Abstract
Background
Implant‐supported overdentures (IODs) have been reported to increase patients' oral health‐related quality of life (OHRQoL) in comparison with conventional dentures (CDs); however, the conclusiveness of evidence on the clinical effectiveness and value for money of IODs versus CDs remains unclear.
Purpose
To review how the added value of IODs is demonstrated in the literature.
Materials and methods
MEDLINE, EMBASE, and the Cochrane Database were searched for randomized control trials, controlled clinical trials, and prospective cohort studies containing evaluations of the economic and health benefits and costs of IODs. Information about the clinical effectiveness, such as magnitude of bite forces or chewing efficacy, OHRQoL, costs, and cost‐effectiveness of IODs, was extracted.
Results
A total of 17 articles were included, reporting 15 economic evaluations: 11 cost‐utility analyses (CUAs), 2 of which were combined with a cost‐effectiveness analysis (CEA), and 2 cost–benefit analyses (CBAs). Seven CUAs used the Oral Health Impact Profile (OHIP) questionnaire while four used satisfaction questionnaires to assess the OHRQoL. One study applied quality‐adjusted prosthesis years (QAPYs) for this purpose. The CBAs expressed both the beneficial outcome and the costs of the IOD in monetary terms. The included studies employed a large variety of economic evaluation methods, which limited cross‐study comparability.
Conclusions
On the basis of existing economic evaluations, IODs have frequently been suggested to be a cost‐efficient treatment alternative to CDs; however, the comparability between the various economic evaluation studies was limited due to the different outcome measures used. In addition, it remains unclear whether the additional health benefits of IODs outweigh the higher costs. This is largely dependent on the decision maker's valuation of oral health outcomes. Future research is encouraged to further elucidate patient willingness to pay for IODs and the societal return on investing in IODs more generally.
Keywords: cost‐effectiveness, edentulous, implants, Oral Health Impact Profile, oral health‐related quality of life, overdenture
What is known
Implant‐supported overdentures (IODs) increase clinical effectiveness: bite forces and chewing efficacy increase.
Implant‐supported overdentures (IODs) also increase patients' oral health‐related quality of life (OHRQoL) as compared to conventional dentures (CDs).
What this study adds
The existing evidence on the added value of IODs and the methodologies used were reviewed.
Cost‐effectiveness analyses (CEAs) and cost‐utility analyses (CUAs) are the proper instruments to calculate the incremental costs (IOD versus CD) in relation to the incremental health improvement.
1. INTRODUCTION
Edentulism (being toothless) can lead to significant functional impairment, as well as unfavorable esthetic and psychological changes in patients. Reported drawbacks include restrictions in diet and a limited ability to eat certain foods,1 speech impairment, and the loss of support for facial musculature, which has an aging effect on appearance.2 Edentulism is even classified as a physical handicap by the World Health Organization.3
Installing dental implants has the potential to mitigate these drawbacks. Many articles corroborate that implant‐supported overdentures (IODs) provide significantly higher satisfaction levels, quality of life, and better mastication than mandibular conventional dentures (CDs).4, 5, 6, 7, 8, 9, 10 As a result of these positive findings, since 2002 it has been recommended that, in case of lack of retention, a mandibular IOD retained by two interforaminal implants (IOD‐2) should be considered the first treatment choice.11 Because of the palate as substantial bearing surface, the CD remains the first step in prosthetic rehabilitation for the edentulous maxilla. Nevertheless, the success of IODs in terms of stability, function, speech, and patient satisfaction has also been shown for the upper jaw.11, 12, 13 An extra advantage of the presence of functioning implants is that clinically significant progressive bone loss is prevented.14 Disadvantages, however, are the invasive treatment, need for maintenance, high costs, and risks for peri‐implantitis.
Despite their benefits, IODs also incur higher treatment costs than CDs, leading to the question of whether IODs provide a reasonable value for money. An economic evaluation means “ensuring that the value of what is gained from an activity outweighs the value of what has to be sacrificed.”15 Such economic calculations can inform patients, healthcare providers, insurers, and policy makers about IOD value for money.16, 17, 18 In order to determine whether the benefits produced by a particular program exceed the opportunity costs of providing that program, a reliable method of measuring and comparing outcomes is required.19 After all, the diversity in included cost‐categories, the various types of economic evaluation used and the different interpretations of it, may complicate the drawing of firm conclusions.
Beneficial aspects for patients can be expressed in terms of clinical effectiveness, such as the number of prosthetic complications, the magnitude of bite forces in newtons, or measuring the masticatory efficacy. In contrast, patient‐reported outcome measures (PROMs) describe patient's perceived health benefits in qualitative terms; for example, patient satisfaction is often scored with the aid of questionnaires asking about general satisfaction and/or masticatory ability with different food types. Another way to identify PROMs is to measure the oral health‐related quality of life (OHRQoL). In dentistry for this purpose the Oral Health Impact Profile (OHIP)‐list is often used.20
Various types of economic evaluation have been presented.21, 22 Both cost‐effectiveness analyses (CEAs) and cost‐utility analyses (CUAs) calculate the incremental costs of a specific treatment in relation to the incremental health improvement. CEAs describe clinical effectiveness, such as number of prosthetic complications or magnitude of bite forces in newtons. CUAs are typically expressed in natural (qualitative) units such as OHRQoL, life years gained, or quality‐adjusted life years (QALYs). Both CEAs and CUAs rely on the incremental cost‐effectiveness ratio (ICER), which compares the difference in costs against the health improvement associated with two or more treatment alternatives.17, 21 CEAs and CUAs are especially suitable for interventions that are more effective than their alternatives but also cost more. In cost–benefit analyses (CBAs), both health outcomes and costs are expressed in monetary terms, thus enabling a direct comparison. For example, it has long been recommended to assess patient preferences in terms of “willingness to pay” (WTP) for different treatments, such as implant placement.23
Conceptually similar to WTP is the concept of WTA (“willingness to accept”), in which patients are asked which amount of money they would accept to go back to their baseline situation, for example, from their IOD to their CD. For nonpatients, this is the maximum amount that they are willing to receive to forgo implant therapy. WTP/WTA are thought to be important in health technology assessments by providing insight into the impact that the risks and benefits of treatments have on society.24
The purpose of this study was to review the existing evidence on the added value of IODs and the methodologies used. It was hypothesized that the information, as available in so far literature, is too diverse to draw firm conclusions.
2. MATERIALS AND METHODS
Thomas Van de Winkel and Laura Heijens conducted a search of the literature written in English and published between January 1995 and August 2020 that compared health outcomes to the involved costs with respect to an IOD. Special attention was focused on the relationship between costs and the extent of the increased OHRQoL.
The MEDLINE, EMBASE, and the Cochrane Database were screened using the following terms: (economic evaluation) and (dental implant) and overdenture. As the search results were minimal, it was decided to choose for the more general terms: cost and (dental implant) and overdenture. As methodology the PICO Principle was used (Table 1).
TABLE 1.
The PICO (population, intervention, control, and outcomes) format as strategy for the research question
| PICO principle | |
|---|---|
| Population | Edentulous patients |
| Intervention | Treatment with IOD |
| Comparison | CD (new or pre‐existing) |
| Outcomes |
(1) Health benefits, such as satisfaction, chewing capacity, OHRQoL (2) Costs, (3) value for money |
Inclusion criteria: for this review, only studies that focused on economic evaluations while providing information about both benefits in OHRQoL and costs of IODs were included, meaning CEAs, CUAs, and CBAs.
Exclusion criteria: case reports, articles which were written in a language other than English, or those involving patients who still had natural teeth were excluded.
Systematic reviews and meta‐analyses obtained from the database search were subsequently perused for other papers on this topic (snowballing). The selected articles were independently evaluated by two reviewers (Laura Heijens and Thomas Van de Winkel). In case of disagreements about inclusion, a consensus discussion was conducted. If no consensus could be reached, Gert Meijer took the final decision. A Cohen's kappa analysis was calculated to determine the interevaluation reliability of the articles included between the two evaluators.25
2.1. Quality assessment
For each selected article, the 24‐item checklist of the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) was used to evaluate whether each item was met. As the aim of the CHEERS list is to optimize the reporting of health economic evaluations, only the quality of reporting is judged, not the quality of conduct.21, 22 The selected randomized controlled trials (RCTs) and clinical controlled trials (CCTs) were evaluated by two independent reviewers (Laura Heijens and Thomas Van de Winkel). In case of disagreement, first a discussion took place to come to a mutual agreement. If no agreement was reached, a final decision was made by Gert Meijer.
To further appraise the risk of bias and the methodological approach of the selected RCTs and CCTs, the respective studies were evaluated by two independent reviewers (Laura Heijens and Thomas Van de Winkel) using Cochrane Risk of Bias Tool.26 Again, in case of dispute, discussions were held to reach agreement, which, if unsuccessful, was followed by a final decision of Gert Meijer. The articles were screened for randomization, blinding of randomization, selective reporting, blinding of staff and participants, blinding of the results, and the presence of incomplete data. Studies were judged to have a “high risk of bias” if one of the items showed a high bias score. If one of the items had an “uncertain risk of bias,” but no “high risk of bias” on the other items, the study was considered as an “uncertain risk of bias.” In cases where all items scored a low risk of bias, the study was categorized as “low risk of bias.” Cohort studies were qualitatively assessed using Form III for assessing a cohort study by the Dutch Cochrane Center (2003). In addition, the articles were screened for the following confounding factors: whether the research was funded by the manufacturer (potential benefit), inclusion or exclusion criteria related to patient factors (disease, mental state), individual factors such as age and number of dental implants, and date of publication with reference to costs.
2.2. Data extraction
For the selected articles, it was first noted if a CEA, CUA, or CBA was included. Furthermore, the following items were recorded: authors, year of publication, inclusion and exclusion criteria regarding the health of the participants, number and age of the participants, follow‐up period, number and location of the implants, outcome measures, and the raw data and conclusions about the increase in OHRQoL. Specifically, information was gathered about the type of costs, such as for IOD fabrication and costs incurred by loss of working time due to travel and attending treatment sessions.
For analytical purposes, it was also noted in which year and in which currency the costs were presented. All raw data were converted to the same currency (US dollars; USD) using the exact exchange rate of the year in which the investigation was performed to allow an optimal comparison.27 If the year in which the costs were incurred was not clear, the relevant author of the article was consulted.
3. RESULTS
A total of 355 studies were identified based on the search terms. After the first screening, which consisted of reading titles and abstracts, 59 and 45 articles were selected, respectively. After intensively evaluating the relevant 45 articles, it appeared that some publications lacked important data, or described the same patient population. Ultimately, 17 studies remained that were suitable for analysis (Figure 1). With respect to the inclusion of the selected articles, between the two reviewers a substantial agreement (Cohen's kappa: 0.72) was measured.25
FIGURE 1.
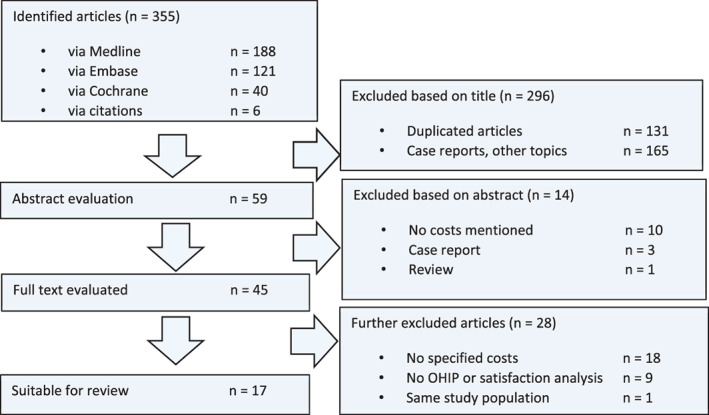
The search strategy used to identify the 17 articles to be reviewed
3.1. Characteristics of included studies
In Tables 2 and 3, the characteristics of all 17 included studies are presented, comprising five RCTs,28, 29, 30, 31, 32 five CCTs,33, 34, 35, 36, 37 two cohort studies,38, 39 and five economic evaluations.40, 41, 42, 43, 44 For a single study population in the Netherlands, Timmerman and colleagues reported the mandibular IOD satisfaction score32 and Stoker and colleagues the mandibular IOD costs.31 Taking these articles together, this randomized study can be labeled a CUA. The same accounts for the two studies presented by Wetzels and colleagues; functional benefits were described in 2016, and costs in 2017. As such, they provided a CEA/CUA analysis of the installation of a mandibular IOD‐2 in patients who have been treated for oral cancer.35, 36
TABLE 2.
Characteristics of the included studies
| Author | Study type | Type of analysis/Score of CHEERS list | Patients (n) | Follow‐up period (months) | Short description of study | Outcome reported |
|---|---|---|---|---|---|---|
| Alfadda and Attard33 | CCT |
CUA 18 (24) 75% |
75 | 168 | Cost analysis for IOD; immediate versus conventional loading | Total costs, OHIP‐20, ICER |
| Attard and colleagues34 | CCT |
CUA 14 (24) 58% |
77 | 12 | Clinical costs, PROMS for immediate‐loading protocol for IOD | Total costs, OHIP‐20, ICER |
| Della Vecchia and colleagues28 | RCT |
CUA 17 (24) 71% |
120 | 6 | Cost‐effectiveness analysis of IOD. MDI versus conventional implants | Total costs, OHIP‐EDENT, ICER |
| Heydecke and colleagues29 | RCT |
CUA 19 (24) 79% |
60 | 60 | Total costs, OHIP‐20, cost‐effectiveness | |
| Jawad and colleagues30 | RCT |
CEA/CUA 14 (24) 58% |
46 | 6 | Cost‐effectiveness analysis of IOD‐2 or IOD‐4 MDIs versus conventional implants | Total costs, OHIP‐20, cost‐effectiveness. Chewing capacity |
| Listl and colleagues40 | EE |
CUA 20 (24) 83% |
833 | 120 | Cost‐effectiveness analysis of 6 and 4 implants in the edentulous maxilla | Manufacturing, maintenance cost, CEAC |
| Matthys and colleagues38 | Cohort study |
CUA 10 (24) 42% |
56 | 60 | Maintenance cost ratios of locators | Cost ratio OHIP‐14 |
| Matthys and colleagues39 | Cohort study |
CUA 17 (24) 71% |
116 | 60 | Initial and maintenance costs for Locator versus Dalla Bona ball implants | Cost‐effectiveness plane, OHIP‐14 |
| Stoker and colleagues31 and Timmerman and colleagues32 |
RCT RCT |
CUA 15 (24) 63% |
110 | 96 | Cost analysis for three types of IOD in lower jaw | Total costs |
| 110 | 12 | Satisfaction for three types of IOD in lower jaw | Satisfaction questionnaire | |||
| Wetzels and colleagues35 and Wetzels and colleagues36 |
CCT CCT |
CEA/CUA 16 (24) 67% |
193 | 60 | Outcome of two implants installed during ablative surgery (DAS protocol) or postponed (P protocol) | Costs, chewing capacity, satisfaction questionnaire |
| Zitzmann and colleagues37 | CCT |
CUA 18 (24) 75% |
60 | 36 | Cost‐effectiveness analysis of two different IODs and CD | Total costs and ICER (QAPYs) |
Abbreviations: CCT, controlled clinical trial; CEA, cost‐effectiveness analysis; CEAC, cost‐effectiveness acceptability curve; CUA, cost‐utility analysis; EE, economic evaluation; ICER, incremental cost‐effectiveness ratio; OHIP, Oral Health Impact Profile; PROM, patient‐related outcome measure; RCT, randomized clinical trial; QAPY, quality‐adjusted prosthesis years.
TABLE 3.
Characteristics of the economic evaluation (EE) studies describing WTP
| Author | Type of analysis/score of the 24‐items CHEERS list | Patients (n) | Follow‐up period (months) | Short description of study | Outcome reported |
|---|---|---|---|---|---|
| Esfandiari and colleagues41 |
CBA 12 (24) 50% |
36 | 24 |
WTP/WTA IOD‐2 |
|
| Sendi and colleagues42 |
CBA 15 (24) 63% |
16 | 60 |
WTP/WTA IOD‐2 |
|
| Srivastava and colleagues43 |
CBA 12 (24) 50% |
38 | N/A |
(partially) dentate were interviewed WTP/WTA IOD‐2 |
|
| Srivastava and colleagues44 |
CBA 12 (24) 50% |
317 | N/A | WTP/WTA (partially) dentate were interviewed about IOD‐2 |
Abbreviations: CBA, cost–benefit analyses; CHEERS, Consolidated Health Economic Evaluation Reporting Standards, WTA, willing to accept; WTP, willing to pay.
Esfandiari and colleagues41 used 2008 Canadian dollars (CAD) in their article (1 CAD = 0.9441 USD).
Sendi and colleagues42 used 2013 Swiss francs (CHF) in their article (1 CHF = 1.0793 USD).
Srivastava and colleagues43 used 2011 Canadian dollars (CAD) in their article (1 CAD = 1.0114 USD).
Srivastava and colleagues44 used 2012 Canadian dollars (CAD) in their article (1 CAD = 1.0002 USD).
In total, nine CUAs28, 29, 31, 33, 34, 37, 38, 39, 40 were presented, plus two combinations of CEA/CUA31, 32, 35, 36 and four CBAs.41, 42, 43, 44 The scores of the 24‐item CHEERS checklist varied between 10 (42%) and 20 (83%).22
Of the RCTs and CCTs, seven studies presented a “high risk of bias,”28, 30, 33, 34, 35, 36, 37 as did the two cohort studies.38, 39 An “uncertain risk of bias” was noted for three studies.29, 31, 32 In summary, the quality of all included RCTs, CCTs, and cohort studies was debatable or low.
3.2. CEA/CUA: Study design
In total, four studies presented a follow‐up period varying between 6 month and 1 year and involved real spend costs.28, 29, 30, 34 Calculated costs were also reported over a 5‐year period39 and over 8 years.31, 32 The only long‐term study involving real costs followed patients for a 14‐year period.33 Although Heydecke and colleagues presented costs over an even longer period (17.9 years), real costs were not included, but calculated based on the Delphi group opinion technique, using an annual price increase of 3%–5%.29, 45 The same accounts for the study of Zitzmann and colleagues: they collected financial data over a 3 years period and estimated costs for a 10‐year period also using an annual price increase of 3%–5%.37 List and colleagues calculated costs that were based on the German private dental insurance fee.40 Within this system, providers' fees can be adjusted by different factors corresponding to the treatment complexity (factor 1: low complexity; factor 2.3: average complexity; factor 3.5: high complexity). One study reported maintenance costs as a percentage of the initial costs.38
Most studies compared the IOD to the original CD.28, 30, 31, 32, 35, 36, 37, 38, 39, 40 In two studies, patients were divided into groups in which patients received a new CD or a IOD‐2.29, 37 In two other studies, first a new CD was manufactured. Subsequently, a few months later implants were installed and the IOD‐2 delivered.33, 34
Most studies focused on IOD's on conventional implants, two studies concentrated on IODs on MDI's.28, 30 All but one study addressed IODs in the lower jaw. Solely, Listl and colleagues calculated costs for an IOD‐4 versus IOD‐6 in the upper jaw.
With respect to the aims, two studies compared an IOD‐2 to a CD,29, 37 two studies compared conventional versus immediate loading,33, 34 and one group compared two surgical protocols in oncology patients.35, 36 And the others compared two treatment modalities, for example, MDIs versus conventional implants,28, 30 ball attachment versus locators,39 and ball attachments versus bar attachment.31, 32
3.3. CEA/CUA: Total costs
In Table 4, the “total costs” of various CD and IOD types for the edentulous lower jaw are shown.
TABLE 4.
Type of construction in relation to total costs
| Type of construction | Time period | Total costs: initial + maintenance + complication + recall + travel time |
|---|---|---|
| CD in mandible | 1 year | $1385a; Heydecke and colleagues29 |
| 17.9 years | $3801a; Heydecke and colleagues29 | |
| 3 years | $2242b; Zitzmann and colleagues37 | |
| IOD on two mandibular implants | 14 years | $4349c; Alfadda and Attard33 (conventional loading) |
| $4022c; Alfadda and Attard33 (immediate loading) | ||
| 1 year | $1983d; Attard and colleagues34 (immediate loading) | |
| $1779d; Attard and colleagues34 (conventional loading) | ||
| 0.5 year | $566e; Della Vechia and colleagues28 | |
| 1 year | $2458a; Heydecke and colleagues29 | |
| 17.9 years | $5960a; Heydecke and colleagues29 | |
| 0.5 year | $1048f; Jawad and colleagues30 | |
| 5 years | $4716g; Matthys39 (ball attachment) | |
| $4302g; Matthys39 (locator attachment) | ||
| 8 years | $3683h; Stoker and colleagues31 (Dalla Bona ball) | |
| $3849h; Stoker and colleagues31 (bar construction) | ||
| 5 years | $3288i; Wetzels and colleagues35 (DAS protocol) | |
| $6108i; Wetzels and colleagues35 (P protocol) | ||
| 3 years | $5413b; Zitzmann and colleagues37 | |
| IOD on four mandibular implants | 8 years | $4912h; Stoker and colleagues31 (bar construction) |
| 3 years | $10881b; Zitzmann and colleagues37 | |
| IOD on mandibular MDIs | 0.5 year | $318e; Della Vechia and colleagues28 (two MDIs) |
| $511e; Della Vechia and colleagues28 (four MDIs) | ||
| $620f; Jawad and colleagues30 (two MDIs) | ||
| IOD‐4 in maxilla | 10 years | $7494j; Listl and colleagues40 (IOD‐4) |
| IOD‐6 in maxilla | $8697j; Listl and colleagues40 (IOD‐6) |
Abbreviations: CD, conventional denture; IOD, implant‐supported overdenture; MDI, mini dental implants.
Note: Conversion table27: https://www.ofx.com/en‐au/forex‐news/historical‐exchange‐rates/yearly‐average‐rates/.
Heydecke and colleagues used 1999–2000 Canadian dollars (CAD) in their article (1 CAD = 0.6733 USD).
Zitzmann and colleagues used 2000 Swiss francs (CHF) in their article (1 CHF = 0.61 USD).
Alfadda and Attard used 2016 Canadian dollars (CAD) in their article (1 CAD = 0.7551 USD).
Attard and colleagues used 2002 Canadian dollars (CAD) in their article (1 CAD = 0.6367 USD).
Della Vecchia and colleagues used 2014 Brazilian reals (BRL) in their article (1 BRL = 0.5720 USD).
Jawad and colleagues used 2017 British pound sterling (GBP) in their article (1 GBP = 1.288 USD).
Matthys and colleagues used 2020 Euros in their article (1 EUR = 1.1290 USD).
Stoker and colleagues used 2000 Euros (EUR) in their article (1 EUR = 1.0850 USD).
Wetzels and colleagues used 2008 Euros (EUR) in their article (1 EUR = 1.4713 USD).
Listl and colleagues used 2014 Euros (EUR) in their article (1 EUR = 13 292 USD).
Initial costs were low in Canada; $627 for a CD versus $1796 for an IOD‐2.29 In Switzerland initial prices were higher: $1540 for a CD and $4230 for an IOD‐2.37 It became clear that an IOD‐2 is 2–3 times more expensive than a CD in terms of initial costs.
After 1 year, Heydecke and colleagues12 calculated $1385 of total costs for a CD, which increased to $3801 after 17.9 years.29 For the IOD‐2 costs were $2458 after 1 year, which went up to $5960 in 17.9 years. Initially, an IOD‐2 was almost 3 times more expensive than a CD; however, after 17.9 years this ratio decreased to less than 2 times.29 Apparently, an IOD‐2 becomes relatively cheaper in time and, however, continues to be more expensive than a CD.29 This outcome was corroborated by Zitzmann and colleagues, who calculated that total costs after 3 years were $2242 and $5413, for a CD and IOD‐2, respectively, resulting in a ratio of 2.4.37
Although the phrase “total costs” was often used, the definitions of this term varied. Initial costs were calculated mostly on an individual basis including, if present, the national dental tariff structure for the purchase of the implants, costs of surgical treatment such as the salary of the clinical workers and supporting personnel, the use of the operating room, and medicines. Costs of the prosthodontic treatment were also included in this category, in addition to laboratory fees. “Maintenance costs” comprised the ongoing costs of the prosthodontic treatment and laboratory fees, such as remakes, relines, hardware replacement, and professional services provided by the prosthodontist and/or the surgeon. Sometimes, the costs of annual recall visits (“recall costs”) were included in the “maintenance costs.” Only few studies included “patient time costs,” corresponding to the loss of income from missing work due to treatment or traveling.33, 34, 37
3.4. CEA/CUA: Patient‐reported outcome measures
PROMs can be expressed using satisfaction questionnaires. For example, the McGill denture satisfaction questionnaire46 was applied by Della Vecchia and colleagues.28 Using a VAS scale (0–100 mm), the following variables were assessed: general satisfaction, ability to speak, and esthetics. In addition, the ability to chew five different foods was recorded: standard‐sized pieces (3 × 1 × 1 cm) of raw apple, bread, raw carrot, cheese, and dry sausage. An alternative is the Denture Satisfaction Scale (DSS),47 as executed by Attard and colleagues,34 which comprises 12 questions and is scored using a 5‐point Likert scale with the following categories: (1) totally satisfied, (2) very satisfied, (3) reasonably satisfied, (4) not very satisfied, and (5) not at all satisfied. Other authors compiled their own questionnaire with different numbers of questions and scales.32, 35, 36, 37, 40, 41, 42
To measure OHRQoL in dentistry, one of the Oral Health Impact Profile (OHIP)‐lists can be used, which focuses solely on toothless patients, such as OHIP‐14, OHIP‐EDENT, and OHIP‐20, which comprise 14, 19, and 20 questions, respectively.48 Similar to the original OHIP‐49, the OHIP‐20 and OHIP‐14 cover the same seven domains: functional limitation, pain, psychological discomfort, physical disability, psychological disability, social disability, and handicap.20 The responses are based on a Likert scale ranging from 0 for “never” to 4 for “very often,” meaning the maximum score for OHIP‐20 is 80; the lower the score, the higher the OHRQoL that is achieved.
The effect of treatment using the OHIP system as PROM is depicted in Table 5. The OHIP‐20 questionnaire was applied in four studies,29, 30, 33, 34 the OHIP‐14 in two studies,38, 39 and the OHIP‐EDENT in one study.28 Sometimes different Likert scales were used; for example, with total scores in the range of 0–8029, 33 or of 20–100.34 Others introduced their own OHIP‐20 version,30 a 6‐point Likert scale varying between 1 and 6, covering nine items: (1) ease of cleaning, (2) general satisfaction, (3) ability to speak, (4) comfort, (5) esthetics, (6) stability, (7) ability to chew, (8) function, and (9) oral condition.30
TABLE 5.
Change in OHIP points as a result of a CD, IOD‐2, IOD‐4, IOD on two MDIs, IOD on four MDIs
| Article | Mandibular IODs versus CDs: OHRQoL scored in three types of OHIP questionnaires; OHIP‐14, OHIP‐20, and OHIP‐EDENT | Effect in QAPYs | ||
|---|---|---|---|---|
| Alfadda and Attard33 | CD “old” (baseline) | 71 OHIP‐20 (Likert 0–4) | ||
| CD new | 51 OHIP‐20 (Likert 0–4) | |||
| IOD‐2 “immediate” loading |
28 OHIP‐20 (Likert 0–4; after 1 year) 25 OHIP‐20 (Likert 0–4; after 5 years) 34 OHIP‐20 (Likert 0–4; after 14 years) |
|||
| Attard and colleagues34 | CD “old” (baseline) | 71 OHIP‐20 (Likert 1–5) | ||
| CD new | 50 OHIP‐20 (Likert 1–5) | |||
| IOD‐2 | 24 OHIP‐20 (Likert 1–5; after 1 year) | |||
| Della Vecchia and colleagues28 | CD “old” (baseline | 14–18 OHIP‐EDENT (Likert 0–2) | ||
| IOD‐2 | 6 OHIP‐EDENT (Likert 0–2; after 0.5 years) | |||
| IOD‐2 on MDIs | 3 OHIP‐EDENT (Likert 0–2; after 0.5 years) | |||
| IOD‐2 on MDIs | 2 OHIP‐EDENT (Likert 0–2; after 0.5 years) | |||
| Heydecke and colleagues29 | CD “old” (baseline) | 56 OHIP‐20 (Likert 0–4) | ||
| CD new | 47 OHIP‐20 (Likert 0–4; after 1 and 17.9 years) | |||
| IOD‐2 | 31 OHIP‐20 (Likert 0–4; after 1 and 17.9 years) | |||
| Jawad and colleagues30 | IOD‐2 | 41 OHIP‐20 (Likert 1–6; after 0.5 years) | ||
| IOD‐2 on MDIs | 56 OHIP‐20 (Likert 1–6; after 0.5 years) | |||
| Matthys and colleagues38 | CD “old” | 20 OHIP‐14 (Likert 0–4; during intake) | ||
| IOD‐2 | 3 OHIP‐14 (Likert 0–4; after 1 and 5 years) | |||
| Matthys and colleagues39 | IOD‐2 (locators) | 9 OHIP‐14 point reduction; after 5 years | ||
| IOD‐2 (ball attachment) | 3 OHIP‐14 points reduction; after 5 years | |||
| Zitzmann and colleagues37 | IOD‐4 |
Dental health state preference VAS 0–1 |
CD “old” (baseline): 0.37 | IOD‐4: 1.57 |
| IOD‐2 | CD “old” (baseline): 0.35 | IOD‐2: 1.46 | ||
| CD new | CD “old” (baseline): 0.52 | CD new: 0.68 | ||
Abbreviations: CD, conventional denture; IOD, implant‐supported overdenture; MDI, mini dental implants; OHIP, Oral Health Impact Profile; QAPYs, quality‐adjusted prosthesis years.
The OHIP‐14 comprises 14 questions, each with a score between 0 (very positive) and 4 (very negative), resulting in a maximum score of 56. This was only used by Matthys and colleagues.38, 39
Della Vecchia and colleagues had a preference for the OHIP‐EDENT, which consists of 19 questions with answers on a Likert scale of 0–2, leading to a maximum score of 38.28 Only four domains were covered: masticatory discomfort, psychological discomfort, social disability, and oral pain/discomfort.49
As alternative measure for PROMs, Zitzmann and colleagues used QAPYs, which corresponds to functioning for 1 year in the best possible prosthetic state.37
3.5. CEA/CUA: Incremental cost‐effectiveness ratios
The cost‐effectiveness of an IOD on four to six maxillary implants was calculated in only one study.40 All others addressed an IOD in the lower jaw.28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39
In a CEA or CUA always an ICER is presented. For a mandibular IOD‐2 versus a new CD, the ICER was $81 per OHIP‐20 point after 1 year34 and $152 per OHIP‐20 point after 17.9 years.29 Costs went up as the years passed: $129, $159, and $362 per OHIP‐20 point after 1, 5, and 14 years, respectively.33
Using the OHIP‐EDENT questionnaire, two studies proved that a mandibular IOD‐2 on two MDIs resulted in a lower ICER score than an IOD on conventional implants: $28 versus $4728 or $17 versus $39.30 Even an IOD‐4 on MDIs was cheaper than an IOD‐2 on conventional implants (ICER $38 vs. $47).28
Zitzmann and colleagues also calculated an ICER, but formulated the measured effect in QAPYs.37 The costs per QAPY were $5551 for an IOD‐2 versus $12078 for an IOD‐4 after 3 years. These amounts reduced in a 10‐year period to $2318 and $4331 for an IOD‐2 versus an IOD‐4, respectively (Table 6).
TABLE 6.
Incremental cost‐effectiveness ratio (ICER) of a CD, IOD‐2, IOD‐4, IOD on two MDIs, IOD on four MDIs in the lower jaw in USD
| Type of prosthesis in the lower jaw | Cost‐effectiveness presented as ICER | |
|---|---|---|
| IOD‐2 implants | Versus new CD | $86b per OHIP‐20 point after 1 year; Attard and colleagues34 |
| $152a per OHIP‐20 point after 17.9 years; Heydecke and colleagues29 | ||
|
$129c per OHIP‐20 point after 1 year; Alfadda and Attard33 $159c per OHIP‐20 point after 5 years; Alfadda and Attard33 $362c per OHIP‐20 point after 14 years; Alfadda and Attard33 | ||
| IOD‐2 | Versus CD “old” (baseline) | $47d per OHIP‐EDENT point after 0.5 year; Della Vecchia and colleagues28 |
| IOD‐2 on MDIs | $28d per OHIP‐EDENT point after 0.5 year; Della Vecchia and colleagues28 | |
| IOD‐4 on MDIs | $38d per OHIP‐EDENT point after 0.5 year; Della Vecchia and colleagues28 | |
| IOD‐2 | Versus CD “old” (baseline) |
$39e per OHIP‐EDENT point after 0.5 year; Jawad and colleagues30 $17e per OHIP‐EDENT point after 0.5 year; Jawad and colleagues30 |
| IOD‐2 MDIs | ||
| Type of prosthesis in the lower jaw | Cost‐effectiveness presented QAPYs | |
|---|---|---|
| IOD‐2 implants | Versus new CD | |
| IOD‐4 implants | ||
Abbreviations: CD, conventional denture; IOD, implant‐supported overdenture; MDI, mini dental implants; OHIP, Oral Health Impact Profile; QAPYs, quality‐adjusted prosthesis years.
Heydecke and colleagues used 1999–2000 Canadian dollars (CAD) in their article (1 CAD = 0.6733 USD).
Attard and colleagues used 2002 Canadian dollars (CAD) in their article (1 CAD = 0.6367 USD).
Alfadda and Attard used 2016 Canadian dollars (CAD) in their article (1 CAD = 0.7551 USD).
Della Vecchia and colleagues used 2014 Brazilian reals (BRL) in their article (1 BRL = 0.5720 USD).
Jawad and colleagues used 2017 British pound sterling (GBP) in their article (1 GBP = 1.288 USD).
Zitzmann and colleagues used 2000 Swiss francs (CHF) in their article (1CHF = 0.61 USD).
3.6. CBA: Willingness to pay
All four included CBA studies (Table 3) focused on the costs of a mandibular IOD‐2. Three studies originated from Canada,41, 43, 44 while one was conducted in Switzerland.42
Esfandiari and colleagues41 interviewed patients who participated 2 years earlier in a RCT8 in which they all received a new CD in their upper jaw, combined with a new CD or an IOD‐2 in their mandible. The authors claimed that about 50% of the participants would pay 3 times more for a mandibular IOD‐2 ($3399) than for a CD ($1133). If payment in monthly installments was allowed, even 96% of the respondents stated that they would pay $3399 for an IOD‐2 (Table 3). An average of 5 years after the mandibular IOD‐2 installation, Sendi and colleagues conducted a telephone interview with the request to answer eight questions. Retrospectively, satisfaction rate was queried for the time of IOD‐2 delivery, after 6 and 24 months, and at the moment of the interview.42 The average WTP price for an IOD‐2 was $4971.
Both studies conducted by Srivastava and colleagues addressed patients who were still dentate. In the first study questionnaires were used, in the second study WTP data were collected through telephone interviews or internet‐based questionnaires. Both studies delivered the same WTP price (about $5500) in one payment on condition of a 90% success rate.43, 44 Patients are willing to prepay $171 as one‐time assurance premium for private dental insurance, meaning that they will be fully covered for a mandibular IOD‐2 if needed in the future based on a 20% chance of becoming toothless.43 In case of a 20% chance to become edentulous, the WTP was $27 as monthly payments for private insurance. The WTP was higher when household income or dental needs were higher.44
In short, both dentate participants,43, 44 as well as edentulous patients who have already been treated with implants41, 42 were asked about WTP. Patient WTP for an IOD‐2 on interforaminal implants varied from $339941 to $4971.42
The WTA numbers are particularly interesting; when asked for how much money they would turn in their IOD‐2 and go back to their original CDs, five patients valued the IOD‐2 state from $26.15742 to priceless41, 42, 43, 44 (Table 3).
4. DISCUSSION
The findings of the present review indicated considerable variation in the type, reporting, and quality of economic evaluation studies on IODs in comparison with their baseline situation, which was always the existing or “old CD.” Different questionnaires, diverse definitions of costs versus health outcome calculation methods, and varying timeframes were applied. With respect to IODs, variations in availability and affordability, pricing policies, level of reimbursement, and the discount rate made a comparison of the selected studies difficult.
Checklists such as CHEERS are commonly used in reviews to standardize the assessment of quality or completeness with respect to the economic evaluations. There is some discussion about how to interpret such checklists; with respect to CHEERS, the minimum reported cutoff for an evaluation to be considered “high quality” was 63%, while the maximum cutoff was 94%.22
In our analysis, most CEA/CUA studies scored 63% or more, and thereby are judged at least as “acceptable.”32, 33, 35, 36, 37, 39, 40, 41, 43, 44, 46
Three studies scored between 42% and 58%; their contribution was “average” or “low.”30, 34, 38
Except for one study (Sendi and colleagues46), scores for CBAs were 50% illustrating that their contribution was “average.”41, 43, 44
4.1. CEA/CUA: Total costs
In Table 4, the “total costs” of various CD and IOD types for the edentulous lower jaw are shown.
Initial costs were low in Canada; $627 for a CD versus $1796 for an IOD‐2.29 In Switzerland initial prices were higher: $1540 for a CD and $4230 for an IOD‐2.37 It became clear that an IOD‐2 is 2–3 times more expensive than a CD in terms of initial costs.
After 1 year, Heydecke and colleagues12 calculated $1385 of total costs for a CD, which increased to $3801 after 17.9 years.29 For the IOD‐2 costs were $2458 after 1 year, which went up to $5960 in 17.9 years. Initially, an IOD‐2 was almost 3 times more expensive than a CD; however, after 17.9 years this ratio decreased to less than 2 times.29 Apparently, an IOD‐2 becomes relatively cheaper in time and, however, continues to be more expensive than a CD.29 This outcome was corroborated by Zitzmann and colleagues, who calculated that total costs after 3 years were $2242 and $5413, for a CD and IOD‐2, respectively, resulting in a ratio of 2.4.37
4.2. Initial costs/total costs
Costs of interventions are not limited to the initial treatment, but also include costs for follow‐up care, maintenance, complications, and patient time lost due to the performance of the treatment working and traveling. In addition, total costs need to be assessed over time. As such, costs should be discounted by an annual set rate.18 Taking all these factors in account, an IOD‐2 is initially 2–3 times more expensive than a CD in terms of initial costs. After 17.9 years this ratio decreased to less than 2 times.29, 37
Costs may differ significantly between patients and between healthcare systems; therefore, economic evaluations should be interpreted in the context of such cost structures. To allow comparison between studies, costs must at least be specified thoroughly within the environment in which they have been conducted.
4.3. Patient‐reported outcome measures
In health‐related economic evaluations of treatments, QALYs are used to express the gain both in the quality and length of life. For nonfatal conditions however, alternatives can be applied, such as satisfaction scores, OHIP indices, or QAPYs.50, 51 However, these differing evaluation methods cannot be compared to QALYs.52
With respect to satisfaction questionnaires, current score lists vary in scoring methodologies: some perform a VAS score, usually on a scale of 0–10 or 0–100, while others use a Likert scale to display their results. The use of the same questionnaire and the same scoring method enables the direct comparison of different studies. It is also crucial to realize that VAS scores are not ratio scale measurements. This implies that, for example, a difference between satisfaction scores of 20 and 40 is not comparable to a difference between scores of 70 and 90.53
A popular method is the use of OHIP question lists; however, these lists are not mutually comparable, and the scale also differs. To interpret, for example, an exact OHIP‐20 score, it is essential to know if a scale of 0–80 or 20–100 was used; however, differences calculated in OHIP‐20 points remain scale‐independent.
As the OHIP‐14 ranges from 0 to 56, and the OHIP‐EDENT from 0 to 38, the results described for the various outcomes are incomparable. To still allow comparison, the concept of a “minimum important difference” (MID) was introduced, which indicates the number of OHIP points that reflect a significant improvement. Locker and colleagues determined the MID for OHIP‐14, which was 5 scale points, or approximately 10% of the scale range of 56 points.54 For the OHIP‐20, a MID‐range was defined between 7 and 10, with a guide value of 9.55
When comparing the “old” CD (71 OHIP‐20 points) versus a mandibular IOD‐2 after 1 year (28 OHIP‐20 points), an improvement of 43 points was detected, which is more than 4 times the MID of 9 points.33 The same trend was seen in the study by Attard and colleagues; an improvement of 47 points was detected for an IOD‐2 versus the “old” CD.34 In comparison with a new CD, 16‐point29 or 26‐point34 differences in OHIP‐20 points were detected for the mandibular IOD‐2, which is about 2–3 times the MID value of 9 points.
Della Vecchia and colleagues reported in OHIP‐EDENT points.28 Although the MID was not established for the OHIP‐DENT, it could be set to four points using the 10% rule. In comparison with the “old” CD, an improvement of 16 points for the IOD‐2 on implants, of 11 points for the IOD‐2 on MDIs, and of 13 points for the IOD‐4 on MDIs was reported, all of which were about 3–4 times the MID; thus, similar to the level of change detected when using one of the other OHIP lists.28 In OHIP‐14 points, Matthys and colleagues presented an improvement of 16 points, which was more than 3 times the MID value of 5 points.38
In short, reported increases in OHRQoL are similar, regardless if the OHIP‐14, OHIP‐20, or OHIP‐EDENT methodology was used. Also, in QAPYs, a mandibular IOD‐2 yielded a satisfaction score 4 times higher than the “old” CD (0.35 vs. 1.46).37
4.4. Economic evaluations (ICERs)
With respect to CUAs delivering ICERs, four studies delivered an ICER comparing IODs with a new CD.29, 33, 34, 37 Another four studies compared IODs with an existing CD.28, 30, 38, 39
Heydecke and colleagues compared “between” two groups receiving either a new CD or a mandibular IOD‐2.29 The ICER for the IOD‐2 versus the new CD after 17.9 years was $152.29 Attard and colleagues measured “within” groups; first new dentures were made, then immediate‐loaded implants were installed and attached to the CDs to deliver an IOD‐2.34 After the first year they produced a lower ICER ($86), as no maintenance and recall costs were involved.
As the years go by, absolute costs increase over time due to continuous maintenance costs: $129 per OHIP‐20 point after 1 year, $159 per OHIP‐20 point after 5 years, and $362 per OHIP‐20 point after 14 years.33 To illustrate the extra value of an ICER, compared with a new CD, a maximum of $362 per OHIP‐20 point for the mandibular IOD‐2 was paid over a 14‐year period.33 In light of the reported improvement of 17 OHIP points over the full 14 years, this represents a total amount of $6154 after 14 years (or $440 per year).
Over the long term, Heydecke and colleagues reported lower costs.29 After 17.9 years they presented an ICER of $152 per OHIP‐20 point versus the new CD. With respect to the assessed decrease of 16 OHIP points, this translates to $136 per year for 17.9 years.29
The higher ICER ($362) and higher annual costs ($440) can be explained by the fact that Alfadda and Attard included the actual maintenance costs, while Heydecke and colleagues made an assumption about the long‐term costs using the Delphi group opinion technique.29, 33
In the short observation period of 0.5 years, it was concluded that a mandibular IOD‐2 on MDIs ($28 per OHIP‐EDENT point) was more cost‐effective than an IOD‐2 on conventional implants ($47 per OHIP‐EDENT point).28 This conclusion was corroborated by Jawad and colleagues, who presented a value of $17 per OHIP‐EDENT point for an IOD‐2 on MDIs versus $39 per OHIP‐EDENT point for an IOD‐2 on conventional implants.30 In both studies, solely edentulous individuals who wore clinically acceptable maxillary and mandibular CDs were included, and only the price of the implants and the attachment system, not the prosthetic costs, were included.
Using the method of calculating QAPYs, “total costs” were calculated for each QAPY. For a mandibular IOD‐2, $5551 per QAPY after 3 years was calculated and $2318 per QAPY after 10 years.37 The latter is in line with the $2432 calculated by Heydecke and colleagues.29 Also applying the QAPY methodology, costs are observed to decrease significantly over time.
WTP values for a mandibular IOD‐2 varied between $3399 and $548141, 44 while WTA values varied from $2615742 to priceless,41, 43, 44 thereby underlining the beneficial effect of an IOD. As WTP surveys patient preferences for an IOD in monetary terms, not only is the appreciation of the IOD itself reflected, but in a way it also clarifies how a patient may endure discomfort, inconvenience, and a loss of time.56 Considerable bias can be introduced by misleading, ambiguous, or inappropriate questions, however.57
5. CONCLUSIONS
Total costs for a mandibular IOD‐2 were associated with 2–3 times higher total costs compared to a CD. Regardless of whether QAPYs or one of the OHIP lists was used, this resulted in a significant improvement in OHRQoL of about 2 times the MID in comparison with a new CD.
Although ICERs give an improved insight into the relationship between incremental costs and increases in OHRQoL, the comparability of the different economic evaluation studies is still complicated by the use of different outcome measures.
Using the same strategy to register outcomes and the same method to present costs would be helpful. However, uncertainty remains as to whether the additional health benefits of an IOD outweigh the higher costs, and this largely depends on the decision maker's valuation of oral health outcomes. As hypothesized, the information available in so far literature seems too diverse to draw firm conclusions. Future research is encouraged to enhance the comparability of oral health outcomes with overall health and wellbeing.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTIONS
Thomas Van de Winkel conceptualized the project idea, conducted the literature search, collected and analyzed the data, and drafted the manuscript. Laura Heijens and Gert Meijer contributed to the data analysis. Laura Heijens, Stefan Listl, and Gert Meijer critically reviewed and revised the manuscript.
Van de Winkel T, Heijens L, Listl S, Meijer G. What is the evidence on the added value of implant‐supported overdentures? A review. Clin Implant Dent Relat Res. 2021;23(4):644–656. 10.1111/cid.13027
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Locker D. The burden of oral disorders in a population of older adults. Community Dent Health. 1992;9(2):109‐124. [PubMed] [Google Scholar]
- 2.Zarb GA. The edentulous milieu. J Prosthet Dent. 1983;49(6):825‐831. [DOI] [PubMed] [Google Scholar]
- 3.WHO . International Classification of Functioning, Disability, and Health: ICF: Version 1.0. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 4.Boerrigter EM, Stegenga B, Raghoebar GM, Boering G. Patient satisfaction and chewing ability with implant‐retained mandibular overdentures: a comparison with new complete dentures with or without preprosthetic surgery. J Oral Maxillofac Surg. 1995;53(10):1167‐1173. [DOI] [PubMed] [Google Scholar]
- 5.Meijer HJ, Raghoebar GM, Van't Hof MA, Geertman ME, Van Oort RP. Implant‐retained mandibular overdentures compared with complete dentures; a 5‐years' follow‐up study of clinical aspects and patient satisfaction. Clin Oral Implants Res. 1999;10(3):238‐244. [DOI] [PubMed] [Google Scholar]
- 6.Naert I, Gizani S, Vuylsteke M, Van Steenberghe D. A 5‐year prospective randomized clinical trial on the influence of splinted and unsplinted oral implants retaining a mandibular overdenture: prosthetic aspects and patient satisfaction. J Oral Rehabil. 1999;26(3):195‐202. [DOI] [PubMed] [Google Scholar]
- 7.Awad MA, Locker D, Korner‐Bitensky N, Feine JS. Measuring the effect of intra‐oral implant rehabilitation on health‐related quality of life in a randomized controlled clinical trial. J Dent Res. 2000;79(9):1659‐1663. [DOI] [PubMed] [Google Scholar]
- 8.Awad MA, Lund JP, Shapiro SH, et al. Oral health status and treatment satisfaction with mandibular implant overdentures and conventional dentures: a randomized clinical trial in a senior population. Int J Prosthodont. 2003;16(4):390‐396. [PubMed] [Google Scholar]
- 9.Klinge B, Flemming T, Cosyn J, et al. The patient undergoing implant therapy. Summary and consensus statements. The 4th EAO consensus conference 2015. Clin Oral Implants Res. 2015;26(suppl 11):64‐67. [DOI] [PubMed] [Google Scholar]
- 10.Vogel R, Smith‐Palmer J, Valentine W. Evaluating the health economic implications and cost‐effectiveness of dental implants: a literature review. Int J Oral Maxillofac Implants. 2013;28(2):343‐356. [DOI] [PubMed] [Google Scholar]
- 11.Feine JS, Carlsson GE, Awad MA, et al. The McGill consensus statement on overdentures. Mandibular two‐implant overdentures as first choice standard of care for edentulous patients. Montreal, Quebec, May 24–25, 2002. Int J Oral Maxillofac Implants. 2002;17(4):601‐602. [PubMed] [Google Scholar]
- 12.Heydecke G, Boudrias P, Awad MA, De Albuquerque RF, Lund JP, Feine JS. Within‐subject comparisons of maxillary fixed and removable implant prostheses: patient satisfaction and choice of prosthesis. Clin Oral Implants Res. 2003;14(1):125‐130. [DOI] [PubMed] [Google Scholar]
- 13.Slot W, Raghoebar GM, Vissink A, Huddleston Slater JJ, Meijer HJ. A systematic review of implant‐supported maxillary overdentures after a mean observation period of at least 1 year. J Clin Periodontol. 2010;37(1):98‐110. [DOI] [PubMed] [Google Scholar]
- 14.von Wowern N, Gotfredsen K. Implant‐supported overdentures, a prevention of bone loss in edentulous mandibles? A 5‐year follow‐up study. Clin Oral Implants Res. 2001;12(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 15.AH W . The economic role of “health indicators.” Paper presented at: Proceedings of a Meeting on Measuring the Social Benefits of Medicine; May 16–19, 1983; Brunel University, London.
- 16.Drummond MF, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford, UK: Oxford Medical Publications; 1987. [Google Scholar]
- 17.Garber AM. Advances in cost‐effectiveness analysis of health interventions. Handbook of Health Economics. Oxford: Elsevier; 2000:181‐221. [Google Scholar]
- 18.Higgins AM, Harris AH. Health economic methods: cost‐minimization, cost‐effectiveness, cost‐utility, and cost‐benefit evaluations. Crit Care Clin. 2012;28(1):11‐24. [DOI] [PubMed] [Google Scholar]
- 19.Listl S, Grytten JI, Birch S. What is health economics? Community Dent Health. 2019;36(4):262‐274. [DOI] [PubMed] [Google Scholar]
- 20.Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dent Health. 1994;11(1):3‐11. [PubMed] [Google Scholar]
- 21.Beikler T, Flemmig TF. EAO consensus conference: economic evaluation of implant‐supported prostheses. Clin Oral Implants Res. 2015;26(suppl 11):57‐63. [DOI] [PubMed] [Google Scholar]
- 22.Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ. 2013;346:f1049. [DOI] [PubMed] [Google Scholar]
- 23.Jönsson B, Karlsson G. Cost‐benefit evaluation of dental implants. Int J Technol Assess Health Care. 1990;6(4):545‐557. [DOI] [PubMed] [Google Scholar]
- 24.Matthews D, Rocchi A, Wang EC, Gafni A. Use of an interactive tool to assess patients' willingness‐to‐pay. J Biomed Inform. 2001;34(5):311‐320. [DOI] [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159‐174. [PubMed] [Google Scholar]
- 26.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; 2011. https://www.handbook.cochrane.org. [Google Scholar]
- 27.OFX . Yearly average rates & forex history data | OFX. https://www.ofx.com/en-au/forex-news/historical-exchange-rates/yearly-average-rates/.
- 28.Della Vecchia MP, Leles CR, Cunha TR, et al. Mini‐implants for mandibular overdentures: cost‐effectiveness analysis alongside a randomized trial. JDR Clin Trans Res. 2018;3(1):47‐56. [DOI] [PubMed] [Google Scholar]
- 29.Heydecke G, Penrod JR, Takanashi Y, Lund JP, Feine JS, Thomason JM. Cost‐effectiveness of mandibular two‐implant overdentures and conventional dentures in the edentulous elderly. J Dent Res. 2005;84(9):794‐799. [DOI] [PubMed] [Google Scholar]
- 30.Jawad S, Barclay C, Whittaker W, Tickle M, Walsh T. A pilot randomised controlled trial evaluating mini and conventional implant retained dentures on the function and quality of life of patients with an edentulous mandible. BMC Oral Health. 2017;17(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoker GT, Wismeijer D, van Waas MA. An eight‐year follow‐up to a randomized clinical trial of aftercare and cost‐analysis with three types of mandibular implant‐retained overdentures. J Dent Res. 2007;86(3):276‐280. [DOI] [PubMed] [Google Scholar]
- 32.Timmerman R, Stoker GT, Wismeijer D, Oosterveld P, Vermeeren JI, van Waas MA. An eight‐year follow‐up to a randomized clinical trial of participant satisfaction with three types of mandibular implant‐retained overdentures. J Dent Res. 2004;83(8):630‐633. [DOI] [PubMed] [Google Scholar]
- 33.Alfadda SA, Attard NJ. A cost analysis of a long‐term prospective study of patients treated with immediately loaded implant‐supported mandibular overdentures. Clin Implant Dent Relat Res. 2017;19(5):944‐951. [DOI] [PubMed] [Google Scholar]
- 34.Attard NJ, Laporte A, Locker D, Zarb GA. A prospective study on immediate loading of implants with mandibular overdentures: patient‐mediated and economic outcomes. Int J Prosthodont. 2006;19(1):67‐73. [PubMed] [Google Scholar]
- 35.Wetzels JW, Koole R, Meijer GJ, de Haan AF, Merkx MA, Speksnijder CM. Functional benefits of implants placed during ablative surgery: a 5‐year prospective study on the prosthodontic rehabilitation of 56 edentulous oral cancer patients. Head Neck. 2016;38(suppl 1):E2103‐E2111. [DOI] [PubMed] [Google Scholar]
- 36.Wetzels JGH, Meijer GJ, Koole R, Adang EM, Merkx MAW, Speksnijder CM. Costs and clinical outcomes of implant placement during ablative surgery and postponed implant placement in curative oral oncology: a five‐year retrospective cohort study. Clin Oral Implants Res. 2017;28(11):1433‐1442. [DOI] [PubMed] [Google Scholar]
- 37.Zitzmann NU, Marinello CP, Sendi P. A cost‐effectiveness analysis of implant overdentures. J Dent Res. 2006;85(8):717‐721. [DOI] [PubMed] [Google Scholar]
- 38.Matthys C, Vervaeke S, Besseler J, De Bruyn H. Five‐year study of mandibular overdentures on stud abutments: clinical outcome, patient satisfaction and prosthetic maintenance‐influence of bone resorption and implant position. Clin Oral Implants Res. 2019;30(9):940‐951. [DOI] [PubMed] [Google Scholar]
- 39.Matthys C, De Vijlder W, Besseler J, Glibert M, De Bruyn H. Cost‐effectiveness analysis of two attachment systems for mandibular overdenture. Clin Oral Implants Res. 2020;31(7):615‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Listl S, Fischer L, Giannakopoulos NN. An economic evaluation of maxillary implant overdentures based on six vs four implants. BMC Oral Health. 2014;14:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esfandiari S, Lund JP, Penrod JR, Savard A, Thomason JM, Feine JS. Implant overdentures for edentulous elders: study of patient preference. Gerodontology. 2009;26(1):3‐10. [DOI] [PubMed] [Google Scholar]
- 42.Sendi P, Bertschinger N, Brand C, Marinello CP, Bucher HC, Bornstein MM. Measuring the monetary value of dental implants for denture retention: a willingness to pay approach. Open Dent J. 2017;11:498‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srivastava A, Feine JS, Esfandiari S. Are people who still have their natural teeth willing to pay for mandibular two‐implant overdentures? J Investig Clin Dent. 2014;5(2):117‐124. [DOI] [PubMed] [Google Scholar]
- 44.Srivastava A, Esfandiari S, Madathil SA, Birch S, Feine JS. Willingness to pay for mandibular overdentures: a societal perspective. JDR Clin Trans Res. 2020;5(1):30‐39. [DOI] [PubMed] [Google Scholar]
- 45.Dalkey NC. The Delphi method: an experimental study of group opinion. In: Dalkey NC, Rourke D, Lewis R, Snyder D, eds. Studies in the Quality of Life: Delphi and Decision‐Making. Lexington, MA: Lexington Books; 1972:13‐54. [Google Scholar]
- 46.de Grandmont P, Feine JS, Taché R, et al. Within‐subject comparisons of implant‐supported mandibular prostheses: psychometric evaluation. J Dent Res. 1994;73(5):1096‐1104. [DOI] [PubMed] [Google Scholar]
- 47.Allen PF, McMillan AS, Walshaw D. A patient‐based assessment of implant‐stabilized and conventional complete dentures. J Prosthet Dent. 2001;85(2):141‐147. [DOI] [PubMed] [Google Scholar]
- 48.Pommer B. Use of the Oral Health Impact Profile (OHIP) in clinical oral implant research. J Dent Oral Craniofac Epidemiol. 2013;1:3‐10.26618183 [Google Scholar]
- 49.de Souza RF, Ribeiro AB, Della Vecchia MP, et al. Mini vs. standard implants for mandibular overdentures: a randomized trial. J Dent Res. 2015;94(10):1376‐1384. [DOI] [PubMed] [Google Scholar]
- 50.Barkun AN, Barkun JS, Sampalis JS, et al. Costs and effectiveness of extracorporeal gallbladder stone shock wave lithotripsy versus laparoscopic cholecystectomy. A randomized clinical trial. McGill Gallstone Treatment Group. Int J Technol Assess Health Care. 1997;13(4):589‐601. [DOI] [PubMed] [Google Scholar]
- 51.Sevick MA, Bradham DD, Muender M, et al. Cost‐effectiveness of aerobic and resistance exercise in seniors with knee osteoarthritis. Med Sci Sports Exerc. 2000;32(9):1534‐1540. [DOI] [PubMed] [Google Scholar]
- 52.Hettiarachchi RM, Kularatna S, Downes MJ, et al. The cost‐effectiveness of oral health interventions: a systematic review of cost‐utility analyses. Community Dent Oral Epidemiol. 2018;46(2):118‐124. [DOI] [PubMed] [Google Scholar]
- 53.Walton JN, Glick N, Macentee MI. A randomized clinical trial comparing patient satisfaction and prosthetic outcomes with mandibular overdentures retained by one or two implants. Int J Prosthodont. 2009;22(4):331‐339. [PubMed] [Google Scholar]
- 54.Locker D, Jokovic A, Clarke M. Assessing the responsiveness of measures of oral health‐related quality of life. Community Dent Oral Epidemiol. 2004;32(1):10‐18. [DOI] [PubMed] [Google Scholar]
- 55.Allen PF, O'Sullivan M, Locker D. Determining the minimally important difference for the Oral Health Impact Profile‐20. Eur J Oral Sci. 2009;117(2):129‐134. [DOI] [PubMed] [Google Scholar]
- 56.Birch S, Sohn W, Ismail AI, Lepkowski JM, Belli RF. Willingness to pay for dentin regeneration in a sample of dentate adults. Community Dent Oral Epidemiol. 2004;32(3):210‐216. [DOI] [PubMed] [Google Scholar]
- 57.MacEntee MI. Measuring the impact of oral health in old age: a qualitative reaction to some quantitative views. Gerodontology. 1996;13(2):76‐81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


