshold value was shown to yield sufficient accuracy, indicating that in this patient cohort LPM is inadequate. In fact, it generates either an excessive risk of recurrence if high specificity is chosen and consequently low sensitivity, or an excessively conservative attitude, if the opposite values are selected.
The main limit of our study is the retrospective design and subsequent risk of recall bias, although it was mitigated by verifying the patient referral of seizure recurrence with objective records whenever possible (eg, clinical records of the emergency department). The long timeframe of the retrospective enrollment might have expanded heterogeneity in the cohort because of a modification of the attitude toward AED discontinuation throughout the years. However, in this period, the patients had been consistently followed by the same neurologists, thus maintaining a standard and uniform clinical approach. In addition, recruitment bias cannot be excluded. This could be related to the loss of patients before treatment interruption and, therefore, before inclusion. It cannot be excluded that some patients might have discontinued AEDs according to the advice of other neurologists. However, we think that if such an event occurred, it should have affected the sample size more than having introduced a recruitment bias; in this case, no reason other than chance can be hypothesized for leaving under treatment the largest epilepsy referral center in the metropolitan area. As for the sample size of this paper (133 patients), it is similar to the two external validations20, 21 and large enough to prove a statistically significant association between a seizure‐free period on therapy shorter than 2 years and seizure recurrence. In addition, according to the high severity of the population under treatment in a third‐level clinical center—rarely discontinuing AEDs—the patient numerosity included should be representative.
Large, multicenter, and multiregional studies are nonetheless needed to generate conclusive data about the recurrence risk after AED withdrawal, and to improve the detection of relevant risk factors. As already proposed by others, the poor accuracy of the LPM herein observed suggests that it should be reviewed and integrated with more informative diagnostic tools as covariates, such as neurophysiological markers of the epileptogenic network (eg, scalp‐recorded high‐frequency oscillations [HFOs]).36, 37, 38, 39, 40
5. ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
6 CONFLICT OF INTEREST
Luca Massacesi, MD, received fees for participation in the advisory board, faculty of teaching courses, or scientific consultation from Novartis, Biogen, Roche, Mylan, Merck‐Serono, and Sanofi‐Genzyme. Educational grants were also received from Merck‐Serono, Teva, Sanofi‐Genzyme, Biogen, Novartis, Roche, and Mylan. Eleonora Rosati, MD, received fees for participation in advisory board or scientific consultation from Eisai, GW, Bial, and UCB. The remaining authors have no conflicts of interest to disclose.
Contento M, Bertaccini B, Biggi M, Magliani M, Failli Y, Rosati E, et al. Prediction of seizure recurrence risk following discontinuation of antiepileptic drugs. Epilepsia. 2021;62:2159–2170. 10.1111/epi.16993
REFERENCES
- 1.Strozzi I, Nolan SJ, Sperling MR, Wingerchuk DM, Sirven J. Early versus late antiepileptic drug withdrawal for people with epilepsy in remission. Cochrane Database Syst Rev. 2015;2015:CD001902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamberink HJ, Otte WM, Geerts AT, Pavlovic M, Ramos‐Lizana J, Marson AG, et al. Individualised prediction model of seizure recurrence and long‐term outcomes after withdrawal of antiepileptic drugs in seizure‐free patients: a systematic review and individual participant data meta‐analysis. Lancet Neurol. 2017;16:523–31. [DOI] [PubMed] [Google Scholar]
- 3.Zou X, Hong Z, Chen J, Zhou D. Is antiepileptic drug withdrawal status related to quality of life in seizure‐free adult patients with epilepsy? Epilepsy Behav. 2014;31:129–35. [DOI] [PubMed] [Google Scholar]
- 4.Eddy CM, Rickards HE, Cavanna AE. The cognitive impact of antiepileptic drugs. Ther Adv Neurol Disord. 2011;4:385–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perucca P, Carter J, Vahle V, Gilliam FG. Adverse antiepileptic drug effects: toward a clinically and neurobiologically relevant taxonomy. Neurology. 2009;72:1223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker GA, Jacoby A, Buck D, Stalgis C, Monnet D. Quality of life of people with epilepsy: a European study. Epilepsia. 1997;38:353–62. [DOI] [PubMed] [Google Scholar]
- 7.Jacoby A, Johnson A, Chadwick D. Psychosocial outcomes of antiepileptic drug discontinuation. The Medical Research Council antiepileptic drug withdrawal Study Group. Epilepsia. 1992;33:1123–31. [DOI] [PubMed] [Google Scholar]
- 8.Auriel E, Landov H, Blatt I, Theitler J, Gandelman‐Marton R, Chistik V, et al. Quality of life in seizure‐free patients with epilepsy on monotherapy. Epilepsy Behav. 2009;14:130–3. [DOI] [PubMed] [Google Scholar]
- 9.Carpay JA, Aldenkamp AP, van Donselaar CA. Complaints associated with the use of antiepileptic drugs: results from a community‐based study. Seizure. 2005;14:198–206. [DOI] [PubMed] [Google Scholar]
- 10.Krauss GL, Gondek S, Krumholz A, Paul S, Shen F. “The Scarlet E”: The presentation of epilepsy in the English language print media. Neurology. 2000;54:1894–8. [DOI] [PubMed] [Google Scholar]
- 11.Siqueira NF, Guerreiro MM, de Souza EAP. Self‐esteem, social support perception and seizure controllability perception in adolescents with epilepsy. Arq Neuropsiquiatr. 2011;69:770–4. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt D, Löscher W. Uncontrolled epilepsy following discontinuation of antiepileptic drugs in seizure‐free patients: a review of current clinical experience. Acta Neurol Scand. 2005;111:291–300. [DOI] [PubMed] [Google Scholar]
- 13.Lamberink HJ, Otte WM, Geleijns K, Braun KPJ. Antiepileptic drug withdrawal in medically and surgically treated patients: a meta‐analysis of seizure recurrence and systematic review of its predictors. Epileptic Disord. 2015;17:211–28. [DOI] [PubMed] [Google Scholar]
- 14.Chadwick DW. Randomized study of antiepileptic drug withdrawal in patients in remission. Medical Research Council Antiepileptic Drug Withdrawal Study Group. Lancet. 1991;337:1175–80. [PubMed] [Google Scholar]
- 15.Lossius MI, Hessen E, Mowinckel P, Stavem K, Erikssen J, Gulbrandsen P, et al. Consequences of antiepileptic drug withdrawal: a randomized, double‐blind study (Akershus Study). Epilepsia. 2008;49:455–63. [DOI] [PubMed] [Google Scholar]
- 16.Dooley J, Gordon K, Camfield P, Camfield C, Smith E. Discontinuation of anticonvulsant therapy in children free of seizures for 1 year: a prospective study. Neurology. 1996;46:969–74. [DOI] [PubMed] [Google Scholar]
- 17.Braathen G, Melander H. Early discontinuation of treatment in children with uncomplicated epilepsy: a prospective study with a model for prediction of outcome. Epilepsia. 1997;38:561–9. [DOI] [PubMed] [Google Scholar]
- 18.Geerts AT, Niermeijer JMF, Peters ACB, Arts WFM, Brouwer OF, Stroink H, et al. Four‐year outcome after early withdrawal of antiepileptic drugs in childhood epilepsy. Neurology. 2005;64:2136–8. [DOI] [PubMed] [Google Scholar]
- 19.Prognostic index for recurrence of seizures after remission of epilepsy. Medical Research Council Antiepileptic Drug Withdrawal Study Group. Br Med J. 1993;306:1374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J, Ding S, Li X, Hua Y, Wang X, He R, et al. External validation and comparison of two prediction models for seizure recurrence after the withdrawal of antiepileptic drugs in adult patients. Epilepsia. 2020;61:115–24. [DOI] [PubMed] [Google Scholar]
- 21.Chu S‐S, Tan G, Wang X‐P, Liu L. Validation of the predictive model for seizure recurrence after withdrawal of antiepileptic drugs. Epilepsy Behav. 2021;114:106987. [DOI] [PubMed] [Google Scholar]
- 22.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross H, Elger EC, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–82. [DOI] [PubMed] [Google Scholar]
- 23.Beghi E, Carpio A, Forsgren L, Hesdorffer DC, Malmgren K, Sander JW, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51:671–5. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, He R, Zheng R, Ding S, Wang Y, Li X, et al. Relative seizure relapse risks associated with antiepileptic drug withdrawal after different seizure‐free periods in adults with focal epilepsy: a prospective, controlled follow‐up study. CNS Drugs. 2019;33:1121–32. [DOI] [PubMed] [Google Scholar]
- 25.Tennison M, Greenwood R, Lewis D, Thorn M. Discontinuing antiepileptic drugs in children with epilepsy. A comparison of a six‐week and a nine‐month taper period. N Engl J Med. 1994;330:1407–10. [DOI] [PubMed] [Google Scholar]
- 26.Serra JG, Montenegro MA, Guerreiro MM. Antiepileptic drug withdrawal in childhood: does the duration of tapering off matter for seizure recurrence? J Child Neurol. 2005;20:624–6. [DOI] [PubMed] [Google Scholar]
- 27.Specchio LM, Tramacere L, La Neve A, Beghi E. Discontinuing antiepileptic drugs in patients who are seizure free on monotherapy. J Neurol Neurosurg Psychiatry. 2002;72:22–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinnar S, Berg AT, Moshé SL, Kang H, O’Dell C, Alemany M, et al. Discontinuing antiepileptic drugs in children with epilepsy: a prospective study. Ann Neurol. 1994;35:534–45. [DOI] [PubMed] [Google Scholar]
- 29.Chadwick D, Taylor J, Johnson T. Outcomes after seizure recurrence in people with well‐controlled epilepsy and the factors that influence it. The MRC Antiepileptic Drug Withdrawal Group. Epilepsia. 1996;37:1043–50. [DOI] [PubMed] [Google Scholar]
- 30.Bouma PAD, Peters ACB, Brouwer OF. Long term course of childhood epilepsy following relapse after antiepileptic drug withdrawal. J Neurol Neurosurg Psychiatry. 2002;72:507–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Chen S, Qin J, She Y, Chen Y, Liu X, et al. Risk of seizure recurrence from antiepileptic drug withdrawal among seizure‐free patients for more than two years. Epilepsy Behav. 2020;113:107485. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt D, Sillanpää M. Stopping epilepsy treatment in seizure remission: Good or bad or both? Seizure. 2017;44:157–61. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt D. AED discontinuation may be dangerous for seizure‐free patients. J Neural Transm. 2011;118:183–6. [DOI] [PubMed] [Google Scholar]
- 34.Del Felice A, Beghi E, Boero G, La Neve A, Bogliun G, De Palo A, et al. Early versus late remission in a cohort of patients with newly diagnosed epilepsy. Epilepsia. 2010;51(1):37–42. [DOI] [PubMed] [Google Scholar]
- 35.Somerville ER. Aggravation of partial seizures by antiepileptic drugs: Is there evidence from clinical trials? Neurology. 2002;59:79–83. [DOI] [PubMed] [Google Scholar]
- 36.Boran E, Sarnthein J, Krayenbühl N, Ramantani G, Fedele T. High‐frequency oscillations in scalp EEG mirror seizure frequency in pediatric focal epilepsy. Sci Rep. 2019;9:16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Klink NEC, Van’t Klooster MA, Leijten FSS, Jacobs J, Braun KPJ, Zijlmans M. Ripples on rolandic spikes: a marker of epilepsy severity. Epilepsia. 2016;57:1179–89. [DOI] [PubMed] [Google Scholar]
- 38.Qian P, Li H, Xue J, Yang Z. Scalp‐recorded high‐frequency oscillations in atypical benign partial epilepsy. Clin Neurophysiol. 2016;127:3306–13. [DOI] [PubMed] [Google Scholar]
- 39.Gong P, Xue J, Qian P, Yang H, Liu X, Cai L, et al. Scalp‐recorded high‐frequency oscillations in childhood epileptic encephalopathy with continuous spike‐and‐wave during sleep with different etiologies. Brain Dev. 2018;40:299–310. [DOI] [PubMed] [Google Scholar]
- 40.Sen OC, Yang RC, Wu RC, Chiang CT, Lin LC. Determination of antiepileptic drugs withdrawal through EEG hjorth parameter analysis. Int J Neural Syst. 2020;30:2050036. [DOI] [PubMed] [Google Scholar]


