Abstract
Purpose
This post‐authorisation safety study estimated the risk of anaphylaxis in patients receiving intravenous (IV) iron in Europe, with interest in iron dextran and iron non‐dextrans. Studies conducted in the United States have reported risk of anaphylaxis to IV iron ranging from 2.0 to 6.8 per 10 000 first treatments.
Methods
Cohort study of IV iron new users, captured mostly through pharmacy ambulatory dispensing, from populations covered by health and administrative data sources in five European countries from 1999 to 2017. Anaphylaxis events were identified through an algorithm that used parenteral penicillin as a positive control.
Results
A total of 304 210 patients with a first IV iron treatment (6367 iron dextran), among whom 13–16 anaphylaxis cases were identified and reported as a range to comply with data protection regulations. The pooled unadjusted incidence proportion (IP) ranged from 0.4 (95% confidence interval [CI], 0.2–0.9) to 0.5 (95% CI, 0.3–1.0) per 10 000 first treatments. No events were identified at first dextran treatments. There were 231 294 first penicillin treatments with 30 potential cases of anaphylaxis (IP = 1.2; 95% CI, 0.8–1.7 per 10 000 treatments).
Conclusion
We found an IP of anaphylaxis from 0.4 to 0.5 per 10 000 first IV iron treatments. The study captured only a fraction of IV iron treatments administered in hospitals, where most first treatments are likely to happen. Due to this limitation, the study could not exclude a differential risk of anaphylaxis between iron dextran and iron non‐dextrans. The IP of anaphylaxis in users of penicillin was consistent with incidences reported in the literature.
Keywords: anaphylaxis, cohort study, dextran, IV iron, multidatabase, severe hypersensitivity reactions
1. INTRODUCTION
Intravenous (IV) iron therapy was introduced in the 1950s for the treatment of severe iron deficiency anaemia.1 In the last decades, the use of IV iron has grown worldwide owing to a better understanding of the management of moderate and severe anaemia related to numerous conditions, including chronic kidney disease, heavy uterine bleeding, pregnancy and postpartum anaemia, and chemotherapy‐induced anaemia.2
Anaphylaxis in IV iron treatment is rare. Hypersensitivity reactions in association with IV iron preparations have been reported in the scientific literature, from spontaneous adverse events–reporting studies and population‐based epidemiologic studies.2, 3, 4, 5, 6, 7 Population‐based studies in the United States have reported anaphylaxis risks of 2.0 to 2.4 per 10 000 first IV iron non‐dextran administrations and 4.0 to 6.8 per 10 000 first IV iron dextran administrations.3, 4 Population‐based studies in Europe are lacking.
This study addressed concerns by the European Medicines Agency regarding the risk of anaphylaxis related to IV iron use in routine clinical practice in European populations, with a particular interest in comparing the risk between iron non‐dextrans and dextran‐containing preparations.
The study was registered in the European Union electronic Register of Post‐Authorisation Studies (EUPAS Number: EUPAS20720) and was conducted under the ENCePP Seal.
2. METHODS
2.1. Study design
The study cohort comprised adults from six data sources in five European countries (Table S1, Supplementary Material): Denmark (Danish National and Regional Linked Registries and Databases), France (Système National des Données de Santé [SNDS]), Germany (German Pharmacoepidemiological Research Database [GePaRD] and Board of Trustees for Dialysis and Kidney Transplantation and its Quality in Nephrology programme [KfH QiN]), the Netherlands (PHARMO Database Network [PHARMO‐NL]), and Sweden (Swedish national registers).
Patients who had a first‐recorded IV iron treatment (new users) during the study period and were registered for at least 12 months before the first‐recorded iron treatment were included in the study (Figure 1). The KfH QiN dialysis registry captured medical and treatment information from the date dialysis is initiated; therefore, the 12‐month lookback period did not apply to this data source. Table 1 shows the IV iron compounds studied. A cohort of parenteral penicillin users in some study data sources was used as a positive control to test the case‐identification algorithm. New users were individuals with a first recorded IV iron treatment or IV penicillin without a record of dispensing/administration of these drugs during the 12 months before the cohort entry date (i.e. the date of the first eligible IV iron or IV penicillin treatment). Users with a second and third or subsequent IV iron treatment meeting the inclusion criteria were included to assess the risk beyond the first treatment.
FIGURE 1.

Study inclusion and exclusion criteria
TABLE 1.
Intravenous iron types and groups
| Type of intravenous iron product [abbreviated namea] | Iron group | Country |
|---|---|---|
| Iron sucrose complex [iron sucrose] | Iron non‐dextrans | Denmark, Germany, Netherlands, Sweden |
| Ferric carboxymaltose complex [iron carboxymaltose] | Iron non‐dextrans | Denmark, France, Germany, Netherlands, Sweden |
| Iron(III) isomaltoside complex [iron isomaltoside] | Iron non‐dextrans | Denmark, Germany, Netherlands, Sweden |
| Sodium ferric gluconate complex [iron gluconate] | Iron non‐dextrans | Germany |
| Iron(III)‐hydroxide dextran complex [iron dextran] | Iron dextran | Denmark, Germany, Netherlands, Sweden |
The name of IV iron products has been abbreviated for in‐text use.
The study period (1999–2017) varied across data sources and was defined as the time between the date of the first eligible dispensing/administration (i.e. treatment) of IV iron and the latest date of data availability in the data source. Patients were followed from the cohort entry date until the date of first occurrence of any of the censoring events: study outcome, death, end of study period, switch between types of IV iron (for main analysis) or disenrollment from the data source.
Diagnosis codes for medical conditions were retrieved from outpatient, inpatient, or emergency department encounters by using International Classification of Diseases (ICD), Ninth or Tenth Revisions, or International Classification of Primary Care codes.8 Medications were retrieved mostly from ambulatory pharmacy dispensing and primary care prescriptions and, in some data sources, from inpatient hospitals' data, hospital outpatient specialists' clinics, and administered treatments in dialysis centres. Medications were identified by using the Anatomical Therapeutic Chemical (ATC) Classification System codes and data source–specific codes. 9
2.2. Outcome
Anaphylaxis events were identified through an adaptation of the algorithm consisting of diagnoses, symptoms and treatment codes developed and validated by Walsh et al.10 (Figure 2), which was based on the clinical criteria by Sampson et al.11 Criterion A used only anaphylaxis diagnosis codes. The symptoms, procedures or treatment codes in Criterion B and Criterion C (Figure 2) were used only in conjunction with anaphylaxis diagnostic codes (Criterion B) or allergic reactions (Criterion C). In a sensitivity analysis, the algorithm was expanded to increase its sensitivity (expansions highlighted in boxes in bold italic font in Figure 2). Outcomes were validated through review of medical records of potential cases in Denmark and in the PHARMO‐NL. The algorithm used in GePaRD‐Germany was indirectly validated through confirmation of potential anaphylaxis events due to any trigger (i.e. not restricted to IV iron) by using data from the Oldenburg University Hospital in Germany.
FIGURE 2.

Main and expanded anaphylaxis algorithms. ICD‐10, International Classification of Diseases, Tenth Revision
2.3. Time at risk
For the main analysis, time at risk was Day 0 (the day of administration of a study drug) for data sources capturing drug administration data. For data sources capturing drug dispensing or lacking an exact date of anaphylaxis diagnosis, the time at risk was Day 0 and Day 1 after dispensing/administration of a study drug (Figure 3). In a sensitivity analysis, an extended risk window of 7 days was considered for data sources capturing drug dispensing or lacking an exact date of anaphylaxis diagnosis (Figure 3).
FIGURE 3.
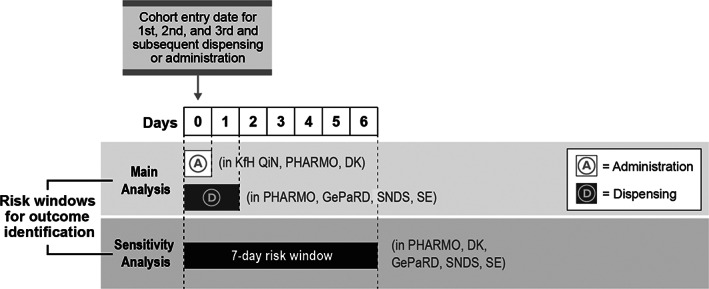
Study follow‐up. DK, Denmark; GePaRD, German Pharmacoepidemiological Research Database; KfH QiN, Board of Trustees for Dialysis and Kidney Transplantation and its Quality in Nephrology programme; PHARMO, PHARMO Database Network; SE, Sweden; SNDS, Système National des Données de Santé (French National Health Care Insurance System Database, previously named SNIIRAM)
2.4. Statistical analysis
Data analyses occurred in two stages: (1) an analysis conducted at each data source and (2) a combined analysis of aggregated data conducted at RTI Health Solutions, the coordinating centre. Descriptive statistics of baseline variables, obtained from the same sources of outcome and exposure data, selected based on their potential for confounding of the association between IV iron treatment and risk of anaphylaxis, were generated for each study cohort.
Incidence proportions (IPs) during the defined time at risk were calculated at each data source as the number of patients with an incident anaphylaxis event divided by the total number of patients/treatments at risk (data not shown). Corresponding 95% confidence intervals (CIs) were derived from the Wilson score method, which has robust coverage for rare events.12 Risk ratio (RR) and risk difference (RD) estimates were calculated, respectively, by dividing and subtracting relevant IP estimates. Corresponding 95% CIs were derived from the Miettinen‐Nurminen method.13 IV iron non‐dextrans were used as the common reference in the IV iron group analyses. Crude pooled analysis and beta‐binomial meta‐regression techniques were employed to integrate the estimates across sources. Beta‐binomial regression methods have been recommended in situations of rare events, particularly when some studies have zero events.14, 15 Beta‐binomial regression was implemented by using the finite mixture model procedure in SAS with default iteration and convergence parameters and the dual quasi‐Newton optimisation technique to obtain maximum likelihood estimates.16 The logit link was used to estimate regression coefficients, and the inverse logit function was applied to these regression coefficients to derive IP point estimates for each compound of interest. For comparative analyses, RR point estimates were derived by dividing corresponding model‐derived IP estimates, and RD point estimates were derived by subtracting corresponding model–derived IP estimates.
Sensitivity analyses were used to calculate the IPs, RRs and RDs of anaphylaxis among the different groups of IV iron compounds assuming different scenarios of risk. These risk scenarios included expansion of the case‐identification algorithm, extension of the risk window from Day 0 until Day 7, risk among IV iron switchers, and risk among IV iron users excluding patients receiving dialysis. Detailed descriptions of these scenarios are presented in Table S1 (Supplementary Material).
For the validation analyses, the positive predictive value (PPV) was computed as the proportion of algorithm‐identified anaphylaxis cases confirmed by medical record review.
For all analyses, owing to the data protection regulations for cell counts below five in Denmark, the exact number of events and IPs for some estimates from the meta‐analyses cannot be disclosed and are reported as minimum and maximum range.
3. RESULTS
3.1. Descriptive data
Overall, 304 210 first IV iron treatments were identified during the study period across all data sources. The number of first IV iron treatments varied from 5825 in PHARMO‐NL to 140 916 in GePaRD‐Germany. IV iron dextran treatments represented 2.1% of all first IV iron treatments (Figure 4). However, in PHARMO‐NL iron dextran represented 41.1% of the first IV iron treatments (Figure 4). There were 148 099 second IV iron treatments across data sources ranging from 1850 treatments in PHARMO‐NL to 67 895 treatments in GePaRD‐Germany (Figure 4). For the third or subsequent IV iron treatments, a total of 3 103 486 treatments in 105 634 patients were identified, of which 2 620 795 (84.4%) were contributed by the KfH QiN dialysis registry and 348 945 (11.2%) from the GePaRD in Germany (Figure 4).
FIGURE 4.
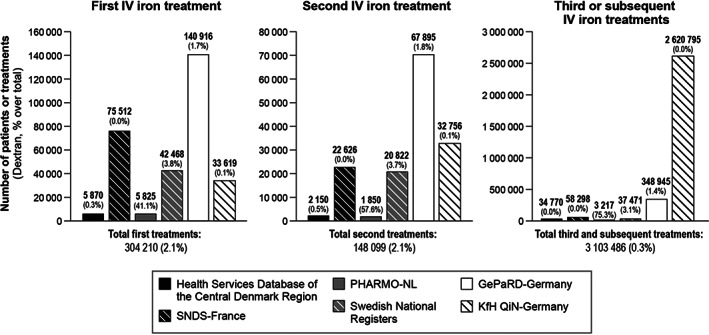
Number of first, second and third or subsequent IV iron treatments stratified by data source and showing the percentage of administrations of iron dextran. Numbers for the Central Denmark Region data were rounded to the nearest 10 to comply with Danish data protection and reporting regulations aimed at prevention of identification of individuals. GePaRD, German Pharmacoepidemiological Research Database; IV, intravenous; KfH QiN, Board of Trustees for Dialysis and Kidney Transplantation and its Quality in Nephrology programme; PHARMO‐NL, PHARMO Database Network in the Netherlands; SNDS, Système National des Données de Santé (French National Health Care Insurance System Database, previously named SNIIRAM)
Selected baseline characteristics of patients by data source are presented in Table S2 (Supplementary Material). The distributions by age and sex were similar in all study populations: mean age (standard deviation) was 57 (19.3) years, 70% were females. The prevalence of the conditions shown in Table 2 varied greatly across study populations, for example, the prevalence of asthma ranged from 1% to 14% and allergies from 3% to 56%, depending on the type of available data (e.g. outpatient diagnoses vs. hospital discharge diagnoses).
TABLE 2.
Selected baseline characteristics of new users of intravenous iron compounds: any intravenous iron by data source
| Characteristics | Danish National and Regional Linked Registries and Databases | SNDS, Francea | PHARMO, Netherlands | Swedish National Registers | GePaRD, Germany | KfH QiN, Germany |
|---|---|---|---|---|---|---|
| Total new users, n | 4817 | 75 680 | 5848 | 42 468 | 153 905 | 33 650 |
| Age at cohort entry date, mean (SD), years | ||||||
| Overall | 57 (19.3) | |||||
| Data source specific | 52 (20) | 57.5 (20.5) | 61 (21) | 54.4 (20.8) | 54.8 (19) | 67.5 (14.9) |
| Female gender, % | ||||||
| Overall | 70 | |||||
| Data source specific | 72 | 69 | 69 | 75 | 73 | 37 |
| Duration of lookback period at cohort entry date, mean (SD), years | 7.7 (2.4) | 2.7 (0.8) | 12.4 (4.5) | 6.5 (2.9) | 5.8 (3.3) | 0.1 (0.5) |
| History of anaphylaxis,b % | 1 | 0.2 | 0.2 | 1 | 1 | 0.1 |
| History of any allergy,b % | 11 | 4 | 3 | 13 | 56 | 3 |
| History of asthma,b % | 7 | 2 | 2 | 7 | 14 | 1 |
| Clinical setting where IV iron was administered at cohort entry, % | ||||||
| Dialysis centre | NA | NA | NA | NA | NA | 100 |
| Other inpatient | 8 | NA | 65 | NAc | NA | NA |
| Outpatient clinic | 92 | 100 | NA | NAc | NA | NA |
| Emergency department | NA | NA | NA | NAc | NA | NA |
| Primary care | NA | NA | 35 | NAc | 100d | NA |
| Gastrointestinal bleeding,e % | 4 | 5 | 6 | 4 | 20 | 3 |
| Genitourinary bleeding (including metrorrhagia), % | 2 | 2 | 3 | 4 | 13 | 3 |
| Chronic kidney disease,f % | 10 | 18 | 11 | 15 | 26 | 100 |
| History of haemodialysis,f % | 1 | 9 | 2 | 1 | 13 | 100 |
| Iron deficiency anaemia,e % | 31 | 21 | 22 | 21 | 46 | 3 |
Abbreviations: GePaRD, German Pharmacoepidemiological Research Database; IV, intravenous; KfH QiN, Board of Trustees for Dialysis and Kidney Transplantation and its Quality in Nephrology programme; NA, not available; PHARMO‐NL, PHARMO Database Network in the Netherlands; SD, standard deviation; SNDS, Système National des Données de Santé (French National Health Care Insurance System Database, previously named SNIIRAM).
Refers to iron carboxymaltose users, the only IV iron compound captured in the SNDS.
Any time before and not including the cohort entry date.
IV iron exposure is captured as dispensed prescriptions; the setting where the drug is administered is not known.
Could be linked either to outpatient care by GP or to specialty physician.
183 days before and including the cohort entry date.
Any time before including the cohort entry date.
3.2. Outcomes
The pooled numbers of potential anaphylaxis events (identified through the main algorithm) and IPs, overall and by iron group (i.e. dextran and non‐dextran), for first IV iron treatments are shown in the first column of Table 3. The number of potential anaphylaxis events, reported as a range to comply with data protection regulations, among patients that had a first exposure to IV iron (N = 304 210 patients) ranged from 13 to 16 events; the IP of anaphylaxis ranged from 0.38 (95% CI, 0.17–0.88) to 0.51 (95% CI, 0.28–0.97) per 10 000 first treatments. All events were identified in iron non‐dextrans. The RD of anaphylaxis between iron dextran and non‐dextrans ranged from −0.44 to −0.55 per 10 000 treatments, favouring the iron dextran. The IP of anaphylaxis for IV penicillins was 1.16 per 10 000 first treatments, based on 30 potential events, whereas at any treatment, the IP was 0.45 per 10 000 treatments (data not shown).
TABLE 3.
Beta‐binomial pooled risk of anaphylaxis after a first IV iron treatment—overall and by IV iron dextran and iron non‐dextrans groups—and parenteral penicillin: main algorithm, expanded algorithm, 7‐day risk window, and exclusion of dialysis patients
| Main analysis | Sensitivity analyses | |||
|---|---|---|---|---|
| Main algorithm | Expanded algorithm | 7‐Day risk window | Exclusion of dialysis patients | |
| Overall IV iron | ||||
| Anaphylaxis events, n a | Min, 13; max, 16 | Min, 19; max, 22 | Min, 24; max, 27 | Min, 13; max, 16 |
| Treatments, n b | 304 210 | 304 210 | 304 210 | 176 261 |
| IP, 95% CI | Min, 0.38 (0.17–0.88); max, 0.51 (0.28–0.97)b | Min, 0.63 (0.38–1.05); max, 2.81 (0.60–13.8)b | Min, 0.74 (0.43–1.29); max, 0.88 (0.56–1.39) | Min, 0.77 (0.41–1.47); max, 1.75 (0.71–4.46) |
| Iron dextran | ||||
| Anaphylaxis events, n | 0 | 3 | 1 | 0 |
| Treatments, n b | 6387 | 6387 | 6387 | 5804 |
| IP, 95% CI | 0 (0 to >9995) | Min, 4.59 (1.43–14.8); max, 4.62 (1.46–14.7) | Min, 1.62 (0.23–11.3); max, 1.61 (0.23–11.2) | Min, 0 (0‐NE); max, 0 (0 to >9995) |
| Iron non‐dextrans | ||||
| Anaphylaxis events, n a | Min, 13; max, 16 | Min, 16; max, 19 | Min, 23; max, 26 | Min, 13; max, 16 |
| Treatments, n b | 297 813 | 297 813 | 297 813 | 170 457 |
| IP, 95% CI | Min, 0.44 (0.16–1.24); max, 0.55 (0.23–1.34) | Min, 0.58 (0.28–1.22); max, 0.70 (0.38–1.31) | Min, 0.77 (0.37–1.62); max, 0.93 (0.50–1.75) | Min, 1.00 (0.42–2.42); max, 1.24 (0.62–2.53) |
| RR, 95% CIc | Min, 0 (0.00 to >9995); max, 0 (0.00 to >9995) | Min, 7.95 (2.05–31.8); max, 6.61 (1.83–24.6) | Min, 2.11 (0.27–17.0); max, 1.74 (0.23–13.4) | Min, 0 (0–NE); max, 0 (0.00 to >9995) |
| RD, 95% CIc | Min, −0.44 (−1.02 to >9995); max, −0.55, (−1.14 to >9995) | Min, 4.02 (0.77–14.3); max, 3.92 (0.68–14.0) | Min, 0.85 (−0.80 to 10.6); max, 0.68 (−0.95 to 10.4) | Min, −1.00 (NE–NE); max, −1.24 (−2.22 to >9995) |
| Penicillin (positive control) | ||||
| Anaphylaxis events, n | 30 | 259 | 48 | NA |
| Treatments, n b | 231 294 | 231 294 | 984 000 | NA |
| IP, 95% CI | 1.16 (0.78–1.73) | 6.45 (4.98–8.42) | 0.53 (0.40–0.71) | NA |
Abbreviations: CI, confidence interval; IP, incidence proportion; IV, intravenous; max, maximum; min, minimum; NA, not applicable; NE, not estimable; RD, risk difference; RR, risk ratio.
The number of events identified in Denmark was between 1 and 4, the exact number cannot be disclosed because of data protection regulations aimed at prevention of identification of individuals. Therefore, number of events and IPs per 10 000 first treatments are reported as minimum and maximum range.
Treatments included the Danish data which were rounded to the nearest 10 to comply with data protection regulations aimed at prevention of identification of individuals.
RRs calculated for iron dextran versus non‐dextrans; RDs calculated for iron dextran minus iron non‐dextrans.
Among patients with second IV iron treatments (N = 148 099 patients), three potential anaphylaxis events were identified, for an IP of anaphylaxis of 0.25 per 10 000 second treatments (Table 4). One event was identified among iron dextran and two events among iron non‐dextrans. The estimated RR of anaphylaxis with iron non‐dextrans as comparator was 13.1 and the RD was 3.08 per 10 000 second treatments, favouring iron non‐dextrans. None of the patients with a second or third IV iron exposure had an anaphylaxis reaction to an earlier dose.
TABLE 4.
Main results for second and third and subsequent IV iron treatments
| Second treatments | Third and subsequent treatments | |
|---|---|---|
| Overall IV iron | ||
| Treatments (patients)a | 148 099 | 3 103 486 (105 634) |
| Anaphylaxis events (n)b | 3 | 10 |
| IP (95% CI)b | 0.25 (0.07–0.94) | 0.02 (0.00–0.13) |
| Iron dextran | ||
| Treatmentsa | 3084 | 9508 |
| Anaphylaxis events (n)b | 1 | 0 |
| IP (95% CI)b | 3.33 (0.48–23.3) | 0 (0 to >9995) |
| Iron non‐dextrans | ||
| Treatmentsa | 145 015 | 3 093 988 |
| Anaphylaxis events (n)b | 2 | 10 |
| IP (95% CI)b | 0.25 (0.06–1.06) | 0.03 (0.00–0.19) |
| RR (95% CI)c | 13.1 (1.26–146) | 0 (0 to >9995) |
| RD (95% CI)c | 3.08 (0.12–23.1) | −0.03 (−0.13 to >9995) |
Abbreviations: CI, confidence interval; IP, incidence proportion; IV, intravenous; RR, risk ratio; RD, risk difference.
Treatments included the Danish data which were rounded to the nearest 10 to comply with data protection regulations aimed at preventing the identification of individuals.
The number of events identified in Denmark was between 1 and 4. The exact number cannot be disclosed because of data protection regulations aimed at preventing the identification of individuals. Therefore, IPs per 10 000 first treatments are reported as a minimum and maximum range.
RRs were calculated for iron dextran vs. non‐dextrans; RDs were calculated for iron dextran minus iron non‐dextrans.
For third or subsequent IV iron treatments (N = 3 103 486 treatments), 10 potential events were identified for an IP of anaphylaxis of 0.02 per 10 000 third or subsequent treatments (Table 4). All events were found among iron non‐dextrans. The RD for iron dextran minus iron non‐dextrans was −0.03 per 10 000 third or subsequent treatments in favour of iron dextran.
The low number of events identified in this study precluded the conduct of adjusted analyses and the interpretation of the results based on groups and types of IV iron.
3.3. Sensitivity analyses
Results of the sensitivity analyses are presented in Table 3. The expanded case‐identification algorithm identified between 19 and 22 potential anaphylaxis events among first IV iron treatments (i.e. 6 additional events compared with the main algorithm), yielding an IP ranging from 0.63 (95% CI, 0.38–1.05) to 2.81 (95% CI, 0.60–13.8) per 10 000 first iron treatments. For the 7‐day risk window scenario, between 24 and 27 anaphylaxis events were identified at first IV iron treatment (i.e. 11 additional events compared with the main risk window), yielding an IP ranging from 0.74 (95% CI, 0.43–1.29) to 0.88 (95% CI, 0.56–1.39) per 10 000 first iron treatments. In the analysis that excluded dialysis patients, between 13 and 16 potential anaphylaxis events were identified in first IV iron treatments, resulting in an IP ranging from 0.77 (95% CI, 0.41–1.47) to 1.75 (95% CI, 0.71–4.46) per 10 000 first iron treatments. When assessing the risk after switching between IV iron groups, no anaphylaxis occurred after a switch from an iron dextran to an iron non‐dextran. However, two potential anaphylaxis events occurred after a first switch from an iron non‐dextran to an iron dextran for an IP of 32.9 per 10 000 first switches (data not shown).
3.4. Validation
The direct validation of the case‐identification algorithms in Denmark yielded a PPV of 70% (95% CI, 50%–86%) based on 42 evaluable potential cases combined across the IV iron and IV penicillin cohorts (cases in the penicillin cohort accounted for more than 90% of all potential cases validated).
In PHARMO‐NL, one evaluable potential anaphylaxis event identified through the main algorithm in the IV penicillin cohort was confirmed: PPV was 100% (95% CI, 2.5%–100%). The expanded algorithm based on 10 evaluable potential cases showed a PPV of 10% (95% CI, 0.25%–45%).
The indirect external validation of the main case‐identification algorithm used in GePaRD‐Germany, showed a PPV of 62.3% (95% CI, 49.8%–73.7%) based on 78 patients with potential anaphylaxis events due to any trigger identified through specific anaphylaxis diagnostic codes captured in the in‐hospital setting at Oldenburg University Hospital in Germany (presented in Figure 2) and 43 confirmed events. No potential outpatient events were identified.
4. DISCUSSION
This study identified 304 210 patients with a first IV iron treatment; 6367 (2.1%) first treatments were iron dextran. The overall IP of anaphylaxis among IV iron users ranged from 0.38 (95% CI, 0.17–0.88) to 0.51 (95% CI, 0.28–0.97) per 10 000 first treatments, corresponding to the maximum and the minimum of the true (masked) number of cases. The IPs of anaphylaxis among repeat users were 0.25 per 10 000 for second treatments and 0.02 per 10 000 for third or subsequent treatments (the latter mostly in dialysis patients). Data on dosing of IV iron was not available. However, for anaphylaxis, dose is not considered critical.17
The first‐use estimates are lower than those reported in the U.S. studies: 2.4 and 6.8 per 10 000 first treatments (IV iron non‐dextrans and iron dextran, respectively) in Wang et al.4 or those by Walsh et al.3: 2.0 and 4.0 per 10 000 first treatments (IV iron non‐dextrans and iron dextran, respectively). One reason for the observed differences in the incidence of anaphylaxis between our study and the U.S. studies3, 4 may be that repeated IV iron use was, potentially, misclassified as new use in our study. The underlying assumption is that the first treatment with IV iron carries the highest risk of anaphylaxis because subsequent treatments are likely to be avoided in patients with a prior hypersensitivity reaction. In our study, the identification of first IV iron treatment was affected by the limited capture of hospital use of IV iron, the setting where first administrations of this drug are most likely to happen. Indeed, data from Sweden suggest that 50%–80% of IV iron treatments occur in hospital.18 In contrast, the U.S. studies3, 4 had ascertainment of treatment with IV iron, irrespective of administration setting, and could therefore determine new‐user status more accurately. However, in Wang et al.,4 the incidence of fatal anaphylaxis among users of IV iron dextran was lower than that among users of IV iron non‐dextrans. This could relate to a differential misclassification of anaphylaxis by type of IV iron and/or to differences in baseline characteristics of users across different IV iron types.
A large proportion (84%) of all third or subsequent IV iron treatments were identified through the KfH QiN dialysis registry in Germany, reflecting the need for repeated iron use in patients undergoing dialysis.
Both U.S. studies excluded dialysis patients. Our study included dialysis patients in the main analysis. However, we conducted a sensitivity analysis excluding dialysis patients to account for the different patterns (i.e. chronic) of use of IV iron and the impossibility of ascertaining new‐user status among these patients, especially in the KfH QiN dialysis registry. This sensitivity analysis showed an IP of anaphylaxis among first IV iron treatments ranging from 0.77 to 1.75 per 10 000 first treatments (compared with a range from 0.38 to 0.51 per 10 000 first IV iron treatments when dialysis patients were included in the main analysis), consistent with a reduced misclassification of first treatment.
Other sensitivity analyses such as the expanded case‐identification algorithm and the 7‐days risk window yielded RRs >1 when comparing the risk of anaphylaxis for iron dextran versus iron non‐dextrans (Table 3); however, these analyses were based on very few cases, all of which had important validity concerns, and therefore, conclusions cannot be drawn.
Another reason to explain the lower risk of anaphylaxis in our study compared with U.S. studies relates to a potential underascertainment of anaphylaxis events. While underascertainment remains a possibility, we think it is unlikely to play a major role because we used an adapted case‐identification algorithm developed and validated by Walsh et al.3 Moreover, the risk of anaphylaxis in our positive control—the penicillin cohort (1.16 per 10 000 first treatments)—was consistent with the published estimates (ranging from 0.1 to 5 per 10 000). In our opinion, this evidence supports the adequateness of the case‐identification algorithm used in our study.
The low number of potential anaphylaxis events identified despite the use of multiple large, population‐based data sources prevented the conduct of adjusted analyses. Beta‐binomial regression meta‐analyses were undertaken instead, which account for the weight of each data source but may be subject to confounding. Differences in risk of anaphylaxis between IV iron types in Europe could be assessed if enough data on first IV iron administration become available.
5. CONCLUSIONS
This study found IPs of anaphylaxis per 10 000 first treatments across all IV iron types ranging from 0.38 (95% CI, 0.17–0.88) to 0.51 (95% CI, 0.28–0.97) and from 0.44 to 0.55 for iron non‐dextrans; IPs were not assessable for iron dextran as no events were identified. These IPs were lower than the estimates of 2 and 6.8 per 10 000 first treatments (IV iron non‐dextrans and iron dextran, respectively) reported in studies in the United States.
Our study identified a large number of IV iron and IV penicillin users in Europe, but it captured only a small fraction of treatments in in‐hospital and specialty clinics, the settings where the most first use of these drugs is likely to happen. Due to this data capture limitation, the study could not exclude a differential risk of anaphylaxis between iron dextran and iron non‐dextrans. However, the results are reassuring for repeat users of IV iron in the ambulatory setting.
CONFLICT OF INTEREST
The study was funded by a consortium of IV iron marketing authorisation holders and was conducted under a contract including the ENCePP Seal granting the research team independent publication rights.
ETHICS STATEMENT
The study was determined by the RTI International institutional review board as research not involving human subjects (RTI‐HS had no interaction with human subjects). Approvals or notifications were obtained/processed from the ethics committees and other bodies as applicable, by participating research centres that contributed to the study according to the applicable requirements for access to data and analysis.
Supporting information
Table S1 Risk scenarios used for sensitivity analyses.
Table S2. Selected characteristics and outcome and variable assessment in study data sources.
ACKNOWLEDGEMENTS
The authors acknowledge the following people for their contributions to this study: Kay Johannes and Nuria Riera, of RTI Health Solutions, for epidemiologic contributions; Brian Samsell, of RTI‐Health Solutions, for technical support; Zoltan Thinsz, of the Centre for Pharmacoepidemiology, Karolinska Institutet, for project and data management support; Alina Ludewig and Inga Schaffer, of the Leibniz Institute for Prevention Research and Epidemiology–BIPS, for data extraction and statistical programming; Federica Edith Pisa, of BIPS, for epidemiologic contributions; Christian F. Christiansen, of Aarhus, for clinical expertise; Uffe Heide‐Jørgensen, at Aarhus University, for statistical assistance of the Danish data; Henriette Kristoffersen and Pia Kjær Kristensen, at Aarhus University, for validation; Irene Bezemer, of PHARMO Institute for Drug Outcomes Research, for project management assistance; C. Kathan‐Selck, of Oldenburg University, for project management assistance; Sanny Kappen, of Oldenburg University, for extracting data for validation; Dominik de Sordi, of Oldenburg University, for data validation analysis; Jan Willem van der Velden, for early scientific orientation and coordination of the effort, and Kathleen Walsh, Principal Investigator of the U.S. Sentinel Study on the risk of IV iron and anaphylaxis, for guidance with the study design and case validation approach. This study, mandated by the European Medicines Agency, was funded by a consortium of IV iron manufacturing companies through a contract with RTI Health Solutions (RTI‐HS), a non‐profit independent research institution that funds all other participating research centres and members of the SAB. This funding also included RTI‐HS medical writing team support for manuscript styling and submission.
Appendix A.
IV Iron Consortium
The Intravenous Iron Consortium is a consortium of 17 iron manufacturing companies sponsoring this Joint post‐authorisation safety study:
Nuno Rodrigues, PharmD, Accord Healthcare Limited; Eva Kopecna, MD, Global Head of Regulatory Affairs, Medical and Pharmacovigilance Acino AG; Sophie Seguin, PharmD, Responsable Pharmacovigilance et Information médicale Arrow Génériques; Örjan Mortimer, MD, EU Qualified Person for Pharmacovigilance (QPPV) Baxter; Rita Ramos, PharmD, Generis Farmacéutica S.A.; Carmen Cortina, MD, and Francisco Ledo, MD, R&D Director, Altan Pharmaceuticals S.A.U; Marian Coquel, Pharm., EU QPPV, Laboratoires Sterop NV; Dieter Fritsch, MD, Pharmacovigilance Manager, Deputy QPPV, Medice Arzneimittel Pütter GmbH & Co. KG; Rachid Sahnoun, MD, Senior Director Pharmacovigilance, Mylan S.A.S.; Lisbeth Aagard Hansen, MSc, Orifarm Generics A/S; Thomas Lajugie, MD, EI‐QPPV/Head of Pharmacovigilance, Panmedica (Panpharma S.A.); Sigal Kaplan, PhD, Director, Pharmacoepidemiology Leader, Pharmachemie BV (Teva); Lars Lykke Thomsen, MD, PhD, DMSc, Chief Medical Officer, Pharmacosmos A/S; Niki Orkopoulou, BSc, Pharmacovigilance Manager/Deputy QPPV, Rafarm S.A.; Stella Böhmert, MD, Head of Global Postmarketing Studies, Sandoz S.A.S.; Denis Granados, MD, MPH, Pharmacoepidemiology Head General Medicine and Consumer Healthcare, Sanofi Aventis Groupe; and Marianne GG Valk‐Cortenraad, MD, EU/International QPPV, Vifor Pharma Nederland BV.
Fortuny J, von Gersdorff G, Lassalle R, et al. Use of intravenous iron and risk of anaphylaxis: A multinational observational post‐authorisation safety study in Europe. Pharmacoepidemiol Drug Saf. 2021;30(10):1447-1457. 10.1002/pds.5319
Intravenous (IV) Iron Consortium members of IV iron manufacturing companies are provided in the Appendix.
Jacques Bénichou, Andreas J. Bircher, E. Garbe and D. S. Rampton are the members of the Scientific Advisory Board (SAB).
Funding information Consortium of IV Iron manufacturing companies
REFERENCES
- 1.Auerbach M, Ballard H. Clinical use of intravenous iron: administration, efficacy, and safety. Hematology Am Soc Hematol Educ Program. 2010;2010:338‐347. [DOI] [PubMed] [Google Scholar]
- 2.Bailie GR, Verhoef JJ. Differences in the reporting rates of serious allergic adverse events from intravenous iron by country and population. Clin Adv Hematol Oncol. 2012. Feb;10(2):101‐108. [PubMed] [Google Scholar]
- 3.Walsh K, Andrade S, Cocoros N, et al. Sentinel assessment report: parenteral iron and anaphylactoid reactions. US Food and Drug Administration; 22 July 2016. https://www.sentinelinitiative.org/sites/default/files/surveillance‐tools/routine‐querying/Sentinel_Parenteral‐Iron‐and‐Anaphylactoid‐Reactions_Report.pdf. Accessed 6 October 2020. [Google Scholar]
- 4.Wang C, Graham DJ, Kane RC, et al. Comparative risk of anaphylactic reactions associated with intravenous iron products. JAMA. 2015;314(19):2062‐2068. [DOI] [PubMed] [Google Scholar]
- 5.Chertow GM, Mason PD, Vaage‐Nilsen O, Ahlmen J. On the relative safety of parenteral iron formulations. Nephrol Dial Transplant. 2004;19(6):1571‐1575. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Mason PD, Vaage‐Nilsen O, Ahlmen J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21(2):378‐382. [DOI] [PubMed] [Google Scholar]
- 7.Bailie GR, Clark JA, Lane CE, Lane PL. Hypersensitivity reactions and deaths associated with intravenous iron preparations. Nephrol Dial Transplant. 2005;20(7):1443‐1449. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . International Statistical Classification of Diseases and Related Health Problems (ICD‐10), 10th Revision. 2016. https://icd.who.int/browse10/2016/en. Accessed 17 August 2020.
- 9.World Health Organization . ATC Classification Index with DDDs. 2019. https://www.whocc.no/atc_ddd_index_and_guidelines/atc_ddd_index/. Accessed 17 August 2020.
- 10.Walsh KE, Cutrona SL, Foy S, et al. Validation of anaphylaxis in the Food and Drug Administration's Mini‐Sentinel. Pharmacoepidemiol Drug Saf. 2013. Nov;22(11):1205‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampson HA, Munoz‐Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391‐397. [DOI] [PubMed] [Google Scholar]
- 12.Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16(2):101‐133. [Google Scholar]
- 13.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4(2):213‐226. [DOI] [PubMed] [Google Scholar]
- 14.Kuss O. Statistical methods for meta‐analyses including information from studies without any events‐add nothing to nothing and succeed nevertheless. Stat Med. 2015;34(7):1097‐1116. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Chu H, Mazumdar M. Meta‐analysis of proportions of rare events—a comparison of exact likelihood methods with robust variance estimation. Commun Stat Simul Comput. 2016;45(8):3036‐3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broyden CG. Quasi‐Newton methods. In: Murray W, ed. Numerical Methods for Unconstrained Optimization. London: Academic Press; 1972:87‐106. [Google Scholar]
- 17.Park B, Kitteringham N, Powell H, Pirmohamed M. Advances in molecular toxicology—towards understanding idiosyncratic drug toxicity. Toxicology. 2000;153(1‐3):39‐60. [DOI] [PubMed] [Google Scholar]
- 18.Swedish Pharmaceutical Statistics. Welcome to the Swedish eHealth Agency. 19 May 2020. https://www.ehalsomyndigheten.se/om-oss/lakemedelsstatistik/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Risk scenarios used for sensitivity analyses.
Table S2. Selected characteristics and outcome and variable assessment in study data sources.


