Abstract
Introduction
Preoperative assessment of deep endometriotic (DE) nodules is necessary to inform patients about the possible treatments and provide informed consent in case of surgery. This study aims to investigate the diagnostic performance of rectal water‐contrast transvaginal ultrasonography (RWC‐TVS) and sonovaginography (SVG) in women with suspicion of posterior DE.
Material and methods
This prospective comparative study (NCT04296760) enrolled women with clinical suspicion of DE at our institution (Piazza della Vittoria 14 SRL, Genoa, Italy). Exclusion criteria were previous diagnosis of DE by imaging techniques or laparoscopy. All patients underwent RWC‐TVS and SVG, independently performed by two gynecological sonologists blinded to the other technique's results. Patients underwent laparoscopic surgery within the following three months; imaging findings were compared with surgical and histological results.
Results
In 208 of 281 (74.0%) patients included, posterior DE was surgically confirmed in rectosigmoid (n = 88), vagina (n = 21), rectovaginal septum (n = 34) and uterosacral ligaments (n = 156). RWC‐TVS and SVG demonstrated similar sensitivity (SE; 93.8% vs 89.4%; p = 0.210) and specificity (SP; 86.3% vs 79.4%; p = 0.481) in diagnosing posterior DE. Specifically, both examinations had similar accuracy in detecting nodules of uterosacral ligaments (p = 0.779), vagina (p = 0.688) and rectovaginal septum (p = 0.824). RWC‐TVS had higher SE (95.2% vs 82.0%; p = 0.003) and similar SP (99.5% vs 98.5%; p = 0.500) in diagnosing rectosigmoid endometriosis and estimated better infiltration of intestinal submucosa (p = 0.039), and distance between these nodules and anal verge (p < 0.001); only RWC‐TVS allowed the estimation of bowel lumen stenosis. A similar proportion of discomfort was experienced during both examinations (p = 0.191), although a statistically higher mean visual analog score was reported during RWC‐TVS (p < 0.001).
Conclusions
Although RWC‐TVS and SVG have similar accuracy in the diagnosis of DE, RWC‐TVS performed better in assessment of the characteristics of rectosigmoid endometriosis.
Keywords: enhanced transvaginal ultrasound, posterior compartment deep endometriosis, rectal water contrast transvaginal ultrasonography, rectosigmoid endometriosis, sonovaginography
Abbreviations
- CI
confidence interval
- CTC
computed tomography‐based virtual colonoscopy
- DE
deep endometriosis
- MRI
magnetic resonance imaging
- NPV
negative predictive value
- PPV
positive predictive value
- RWC‐TVS
rectal water‐contrast transvaginal ultrasonography
- SE
sensitivity
- SP
specificity
- SVG
sonovaginography
- TVS
transvaginal ultrasonography
- VAS
visual analog scale
- VOCAL
virtual organ computer‐aided analysis
Key message.
Until now, no study has compared enhanced techniques in diagnosing posterior compartment deep endometriosis (DE). Rectal water‐contrast transvaginal ultrasonography (RWC‐TVS) and sonovaginography have similar accuracy in diagnosing posterior DE; nevertheless, RWC‐TVS performed better in detection of rectosigmoid endometriosis.
1. INTRODUCTION
Deep infiltrating endometriosis (DE), characterized by foci of endometrial‐like and fibrotic tissues deeper than 5 mm below the peritoneum, is considered the most severe form of endometriosis.1 It occurs in 15%–30% of patients with endometriosis and, when located in the posterior pelvic compartment, may involve uterosacral ligaments, rectosigmoid colon, the rectovaginal space and vagina.2
Since the diagnosis of DE cannot be reliably performed based on symptoms and clinical examination,3 imaging techniques have a relevant role in the diagnostic workup of patients with suspicion of endometriosis. Preoperative assessment of the presence, location and characteristics of vaginal or rectal wall infiltration is crucial for planning surgical treatment for posterior DE. Infiltration of the rectosigmoid colon is essential for providing the women with informed consent on benefits and risks of medical and surgical treatment and scheduling the presence of an adequate multidisciplinary team, including the colorectal surgeon.4, 5
Nowadays, transvaginal ultrasonography (TVS) is considered the first‐line investigation in patients with suspicion of DE.6 Compared with other imaging techniques, TVS has the advantage of being relatively inexpensive and being directly performed by gynecological sonologists, who usually manage patients with endometriosis. TVS allows a dynamic evaluation of pelvic structures, it enables the characterization of endometriotic cysts and DE implants, and it is well tolerated by the patients.7 However, the performance of TVS in diagnosing DE is highly dependent on the examiner's experience.8
Enhanced ultrasonographic techniques have been proposed to improve the diagnosis of DE.9 These techniques include sonovaginography (SVG), based on the distention of the vagina, and rectal water contrast‐transvaginal ultrasonography (RWC‐TVS), based on the distention of the rectosigmoid. These distention media, usually consisting of saline solution or ultrasonographic gel, aim to create artificial acoustic windows, facilitating the detection of DE nodules by delineating the margins of pelvic spaces and organs. In particular, RWC‐TVS and SVG have been primarily employed to describe the presence and characteristics of rectosigmoid10, 11, 12, 13, 14 and rectovaginal endometriosis.15, 16, 17, 18 A recent meta‐analysis showed that TVS and enhanced TVS techniques have similar accuracy in diagnosing rectosigmoid endometriosis; however, enhanced techniques such as RWC‐TVS give additional useful information in the preoperative evaluation of these patients, such as estimation of bowel stenosis.19
This study compared the performance of RWC‐TVS and SVG in diagnosing DE located in the posterior pelvic compartment.
2. MATERIAL AND METHODS
This was a single‐center prospective study including consecutive women referred to our institution (Piazza della Vittoria 14 SRL, Genoa, Italy) between November 2017 and January 2020 undergoing surgical treatment because of pain and intestinal symptoms suggestive of posterior compartment DE.
The primary objective of the study was to compare the performance of RWC‐TVS and SVG in the diagnosis of posterior DE. The secondary objectives were to evaluate the accuracy of both techniques in estimating the size of nodules, and, in the case of rectosigmoid endometriosis, the precision estimating the infiltration of the intestinal submucosa, the distance between the lower margin of the nodules and the anal verge, and the presence of multifocal disease (presence of one or more lesions affecting the rectosigmoid colon that are associated with a primary colorectal lesion). Another secondary objective was to compare the tolerability of both examinations.
Exclusion criteria for this study included: previous surgical diagnosis of DE; previous radiological diagnosis of DE including techniques used to diagnose DE, including intestinal endometriosis (such as magnetic resonance [MRI], computed tomography‐based virtual colonoscopy [CTC] or double‐contrast barium enema); history of colorectal surgery (except appendectomy); contraindications to bowel preparation or distending the rectosigmoid (such as rectal malformations); previous bilateral ovariectomy; psychiatric disorders.
A power analysis calculation demonstrated that at least 150 patients would need to be recruited to obtain a statistically significant difference in sensitivity (SE) and specificity (SP) between 80% and 95% (by considering a type I error a = 0.05 and a type II error b = 0.05).
RWC‐TVS and SVG were performed by two gynecological sonologists (F.B. and S.F.) with extensive experience in diagnosing DE (over 500 transvaginal sonograms for DE). Both examinations were performed using Voluson E6 and E10 machines equipped with a transvaginal transducer (GE Medical Systems, Zipf, Austria). The scans were independently performed by the two gynecological sonologists blinded to the other technique's results. All participants initially underwent RWC‐TVS and then SVG, which was performed within an interval of 1 week to 2 months. The two gynecological sonologists alternatively performed each exam. Subsequently, patients underwent laparoscopy within 3 months from the last ultrasonographic exam.
Number, size and anatomical localization of posterior DE nodules were described according to IDEA criteria (20), evaluating the following localizations: uterosacral ligaments, rectovaginal septum, vagina, rectosigmoid (anterior lower and upper rectum, rectosigmoid junction and sigmoid colon).
According to previous authors,17 contiguous DE lesions involving more than one pelvic structure were considered separately in multiple localizations, ie nodules of the vagina and/or rectum extending to rectovaginal septum were also considered rectovaginal nodules.
At TVS, DE nodules in the uterosacral ligaments are characterized by nodules with regular or irregular margins and often hyperechoic points, or a linear hypoechoic thickening with regular or irregular margins; DE nodules in the rectovaginal septum appear as lesions below a horizontal plane that passes along the lower margin of the posterior lip of the cervix, under the peritoneum. DE nodules in the vagina should be suspected when the posterior vaginal fornix is thickened, with or without surrounding cystic anechoic areas. Rectosigmoid endometriosis often consists of hypoechoic thickening of bowel muscularis propria, eventually characterized by hyperechoic foci with blurred margins20; these rectosigmoid nodules can even replace the typical appearance of the intestinal muscularis propria; retraction and/or adhesions can be concomitantly present.20, 21
The distance between the lowest rectosigmoid nodule and the anal verge was measured according to the IDEA consensus.20 For DE of the rectovaginal septum, the caudal part of the nodule was identified, and an index finger was placed on the transvaginal probe at the level of the anal verge. The probe was then withdrawn, and the distance from the tip of the transvaginal probe down to the index finger was measured using a ruler. For DE above the rectovaginal septum, the distance between the caudal part of the lesion and the lower lip of the posterior cervix was measured in a frozen image, and the distance between the lower lip of the posterior cervix down to the anal verge was measured as described for DE at the level of the rectovaginal septum; these two measurements were summed.
Three‐dimensional (3D) reconstruction of coronal, sagittal and parasagittal planes complemented the standard ultrasonographic protocol for detecting and describing DE nodules. For acquiring the 3D volume datasets, a frequency of 6–9 MHz, a scanning angle of 120, and an average scanning speed were employed. Images were acquired with 3D multiplanar mode and rendering mode; two specific quality enhancement tools, advanced Speckle Reduction Imaging (SRI) and CrossXBeamCRITM (GE Medical Systems), were applied during 3D rendering. On the 3D rendering, DE lesions typically appear as spiculated lesions with a retracting line all around the nodule.22 The volume of the nodules was estimated using virtual organ computer‐aided analysis (VOCAL; GE Medical Systems), specifically, obtaining a sequence of 20 sections of each endometriotic nodule around a fixed axis, each after 9° rotation from the previous section. As recently described, each nodule contour was drawn manually using the rollerball cursor of the ultrasound machine.23 At RWC‐TVS, the bowel stenosis due to DE nodule was evaluated by transversal post‐acquisition 3D reconstruction of the intestinal lumen. The percentage of stenosis was estimated by subtracting the mean area of the endometriotic nodule from the mean area of healthy bowel lumen (Figure 1).
FIGURE 1.
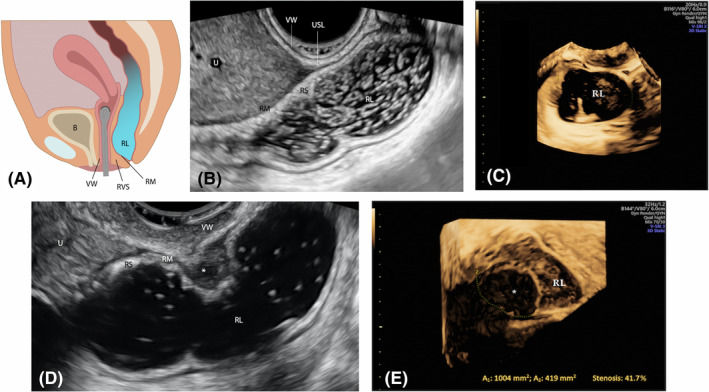
Rectal water contrast‐transvaginal ultrasonography. Schematic draw (A). Sagittal plane showing a healthy rectum (B) and the presence of an upper rectal endometriotic nodule (*) bulging to the intestinal lumen (D). Transversal post‐acquisition 3D reconstruction of intestinal lumen without (C) and with (E) the endometriotic nodule. The area of the endometriotic nodule has been subtracted from the area of bowel lumen to obtain the proportion (%) of stenosis. B, bladder; RL, rectal lumen; RM, rectal muscularis; RS, rectal submucosa; RVS, rectovaginal space; U, uterus; USL, uterosacral ligament; VW, vaginal wall
2.1. Rectal water contrast–transvaginal sonography
A rectal enema (133 ml of monobasic sodium phosphate anhydrous; Clisma Lax; Sofar, Milan, Italy) was administered a few hours before ultrasonographic examinations to favor the cleaning of the rectosigmoid colon of any fecal residue. Approximately 300 mL of saline solution was employed to distend the rectosigmoid under ultrasonographic control. The rectal distension was performed by a catheter connected to a 100‐mL sterile syringe introduced in the rectum, up to a distance of approximately 15 cm from the anal verge, as previously described.12, 14 A topical 2% lidocaine gel (Luan; Molteni & C.) was used to minimize the discomfort caused by the catheter's passage.
2.2. Sonovaginography
Approximately 40 ml of ultrasound gel (Ultragel; G.P.S. S.r.l.) distended the vagina, being introduced in the posterior vaginal fornix by a 50‐ml plastic syringe before inserting the transvaginal probe. The gel was loaded carefully into the syringe, ensuring minimal air bubbles/pockets within the gel. The syringe was entirely filled so that the plunger came in direct contact with the gel, thus further reducing the number of air pockets during the injection of contrast medium into the vagina (Figure 2).
FIGURE 2.
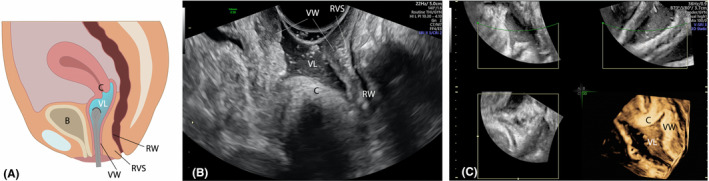
Sonovaginography. Schematic draw (A). Sagittal plane (B) and 3D sagittal reconstruction (C) of rectovaginal area; B, bladder; C, cervix; RVS, rectovaginal space; RW, rectal wall; VL, vaginal lumen; VW, vaginal wall
2.3. Tolerability of radiological examinations
After 1 h from the exam, the women judged the intensity of the pain perceived on a 100‐mm visual analog scale (VAS). Patients were also asked to qualitatively rate the discomfort perceived during the exam using a 5‐point Likert scale (very tolerable, tolerable, neutral, painful, very painful).
2.4. Surgery
Before laparoscopy, the surgeons evaluated the reports and the images from both enhanced diagnostics ultrasonographies. The procedures were performed by a team of gynecological and colorectal surgeons with extensive experience in the surgical treatment of DE. The diagnosis of endometriosis was confirmed by the pathological analysis of nodules excised at the surgery. The distance between rectosigmoid endometriotic nodules and anal verge was estimated using a 20‐F soft rectal catheter introduced from the rectum up to the level of the intestinal lesion.
Bowel specimens were sent unfixed for pathological analysis, which was done in a standardized fashion. The specimens were fixed in 10% buffered formalin for 12–18 h, embedded in paraffin blocks, and cut with a rotative microtome to obtain 3‐µm‐thick histologic slides of the large bowel that were stained with hematoxylin and eosin. Endometriosis was identified by the presence of endometrial‐like epithelium and stroma. The depth of infiltration of endometriosis in the intestinal wall was assessed. The maximal length of the largest endometriotic nodule, the bowel lumen diameter in proximity to the nodule, above and below, was measured in millimeters by eyepiece.
2.5. Statistical analyses
Accuracy, SE, SP, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+), and negative likelihood ratio (LR–) were evaluated for RWC‐TVS and SVG for predicting the presence of at least one DE nodule in the posterior compartment as well as in the above‐described four localizations of the posterior compartment. Efficacy parameters were calculated with 95% confidence intervals (CIs). The accuracy of both examinations in the diagnosis of posterior DE was compared using McNemar's test with the Yates continuity correction. The precision of the measurements of largest diameter and volume of nodules and, in the case of rectosigmoid endometriosis, their distance from the anal verge was estimated by the mean bias of the difference between the measurements of the imaging techniques and those of surgery/histology. Limits of agreement were calculated as mean difference ±2 SD of the difference. The normality of distribution of continuous variables was evaluated by the Kolmogorov–Smirnov normality test. The pain intensity experienced by the patients during both examinations was evaluated by the nonparametric Mann–Whitney test. Data were analyzed using the SPSS software version 24.0 (SPSS Science). The p values <0.05 were considered statistically significant.
2.6. Ethical approval
The local ethics committee approved the study protocol (CE Regione Liguria prot. 10074, 9 December 2019). Patients participating in the study provided written informed consent. This study was registered in Clinicaltrial.gov (NCT04296760). This study followed the STARD stands for “Standards for Reporting Diagnostic accuracy studies” (File S1).24
3. RESULTS
3.1. Characteristics of the study population and endometriotic nodules
The analysis of study was done on 281 consecutive women referred to our institution because of symptoms suggestive of posterior compartment DE and undergoing surgical treatment. The study flow is available as Figure 3. The mean (±SD) age of these patients was 35.3 ± 4.7 years. At the time of the study, 221 patients (78.6%, 95% CI 73.4%–83.3%) were using hormonal therapies. Table 1 reports the other demographic characteristics of the study population.
FIGURE 3.

Flow chart of the study
TABLE 1.
Demographic characteristics of the study population
| Total n = 281 | |
|---|---|
| Age, years (mean ± SD) | 33.4 ± 5.8 |
| Body mass index, kg/m2 (mean ± SD) | 24.3 ± 3.2 |
| Smokers, n (%) | 56 (19.9) |
| Race, n (%) | |
| White | 264 (94.0) |
| African | 12 (4.3) |
| Asiatic | 5 (1.7) |
| Level of education, n (%) | |
| Primary school | 4 (1.4) |
| Secondary school | 36 (12.8) |
| High school | 117 (41.6) |
| University | 124 (44.2) |
| Parous women, n (%) | 79 (28.1%) |
| Previous surgery for endometriosis, n (%) | 96 (34.2%) |
| Concomitant endometriomas, n (%) | 108 (38.4%) |
| Use of hormonal therapies at the time of study inclusion, n (%) | 215 (76.5%) |
| Oral estroprogestin pill | 106 |
| Contraceptive vaginal ring | 13 |
| Desogestrel | 8 |
| Norethindrone acetate | 48 |
| Dienogest | 22 |
| Etonogestrel‐releasing implant | 8 |
| Levonorgestrel‐releasing intrauterine device | 6 |
| Gonadotropin‐releasing hormone analog | 4 |
| Symptoms, n (%) | |
| Dysmenorrhea | 51/78a (65.4) |
| Deep dyspareunia | 146/237b (61.6) |
| Non‐menstrual pelvic pain | 132 (47.0) |
| Dyschezia | 98 (34.9) |
| Diarrhea | 44 (15.7) |
| Constipation | 38 (13.5) |
| Abdominal bloating | 62 (22.1) |
| Intestinal cramping | 67 (23.8) |
| Passage of mucus | 51 (19.2) |
All the other patients were using hormonal therapies causing amenorrhea.
Seven patients were not sexually active.
In 208 patients (74.0%, 95% CI 68.4%–79.1%) posterior DE was confirmed at laparoscopy; 299 nodules were diagnosed in the following localizations, namely, bowel (n = 88; 29.4%), uterosacral ligaments (156; 52.2%), rectovaginal septum (n = 34; 11.4%) and vagina (n = 21; 7.0%). Eighty‐nine patients (42.9%) had more than one nodule in the previous localizations of posterior pelvic compartment. The mean (±SD) largest diameter of endometriotic nodule at histology was 13.6 (± 9.7) mm.
Twenty‐two of 156 lesions of uterosacral ligaments were located at uterine torus (14.1%), 70 (44.9%) and 64 (41.0%) in the left and right ligaments, respectively. POD obliteration was described in 15.3% of patients (n = 32).
Eighty‐three patients had intestinal nodules; among them, five patients (6.0%) showed evidence of multifocal intestinal disease. Nodules were located on the sigmoid in 20.5% (n = 18), on the rectosigmoid junction in 26.2% (n = 23), on the anterior upper rectum in 43.1% (n = 38) and on the anterior lower rectum in 10.2% (n = 9) of patients. Overall, 31 women (37.3%) underwent shaving of the colorectal nodules, 25 (30.2%) underwent discoid excision and 27 (32.5%) underwent segmental colorectal resection. In four of five patients with multifocal disease, segmental colorectal resection was performed; one woman underwent shaving of two intestinal nodules. In patients undergoing segmental colorectal resection, the mean (± SD) length of the resected bowel specimen was 115 ± 49 mm. Concerning the depth of infiltration of endometriosis in the intestinal wall, at histology, 65 nodules (73.9%) infiltrated only the muscularis propria, 19 nodules (21.6%) the submucosa and four nodules the mucosa (4.5%).
3.2. Accuracy of examinations for diagnosis posterior compartment DE
RWC‐TVS and SVG had similar SE (93.8% vs 89.4%; p = 0.210) and SP (86.3% vs 79.4%; p = 0.481) in diagnosing DE of the pelvic posterior compartment. Their global accuracy was not different (91.8% vs 86.8%; p = 0.775) (Table 2, Table S1). The examinations were similarly precise in estimating the largest diameter of the main endometriotic nodule (p = 0.066). When compared with histology, the mean bias was –0.1 (±4.3; limits of agreement, –8.7 to 8.4) at RWC‐TVS and 0.2 (±2.3; limits of agreement, –10.1 to 10.5) at SVG (Table 3). Scatterplots displaying the correlation between diameter and volume estimated using RWC‐TVS and SVG are shown in Figure S1.
TABLE 2.
Diagnostic performance of imaging techniques in the diagnosis of posterior DE
| SE | SP | PPV | NPV | LR+ | LR– | ACC | |
|---|---|---|---|---|---|---|---|
| All localizations | |||||||
| RWC‐TVS | 93.8 (89.6–96.6) | 86.3 (76.3–93.3) | 95.1 (91.6–97.2) | 82.9 (74.0–89.2) | 6.8 (3.8–12.2) | 0.07 (0.04–12) | 91.8 (88.0–94.7) |
| SVG | 89.4 (84.4–93.3) | 79.4 (68.4–88.0) | 93.5 (88.7–95.1) | 72.5 (63.6–79.9) | 4.4 (2.8–6.9) | 0.1 (0.09–0.2) | 86.8 (82.3–90.6) |
| Rectosigmoid | |||||||
| RWC‐TVS | 95.2 (88.1–98.7) | 99.5 (97.2–100.0) | 98.8 (91.8–99.8) | 98.0 (95.0–99.2) | 188.5 (26.7–1332.1) | 0.05 (0.02–0.1) | 98.2 (95.9–99.4) |
| SVG | 82.0 (72.0– 89.5) | 98.5 (95.6–99.7) | 95.8 (88.0 to 98.4) | 92.9 (89.1–95.4) | 54.1 (17.5–167.0) | 0.2 (0.1–0.3) | 93.6 (90.1– 96.2) |
| Vagina | |||||||
| RWC‐TVS | 85.7 (63.7– 97.0) | 98.5 (96.2–99.6) | 81.8 (62.8 to 92.4) | 98.8 (96.8–99.6) | 55.7 (20.7–149.6) | 0.2 (0.05–0.4) | 97.5 (94.9– 99.0) |
| SVG | 95.2 (76.2–99.9) | 99.6 (97.9–100.0) | 95.2 (73.8–99.3) | 99.3 (97.5–99.9) | 247.6 (0.01–1755.4) | 0.05 (0.01–0.3) | 99.3 (97.5–99.9) |
| Rectovaginal septum | |||||||
| RWC‐TVS | 85.3 (68.9–95.1) | 96.4 (93.2–98.3) | 76.3 (62.6–86.1) | 97.9 (95.5–99.1) | 23.4 (12.1–45.1) | 0.2 (0.07–0.3) | 95.0 (91.8–97.3) |
| SVG | 91.2 (76.3–98.1) | 97.6 (94.8–99.1) | 83.8 (70.0–92.0) | 98.8 (96.5–99.6) | 37.5 (16.9–83.3) | 0.09 (0.03–0.3) | 96.8 (94.0–98.5) |
| Uterosacral ligaments | |||||||
| RWC‐TVS | 80.1 (73.0–86.1) | 92.8 (86.8–96.7) | 93.3 (88.1–96.3) | 78.9 (73.1–83.7) | 11.1 (5.9–21.0) | 0.2 (0.2–0.3) | 85.8 (81.1–89.6) |
| SVG | 77.6 (70.2–83.9) | 88.8 (81.9–93.7) | 89.6 (84.0–93.5) | 76.0 (70.2–81.0) | 6.9 (4.2–11.4) | 0.3 (0.2–0.3) | 82.6 (77.6–86.8) |
The values are reported as % (95% CI).
Abbreviations: SE, sensitivity; SP, specificity; LR+, positive likelihood ratio; LR–, negative likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; RWC‐TVS, rectal water contrast‐transvaginal ultrasonography; SVG, sonovaginography.
TABLE 3.
Difference between the size of nodule estimated by imaging techniques vs measured on histopathology in patients with posterior compartment DE
| Location | Length on histology (mm, mean ±SD) | RWC‐TVS | SVG | p | ||
|---|---|---|---|---|---|---|
| Bias (SD)a | Limits of agreementb | Bias (SD)a | Limits of agreementb | |||
| All (n c = 299) | 10.6 ± 6.9 | –0.1 (±4.3) | –8.7 to 8.4 | 0.2 (±5.2) | –10.1 to 10.5 | 0.066 |
| Recto sigmoid (n c = 88) | 17.3 ± 7.8 | 0.8 (±5.7) | –10.2 to 11.9 | 1.0 (±7.0) | –12.8 to 14.9 | 0.069 |
| Rectovaginal septum (n c = 34) | 11.0 ± 4.3 | –1.1 (±2.0) | –5.1 to 2.8 | –0.6 (±2.1) | –4.8 to 3.5 | 0.169 |
| Uterosacral ligaments (n c = 156) | 6.7 ± 3.4 | –1.1 (±1.8) | –4.8 to 2.6 | –0.6 (±2.6) | –5.6 to 4.4 | 0.803 |
| Vagina (n c = 21) | 10.9 ± 3.4 | 0.8 (±5.6) | –10.2 to 11.9 | 1.0 (±7.1) | –12.8 to 14.9 | 0.434 |
Abbreviations: RWC‐TVS: Rectal water contrast‐transvaginal ultrasonography; SVG: sonovaginography.
Calculated by subtracting the size of nodule measured by imaging technique from the size of nodule measured on histology.
Limits of agreement calculated as mean difference ±2 SD of the difference.
Number of nodules.
Concerning specific DE localization, RWC‐TVS and SVG had similar accuracy in detecting nodules of uterosacral ligaments (p = 0.779), vagina (p = 0.688) and rectovaginal septum (p = 0.824). RWC‐TVS had higher SE (95.2% vs 82.0%; p = 0.003) and similar SP (99.5% vs 98.5%; p = 0.500) in diagnosing rectosigmoid endometriosis, although both examinations correctly diagnosed the presence of all the lower rectal nodules (n = 9/9) (Figures 4 and 5). RWC‐TVS provided a better estimation of the infiltration of the intestinal submucosa better (p = 0.039) (Table 4, Table S2) and was more accurate than SVG in estimating the largest diameter of the intestinal nodule of the rectosigmoid junction and sigmoid (p = 0.014; Figure S2). RWC‐TVS provided a better estimation of the distance between its lower margin and the anal verge (p < 0.001) with a mean bias of 3.6 (±31.0; limits of agreement, –57.0 to 64.0) at RWC‐TVS and 8.6 (±23.0; limits of agreement, –36.0 to 53.0) at SVG (Figure 6). Both examinations similarly detected the presence of multifocal intestinal disease (n = 4/5 patients; p = 1.000).
FIGURE 4.
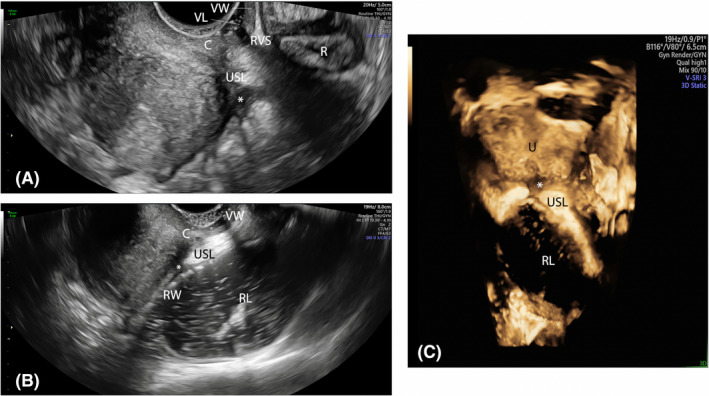
Sagittal plane showing an endometriotic nodule (*) of the right uterosacral ligament at sonovaginography (A) and rectal water contrast‐transvaginal ultrasonography (B). Transversal post‐acquisition 3D reconstruction (C) showing that the endometriotic nodule does not infiltrate the intestinal wall. C, cervix; R, rectum, RL, rectal lumen; RVS, rectovaginal space; RW, rectal wall; USL, uterosacral ligament; VL, vaginal lumen; VW, vaginal wall
FIGURE 5.
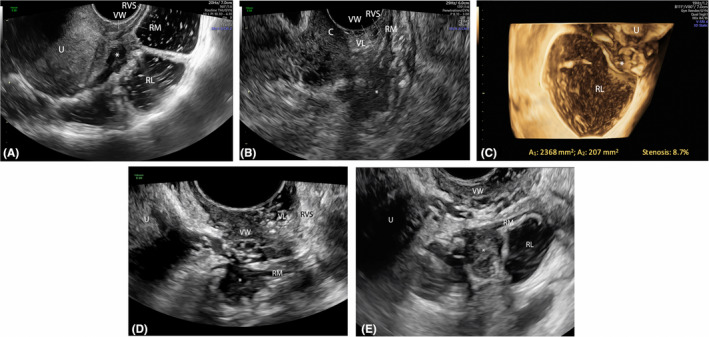
Sagittal plane showing an endometriotic nodule (*) of the upper rectum at rectal water contrast‐transvaginal ultrasonography (RWC‐TVS) (A) and sonovaginography (SVG) (B). 3D coronal reconstruction of the intestinal lumen at the level of the nodule (estimated stenosis: 8.7%. (C). Another endometriotic nodule (*) of the upper rectum at RWC‐TVS (D) and SVG (E). C, cervix; RL, rectal lumen; RM, rectal muscularis; RVS, rectovaginal space; U, uterus; VL, vaginal lumen; VW, vaginal wall
TABLE 4.
Diagnostic performance of imaging techniques in the diagnosis of intestinal submucosal/mucosal infiltration of rectosigmoid endometriosis (n = 88 nodules)
| SE | SP | PPV | NPV | LR+ | LR– | ACC | |
|---|---|---|---|---|---|---|---|
| RWC‐TVS | 87.0 (66.4–97.2) | 96.9 (89.3–99.6) | 91.0 (71.7–97.5) | 95.5 (88.0–98.4) | 28.3 (7.2–111.6) | 0.1 (0.1–04) | 94.3 (87.2–98.1) |
| SVG | 56.5 (34.5–76.8) | 78.5 (66.5–87.7) | 48.2 (34.1–62.5) | 83.6 (75.9–89.2) | 2.6 (1.5–4.8) | 0.6 (0.3–0.9) | 72.7 (62.2–81.7) |
The values are reported as % (95% CI).
Abbreviations: SE, sensitivity; SP, specificity; LR+, positive likelihood ratio; LR–, negative likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; RWC‐TVS, rectal water contrast‐transvaginal ultrasonography; SVG, sonovaginography.
FIGURE 6.
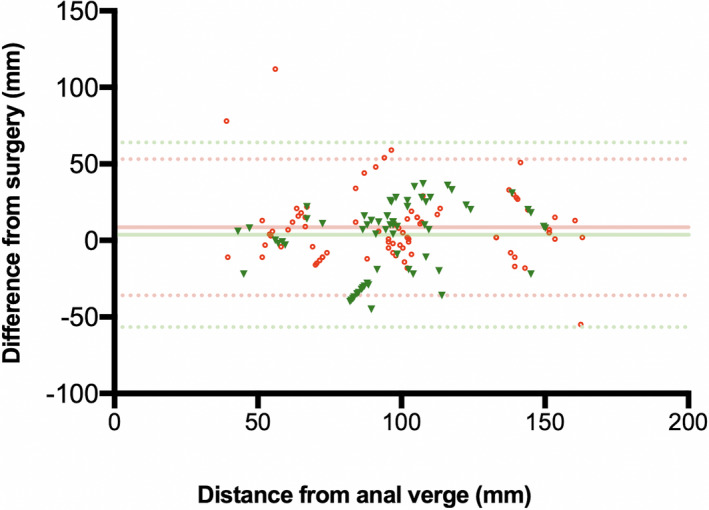
Bland–Altman plot displaying the difference in measurement of the lesion‐to‐anal‐verge distance between rectal water contrast‐transvaginal ultrasonography (red circles) and sonovaginography (green triangles) in women with rectosigmoid DE. Mean (‐) and 95% limits of agreement (‐‐‐) are shown
At the pathological examination in patients undergoing segmental resection, the degree of bowel lumen stenosis was 55.8 ± 24.4%. Only RWC‐TVS allowed bowel lumen stenosis to be evaluated, with a mean bias in the estimation of 13.2% (± 6.1, limits of agreement, –2.3 to 7.9) in comparison with pathological examination.
3.3. Tolerability of examinations
A similar proportion of women complained of pain during RWC‐TVS and SVG (patients experiencing a painful/very painful exam n = 5, 1.8% vs n = 8, 2.8%; p = 0.191); however, the mean (±SD) intensity of pain experienced was higher during RWC‐TVS than SVG (VAS, 22.4 ± 13.7 vs. 10.1 ± 15.8 mm; p < 0.001).
4. DISCUSSION
This prospective study showed that RWC‐TVS and SVG have good comparable diagnostic accuracy for detecting DE (91.8% vs 86.8%; p = 0.775), as both examinations were able to detect implants correctly in uterosacral ligaments, vagina and rectovaginal septum to a similar extent. However, RWC‐TVS showed higher performance in detecting the presence of rectosigmoid endometriosis (SE: 95.2% vs 82.0%; p = 0.003) and describing its characteristics.
Over the last 10 years, several enhanced ultrasonographic techniques have been proposed to improve the diagnosis of DE (27); until now, no study has compared the performance of different enhanced techniques in diagnosing posterior compartment DE.25, 26
Several studies showed that RWC‐TVS is accurate in diagnosing rectosigmoid endometriosis, and its performance has been compared with that of other radiological examinations, such as multidetector computerized tomography enema (MDCT‐e), CTC and MR‐enema.10, 11, 14, 22 Bowel cleansing is usually recommended before RWC‐TVS, despite there not being any evidence that it improves the diagnostic accuracy of this exam in predicting the presence and characteristics of rectosigmoid endometriosis.27 RWC‐TVS allows an accurate estimation of the depth of infiltration of endometriosis in the intestinal wall (notably, the infiltration of the submucosa) and the largest diameter of the nodules; nevertheless, other radiological techniques, such as CTC, may be more precise in estimating the distance between the lowest endometriotic nodule and the anal verge, particularly in the case of upper rectosigmoid nodules.10, 22 Lastly, the degree of intestinal stenosis due to endometriotic nodules may be estimated by RWC‐TVS22; preoperative estimation of the stenosis of bowel lumen may be helpful not only during the preoperative surgical planning but also to evaluate the need of surgical treatment in infertile women who may have a higher risk of bowel occlusion during ovarian stimulation and pregnancy.28
Data from the current literature showed that TVS has a lower SE for detecting DE nodules located in the rectovaginal septum (49%–59%) and vagina (58%) compared with other pelvic locations.29, 30 SVG is based on the introduction of saline solution or ultrasonographic gel in the vagina, which creates an acoustic window between the probe and the surrounding structures. Additionally, it exerts pressure that can distend the vaginal walls. These characteristics aim to improve visualization of the structures of the posterior pelvic compartment.27 Previous studies evaluated the use of SVG for diagnosing DE.15, 17, 18, 31, 32, 33, 34 A large Italian prospective study including 102 women, investigated SVG with saline solution compared with TVS and MRI to diagnose posterior DE.18 The SE of SVG and MRI, respectively, was 97.1% and 94.3% for endometriotic involvement of vaginal fornix, 95.6% and 95.6% for uterosacral ligaments, and 100% and 77.8% for the rectovaginal septum. In the diagnosis of rectal endometriosis, SVG and MRI obtained a superimposable SE of 66.7%; otherwise, these examinations had an SP of 93.8% and 95.8%, respectively; the evaluation of diagnostic accuracy for rectal endometriosis was limited by the small number of women (only six with rectosigmoid endometriosis). Another multicenter prospective study investigated the performance of SVG with gel17 in 189 consecutive women with clinical suspicion of posterior DE who subsequently underwent laparoscopic treatment of endometriosis. The findings of that study revealed that 57 (30.1%) women had a surgical diagnosis of posterior DE and 43 (22.8%) showed rectosigmoid DE. For posterior vaginal wall (n = 11 women) and rectovaginal septum DE (n = 11 women), respectively, SVG had an accuracy of 94.7% and 95.2%, SE was 18.2% and 18.2%, SP was 99.4% and 100%, PPV was 66.7% and 100% and NPV was 95.2% and 95.2%, respectively (p = 0.009 and p = 0.003). The low SE for detecting these nodules may be influenced by the low prevalence of vaginal and rectovaginal septum DE in the authors’ samples. Nevertheless, similarly to our findings, Reid et al.17 obtained an accuracy of 92.1%, an SE of 88.4%, an SP of 93.2%, a PPV of 79.2% and an NPV of 96.5% (p < 0.001) for predicting the presence of rectosigmoid/anterior rectal DE (n = 43 women).
In our study, we employed ultrasonographic gel instead of saline solution as distending media when performing SVG; the use of gel has the advantage that one operator can at the same time insert the distension media and perform the examination. In agreement with the current literature,17, 18 our findings confirm that SVG has a high SE for detecting vaginal endometriotic implants (95.2%; Figure 7). However, the similar accuracy between RWC‐TVS and SVG for vaginal DE (p = 0.688) may be likely due to the low prevalence (6.0%) of vaginal lesions in our study group. Furthermore, it has been reported previously that confirmation of DE in the rectovaginal septum by SVG may be more difficult in the case of the presence of a contiguous anterior rectal wall lesion, which may infiltrate and/or obliterate the rectovaginal space.17 Our study did not confirm these data, as SVG could detect the lower anterior rectal DE nodules in all the cases (n = 9/9). However, the low prevalence of patients with rectovaginal septum nodules infiltrating the anterior rectal wall may limit interpretation of this finding. Otherwise, the accuracy of SVG was lower in describing the presence and characteristics (largest diameter, distance from the anal verge) of upper intestinal nodules, mainly when located above the upper rectum. This may be explained by the acoustic shadowing related to even minimal air bubbles/pockets in the vagina distending gel, limiting penetration of ultrasound in deeper pelvic fields of interest.
FIGURE 7.
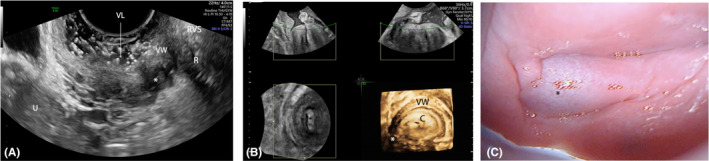
Endometriotic nodule (*) of the lower part of the lateral vaginal fornix at sonovaginography: sagittal plane (A) and 3D reconstruction (B); colposcopic view of the nodule (C). C, cervix; R, rectum; RVS, rectovaginal space; U, uterus; VL, vaginal lumen; VW, vaginal wall
In our study, 3D reconstruction was employed during the enhanced ultrasonographic examinations; these acquisitions have been investigated to characterize DE nodules, as they have a wide spatial orientation and allow to obtain a range of different displays of the images in the three orthogonal planes.35 Moreover, 3D acquisitions, being assessed off‐line by a dedicated post‐processing software, can be manipulated, rotated or scrolled through, in fascinating virtual navigation.22 It has recently been reported that 3D reconstruction of the coronal plane may allow for better visualization of pelvic structures, such as the posterior vaginal fornix and rectovaginal septum.36 According to our experience, 373D modality should be complementary to 2D scan and be done dynamically during ultrasonographic examination. However, we deem that the main advantages of using 3D reconstructions are the estimation of the volume of DE nodules by VOCAL technique and the contribution in evaluating the degree of the stenosis of the bowel lumen in patients with rectosigmoid nodules at RWC‐TVS (Figure 1 and Figure S2); obviously, this was not possible by SVG, which is not based on the distention of the bowel lumen.
Like conventional TVS, a limitation of enhanced TVS techniques is that they cannot diagnose endometriotic nodules located above the rectosigmoid, as they are beyond the field of view of ultrasonography. Therefore, when multicentric disease (endometriotic nodules in different bowel segments) is suspected, other radiological examinations, such as CTC, should be employed to investigate the whole colon.10 Furthermore, RWC‐TVS and SVG may cause some pain because of distention of the rectosigmoid and vagina, respectively. Our results showed a similar proportion of discomfort during both examinations, although the mean VAS score during RWC‐TVS was statistically higher. However, these examinations are generally well tolerated.26
To sum up, in clinical practice, TVS should be considered the first‐line approach for evaluating patients with suspicion of DE, when performed by a sonographer expert in endometriosis.6 The findings of our study indicate that enhanced ultrasonographic examinations can be helpful in the preoperative evaluation of patients with DE nodules in the posterior pelvic compartment, particularly when localized in the rectosigmoid (RWC‐TVS) and rectovaginal space (RWC‐TVS and SVG). The preoperative detection of the presence, location and characteristics of nodules is crucial to adequately plan the surgical treatment of patients with posterior DE nodules. Moreover, it is essential for providing the women with informed consent on benefits and risks of surgical treatment.
The main strengths of this study are the prospective design including consecutive women undergoing preoperative imaging evaluation by two independent, blinded readers and the large sample size, which was adequate for detecting differences between the diagnostic techniques. Additionally, both RWC‐TVS and SVG have been clearly described, following previous studies10, 12, 17 and could be systematically reproduced. Lastly, DE nodules were classified following current international IDEA consensus.20
This study has some limitations. Indeed, the extensive experience in performing RWC‐TVS and SVG of the two gynecological sonologists, respectively, may have influenced the accuracy of these techniques in diagnosing posterior DE. Moreover, a high prevalence of rectosigmoid endometriosis can be partly attributed to a study population with a high rate of intestinal symptoms; nevertheless, this issue might potentially have influenced the diagnostic performance of RWC‐TVS and SVG. In our study, the surgeons, skilled in endometriosis surgery, examined RWC‐TVS and SVG reports before the surgical approach; this may be considered a source of bias, but it would be unethical not to inform the surgeons about the preoperative imaging findings. Therefore, the surgeons decided to perform the surgical excision of DE nodules after doing an accurate intraoperative evaluation, establishing the most appropriate operative approach. Lastly, although these enhanced techniques accurately detected the infiltration of the vaginal and rectal walls, the definitive diagnosis of absence of infiltration was based only on the fact that no intestinal or vaginal resection procedures were required at surgery; this diagnostic parameter is strictly subjective, based on the surgeon's diagnostic suspicion, and may have excluded cases of a minimal infiltration of the rectal or vaginal wall. In the near future, intra‐/interobserver reproducibility studies are needed to confirm the diagnostic accuracy and clinical applicability of RWC‐TVS and SVG to predict posterior compartment DE.
5. CONCLUSION
In patients undergoing surgical treatment of DE, RWC‐TVS and SVG had similar SE and SP in diagnosing the posterior pelvic compartment nodules. The examinations had similar accuracy in detecting nodules of uterosacral ligaments, vagina and rectovaginal septum. RWC‐TVS had higher diagnostic accuracy in diagnosing rectosigmoid endometriosis, providing better estimates of the infiltration of intestinal submucosa, and the distance between the nodule and anal verge; only RWC‐TVS allowed estimation of bowel lumen stenosis.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
SF: protocol/project development, writing/editing. FB and SF: investigation and data collection. FA and CAS: software. GE: figure development, revision of manuscript. CAS and ULRM: formal analysis. FB: writing/editing manuscript. SF: revision of manuscript. CES: supervision.
Supporting information
File S1
Fig S1
Fig S2
Fig S3
Table S1
Table S2
Barra F, Leone Roberti Maggiore U, Evangelisti G, et al. A prospective study comparing rectal water contrast‐transvaginal ultrasonography with sonovaginography for the diagnosis of deep posterior endometriosis. Acta Obstet Gynecol Scand. 2021;100:1700–1711. 10.1111/aogs.14209
REFERENCES
- 1.Koninckx PR, Ussia A, Adamyan L, Wattiez A, Donnez J. Deep endometriosis: definition, diagnosis, and treatment. Fertil Steril. 2012;98:564‐571. [DOI] [PubMed] [Google Scholar]
- 2.Bazot M, Darai E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. 2017;108:886‐894. [DOI] [PubMed] [Google Scholar]
- 3.Chapron C, Dubuisson JB, Pansini V, et al. Routine clinical examination is not sufficient for diagnosing and locating deeply infiltrating endometriosis. J Am Assoc Gynecol Laparosc. 2002;9:115‐119. [DOI] [PubMed] [Google Scholar]
- 4.Donnez O, Roman H. Choosing the right surgical technique for deep endometriosis: shaving, disc excision, or bowel resection? Fertil Steril. 2017;108:931‐942. [DOI] [PubMed] [Google Scholar]
- 5.Berlanda N, Somigliana E, Frattaruolo MP, Buggio L, Dridi D, Vercellini P. Surgery versus hormonal therapy for deep endometriosis: is it a choice of the physician? Eur J Obstet Gynecol Reprod Biol. 2017;209:67‐71. [DOI] [PubMed] [Google Scholar]
- 6.Guerriero S, Condous G, Van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318‐332. [DOI] [PubMed] [Google Scholar]
- 7.Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16‐24. [DOI] [PubMed] [Google Scholar]
- 8.Guerriero S, Pascual MA, Ajossa S, et al. Learning curve for ultrasonographic diagnosis of deep infiltrating endometriosis using structured offline training program. Ultrasound Obstet Gynecol. 2019;54:262‐269. [DOI] [PubMed] [Google Scholar]
- 9.Ferrero S, Leone Roberti Maggiore U, Barra F, Scala C. Modified ultrasonographic techniques. In: Guerriero S, Condous G, Alcazar JL, eds. How to Perform Ultrasonography in Endometriosis. Springer; 2018:133‐145. [Google Scholar]
- 10.Ferrero S, Biscaldi E, Vellone VG, Venturini PL, Leone Roberti Maggiore U. Computed tomographic colonography vs rectal water‐ contrast transvaginal sonography in diagnosis of rectosigmoid endometriosis: a pilot study. Ultrasound Obstet Gynecol. 2017;49:515‐523. [DOI] [PubMed] [Google Scholar]
- 11.Leone Roberti Maggiore U, Biscaldi E, Vellone VG, Venturini PL, Ferrero S. Magnetic resonance enema vs rectal water‐contrast transvaginal sonography in diagnosis of rectosigmoid endometriosis. Ultrasound Obstet Gynecol. 2017;49:524‐532. [DOI] [PubMed] [Google Scholar]
- 12.Valenzano Menada M, Remorgida V, Abbamonte LH, Nicoletti A, Ragni N, Ferrero S. Does transvaginal ultrasonography combined with water‐contrast in the rectum aid in the diagnosis of rectovaginal endometriosis infiltrating the bowel? Hum Reprod. 2008;23:1069‐1075. [DOI] [PubMed] [Google Scholar]
- 13.Menada MV, Remorgida V, Abbamonte LH, Fulcheri E, Ragni N, Ferrero S. Transvaginal ultrasonography combined with water‐contrast in the rectum in the diagnosis of rectovaginal endometriosis infiltrating the bowel. Fertil Steril. 2008;89:699‐700. [DOI] [PubMed] [Google Scholar]
- 14.Ferrero S, Biscaldi E, Morotti M, et al. Multidetector computerized tomography enteroclysis vs. rectal water contrast transvaginal ultrasonography in determining the presence and extent of bowel endometriosis. Ultrasound Obstet Gynecol. 2011;37:603‐613. [DOI] [PubMed] [Google Scholar]
- 15.Reid S, Bignardi T, Lu C, Lam A, Condous G. The use of intra‐operative saline sonovaginography to define the rectovaginal septum in women with suspected rectovaginal endometriosis: a pilot study. Australas J Ultrasound Med. 2011;14:4‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dessole S, Farina M, Rubattu G, Cosmi E, Ambrosini G, Nardelli GB. Sonovaginography is a new technique for assessing rectovaginal endometriosis. Fertil Steril. 2003;79:1023‐1027. [DOI] [PubMed] [Google Scholar]
- 17.Reid S, Lu C, Hardy N, et al. Office gel sonovaginography for the prediction of posterior deep infiltrating endometriosis: a multicenter prospective observational study. Ultrasound Obstet Gynecol. 2014;44:710‐718. [DOI] [PubMed] [Google Scholar]
- 18.Saccardi C, Cosmi E, Borghero A, Tregnaghi A, Dessole S, Litta P. Comparison between transvaginal sonography, saline contrast sonovaginography and magnetic resonance imaging in the diagnosis of posterior deep infiltrating endometriosis. Ultrasound Obstet Gynecol. 2012;40:464‐469. [DOI] [PubMed] [Google Scholar]
- 19.Gerges B, Li W, Leonardi M, Mol BW, Condous G. Meta‐analysis and systematic review to determine the optimal imaging modality for the detection of rectosigmoid deep endometriosis. Ultrasound Obstet Gynecol. 2020; 10.1002/uog.23148. [DOI] [PubMed] [Google Scholar]
- 20.Guerriero S, Condous G, van den Bosch T , et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48:318‐332. [DOI] [PubMed] [Google Scholar]
- 21.Benacerraf BR, Groszmann Y, Hornstein MD, Bromley B. Deep infiltrating endometriosis of the bowel wall: the comet sign. J Ultrasound Med. 2015;34:537‐542. [DOI] [PubMed] [Google Scholar]
- 22.Barra F, Biscaldi E, Scala C, et al. A prospective study comparing three‐dimensional rectal water contrast transvaginal ultrasonography and computed tomographic colonography in the diagnosis of rectosigmoid endometriosis. Diagnostics (Basel). 2020;10:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barra F, Scala C, Leone Roberti Maggiore U, Ferrero S. Long‐term administration of dienogest for the treatment of pain and intestinal symptoms in patients with rectosigmoid endometriosis. J Clin Med. 2020;9:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrero S, Barra F, Scala C, Condous G. Ultrasonography for bowel endometriosis. Best Pract Res Clin Obstet Gynaecol. 2021;71:38‐50. [DOI] [PubMed] [Google Scholar]
- 26.Ferrero S, Barra F, Scala C, Rolla M, León M. Enhanced ultrasonographic techniques. In: Ferrero S, Ceccaroni M, eds. Clinical Management of Bowel Endometriosis: From Diagnosis to Treatment. Cham: Springer International Publishing; 2020:53‐64. [Google Scholar]
- 27.Ferrero S, Barra F, Stabilini C, Vellone VG, Leone Roberti Maggiore U, Scala C. Does bowel preparation improve the performance of rectal water contrast transvaginal ultrasonography in diagnosing rectosigmoid endometriosis? J Ultrasound Med. 2019;38:1017‐1025. [DOI] [PubMed] [Google Scholar]
- 28.Barra F, Mikhail E, Villegas‐Echeverri JD, Ferrero S. Infertility in patients with bowel endometriosis. Best Pract Res Clin Obstet Gynaecol. 2021;71:161‐171. [DOI] [PubMed] [Google Scholar]
- 29.Guerriero S, Ajossa S, Minguez JA, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2015;46:534‐545. [DOI] [PubMed] [Google Scholar]
- 30.Guerriero S, Saba L, Pascual MA, et al. Transvaginal ultrasound vs magnetic resonance imaging for diagnosing deep infiltrating endometriosis: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2018;51:586‐595. [DOI] [PubMed] [Google Scholar]
- 31.Bratila E, Comandasu DE, Coroleuca C, et al. Diagnosis of endometriotic lesions by sonovaginography with ultrasound gel. Med Ultrason. 2016;18:469‐474. [DOI] [PubMed] [Google Scholar]
- 32.Leon M, Vaccaro H, Alcazar JL, et al. Extended transvaginal sonography in deep infiltrating endometriosis: use of bowel preparation and an acoustic window with intravaginal gel: preliminary results. J Ultrasound Med. 2014;33:315‐321. [DOI] [PubMed] [Google Scholar]
- 33.Cruz J, Moreira C, Cunha R, Ferreira J, Martinho M, Beires J. Diagnostic accuracy of sonovaginography for deep infiltrating endometriosis. Acta Obstet Ginecol Port. 2018;12:190‐194. [Google Scholar]
- 34.Venkatesh S, Anjali M, Vasudeva A, Kumar P. sliding sign and gel sonovaginography: a sneak peek prior to laparoscopy in patients with endometriosis. J Hum Reprod Sci. 2020;13:26‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerriero S, Saba L, Ajossa S, et al. Three‐dimensional ultrasonography in the diagnosis of deep endometriosis. Hum Reprod. 2014;29:1189‐1198. [DOI] [PubMed] [Google Scholar]
- 36.Cossi P, Schor E, Goncalves LF, Werner H. Assessment of rectovaginal endometriosis using three‐dimensional gel‐infusion sonovaginography. Ultrasound Obstet Gynecol. 2019;53:558‐560. [DOI] [PubMed] [Google Scholar]
- 37.Barra F, Alessandri F, Scala C, Ferrero S. Ultrasonographic 3D evaluation in the diagnosis of bladder endometriosis: a prospective comparative diagnostic accuracy study. Gynecol Obstet Invest. 2021;1–8. 10.1159/000516634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1
Fig S1
Fig S2
Fig S3
Table S1
Table S2


