Abstract
Immunoglobulin G (IgG) therapy is an established long‐term treatment in chronic inflammatory demyelinating polyneuropathy (CIDP) that is commonly administered intravenously (IVIg). The subcutaneous immunoglobulin (SCIg) administration route is a safe and effective alternative option, approved by the United States Food and Drug Administration (FDA) in 2018, for maintenance treatment of adults with CIDP. Physicians and patients alike need to be aware of all their treatment options in order to make informed decisions and plan long‐term treatment strategies. In this review, we collate the evidence for SCIg in CIDP from all published studies and discuss their implications and translation to clinical practice. We also provide guidance on the practicalities of how and when to transition patients from IVIg to SCIg and ongoing patient support. Evidence suggests that IVIg and SCIg have comparable long‐term efficacy in CIDP. However, SCIg can provide additional benefits for some patients, including no requirement for venous access or premedication, and reduced frequency of systemic adverse events. Local‐site reactions are more common with SCIg than IVIg, but these are mostly well‐tolerated and abate with subsequent infusions. Data suggest that many patients prefer SCIg following transition from IVIg. SCIg preference may be a result of the independence and flexibility associated with self‐infusion, whereas IVIg preference may be a result of familiarity and reliance on a healthcare professional for infusions. In practice, individualizing maintenance dosing based on disease behavior and determining the minimally effective IgG dose for individuals are key considerations irrespective of the administration route chosen.
Keywords: CIDP, immunoglobulin therapy, IVIg, SCIg, transition
Abbreviations
- AE
adverse event
- CIDP
chronic inflammatory demyelinating polyneuropathy
- COVID‐19
coronavirus disease 2019
- EFNS
European Federation of Neurological Societies
- EQ‐5D
EuroQoL 5 Dimension
- EU
European Union
- FDA
United States Food and Drug Administration
- fSCIG
facilitated SCIg
- HCP
healthcare professional
- ICE
Immunoglobulin Intravenous CIDP Efficacy (trial)
- IgG
immunoglobulin G
- INCAT
Inflammatory Neuropathy Cause and Treatment
- I‐RODS
Inflammatory Neuropathy‐Rasch‐Built Overall Disability Scale
- ITT
intention‐to‐treat
- IVIg
intravenous immunoglobulin
- LQI
Life Quality Index
- MG
myasthenia gravis
- MMN
multifocal motor neuropathy
- MRC
Medical Research Council
- ODSS
Overall Disability Sum Score
- OLE
open‐label extension
- PATH
Polyneuropathy and Treatment with Hizentra
- PFS
pre‐filled syringe
- PID
primary immunodeficiency
- PNS
Peripheral Nerve Society
- QoL
quality of life
- SC
subcutaneous
- SCIg
subcutaneous immunoglobulin
- SmPC
Summary of Product Characteristics
- TEE
thromboembolic event
- TRF
treatment‐related fluctuation
- US
United States
- USPI
United States Prescribing Information
- VAS
Visual Analog Scale
1. INTRODUCTION
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an immune‐mediated neurological disorder causing demyelination of the peripheral nerves.1 There are several variants and disease courses (progressive, relapsing–remitting or monophasic), but typically CIDP is characterized by symmetrical muscle weakness and impaired sensory function in distal and proximal limbs.1, 2 Unlike most other neuropathies, CIDP is treatable and reversible.3 The aim of treatment is to reduce symptoms and improve muscle and sensory function.4 Approximately 30% of CIDP patients can be cured (stable and off treatment for 5 or more years) or enter remission (stable and off any treatment for less than 5 y).5 It remains a challenge to balance maintaining long‐term remission without overtreating the patient, versus the risk of relapse if treatment is stopped or reduced prematurely.4, 6
Established first‐line induction therapy options include intravenous immunoglobulin (IVIg), corticosteroids, and plasma exchange.7 Studies have shown that corticosteroids and plasma exchange can effectively treat CIDP on a short‐term basis.7 Short‐term corticosteroid use has in some cases led to periods of drug‐free remission (up to 12 mo).8 Evidence suggests corticosteroids may be effective longer term,8, 9 however, intolerance and side effects often prevent their long‐term use.7 IVIg has demonstrated long‐term efficacy in CIDP maintenance therapy, but IV administration can pose challenges for some patients.10 Subcutaneous immunoglobulin (SCIg) has been used for decades in other areas, but it is a relatively recent mode of immunoglobulin G (IgG) therapy in CIDP and requires a certain level of familiarity with dosing and administration unique to the subcutaneous (SC) route of administration. Particularly in the coronavirus disease 2019 (COVID‐19) environment, SCIg self‐administration at home can provide more patient autonomy and potentially less inadvertent risk of exposure compared with nurse‐administered IVIg and/or attendance at an infusion center. Factors dictating the choice of maintenance treatment are varied and will ultimately impact patient decision. Therefore, it is crucial to outline all the treatment options as early as possible to allow informed decisions.
In this review, we consolidate the findings of SCIg studies in CIDP with a focus on the practical application of the data. We aim to provide an overview for clinicians including when to consider SCIg, initiating the transition from IVIg to SCIg, dose adjustments, and long‐term patient support and retention.
2. COMPARISON OF IVIg AND SCIg THERAPY
IVIg has been approved for CIDP treatment in the United States (US) since 2008 following the randomized, double‐blind, placebo‐controlled Immunoglobulin Intravenous CIDP Efficacy (ICE) study.11, 12 Results showed a clinically meaningful improvement in disability (assessed by the Inflammatory Neuropathy Cause and Treatment [INCAT]) at 24 wk in 54% of patients who received IVIg versus 21% of patients who received placebo.11 Efficacy of IVIg for up to 52 wk was also seen in a single‐arm, open trial, although a higher frequency of adverse events (AEs; 94%) was observed in this trial compared with the 75% seen in the ICE study (75%).11, 13 In a recent online survey of 100 US community neurologists, nearly half reported using IVIg alone as their first treatment of choice for CIDP.4 The 2010 European Federation of Neurological Societies/Peripheral Nerve Society (EFNS/PNS) guidelines recommend that IVIg should be individualized to achieve the lowest effective maintenance dose and periodically reduce the dose, or stop IVIg, to determine the need for ongoing therapy.14 Guidelines from the EFNS/PNS, currently under development, should provide more clarity on clinical definitions, electrophysiologic criteria, implications of nodal and paranodal antibodies, individualizing treatment, and inclusion of SCIg as an alternative option to maintain patients and optimize treatment.15
SCIg was first approved by the FDA in 2018 for maintenance therapy in adult patients with CIDP based on the Polyneuropathy and Treatment with Hizentra (PATH) study findings,16 but has been used successfully in other conditions such as primary immunodeficiency (PID) for over two decades.17 Collective experience with over 300 patients with CIDP, treated with various SCIg products over the past 15 y, supports the use of SCIg as a maintenance therapy for CIDP (Table 1). Additionally, long‐term SCIg data are now available supporting continued stabilization and, in some cases, improvements in function and quality of life (QoL), for up to 7 y post initiation of SCIg therapy (N = 17, SCIg mean duration was 4.8 y [2–7 y] and an average dose of 18.5 g/wk).18
TABLE 1.
Summary of main findings from SCIg studies in CIDP
| Study | Study design | No. patients | Follow‐up (mo) | Main findings |
|---|---|---|---|---|
| Köller, et al. 200621 | Case report | 1 CIDP; 2 MMN | 6 | SCIg was well tolerated with high patient satisfaction. CIDP patient improved by INCAT disability score and MRC sum score. |
| Lee, et al. 200822 | Case report | 2 CIDP | 8–24 | SCIg was well tolerated, easy to manage and stabilization disease course. No systemic or serious side effects were reported, only mild swelling at the infusion site. |
| Cocito, et al. 201123 | Prospective, open‐label, longitudinal | 5 CIDP | 6 | SCIg efficacy, QoL, and patient satisfaction were comparable with previous IVIg—4/5 patients preferred SCIg to IVIg. A reduction in side effects was observed with SCIg and no need for pre‐medication. |
| Markvardsen, et al. 201324 | Randomized, double‐blind, placebo‐controlled | 29 CIDP (14 SCIg vs 15 placebo) | 3 | Muscle strength and disability were improved with SCIg—70% of patients preferred SCIg to IVIg. Side effects were limited to mild infusion site reactions. |
| Cocito, et al. 201325 | Open label (SCIG 16% vs SCIG 20%) | 10 CIDP | 3 + 3 | LQI score was higher with SCIg 20% versus SCIg 16% (most likely due to less frequent infusions versus SCIg 16%) |
| Markvardsen, et al. 201426 | Prospective, open‐label extension study | 17 CIDP (from previous 2013 study) | 12 | Muscle strength and disability were preserved after 1 y receiving SCIg. |
| Cocito, et al. 201427 | Prospective, multicenter case series | 66 CIDP; 21 MMN | 4 | ONLS was significantly improved with SCIg; MRC score was minimally improved |
| Hadden, et al. 201528 | Partially prospective case series | 4 CIDP; 4 MMN | 33 (mean) | Tolerability and patient satisfaction were improved with SCIg; patients also remained clinically stable (based on MRC and ONLS scores) |
| Yoon, et al. 201529 | Retrospective case series | 3 CIDP; 1 MMN; 1 MG | 39 (mean) | Patients remained stable with no serious side effects; SCIg was well‐tolerated and preferred to IVIg by all patients |
| Cocito, et al. 201630 | Prospective, multicenter case‐series | 45 CIDP; 21 MMN (from previous 2014 study) | 24 | Adherence to SCIg was 76% at 2 y and patient satisfaction was significantly increased |
| Markvardsen, et al. 201731 | Randomized, single blind, cross‐over | 19 CIDP (treatment‐naïve) | 5 | Similar efficacy, but maximal improvement in muscle strength was by 5 wk with SCIg versus 2 wk with IVIg |
| Van Schaik, et al. 201816 | Randomized, double‐blind, placebo‐controlled | 172 CIDP (115 SCIg vs 57 placebo) | 6 | Two doses of SCIg (0.2 and 0.4 g/kg) were efficacious and well‐tolerated. Over half preferred SCIg to their previous IVIg. |
| Cirillo, et al. 201832 | Prospective, open‐label cohort | 16 CIDP | 24 | Primary demyelinating features of nerve conduction, and clinical variables (MRC sum, INCAT, ODSS) were significantly improved with SCIg |
| Van Schaik, et al. 201933 | Prospective, open‐label extension study | 82 CIDP (from previous 2018 study) | 12 | SCIg demonstrated long‐term efficacy and safety at both doses, although lower relapse rates were reported on the 0.4 g/kg dose |
| Gentile, et al. 202018 | Retrospective, case series | 17 CIDP | 84 | Strength and motor functions remained stable or improved with long‐term SCIg |
Abbreviation: ONLS, Overall Neuropathy Limitation Scale.
Currently, there are no data available from head‐to‐head trials comparing relapse rates in maintenance therapy with IVIg and SCIg.19 Findings from the studies outlined in Table 1 suggest similar efficacy. A 2017 meta‐analysis of eight studies concluded that the efficacy (measured by muscle strength) of SCIg is comparable with IVIg in the treatment of CIDP (n = 88) and multifocal motor neuropathy (MMN) (n = 50).20 IVIg and SCIg therapy have distinguishing attributes that will appeal to patients differently depending on their circumstances and lifestyle (Table 2). SCIg can offer important safety and QoL advantages compared with IVIg, such as avoidance of regular venous access and reduced systemic AEs. However, disadvantages of SCIg for some patients can be the need for weekly infusions and the potential for local‐site reaction.
TABLE 2.
Comparison of IVIg and SCIg
| IVIg (10%) | SCIg (20%) | |
|---|---|---|
| Infusion regimen | ||
| Dose33, 34, 35 |
Induction: 2 g/kg body weight (20 mL/kg) in divided doses over 2–5 consecutive days Maintenance: 1 g/kg (10 mL/kg) administered In 1 or 2 infusions on consecutive days |
Induction: Not approved for induction therapy Maintenance: 0.2 g/kg (1 mL/kg) in 1 or 2 sessions. Higher doses up to 0.4 g/kg (2 mL/kg) may be considered |
| Infusion rate and volume33, 34, 35 |
Initial: 0.5–5 mg/kg/min Maintenance: 8 mg/kg/min |
Initial volume: ≤20 mL/site Max. Volume: ≤50 mL/site Initial rate: ≤20 mL/hr/site Max. rate: ≤50 mL/hr/site Maximum infusion sites: ≤8 |
| Frequency of infusions33, 34, 35 |
Every 3‐4 wk (can be administered more frequently) |
Weekly |
| No. of infusion sites34 | 1 | 1–8 |
| Overall infusion time18 a | 3–5 h/mo | 1–1.5 h/wk |
| Onset of action11, 16, 30 | 1–2 wk | Relatively slow—4 wk (if not started 1 wk after last IVIg dose) |
| IgG level profile36 | Cyclical, troughs and peaks | Near steady‐state |
| Other factors | ||
| Setting18 | Hospital, infusion clinic, or at home with an infusion nurse | Home, work, school etc. |
| HCP required37 | Yes | No |
| Systemic AEs2 | Yes | Reduced |
| Local AEs | Rarely | Common |
| Need for premedication16 | Common | Rarely |
| Venous access required | Yes | No |
| TRFs36 | Wear‐off effects can occur between doses | Potentially improved due to more regular/more frequent infusions compared with IV |
| Bioavailability2 | Higher than SCIg | Estimated to be ≈ 85% compared with IVIg |
| High‐dose requirementb | High doses relatively unaffected by BMI or tissue volume as delivered intravenously | High doses can be limited by available subcutaneous tissue volume in low BMI patients; may require a higher number of infusion sites |
| Patient satisfaction38 | Mixed | Mixed, but generally improved versus IVIg |
| Cost2 | High acquisition cost | High acquisition cost, but may be associated with reduced overall costs in the long term due to less hospital visits and HCP resource |
Abbreviation: BMI, body mass index.
Estimated range; actual infusion times will vary depending on infusion regimen and patient tolerability. Infusion regimens here are based on US prescribing information and may vary by country. For more information, please refer to your local prescribing information.
Obese patients (BMI > 30 kg/m2) are more likely to have cardiovascular risk factors and have a higher risk of AEs as a result of higher IgG doses; therefore, caution is recommended.39
3. KEY FINDINGS FROM THE PATH AND OPEN‐LABEL EXTENSION STUDIES
The study demonstrated that SCIg was efficacious and well tolerated in patients with CIDP previously stabilized on IVIg and treated for 24 wk.16 Patients were randomized to receive 24 wk of weekly SCIg doses at 0.2 g/kg (n = 57), 0.4 g/kg (n = 58), or placebo (n = 57).16 Fewer patients treated with SCIg relapsed compared with placebo. Most patients showed improvements in their INCAT total score, grip strength, and Medical Research Council (MRC) sum scores compared with the placebo group, with no statistically significant difference observed between the two SCIg doses. However, the 0.4 g/kg dose (but not the 0.2 g/kg dose) was significantly more effective than placebo in preventing deterioration in overall disability, assessed by I‐RODS. The most common AEs recorded were local‐site reactions which were predominantly mild or moderate in intensity.
The open‐label extension (OLE) provided Class IV evidence supporting maintenance treatment with SCIg in patients with CIDP for up to an additional 48 wk.33 A summary of the key findings is provided below, but further details of the study designs and outcomes have been published previously.16, 33, 40 Eligible patients could continue on SCIg for up to an additional 48 wk.33 Eighty‐two patients were enrolled (n = 62 starting on 0.4 g/kg; n = 20 starting on 0.2 g/kg). The study highlighted that relapse rates differed depending on treatment allocation in the original study with the highest probability of clinical stability seen with the 0.4 g/kg dose, although some patients could be maintained on 0.2 g/kg without relapse (Figure SS1).41 This disparity portrays the inter‐patient heterogeneity in terms of IgG threshold and the importance of determining the minimum effective dose on a case‐by‐case basis.
4. PRACTICALITIES OF USING SCIg IN CIDP
4.1. Induction and maintenance therapy
SCIg is currently not approved for induction therapy in CIDP, where typically a higher dose is used to stabilize patients starting IgG therapy.35 IVIg is delivered directly into the bloodstream resulting in a rapid rise in IgG levels which has been shown to lead to a faster onset of action, quicker improvements in disability, and (in many patients) “an energy boost”.42 In comparison, SCIg had a slower onset of action due to slower absorption into the bloodstream. SCIg has previously been investigated as a first‐line treatment option in patients with CIDP.31 Markvardsen et al. demonstrated that SCIg (0.4 g/kg/wk) for 5 weeks had similar efficacy to IVIg (0.4 g/kg/day) for 5 days as a first‐line treatment in treatment‐naïve CIDP patients.31 However, the maximal improvement was reached sooner with IVIg (IVIg, 2 wk vs SCIg, 5 wk).31 Newly diagnosed patients will often benefit from a quicker stabilization of their disease. An IVIg induction dose followed by IVIg maintenance doses to stabilize the patient should take place before considering transition to SCIg for longer term maintenance therapy.35 However, we recommend introducing patients early on, and simultaneously, to the concepts of IVIg and SCIg, the same treatment administered via two different routes.
4.2. Transitioning from IVIg maintenance to SCIg maintenance
Differences in symptom control and systemic side effects may be attributed to differences in pharmacokinetics between IVIg and SCIg.33, 42 IVIg is administered as a large intermittent bolus, typically every 3–4 wk, whereas SCIg is administered in more frequent (typically weekly) smaller doses.37 Low IgG trough levels toward the end of IVIg dosing intervals may lead to cyclic fluctuations in disability and a return of symptoms in some patients referred to as “wear‐off” effects37 (Figure 1). Weekly SCIg dosing results in a steadier IgG concentration that is consistent between infusions, but with a peak serum IgG concentration that is lower than that achieved with IVIg.19, 37
FIGURE 1.
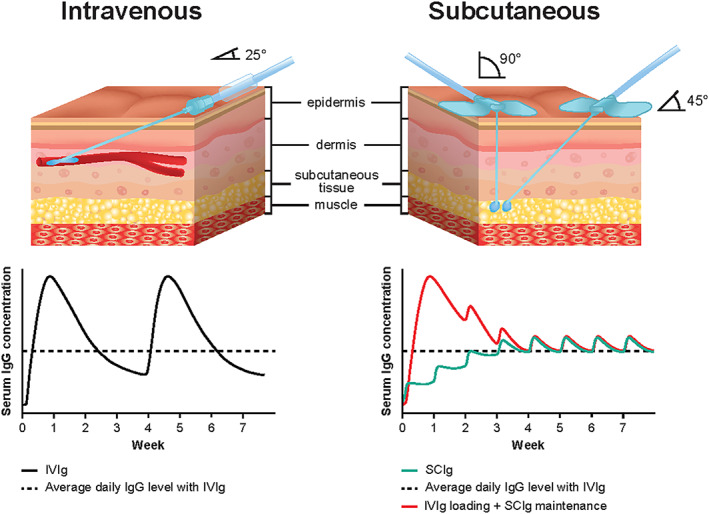
Schematic of intravenous vs subcutaneous infusions and the impact on IgG concentration. Dependent on infusion site location and patient preference, subcutaneous infusions can be conducted at a 90‐ or 45° angle. Red line indicates where IVIg dose is followed 1 wk later by SCIg maintenance doses
While country‐specific labels have varying recommendations for the SCIg starting dose in CIDP, the evidence supports doses between 0.2–0.4 g/kg body weight (bw) weekly. The best approach to transition patients from IVIg to SCIg remains unclear and will vary between patients.19 A variety of successful transition protocols have been reported in CIDP and MMN.43, 44 However, SCIg should be initiated 1 wk after the last IVIg infusion35, 45, 46 so that the serum IgG concentration remains high enough to smoothly transition to a stable steady state.19 In the Unites States (US), the recommended starting dose for SCIg is 0.2 g/kg bw/wk (with up to eight simultaneous infusion sites); the dose can be increased up to 0.4 g/kg if necessary. In Europe and Canada 0.2–0.4 g/kg bw/wk (no limit of infusion sites) is recommended.34, 35, 46 The starting dose range of 0.2–0.4 g/kg/wk in the European Union (EU) and Canadian labels provides more flexibility to start patients on a dose based on a 1:1 conversion from their previous IVIg treatment (typically this would be 0.33 g/kg based on a 1 g/kg IVIg maintenance dose every 3 wk) and reduces the risk of underdosing in patients with higher IVIg requirements. It also provides the option of treating at 0.4 g/kg and down‐titrating to determine the minimum requirement. Patients fearful of relapse may prefer this approach as opposed to transitioning to 0.2 g/kg, which may lead to lower IgG concentrations than their current IVIg dose and contribute to a greater risk of treatment failure. Moreover, lower IgG concentrations can arise due to the decreased bioavailability of SCIg, which may be up to 30% lower relative to IVIG.42, 47 Most reports adopt an initial SCIg dose calculated using a 1:1 conversion from the previous IVIg dose divided per week.28, 30, 48
4.3. Dose adjustments—Stabilized patients
Physicians may want to reduce a patient's dose after a period of clinical stability.33 Once patients treated with IgG products improve to a good baseline and do not fluctuate with therapy, tapering or discontinuation of IgG therapy should be considered to assess whether the patient is in remission. This approach may be considered in responders as early as 6–12 mo after IgG therapy initiation. There are a variety of approaches to discontinue IgG therapy once the physician deems the patient is a candidate. For IVIg, the most common approach is to progressively taper the dose by approximately 20%–25% or to extend the interval between infusions. However, some have suggested an alternate individualized approach of abrupt interruption of IVIg after two loading doses to determine response.49 Once a responder deteriorates, the IVIg dosing interval is determined and then dosage is tapered.49 Clinically stable patients with CIDP may have low variability in their IgG levels, however, it is not clear if using IgG levels to guide dosing can be widely adopted with IVIg therapy since IgG levels in unstable patients have not been yet evaluated.50 For stable SCIg responders, weekly dosage tapering every month or so by 20%–25% is a sensible strategy. Alternatively, although data are lacking, abrupt discontinuation may be considered in select patients based on CIDP disease status and physician‐patient discussion. The flexibility of SCIg allows minor dosing adjustments without the need for complicated scheduling challenges of clinic (or HCP)‐based infusions to maximize the clinical dose–response relationship. CIDP dose adjustments are often made on the basis of the neurological examination and close monitoring of the clinical response using functional and disability assessments (INCAT, I‐RODs, and grip strength etc.), with the aim of titrating to the lowest effective dose.51 Patients may require lower or higher doses depending on factors such as IgG threshold, disease severity/course and previous treatment.16, 33, 52 There are anecdotal reports of IVIg combination therapy with steroids during IgG dose tapering.53, 54 While there are no data to support the effectiveness of SCIg combination therapy with steroids, this approach might be a practical consideration for CIDP patients experiencing worsening or partial response while on SCIg monotherapy. Additional controlled studies are required to determine the optimal maintenance and dose‐tapering strategies for IVIg and SCIg.
4.4. Dose adjustments—Following clinical deterioration
In the case of clinical deterioration following transition to SCIg, the EU label (Summary of Product Characteristics [SmPC]) and US Prescribing Information (USPI) recommend increasing the dose to 0.4 g/kg bw/wk.35, 46 Following a relapse, patients may be more reluctant to remain on SCIg. Data suggest a good probability of recovery following up‐titration with SCIg and can be cited to reassure patients.33 For example, van Schaik et al. reported over 90% of patients who relapsed on 0.2 g/kg SCIg recovered to previous clinical levels when switched to 0.4 g/kg SCIg.33 Up‐titration of the SCIg dose can be a successful method to re‐stabilize a patient following relapse as an alternative to using IVIg as rescue therapy.33, 46 As patients tend to show a preference for SCIg, they may prefer to remain on SCIg during a period of dose adjustment following a relapse rather than changing IgG treatment route. The USPI recommends reinitiating IVIg, while discontinuing SCIg, if CIDP symptoms worsen on 0.4 g/kg/wk.35 Based on author experience, if a patient mildly worsens while on SCIg 0.4 g/kg/wk, dosage escalation to 0.5–0.6 g/kg/wk, although not rigorously studied, may be a practical approach with careful monitoring. Patients should always be assessed on a case‐by‐case basis to determine the best approach, and long‐term maintenance therapy beyond 12–18 mo should be individualized based upon the patient's response and need for continued therapy.35, 46
4.5. Supporting patients with self‐administration
To self‐administer SCIg, patients should be adequately trained and monitored by a healthcare professional (HCP). Patients often report that learning self‐administration is easy.16 Discomfort with the technique can be an issue for some patients. SCIg (IgPro20) is available in either a vial (5, 10, 20, 50 mL) or more recently single‐use pre‐filled syringes (PFS) (5, 10, 20 mL [USA, EU, and Canada]).35, 45, 46 As patients with CIDP are often elderly and/or can have muscle weakness in distal limbs, using PFS may be beneficial for patients with decreased dexterity, vision, or coordination.1, 55 Studies in other disease settings show that PFS can reduce preparation time, medication errors, and drug wastage and are often preferred by patients as a simplified self‐administration method.55
SCIg infusion time is typically around 1 h.16 It has been reported that patients tolerated volumes up to 50 mL per site and infusion rates up to 50 mL/h/site.16 This is consistent with recommendations in the USPI, but the SmPC recommends an initial infusion rate of 20 mL/h/site and two further tolerated infusions up to 35 mL/h/site for device‐assisted infusions. An increase in the infusion rate for successive infusions may be considered at the discretion of the patient and based on the HCP's judgement. The SmPC recommends infusion rates up to 120 mL/h/site for manual infusion.35, 46 SCIg infuson via an infusion pump has been the traditional method for IgG delivery. However, manual infusion (≤60 mL per injection site and flow rates of >60 mL/h per injection site for SCIg) is a simpler method that has been growing in popularity and is used successfully in patients with PID56, 57, 58, 59 and the benefits may be translatable to the CIDP population.
Setting patient expectations prior to initiation of SCIg therapy is important. Patients should be informed that mild to moderate local infusion‐site reactions are a common side effect that improves over time.35, 46 Local infusion‐site reactions can be alleviated by massage and the use of mild analgesics.10 Watkins et al., provides a comprehensive list of strategies that patients and nurses can employ to mitigate local reactions.60 Patients should consider rotating their infusion‐site location, adjusting volume per site, needle gauge, and/or rate of infusion based on how they are tolerating infusions.35, 46 Changes to the infusion regimen or ancillary supplies should be done one at a time to allow assessment of each change. Once volume per site is optimized, the rate of infusion can be increased. Optimizing infusions will require adjustments over a period of time and with support from an HCP. Educating patients is extremely important during SCIg initiation and self‐administration training and enables patients to better manage their treatment. Nursing support and good communication are essential for helping patients to successfully transition from IVIg to SCIg, along with patient factors such as motivation, ability to learn, dexterity, compliance, and caregiver availability.60
4.6. Patient preference and QoL
SCIg may be a preferred route of IgG administration for many patients with CIDP due to its ease of use, safety profile, and patient independence.61 Often, newly diagnosed patients with CIDP and those experiencing issues with their current treatment may be more receptive to SCIg, but all patients, whether new or established, should be made aware of their options with IgG therapy and the potential pros and cons associated with both IVIg and SCIg use. Table 3 provides examples of potential candidates who may prefer to remain on IVIg, and Table 4 provides example potential candidates for SCIg. These tables are intended to provide discussion points to consider with the patient.
TABLE 3.
Example CIDP candidates for IVIg
| Potential IVIg candidate examplesa | Considerations to discuss with patient |
|---|---|
| Patient lacking organizational skill or drive to self‐administer | Some patients may be unwilling, or lack the skills, necessary to take on an element of their own disease management. Setting appropriate patient expectations when discussing SCIg is important to outline the responsibilities of self‐administration and to help assess whether the patient would derive more benefit from an HCP‐assisted mode of administration |
| Patient unable to self‐administer due to poor dexterity, fear of needles or no reliable support network | Patients with poor manual dexterity or fear of needles may struggle with aspects of the self‐administration technique (drawing solution from vial into syringe, etc.). A reliable caregiver (eg, spouse, family member, or friend) nearby to provide support or assist with the infusion can be considered if available. The use of a SCIg pre‐filled syringe to simplify the process can also be an option. In the absence of the above options, remaining on IVIg may be a more appropriate plan |
| Patient preferring treatment in a clinic setting or administered by an HCP | Some patients may prefer a clinic setting for their infusions due to the confidence and relationships built with staff. IVIg has the advantage of access to laboratory testing at the time of initiating the IV line. There is also potentially less risk of dosing errors in treatment administered by an HCP and the reliance on an HCP to monitor for side effects |
| Patient familiarity and extensive history with IVIg | For established patients with CIDP, they may have received IVIg for a long time and feel comfortable with a therapy they know and trust. Patients may not get as much subjective benefit from SCIg compared with IVIg due to the route of administration and slower absorption. Physicians should periodically reassess the patient's perception of SCIg as the attributes which did not initially appeal may be viewed more favorably with changing patient circumstances |
| Patient preferring more infrequent infusions | Some patients may find the infrequent infusion schedule (and potentially fewer disease reminders) associated with IVIg every 3 or more weeks fits in better with their lifestyle. As above, periodic discussions on the most suitable mode of administration should take place to reflect changing circumstances |
| Low BMI patients requiring large IgG doses | Self‐administration of large volumes of SCIg may be unappealing or challenging for low BMI patients who have less available subcutaneous tissue for infusions. Often a SCIg infusion regimen can be designed to accommodate these patients, but this may require a higher number of infusion sites |
Abbreviation: BMI, body mass index.
Table does not provide an exhaustive list of potential candidates, but rather highlights some of the considerations to factor in during discussions with patients.
TABLE 4.
Example CIDP candidates for SCIg
| Potential SCIg candidate examplesa | Considerations to discuss with patient |
|---|---|
| Patient with venous access concerns | In patients requiring a port there is an added safety risk with infections and device maintenance. In most cases SCIg should be recommended rather than fitting a port |
| Patient experiencing wear‐off effects between IVIg infusions | Reducing the interval between IVIg infusions can be attempted to minimize wear‐off effects. However, weekly SCIg can be a practical solution to provide improved steady‐state IgG levels and reduce TRFs |
| Patient experiencing intolerable side effects with IVIg | Some IVIg‐related AEs can be managed with premedication. However, for most patients the frequency of systemic AEs is reduced with SCIg and premedication requirements are rare |
| Patient with scheduling and/or logistical issues attending infusion clinics | Those who live far away from their infusion facility or with demanding work/home life schedules may have options to try IVIg at home, although this still requires an HCP visit to conduct infusions. SCIg can be a practical alternative to ease logistical challenges |
| Patient desiring more independence and autonomy due to lifestyle | Once properly trained a patient can infuse SCIg in many locations including work, school, and while on vacation or travelling etc. The importance of good sterile technique and keeping detailed infusion records should be emphasized |
| Patient with comorbidities | IVIg is associated with some serious, but rare, side effects. SCIg may be considered as a preferred treatment to IVIg in patients with any existing conditions increasing their risk of renal dysfunction, TEEs, hemolysis, or aseptic meningitis. These conditions are warnings in the SCIg USPI, although their occurrence is rarer than in IVIg |
| Patient preferring to avoid risk of infection exposure during pandemics | In light of recent events with the COVID‐19 pandemic, many patients may feel more comfortable conducting their infusions independently and at home to reduce their exposure risk and limit reliance on HCP resource—Although nurse follow‐up is still required, this can be conducted via video calls or over the phone if necessary |
Table does not provide an exhaustive list of potential candidates, but rather highlights some of the considerations to factor in during discussions with patients.
Results from a systematic review indicate that SCIg may improve QoL over IVIg in patients with inflammatory neuropathies.62 Many patients with CIDP have shown a preference for SCIg compared with their previous IVIg.24, 28, 30, 33, 39 Patient preference for SCIg may be related to the convenience of self‐administering at home, lower infusion volumes, fewer systemic AEs, and reduced treatment‐related fluctuations (TRFs).37, 63 For example, in the recent van Schaik et al. extension study, 82% of patients preferred SCIg to their previous IVIg citing greater independence, reduced administration time, and fewer side effects as the main reasons.33 It can be noted that patients opting to transition to SCIg in published reports may have more reason to be unsatisfied with IVIg and, therefore, will experience a greater improvement in QoL after transitioning. In a treatment preference questionnaire, 53% of responders receiving SCIg preferred it over their previous IVIg treatment compared with 18% who preferred IVIg16 (Figure 2). Consequently, physicians should thoroughly evaluate and discuss with patients before transitioning them between IgG therapies as some may need additional support with self‐administration or have other reasons for preferring to remain on IVIg.17
FIGURE 2.
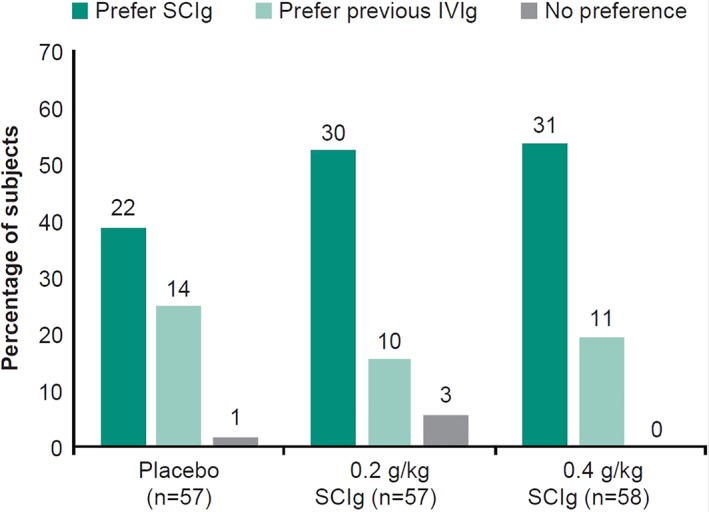
Subject preference for SCIg and IVIg. Not all subjects in the clinical trial responded to the preference questionnaire. Numbers above bars are actual numbers of subjects who responded
4.7. Long‐term adherence
Although patients often prefer the convenience of home‐based IgG administration, Ness et al. highlights that there is some variation in patient preference with regard to clinic versus home‐based therapy.38 Some patients may feel overwhelmed by the number of injections associated with SCIg, the idea of managing their own treatment or the loss of a regular HCP touchpoint; these patients may prefer treatment to be administered by a HCP.37 A support program for patients opting to transition to SCIg is important to build confidence with self‐administration for successful long‐term adherence.64 In one study, a nurse‐led individualized program that included teaching sessions, written materials, and a clear care plan helped to successfully transition patients with neurological disorders from IVIg to SCIg. In this study, SCIg retention rates were 90% (n = 17/19) at 6 mo and 79% (n = 15/19) at 12 mo.64 It is important to highlight to patients the importance of treatment adherence to maintain therapeutic IgG concentrations and to keep accurate records of each infusion. Nurses and pharmacists are also important in providing a continued touchpoint for patient support. Additional support can encompass areas such as patient education, refresher self‐administration training, assessing treatment response in between clinic appointments, identifying treatment barriers, helping patients understand and manage local reactions, monitoring adherence, and reporting back to the treating physician any issues.60
5. SAFETY PROFILE
5.1. Systemic adverse events
Similar to other plasma‐derived IgG products marketed in the United States, SCIg carries a US FDA‐mandated “black box” class warning for thrombosis.35, 37 IVIg is associated with rare but potentially serious AEs, such as thromboembolic events (TEEs), aseptic meningitis, hemolysis, and renal dysfunction.65 Although less common, these serious AEs can also still occur with SCIg. IVIg is also associated with systemic AEs such as headache, nausea, and flu‐like symptoms, which may be due to the high infusion volumes required and rapid rise in IgG concentration following infusion.42, 65 Many patients require premedication to tolerate IVIg infusions. In contrast, SCIg administration is associated with a lower rate of systemic AEs and less need for premedication.33 The Racosta et al. meta‐analysis demonstrated a 28% reduction in relative risk of moderate and / or systemic AEs with SCIg versus IVIg.20 Van Schaik et al. reported that the rate of headaches was low for SCIg (7%) and this was maintained during the OLE study (5%).16, 33 In comparison, headaches associated with IVIg have been reported in other studies as ranging between 32% and 62% of patients.11, 66 Performing an early assessment of patient risk factors for IVIg‐associated AEs can help determine if switching to SCIg would be beneficial.
5.2. Local‐site reactions
Local‐site reactions tend to be the most common AE reported by patients receiving SCIg.16, 24, 33, 67 Local‐site reactions are usually mild to moderate in intensity and have been reported to significantly decrease with subsequent infusions.16 The decline in local‐site reactions is potentially a result of improving patient self‐administration technique and habituation to subcutaneous infusions. Local site reactions are rarely reported with IVIg infusions, although bruising can occur at the site of infusion.
5.3. Venous access
Another important difference of SCIg is that it does not require venous access. The majority of patients with CIDP are over 60 y of age and may need treatment for many years.68 The ability to establish peripheral venous access that remains viable throughout the infusion can become progressively more difficult, and a central venous line may need to be inserted, carrying additional risks.69 SCIg should always be considered before resorting to a port. Eliminating the need for venous access with SCIg therapy may also provide more treatment flexibility as patients are able to self‐administer at a variety of locations at their own convenience, which may be even more beneficial in the context of the COVID‐19 pandemic.
6. ECONOMIC IMPACT AND FUTURE OF IgG THERAPY IN CIDP
Studies comparing the economic burden of SCIg vs IVIg in CIDP report mixed results and have primarily been conducted in European settings.70, 71, 72 In general, studies agree that home‐based infusions vs hospital‐based result in cost reductions irrespective of the route of administration.19, 71, 72 The primary cost driver is often the product itself and, in turn, the dose requirement. However, comparisons are complicated by the indirect costs associated with site of care, HCP resource, and long‐term requirement for hospitalizations due to AEs or other disease‐related complications. In reality, it is often unclear which route of administration will prove most cost effective, as the slightly more expensive cost of the SCIg product can eventually be offset indirectly by associated infusion cost savings and reduce productivity loss for patients as a result of hospital/infusion‐related absenteeism.28, 73
Currently, only one 20% SCIg solution is approved for use in adult patients with CIDP,35 but other 10%–20% SC formulations are in various phases of development. In addition, an ongoing trial is exploring the tolerability and safety of hyaluronidase facilitated SCIg (fSCIg) in CIDP (NCT02955355). This method utilizes 10% SCIg and hyaluronidase in a two‐step infusion, which can theoretically deliver volumes greater than 700 mL compared with the recommended maximum of 50 mL per site with conventional SCIg.35, 74 fSCIg allows infusions at similar rates and volumes to IVIg, but with potential reductions in systemic AEs comparable with SCIg. Data on long‐term safety and cost comparisons for fSCIg are lacking and there is limited evidence to suggest any differences in QoL between SCIg and fSCIg in either CIDP or other neuromuscular disorders.75, 76, 77
To date, the United States has the highest IgG usage per capita, followed by Canada, Australia, and some European countries.78 Uptake of SCIg stands at 15% of total IgG use in the US market (of which currently 61% is used in PID).78 The use of IgG in neurological indications is growing and is anticipated to continue with high‐dose neurological indications, such as CIDP. Given the expanding IgG therapy options in CIDP and the high cost of treatment, exploring opportunities for cost minimization are important.
To conclude, there is a role for both IVIg and SCIg in CIDP maintenance therapy. The most appropriate route of administration should be individualized and will be determined by the patient. Ensuring patients are familiar with the benefits of each route is important to aid in treatment optimization and provide a better chance of long‐term adherence and success.
CONFLICT OF INTEREST
Mazen Dimachkie serves, or recently served, as a consultant for ArgenX, Catalyst, Cello, CSL Behring, EcoR1, Kezar, Momenta, NuFactor, Octapharma, RaPharma/UCB, RMS Medical, Sanofi Genzyme, Shire Takeda, Spark Therapeutics and UCB Biopharma. Dr. Dimachkie received grants from Alexion, Alnylam Pharmaceuticals, Amicus, Biomarin, Bristol‐Myers Squibb, Catalyst, Corbus, CSL‐Behring, FDA/OOPD, GlaxoSmithKline, Genentech, Grifols, Kezar, Mitsubishi Tanabe Pharma, MDA, NIH, Novartis, Octapharma, Orphazyme Ra Pharma/UCB, Sanofi Genzyme, Sarepta Therapeutics, Shire Takeda, Spark Therapeutics, UCB Biopharma, Viromed/Healixmith & TMA. Namita Goyal has received research support from Brainstorm Cell Therapeutics, Cytokinetics, Fulcrum, Kezar, Octapharma, Orion, Orphazyme. Dr. Goyal has served on Advisory Boards for Acceleron, Alexion, Argenx, Biogen, CSL Behring, Cytokinetics, MT Pharma, Sanofi Genzyme, Sarepta. In relation to these activities, she has received travel reimbursement and honoraria. She has also served on the speaker's bureau for CSL. The remaining authors have no conflicts of interest. Kazim Sheikh has received personal compensation for speaking engagements from CSL Behring and research/grant support from the Department of Defense (W81XWH‐18‐1‐0422) and the National Institute of Neurological Disorders and Stroke (R21NS107961). Chafic Karam has consulted for Acceleron Pharma, Inc; Akcea Therapeutics; Alnylam Pharmaceuticals, Inc; Argenx; Biogen; CSL Behring; and Sanofi Genzyme. Dr Karam has received personal compensation for speaking engagements from Akcea Therapeutics; Alnylam Pharmaceuticals, Inc; CSL Behring; and Sanofi Genzyme and research/grant support from Akcea Therapeutics and Sanofi Genzyme.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Figure S1. Relapse rates across all groups.
ACKNOWLEDGMENTS
We thank Prof. van Schaik for providing a critical review of this manuscript. Editorial assistance was provided by Meridian HealthComms Ltd, funded by CSL Behring. All medical writing assistance was guided by the authors, and all authors reviewed and approved each draft.
Goyal NA, Karam C, Sheikh KA, Dimachkie MM. Subcutaneous immunoglobulin treatment for chronic inflammatory demyelinating polyneuropathy. Muscle & Nerve. 2021;64(3):243‐254. 10.1002/mus.27356
The objectives of this activity are to: 1) Choose patients appropriately for treatment with subcutaneous immunoglobulin; 2) Calculate and order induction and maintenance therapy dosing correctly; 3) Make informed choices of subcutaneous vs. intravenous immunoglobulin therapy.I have no conflicts of interest
Answer questions and earn CME https://education.aanem.org/URL/JR85.
DATA AVAILABILITY STATEMENT
All data included in this manuscript is publicly available. The authors will consider requests for additional data from genuine researchers on an individual basis.
REFERENCES
- 1.Mathey EK, Park SB, Hughes RA, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry. 2015;86(9):973‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamb YN, Syed YY, Dhillon S. Immune globulin subcutaneous (human) 20% (Hizentra[®]): a review in chronic inflammatory demyelinating polyneuropathy. CNS Drugs. 2019;33(8):831‐838. [DOI] [PubMed] [Google Scholar]
- 3.Dimachkie MM, Barohn RJ. Chronic inflammatory demyelinating polyneuropathy. Curr Treat Options Neurol. 2013;15(3):350‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelinas D, Katz J, Nisbet P, England JD. Current practice patterns in CIDP: a cross‐sectional survey of neurologists in the United States. J Neurol Sci. 2019;397:84‐91. [DOI] [PubMed] [Google Scholar]
- 5.Gorson KC, van Schaik IN, Merkies ISJ, et al. Chronic inflammatory demyelinating polyneuropathy disease activity status: recommendations for clinical research standards and use in clinical practice. J Peripher Nerv Syst. 2010;15(4):326‐333. [DOI] [PubMed] [Google Scholar]
- 6.Barnett C, Sadeghian H. Evidence of persistent improvements with long‐term subcutaneous immunoglobulin in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2019;60(6):643‐644. [DOI] [PubMed] [Google Scholar]
- 7.Oaklander AL, Lunn MP, Hughes RA, van Schaik IN, Frost C, Chalk CH. Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): an overview of systematic reviews. Cochrane Database Syst Rev. 2017;1(1):Cd010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Schaik IN, Eftimov F, van Doorn PA, et al. Pulsed high‐dose dexamethasone versus standard prednisolone treatment for chronic inflammatory demyelinating polyradiculoneuropathy (PREDICT study): a double‐blind, randomised, controlled trial. Lancet Neurol. 2010;9(3):245‐253. [DOI] [PubMed] [Google Scholar]
- 9.Nobile‐Orazio E, Cocito D, Jann S, et al. Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: a randomised controlled trial. Lancet Neurol. 2012;11(6):493‐502. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Wang C, Xu F, Ming F, Zhang H. Efficacy and tolerability of intravenous immunoglobulin and subcutaneous immunoglobulin in neurologic diseases. Clin Ther. 2019;41(10):2112‐2136. [DOI] [PubMed] [Google Scholar]
- 11.Hughes RA, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate‐chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo‐controlled trial. Lancet Neurol. 2008;7(2):136‐144. [DOI] [PubMed] [Google Scholar]
- 12.Farmakidis C, Dimachkie MM, Pasnoor M, Barohn RJ. Immunosuppressive and immunomodulatory therapies for neuromuscular diseases. Part I: Traditional agents. Muscle Nerve. 2020;61(1):5‐16. [DOI] [PubMed] [Google Scholar]
- 13.Kuwabara S, Mori M, Misawa S, et al. Intravenous immunoglobulin for maintenance treatment of chronic inflammatory demyelinating polyneuropathy: a multicentre, open‐label, 52‐week phase III trial. J Neurol Neurosurg Psychiatry. 2017;88(10):832‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van den Bergh PY, Hadden RD, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society ‐ first revision. Eur J Neurol. 2010;17(3):356‐363. [DOI] [PubMed] [Google Scholar]
- 15.Allen JA. Diagnostic Snares in Chronic Inflammatory Demyelinating Polyneuropathy ‐ Medscape ‐ Jan 06, 2020. New York, NY: WebMD, LLC; 2020. [Google Scholar]
- 16.van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Neurol. 2018;17(1):35‐46. [DOI] [PubMed] [Google Scholar]
- 17.Farmakidis C, Dimachkie MM, Pasnoor M, Barohn RJ. Immunosuppressive and immunomodulatory therapies for neuromuscular diseases. Part II: new and novel agents. Muscle Nerve. 2020;61(1):17‐25. [DOI] [PubMed] [Google Scholar]
- 18.Gentile L, Mazzeo A, Russo M, Arimatea I, Vita G, Toscano A. Long‐term treatment with subcutaneous immunoglobulin in patients with chronic inflammatory demyelinating polyradiculoneuropathy: a follow‐up period up to 7 years. Sci Rep. 2020;10(1):7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen JA, Gelinas DF, Freimer M, Runken MC, Wolfe GI. Immunoglobulin administration for the treatment of CIDP: IVIG or SCIG? J Neurol Sci. 2020;408:116497. [DOI] [PubMed] [Google Scholar]
- 20.Racosta JM, Sposato LA, Kimpinski K. Subcutaneous versus intravenous immunoglobulin for chronic autoimmune neuropathies: a meta‐analysis. Muscle Nerve. 2017;55(6):802‐809. [DOI] [PubMed] [Google Scholar]
- 21.Köller H, Schroeter M, Feischen H, Hartung HP, Kieseier BC. Subcutaneous self‐infusions of immunoglobulins as a potential therapeutic regimen in immune‐mediated neuropathies. J Neurol. 2006;253(11):1505‐1506. [DOI] [PubMed] [Google Scholar]
- 22.Lee DH, Linker RA, Paulus W, Schneider‐Gold C, Chan A, Gold R. Subcutaneous immunoglobulin infusion: a new therapeutic option in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2008;37(3):406‐409. [DOI] [PubMed] [Google Scholar]
- 23.Cocito D, Serra G, Falcone Y, Paolasso I. The efficacy of subcutaneous immunoglobulin administration in chronic inflammatory demyelinating polyneuropathy responders to intravenous immunoglobulin. J Peripher Nerv Syst. 2011;16(2):150‐152. [DOI] [PubMed] [Google Scholar]
- 24.Markvardsen LH, Debost JC, Harbo T, et al. Subcutaneous immunoglobulin in responders to intravenous therapy with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2013;20(5):836‐842. [DOI] [PubMed] [Google Scholar]
- 25.Cocito D, Paolasso I, Peci E, Spagone E, Lopiano L. Improvement of quality of life in patients with chronic inflammatory demyelinating polyneuropathy shifting from 16 to 20% subcutaneous immunoglobulins. Neurol Sci. 2013;34(11):2061‐2062. [DOI] [PubMed] [Google Scholar]
- 26.Markvardsen LH, Harbo T, Sindrup SH, Christiansen I, Andersen H, Jakobsen J. Subcutaneous immunoglobulin preserves muscle strength in chronic inflammatory demyelinating polyneuropathy. Eur J Neurol. 2014;21(12):1465‐1470. [DOI] [PubMed] [Google Scholar]
- 27.Cocito D, Merola A, Peci E, et al. Subcutaneous immunoglobulin in CIDP and MMN: a short‐term nationwide study. J Neurol. 2014;261(11):2159‐2164. [DOI] [PubMed] [Google Scholar]
- 28.Hadden RD, Marreno F. Switch from intravenous to subcutaneous immunoglobulin in CIDP and MMN: improved tolerability and patient satisfaction. Ther Adv Neurol Disord. 2015;8(1):14‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon MS, Gold R, Kerasnoudis A. Subcutaneous immunoglobulin in treating inflammatory neuromuscular disorders. Ther Adv Neurol Disord. 2015;8(4):153‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cocito D, Merola A, Romagnolo A, et al. Subcutaneous immunoglobulin in CIDP and MMN: a different long‐term clinical response? J Neurol Neurosurg Psychiatry. 2016;87(7):791‐793. [DOI] [PubMed] [Google Scholar]
- 31.Markvardsen LH, Sindrup SH, Christiansen I, Olsen NK, Jakobsen J, Andersen H. Subcutaneous immunoglobulin as first‐line therapy in treatment‐naive patients with chronic inflammatory demyelinating polyneuropathy: randomized controlled trial study. Eur J Neurol. 2017;24(2):412‐418. [DOI] [PubMed] [Google Scholar]
- 32.Cirillo G, Todisco V, Tedeschi G. Long‐term neurophysiological and clinical response in patients with chronic inflammatory demyelinating polyradiculoneuropathy treated with subcutaneous immunoglobulin. Clin Neurophysiol. 2018;129(5):967‐973. [DOI] [PubMed] [Google Scholar]
- 33.van Schaik IN, Mielke O, Bril V, et al. Long‐term safety and efficacy of subcutaneous immunoglobulin IgPro20 in CIDP: PATH extension study. Neurol Neuroimmunol Neuroinflammation. 2019;6(5):e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Food US and Administration Drug. 2020. ‘Gamunex‐C Prescribing Information’. https://www.gamunex-c.com/documents/27482625/27482925/Gamunex-C+Prescribing+Information.pdf/9258bd0f-4205-47e1-ab80-540304c1ff8e. Accessed May 19, 2020.
- 35.Food US and Administration Drug. 2021. ‘Hizentra Prescribing Information’. https://labeling.cslbehring.com/PI/US/Hizentra/EN/Hizentra-Prescribing-Information.pdf. Accessed April 27, 2021.
- 36.Food US and Administration Drug 2019. ‘Privigen Prescribing Information’. http://cslbehring.vo.llnwd.net/o33/u/central/PI/US/Privigen/EN/Privigen-Prescribing-Information.pdf. Accessed May 19, 2020.
- 37.Berger M, Harbo T, Cornblath DR, Mielke O. IgPro20, the polyneuropathy and treatment with Hizentra([R]) study (PATH), and the treatment of chronic inflammatory demyelinating polyradiculoneuropathy with subcutaneous IgG. Immunotherapy. 2018;10(11):919‐933. [DOI] [PubMed] [Google Scholar]
- 38.Ness S. Differentiating characteristics and evaluating intravenous and subcutaneous immunoglobulin. Am J Manag Care. 2019;25(6 Suppl):S98‐s104. [PubMed] [Google Scholar]
- 39.Sala TP, Crave JC, Duracinsky M, et al. Efficacy and patient satisfaction in the use of subcutaneous immunoglobulin immunotherapy for the treatment of auto‐immune neuromuscular diseases. Autoimmun Rev. 2018;17(9):873‐881. [DOI] [PubMed] [Google Scholar]
- 40.van Schaik IN, van Geloven N, Bril V, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (the PATH study): study protocol for a randomized controlled trial. Trials. 2016;17(1):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Schaik IN, Mielke O, Bril V, van Geloven N, Hartung HP, Lewis RA, et al. Long‐term Safety and Efficacy of Subcutaneous Immunoglobulin IgPro20 in CIDP: the PATH Extension Study. Peripheral Nerve Society (PNS) Annual Meeting; Baltimore, MD 2018. [DOI] [PMC free article] [PubMed]
- 42.Berger M, Jolles S, Orange JS, Sleasman JW. Bioavailability of IgG administered by the subcutaneous route. J Clin Immunol. 2013;33(5):984‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasutis VM, Katzberg HD, Bril V. High‐dose subcutaneous immunoglobulin in patients with multifocal motor neuropathy: a nursing perspective. J Infus Nurs. 2017;40(5):305‐312. [DOI] [PubMed] [Google Scholar]
- 44.Misbah SA, Baumann A, Fazio R, et al. A smooth transition protocol for patients with multifocal motor neuropathy going from intravenous to subcutaneous immunoglobulin therapy: an open‐label proof‐of‐concept study. J Peripher Nerv Syst. 2011;16(2):92‐97. [DOI] [PubMed] [Google Scholar]
- 45.Canada Product Monograph HIzentra subcutaneous immunoglobulin (Human) 20% solution. Accessed May 22, 2020.
- 46.European Medicines Agency . 2020. ‘Hizentra Summary of Product Characteristics’. https://www.ema.europa.eu/en/documents/product-information/hizentra-epar-product-information_en.pdf. Accessed January 15, 2021.
- 47.Berger M, Rojavin M, Kiessling P, Zenker O. Pharmacokinetics of subcutaneous immunoglobulin and their use in dosing of replacement therapy in patients with primary immunodeficiencies. Clin Immunol. 2011;139(2):133‐141. [DOI] [PubMed] [Google Scholar]
- 48.Markvardsen LH, Christiansen I, Jakobsen J. Improvement of hemoglobin levels after a switch from intravenous to subcutaneous administration of immunoglobulin in chronic inflammatory demyelinating polyneuropathy and multifocal motor neuropathy. Transfusion. 2016;56(10):2443‐2448. [DOI] [PubMed] [Google Scholar]
- 49.Lunn MP, Ellis L, Hadden RD, Rajabally YA, Winer JB, Reilly MM. A proposed dosing algorithm for the individualized dosing of human immunoglobulin in chronic inflammatory neuropathies. J Peripher Nerv Syst. 2016;21(1):33‐37. [DOI] [PubMed] [Google Scholar]
- 50.Kuitwaard K, van Doorn PA, Vermeulen M, et al. Serum IgG levels in IV immunoglobulin treated chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2013;84(8):859‐861. [DOI] [PubMed] [Google Scholar]
- 51.Doneddu PE, Hadden RDM. Daily grip strength response to intravenous immunoglobulin in chronic immune neuropathies. Muscle Nerve. 2020;62(1):103‐110. [DOI] [PubMed] [Google Scholar]
- 52.Dyck PJB, Tracy JA. History, diagnosis, and Management of Chronic Inflammatory Demyelinating Polyradiculoneuropathy. Mayo Clin Proc. 2018;93(6):777‐793. [DOI] [PubMed] [Google Scholar]
- 53.Adrichem ME, Bus SR, Wieske L, et al. Combined intravenous immunoglobulin and methylprednisolone as induction treatment in chronic inflammatory demyelinating polyneuropathy (OPTIC protocol): a prospective pilot study. Eur J Neurol. 2020;27(3):506‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hahn AF, Bolton CF, Zochodne D, Feasby TE. Intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy. A double‐blind, placebo‐controlled, cross‐over study. Brain. 1996;119(Pt 4):1067‐1077. [DOI] [PubMed] [Google Scholar]
- 55.Kafal AR, Vinh DC, Langelier MJ. Prefilled syringes for immunoglobulin G (IgG) replacement therapy: clinical experience from other disease settings. Expert Opin Drug Deliv. 2018;15(12):1199‐1209. [DOI] [PubMed] [Google Scholar]
- 56.Bienvenu B, Cozon G, Mataix Y, et al. Rapid push vs pump‐infused subcutaneous immunoglobulin treatment: a randomized crossover study of quality of life in primary immunodeficiency patients. J Clin Immunol. 2018;38(4):503‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shapiro RS. Subcutaneous immunoglobulin: rapid push vs. infusion pump in pediatrics. Pediatr Allergy Immunol. 2013;24(1):49‐53. [DOI] [PubMed] [Google Scholar]
- 58.Shapiro RS. Subcutaneous immunoglobulin therapy given by subcutaneous rapid push vs infusion pump: a retrospective analysis. Ann Allergy Asthma Immunol 2013;111(1):51–5. [DOI] [PubMed] [Google Scholar]
- 59.Shapiro R. Subcutaneous immunoglobulin (16 or 20%) therapy in obese patients with primary immunodeficiency: a retrospective analysis of administration by infusion pump or subcutaneous rapid push. Clin Exp Immunol. 2013;173(2):365‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watkins JM, Dimachkie MM, Riley P, Murphy E. Subcutaneous immunoglobulin therapy for chronic inflammatory demyelinating polyneuropathy: a nursing perspective. J Neurosci Nurs. 2019;51(4):198‐203. [DOI] [PubMed] [Google Scholar]
- 61.van Schaik IN, Bril V, van Geloven N, Hartung HP, Lewis RA, Sobue G, et al. Practical Application of Subcutaneous Immunoglobulin for Maintenance Treatment in Chronic Inflammatory Demyelinating Polyneuropathy – The PATH Study. Peripheral Nerve Society (PNS) Annual Meeting; Baltimore, MD 2018.
- 62.Rajabally YA, Cavanna AE. Health‐related quality of life in chronic inflammatory neuropathies: a systematic review. J Neurol Sci. 2015;348(1–2):18‐23. [DOI] [PubMed] [Google Scholar]
- 63.Hartung HP, Mallick R, Bril V, et al. Patient‐reported outcomes with subcutaneous immunoglobulin in chronic inflammatory demyelinating polyneuropathy: the PATH study. Eur J Neurol. 2020;27(1):196‐203. [DOI] [PubMed] [Google Scholar]
- 64.Suleman A, Theoret L, Bourque P, Pringle E, Cameron DW, Cowan J. Evaluation of a personalized subcutaneous immunoglobulin treatment program for neurological patients. Can J Neurol Sci. 2019;46(1):38‐43. [DOI] [PubMed] [Google Scholar]
- 65.Guo Y, Tian X, Wang X, Xiao Z. Adverse effects of immunoglobulin therapy. Front Immunol. 2018;9:1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuitwaard K, van den Berg LH, Vermeulen M, et al. Randomised controlled trial comparing two different intravenous immunoglobulins in chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Neurosurg Psychiatry. 2010;81(12):1374‐1379. [DOI] [PubMed] [Google Scholar]
- 67.Markvardsen LH, Harbo T. Subcutaneous immunoglobulin treatment in CIDP and MMN. Efficacy, treatment satisfaction and costs. J Neurol Sci. 2017;378:19‐25. [DOI] [PubMed] [Google Scholar]
- 68.Laughlin RS, Dyck PJ, Melton LJ 3rd, Leibson C, Ransom J, Dyck PJ. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology. 2009;73(1):39‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel AR, Patel AR, Singh S, Singh S, Khawaja I. Central line catheters and associated complications: a review. Cureus. 2019;11(5):e4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perraudin C, Bourdin A, Vicino A, Kuntzer T, Bugnon O, Berger J. Home‐based subcutaneous immunoglobulin for chronic inflammatory demyelinating polyneuropathy patients: a Swiss cost‐minimization analysis. PLoS One. 2020;15(11):e0242630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Le Masson G, Solé G, Desnuelle C, et al. Home versus hospital immunoglobulin treatment for autoimmune neuropathies: a cost minimization analysis. Brain Behav. 2018;8(2):e00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lazzaro C, Lopiano L, Cocito D. Subcutaneous vs intravenous administration of immunoglobulin in chronic inflammatory demyelinating polyneuropathy: an Italian cost‐minimization analysis. Neurol Sci. 2014;35(7):1023‐1034. [DOI] [PubMed] [Google Scholar]
- 73.Owens GM. The economic burden and managed care implications of chronic inflammatory demyelinating polyneuropathy. Am J Manag Care. 2018;24(17 Suppl):S380‐s4. [PubMed] [Google Scholar]
- 74.Jolles S. Hyaluronidase facilitated subcutaneous immunoglobulin in primary immunodeficiency. Immunotargets Ther. 2013;2:125‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hasan S, Duff K, Wisseh S, Youssef A, Chavan S. Rationale and Design of a Phase 3b study of the long‐term tolerability and safety of HyQvia in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): ADVANCE‐CIDP 3 (4331). Neurology. 2020;94(15 Supplement):4331. [Google Scholar]
- 76.Herraets IJT, Bakers JNE, van Eijk RPA, Goedee HS, van der Pol WL, van den Berg LH. Human immune globulin 10% with recombinant human hyaluronidase in multifocal motor neuropathy. J Neurol. 2019;266(11):2734‐2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al‐Zuhairy A, Jakobsen J, Andersen H, Sindrup SH, Markvardsen LK. Randomized trial of facilitated subcutaneous immunoglobulin in multifocal motor neuropathy. Eur J Neurol. 2019;26(10):1289‐e82. [DOI] [PubMed] [Google Scholar]
- 78.Farrugia A, Grazzini G, Quinti I, Candura F, Profili S, Liumbruno GM. The growing importance of achieving national self‐sufficiency in immunoglobulin in Italy. The emergence of a national imperative. Blood Transfus. 2019;17(6):449‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Relapse rates across all groups.
Data Availability Statement
All data included in this manuscript is publicly available. The authors will consider requests for additional data from genuine researchers on an individual basis.


