Abstract
The influence of climate change on wildlife disease dynamics is a burgeoning conservation and human health issue, but few long‐term studies empirically link climate to pathogen prevalence. Polar bears (Ursus maritimus) are vulnerable to the negative impacts of sea ice loss as a result of accelerated Arctic warming. While studies have associated changes in polar bear body condition, reproductive output, survival, and abundance to reductions in sea ice, no long‐term studies have documented the impact of climate change on pathogen exposure. We examined 425 serum samples from 381 adult polar bears, collected in western Hudson Bay (WH), Canada, for antibodies to selected pathogens across three time periods: 1986–1989 (n = 157), 1995–1998 (n = 159) and 2015–2017 (n = 109). We ran serological assays for antibodies to seven pathogens: Toxoplasma gondii, Neospora caninum, Trichinella spp., Francisella tularensis, Bordetella bronchiseptica, canine morbillivirus (CDV) and canine parvovirus (CPV). Seroprevalence of zoonotic parasites (T. gondii, Trichinella spp.) and bacterial pathogens (F. tularensis, B. bronchiseptica) increased significantly between 1986–1989 and 1995–1998, ranging from +6.2% to +20.8%, with T. gondii continuing to increase into 2015–2017 (+25.8% overall). Seroprevalence of viral pathogens (CDV, CPV) and N. caninum did not change with time. Toxoplasma gondii seroprevalence was higher following wetter summers, while seroprevalences of Trichinella spp. and B. bronchiseptica were positively correlated with hotter summers. Seroprevalence of antibodies to F. tularensis increased following years polar bears spent more days on land, and polar bears previously captured in human settlements were more likely to be seropositive for Trichinella spp. As the Arctic has warmed due to climate change, zoonotic pathogen exposure in WH polar bears has increased, driven by numerous altered ecosystem pathways.
Keywords: Arctic, climate change, serology, Ursus maritimus, wildlife disease, zoonosis
Western Hudson Bay (WH) polar bear serum samples were analysed for antibodies to seven pathogens across three time periods: 1986–1989, 1995–1998 and 2014–2017. Antibodies to zoonotic pathogens increased between the 1980s and all other time periods, and were driven by environmental variables undergoing climate change. The increase in antibodies was greatest between 1986–1989 and 1995–1998, a period during which WH polar bears experienced declines in abundance, body condition and reproductive output.

1. INTRODUCTION
Pathogens can impact the health of wildlife in a range of ways, from sublethal effects to eruptive disease outbreaks causing significant population declines (Alexander & Appel, 1994; Sasan et al., 2019; Rijks et al., 2005). Concomitant exposure and multi‐pathogen infections can also alter host response, potentially compounding the burden on the immune system (Cox, 2001; Pedersen & Fenton, 2007). Therefore, factors that influence pathogen persistence are important considerations for conservation management. This is imperative with respect to zoonotic pathogens, which can cause significant health challenges in humans (Shereen et al., 2020), with cascading impacts on the conservation of wildlife (Hockings et al., 2020; Lindsey et al., 2020).
Climate change is expected to amplify some pathogens in wildlife, with warming temperatures and changing precipitation regimes potentially causing an increase in pathogen persistence, prevalence, emergence, and transmission (Altizer et al., 2013). However, host–pathogen ecology, including density‐dependent processes and vectors, needs careful examination for the role of climate (Harvell et al., 2009; McDonald et al., 2016; Ogden & Lindsay, 2016). Despite the recent rise in climate–disease interaction studies (Altizer et al., 2013), and the formation of a crisis discipline (‘Conservation Medicine’; Aguirre et al., 2002), there remains a paucity of long‐term empirical studies associating the prevalence of wildlife disease with climatic factors. Because not all pathogens respond to changes in climate in the same way (Karvonen et al., 2010; Lafferty, 2009; Menge et al., 2016), further empirical study is necessary to improve forecasting models.
The Arctic is an important ecosystem for monitoring climate–disease interactions because it is warming twice as fast as the rest of the planet (Cohen et al., 2014), is relatively simple with low species diversity (Post et al., 2013) and many northern people depend on the traditional harvest of wildlife which can lead to zoonotic exposure risk (McDonald et al., 1990; Møller et al., 2010). Recent Arctic studies have shown linkages between climate change and the life cycles of endoparasites (Hoar et al., 2012; Kutz et al., 2005), ectoparasites (Descamps, 2013; Larsson et al., 2007) and biting insects (Culler et al., 2015). Although there has been a long history of investigations into disease prevalence in Arctic wildlife (Connell, 1949; Elton, 1931), the majority have been cross‐sectional rather than longitudinal (Carlsson et al., 2019; Clausen & Hjort, 1986; Dick & Belosevic, 1978; Elmore et al., 2016). While calls have been made for Arctic climate–disease interaction studies (Bradley et al., 2005; Burek et al., 2008), logistical challenges have been a major barrier to long‐term, repeat sample collection.
Polar bears (Ursus maritimus) are a widely dispersed apex carnivore, with a species range covering the circumpolar Arctic and extending across more than 35 degrees of latitude (PBSG, 2018). Given their range and trophic position, polar bears may be a sentinel species for changing disease dynamics in the Arctic (Stirling & Derocher, 2012). Jensen et al. (2010) reported an increase in the seroprevalence of Toxoplasma gondii in polar bears from Svalbard, Norway from 24.3% in the 1990s (Oksanen et al., 2009), to 47.6% in 2006–2008. Jensen et al. (2010) speculated that the increase was linked to enhanced survival of oocysts in warmer water from the North Atlantic Current. Atwood et al. (2017) documented an increase in the seroprevalence of T. gondii and Coxiella burnetii in polar bears from the southern Beaufort Sea, 2007–2014. Polar bears that spent summer and fall on land were found to be seven times more likely to be seropositive for T. gondii than those that stayed on the sea ice (Atwood et al., 2017), and to have a heightened immune system response (Whiteman et al., 2019). As increased use of land was related to sea ice loss as a consequence of climate change (Atwood, Peacock, et al., 2016; Gleason & Rode, 2009), Atwood et al. (2017) was the first polar bear study to link an ecosystem pathway for increased pathogen prevalence to climate change.
Polar bears in western Hudson Bay (WH), Canada migrate to and from land annually, following the melt and refreeze patterns of sea ice in the Bay (Derocher & Stirling, 1990a). Between 1979 and 2014, the number of ice‐free days in WH increased 8.6 days/decade (Stern & Laidre, 2016), altering polar bear migratory phenology (Castro de la Guardia et al., 2017; Cherry et al., 2013). While on land, polar bears forage opportunistically on a variety of terrestrial foods (Derocher et al., 1993; Russell, 1975), but lose approximately 1 kg of mass per day (Pilfold et al., 2016). As a result, longer ice‐free seasons in Hudson Bay are correlated with decreases in body condition, reproductive output and survival of polar bears (Derocher & Stirling, 1995; Lunn et al., 2016; Regehr et al., 2007; Sciullo et al., 2016). Additionally, increased time on land is positively correlated with human–polar bear interactions in communities (Towns et al., 2009). However, the consequences of climate change and longer ice‐free seasons on pathogen exposure in WH polar bears are unknown.
The objectives of this study were to examine whether the seroprevalence of seven pathogens in WH polar bears has changed over a 32‐year period (1986–2017), and assess any temporal trends in seropositivity against biotic and abiotic variables for potential influential drivers of changes in pathogen exposure. It has been shown that sex and age can influence seroprevalence to some pathogens in polar bears (Jensen et al., 2010; Oksanen et al., 2009; Rah et al., 2005). Additionally, while sea ice has been the primary focus for climate interaction studies in polar bears (Stirling & Derocher, 2012), the Hudson Bay ecosystem is also undergoing changes in air temperature and precipitation regimes (Macrae et al., 2014; Figure 1). We aimed to infer which factors are most influential to changing pathogen exposure in polar bears through a serological analysis from the longest continuously studied polar bear population in the world.
FIGURE 1.
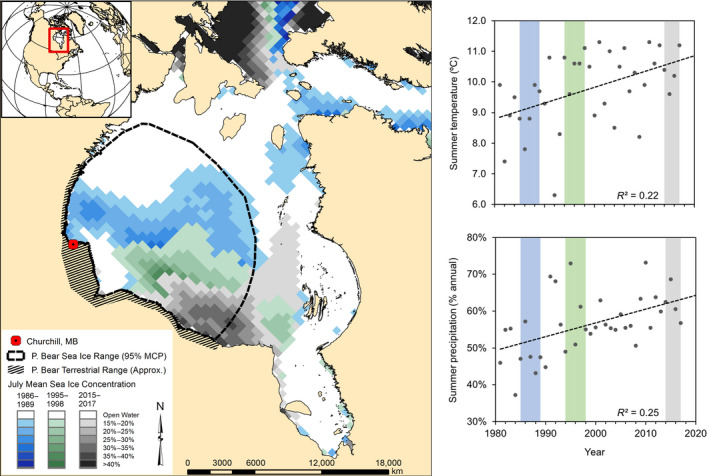
Study area of western Hudson Bay, Canada. Hudson Bay has undergone significant changes in sea ice cover, summer air temperatures and precipitation regimes, 1986–2017. The thick dashed black line is the mean on‐ice home range of polar bears based on adult female movement (McCall et al., 2014), with July mean sea ice concentrations during study periods overlaid from oldest to newest. The hatched polygon represents the approximate terrestrial range of polar bears during ice‐free months
2. MATERIALS AND METHODS
2.1. Study area
Hudson Bay is a shallow inland sea (Jones & Anderson, 1994), with a seasonal sea ice regime that influences the climate dynamics of the ecosystem (Rouse, 1998). Between 1986 and 2017, annual mean (±SE) air temperature as recorded at Churchill Airport, Manitoba (Churchill A, Station ID 5060600, 58°44'24.0"N, 94°04'12.0"W, Environment Canada, 2020) was –6.30 ± 0.07°C, with a mean air temperature of 9.90 ± 0.06°C in summer (June–September) and –22.7 ± 0.1°C in winter (December–March). Mean annual precipitation between 1986 and 2017 was 412.2 ± 3.1 mm. The proportion of precipitation falling during summer increased over the study period, along with air temperature (Figure 1).
During the winter, Hudson Bay is ice‐covered. WH polar bears occupy and travel over much of the Bay, with mean annual home ranges for adult females of approximately 230,000–380,000 km2 (McCall et al., 2014; Figure 1). Sea ice break‐up begins in May and the Bay is ice‐free by August, forcing WH bears to spend the summer and fall onshore. WH polar bears congregate during the ice‐free months along the western coastline of Hudson Bay in the lowlands (Derocher & Stirling, 1990a; Stirling et al., 2004), which are dominated by poorly drained peat bog plateaus and channel fens, with a mixture of open‐canopy spruce‐lichen woodlands (Macrae et al., 2014). During the ice‐free period, WH polar bears can also be found near human settlements, in particular Churchill, MB, which employs the Polar Bear Alert Program to minimize human–polar bear conflict by intercepting bears that come close to town (Kearney, 1989; Towns et al., 2009).
2.2. Sample collection
Samples were collected in 1986–1989, 1995–1998 and 2015–2017 as part of ongoing, long‐term studies of WH polar bears (Lunn et al., 2016). Bears were located by helicopter and chemically immobilized via remote injection following standard protocols (Stirling et al., 1989). A vestigial premolar was extracted for age estimation (Calvert & Ramsay, 1988) from previously unmarked bears older than 1 year. Blood was drawn from a femoral vein using a 30 or 60 ml syringe within half an hour of capture, immediately transferred into red/grey SST tubes and kept in a cooler until it was spun in a centrifuge to separate the serum at the end of each day. All capture and handling methods were reviewed and approved annually by the Environment and Climate Change Canada Western and Northern Animal Care Committee.
Sera were stored at –70°C until needed. Freezers were monitored with alarm systems to ensure serum samples did not undergo freeze–thaw cycles. While we did not analyse for the possible influence of sample degradation over time, we were confident that storage at –70°C prevented proteolysis and had negligible impact on our ability to detect antibodies (Cecchini et al., 1992; Dard et al., 2017). We focused the analysis on sera from adult polar bears (≥5 years) and when possible, balanced samples between males and females, within and over years (Table 1). Negative control sera were obtained in 2019 from two adult male polar bears (aged 5 and 7 years) born and raised in captivity at the Toronto Zoo.
TABLE 1.
Sera sample sizes from adult polar bears of the Western Hudson Bay, Canada, 1986–2017
| Year | Female | Male | Total |
|---|---|---|---|
| 1986 | 17 | 17 | 34 |
| 1987 | 20 | 21 | 41 |
| 1988 | 19 | 20 | 39 |
| 1989 | 21 | 22 | 43 |
| 1995 | 20 | 20 | 40 |
| 1996 | 20 | 20 | 40 |
| 1997 | 20 | 19 | 39 |
| 1998 | 20 | 20 | 40 |
| 2015 | 17 | 15 | 32 |
| 2016 | 18 | 20 | 38 |
| 2017 | 19 | 20 | 39 |
| Total | 211 | 214 | 425 |
2.3. Serological analyses
We ran serological assays to detect exposure to seven pathogens (Table 2): T. gondii, Neospora caninum, Trichinella spp., Francisella tularensis, Bordetella bronchiseptica, canine morbillivirus (CDV) and canine parvovirus (CPV). We selected these pathogens in order to have representation from parasitic, bacterial and viral diseases. We selected pathogens well‐documented in polar bears for comparison of seroprevalence across their range (T. gondii, Trichinella spp., CDV), pathogens with minimal documentation for follow‐up (N. caninum, F. tularensis) and pathogens that have not been surveyed before (B. bronchiseptica, CPV) to provide a baseline for future monitoring.
TABLE 2.
Serology for selected diseases in adult polar bears of Western Hudson Bay, Canada, 1986–2017
| Pathogen | Type | Zoonotic | Test | Kit | Cut‐off | Antibodies detected | Negative control | Positive control |
|---|---|---|---|---|---|---|---|---|
| Toxoplasma gondii | Protozoan | Yes | iELISA | ID Screen® Toxoplasmosis Indirect Multi‐species | S/P% ≥ 50 = Positive | IgG | Kit control | Kit control |
| Neospora caninum | Protozoan | No | cELISA | Neospora Caninum Antibody Test Kit | % I > 30 = Positive | IgG, IgM | Kit control | Kit control |
| Trichinella spp. | Helminth | Yes | iELISA | Developed at the CFAP, CFIA | PP ≥13.15% = Positive | IgG, IgM | Polar bear sera | Polar bear serum |
| cELISA | Developed at the CFAP, CFIA | Not establisheda | IgG, IgM | Polar bear sera | Infected pig serum | |||
| Francisella tularensis | Bacteria | Yes | MAT | Sato et al. (1990) | 1:128 | IgG, IgM | Arctic fox sera | Arctic fox sera |
| Bordetella bronchiseptica | Bacteria | Yes | ELISA | Ellis et al. (2011, 2021) | 15 OD units | IgG | Dog sera | Dog sera |
| Canine parvovirus | Virus | No | ELISA | Developed at the WCVM, University of Saskatchewan | 20 OD units | IgG | Dog sera | Dog sera |
| Canine morbillivirus | Virus | No | ELISA | Soma et al. (2001) | 1/0 | IgG | Dog sera | Dog sera |
Test values of the negative control are used for the normalization of test results in cELISA. Samples from animals with infection‐free status confirmed by a gold standard method are required for establishing the cELISA cut‐off. Matching muscle tissue samples were not available in this study to confirm the presumed negative Trichinella infection status of the negative controls by the artificial digestion method.
2.3.1. Toxoplasma gondii
IgG antibodies against T. gondii were detected using a commercial indirect‐ELISA (iELISA) kit (ID Screen® Toxoplasmosis Indirect Multi‐species, IDvet Innovative Diagnostics) that has shown high specificity for T. gondii and non‐cross reactivity with N. caninum (ID Vet, 2020). In addition to kit controls and negative controls from captive polar bears, serum samples previously collected from wolverines and known to be positive and/or negative by molecular methods (Sharma et al., 2019) were used as internal controls. Results were obtained as sample/positive percentage (S/P%), calculated using the optical density (OD) of the positive and negative kit controls, and the OD of test samples using the following formula: S/P% = [(OD sample – OD negative control)/(OD positive control – OD negative control)] ×100. S/P% values of ≥50 were considered positive.
2.3.2. Neospora caninum
Serum samples were tested for the presence of antibodies to N. caninum using a commercially available competitive‐ELISA (cELISA) kit (Neospora Caninum Antibody Test Kit cELISA, Veterinary Medical Research & Development), in which both the antigen and monoclonal antibody conjugate have shown high specificity to N. caninum and non‐cross reactivity with T. gondii or Sarcocystis spp. (Baszler et al., 1996). The test was performed following the manufacturer's instructions. Results were calculated and expressed as % Inhibition (% I) using the formula % I = 100 × [1 − (Sample OD/negative control OD)]. Samples were considered positive if the test sample produced ≥0% inhibition and negative if the test sample produced <30% inhibition.
2.3.3. Trichinella spp.
Antibodies to Trichinella spp. were detected using an in‐house cELISA and iELISA at the Canadian Food Inspection Agency's Centre for Food‐borne and Animal Parasitology. Both assays utilized Nunc MaxiSorp 96‐well plates (Thermo Fisher Scientific) coated overnight at 2°C–8°C with excretory‐secretory (E‐S) antigen of T. spiralis first‐stage larvae diluted in carbonate/bicarbonate buffer (pH 9.6). Washing of ELISA plates was performed with Tris‐buffered saline containing 0.05% Tween‐20 (TBST). After coating with antigen, the plates were washed, then blocked for 2 h at room temperature with 2% bovine serum albumin (BSA; Sigma‐Aldrich) in Tris‐buffered saline.
All polar bear sera used in this study were initially tested by cELISA using plates coated with 50 ng/well of the E‐S antigen. Immune serum from a pig experimentally infected with T. spiralis served as a positive repeatability control. Sera were diluted 1/50 in TBST containing 1% BSA and the working dilution of an in‐house produced mouse monoclonal antibody (M3963, IgG1 isotype) specific to TSL‐1 (immunodominant carbohydrate epitope containing β‐tyvelose), that was determined by checkerboard titration. Positive control and test sera were loaded in duplicate, whereas the negative controls were tested in triplicate. Since the two negative control captive polar bear sera produced very similar test values in every assay run, absorbance values for only one of them were used to calculate % I. The plates were incubated for 1 h at room temperature, with shaking (450 rpm). After three washes with TBST, the plates were incubated for 30 min as above with cross‐adsorbed anti‐mouse IgG (Fc):HRP (Thermo Fisher Scientific) diluted 1/5000 in TBST‐1% BSA. The plates were washed six times and incubated for 15 min at room temperature with 3,3’,5,5'‐tetramethylbenzidine (TMB; Seracare). The reaction was stopped using TMB BlueSTOP solution (Seracare) and the plates were read at 650 nm using a SpectraMax Plus 384 microplate spectrophotometer and SoftMax Pro 6.2 software (Molecular Devices). The results were expressed as % I using the same equation described above for N. caninum cELISA. Testing by cELISA enabled selection of a subset of positive polar bear sera with high % I values that were instrumental in developing the iELISA. To produce bear‐specific conjugate for iELISA, goat anti‐bear serum (Forensic; MP Biomedicals) was purified using the NAb Protein A/G Spin Kit (Thermo Scientific). The eluted antibodies were transferred into 20 mM HEPES, 300 mM NaCl using Sephadex G‐25 Desalting Columns (GE Healthcare). Three hundred microlitres of this preparation containing 4 mg of purified IgG was conjugated to HRP using a Lightning‐Link HRP Labelling Kit (Novus Biologicals). The conjugate was diluted twofold in an equal volume of sterile glycerol and stored at −20ºC. To perform the assay, ELISA plates were coated with 100 ng/well of the E‐S antigen. A polar bear serum sample that exhibited 92.2% inhibition on preliminary testing by cELISA (above) was used as a positive control. Test and control sera were diluted 1/200 in TBST containing 1% BSA and 5% skim milk and incubated in duplicate for 1 h at room temperature, with shaking (450 rpm). This was followed by incubation for 1 h as above with the anti‐bear HRP conjugate diluted 1/5000 in TBST‐1% BSA. After the final wash, incubation with the TMB substrate was carried out for 10 min. The subsequent steps were as those for cELISA. The absorbance values in iELISA were normalized as per cent of positive control (PP). The cut‐off for iELISA was calculated as four times the mean of PP values of the two negative control sera. There was a strong correlation between both Trichinella assays (Pearson correlation coefficient: r = 0.93).
2.3.4. Franciscella tularensis
A microagglutination assay (MAT) was used to detect IgM and IgG antibodies for F. tularensis (Sato et al., 1990). Serum samples from Arctic foxes (Vulpes lagopus) that were previously tested with a MAT were used as positive and negative controls. A high positive control (1:1024), low positive control (1:128) and negative control were used for each run. Titres ≥1:128 were considered positive.
2.3.5. Bordetella bronchiseptica
An ELISA for B. bronchiseptica‐reactive IgG antibodies was performed as previously described using anti‐canine IgG as the conjugate (Ellis et al., 2011, 2021). A previously determined cut‐off value of 15 OD units, which was based on the testing of positive and negative dog sera, was used as an indicator of biologically significant antibody response after exposure to B. bronchiseptica antigens (Ellis et al., 2011, 2021).
2.3.6. Canine parvovirus
Serum antibodies reactive with CPV antigens were measured using an ELISA that was developed using standard procedures. Briefly, single‐component, high titre (>1/8000 haemagglutination titre), low‐passage CPV 2a vaccine (Vanguard Plus CPV, Zoetis, kindly provided by Dr J. Wu) was used as antigen to coat plates. Optimal dilution of this antigen and secondary sheep anti‐canine IgG was determined in a checkboard titration using known CPV positive and negative canine sera. A 1/500 dilution of antigen was used and sera were tested at a 1/100 final dilution. Units of activity were determined by comparison with the OD values of the canine positive control serum.
2.3.7. Canine morbillivirus
Serum antibodies reactive with CDV were detected in an immunoperoxidase plaque staining assay that was conducted according to previously described methods (Soma et al., 2001), with minor modifications. Briefly, Vero cells were infected in suspension with approximately 0.1 multiplicity of infection of the Onderstepoort strain of CDV. Approximately 105 cells were plated into 96‐well tissue culture plates. Monolayers were fixed in acetone 72 h later, and reacted with a 1/50 dilution of polar bear sera or CDV‐positive or ‐negative canine sera, followed by peroxidase‐conjugated sheep anti‐canine IgG antiserum. Individual wells were scored as positive or negative.
2.4. Statistical analyses
Seroprevalence of each pathogen between 1986–1989 (n = 157), 1995–1998 (n = 159), and 2015–2017 (n = 109), and co‐occurrence between pathogens, were compared using a Pearson chi‐square. Polar bears were sampled a maximum of once per year, but some individuals were sampled multiple times within a time period (1986–1989, 1995–1998, 2015–2017). To minimize overestimates resulting from enduring antibodies, individuals that were sampled multiple times (27/41 total repeats) were only counted on their first positive result, not subsequent positive results within the time period. This resulted in only 3.7% (14/381) of the seroprevalence dataset from the same bears repeatedly sampled across time periods. Any individual seroconversions within a time period were recorded as independent observations. Because the number of repeat positives removed varied by pathogen, sample sizes for seroprevalence estimates are unequal. We assessed mean age of bears in each time period with a one‐way ANOVA, to exclude the explanation of an age‐based bias influencing seroprevalence changes over time.
To examine which factors were influential to changes in pathogen exposure in polar bears, we considered potentially influential sets of biological and climatic variables on an annual time scale (Tables 3 and 4). Biological variables included age, sex, weight, body condition and conflict history of the bear. Briefly, the variable ‘Age’ was estimated from the cementum layers of an extracted vestigial premolar (Calvert & Ramsay, 1988), while ‘Sex’ was determined in the field. Polar bears were given a subjective fatness rating of 1–5 at the time of capture (Stirling et al., 2008); bears rated 1–2 were considered in ‘Poor’ condition, bears rated 3 were ‘Average’ and bears rated 4–5 were considered in ‘Good’ condition. Prior to modelling, categories of body condition were dummy coded with ‘Average’ forming the reference category. The variable ‘Weight’ was estimated from measurements of straight‐line length and axillary girth during handling (Thiemann et al., 2011). Lastly, polar bears with any history of capture as part of the Polar Bear Alert Program prior to sampling were categorized as ‘Conflict’ bears.
TABLE 3.
Covariates used to model the likelihood of pathogen seropositivity in adult polar bears of Western Hudson Bay, Canada, 1986–2017
| Name | Range | Description and source |
|---|---|---|
| Biological | ||
| Age | 5–31 | Age of polar bear via tooth histology (Calvert & Ramsay, 1988) |
| Sex | 1/0 | Field determination with females as reference category (0) |
| Poora | 1/0 | Polar bears rated 1 or 2 on five‐point body condition index (Stirling et al., 2008) |
| Gooda | 1/0 | Polar bears rated 4 or 5 on five‐point body condition index (Stirling et al., 2008) |
| Weightb | 136–602 kg | Calculated weight following Thiemann et al. (2011) matched to temporal equations for WH |
| Conflict | 1/0 | Polar bears captured by Manitoba Conservation in the town of Churchill, MB (Kearney, 1989; Towns et al., 2009) prior to sample collection |
| Climatic c | ||
| IceFree | 110–152 days | Number of days of sea ice concentration <15% as determined by SSM/I (Cavalieri et al., 1996), within 95% minimum convex polygon polar bear home range (McCall et al., 2014) |
| STemp | 7.8°C–10.8°C | Mean air temperature June–September as measured at Churchill Airport, MB (Environment Canada, 2020) |
| SPrecip | 169.0–310.6 mm | Total precipitation June–September as measured at Churchill Airport, MB (Environment Canada, 2020) |
| WMinTemp | −30.0°C to −24.9°C | Mean minimum air temperature December–March as measured at Churchill Airport, MB (Environment Canada, 2020) |
| ATemp | −7.4°C to −5.2°C | Mean annual air temperature as measured at Churchill Airport, MB (Environment Canada, 2020) |
| APrecip | 344.7–507.8 mm | Total annual precipitation as measured at Churchill Airport, MB (Environment Canada, 2020) |
Five‐point body condition index dummy‐coded, with ‘Average’ (3) forming the reference category.
Weight mean centred within sex prior to modelling.
All climate variables measured the year prior to serum sample.
TABLE 4.
Mean values of climatic variables in each time period of investigation in the Hudson Bay, Canada, 1986–2017. See Table 3 for definitions of each variable
| Units | 1986–1989 | 1995–1998 | 2015–2017 | |
|---|---|---|---|---|
| IceFree | days | 114 ± 6 | 141 ± 16 | 145 ± 7 |
| STemp | °C | 9.1 ± 0.9 | 10.5 ± 0.6 | 10.3 ± 0.9 |
| SPrecip | mm | 212.5 ± 49.5 | 274.1 ± 42.4 | 226.0 ± 16.0 |
| WMinTemp | °C | −27.5 ± 2.0 | −27.2 ± 1.9 | −25.9 ± 1.3 |
| ATemp | °C | −7.0 ± 1.1 | −6.3 ± 1.2 | −5.7 ± 1.3 |
| APrecip | mm | 431.7 ± 56.9 | 458.0 ± 45.4 | 366.4 ± 38.3 |
Climatic variables included number of ice‐free days, summer temperature, winter minimum temperature, annual temperature, summer precipitation and annual precipitation. Summer was defined as June–September, while winter was defined as December–March. All climate variables, with the exception of sea ice, were measured at the weather station at Churchill Airport, Manitoba (Environment Canada, 2020). All WH polar bears migrate to land during the ice‐free period, and migration phenology correlates with thresholds of sea ice concentration (Cherry et al., 2013; Stirling et al., 1999). The variable ‘IceFree’ was the number of days in a year of less than 15% sea ice concentration as determined by SSM/I with a spatial grain of 25 km2 (Cavalieri et al., 1996), within the on‐ice home range of WH polar bears (McCall et al., 2014). We used a threshold of 15% sea ice concentration as it aligns with the climatic definition of ‘ice‐free’ (Parkinson et al., 1999), and is a conservative measure of the total number of days polar bears likely spent on land. Lastly, all climatic measurements were taken from the year prior to the year of the serum sample. Temporal lags were tested out to 3 years from year of sample, and a 1‐year lag resulted in the best fit (Table S1).
We related the seroprevalence (1/0) of pathogens that showed significant change over time to variables using binomial (logit link) generalized linear mixed models, and the same constrained set of a priori models for each pathogen, balanced for equal representation of all variables (Tables S2 and S3). We included Bear ID as a random effect to control for repeat samples from the same individuals. Prior to modelling, we mean‐centred continuous covariates about zero, and examined Pearson correlation coefficients for any pairs of factors that had a coefficient >0.7. To assess the relative influence of biological and climatic factors, we used Akaike's information criterion for small samples (AICC; Burnham & Anderson, 2002). We evaluated sets of biological and climatic factors separately, and identified top factors for each as having an AICC wi ≥ 0.60. To assess the comparative strength of biological and climatic factors on their influence on disease exposure, we combined top biological and climatic factors into one model, and used log‐likelihood ratio tests and conditional‐R 2 (Nakagawa & Schielzeth, 2013) to examine model improvement. All analyses were conducted in R (Version 3.3.3; R Development Core Team, 2017); all reported variances are 95% confidence intervals, and the alpha cut‐off value was set to 0.05 for all significance tests.
3. RESULTS
3.1. Seroprevalence
The mean age of adult females and males sampled was 14.1 ± 0.8 (n = 211) and 12.4 ± 0.7 (n = 214), respectively, and there was no significant difference in mean age between the time periods (F 2,422 = 0.60, p = 0.55). Mean seroprevalence was 55.9% (228/408) for T. gondii, 9.7% (41/424) for N. caninum, 66.0% (270/409) for Trichinella spp., 52.3% (216/413) for F. tularensis, 68.4% (281/411) for B. bronchiseptica, 24.0% (100/417) for CDV and 7.0% (28/400) for CPV. Co‐occurrence between pathogens did not significantly vary from random (Table 5), with the exception of T. gondii and N. caninum (χ 2 = 13.5, df =1, p < 0.001), in which all seropositive cases of N. caninum were observed in bears also seropositive for T. gondii.
TABLE 5.
Co‐occurrence of seropositive assays for pathogens examined in adult polar bears of Western Hudson Bay, Canada, 1986–2017
| Neospora caninum | Trichinella spp. | Francisella tularensis | Bordetella bronchiseptica | Canine parvovirus | Canine morbillivirus | |
|---|---|---|---|---|---|---|
| Toxoplasma gondii | 9.88% | 40.24% | 30.12% | 40.71% | 3.99% | 13.41% |
| Neospora caninum | 5.88% | 4.71% | 7.53% | 0.50% | 1.41% | |
| Trichinella spp. | 35.53% | 48.24% | 4.49% | 18.59% | ||
| Francisella tularensis | 38.35% | 3.24% | 14.59% | |||
| Bordetella bronchiseptica | 5.49% | 18.82% | ||||
| Canine parvovirus | 0.94% |
Bold value indicates co‐occurrences significantly different than expected by chance (p < 0.05).
3.2. Seroprevalence over time
Seroprevalence varied significantly between time periods for T. gondii (χ 2 = 17.4, df =2, p < 0.001), Trichinella spp. (χ 2 = 13.4, df =2, p = 0.001), F. tularensis (χ 2 = 9.9, df =2, p = 0.007) and B. bronchiseptica (χ 2 = 23.1, df =2, p < 0.001). The seroprevalence of all four pathogens increased between 1986–1989 and 1995–1998 (Figure 2). Only T. gondii seroprevalence continued to increase significantly between 1995–1998 and 2015–2017, increasing by an overall total of 25.8% (Figure 2a). Seroprevalence of N. caninum (χ 2 = 4.4, df =2, p = 0.11), and viral pathogens CDV (χ 2 = 0.4, df =2, p = 0.83) and CPV (χ 2 = 2.7, df =2, p = 0.26) did not significantly vary between time periods. Of the 381 individual polar bears sampled, 38 individuals were sampled twice and three individuals were sampled three times. The number of observed seroconversions varied by pathogen, with only one observation for T. gondii and CPV, and 15 observations for B. bronchiseptica, with a range of 1–21 years between observations (Table 6).
FIGURE 2.

Temporal trends in the seroprevalence of pathogens (a) Toxoplasma gondii, (b) Trichinella spp., (c) Neospora caninum, (d) Francisella tularensis, (e) Bordetella bronchiseptica, (f) canine parvovirus and (g) canine morbillivirus for adult polar bears in Western Hudson Bay, 1986–2017. Bold indicates values that are significantly different from all others (p < 0.05)
TABLE 6.
Observations of seroconversion for pathogens examined for adult polar bears of Western Hudson Bay, Canada, with repeat samples (n = 41)
| Seroconversion | Years apart | ||||
|---|---|---|---|---|---|
| Positive | Negative | Total | Minimum | Maximum | |
| Toxoplasma gondii | 1 | 0 | 1 | 8 | 8 |
| Neospora caninum | 1 | 2 | 3 | 1 | 9 |
| Trichinella spp. | 9 | 0 | 9 | 1 | 9 |
| Francisella tularensis | 4 | 7 | 11 | 1 | 11 |
| Bordetella bronchiseptica | 10 | 5 | 15 | 1 | 21 |
| Canine parvovirus | 1 | 0 | 1 | 2 | 2 |
| Canine morbillivirus | 1 | 2 | 3 | 2 | 7 |
3.3. Biological factors
Sex was a top factor in the seroprevalence of both Trichinella spp. (AICC wi = 0.83) and B. bronchiseptica (AICC wi = 1.00). Males were 1.82 (CI95 = 1.03–3.23) times more likely to be seropositive for Trichinella spp. than females, while females were 4.95 (CI95 = 2.53–9.71) times more likely to be seropositive for B. bronchiseptica. Age was a top factor in the seroprevalences of Trichinella spp. (AICC wi = 0.92) and B. bronchiseptica (AICC wi = 0.98). The likelihood of being seropositive for both Trichinella spp. (β = 0.06, CI95 ± 0.05) and B. bronchiseptica (β = 0.09, CI95 ± 0.06) increased with age. Polar bears previously captured in human settlements (‘Conflict’) was a top factor for Trichinella spp. (AICC wi = 0.81) and F. tularensis (AICC wi = 0.74). Conflict polar bears were 2.24 (CI95 = 1.11–4.54) times more likely to be seropositive for Trichinella spp., and 1.97 (CI95 = 1.16–3.32) times less likely for F. tularensis. Body condition was a top factor for seroprevalence of F. tularensis (AICC wi = 0.70), with bears rated in good condition 1.84 (CI95 = 1.07–3.19) times more likely to be seropositive for F. tularensis. No biological factors had a ranking AICC wi ≥ 0.60 for T. gondii. In all cases, the addition of top biological factors significantly improved overall model fit for all pathogens modelled (Table 7).
TABLE 7.
Comparison of model fit for top biological and climatic factors to the likelihood of seroprevalence of Toxoplasma gondii, Trichinella spp., Francisella tularensis and Bordetella bronchiseptica in adult polar bears of western Hudson Bay, Canada, 1986–2017. Models were fit using a binomial logit‐link generalized linear mixed model, with individual Bear ID as a random effect (1|BearID). For T. gondii, no biological covariates made the cut‐off for inclusion, therefore the combined model is identical to the climatic model. For Trichinella spp., F. tularensis and B. bronchiseptica, all top models significantly improved fit with inclusion of both biological and climatic covariates (LL ratio test; p ≤ 0.02)
| Rank | Type | Model | k | LL | AICC | ΔAICC | R 2 Cond |
|---|---|---|---|---|---|---|---|
| Toxoplasma gondii | |||||||
| 1 | Combined | (1|BearID) + SPrecip + APrecip | 4 | −274.99 | 558.08 | 0.00 | 0.39 |
| 2 | Null | (1|BearID) | 2 | −284.60 | 573.24 | 15.16 | 0.35 |
| Trichinella spp. | |||||||
| 1 | Combined | (1|BearID) + Sex + Age + Conflict + STemp + WMinTemp | 7 | −250.66 | 515.58 | 0.00 | 0.37 |
| 2 | Biological | (1|BearID) + Sex + Age + Conflict | 5 | −258.81 | 524.64 | 9.05 | 0.32 |
| 3 | Climatic | (1|BearID) + STemp + WMinTemp | 4 | −257.25 | 525.72 | 10.13 | 0.32 |
| 4 | Null | (1|BearID) | 2 | −266.27 | 536.57 | 20.99 | 0.25 |
| Francisella tularensis | |||||||
| 1 | Combined | (1|BearID) + Good + Conflict + IceFree | 5 | −282.06 | 574.27 | 0.00 | 0.19 |
| 2 | Climatic | (1|BearID) + IceFree | 3 | −287.43 | 580.91 | 6.64 | 0.16 |
| 3 | Biological | (1|BearID) + Good + Conflict | 4 | −291.35 | 582.74 | 8.46 | 0.16 |
| 4 | Null | (1|BearID) | 2 | −292.27 | 588.56 | 14.29 | 0.13 |
| Bordetella bronchiseptica | |||||||
| 1 | Combined | (1|BearID) + Sex + Age + STemp | 5 | −222.27 | 454.68 | 0.00 | 0.42 |
| 2 | Biological | (1|BearID) + Sex + Age | 4 | −236.79 | 481.67 | 26.99 | 0.31 |
| 3 | Climatic | (1|BearID) + STemp | 3 | −247.72 | 501.50 | 46.82 | 0.24 |
| 4 | Null | (1|BearID) | 2 | −260.65 | 525.32 | 70.64 | 0.12 |
3.4. Climate factors
Mean summer temperature was a top factor in the seroprevalence of Trichinella spp. (AICC wi = 0.72) and B. bronchiseptica (AICC wi = 0.76). The likelihood of being seropositive for Trichinella spp. (β = 0.51, CI95 ± 0.35) and B. bronchiseptica (β = 0.78, CI95 ± 0.35) increased with warmer summers. In addition, mean minimum winter temperature was a top factor for Trichinella spp. (AICC wi = 0.73), with bears more likely to be seropositive following warmer winters (β = 0.20, CI95 ± 0.18). Total precipitation in the summer months (AICC wi = 0.85) and annually (AICC wi = 0.85) were top factors in the seroprevalence of T. gondii. Wetter summers (β = 0.12, CI95 ± 0.08) within dryer years overall (β = −0.13, CI95 ± 0.07) increased the likelihood of polar bears seropositive for T. gondii. The number of ice‐free days was a top factor in the seroprevalence of F. tularensis (AICC wi = 0.77), with increased likelihood of being seropositive following years with more ice‐free days (β = 0.03, CI95 ± 0.02). In all cases, the addition of top climatic factors significantly improved overall model fit for all diseases modelled (Table 7).
4. DISCUSSION
Climate change is expected to increase the severity and scope of outbreaks of some diseases in wildlife, as warmer and wetter conditions are hypothesized to facilitate the transmission and survival of pathogens (Altizer et al., 2013), including expanding pathogen range into latitudes not previously observed (Baker‐Austin et al., 2013; Dudley et al., 2015). However, empirical evidence of climate‐mediated change in disease prevalence, even in Arctic systems with accelerated warming, has been lacking. We detected increases in the seroprevalence of four of the seven pathogens we surveyed in WH polar bears between at least two time periods. Seroprevalences increased for zoonotic parasites (T. gondii and Trichinella spp.) and zoonotic bacterial pathogens (F. tularensis and B. bronchiseptica), but remained unchanged for viruses (CDV and CPV) and N. caninum. We also found support for climate‐mediated changes in the prevalence of each of the four zoonotic pathogens surveyed.
The protozoan parasite T. gondii increased in seroprevalence between all time periods, peaking at 69.6% in 2015–2017. Our findings are higher than any previous report for adult polar bears (Atwood et al., 2017; Jensen et al., 2010; Kirk et al., 2010; Naidenko et al., 2013; Rah et al., 2005). Polar bears in Hudson Bay have the longest on‐land period relative to other subpopulations (PBSG, 2018), suggesting that terrestrial exposure may be an important factor in T. gondii seroprevalence (Atwood et al. 2017). Toxoplasma gondii seroprevalence did not vary with sex or across age, but rather was associated with changing precipitation patterns in Hudson Bay during the period in which polar bears are on land.
Wetter summers, within dryer years overall, were correlated with a higher seroprevalence of T. gondii for WH polar bears. The positive correlation between precipitation and T. gondii seroprevalence reflects patterns documented in other regions with definitive felid hosts (Afonso et al., 2010), intermediate wildlife host species (e.g. crows Covus spp. Salant et al., 2013; roe deer Capreolus capreolus Gamarra et al., 2008; wild rabbits Oryctolagus cuniculus Almeria et al., 2004), and is consistent with the expectations of T. gondii transmission under future climate scenarios (Meerburg & Kijlstra, 2009). Potential terrestrial T. gondii exposure includes polar bears ingesting surface water contaminated with oocysts shed by domestic or wild felid hosts, with wetter conditions during summer months favouring sporulation and spread (Dubey, 2010). In Churchill, domestic cats are kept as pets, but bears that were previously captured there were not more likely to have T. gondii antibodies. Canada lynx (Lynx canadensis) are suspected to serve as definitive hosts of T. gondii, and seropositive lynx have been documented in the surrounding Hudson Bay watershed (Labelle et al., 2001; Simon et al., 2013). However, while Canada lynx range includes the Hudson Bay lowlands (Vashon, 2016), the current status of lynx in the WH study area is unknown. Observations by trappers have been rare (L. Fishback, personal communication, August 11, 2020), and lynx are the only endemic carnivore not detected by trail cameras that have been operating along the coast of Wapusk National Park since 2011 (D. Clark, personal communication, August 11, 2020; Clark et al., 2019).
Like other Arctic carnivores, polar bears may also be exposed to T. gondii through the consumption of tissue cysts in intermediate host species, and precipitation may modulate exposure. On the sea ice, ringed seals (Pusa hispida) are the primary prey for WH polar bears (Thiemann et al., 2008), and ringed seals in Canada have been documented with T. gondii seroprevalences ranging from 10% to 26% (Bachand et al., 2019; Reiling et al., 2019; Simon et al., 2011). Surface run‐off contaminated with T. gondii oocysts into coastal marine environments is a suspected source of infection for marine mammals in the Arctic (Simon et al., 2013). Higher precipitation during the summer months coincides with the hyperphagic feeding period for ringed seals (Young & Ferguson, 2013), which may increase exposure to T. gondii oocysts lodged in the alimentary canal of filter feeding fish (Massie et al., 2010). Alternatively, T. gondii exposure for polar bears could involve the consumption of terrestrial intermediate hosts such as Arctic fox (V. lagopus; Prestrud et al., 2007) or Arctic‐nesting migratory lesser snow geese (Chen caerulescens; Bachand et al., 2019; Elmore et al., 2015). While observations of Arctic fox consumption are very rare in Hudson Bay (but see Richardson & Brook, 2004), snow geese are preyed upon in the summer months by some polar bears in WH (Gormezano & Rockwell, 2013). In years with higher summer precipitation, plant growth is enhanced and nesting survival of snow geese improves (Lecomte et al., 2009), which may bolster goose populations and encounter rates for polar bears.
Neospora caninum is a tissue‐dwelling coccidian parasite closely related to T. gondii, but neosporosis has only been recognized for about 30 years (Dubey et al., 2007). Atwood et al. (2017) was the first to report N. caninum antibodies in polar bears, recorded in 3.7% of Southern Beaufort Sea polar bears, 2007–2014. Comparatively, mean N. caninum seroprevalence in WH was more than double the Southern Beaufort Sea subpopulation. Additionally, all WH polar bears with N. caninum antibodies also had antibodies to T. gondii, potentially reflecting a common route of exposure. Grey wolves (Canis lupus) are the only definitive hosts for N. caninum (Dubey et al., 2011) to overlap with polar bears in WH. As intermediate hosts, polar bears are most likely infected by consuming oocysts shed by canids into the environment.
Transmission by carnivory among intermediate hosts, common for terrestrial mammalian transmission of T. gondii (Dubey, 2010), to our knowledge has not been demonstrated for N. caninum. If this was a potential route of exposure for bears, caribou (Rangifer tarandus) and ringed seals may contribute to exposure. Caribou were found in 10% of WH polar bear scats collected on land (Gormezano & Rockwell, 2013), and ringed seals are the main prey on the sea ice. Neospora caninum‐like DNA was reported in the tissue of 26% ringed seals in eastern Hudson Bay (Reiling et al., 2019), while more than 80% of the Quaminuriaq caribou herd of Hudson Bay were reported as N. caninum seropositive (Carlsson et al., 2019). However, if feeding on intermediate hosts exposed polar bears to N. caninum, we would expect a seroprevalence comparable to T. gondii. The N. caninum seroprevalence we observed aligns with the generally lower oocyst shedding pattern observed in canids (Dubey & Schares, 2011), and likely reflects the overall level circulating in the Hudson Bay ecosystem.
Trichinella spp. have a long history of documentation in polar bears, and previous studies found prevalences comparable to our study (Asbakk et al., 2010; Larsen & Kjos‐Hanssen, 1983; Naidenko et al., 2013; Rah et al., 2005). Our results are consistent with previous findings that Trichinella spp. seroprevalence increases with age in polar bears, although we did not find uniformity between sexes (Asbakk et al., 2010; Rah et al., 2005); rather, males had higher seroprevalence than females. Due to the low Trichinella spp. prevalence in arctic marine mammals (Jenkins et al., 2013), transmission to polar bears likely relies on hunting or scavenging terrestrial carnivores (Richardson & Brook, 2004) or cannibalism (Forbes, 2000; Larsen & Kjos‐Hanssen, 1983). The finding that older males are more likely to be seropositive supports cannibalism as a mode of transmission, as cannibalism is more frequently observed in adult males (Taylor et al., 1985). Dietary studies from scat remains also suggest that WH polar bears more frequently cannibalize their own than consume other terrestrial carnivores (Derocher et al., 1993; Gormezano & Rockwell, 2013; Russell, 1975).
Trichinella spp. seroprevalence in WH polar bears was positively correlated to both warmer summer and winter temperatures across all time periods, but was not correlated to annual temperatures, suggesting a season‐specific response. Although serology cannot differentiate amongst Trichinella spp., it is most probable that the species in this study are Trichinella nativa and/or Trichinella‐T6, which are freeze‐tolerant and the most common sylvatic taxa found in wildlife in Canada (Gajadhar & Forbes, 2010). Mean annual minimum winter temperatures during our study ranged between –30.0°C and –24.9°C. Environmental exposure to temperatures below –20°C is suggested to reduce the survival of T. nativa and Trichinella‐T6 larvae in muscle tissue, with optimal long‐term survival in the range of 0°C to –20°C (Pozio, 2016). It is therefore possible that under reduced extreme winter cold as seen in our study, the survival of Trichinella spp. increased. Furthermore, carcasses freeze more quickly in extreme cold, likely reducing the consumption of muscle tissue by scavengers. Stirling and Øritsland (1995) observed that in –25°C weather, polar bears will generally abandon a seal carcass after the fat is stripped. Under reduced extreme cold in winter, Trichinella parasites may survive longer in the muscle tissue of infected carcasses, and carcasses may remain available for consumption longer, potentially infecting a greater number of bears.
Arctic research has focused on the cold tolerance of Trichinella spp. as a limit to their range (Masuoka et al., 2009; Pozio, 2016), but there has been little research on the effect of summer temperature. Warmer summer air temperatures in Hudson Bay likely increase the probability of heat stress for polar bears (Øritsland et al., 1974) that are near the southern end of their range. Heat stress in endotherms alters the endocrine system, increasing corticoids and depressing the inflammatory response (Morley & Lewis, 2014), which may compromise immunity to parasite invasion. Although long‐term survival of cold‐tolerant T. nativa and Trichinella‐T6 may decrease with warming, host immune response may also subsequently diminish when exposed to warming beyond their thermal tolerance. We suggest future studies of Trichinella spp. in wildlife include a measure of thermal stress to establish its potential role in pathogen transmission.
Both of the bacterial pathogens we surveyed, F. tularensis and B. bronchiseptica, increased in seroprevalence between 1986–1989 and 1995–1998. Little research has been conducted for either of these pathogens in polar bears. Atwood et al. (2017) reported low seroprevalences of F. tularensis in Southern Beaufort Sea polar bears, with less than 5% of bears sampled having antibodies. In our study, prevalence was 8–12 times higher, with more than 60% of polar bears seropositive in 2015–2017. The terrestrial life cycle of F. tularensis involves transmission by biting insects such as ticks, mosquitoes and flies (Feldman, 2003), lagomorph and rodent reservoirs such as muskrats (Ondatra zibethicus; Martin et al., 1982) and/or through water‐borne transmission in wetland areas (Eliasson et al., 2006). All WH polar bears spend at least 3–4 months on land annually, whereas only 20% of Beaufort Sea polar bears come to shore, and stay on average for 2 months (Atwood, Marcot, et al., 2016). The differences in migration ecology between WH and Southern Beaufort Sea polar bears may partly explain the large regional difference in F. tularensis seroprevalence.
During summer, WH polar bears are found along the coast of Hudson Bay (Derocher & Stirling, 1990a), with bears sighted around the town of Churchill on the coastline, and farther inland in Wapusk National Park (Stapleton et al., 2014). Francisella tularensis seroprevalence was lower for WH polar bears that had been previously captured near the town of Churchill. It is possible that polar bears in closer proximity to the coast find refuge from deer flies and horseflies (Tabanidae), which use peatland habitats for key aspects of their life cycle (McElligott & Lewis, 1996). However, although polar bears demographically segregate, with males staying along the coast (Derocher & Stirling, 1990b) and adult females using inland areas (Derocher & Stirling, 1990a), we did not find any significant difference in F. tularensis seroprevalence of males (49%) and females (55%).
Increased time on land as a result of more ice‐free days in Hudson Bay was positively correlated with F. tularensis seroprevalence. In the Hudson Bay lowlands, mosquitoes (Culicidae) emerge in June and peak in mid‐July, whereas horseflies and deer flies begin to emerge in July and peak in early August (Park, 2017; Twinn et al., 1948). Longer time on land may increase seasonal exposure to biting insects, and polar bears forced onto land earlier may overlap with peak abundances of mosquitoes. Additionally, more time on land likely increases use of terrestrial water bodies, which may expose polar bears to water‐borne F. tularensis or facilitate close contact with other reservoir hosts, such as rodents. Francisella tularensis antibodies were also more likely in polar bears in better body condition. As body condition and immune function are positively correlated in polar bears (Whiteman et al., 2019), we may be detecting increased antibodies to F. tularensis in bears in better condition. Alternatively, these bears could have been more likely to survive exposure without developing clinical disease. As longer ice‐free periods are expected to reduce polar bear body condition (Castro de la Guardia et al., 2013), future declines in sea ice may hinder antibody response against F. tularensis and/or lead to more clinical tularaemia, which can be fatal.
Bordetella bronchiseptica is a highly infectious respiratory pathogen well documented in laboratory and domestic animals (Goodnow, 1980), but little is known about its presence in wildlife. The high seroprevalence of antibodies to B. bronchiseptica in WH polar bears, including the most frequent seroconversion out of the pathogens surveyed, suggests regular exposure. Bordetella bronchiseptica has been shown to be common in domestic dogs (Canis familiaris) from remote communities in Canada (Bryan et al., 2011), and although dogs are kept in Churchill, we found no correlation with bears previously captured in human settlements. Bordetella bronchiseptica spreads by aerosol transmission or direct contact; thus transmission through close contact among polar bears is a possible route of infection and maintenance in the population (Ellis et al., 2021). While adult males tend to aggregate in the summer, most adult females are solitary except for those accompanied by cubs (Derocher & Stirling, 1990b). However, our results are inconsistent with transmission through social aggregation, as B. bronchiseptica seroprevalence was five times more likely to occur in females. Environmentally transmitted bacteria are suggested to benefit from a warming Arctic (Bradley et al., 2005), and we found support for this hypothesis, as B. bronchiseptica seroprevalence in WH polar bears increased following warmer summers. We recommend that more wildlife studies include serological and direct testing (culture and/or DNA‐based methods) for B. bronchiseptica to confirm species identity and host range, understand how Bordetella spp. are maintained and spread in natural systems, and determine the potential for transmission within and among wildlife, domestic animals and people.
Seroprevalence of the viruses we surveyed (CDV and CPV) did not change over time. CDV has been commonly studied in polar bears, including one previous WH study reporting 31% seroprevalence (Cattet et al., 2004). Our study is the first to document CPV seroprevalence in polar bears, and WH polar bears had a relatively low prevalence (7%). Both CDV and CPV are cold‐tolerant viruses which can remain viable for months in cool, moist environments, and for years if frozen (Appel & Gillespie, 1972; Gordon & Angrick, 1986). The steady CDV and CPV seroprevalence over time may reflect the optimal conditions in Hudson Bay for long‐term virus survival, creating a stable endemic condition, rather than eruptive outbreaks. There is some evidence from other ecosystems of the influence of climate on the prevalence and virulence of CDV and CPV (Kelman et al., 2020; Munson et al., 2008); however, evidence for environmental regulation remains scant, warranting further investigation. The source of CDV and CPV in polar bears remains unclear and both exist as quasispecies, suggesting they could be of ursine origin, or derived from other carnivores, such as canids, wild or domestic.
Between 1987 and 2011, the WH polar bear population declined from 1187 individuals to 806 (Lunn et al., 2016). However, the change was not linear. Rapid decline occurred between the mid‐1980s and mid‐1990s, when several measures of polar bear health including body condition, reproductive output and survival decreased (Derocher & Stirling, 1995; Regehr et al., 2007). These indices subsequently stabilized in the mid‐2000s (Lunn et al., 2016). Additionally, Boonstra et al. (2020) found a shift in the stress axis of polar bears between 1983–1990 and 1991–2015. In our study, we found a similar temporal pattern in the change in the seroprevalence of T. gondii, F. tularensis and B. bronchiseptica. We do not have direct evidence for the virulence of these pathogens in polar bears, and therefore cannot associate pathogen exposure with survival. However, some exposure patterns suggest the potential for negative impacts on the population if exposure leads to disease. For example, co‐occurrence between T. gondii and N. caninum was higher than expected by chance. Both protozoan parasites can cause spontaneous abortions in some species (Dubey, 2010; Dubey et al., 2007), suggesting a potential impact on the reproductive output of polar bears. In addition, these parasites can cause clinical neurological disease and more subtle behavioural changes. We suggest that these protozoans have potential roles in the population health of polar bears, and should be included in conservation management considerations (Atwood, Marcot, et al., 2016).
All of the zoonotic pathogens we surveyed increased in seroprevalence between 1986–1989 and 1995–1998. By 2015–2017, on average, each polar bear in our study had antibodies to three of four zoonotic pathogens. We consider polar bears to be sentinel species for all pathogens, and a potential source of human exposure for F. tularensis, Trichinella spp. and T. gondii, all of which have been recorded in communities in northern Canada (MacLean et al., 1989; McDonald et al., 1990; Messier et al., 2012). In particular, 70% of bears had antibodies to Trichinella spp. in 2015–2017, supporting the risk for human exposure if polar bear meat is consumed raw or undercooked (Dupouy‐Camet et al., 2017). Adult male polar bears that were previously captured in human settlements were four times more likely to be Trichinella spp. seropositive. All samples were collected during a period when male bears were preferentially targeted in subsistence hunts as part of a 2:1, male‐biased harvest management plan (Taylor et al., 2008). Congruently, male polar bears that enter human settlements in the Arctic are at an increased risk of being harvested (Dyck, 2006). We note that serology only indicates exposure rather than active infection; however, larvae of Trichinella can survive for years in muscle tissue, and bears are a known source of human exposure to Trichinella (Rostami et al., 2017). Therefore, we recommend Hudson Bay communities employ precautionary measures to reduce the risk of exposure to zoonotic pathogens when handling and consuming polar bears, including, but not limited to, handling carcasses with gloves and/or washing hands after handling, disinfecting harvesting tools following use, ensuring that meat remains frozen to –20°C or colder for at least 3 days prior to consumption (to inactive cysts of T. gondii) and thoroughly cooking the meat before eating (the only reliable means to inactivate freeze‐tolerant Trichinella spp.).
The potential for increased exposure to zoonotic pathogens for both people and animals highlights the utility of a One Health approach in the Arctic (Ruscio et al., 2015), which considers the interconnectedness between people, wildlife and ecosystem change. Globally, the majority of emerging infectious diseases affecting people are zoonotic and are increasing with time (Jones et al., 2008), mainly as a result of agriculture, forestry, urbanization and other land‐use changes (Gibb et al., 2020; Keesing et al., 2010). In the Arctic, direct anthropogenic land‐use change is minimal, while the effects of climate change are accelerated. The increases in seroprevalence of zoonotic pathogens in polar bears were all associated with environmental conditions undergoing climate change in both the terrestrial and marine ecosystems. This suggests that climate change can alter zoonotic pathogen prevalence in the absence of land‐use change, especially in depauperate systems. As the pathogens and ecosystem pathways we monitored are common to many species globally, polar bears may once again be a harbinger of the coming impacts of climate change to wildlife health.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
Financial support for the serological analyses was provided by the McBeth Foundation. Financial and logistic support for the fieldwork was provided by the Canadian Association of Zoos and Aquariums, the Churchill Northern Studies Centre, Canadian Wildlife Federation, Care for the Wild International, Earth Rangers Foundation, Hauser Bears, the Isdell Family Foundation, Manitoba Sustainable Development, Natural Sciences and Engineering Research Council of Canada, Parks Canada Agency, Polar Bears International, Quark Expeditions, Schad Foundation, Wildlife Media Inc. and World Wildlife Fund (Canada). We thank Dr Stephen Peterson and the Assiniboine Park Zoo and the Toronto Zoo (Kerri Chase, Dr Chris Dutton, Malcolm Glennie and Dawn Mihailovic) for providing serum samples. We acknowledge the logistical support of Dr Megan Jones, and the fieldwork support of staff and graduate students, especially Ian Stirling, Andrew Derocher and Dennis Andriashek, who collected the samples over many years as part of Environment and Climate Change Canada’s long‐term research, without which this study would not have been possible.
[Correction added on 13 August 2021, after first online publication: Affiliation details for Nicholas W. Pilfold, Megan A. Owen and Bruce Rideout have been updated.]
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Afonso, E., Thulliez, P., & Gilot‐Fromont, E. (2010). Local meteorological conditions, dynamics of seroconversion to Toxoplasma gondii in cats (Felis catus) and oocyst burden in a rural environment. Epidemiology and Infection, 138(8), 1105–1113. 10.1017/S0950268809991270 [DOI] [PubMed] [Google Scholar]
- Aguirre, A. A., Ostfeld, R. S., Tabor, G. M., House, C., & Pearl, M. C. (2002). Conservation medicine: Ecological health in practice. Oxford University Press. [Google Scholar]
- Alexander, K. A., & Appel, M. J. G. (1994). African wild dogs (Lycaon pictus) endangered by a canine distemper epizootic among domestic dogs near the Masai Mara National Reserve, Kenya. Journal of Wildlife Diseases, 30(4), 481–485. 10.7589/0090-3558-30.4.481 [DOI] [PubMed] [Google Scholar]
- Almeria, S., Calvete, C., Pages, A., Gauss, C., & Dubey, J. P. (2004). Factors affecting the seroprevalence of Toxoplasma gondii infection in wild rabbits (Oryctolagus cuniculus) from Spain. Veterinary Parasitology, 123(3–4), 265–270. 10.1016/j.vetpar.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Altizer, S., Ostfeld, R. S., Johnson, P. T. J., Kutz, S., & Harvell, C. D. (2013). Climate change and infectious diseases: From evidence to a predictive framework. Science, 341(6145), 514–519. 10.1126/science.1239401 [DOI] [PubMed] [Google Scholar]
- Appel, M. J., & Gillespie, J. H. (1972). Canine distemper virus. In Kingsbury D. W. & Zur Hausen H. (Eds.), Canine Distemper Virus. Virology Monographs / Die Virusforschung in Einzeldarstellungen (Handbook of Virus Research / Handbuch der Virusforschung) (Vol. 11, pp. 1–96). Springer. [Google Scholar]
- Åsbakk, K., Aars, J., Derocher, A. E., Wiig, Ø., Oksanen, A., Born, E. W., Dietz, R., Sonne, C., Godfroid, J., & Kapel, C. M. O. (2010). Serosurvey for Trichinella in polar bears (Ursus maritimus) from Svalbard and the Barents Sea. Veterinary Parasitology, 172(3–4), 256–263. 10.1016/j.vetpar.2010.05.018 [DOI] [PubMed] [Google Scholar]
- Atwood, T. C., Duncan, C., Patyk, K. A., Nol, P., Rhyan, J., McCollum, M., McKinney, M. A., Ramey, A. M., Cerqueira‐Cézar, C. K., Kwok, O. C. H., Dubey, J. P., & Hennager, S. (2017). Environmental and behavioral changes may influence the exposure of an Arctic apex predator to pathogens and contaminants. Scientific Reports, 7(1), 13193. 10.1038/s41598-017-13496-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood, T. C., Marcot, B. G., Douglas, D. C., Amstrup, S. C., Rode, K. D., Durner, G. M., & Bromaghin, J. F. (2016). Forecasting the relative influence of environmental and anthropogenic stressors on polar bears. Ecosphere, 7(6), e01370. 10.1002/ecs2.1370 [DOI] [Google Scholar]
- Atwood, T. C., Peacock, E., McKinney, M. A., Lillie, K., Wilson, R., Douglas, D. C., Miller, S., & Terletzky, P. (2016). Rapid environmental change drives increased land use by an Arctic marine predator. PLoS One, 11(6), e0155932. 10.1371/journal.pone.0155932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachand, N., Ravel, A., Leighton, P., Stephen, C., Ndao, M., Avard, E., & Jenkins, E. (2019). Serological and molecular detection of Toxoplasma gondii in terrestrial and marine wildlife harvested for food in Nunavik, Canada. Parasites & Vectors, 12(1), 155. 10.1186/s13071-019-3408-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker‐Austin, C., Trinanes, J. A., Taylor, N. G. H., Hartnell, R., Siitonen, A., & Martinez‐Urtaza, J. (2013). Emerging Vibrio risk at high latitudes in response to ocean warming. Nature Climate Change, 3(1), 73–77. 10.1038/nclimate1628 [DOI] [Google Scholar]
- Baszler, T. V., Knowles, D. P., Dubey, J. P., Gay, J. M., Mathison, B. A., & McElwain, T. F. (1996). Serological diagnosis of bovine neosporosis by Neospora caninum monoclonal antibody‐based competitive inhibition enzyme‐linked immunosorbent assay. Journal of Clinical Microbiology, 34(6), 1423–1428. 10.1128/JCM.34.6.1423-1428.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra, R., Bodner, K., Bosson, C., Delehanty, B., Richardson, E. S., Lunn, N. J., Derocher, A. E., & Molnár, P. K. (2020). The stress of Arctic warming on polar bears. Global Change Biology, 26(8), 4197–4214. 10.1111/gcb.15142 [DOI] [PubMed] [Google Scholar]
- Bradley, M. J., Kutz, S. J., Jenkins, E., & O’Hara, T. M. (2005). The potential impact of climate change on infectious diseases of Arctic fauna. International Journal of Circumpolar Health, 64(5), 468–477. 10.3402/ijch.v64i5.18028 [DOI] [PubMed] [Google Scholar]
- Bryan, H. M., Darimont, C. T., Paquet, P. C., Ellis, J. A., Goji, N., Gouix, M., & Smits, J. E. (2011). Exposure to infectious agents in dogs in remote coastal British Columbia: Possible sentinels of diseases in wildlife and humans. Canadian Journal of Veterinary Research, 75(1), 11–17. [PMC free article] [PubMed] [Google Scholar]
- Burek, K. A., Gulland, F. M. D., & O'Hara, T. M. (2008). Effects of climate change on marine mammal health. Ecological Applications, 18(sp2), S126–S134. 10.1890/06-0553.1 [DOI] [PubMed] [Google Scholar]
- Burnham, K. P., & Anderson, D. R. (2002). Model selection and multimodel inference: A practical information‐theoretic approach (2nd ed.). Springer. [Google Scholar]
- Calvert, W., & Ramsay, M. A. (1988). Evaluation of age determination of polar bears by counts of cementum growth layer groups. Ursus, 10, 449–453. [Google Scholar]
- Carlsson, A. M., Curry, P., Elkin, B., Russell, D., Veitch, A., Branigan, M., Campbell, M., Croft, B., Cuyler, C., Côté, S. D., Leclerc, L.‐M., Tryland, M., Nymo, I. H., & Kutz, S. J. (2019). Multi‐pathogen serological survey of migratory caribou herds: A snapshot in time. PLoS One, 14(7), e0219838. 10.1371/journal.pone.0219838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro de la Guardia, L., Derocher, A. E., Myers, P. G., Terwisscha van Scheltinga, A. D., & Lunn, N. J. (2013). Future sea ice conditions in Western Hudson Bay and consequences for polar bears in the 21st century. Global Change Biology, 19(9), 2675–2687. 10.1111/gcb.12272 [DOI] [PubMed] [Google Scholar]
- Castro de la Guardia, L., Myers, P. G., Derocher, A. E., Lunn, N. J., & Terwisscha van Scheltinga, A. D. (2017). Sea ice cycle in western Hudson Bay, Canada, from a polar bear perspective. Marine Ecology Progress Series, 564, 225–233. 10.3354/meps11964 [DOI] [Google Scholar]
- Cattet, M. R. L., Duignan, P. J., House, C. A., & St. Aubin, D. J. (2004). Antibodies to Canine distemper and Phocine distemper viruses in polar bears from the Canadian Arctic. Journal of Wildlife Diseases, 40(2), 338–342. 10.7589/0090-3558-40.2.338 [DOI] [PubMed] [Google Scholar]
- Cavalieri, D., Parkinson, C., Gloersen, P., & Zwally, H. (1996). Sea ice concentrations from Nimbus‐7 SMMR and DMSP SSM/I‐SSMIS passive microwave data. NASA DAAC at the National Snow and Ice Data Center. 10.5067/8GQ8LZQVL0VL [DOI] [Google Scholar]
- Cecchini, G., Bekele, T., & Kasali, O. B. (1992). The effect of repeated freezing and thawing of serum on the activity of antibodies. Veterinary Research Communications, 16(6), 425–428. 10.1007/BF01839019 [DOI] [PubMed] [Google Scholar]
- Cherry, S. G., Derocher, A. E., Thiemann, G. W., & Lunn, N. J. (2013). Migration phenology and seasonal fidelity of an Arctic marine predator in relation to sea ice dynamics. Journal of Animal Ecology, 82(4), 912–921. 10.1111/1365-2656.12050 [DOI] [PubMed] [Google Scholar]
- Clark, D. A., Brook, R., Oliphant‐Reskanski, C., Laforge, M. P., Olson, K., & Rivet, D. (2019). Novel range overlap of three ursids in the Canadian subarctic. Arctic Science, 5(1), 62–70. 10.1139/as-2018-0013 [DOI] [Google Scholar]
- Clausen, B., & Hjort, P. (1986). Survey for antibodies against various infectious disease agents in muskoxen (Ovibos moschatus) from Jamesonland, Northeast Greenland. Journal of Wildlife Diseases, 22(2), 264–266. [DOI] [PubMed] [Google Scholar]
- Cohen, J., Screen, J. A., Furtado, J. C., Barlow, M., Whittleston, D., Coumou, D., Francis, J., Dethloff, K., Entekhabi, D., Overland, J., & Jones, J. (2014). Recent Arctic amplification and extreme mid‐latitude weather. Nature Geoscience, 7(9), 627–637. 10.1038/ngeo2234 [DOI] [Google Scholar]
- Connell, F. H. (1949). Trichinosis in the Arctic: A review. Arctic, 2(2), 98–107. [Google Scholar]
- Cox, F. E. G. (2001). Concomitant infections, parasites and immune responses. Parasitology, 122(S1), S23–S38. 10.1017/S003118200001698X [DOI] [PubMed] [Google Scholar]
- Culler, L. E., Ayres, M. P., & Virginia, R. A. (2015). In a warmer Arctic, mosquitoes avoid increased mortality from predators by growing faster. Proceedings of the Royal Society B, 282(1815), 10.1098/rspb.2015.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dard, C., Bailly, S., Drouet, T., Fricker‐Hidalgo, H., Brenier‐Pinchart, M. P., & Pelloux, H. (2017). Long‐term sera storage does not significantly modify the interpretation of toxoplasmosis serologies. Journal of Microbiological Methods, 134, 38–45. 10.1016/j.mimet.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Derocher, A. E., Andriashek, D., & Stirling, I. (1993). Terrestrial foraging by polar bears during the ice‐free period in western Hudson Bay. Arctic, 46(3), 251–254. [Google Scholar]
- Derocher, A. E., & Stirling, I. (1990a). Distribution of polar bears (Ursus maritimus) during the ice‐free period in western Hudson Bay. Canadian Journal of Zoology, 68(7), 1395–1403. 10.1139/z90-208 [DOI] [Google Scholar]
- Derocher, A. E., & Stirling, I. (1990b). Observations of aggregating behavior in adult male polar bears (Ursus maritimus). Canadian Journal of Zoology, 68(7), 1390–1394. 10.1139/z90-207 [DOI] [Google Scholar]
- Derocher, A. E., & Stirling, I. (1995). Temporal variation in reproduction and body mass of polar bears in western Hudson Bay. Canadian Journal of Zoology, 73(9), 1657–1665. 10.1139/z95-197 [DOI] [Google Scholar]
- Descamps, S. (2013). Winter temperature affects the prevalence of ticks in an Arctic seabird. PLoS One, 8(6), e65374. 10.1371/journal.pone.0065374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick, T. A., & Belosevic, M. (1978). Observations on a Trichinella spiralis isolate from a polar bear. The Journal of Parasitology, 64(6), 1143–1145. 10.2307/3279756 [DOI] [PubMed] [Google Scholar]
- Dubey, J. P. (2010). Toxoplasmosis of animals and humans (2nd ed.). CRC Press. [Google Scholar]
- Dubey, J. P., Jenkins, M. C., Rajendran, C., Miska, K., Ferreira, L. R., Martins, J., Kwok, O., & Choudhary, S. (2011). Gray wolf (Canis lupus) is a natural definitive host for Neospora caninum . Veterinary Parasitology, 181(2), 382–387. 10.1016/j.vetpar.2011.05.018 [DOI] [PubMed] [Google Scholar]
- Dubey, J. P., & Schares, G. (2011). Neosporosis in animals – The last five years. Veterinary Parasitology, 180(1), 90–108. 10.1016/j.vetpar.2011.05.031 [DOI] [PubMed] [Google Scholar]
- Dubey, J. P., Schares, G., & Ortega‐Mora, L. M. (2007). Epidemiology and control of neosporosis and Neospora caninum . Clinical Microbiology Reviews, 20(2), 323–367. 10.1128/CMR.00031-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, J., Hoberg, E., Jenkins, E., & Parkinson, A. (2015). Climate change in the North American arctic: A one health perspective. EcoHealth, 12(4), 713–725. 10.1007/s10393-015-1036-1 [DOI] [PubMed] [Google Scholar]
- Dupouy‐Camet, J., Bourée, P., & Yera, H. (2017). Trichinella and polar bears: A limited risk for humans. Journal of Helminthology, 91(4), 440–446. 10.1017/S0022149X17000219 [DOI] [PubMed] [Google Scholar]
- Dyck, M. G. (2006). Characteristics of polar bears killed in defense of life and property in Nunavut, Canada, 1970–2000. Ursus, 17(1), 52–62. [Google Scholar]
- Eliasson, H., Broman, T., Forsman, M., & Back, E. (2006). Tularemia: current epidemiology and disease management. Infectious Disease Clinics of North America, 20(2), 289–311. 10.1016/j.idc.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Ellis, J., Anseeuw, E., Gow, S., Bryan, H., Salb, A., Goji, N., Rhodes, C., La Coste, S., & Smits, J. Kutz, S. (2011). Seroepidemiology of respiratory (group 2) canine coronavirus, canine parainfluenza virus, and Bordetella bronchiseptica infections in urban dogs in a humane shelter and in rural dogs in small communities. The Canadian Veterinary Journal, 52(8), 861–868. [PMC free article] [PubMed] [Google Scholar]
- Ellis, J., Gow, S., Pilfold, N. W., Lacoste, S., Lunn, N. J., Richardson, E., McGeachy, D., Owen, M. A., & Rideout, B. (2021). Bordetella bronchiseptica‐reactive antibodies in Canadian polar bears. The Canadian Veterinary Journal, in press. [PMC free article] [PubMed] [Google Scholar]
- Elmore, S. A., Samelius, G., Al‐Adhami, B., Huyvaert, K. P., Bailey, L. L., Alisauskas, R. T., & Jenkins, E. J. (2016). Estimating Toxoplasma gondii exposure in Arctic foxes (Vulpes lagopus) while navigating the imperfect world of wildlife serology. Journal of Wildlife Diseases, 52(1), 47–56. 10.7589/2015-03-075 [DOI] [PubMed] [Google Scholar]
- Elmore, S. A., Samelius, G., Fernando, C., Alisauskas, R. T., & Jenkins, E. J. (2015). Evidence for Toxoplasma gondii in migratory vs. nonmigratory herbivores in a terrestrial arctic ecosystem. Canadian Journal of Zoology, 93(8), 671–675. 10.1139/cjz-2015-0078 [DOI] [Google Scholar]
- Elton, C. (1931). Epidemics among sledge dogs in the Canadian Arctic and their relation to disease in the Arctic fox. Canadian Journal of Research, 5(6), 673–692. 10.1139/cjr31-106 [DOI] [Google Scholar]
- Environment Canada . (2020). National climate data and information archive. https://climate.weather.gc.ca/index_e.html [Google Scholar]
- Feldman, K. A. (2003). Tularemia. Journal of the American Veterinary Medical Association, 222(6), 725–730. 10.2460/javma.2003.222.725 [DOI] [PubMed] [Google Scholar]
- Fereidouni, S., Freimanis, G. L., Orynbayev, M., Ribeca, P., Flannery, J., King, D. P., Zuther, S., Beer, M., Höper, D., Kydyrmanov, A., Karamendin, K., & Kock, R. (2019). Mass die‐off of saiga antelopes, Kazakhstan, 2015. Emerging Infectious Diseases, 25(6), 1169–1176. 10.3201/eid2506.180990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes, L. B. (2000). The occurrence and ecology of Trichinella in marine mammals. Veterinary Parasitology, 93(3), 321–334. 10.1016/S0304-4017(00)00349-6 [DOI] [PubMed] [Google Scholar]
- Gajadhar, A. A., & Forbes, L. B. (2010). A 10‐year wildlife survey of 15 species of Canadian carnivores identifies new hosts or geographic locations for Trichinella genotypes T2, T4, T5, and T6. Veterinary Parasitology, 168(1), 78–83. 10.1016/j.vetpar.2009.10.012 [DOI] [PubMed] [Google Scholar]
- Gamarra, J. A., Cabezón, O., Pabón, M., Arnal, M. C., Luco, D. F., Dubey, J. P., Gortázar, C., & Almeria, S. (2008). Prevalence of antibodies against Toxoplasma gondii in roe deer from Spain. Veterinary Parasitology, 153(1–2), 152–156. 10.1016/j.vetpar.2008.01.028 [DOI] [PubMed] [Google Scholar]
- Gibb, R., Redding, D. W., Chin, K. Q., Donnelly, C. A., Blackburn, T. M., Newbold, T., & Jones, K. E. (2020). Zoonotic host diversity increases in human‐dominated ecosystems. Nature, 584(7821), 398–402. 10.1038/s41586-020-2562-8 [DOI] [PubMed] [Google Scholar]
- Gleason, J. S., & Rode, K. D. (2009). Polar bear distribution and habitat association reflect long‐term changes in fall sea ice conditions in the Alaskan Beaufort Sea. Arctic, 62(4), 405–417. [Google Scholar]
- Goodnow, R. A. (1980). Biology of Bordetella bronchiseptica . Microbiological Reviews, 44(4), 722–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, J. C., & Angrick, E. J. (1986). Canine parvovirus: Environmental effects on infectivity. American Journal of Veterinary Research, 47(7), 1464–1467. [PubMed] [Google Scholar]
- Gormezano, L. J., & Rockwell, R. F. (2013). What to eat now? Shifts in polar bear diet during the ice‐free season in western Hudson Bay. Ecology and Evolution, 3(10), 3509–3523. 10.1002/ece3.740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvell, D., Altizer, S., Cattadori, I. M., Harrington, L., & Weil, E. (2009). Climate change and wildlife diseases: When does the host matter the most? Ecology, 90(4), 912–920. 10.1890/08-0616.1 [DOI] [PubMed] [Google Scholar]
- Hoar, B. M., Ruckstuhl, K., & Kutz, S. (2012). Development and availability of the free‐living stages of Ostertagia gruehneri, an abomasal parasite of barrenground caribou (Rangifer tarandus groenlandicus), on the Canadian tundra. Parasitology, 139(8), 1093–1100. 10.1017/S003118201200042X [DOI] [PubMed] [Google Scholar]
- Hockings, M., Dudley, N., Ellio, W., Ferreira, M. N., MacKinnon, K., Pasha, M., Phillips, A., Stolton, S., Woodley, S., Appleton, M., Chassot, O., Fitzsimons, J., Galliers, C., Kroner, R. G., Goodrich, J., Hopkins, J. O., Jackson, W., Jonas, H., Long, B., … Yang, A. (2020). Editorial essay: COVID‐19 and protected and conserved areas. Parks, 26(1), 7–24. 10.2305/IUCN.CH.2020.PARKS-26-1MH.en [DOI] [Google Scholar]
- ID Vet . (2020). ID screen® toxoplasmosis indirect multi‐species. https://www.id‐vet.com/produit/id‐screen‐toxoplasmosis‐indirect‐multi‐species/
- Jenkins, E. J., Castrodale, L. J., de Rosemond, S. J., Dixon, B. R., Elmore, S. A., Gesy, K. M.,Hoberg, E. P., Polley, L., Schurer, J. M., Simard, M., & Thompson, R. C. (2013). Tradition and transition: Parasitic zoonoses of people and animals in Alaska, northern Canada, and Greenland. Advances in Parasitology, 82, 33–204. 10.1016/B978-0-12-407706-5.00002-2 [DOI] [PubMed] [Google Scholar]
- Jensen, S. K., Aars, J., Lydersen, C., Kovacs, K. M., & Åsbakk, K. (2010). The prevalence of Toxoplasma gondii in polar bears and their marine mammal prey: Evidence for a marine transmission pathway? Polar Biology, 33(5), 599–606. 10.1007/s00300-009-0735-x [DOI] [Google Scholar]
- Jones, E. P., & Anderson, L. G. (1994). Northern Hudson Bay and Foxe Basin: Water masses, circulation and productivity. Atmosphere‐Ocean, 32(2), 361–374. 10.1080/07055900.1994.9649502 [DOI] [Google Scholar]
- Jones, K. E., Patel, N. G., Levy, M. A., Storeygard, A., Balk, D., Gittleman, J. L., & Daszak, P. (2008). Global trends in emerging infectious diseases. Nature, 451(7181), 990–993. 10.1038/nature06536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvonen, A., Rintamaki, P., Jokela, J., & Valtonen, E. T. (2010). Increasing water temperature and disease risks in aquatic systems: Climate change increases the risk of some, but not all, diseases. International Journal of Parasitology, 40(13), 1483–1488. 10.1016/j.ijpara.2010.04.015 [DOI] [PubMed] [Google Scholar]
- Kearney, S. (1989). The polar bear alert program at Churchill, Manitoba. Paper presented at the Bear‐People Conflict: Proceedings of a Symposium on Management Strategies, Yellowknife, Northwest Territories Department of Renewable Resources. [Google Scholar]
- Keesing, F., Belden, L. K., Daszak, P., Dobson, A., Harvell, C. D., Holt, R. D., Hudson, P., Jolles, A., Jones, K. E., Mitchell, C. E., Myers, S. S., Bogich, T., & Ostfeld, R. S. (2010). Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature, 468(7324), 647–652. 10.1038/nature09575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelman, M., Barrs, V. R., Norris, J. M., & Ward, M. P. (2020). Socioeconomic, geographic and climatic risk factors for canine parvovirus infection and euthanasia in Australia. Preventive Veterinary Medicine, 174, 104816. 10.1016/j.prevetmed.2019.104816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk, C. M., Amstrup, S., Swor, R., Holcomb, D., & O'Hara, T. M. (2010). Morbillivirus and Toxoplasma exposure and association with hematological parameters for southern Beaufort Sea polar bears: Potential response to infectious agents in a sentinel species. EcoHealth, 7(3), 321–331. 10.1007/s10393-010-0323-0 [DOI] [PubMed] [Google Scholar]
- Kutz, S. J., Hoberg, E. P., Polley, L., & Jenkins, E. J. (2005). Global warming is changing the dynamics of Arctic host‐parasite systems. Proceedings of the Royal Society B, 272(1581), 2571–2576. 10.1098/rspb.2005.3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle, P., Dubey, J. P., Mikaelian, I., Blanchette, N., Lafond, R., St‐Onge, S., & Martineau, D. (2001). Seroprevalence of antibodies to Toxoplasma gondii in lynx (Lynx canadensis) and bobcats (Lynx rufus) from Québec, Canada. The Journal of Parasitology, 87(5), 1194–1196. 10.2307/3285264 [DOI] [PubMed] [Google Scholar]
- Lafferty, K. D. (2009). The ecology of climate change and infectious diseases. Ecology, 90(4), 888–900. 10.1890/08-0079.1 [DOI] [PubMed] [Google Scholar]
- Larsen, T., & Kjos‐Hanssen, B. (1983). Trichinella sp. in polar bears from Svalbard, in relation to hide length and age. Polar Research, 1(1), 89–96. 10.3402/polar.v1i1.6973 [DOI] [Google Scholar]
- Larsson, C., Comstedt, P., Olsen, B., & Bergström, S. (2007). First record of lyme disease Borrelia in the Arctic. Vector‐Borne and Zoonotic Diseases, 7(3), 453–456. 10.1089/vbz.2006.0644 [DOI] [PubMed] [Google Scholar]
- Lecomte, N., Gauthier, G., & Giroux, J.‐F. (2009). A link between water availability and nesting success mediated by predator–prey interactions in the Arctic. Ecology, 90(2), 465–475. 10.1890/08-0215.1 [DOI] [PubMed] [Google Scholar]
- Lindsey, P., Allan, J., Brehony, P., Dickman, A., Robson, A., Begg, C., Bhammar, H., Blanken, L., Breuer, T., Fitzgerald, K., Flyman, M., Gandiwa, P., Giva, N., Kaelo, D., Nampindo, S., Nyambe, N., Steiner, K., Parker, A., Roe, D., … Tyrrell, P. (2020). Conserving Africa’s wildlife and wildlands through the COVID‐19 crisis and beyond. Nature Ecology & Evolution, 4(10), 1300–1310. 10.1038/s41559-020-1275-6 [DOI] [PubMed] [Google Scholar]
- Lunn, N. J., Servanty, S., Regehr, E. V., Converse, S. J., Richardson, E., & Stirling, I. (2016). Demography of an apex predator at the edge of its range: Impacts of changing sea ice on polar bears in Hudson Bay. Ecological Applications, 26(5), 1302–1320. 10.1890/15-1256 [DOI] [PubMed] [Google Scholar]
- MacLean, J. D., Viallet, J., Law, C., & Staudt, M. (1989). Trichinosis in the Canadian Arctic: Report of five outbreaks and a new clinical syndrome. The Journal of Infectious Diseases, 160(3), 513–520. 10.1093/infdis/160.3.513 [DOI] [PubMed] [Google Scholar]
- Macrae, M. L., Brown, L. C., Duguay, C. R., Parrott, J. A., & Petrone, R. M. (2014). Observed and projected climate change in the Churchill region of the Hudson Bay lowlands and implications for pond sustainability. Arctic, Antarctic, and Alpine Research, 46(1), 272–285. 10.1657/1938-4246-46.1.272 [DOI] [Google Scholar]
- Martin, T., Holmes, I. H., Wobeser, G. A., Anthony, R. F., & Greefkes, I. (1982). Tularemia in Canada with a focus on Saskatchewan. Canadian Medical Association Journal, 127(4), 279–282. [PMC free article] [PubMed] [Google Scholar]
- Massie, G. N., Ware, M. W., Villegas, E. N., & Black, M. W. (2010). Uptake and transmission of Toxoplasma gondii oocysts by migratory, filter‐feeding fish. Veterinary Parasitology, 169(3–4), 296–303. 10.1016/j.vetpar.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Masuoka, P. M., Burke, R., Colaccico, M., Razuri, H., Hill, D., & Murrell, K. D. (2009). Predicted geographic ranges for North American sylvatic Trichinella species. Journal of Parasitology, 95(4), 829–837. 10.1645/GE-1952.1 [DOI] [PubMed] [Google Scholar]
- McCall, A. G., Derocher, A. E., & Lunn, N. J. (2014). Home range distribution of polar bears in western Hudson Bay. Polar Biology, 38(3), 343–355. 10.1007/s00300-014-1590-y [DOI] [Google Scholar]
- McDonald, J. L., Bailey, T., Delahay, R. J., McDonald, R. A., Smith, G. C., & Hodgson, D. J. (2016). Demographic buffering and compensatory recruitment promotes the persistence of disease in a wildlife population. Ecology Letters, 19(4), 443–449. 10.1111/ele.12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, J. C., Gyorkos, T., Alberton, B., MacLean, J. D., Richer, G., & Juranek, D. (1990). An outbreak of toxoplasmosis in pregnant women in northern Québec. The Journal of Infectious Diseases, 161(4), 769–774. 10.1093/infdis/161.4.769 [DOI] [PubMed] [Google Scholar]
- McElligott, P. E. K., & Lewis, D. J. (1996). Distribution and abundance of immature Tabanidae (Diptera) in a subarctic Labrador peatland. Canadian Journal of Zoology, 74(7), 1364–1369. 10.1139/z96-149 [DOI] [Google Scholar]
- Meerburg, B. G., & Kijlstra, A. (2009). Changing climate‐changing pathogens: Toxoplasma gondii in North‐Western Europe. Parasitology Research, 105(1), 17–24. 10.1007/s00436-009-1447-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge, B. A., Cerny‐Chipman, E. B., Johnson, A., Sullivan, J., Gravem, S., & Chan, F. (2016). Sea star wasting disease in the keystone predator Pisaster ochraceus in Oregon: Insights into differential population impacts, recovery, predation rate, and temperature effects from long‐term research. PLoS One, 11(5), e0153994. 10.1371/journal.pone.0153994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier, V., Lévesque, B., Proulx, J.‐F., Rochette, L., Serhir, B., Couillard, M., Ward, B. J., Libman, M. D., Dewailly, É., & Déry, S. (2012). Seroprevalence of seven zoonotic infections in Nunavik, Quebec (Canada). Zoonoses & Public Health, 59(2), 107–117. 10.1111/j.1863-2378.2011.01424.x [DOI] [PubMed] [Google Scholar]
- Møller, L. N., Koch, A., Petersen, E., Hjuler, T., Kapel, C. M., Andersen, A., & Melbye, M. (2010). Trichinella infection in a hunting community in East Greenland. Epidemiology and Infection, 138(9), 1252–1256. 10.1017/S0950268810000282 [DOI] [PubMed] [Google Scholar]
- Morley, N. J., & Lewis, J. W. (2014). Temperature stress and parasitism of endothermic hosts under climate change. Trends in Parasitology, 30(5), 221–227. 10.1016/j.pt.2014.01.007 [DOI] [PubMed] [Google Scholar]
- Munson, L., Terio, K. A., Kock, R., Mlengeya, T., Roelke, M. E., Dubovi, E., Summers, B., Sinclair, A. R. E., & Packer, C. (2008). Climate extremes promote fatal co‐infections during Canine distemper epidemics in African lions. PLoS One, 3(6), e2545. 10.1371/journal.pone.0002545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidenko, S. V., Ivanov, E. A., Mordvintsev, I. N., Platonov, N. G., Ershov, R. V., & Rozhnov, V. V. (2013). Seropositivity for different pathogens in polar bears (Ursus maritimus) from Barents Sea Islands. Biology Bulletin, 40(9), 779–782. 10.1134/s1062359013090082 [DOI] [Google Scholar]
- Nakagawa, S., & Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed‐effects models. Methods in Ecology and Evolution, 4(2), 133–142. 10.1111/j.2041-210x.2012.00261.x [DOI] [Google Scholar]
- Ogden, N. H., & Lindsay, L. R. (2016). Effects of climate and climate change on vectors and vector‐borne diseases: Ticks are different. Trends in Parasitology, 32(8), 646–656. 10.1016/j.pt.2016.04.015 [DOI] [PubMed] [Google Scholar]
- Oksanen, A., Åsbakk, K., Prestrud, K. W., Aars, J., Derocher, A. E., Tryland, M., Wiig, Ø., Dubey, J. P., Sonne, C., Dietz, R., Andersen, M., & Born, E. W. (2009). Prevalence of antibodies against Toxoplasma gondii in polar bears (Ursus maritimus) from Svalbard and east Greenland. Journal of Parasitology, 95(1), 89–94. 10.1645/GE-1590.1 [DOI] [PubMed] [Google Scholar]
- Øritsland, N. A., Lentfer, J. W., & Ronald, K. (1974). Radiative surface temperatures of the polar bear. Journal of Mammalogy, 55(2), 459–461. 10.2307/1379018 [DOI] [PubMed] [Google Scholar]
- Park, J. S. (2017). A race against time: habitat alteration by snow geese prunes the seasonal sequence of mosquito emergence in a subarctic brackish landscape. Polar Biology, 40(3), 553–561. 10.1007/s00300-016-1978-y [DOI] [Google Scholar]
- Parkinson, C. L., Cavalieri, D. J., Gloersen, P., Zwally, H. J., & Comiso, J. C. (1999). Arctic sea ice extents, areas, and trends, 1978–1996. Journal of Geophysical Research: Oceans, 104(C9), 20837–20856. 10.1029/1999JC900082 [DOI] [Google Scholar]
- PBSG . (2018). 2016 Status report on the world’s polar bear subpopulations. IUCN. [Google Scholar]
- Pedersen, A. B., & Fenton, A. (2007). Emphasizing the ecology in parasite community ecology. Trends in Ecology & Evolution, 22(3), 133–139. 10.1016/j.tree.2006.11.005 [DOI] [PubMed] [Google Scholar]
- Pilfold, N. W., Hedman, D., Stirling, I., Derocher, A. E., Lunn, N. J., & Richardson, E. (2016). Mass loss rates of fasting polar bears. Physiological and Biochemical Zoology, 89(5), 377–388. 10.1086/687988 [DOI] [PubMed] [Google Scholar]
- Post, E., Bhatt, U. S., Bitz, C. M., Brodie, J. F., Fulton, T. L., Hebblewhite, M., Kerby, J., Kutz, S. J., Stirling, I., & Walker, D. A. (2013). Ecological consequences of sea‐ice decline. Science, 341(6145), 519–524. 10.1126/science.1235225 [DOI] [PubMed] [Google Scholar]
- Pozio, E. (2016). Adaptation of Trichinella spp. for survival in cold climates. Food and Waterborne Parasitology, 4, 4–12. 10.1016/j.fawpar.2016.07.001 [DOI] [Google Scholar]
- Prestrud, K. W., Åsbakk, K., Fuglei, E., Mørk, T., Stien, A., Ropstad, E., Tryland, M., Gabrielsen, G. W., Lydersen, C., Kovacs, K. M., Loonen, M. J. J. E., Sagerup, K., & Oksanen, A. (2007). Serosurvey for Toxoplasma gondii in arctic foxes and possible sources of infection in the high Arctic of Svalbard. Veterinary Parasitology, 150(1–2), 6–12. 10.1016/j.vetpar.2007.09.006 [DOI] [PubMed] [Google Scholar]
- R Development Core Team . (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R‐project.org/ [Google Scholar]
- Rah, H., Chomel, B. B., Kasten, R. W., Hew, C. H., Farver, T. B., Follmann, E. H., Garner, G. W., & Amstrup, S. C. (2005). Serosurvey of selected zoonotic agents in polar bears (Ursus maritimus). Veterinary Record, 156(1), 7–13. 10.1136/vr.156.1.7 [DOI] [PubMed] [Google Scholar]
- Regehr, E. V., Lunn, N. J., Amstrup, S. C., & Stirling, I. (2007). Effects of earlier sea ice breakup on survival and population size of polar bears in western Hudson Bay. Journal of Wildlife Management, 71(8), 2673–2683. 10.2193/2006-180 [DOI] [Google Scholar]
- Reiling, S. J., Measures, L., Feng, S., Boone, R., Merks, H., & Dixon, B. R. (2019). Toxoplasma gondii, Sarcocystis sp. and Neospora caninum‐like parasites in seals from northern and eastern Canada: Potential risk to consumers. Food and Waterborne Parasitology, 17, e00067. 10.1016/j.fawpar.2019.e00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, E. S., & Brook, R. K. (2004). Excavation of an Arctic Fox, Alopex lagopus, den by a Polar Bear, Ursus maritimus . The Canadian Field‐Naturalist, 118(4), 602–603. [Google Scholar]
- Rijks, J. M., Van de Bildt, M. W. G., Jensen, T., Philippa, J. D. W., Osterhaus, A. D. M. E., & Kuiken, T. (2005). Phocine distemper outbreak, The Netherlands, 2002. Emerging Infectious Diseases, 11(12), 1945–1948. 10.3201/eid1112.050596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami, A., Gamble, H. R., Dupouy‐Camet, J., Khazan, H., & Bruschi, F. (2017). Meat sources of infection for outbreaks of human trichinellosis. Food Microbiology, 64, 65–71. 10.1016/j.fm.2016.12.012 [DOI] [PubMed] [Google Scholar]
- Rouse, W. R. (1998). A water balance model for a subarctic sedge fen and its application to climatic change. Climatic Change, 38, 207–234. 10.1023/A:1005358017894 [DOI] [Google Scholar]
- Ruscio, B. A., Brubaker, M., Glasser, J., Hueston, W., & Hennessy, T. W. (2015). One Health – A strategy for resilience in a changing arctic. International Journal of Circumpolar Health, 74(1), 27913. 10.3402/ijch.v74.27913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, R. H. (1975). The food habits of polar bears of James Bay and Southwest Hudson Bay in summer and autumn. Arctic, 28, 117–129. [Google Scholar]
- Salant, H., Hamburger, J., King, R., & Baneth, G. (2013). Toxoplasma gondii prevalence in Israeli crows and Griffon vultures. Veterinary Parasitology, 191(1–2), 23–28. 10.1016/j.vetpar.2012.07.029 [DOI] [PubMed] [Google Scholar]
- Sato, T., Fujita, H., Ohara, Y., & Homma, M. (1990). Microagglutination test for early and specific serodiagnosis of tularemia. Journal of Clinical Microbiology, 28(10), 2372–2374. 10.1128/JCM.28.10.2372-2374.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciullo, L., Thiemann, G. W., & Lunn, N. J. (2016). Comparative assessment of metrics for monitoring the body condition of polar bears in western Hudson Bay. Journal of Zoology, 300(1), 45–58. 10.1111/jzo.12354 [DOI] [Google Scholar]
- Sharma, R., Parker, S., Al‐Adhami, B., Bachand, N., & Jenkins, E. (2019). Comparison of tissues (heart vs. brain) and serological tests (MAT, ELISA and IFAT) for detection of Toxoplasma gondii in naturally infected wolverines (Gulo gulo) from the Yukon. Canada. Food and Waterborne Parasitology, 15, e00046. 10.1016/j.fawpar.2019.e00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen, M. A., Khan, S., Kazmi, A., Bashir, N., & Siddique, R. (2020). COVID‐19 infection: Origin, transmission, and characteristics of human coronaviruses. Journal of Advanced Research, 24, 91–98. 10.1016/j.jare.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, A., Bigras Poulin, M., Rousseau, A. N., Dubey, J. P., & Ogden, N. H. (2013). Spatiotemporal dynamics of Toxoplasma gondii infection in Canadian lynx (Lynx canadensis) in western Quebec, Canada. Journal of Wildlife Diseases, 49(1), 39–48. 10.7589/2012-02-048 [DOI] [PubMed] [Google Scholar]
- Simon, A., Chambellant, M., Ward, B. J., Simard, M., Proulx, J. F., Levesque, B., & Ogden, N. H. (2011). Spatio‐temporal variations and age effect on Toxoplasma gondii seroprevalence in seals from the Canadian Arctic. Parasitology, 138(11), 1362–1368. 10.1017/S0031182011001260 [DOI] [PubMed] [Google Scholar]
- Simon, A., Rousseau, A. N., Savary, S., Bigras‐Poulin, M., & Ogden, N. H. (2013). Hydrological modelling of Toxoplasma gondii oocysts transport to investigate contaminated snowmelt runoff as a potential source of infection for marine mammals in the Canadian Arctic. Journal of Environmental Management, 127, 150–161. 10.1016/j.jenvman.2013.04.031 [DOI] [PubMed] [Google Scholar]
- Soma, T., Ishii, H., Hara, M., Yamamoto, S., Yoshida, T., Kinoshita, T., & Nomura, K. (2001). Comparison of immunoperoxidase plaque staining and neutralizing tests for canine distemper virus. Veterinary Research Communications, 25(4), 311–325. 10.1023/A:1010682726245 [DOI] [PubMed] [Google Scholar]
- Stapleton, S., Atkinson, S., Hedman, D., & Garshelis, D. (2014). Revisiting Western Hudson Bay: Using aerial surveys to update polar bear abundance in a sentinel population. Biological Conservation, 170, 38–47. 10.1016/j.biocon.2013.12.040 [DOI] [Google Scholar]
- Stern, H., & Laidre, K. L. (2016). Sea‐ice indicators of polar bear habitat. The Cryosphere, 10, 1–15. 10.5194/tc-10-1-2016 [DOI] [Google Scholar]
- Stirling, I., & Derocher, A. E. (2012). Effects of climate warming on polar bears: A review of the evidence. Global Change Biology, 18(9), 2694–2706. 10.1111/j.1365-2486.2012.02753.x [DOI] [PubMed] [Google Scholar]
- Stirling, I., Lunn, N. J., & Iacozza, J. (1999). Long‐term trends in the population ecology of polar bears in western Hudson Bay in relation to climatic change. Arctic, 52(3), 294–306. [Google Scholar]
- Stirling, I., Lunn, N. J., Iacozza, J., Elliott, C., & Obbard, M. (2004). Polar bear distribution and abundance on the southwestern Hudson Bay Coast during open water season, in relation to population trends and annual ice patterns. Arctic, 57(1), 15–26. [Google Scholar]
- Stirling, I., & Øritsland, N. A. (1995). Relationships between estimates of ringed seal (Phoca hispida) and polar bear (Ursus maritimus) populations in the Canadian Arctic. Canadian Journal of Fisheries and Aquatic Sciences, 52(12), 2594–2612. [Google Scholar]
- Stirling, I., Spencer, C., & Andriashek, D. (1989). Immobilization of polar bears (Ursus maritimus) with Telazol® in the Canadian Arctic. Journal of Wildlife Diseases, 25(2), 159–168. [DOI] [PubMed] [Google Scholar]
- Stirling, I., Thiemann, G. W., & Richardson, E. (2008). Quantitative support for a subjective fatness index for immobilized polar bears. Journal of Wildlife Management, 72(2), 568–574. 10.2193/2007-123 [DOI] [Google Scholar]
- Taylor, M., Larsen, T., & Schweinsburg, R. E. (1985). Observations of intraspecific aggression and cannibalism in polar bears (Ursus maritimus). Arctic, 38(4), 303–309. [Google Scholar]
- Taylor, M. K., McLoughlin, P. D., & Messier, F. (2008). Sex‐selective harvesting of polar bears Ursus maritimus . Wildlife Biology, 14(1), 52–60.10.2981/0909‐6396(2008)14[52:SHOPBU]2.0.CO;2 [Google Scholar]
- Thiemann, G. W., Iverson, S. J., & Stirling, I. (2008). Polar bear diets and arctic marine food webs: Insights from fatty acid analysis. Ecological Monographs, 78(4), 591–614. [Google Scholar]
- Thiemann, G. W., Lunn, N. J., Richardson, E. S., & Andriashek, D. S. (2011). Temporal change in the morphometry–body mass relationship of polar bears. The Journal of Wildlife Management, 75(3), 580–587. 10.1002/jwmg.112 [DOI] [Google Scholar]
- Towns, L., Derocher, A. E., Stirling, I., Lunn, N. J., & Hedman, D. (2009). Spatial and temporal patterns of problem polar bears in Churchill, Manitoba. Polar Biology, 32(10), 1529–1537. 10.1007/s00300-009-0653-y [DOI] [Google Scholar]
- Twinn, C. R., Hocking, B., McDuffie, W. C., & Cross, H. F. (1948). A preliminary account of the biting flies at Churchill, Manitoba. Canadian Journal of Research, 26d(6), 334–357. 10.1139/cjr48d-025 [DOI] [PubMed] [Google Scholar]
- Vashon, J. (2016). Lynx canadensis. The IUCN Red List of Threatened Species 2016. e.T12518A101138963. [Google Scholar]
- Whiteman, J. P., Harlow, H. J., Durner, G. M., Regehr, E. V., Amstrup, S. C., & Ben‐David, M. (2019). Heightened immune system function in polar bears using terrestrial habitats. Physiological and Biochemical Zoology, 92(1), 1–11. 10.1086/698996 [DOI] [PubMed] [Google Scholar]
- Young, B. G., & Ferguson, S. H. (2013). Seasons of the ringed seal: Pelagic open‐water hyperphagy, benthic feeding over winter and spring fasting during molt. Wildlife Research, 40(1), 52–60. 10.1071/wr12168 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


