Abstract
Aim
To identify invasive dental procedures as a risk factor for postoperative spinal infection (PSI) and evaluate the effectiveness of antibiotic prophylaxis.
Materials and Methods
We analysed 229,335 patients who underwent spinal surgery with instrumentation from 2010 to 2017, using the nationwide database. The incidence of spinal infection 2 years after surgery was determined. Invasive dental procedures as a risk factor for PSI and the effects of antibiotic prophylaxis during this period were also analysed.
Results
A total of 15,346 patients (6.69%) were diagnosed with PSI. It was found that advanced age, male sex, and a high Charlson Comorbidity Index were risk factors for PSI. The risk of PSI did not increase following dental procedures (adjusted hazard ratio [HR] 0.850; 95% confidence interval [CI], 0.793–0.912) and was not affected by antibiotics (adjusted HR 1.097; 95% CI, 0.987–1.218). Patients who received dental treatment as early as 3 months after spinal surgery had the lowest risk of postoperative infection (adjusted HR 0.869; 95% CI, 0.795–0.950).
Conclusions
Invasive dental procedure does not increase the risk of PSI, and antibiotic prophylaxis before dental procedure was not effective in preventing spinal infection.
Keywords: antibiotic prophylaxis, invasive dental procedure, nationwide database, postoperative spinal infection, spinal surgery
Clinical Relevance.
Scientific rationale for study: Antibiotic prophylaxis before invasive dental procedure has been dependent only on expert opinion until now. This study used the nationwide database to investigate whether invasive dental procedure is a risk factor of postoperative spinal infection and whether antibiotic prophylaxis is effective.
Principal findings: Invasive dental procedure was not identified as a risk factor for postoperative infection after spinal surgery with instrumentation. In addition, it was identified that antibiotic prophylaxis for dental procedure was not effective in preventing postoperative infection.
Practical implications: These findings will help establish an evidence‐based guideline for dental treatment in patients undergoing spinal surgery.
1. INTRODUCTION
Invasive dental procedures that manipulate gingival tissue cause temporary bacteraemia and are known as risk factors for infective endocarditis (Wilson et al., 2007). Although it is controversial whether antibiotic administration can effectively prevent infective endocarditis after dental procedures (Hirsh et al., 1948; van der Meer et al., 1992; Lockhart & Durack, 1999), the American Heart Association (AHA) guidelines (2017) recommend antibiotic prophylaxis for dental procedures in high‐risk patients with prosthetic cardiac valves, a history of infective endocarditis, or congenital heart disease (Wilson et al., 2007). There are several known pathogeneses describing why patients with prosthetic valve are at high risk of developing endocarditis. It has been suggested that tissue damage after surgery, turbulent blood flow due to implants, and bacterial aggregation on the implant surface may contribute to an increased risk of endocarditis (Nord & Heimdahl, 1990; Wang et al., 2018).
Spinal infections are mostly caused by hematogenous spread (Gouliouris et al., 2010; Lener et al., 2018). In patients who have undergone spinal fusion surgery, the implant is usually not removed after complete fusion, except in certain circumstances such as infection and the occurrence of implant‐related pain (Stavridis et al., 2010). Therefore, it can be hypothesized that bacteria adhering to the implant might cause spinal infection if bacteraemia occurs after invasive dental procedures, similar to the development of endocarditis after dental treatment in patients with prosthetic valves (Kasliwal et al., 2013; Yin et al., 2018).
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) proposed a guideline in 2012 for the prevention of orthopaedic implant infection after dental procedures (Hamedani, 2013). They recommended against prescribing prophylactic antibiotics before dental procedures and advised oral hygiene. However, this guideline can be applied only to patients with hip and knee prosthetic joint implants, and an evidence‐based guideline for cases of spinal surgery with instrumentation has not been proposed yet (Martin et al., 2020). There have been no objective studies on the effectiveness of antibiotic prophylaxis in these patients. There has been only one expert survey that revealed that most doctors did not recommend the routine use of prophylactic antibiotics for invasive dental procedures after spinal fusion (Lewkonia et al., 2016).
Therefore, this study aimed to identify whether invasive dental procedures are risk factors for postoperative spinal infection (PSI) and to evaluate the effect of antibiotic prophylaxis following dental procedures using a nationwide database.
2. MATERIALS AND METHODS
This study was approved by the institutional review board of the corresponding author's hospital (4‐2019‐0915). The requirement for informed consent was waived as the study involved the retrospective use of anonymized and publicly available data.
2.1. Sources of data and data selection
The National Health Insurance (NHI) system of South Korea covers almost the entire national population (over 98%) (Kim et al., 2014). The Health Insurance Review and Assessment (HIRA) Service is a public agency that evaluates the appropriateness of insurance claims. The claims data collected by HIRA comprise extensive and detailed information of insured patients, including the data of not only prescribed medications and performed procedures but also diagnoses, demographic data, and comorbidities (Kim et al., 2014).
Insurance claims data of patients aged over 50 years who underwent spinal surgeries with instrumentation from 1 January 2010 to 31 December 2017 were collected. The procedural codes for patient screening used in this study are described in Table 1. Patients who underwent invasive dental procedures within the 12 weeks prior to spinal surgery were excluded, to rule out the possibility that temporary bacteraemia caused by dental procedures could affect the occurrence of postoperative infection (Chen et al., 2015). In addition, a wash‐out period of at least 2 years before surgery was set to exclude patients with spinal infections during this period; patients who had undergone multiple spinal surgeries were also excluded (Figure 1).
TABLE 1.
Korea Informative Classification of Diseases procedural codes for spinal surgeries with instrumentation in the study
| Level | Procedural codes | Procedures |
|---|---|---|
| Cervical | N2463, N2462, N2461, N0464 | Arthrodesis of spine—cervical spine (anterior technique) |
| N0451 | Vertebral corpectomy (cervical spine) | |
| N2469, N2468, N2467 | Arthrodesis of spine—cervical spine (posterior technique) | |
| N2491, N2492 | Cervical spine laminoplasty | |
| Thoracic | N2466, N2465, N2464 | Arthrodesis of spine—thoracic spine (anterior technique) |
| N0452 | Vertebral corpectomy (thoracic spine) | |
| N0468 | Arthrodesis of spine—thoracic spine (posterior technique) | |
| Lumbo‐sacral | N0466, N1466 | Arthrodesis of spine—lumbar spine (anterior technique) |
| N0453 | Vertebral corpectomy (lumbar spine) | |
| N1460, N2470 | Posterior lumbar interbody fusion | |
| N0469, N1469 | Arthrodesis of spine—lumbar spine (posterior technique) | |
| Multilevel | N0444, N0445 | Arthrodesis for spinal deformity (anterior technique) |
| N0446, N0447 | Arthrodesis for spinal deformity (posterior technique) | |
| When procedural codes of two or more sites are registered at the same surgery | ||
FIGURE 1.
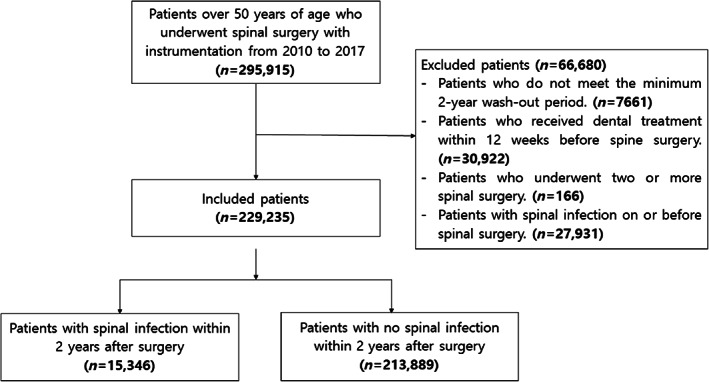
Diagram representing the nationwide scoliosis investigation based on data from the Health Insurance Review and Assessment service
2.2. Identification of invasive dental procedures and antibiotic prophylaxis
Dental procedures were considered “invasive” if they could cause temporary bacteraemia by inducing oral cavity bleeding or involving manipulation of the gingival or periapical region of the teeth or perforation of the oral mucosa, based on the guidelines the European Society of Cardiology and AHA (Table 2) (Wilson et al., 2007; Habib et al., 2015). Use of antibiotic prophylaxis before the dental procedure was confirmed through the registered prescription code. A dental procedure with antibiotic prophylaxis was defined when an antibiotic recommended by the AHA guidelines was prescribed by the dentist who performed the dental procedure on the day of the procedure or within a week before the dental procedure (Wilson et al., 2007). Those who were prescribed with postoperative antibiotics were excluded.
TABLE 2.
Korea Informative Classification of Diseases procedural codes of invasive dental procedures included in the study
| Procedures | Code(s) | |
|---|---|---|
| Non‐surgical periodontal and endodontic treatment | One‐visit endodontics | U0001, U0002, U0074, U0075 |
| Removal of fractured tooth fragment | U0012 | |
| Access cavity preparation | U0050 | |
| Root canal enlargement | U0116 | |
| Pulpotomy | U0090 | |
| Pulp extirpation | U0101 | |
| Emergency pulp treatment | U0210 | |
| Scaling and root planing | U2232, U2233, U2240 | |
| Surgical periodontal and endodontic treatment | Subgingival curettage | U1010 |
| Excisional new attachment procedure | U1020 | |
| Gingivoplasty | U1030 | |
| Gingivectomy | U1040 | |
| Periodontal flap operation | U1051, U1052 | |
| Bone graft for alveolar bone defects | U1071, U1072, U1073 | |
| Guided tissue regeneration | U1081, U1082, U1083 | |
| Removal of barrier membrane | U1090 | |
| Laterally positioned flap, coronally positioned flap | U1100 | |
| Gingival graft | U1110 | |
| Root resection | U1131, U1132 | |
| Crown lengthening | UY101, UY102, UY103 | |
| Bicuspidization | UX102 | |
| Gingival depigmentation | UZ111 | |
| Aesthetic crown lengthening | UZ112, UZ113 | |
| Apicoectomy | U4591, U4592 | |
| Oral surgery | Tooth extraction | U4411, U4412, U4413, U4414, U4415, U4416, U4417 |
| Recurrettage of extracted socket | U4420 | |
| Alveoloplasty | U4430 | |
| Intraoral antiphlogosis | U4454, U4455, U4456, U4457 | |
| Extraoral antiphlogosis | U4464, U4465, U4467 | |
| Closure of intraoral laceration | U4474, U4475, U4476, U4477 | |
| Buccal and labial frenectomy | U4501, U4502 | |
| Lingual frenectomy | U4511, U4512 | |
| Incision of peri‐tonsillar abscess | U4520 | |
| Surgery of osteomyelitis of mandible or maxilla | U4533, U4534, U4535 | |
| Operation of ameloblastoma | U4551, U4552, U4553 | |
| Radicular cyst enucleation | U4561, U4562, U4563, U4564 | |
| Intermaxillary fixation | U2320 | |
| Orofacial fistula closure | U4610 | |
| Oroantral fistula closure | U4621, U4622 | |
| Replantation | U4630 | |
| Reconstruction of mandible | U4640 | |
| Operculectomy | U4660 | |
| Excision of lesion or benign tumour of gingiva or alveolar portion | U4670 | |
| Reduction of luxated teeth | U4690 | |
| Open reduction of alveolar fracture | U4721, U4722 | |
| Excision of torus | U4731, U4732 | |
| Reduction of zygomatic bone fracture | U4741, U4742 | |
| Corrective osteotomy of malunioned zygomatic bone | U4750 | |
| Coronoidectomy | U4760 | |
| Open reduction of maxillary fracture | U4781, U4782, U4783 | |
| Circumzygomatic suspension wiring | U4784 | |
| Craniomaxillary suspension wiring | U4785 | |
| Maxillectomy | U4791, U4792 | |
| Resection of benign tumour of maxilla | U4801, U4802 | |
| Resection of malignant tumour of maxilla | U4811, U4812 | |
| Closed reduction of mandibular fracture | U4830 | |
| Open reduction of mandibular fracture | U4841, U4842 | |
| Circummandibular wiring | U4843 | |
| Corrective osteotomy of mal‐unioned mandibular fracture | U4850 | |
| Mandibulectomy | U4861, U4862 | |
| Resection of benign tumour of mandible | U4871, 4872, U4873 | |
| Resection of malignant tumour of mandible | U4881, U4882, U4883 | |
| Open reduction of temporomandibular joint dislocation | U4910 | |
| Temporomandibular joint meniscoplasty | U4930 | |
| Arthroplasty of temporomandibular joint | U4940 | |
| Substitution of temporomandibular Joint | U4950 | |
| Mandibular condylectomy | U4960 | |
| Removal of implant for internal fixation | U4971, U4972, U4973, U4974, U4975 | |
| Dental implant removal | U4981, U4982 | |
| Osteoplasty of the jaw | UY042, UY043, UY044, UY045, UY046, UY047, UY048 | |
| Surgical uncovering | UX041 | |
| Temporomandibular joint arthrocentesis | UX044 | |
| Corticotomy for orthodontic treatment | UZ081 | |
| Tooth autotransplantation | UZ082 | |
| Autogenous bone graft | UZ083 |
2.3. Incidence and risk factors of postoperative infection after spinal surgery
Although there is no standardized definition of postoperative infection in patients undergoing spinal surgery with instrumentation, many studies have considered infection occurring within 1 year of surgery as postoperative infection (Aydinli et al., 1999; Kasliwal et al., 2013). The diagnosis of spinal infection is often delayed because symptoms usually appear slowly and diagnosis is not easy (Collins et al., 2008; Gerometta et al., 2012). One‐quarter of surgical site infections were reported after more than 1 year after surgery (Collins et al., 2008). In this study, a period of 2 years after spinal surgery was set as the follow‐up period to include delayed infections and determine the causal relationship between postoperative infection and dental procedures. Patients registered with the diagnostic codes shown in Table 3 were assumed to have spinal infection.
TABLE 3.
Korea Informative Classification of Disease diagnostic codes for postoperative spinal infection included in the study
| Diagnostic codes | Diagnosis |
|---|---|
| M00.08, M00.18, M00.28, M00.98 | Pyogenic arthritis, osteomyelitis, vertebral column |
| M00.09, M00.19, M00.29, M00.99 | Pyogenic arthritis, osteomyelitis, site unspecified |
| M86.0–M86.9 | Osteomyelitis |
| M46.20–M46.29 | Osteomyelitis of vertebra |
| M46.30–M46.39 | Infection of intervertebral disc (pyogenic) |
| M46.50–M46.59 | Other infective spondylopathies |
| T81.4 | Infection following a procedure |
| T84.2–T84.9, T85.7 | Infection and inflammatory reaction due to internal orthopaedic prosthetic devices |
Several risk factors were evaluated to identify the factors associated with postoperative infection after spinal surgery with instrumentation. The Charlson Comorbidity Index (CCI) was used to identify underlying diseases. To identify the relationship between dental treatment and postoperative infection, we investigated whether patients who underwent spinal surgery received dental treatment within 1 year of surgery. If they did, we examined the time from spinal surgery to the first dental treatment and whether they received antibiotic prophylaxis. Time‐dependent Cox regression analysis was performed to identify the variables associated with an increased risk of PSI, including dental treatment and antibiotic prophylaxis.
2.4. Statistical analysis
The data were checked for normality, and nonparametric tests were used. Continuous variables were expressed as the median and quartiles and compared using the Mann–Whitney U‐test. Categorical variables were expressed as numbers and were compared using the chi‐squared test or Fisher's exact test. Univariable and multivariable Cox regression analyses were performed to identify factors associated with the risk of infection after surgery. Dental treatment and antibiotic prophylaxis were treated as time‐dependent variables. In the multivariable Cox regression, age, sex, and CCI were included as potential confounding factors to be adjusted for. All statistical tests were two‐sided, and p‐values less than .05 were considered statistically significant. SAS 9.4 software (SAS Institute, Cary, NC) was used for statistical analyses.
3. RESULTS
A total of 295,915 patients over 50 years of age underwent spinal surgery from 2010 to 2017. Among 229,235 patients (excluding those who met the exclusion criteria), 15,346 patients developed postoperative infection within 2 years of surgery and the incidence rate was 6.69% (Figure 1). The mean time from surgery to the development of postoperative infection was 162.50 ± 200.00 days. Lumbar surgery (72.31%) was the most common procedure, and the infection rate of multilevel surgery was the highest (9.67%, p < .0001) (Table 4). The percentages of other procedures are listed in Table 4.
TABLE 4.
Incidence rate of postoperative spinal infections by the site of spinal surgery
| Site | No infection (n = 213,889) (%) | Infection (n = 15,346) (%) | Total (n = 229,235) (%) | p‐Value |
|---|---|---|---|---|
| Lumbar | 153,917 (92.85) | 11,844 (7.15) | 165,761 (72.31) | <.0001 |
| Cervical | 50,461 (95.03) | 2637 (4.97) | 53,098 (23.16) | |
| Thoracic | 6988 (92.15) | 595 (7.85) | 7583 (3.31) | |
| Multilevel | 2523 (90.33) | 270 (9.67) | 2793 (1.22) |
Patients who developed postoperative infection after spinal surgery were older than those who did not (66 [59–72] vs. 64 [57–71], p < .0001) (Table 5). The male‐to‐female ratio was higher in patients with postoperative infection than in those without (0.812 vs. 0.726, p < .0001). The CCI score was higher in patients with infection than in those without (3 [2–5] vs. 3 [1–5], p < .0001), and the prevalence of all diseases constituting the CCI score was higher in patients with infection.
TABLE 5.
Patient characteristics comorbidities and factors related to dental treatment according to presence of postoperative spinal infection
| No infection (n = 213,889) (%) | Infection (n = 15,346) (%) | p‐Value | |
|---|---|---|---|
| Agea | 64 (57–71) | 66 (59–72) | <.0001 |
| CCI scorea | 3 (1–5) | 3 (2–5) | <.0001 |
| Sex | <.0001 | ||
| Male | 89,960 (42.06) | 6877 (44.81) | |
| Female | 123,929 (57.94) | 8469 (55.19) | |
| CCI | |||
| Myocardial infarction | 4050 (1.89) | 365 (2.38) | <.0001 |
| Congestive heart failure | 16,770 (7.84) | 1555 (10.13) | <.0001 |
| Peripheral vascular disease | 67,444 (31.53) | 5525 (36.00) | <.0001 |
| Cerebrovascular disease | 38,471 (17.99) | 3211 (20.92) | <.0001 |
| Dementia | 11,591 (5.42) | 1043 (6.80) | <.0001 |
| Chronic pulmonary disease | 101,773 (47.58) | 7864 (51.24) | <.0001 |
| Rheumatic disease | 28,210 (13.19) | 2554 (16.64) | <.0001 |
| Peptic ulcer disease | 107,599 (50.31) | 8506 (55.43) | <.0001 |
| Mild liver disease | 83,845 (39.20) | 6694 (43.62) | <.0001 |
| Diabetes without chronic complication | 77,518 (36.24) | 6345 (41.35) | <.0001 |
| Diabetes with chronic complication | 33,107 (15.48) | 2893 (18.85) | <.0001 |
| Hemiplegia or paraplegia | 5146 (2.41) | 442 (2.88) | <0.0001 |
| Renal disease | 5805 (2.71) | 596 (3.88) | <.0001 |
| Any malignancy | 19,705 (9.21) | 1452 (9.46) | <.0001 |
| Moderate or severe liver disease | 1916 (0.90) | 151 (0.98) | <0.0001 |
| Metastatic solid tumour | 3016 (1.41) | 225 (1.47) | <.0001 |
| AIDS/HIV | 42 (0.02) | 6 (0.04) | <.0001 |
| Dental treatment within 1 year after surgery | <.0001 | ||
| No | 147,623 (69.02) | 13,815 (90.02) | |
| Yes | 66,266 (30.98) | 1531 (9.98) | |
| Duration from surgery to first dental treatment | <.0001 | ||
| No | 147,623 (69.02) | 13,815 (90.02) | |
| ~3 months | 15,309 (7.16) | 518 (3.38) | |
| 3–6 months | 19,504 (9.12) | 476 (3.10) | |
| 6–9 months | 16,557 (7.74) | 326 (2.12) | |
| 9–12 months | 14,896 (6.96) | 211 (1.37) | |
| Mean duration from surgery to first dental treatmenta | 172 (96–260) | 134 (69–226) | <.0001 |
| Number of dental treatmenta | 2 (1–3) | 1 (1–3) | .0039 |
| Prophylactic antibiotics for dental procedure | |||
| No | 190,185 (88.92) | 14,786 (96.35) | <.0001 |
| Yes | 23,704 (11.08) | 560 (3.65) |
Abbreviation: CCI, Charlson Comorbidity Index.
Bold values denote statistical significance at the p < 0.05 level.
The data are presented as median (first quartile to third quartile), and Mann–Whitney U‐test was used to calculate the p‐values.
Patients without infection had a higher rate of receiving dental treatment within 1 year of spinal surgery (9.98% vs. 30.98%, p < .0001). In contrast, the median duration from spinal surgery to the first dental treatment was 38 days shorter in patients with postoperative infection (134 [69–226] vs. 172 [96–260] days, p < .0001). The average number of invasive dental procedures was lower in patients with postoperative infection than in those without (1 [1–3] vs. 2 [1–3], p = .0039). The proportion of patients who received antibiotic prophylaxis was not significantly different between the two groups (36.58% vs. 35.77%, p = .5498).
3.1. Univariable Cox regression analysis
In univariable analysis, the risk of infection increased with age (hazard ratio [HR] 1.013; 95% confidence interval [CI], 1.011–1.015), and it was confirmed that men were at a higher risk than women (HR 1.126; 95% CI, 1.090–1.162). A one‐point increase in the CCI score was found to increase the risk of postoperative infection by 1.070 times (95% CI, 1.063–1.078), and all comorbidities contributing to the CCI score, except for liver disease and acquired immune deficiency syndrome, increased the risk of postoperative infection (Table 6). There was a negative correlation between dental procedures and PSI (HR 0.908; 95% CI, 0.857–0.961). Patients who received dental treatment within 3 months of spinal surgery had a particularly low risk of postoperative infection (HR 0.869; 95% CI, 0.795–0.950). Antibiotic prophylaxis before the dental procedure was not associated with the occurrence of postoperative infection (HR 0.995; 95% CI, 0.912–1.085).
TABLE 6.
Risk factors for postoperative spinal infection using time‐dependent Cox regression analysis
| Crude HR (95% CI) | p‐Value | Adjusted HR (95% CI)a | p‐Value | |
|---|---|---|---|---|
| Age | 1.013 (1.011–1.015) | <.0001 | 1.008 (1.006–1.010) | <.0001 |
| CCI score | 1.070 (1.063–1.078) | <.0001 | ||
| Sex | ||||
| Male | 1 (ref) | 1 (ref) | ||
| Female | 0.888 (0.861–0.917) | <.0001 | 0.847 (0.820–0.875) | <.0001 |
| CCI | ||||
| Myocardial infarction | 1.262 (1.137–1.400) | <.0001 | 1.052 (0.947–1.169) | .3462 |
| Congestive heart failure | 1.317 (1.250–1.388) | <.0001 | 1.128 (1.068–1.192) | <.0001 |
| Peripheral vascular disease | 1.209 (1.170–1.250) | <.0001 | 1.093 (1.056–1.131) | <.0001 |
| Cerebrovascular disease | 1.200 (1.154–1.247) | <.0001 | 1.043 (1.001–1.088) | .0450 |
| Dementia | 1.265 (1.188–1.347) | <.0001 | 1.073 (1.005–1.146) | .0356 |
| Chronic pulmonary disease | 1.152 (1.116–1.189) | <.0001 | 1.056 (1.022–1.091) | .0011 |
| Rheumatic disease | 1.295 (1.242–1.352) | <.0001 | 1.231 (1.179–1.285) | <.0001 |
| Peptic ulcer disease | 1.218 (1.180–1.258) | <.0001 | 1.135 (1.098–1.173) | <.0001 |
| Mild liver disease | 1.193 (1.156–1.232) | <.0001 | 1.090 (1.054–1.127) | <.0001 |
| Diabetes without chronic complication | 1.233 (1.194–1.273) | <.0001 | 1.077 (1.038–1.117) | <.0001 |
| Diabetes with chronic complication | 1.258 (1.208–1.310) | <.0001 | 1.073 (1.025–1.123) | .0023 |
| Hemiplegia or paraplegia | 1.204 (1.095–1.323) | .0001 | 1.076 (0.978–1.184) | .1344 |
| Renal disease | 1.446 (1.332–1.569) | <.0001 | 1.209 (1.112–1.315) | <.0001 |
| Any malignancy | 1.062 (1.006–1.121) | .0302 | 0.954 (0.901–1.011) | .1108 |
| Moderate or severe liver disease | 1.119 (0.953–1.314) | .1692 | 0.976 (0.831–1.147) | .7691 |
| Metastatic solid tumour | 1.247 (1.093–1.423) | .0010 | 1.244 (1.082–1.431) | .0022 |
| AIDS/HIV | 1.872 (0.841–4.166) | .1246 | 1.805 (0.811–4.018) | .1482 |
| Dental treatment within 1 year after surgery | ||||
| No | 1 (ref) | 1 (ref) | ||
| Yes | 0.908 (0.857–0.961) | .0009 | 0.850 (0.793–0.912) | <.0001 |
| Duration from surgery to first dental treatment | ||||
| No | 1 (ref) | |||
| ~3 months | 0.869 (0.795–0.950) | .0020 | ||
| 3–6 months | .1456 | |||
| 6–9 months | 0.960 (0.857–1.076) | .4817 | ||
| 9–12 months | 0.889 (0.773–1.023) | .0995 | ||
| Prophylactic antibiotics for dental procedure | ||||
| No | 1 (ref) | 1 (ref) | ||
| Yes | 0.995 (0.912–1.085) | .9117 | 1.097 (0.987–1.218) | .0853 |
Abbreviations: CCI, Charlson Comorbidity Index; CI, confidence interval; HR, hazard ratio.
Bold values denote statistical significance at the p < 0.05 level.
The multivariable Cox regression model included age, sex, CCI, dental treatment, and antibiotic prophylaxis.
3.2. Multivariable Cox regression analysis
Age (adjusted HR 1.008; 95% CI, 1.006–1.010) and sex (adjusted HR 0.847; 95% CI, 0.820–0.875) were independent risk factors (Table 6). Congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, mild liver disease, diabetes, renal disease, any malignancy, and metastatic solid tumours were identified as risk factors. The dental procedure was negatively associated with the risk of postoperative infection (adjusted HR 0.850; 95% CI, 0.793–0.912), and the antibiotic prophylaxis before the dental procedure was not identified as a risk factor (adjusted HR 1.097; 95% CI, 0.987–1.218) even after adjustment for age, sex, and CCI.
4. DISCUSSION
Analysis of data of patients undergoing spinal surgery with instrumentation over an 8‐year period, extracted from a nationwide database, showed that invasive dental procedures did not increase the risk of postoperative infection within 2 years of spinal surgery. Antibiotic prophylaxis for invasive dental procedures did not prevent postoperative infections after spinal surgery, and receiving dental treatment as early as 3 months after spinal surgery did not increase the risk of postoperative infection. These findings can be helpful in establishing an evidence‐based guideline for antibiotic prophylaxis before dental treatment in patients who have undergone spinal surgery (Martin et al., 2020). Older age, male sex, congestive heart failure, peripheral vascular disease, cerebrovascular disease, peptic ulcer disease, mild liver disease, diabetes, renal disease, malignancy, and metastatic solid tumour were other risk factors for PSI.
Infective endocarditis is a rare but life‐threatening disease, with a mortality rate of 25% (Wallace et al., 2002). Therefore, the use of prophylactic antibiotics is recommended during procedures that pose a risk of endocarditis in patients with certain underlying diseases (Wilson et al., 2007). However, there is a controversy regarding the clinical effects of prophylactic antibiotics in preventing endocarditis, and continuous efforts are being made to reduce the overuse of antibiotics (Durack, 1998; Oliver et al., 2004; Duval & Leport, 2008). Therefore, recent guidelines tend to minimize the indications for antibiotic prophylaxis, and the 2007 AHA guideline recommends prophylaxis only for those with high‐risk heart diseases, which could lead to the worst outcomes (Wilson et al., 2007). Thus, antibiotic prophylaxis is recommended only when patients with a history of endocarditis, congenital heart diseases, or prosthetic valves undergo procedures involving the oral or respiratory mucosa, such as dental treatment and procedures involving the manipulation of infected tissues (Wilson et al., 2007).
Postoperative infection is one of the most common and serious complications following spinal surgery. The incidence of this complication reported in recent studies ranged from 0.7% to 12% (Olsen et al., 2008; Ter Gunne & Cohen, 2009; Veeravagu et al., 2009; Fei et al., 2016; Lenz et al., 2021). Known risk factors vary, including old age, male sex, and long operation time, and it has been reported that the postoperative infection rate is 1.24–2.15 times higher in cases involving the use of instrumentation than in cases not involving the use of instrumentation (Veeravagu et al., 2009; Fei et al., 2016). The factors related to instrumentation surgery, such as longer surgical time, more extensive exposure, excessive retraction and injury of the paraspinal muscle, large dead space, and exposure of the implant to air, contribute to the higher infection rate (Gerometta et al., 2012; Oikonomidis et al., 2021). In addition, bacteria from the blood can adhere to the surface of the inserted implant and form a biofilm. Biofilms protect microorganisms from antibiotics, phagocytes, and other immune reactions, making it difficult to treat infections (Donlan, 2001; Costerton, 2005). This is similar to the pathogenesis of prosthetic valve endocarditis, wherein endothelial damage due to surgery, turbulent blood flow, and bacterial adhesion and biofilm formation on the implant surface contribute to the occurrence of endocarditis.
For these reasons, as in the case of patients with prosthetic heart valves, there have been some discussions on the effectiveness of antibiotic prophylaxis before procedures that can induce bacteraemia in patients who have undergo spinal surgery. However, due to the lack of clinical evidence, it has been dependent only on expert opinion until now. The majority of spinal surgeons recommend against prescribing prophylactic antibiotics before the dental procedure to patients with uncomplicated lumbar fusion (Lewkonia et al., 2016; Martin et al., 2020). However, in actual clinical practice, dentists prescribe antibiotics before the procedure at a fairly high rate. In fact, this study identified that 35.79% (24,264 of 67,797) of patients who underwent spinal surgery received antibiotic prophylaxis before dental procedures. This may due to the fact that dentists consider it safer to prescribe antibiotics in uncertain circumstances without solid evidence or concrete guidelines. However, the overuse and misuse of antibiotics contributes to the emergence of drug‐resistant pathogens and exposure to the risk of Clostridium difficile infection (Sung et al., 2020).
Similar to that in patients undergoing spinal surgery, the prosthesis is barely removed in patients undergoing total joint arthroplasty. Periprosthetic joint infection is a devastating complication of total joint arthroplasty, which in most cases requires revision surgery with prolonged treatment. For this reason, research on the effectiveness of prophylactic antibiotics before dental procedures is being conducted more actively in patients undergoing arthroplasty than in those undergoing spinal surgery. ADA and AAOS recommended in 2012 that clinicians should consider discontinuing the routine prescription of prophylactic antibiotics for patients with prosthetic joint implants undergoing dental treatment (Watters et al., 2013). In addition, Kao et al. reported that there was no association between antibiotic prophylaxis before dental procedures and periprosthetic joint infection (Kao et al., 2017).
To achieve sufficient statistical power and minimize the possibility of type II error, 229,235 patients were analysed in this study. The NHI database covers over 98% of the South Korean population, and therefore the sample group was considered to adequately represent the entire population. Using this large nationwide database, we identified that dental treatment received within 1 year of spinal surgery did not increase the incidence of postoperative infection, and antibiotic prophylaxis was not effective in preventing infection. Rather, an interesting result was found: dental treatment had a negative correlation with postoperative infection (adjusted HR 0.850; 95% CI, 0.793–0.912). A possible explanation for this result is that patients who receive dental treatment early after surgery are more likely to be in better health than those who are still recovering from the surgery. In addition, patients who actively visit the dental clinic have good oral hygiene, which may have an effect in preventing bacteraemia and infection (Lockhart et al., 2009).
This study has several limitations. The NHI and HIRA databases contain the data of only prescription and diagnostic codes, but not laboratory data, pathologic results, or medical records. Therefore, the diagnosis could not be confirmed by reviewing the medical records, and the clinical progress of patients who developed infection could not be investigated. In addition, the risk factor analysis did not include surgical factors such as operation time, fixation level, surgical approach, and blood loss, because data on these variables could be obtained only from medical record review. Many experts have emphasized that it is more important to maintain oral hygiene than to use prophylactic antibiotics with dental treatment. However, we could not identify underlying dental diseases or the patient's oral condition using the claim data. We also could not investigate the impact of different types of dental procedures on the risk of infection. It was also not possible to analyse the outcomes (surgery rate, mortality, and medical cost) of patients who developed spinal infection after dental procedures.
5. CONCLUSION
Invasive dental procedures in patients who have undergone spinal surgery with instrumentation are not risk factors for postoperative infection. It was also confirmed that early dental treatment (within 3 months after surgery) was not associated with the risk of postoperative infection. In addition, antibiotic prophylaxis following dental procedures was not effective in preventing spinal infection. Therefore, we believe that patients undergoing spinal surgery with instrumentation should not avoid dental treatment after surgery, and prophylactic antibiotics may not be necessary.
CONFLICT OF INTEREST
The authors declare that they have no competing interest.
ETHICS STATEMENT
This study was approved by the institutional review board of the author's hospital (4‐2019‐0915). The requirement for informed consent was waived as the study involved the retrospective use of anonymized and publicly available data.
AUTHOR CONTRIBUTIONS
Sahyun Sung: Conceptualization; methodology; investigation; writing—original draft preparation; read and agreed to the published version of the manuscript. Eun Hwa Kim: Software; formal analysis; visualization; read and agreed to the published version of the manuscript. Ji‐Won Kwon: Validation; visualization; read and agreed to the published version of the manuscript. Jung‐Seok Lee: Validation; writing—review and editing; read and agreed to the published version of the manuscript. Soo‐Bin Lee: Validation; visualization; read and agreed to the published version of the manuscript. Seong‐Hwan Moon: Resources; read and agreed to the published version of the manuscript. Hwan‐Mo Lee: Resources; data curation; read and agreed to the published version of the manuscript. Inkyung Jung: Methodology; software; formal analysis; writing—review and editing; read and agreed to the published version of the manuscript. Byung Ho Lee: Conceptualization; methodology; investigation; resources; writing—review and editing; supervision; read and agreed to the published version of the manuscript.
Sung, S., Kim, E. H., Kwon, J.‐W., Lee, J.‐S., Lee, S.‐B., Moon, S.‐H., Lee, H.‐M., Jung, I., & Lee, B. H. (2021). Invasive dental procedures as risk factors for postoperative spinal infection and the effect of antibiotic prophylaxis. Journal of Clinical Periodontology, 48(9), 1270–1280. 10.1111/jcpe.13514
Sahyun Sung and Eun Hwa Kim contributed equally as co‐first authors.
Contributor Information
Inkyung Jung, Email: ijung@yuhs.ac.
Byung Ho Lee, Email: bhlee96@yuhs.ac.
DATA AVAILABILITY STATEMENT
The authors are restricted from sharing the data underlying this study because The Korean National Health Insurance Service (NHIS) owns the data. Researchers can request access on the NHIS website (https://nhiss.nhis.or.kr).
REFERENCES
- Aydinli, U., Karaeminoǧullary, O., & Tiskaya, K. (1999). Postoperative deep wound infection in instrumented spinal surgery. Acta Orthopaedica Belgica, 65, 182–187. [PubMed] [Google Scholar]
- Chen, P. C., Tung, Y. C., Wu, P. W., Wu, L. S., Lin, Y. S., Chang, C. J., Kung, S., & Chu, P. H. (2015). Dental procedures and the risk of infective endocarditis. Medicine (Baltimore), 94(43), e1826. 10.1097/MD.0000000000001826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, I., Wilson‐MacDonald, J., Chami, G., Burgoyne, W., Vinayakam, P., Berendt, T., & Fairbank, J. (2008). The diagnosis and management of infection following instrumented spinal fusion. European Spine Journal, 17(3), 445–450. 10.1007/s00586-007-0559-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton, J. W. (2005). Biofilm theory can guide the treatment of device‐related orthopaedic infections. Clinical Orthopaedics and Related Research, 437, 7–11. [DOI] [PubMed] [Google Scholar]
- Donlan, R. M. (2001). Biofilm formation: A clinically relevant microbiological process. Clinical Infectious Diseases, 33(8), 1387–1392. [DOI] [PubMed] [Google Scholar]
- Durack, D. T. (1998). Antibiotics for prevention of endocarditis during dentistry: Time to scale back? Annals of Internal Medicine, 129(10), 829–831. [DOI] [PubMed] [Google Scholar]
- Duval, X., & Leport, C. (2008). Prophylaxis of infective endocarditis: Current tendencies, continuing controversies. The Lancet Infectious Diseases, 8(4), 225–232. 10.1016/s1473-3099(08)70064-1 [DOI] [PubMed] [Google Scholar]
- Fei, Q., Li, J., Lin, J., Li, D., Wang, B., Meng, H., Wang, Q., Su, N., & Yang, Y. (2016). Risk factors for surgical site infection after spinal surgery: A meta‐analysis. World Neurosurgery, 95, 507–515. [DOI] [PubMed] [Google Scholar]
- Gerometta, A., Rodriguez Olaverri, J. C., & Bitan, F. (2012). Infections in spinal instrumentation. International Orthopaedics, 36(2), 457–464. 10.1007/s00264-011-1426-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouliouris, T., Aliyu, S. H., & Brown, N. M. (2010). Spondylodiscitis: Update on diagnosis and management. The Journal of Antimicrobial Chemotherapy, 65(Suppl. 3), iii11–iii24. 10.1093/jac/dkq303 [DOI] [PubMed] [Google Scholar]
- Habib, G., Lancellotti, P., Antunes, M. J., Bongiorni, M. G., Casalta, J.‐P., Del Zotti, F., Dulgheru, R., El Khoury, G., Erba, P. A., Iung, B., Miro, J. M., Mulder, B. J., Plonska‐Gosciniak, E., Price, S., Roos‐Hesselink, J., Snygg‐Martin, U., Thuny, F., Mas, P. T., Vilacosta, I., … ESC Scientific Document Group . (2015). 2015 ESC guidelines for the management of infective endocarditis: The task force for the management of infective endocarditis of the European Society of Cardiology (ESC) endorsed by: European Association for Cardio‐Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). European Heart Journal, 36(44), 3075–3128. [DOI] [PubMed] [Google Scholar]
- Hamedani, S. (2013). A clinical practice update on the latest AAOS/ADA guideline (December 2012) on prevention of orthopaedic implant infection in dental patients. Journal of Dentistry, 14(1), 49–52. [PMC free article] [PubMed] [Google Scholar]
- Hirsh, H. L., Vivino, J. J., Merril, A., & Dowling, H. F. (1948). Effect of prophylactically administered penicillin on incidence of bacteremia following extraction of teeth; results in patients with healed rheumatic and bacterial endocarditis. Archives of Internal Medicine (Chicago, Ill.), 81(6), 868–878. 10.1001/archinte.1948.00220240077005 [DOI] [PubMed] [Google Scholar]
- Kao, F. C., Hsu, Y. C., Chen, W. H., Lin, J. N., Lo, Y. Y., & Tu, Y. K. (2017). Prosthetic joint infection following invasive dental procedures and antibiotic prophylaxis in patients with hip or knee arthroplasty. Infection Control and Hospital Epidemiology, 38(2), 154–161. 10.1017/ice.2016.248 [DOI] [PubMed] [Google Scholar]
- Kasliwal, M. K., Tan, L. A., & Traynelis, V. C. (2013). Infection with spinal instrumentation: Review of pathogenesis, diagnosis, prevention, and management. Surgical Neurology International, 4(Suppl 5), S392–S403. 10.4103/2152-7806.120783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, L., Kim, J. A., & Kim, S. (2014). A guide for the utilization of health insurance review and assessment service national patient samples. Epidemiology and Health, 36, e2014008. 10.4178/epih/e2014008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lener, S., Hartmann, S., Barbagallo, G. M. V., Certo, F., Thome, C., & Tschugg, A. (2018). Management of spinal infection: A review of the literature. Acta Neurochirurgica, 160(3), 487–496. 10.1007/s00701-018-3467-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz, M., Mohamud, K., Bredow, J., Oikonomidis, S., Eysel, P., & Scheyerer, M. J. (2021). Comparison of different approaches in lumbosacral spinal fusion surgery: A systematic review and meta‐analysis. Asian Spine J. 10.31616/asj.2020.0405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkonia, P., DiPaola, C., & Street, J. (2016). Incidence and risk of delayed surgical site infection following instrumented lumbar spine fusion. Journal of Clinical Neuroscience, 23, 76–80. 10.1016/j.jocn.2015.05.039 [DOI] [PubMed] [Google Scholar]
- Lockhart, P. B., Brennan, M. T., Thornhill, M., Michalowicz, B. S., Noll, J., Bahrani‐Mougeot, F. K., & Sasser, H. C. (2009). Poor oral hygiene as a risk factor for infective endocarditis–related bacteremia. The Journal of the American Dental Association, 140(10), 1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart, P. B., & Durack, D. T. (1999). Oral microflora as a cause of endocarditis and other distant site infections. Infectious Disease Clinics of North America, 13(4), 833–850, vi. 10.1016/s0891-5520(05)70111-2 [DOI] [PubMed] [Google Scholar]
- Martin, P., Hundal, R., Matulich, K., Porta, M., Patel, R., & Aleem, I. (2020). Is dental prophylaxis required following spinal fusion?‐A systematic review and call for evidence. Journal of Spine Surgery, 6(1), 13–17. 10.21037/jss.2020.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord, C. E., & Heimdahl, A. (1990). Cardiovascular infections: Bacterial endocarditis of oral origin. Pathogenesis and prophylaxis. Journal of Clinical Periodontology, 17(7 (Pt 2)), 494–496. 10.1111/j.1365-2710.1992.tb01221.x [DOI] [PubMed] [Google Scholar]
- Oikonomidis, S., Altenrath, L., Westermann, L., Bredow, J., Eysel, P., & Scheyerer, M. J. (2021). Implant‐associated infection of long‐segment spinal instrumentation: A retrospective analysis of 46 consecutive patients. Asian Spine Journal, 15(2), 234–243. 10.31616/asj.2019.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, R. J., Roberts, G. J., & Hooper, L. (2004). Penicillins for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database of Systematic Reviews, 2, CD003813. [DOI] [PubMed] [Google Scholar]
- Olsen, M. A., Nepple, J. J., Riew, K. D., Lenke, L. G., Bridwell, K. H., Mayfield, J., & Fraser, V. J. (2008). Risk factors for surgical site infection following orthopaedic spinal operations. JBJS, 90(1), 62–69. [DOI] [PubMed] [Google Scholar]
- Stavridis, S. I., Bucking, P., Schaeren, S., Jeanneret, B., & Schnake, K. J. (2010). Implant removal after posterior stabilization of the thoraco‐lumbar spine. Archives of Orthopaedic and Trauma Surgery, 130(1), 119–123. 10.1007/s00402-009-0962-1 [DOI] [PubMed] [Google Scholar]
- Sung, S., Kwon, J.‐W., Lee, S.‐B., Lee, H.‐M., Moon, S.‐H., & Lee, B. H. (2020). Risk factors of Clostridium difficile infection after spinal surgery: National Health Insurance Database. Scientific Reports, 10(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Gunne, A. F. P., & Cohen, D. B. (2009). Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine, 34(13), 1422–1428. [DOI] [PubMed] [Google Scholar]
- van der Meer, J. T., Thompson, J., Valkenburg, H. A., & Michel, M. F. (1992). Epidemiology of bacterial endocarditis in The Netherlands. II. Antecedent procedures and use of prophylaxis. Archives of Internal Medicine, 152(9), 1869–1873. 10.1001/archinte.152.9.1869 [DOI] [PubMed] [Google Scholar]
- Veeravagu, A., Patil, C. G., Lad, S. P., & Boakye, M. (2009). Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine, 34(17), 1869–1872. [DOI] [PubMed] [Google Scholar]
- Wallace, S., Walton, B., Kharbanda, R., Hardy, R., Wilson, A., & Swanton, R. (2002). Mortality from infective endocarditis: Clinical predictors of outcome. Heart, 88(1), 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, A., Gaca, J. G., & Chu, V. H. (2018). Management considerations in infective endocarditis: A review. JAMA, 320(1), 72–83. 10.1001/jama.2018.7596 [DOI] [PubMed] [Google Scholar]
- Watters, W., 3rd, Rethman, M. P., Hanson, N. B., Abt, E., Anderson, P. A., Carroll, K. C., Futrell, H. C., Garvin, K., Glenn, S. O., Hellstein, J., Hewlett, A., Kolessar, D., Moucha, C., O'Donnell, R. J., O'Toole, J. E., Osmon, D. R., Evans, R. P., Rinella, A., Steinberg, M. J., … American Academy of Orthopedic Surgeons; American Dental Association . (2013). Prevention of orthopaedic implant infection in patients undergoing Dental procedures. The Journal of the American Academy of Orthopaedic Surgeons, 21(3), 180–189. 10.5435/JAAOS-21-03-180 [DOI] [PubMed] [Google Scholar]
- Wilson, W., Taubert, K. A., Gewitz, M., Lockhart, P. B., Baddour, L. M., Levison, M., Bolger, A., Cabell, C. H., Takahashi, M., Baltimore, R. S., Newburger, J. W., Strom, B. L., Tani, L. Y., Gerber, M., Bonow, R. O., Pallasch, T., Shulman, S. T., Rowley, A. H., Burns, J. C., … Quality of Care and Outcomes Research Interdisciplinary Working Group . (2007). Prevention of infective endocarditis: Guidelines from the American Heart Association: A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation, 116(15), 1736–1754. 10.1161/CIRCULATIONAHA.106.183095 [DOI] [PubMed] [Google Scholar]
- Yin, D., Liu, B., Chang, Y., Gu, H., & Zheng, X. (2018). Management of late‐onset deep surgical site infection after instrumented spinal surgery. BMC Surgery, 18(1), 121. 10.1186/s12893-018-0458-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors are restricted from sharing the data underlying this study because The Korean National Health Insurance Service (NHIS) owns the data. Researchers can request access on the NHIS website (https://nhiss.nhis.or.kr).


