Abstract
Background and Aims
Nonalcoholic fatty liver disease, especially nonalcoholic steatohepatitis (NASH), has become a major cause of liver transplantation and liver‐associated death. NASH is the hepatic manifestation of metabolic syndrome and is characterized by hepatic steatosis, inflammation, hepatocellular injury, and different degrees of fibrosis. However, there is no US Food and Drug Administration–approved medication to treat this devastating disease. Therapeutic activators of the AMP‐activated protein kinase (AMPK) have been proposed as a potential treatment for metabolic diseases such as NASH. Cordycepin, a natural product isolated from the traditional Chinese medicine Cordyceps militaris, has recently emerged as a promising drug candidate for metabolic diseases.
Approach and Results
We evaluated the effects of cordycepin on lipid storage in hepatocytes, inflammation, and fibrosis development in mice with NASH. Cordycepin attenuated lipid accumulation, inflammation, and lipotoxicity in hepatocytes subjected to metabolic stress. In addition, cordycepin treatment significantly and dose‐dependently decreased the elevated levels of serum aminotransferases in mice with diet‐induced NASH. Furthermore, cordycepin treatment significantly reduced hepatic triglyceride accumulation, inflammatory cell infiltration, and hepatic fibrosis in mice. In vitro and in vivo mechanistic studies revealed that a key mechanism linking the protective effects of cordycepin were AMPK phosphorylation–dependent, as indicated by the finding that treatment with the AMPK inhibitor Compound C abrogated cordycepin‐induced hepatoprotection in hepatocytes and mice with NASH.
Conclusion
Cordycepin exerts significant protective effects against hepatic steatosis, inflammation, liver injury, and fibrosis in mice under metabolic stress through activation of the AMPK signaling pathway. Cordycepin might be an AMPK activator that can be used for the treatment of NASH.
Abbreviations
- ACCα
acetyl‐CoA carboxylase
- Actb
β‐actin‐encoding gene
- ALT
alanine aminotransferase
- AMPK
AMP‐activated protein kinase
- AST
aspartate aminotransferase
- CC
Compound C
- Ccl2/5
chemokine (C‐C motif) ligand 2/5
- CD
cluster of differentiation
- Col1a1
collagen type I α 1
- Col3a1
collagen type III α 1
- Cpt1α
carnitine palmitoyltransferase 1α
- Ctgf
connective tissue growth factor
- Cxcl10
chemokine (C‐X‐C motif) ligand 10
- Fabp
fatty acid binding protein
- Fasn
fatty acid synthase
- GAPDH
glyceraldehyde 3‐phosphate dehydrogenase
- GSEA
gene set enrichment analysis
- H&E
hematoxylin and eosin
- HFD
high‐fat diet
- HFHC
high‐fat/high‐cholesterol
- Hmgcr
3‐hydroxy‐3‐methyl‐glutaryl‐CoA reductase
- IKBα
inhibitor of NF‐κB α
- IKKβ
inhibitor of NF‐κB kinase subunit β
- IL
interleukin
- LW
liver weight
- LW/BW
LW‐to‐body weight ratio
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
nonalcoholic steatohepatitis
- OA
oleic acid
- p‐
phosphorylated
- PA
palmitic acid
- PCA
principal component analysis
- PO
PA + OA
- Pparγ
peroxisome proliferator–activated receptor γ
- PSR
picrosirius red
- RNA‐seq
RNA sequencing
- Scd1
stearoyl‐CoA desaturase 1
- α‐SMA
α‐smooth muscle actin
- Smad
mothers against decapentaplegic
- Srebp
sterol regulatory element binding protein
- TC
total cholesterol
- TG
triglyceride
- TGFβ
transforming growth factor‐β
- Timp1
tissue inhibitor of metalloproteinase 1
- TNFα
tumor necrosis factor‐α
Nonalcoholic fatty liver disease (NAFLD) is a disease spectrum that ranges from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH) with progressive fibrosis.( 1 ) Overnutrition‐induced hepatic steatosis and chronic, low‐grade inflammation lead to liver damage in the context of NASH.( 2, 3 ) Although often clinically silent, NASH can progress over time to cirrhosis or end‐stage liver disease and may cause patients to need a liver transplant.( 2 ) The global prevalence of NAFLD in adults is estimated to be approximately 25%.( 4 ) Although NAFLD is highly prevalent in the general population, its progressive subtype of NASH is more clinically relevant than the NAFL subtype.( 4, 5 ) In the United States, an estimated 1.5%‐6.45% of the general population has NASH.( 6 ) With rapid lifestyle transitions, the increasing burden of NAFLD in China has emerged as a major public health issue. From 1999 to 2018, the prevalence of NAFLD in China increased by 8%‐9%, with the current overall prevalence reaching 29.1%.( 7 ) The Chinese population may have a higher hereditary risk of NAFLD due to more frequent nonsynonymous mutations in genes regulating lipid metabolism. China has experienced an unexpected rapid increase in the burden of NAFLD over a short period.( 8 )
The establishment of anti‐inflammatory and antifibrotic therapies is one of the major clinical needs for the treatment of liver fibrosis, especially NASH. Pharmacological approaches targeting NASH should ideally block progression and reverse liver injury. However, despite the very large investment by the pharmacological industry in recent years, there are still no approved therapies targeting NASH.( 9 ) Currently, only weight loss due to bariatric surgery and nonpharmacological management through a healthy lifestyle/diet and/or physical activity can be effective.( 10, 11 ) Thus, the development of medicines for NAFLD, especially incurable NASH, is an unmet medical need.
Cordycepin (3′‐deoxyadenosine) is a major bioactive component of the fungus Cordyceps militaris.( 12 ) Cordycepin has a broad spectrum of biological activities, such as anti‐inflammatory,( 13 ) antioxidant,( 14 ) antifibrotic,( 15 ) antiadipogenic,( 16 ) and antitumor( 17 ) activities. Furthermore, cordycepin prevents hyperlipidemia in hamsters( 18 ) and reduces the weight of rats with high‐fat diet (HFD)–induced obesity.( 16, 19 ) Cordycepin not only prevents D‐galactosamine/lipopolysaccharide (LPS)–induced acute liver injury( 20 ) but also HFD‐induced or alcohol‐induced chronic hepatotoxicity.( 18, 21 ) Cordycepin suppresses the migration and invasion of human liver cancer cells.( 22 ) However, the protective effects of cordycepin against the progression of NASH and the underlying mechanisms of action of these effects remain unknown.
The goals of the present study were to determine the effects of cordycepin on the development of NASH using a murine model established by HFD or high‐fat/high‐cholesterol (HFHC) diet feeding that recapitulates the human NASH metabolic profile, which is characterized by lipid accumulation, inflammation, and fibrosis, and to corroborate the direct effects of cordycepin in hepatocytes in the presence or absence of free fatty acids. We found that cordycepin inhibits hepatic triglyceride (TG) accumulation and protects against hepatic inflammation and fibrosis in mice with NASH. Our study identifies cordycepin as a promising mitigator that acts as a natural activator through direct interaction with the α‐subunit of AMP‐activated protein kinase (AMPK).
Materials and Methods
During the experiments, all animal protocols were approved by the Animal Care and Use Committee of Renmin Hospital of Wuhan University. All animals received humane care according to the criteria outlined in the NIH “Guide for the Care and Use of Laboratory Animals.” The detailed materials and methods are provided in the Supporting Information.
Results
Cordycepin Attenuates Palmitic Acid–Induced Lipid Accumulation, Inflammation, and Lipotoxicity in Hepatocytes
To investigate the effects of cordycepin on lipid accumulation and inflammation in hepatocytes under metabolic stress, L02 cells were treated with cordycepin (50 and 100 μM) in the presence or absence of palmitic acid (PA) and oleic acid (OA) (PA + OA [PO]) stimulation for 12 hours. Oil red O staining showed that cellular lipid droplet accumulation was notably decreased by cordycepin (Fig. 1A). The lipid‐lowering effect of cordycepin was confirmed by measurement of cellular TG and total cholesterol (TC) levels (Fig. 1B). Furthermore, cordycepin significantly attenuated PA‐induced up‐regulation of fatty acid synthesis genes (fatty acid synthase [Fasn], stearoyl‐CoA desaturase 1 [Scd1], acetyl‐CoA carboxylase alpha [Accα], and peroxisome proliferator–activated receptor gamma [Pparγ]) and inflammatory genes (interleukin‐6 [Il6], tumor necrosis factor‐α [Tnfα], chemokine [C‐C motif] ligand 5 [Ccl5], and chemokine [C‐X‐C motif] ligand 10 [Cxcl10]). In contrast, cordycepin reversed the PA‐induced down‐regulation of fatty acid β‐oxidation gene (Pparα) in L02 cells (Fig. 1C). Similar results were observed in primary hepatocytes treated with PA or PO (Supporting Fig. S1A‐C). Treatment with PO for 12 hours reduced the accumulation of lipids in cordycepin‐treated primary hepatocytes (Supporting Fig. S1A,B). Furthermore, cordycepin attenuated PA‐mediated induction of fatty acid synthesis genes (Fasn, Scd1, Accα, and sterol regulatory element binding protein [Srebp]) in primary hepatocytes (Supporting Fig. S1C). Additionally, cordycepin significantly diminished the induction of inflammatory genes (Il1b, Il6, Tnfα, Ccl5, and Cxcl10) by PA (Supporting Fig. S1C).
FIG. 1.
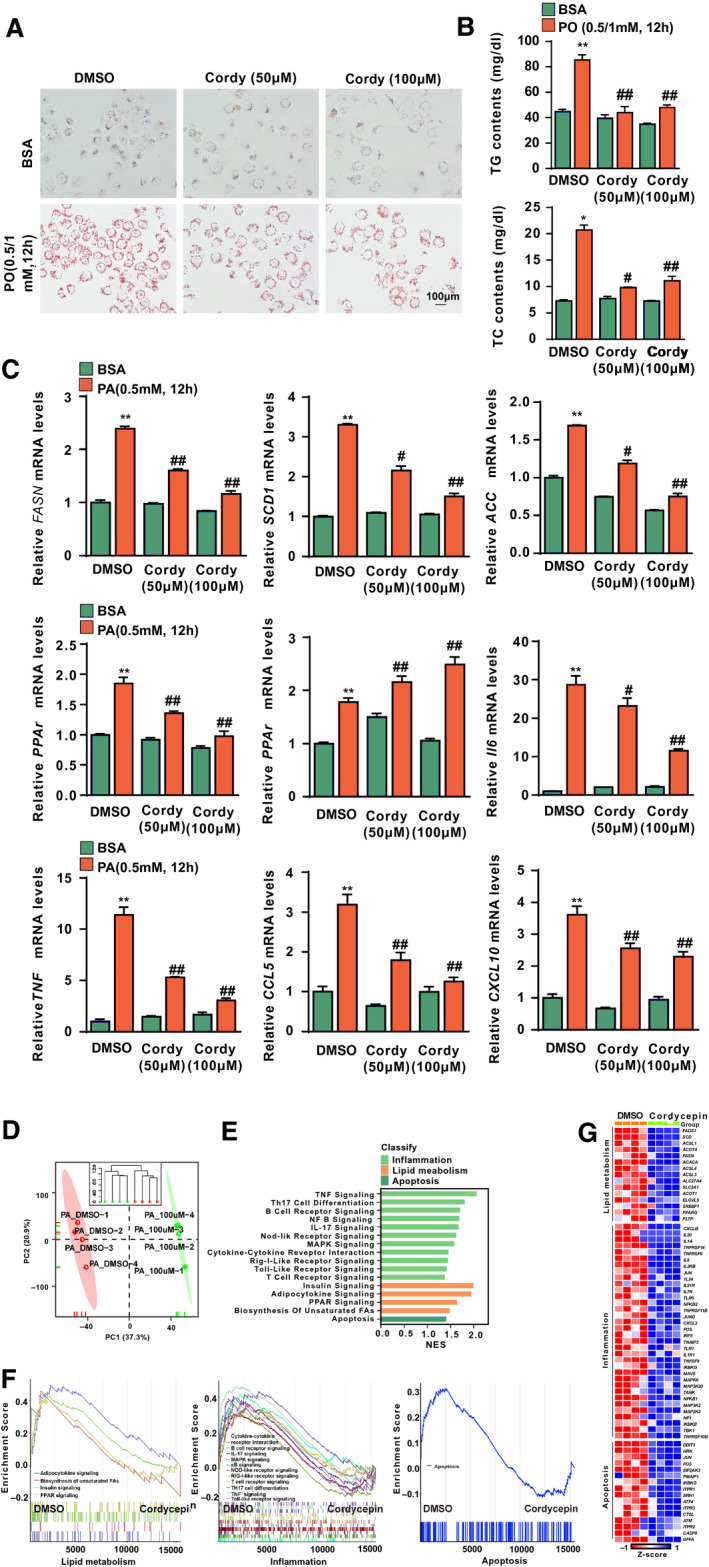
Effects of cordycepin on lipid accumulation and inflammation in hepatocytes. (A) Oil red O staining showing the degrees of lipid accumulation in L02 cells treated with DMSO, 50 μM or 100 μM cordycepin in response to BSA or PO (0.5 mM PA and 1.0 mM OA) stimulation for 12 hours. (B) TG and TC contents in L02 cells in the indicated groups stimulated with BSA or PO (0.5 mM PA and 1.0 mM OA) for 12 hours. n = 3 per group. The data are presented as the mean ± SD. Significant differences between the DMSO‐BSA group and the DMSO‐PO group: *P < 0.05, **P < 0.01; significant differences between the DMSO‐PO group and the cordycepin‐PO group: # P < 0.05, ## P < 0.01. (C) Relative mRNA levels of the indicated lipid metabolism–related genes (Fasn, Scd1, Accα, Pparγ, and Pparα) and inflammatory factors (Il6, Tnfα, Ccl5, and Cxcl10) and in L02 cells treated with PA (0.5 mM). The relative mRNA expression was normalized to that of the β‐actin‐encoding gene Actb. n = 3 per group. The data are presented as the mean ± SD. Significant differences between the DMSO‐BSA group and the DMSO‐PA group: **P < 0.01; significant differences between the DMSO‐PA group and the cordycepin‐PA group: # P < 0.05, ## P < 0.01. (D) PCA of RNA‐seq data for L02 cells treated with PA and cordycepin. (E,F) GSEA of pathways related to inflammation, lipid metabolism, and apoptosis. The font colors of the pathways related to inflammation and lipid metabolism are blue and yellow, respectively. n = 4 per group. (G) Heatmap of lipid metabolism–related, inflammation‐related, and apoptosis‐related gene expression profiles based on the RNA‐seq data set. Abbreviations: BSA, bovine serum albumin; Cordy, cordycepin; NES, normalized enrichment score; PC, principal component.
To systematically investigate the effects of cordycepin on lipid metabolism, inflammation, and lipotoxicity, L02 cells treated with cordycepin and DMSO controls under PA‐induced metabolic stress were evaluated by RNA‐sequencing (RNA‐seq) analysis. Principal component analysis (PCA) and unsupervised hierarchical clustering clearly separated the samples into two clusters (Fig. 1D). Next, the therapeutic effect of cordycepin on the main signaling pathways involved in NASH was also investigated. Gene set enrichment analysis (GSEA) pathway enrichment results revealed that pathways related to inflammation, lipid metabolism, and apoptosis were enriched (Fig. 1E) and significantly down‐regulated by cordycepin treatment compared with the DMSO control (Fig. 1F). A heatmap based on the GSEA results revealed that genes related to inflammation, lipid metabolism, and apoptosis pathways were significantly down‐regulated by cordycepin treatment in PA‐treated hepatocytes (Fig. 1G).
Cordycepin Attenuates HFD‐Induced Hepatic Steatosis and Inflammation
Given the antilipotoxic protective effects of cordycepin in in vitro assays, we next examined whether this compound attenuates hepatic steatosis in mice fed the HFD diet. Mice were fed the HFD for 16 weeks and then administered cordycepin (100 and 200 mg/kg/day) while continuing to receive the HFD for an additional 8 weeks (Supporting Fig. S2A). Compared to those of the normal chow control mice, the body weights of the treated mice significantly increased over time. However, there were no significant differences between the HFD‐fed mice treated with cordycepin and the HFD‐fed mice treated with the vehicle control (control‐HFD mice) (Supporting Fig. S2B). After 24 weeks of HFD feeding, the liver weights (LWs) and LW‐to‐body weight (LW/BW) ratios of the HFD‐fed mice were significantly increased. In contrast, the LWs and LW/BW ratios were significantly decreased in mice treated with cordycepin (Fig. 2A,B). However, the white adipose weights and the ratios of white adipose weight to body weight did not differ between the cordycepin and vehicle treatment groups after 24 weeks of HFD feeding (Supporting Fig. S2C,D). Compared with control‐HFD mice, cordycepin‐treated mice fed the HFD for 24 weeks exhibited significantly lower hepatic and serum lipid (TG and TC) levels (Fig. 2C; Supporting Fig. S2E). Hematoxylin and eosin (H&E) staining and NAFLD activity score (NAS) showed that hepatocyte damage induced by lipotoxicity was observed in HFD‐fed mice; however, cordycepin treatment significantly reduced the HFD‐induced hepatic steatosis and ballooning in the mouse livers. In addition, oil red O staining showed that cordycepin treatment significantly slowed hepatic lipid droplet accumulation in mice fed the HFD (Fig. 2D).
FIG. 2.
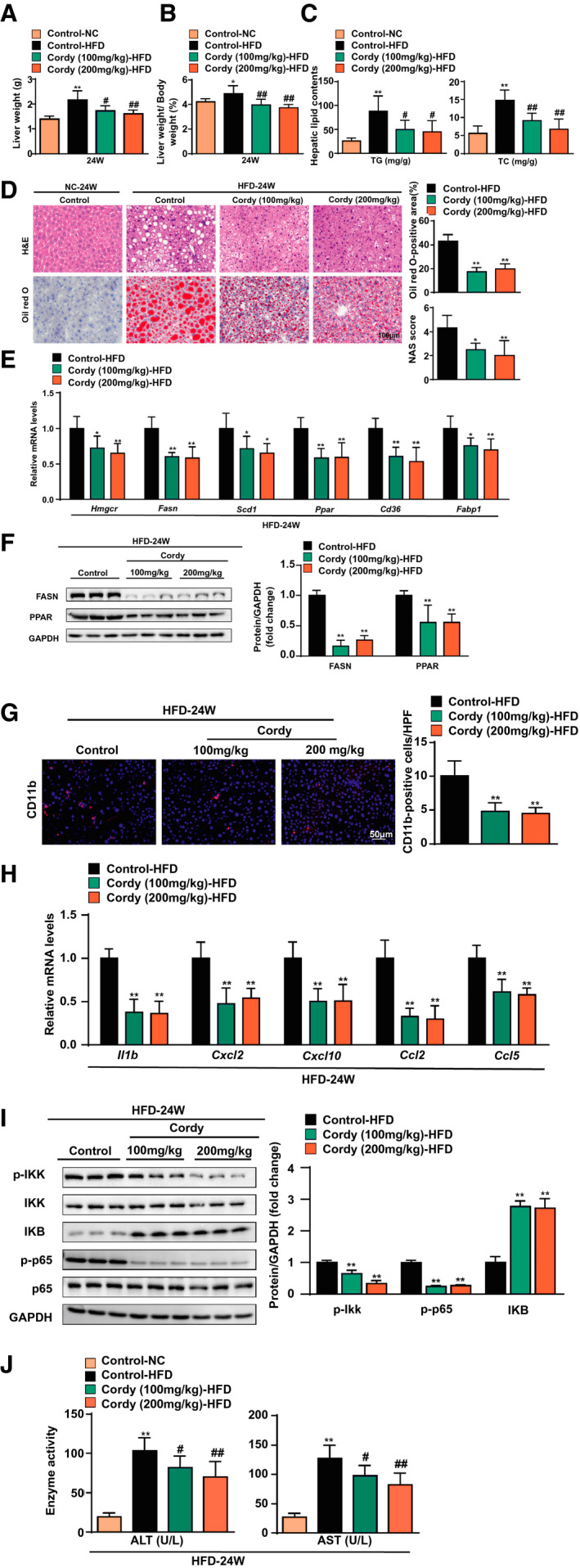
HFD‐induced hepatic steatosis and inflammation are alleviated in mice treated with cordycepin. (A) LWs and (B) LW/BW ratios of the mice. n = 10 per group. (C) Hepatic lipid (TG and TC) levels. n = 10 per group. (D) Hepatic steatosis and lipid accumulation were measured by H&E, oil red O staining, and NAFLD activity score in the indicated groups. n = 6 per group. Scale bar, 100 µm. (E) Quantitative real‐time PCR analysis of the transcript levels of genes related to lipid metabolism (Hmgcr, Fasn, Scd1, Pparγ, Cd36, and Fabp1). Gene expression was normalized to Actb mRNA levels. n = 6 per group. (F) Western blotting of proteins involved in lipid metabolism in the livers of the mice. GAPDH served as a loading control. n = 3 per group. (G) Immunofluorescence staining of Cd11b (red) in the livers of HFD‐fed mice. Nuclei were labeled with DAPI (blue). n = 4 per group. Scale bar, 50 µm. (H) Quantitative real‐time PCR analysis of the transcript levels of genes related to inflammation (Il1b, Cxcl2, Cxcl10, Ccl2, and Ccl5). Gene expression was normalized to Actb mRNA levels. n = 6 per group. (I) Western blotting of proteins associated with inflammation: p‐IKKβ, IKKβ, IKBα, p‐p65, p65, and GAPDH. GAPDH served as a loading control. n = 3 per group. (J) Levels of serum ALT and AST were measured in mice after 24 weeks of normal chow or HFD feeding. n = 10 per group. The data are presented as the mean ± SD. Significant differences between the control‐normal chow group and the control‐HFD group for (A‐C) and (J): *P < 0.05, **P < 0.01; significant differences between the control‐HFD group and the cordycepin‐HFD group: # P < 0.05, ## P < 0.01; significant differences between the control‐HFD group and the cordycepin‐HFD group for (D‐F): *P < 0.05, **P < 0.01. Abbreviations: Cordy, cordycepin; HPF, high‐power field; NC, normal chow.
The presence of lipid droplets containing TGs in hepatocytes is a hallmark of NAFLD, and multiple genes involved in lipogenesis, fatty acid uptake, and oxidation contribute to this phenomenon.( 23 ) We next examined representative genes by quantitative real‐time PCR assays and found that hepatic expression of genes related to fatty acid synthesis and uptake (3‐hydroxy‐3‐methyl‐glutaryl‐CoA reductase [Hmgcr], Fasn, Scd1, Pparγ, cluster of differentiation 36 [Cd36], and fatty acid binding protein 1 [Fabp1]) was lower in cordycepin‐treated mice than in control mice after 24 weeks of HFD feeding (Fig. 2E). In addition, the down‐regulation of FASN and PPARγ expression by cordycepin was further confirmed by western blot assay (Fig. 2F). CD11b immunofluorescence staining showed that cordycepin significantly decreased inflammatory cell infiltration in the livers of mice fed the HFD (Fig. 2G). Furthermore, quantitative real‐time PCR assays showed that inflammatory genes (Il1b, Cxcl2, Cxcl10, Ccl2, and Ccl5) were repressed by cordycepin treatment (Fig. 2H). Additionally, western blot analysis showed that phosphorylated inhibitor of NF‐κB kinase subunit β (p‐IKKβ) and p‐p65 levels were significantly reduced by cordycepin treatment. In contrast, inhibitor of NF‐κB α (IKBα) expression was significantly induced by cordycepin, suggesting that cordycepin inhibits NF‐κB activation in the livers of mice fed the HFD (Fig. 2I). Lipotoxicity eventually leads to hepatocyte injury or even death( 24 ); thus, serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured. Consistently, the serum levels of ALT and AST were lower in cordycepin‐treated HFD‐fed mice than in control‐HFD mice (Fig. 2J). Collectively, these data suggest that cordycepin treatment protects mice against hepatic lipid dysfunction and inflammation induced by HFD feeding.
Cordycepin Prevents HFHC Diet–Induced Hepatic Steatosis, Inflammation, and Fibrosis
To further investigate the protective effects of cordycepin against NASH in vivo, we established an HFHC diet–induced mouse NASH model that exhibited more profound inflammatory responses and fibrosis symptoms than the HFD‐induced model.( 25 ) Mice were fed the HFHC diet for 8 weeks and then administered cordycepin while continuing to receive the HFHC diet for an additional 8 weeks (Supporting Fig. S3A). Similar to the results obtained with the HFD NASH model, no obvious differences in body weight were observed between the control mice and the cordycepin‐treated mice during HFHC diet administration (Supporting Fig. S3B). Compared to control mice, cordycepin‐treated mice exhibited significantly lower LWs and LW/BW ratios after HFHC diet feeding for 16 weeks (Fig. 3A). Similar to the situation for the HFD model, the white adipose weights and the white adipose weight/body weight ratios did not differ between the cordycepin treatment and control groups after 16 weeks of HFHC diet feeding (Supporting Fig. S3C). Cordycepin significantly decreased the hepatic and serum lipid (TG and TC) levels in mice fed the HFHC diet for 16 weeks (Fig. 3B; Supporting Fig. S3D). H&E staining and NAS showed that hepatocyte damage induced by lipotoxicity was observed in HFHC diet–fed mice. In addition, oil red O staining showed that cordycepin treatment significantly slowed hepatic lipid droplet accumulation in mice fed the HFHC diet (Fig. 3C). Cordycepin also significantly attenuated HFHC diet–induced lipid‐related gene (Hmgcr, Srebp1, Fasn, Scd1, Pparγ, Cd36, and Fabp1) expression (Fig. 3D). The abnormal protein expression of FASN and PPARγ induced by the HFHC diet was also significantly reduced by cordycepin treatment (Fig. 3E). Combined with the results obtained with the HFD model, these data suggest that cordycepin attenuates hepatic steatosis under conditions of metabolic stress.
FIG. 3.
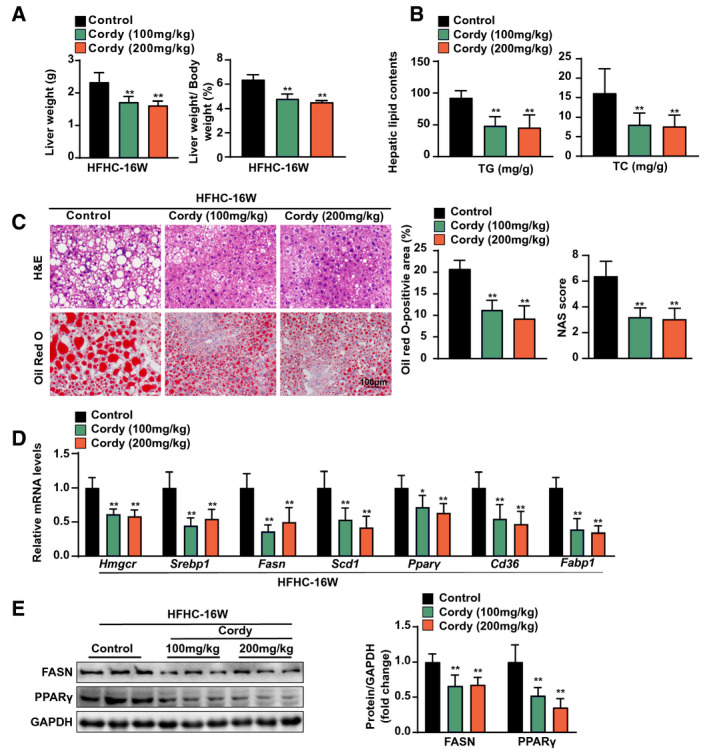
Cordycepin attenuated hepatic steatosis and injury in mice fed the HFHC diet. (A) LW and LW/BW ratios of the mice. n = 10 per group. (B) Hepatic lipid (TG and TC) levels. n = 10 per group. (C) Hepatic steatosis and lipid accumulation were measured by H&E, oil red O staining, and NAFLD activity score in the indicated groups. n = 6 per group. Scale bar, 100 µm. (D) Quantitative real‐time PCR analysis of the transcript levels of genes related to lipid metabolism (Hmgcr, Srebp1, Fasn, Scd1, Pparγ, Cd36, and Fabp1). Gene expression was normalized to Actb mRNA levels. n = 6 per group. (E) Western blotting of proteins involved in lipid metabolism (FASN and PPARγ). GAPDH served as a loading control. The data are presented as the mean ± SD. Significant differences between the control group and the cordycepin group: *P < 0.05, **P < 0.01. Abbreviation: Cordy, cordycepin.
Next, we examined the effects of cordycepin on NASH‐associated inflammation and fibrosis. A quantitative real‐time PCR assay showed that cordycepin treatment significantly attenuated the increases in the expression of inflammatory genes (Tnfα, Il1b, Cxcl10, Ccl2, and Ccl5) in mice with NASH induced by HFHC diet administration for 16 weeks (Fig. 4A). An anti‐inflammatory effect of cordycepin treatment was also indicated by reductions in inflammatory cell accumulation in the liver, as demonstrated by immunofluorescence staining for the macrophage marker CD11b (Fig. 4B). The protein levels of p‐IKKβ and p‐p65 were significantly down‐regulated by cordycepin treatment, whereas those of IKBα were up‐regulated by cordycepin (Fig. 4C), suggesting that cordycepin inhibits the activation of NF‐κB signaling and inflammation. The expression of profibrotic genes (collagen type I α 1 [Col1a1], collagen type III α 1 [Col3a1], α‐smooth muscle actin [α‐Sma], connective tissue growth factor [Ctgf], transforming growth factor‐β [Tgfb], and tissue inhibitor of metalloproteinase 1 [Timp1]) in HFHC diet–induced NASH mice was suppressed by cordycepin treatment (Fig. 4D). Picrosirius red (PSR) staining was markedly increased in NASH mice, but it was significantly decreased by cordycepin treatment (Fig. 4E). Furthermore, the phosphorylation of mothers against decapentaplegic 2/3 (Smad 2/3) was suppressed in cordycepin‐treated mice (Fig. 4F). These results suggested that cordycepin suppresses hepatic inflammation and fibrosis in mice with NASH induced by 16 weeks of HFHC diet administration. Consistently, the serum levels of ALT and AST in mice with HFHC diet‐induced NASH were significantly decreased by cordycepin (Fig. 4G).
FIG. 4.
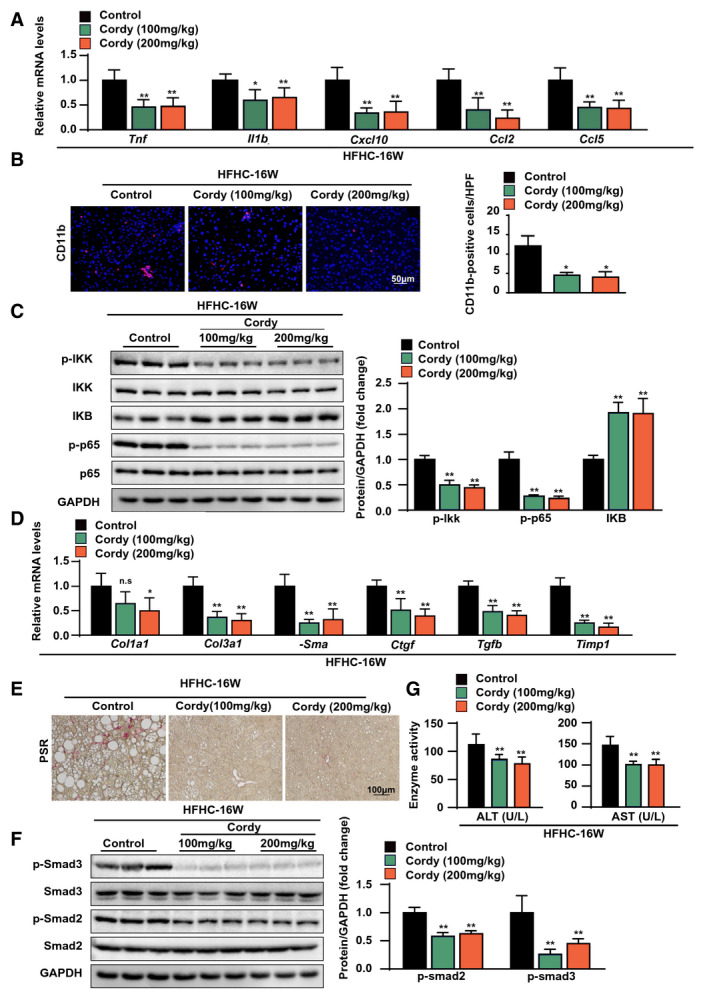
Cordycepin attenuated hepatic inflammation and fibrosis in mice fed the HFHC diet. (A) Relative mRNA levels of inflammatory genes (Tnfα, Il1b, Cxcl10, Ccl2, and Ccl5) in the livers of the indicated mice fed the HFHC diet for 16 weeks. n = 6 mice per group. (B) Immunofluorescence staining of Cd11b (red) in the livers of the indicated mice fed the HFHC diet for 16 weeks. Nuclei were labeled with DAPI (blue). n = 4 mice per group. Scale bar, 50 μm. (C) Immunoblot analyses of IKBα, GAPDH, and total and phosphorylated IKKβ and p65 protein in the liver tissues of vehicle‐treated or cordycepin‐treated mice fed the HFHC diet for 16 weeks (n = 3 mice per group). (D) Relative mRNA levels of profibrotic genes (Cola1, Col3a1, a‐Sma, Ctgf, Tgfb, and Timp1) in the livers of the indicated mice fed the HFHC diet for 16 weeks. The relative mRNA expression was normalized to that of Actb. n = 6 mice per group. (E) Representative images showing PSR staining in the livers of the indicated mice fed the HFHC diet for 16 weeks. n = 6 mice per group. Scale bar, 100 μm. (F) Immunoblot analyses of total and phosphorylated Smad2 and Smad3 proteins in the liver tissues of vehicle‐treated and cordycepin‐treated mice fed the HFHC diet for 16 weeks (n = 3 mice per group). (G) Serum ALT and AST levels of the mice in the indicated groups. n = 10 per group. The data are presented as the means ± SD. Significant differences between the control group and the cordycepin group: *P < 0.05, **P < 0.01. Abbreviations: Cordy, cordycepin; HPF, high‐power field; n.s., no significant difference between the control group and the cordycepin group (P > 0.05).
Cordycepin Protects Mice From HFHC Diet–Induced NASH by Systematically Suppressing Lipid Metabolism, Inflammation, and Fibrosis‐Related Gene Expression
To further systematically investigate how cordycepin suppresses NASH induced by HFHC diet feeding, we performed RNA‐seq and proteomics analyses on the livers of control and cordycepin‐treated mice. PCA and hierarchical clustering clearly separated the samples from the control mice fed the HFHC diet for 16 weeks and the HFHC diet‐fed mice treated with cordycepin into two clusters (Fig. 5A). GSEA systematically revealed that cellular signaling pathways related to inflammation (such as the mitogen‐activated protein kinase signaling and leukocyte transendothelial migration pathways), lipid metabolism (such as the fatty acid elongation and insulin signaling pathways), apoptosis (the P53 signaling pathway), and fibrosis (the extracellular matrix–receptor interaction and TGFβ signaling pathways) were enriched (Fig. 5B) and significantly down‐regulated by cordycepin treatment (Fig. 5C). Furthermore, a heatmap based on GSEA revealed that the expression of hepatic genes related to inflammation, lipid metabolism, apoptosis, and fibrosis pathways was significantly down‐regulated by cordycepin treatment in mice administered the HFHC diet for 16 weeks (Fig. 5D).
FIG. 5.
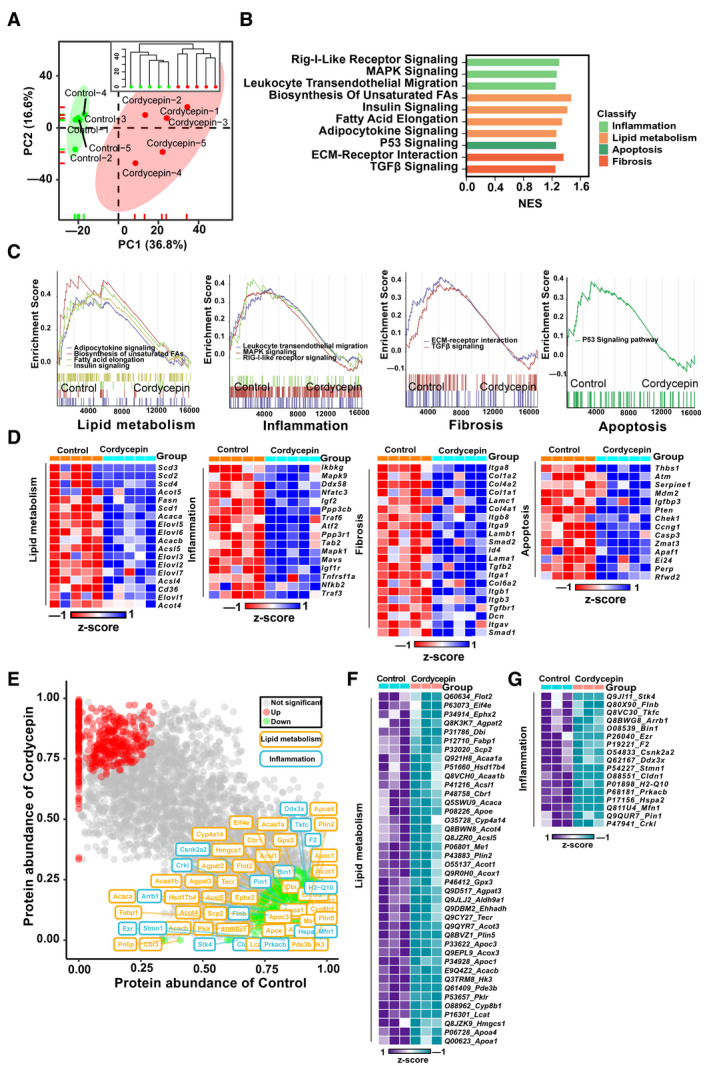
RNA‐seq and proteomics analyses revealed key differential targets between the vehicle‐treated and cordycepin‐treated HFHC diet–fed mice. (A) PCA and unsupervised hierarchical clustering analysis of the RNA‐seq data from the mice fed the HFHC diet (n = 5 mice per group) for 16 weeks. (B,C) GSEA pathway enrichment analysis of pathways related to inflammation, lipid metabolism, apoptosis, and fibrosis. (D) Heatmap of lipid metabolism–related, fibrosis‐related, inflammation‐related, and apoptosis‐related gene expression profiles based on the RNA‐seq data set. n = 5 per group. (E) Protein levels in the livers of HFHC diet–fed mice treated with vehicle and cordycepin based on a proteomics assay. The font colors of the protein related to lipid metabolism and inflammation are yellow and blue, respectively. (F,G) Heatmap of representative differentially expressed proteins related to lipid metabolism and inflammation in the livers of HFHC diet–fed mice treated with vehicle and cordycepin based on a proteomics assay. Abbreviations: ECM, extracellular matrix; FA, fatty acid; MAPK, mitogen‐activated protein kinase; NES, normalized enrichment score; PC, principal component; Rig‐I, retinoic acid–inducible gene I.
Next, we adopted a nonbiased approach to identify and characterize the key proteins mediating the effect of cordycepin on the progression of NASH in mice. To this end, we performed proteomics analysis by mass spectrometry on livers from HFHC diet–fed mice treated with cordycepin or the vehicle control. Unbiased Ingenuity Pathway Analysis of the proteomics data identified lipid metabolism and inflammation among the top represented canonical pathways, particularly in livers from cordycepin‐treated mice compared to livers from vehicle‐treated mice after 16 weeks of HFHC diet feeding (Fig. 5E). Representative differentially expressed proteins related to lipid metabolism and inflammation are presented in heatmaps (Fig. 5F,G). Taken together, consistent with the results obtained with the HFHC model, these data strongly demonstrate that cordycepin attenuates hepatic steatosis, inflammation, and fibrosis under conditions of metabolic stress, exerting potent anti‐NASH effects.
Cordycepin Activates AMPK in Hepatocytes and Mice Under Metabolic Stress
To further explore the mechanism by which cordycepin protects against NASH, we analyzed the transcriptomes of L02 cells treated with cordycepin after PA stimulation and mice treated with cordycepin after HFHC for 16 weeks. RNA‐seq analysis showed that cordycepin treatment affected various signaling pathways related to metabolism, inflammation, and fibrosis both in vitro and in vivo (Fig. 6A). Subsequently, we analyzed the data in combination with the transcriptomes of cells and mice and identified that six signaling pathways were consistently affected and that the scores of the AMPK signaling pathway were superior to those of other pathways (Fig. 6B). Multi‐omics (transcriptomic, proteomic, and phosphoproteomic) analyses showed that the core kinases in the AMPK pathway based on the available kinase‐substrate databases were significantly correlated with the targets of lipid metabolism, inflammation, fibrosis, and apoptosis (Fig. 6C). AMPK is a sensor of cellular energy status that exists in heterotrimeric complexes comprising a catalytic α‐subunit and regulatory β‐subunits and γ‐subunits.( 26 ) Phosphorylation of Thr172 in its α‐subunit activates the kinase domain of AMPK, which in turn phosphorylates other target proteins. ACC is a major substrate for AMPK; as such, the phosphorylation status of ACC is often assayed as a proxy for AMPK activation. Thus, to determine whether cordycepin prevents NASH by regulating AMPK activity, we first measured the levels of p‐AMPK (Thr172) and p‐ACC in hepatocytes. Phosphorylation of AMPK on its autophosphorylation site in the α‐subunit was induced by cordycepin treatment in hepatocytes (Fig. 6D). Furthermore, cordycepin treatment also caused substantial increases in the levels of p‐ACC, suggesting that cordycepin is able to activate AMPK signaling in hepatocytes subjected to PA stimulation (Fig. 6D). We also found that treatment with cordycepin (100 and 200 mg/kg) significantly increased the levels of p‐AMPK and p‐ACC in liver tissues of mice fed the HFD for 24 weeks (Fig. 6E) and the HFHC diet for 16 weeks (Fig. 6F), respectively.
FIG. 6.
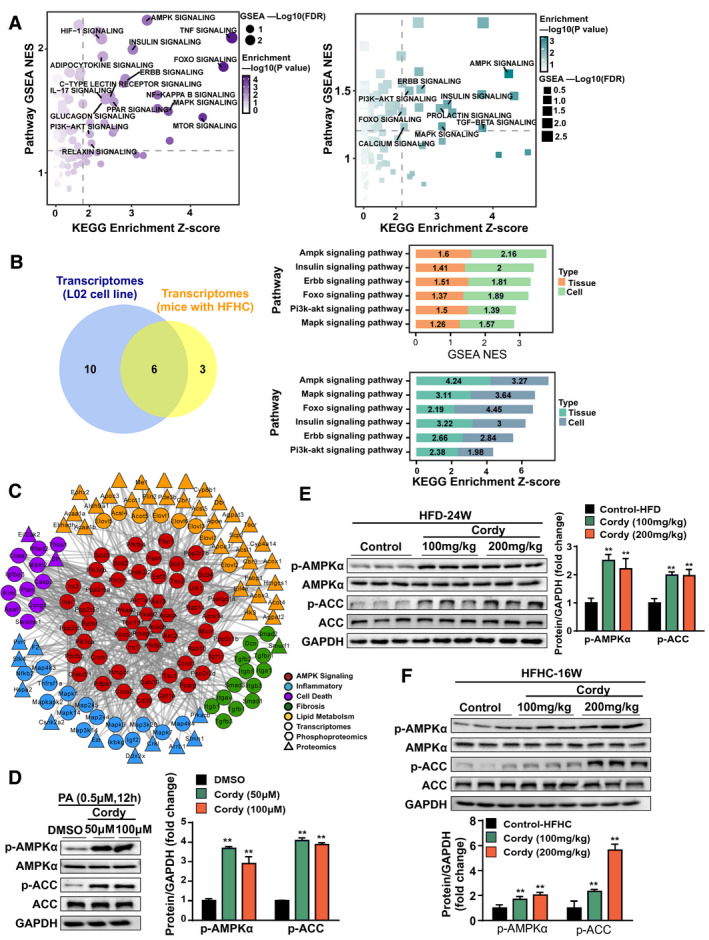
Association analysis of the transcriptomes and phosphoproteomics revealed that the AMPK pathway may be the target of cordycepin. (A) GSEA and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses of the transcriptomes of cells (in purple) and HFHC diet–fed mouse liver samples (in green). (B) Venn diagram and the corresponding scores of the intersecting pathways based on the transcriptomic data from L02 cell and HFHC diet–fed mouse liver samples. (C) Core kinases in the AMPK pathway based on the available kinase‐substrate databases. (D) Western blotting of proteins involved in the AMPKα‐mediated signaling cascade in cells. (E,F) Western blotting of proteins involved in the AMPKα‐mediated signaling cascade in HFD‐fed and HFFC‐fed mice, respectively. GAPDH served as a loading control. The data are presented as the mean ± SD. Significant differences between the DMSO/control group and the cordycepin group: *P < 0.05, **P < 0.01. Abbreviations: Cordy, cordycepin; ERBB, erythroblastic leukemia viral oncogene homolog; FDR, false discovery rate; FOXO, forkhead box O; HIF‐1, hypoxia‐inducible factor 1; KEGG, Kyoto Encyclopedia of Genes and Genomes; MAPK, mitogen‐activated protein kinase; MTOR, mammalian target of rapamycin; NES, normalized enrichment score; PI3K, phosphoinositide 3‐kinase.
Inhibition of AMPK Phosphorylation Abolishes Cordycepin‐Mediated Suppression of Lipid Accumulation and Inflammation in Hepatocytes Treated With PO
To investigate whether cordycepin mediates lipid accumulation dependent on regulation of AMPK activation, we tested the effect of the AMPK inhibitor Compound C (CC) on the phosphorylation of AMPK and its downstream target ACC. Oil red O staining showed that the ability of cordycepin (50 μM) to reduce lipid accumulation in hepatocytes treated with PO was almost abolished by pretreatment with CC (Fig. 7A). In addition, treatment with cordycepin significantly decreased the intracellular TG and TC levels, but these effects were impaired by pretreatment with CC (Fig. 7B). To further investigate whether AMPKα is required for the hypolipidemic and anti‐inflammatory effects of cordycepin in PA‐treated hepatocytes, quantitative real‐time PCR assays were performed to examine the mRNA levels of metabolic lipids and inflammatory genes in cordycepin‐treated hepatocytes in the presence or absence of CC. The down‐regulation of fatty acid synthesis and uptake genes (Fasn, Cd36, Scd1, and Pparγ) caused by cordycepin was reversed by CC. Similarly, the up‐regulation of fatty acid β‐oxidation and transportation genes (Pparα and carnitine palmitoyltransferase 1α [Cpt1α]) caused by cordycepin was attenuated by CC (Fig. 7C). Furthermore, the reductions in the expression of inflammatory genes (Il6, Tnfα, Il1b, and Ccl2) mediated by cordycepin (50 μM) were also abrogated by CC (Fig. 7C). Moreover, pretreatment with CC almost completely suppressed the cordycepin‐induced phosphorylation of AMPK and ACC without changing the expression levels of total AMPK, ACC, and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) in hepatocytes cultured with PA for 12 hours (Fig. 7D). In vitro assays further confirmed that activation of the AMPK pathway and attenuation of lipid accumulation by cordycepin were almost abrogated in AMPK knockout L02 cells (Supporting Fig. S4A,B). These data suggest that cordycepin regulates genes associated with lipid metabolism and inflammation in hepatocytes through an AMPKα‐mediated pathway.
FIG. 7.
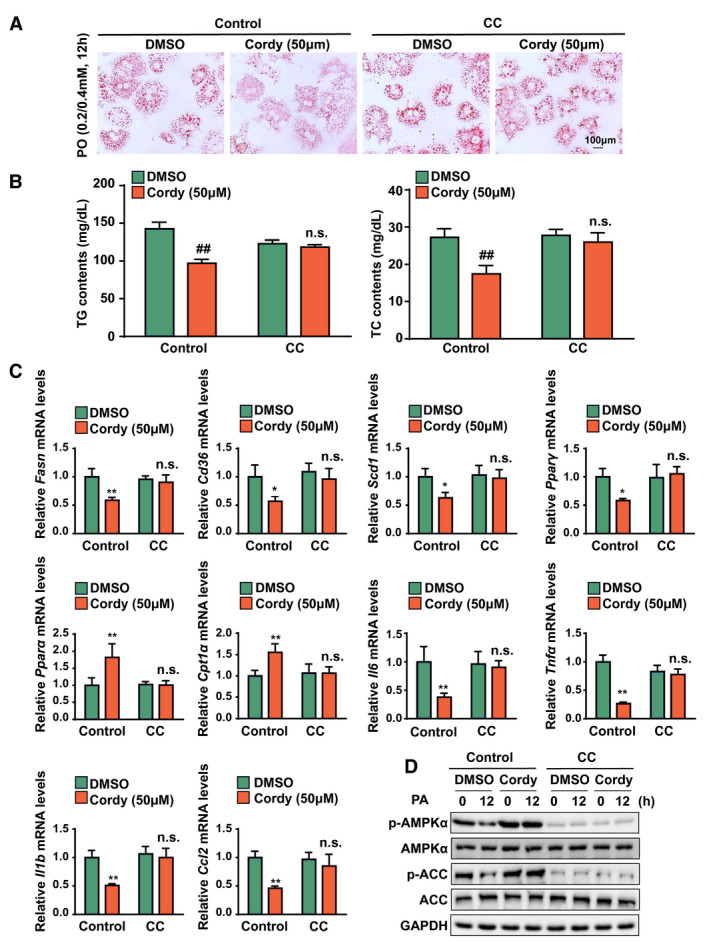
AMPK activation is responsible for the protective effects of cordycepin on lipid accumulation and inflammation in hepatocytes under metabolic stress. (A) Oil red O staining showing the degrees of lipid accumulation in DMSO‐treated and cordycepin (50 μM)–treated primary hepatocytes after PO (0.2 mM PA and 0.4 mM OA) stimulation for 12 hours in the presence or absence of CC. (B) TC and TG levels in primary hepatocytes in the indicated groups stimulated with PO (0.5 mM PA and 1.0 mM OA) for 12 hours. n = 3 per group. (C) Relative mRNA levels of the indicated lipid metabolism–related genes (Fasn, Cd36, Scd1, Pparγ, Ppara, and Cpt1a) and inflammatory factors (Il6, Tnfα, Il1b, and Ccl2) in primary hepatocytes treated with PA. The relative mRNA expression was normalized to that of Actb. n = 4 per group. (D) Western blot analysis of the total and phosphorylated protein levels of AMPKα and ACC after treatment with PA for 0 or 12 hours. GAPDH was the loading control for (D). n = 3 per group. The data are presented as the mean ± SD. Significant difference between the control‐DMSO group and the control‐cordycepin group for (B), ## P < 0.01; n.s., no significant difference between the CC‐DMSO group and the CC‐cordycepin group for (B); significant difference between the control‐DMSO group and the control‐cordycepin group for (C): *P < 0.05, **P < 0.01; n.s., no significant difference between the CC‐DMSO group and the CC‐cordycepin group for (C). Abbreviation: Cordy, cordycepin.
AMPK Pathway Activation is Required For Cordycepin‐Mediated Improvement of HFD‐Induced Hepatic Steatosis and Inflammation in Mice
To test whether activation of the AMPK pathway is required for the protective effects of cordycepin against hepatic steatosis and inflammation, 24‐week HFD‐fed mice were administered CC at week 15 for 1 week and then cotreated with cordycepin for an additional 8 weeks (Supporting Fig. S5A). Body weight did not differ among the different groups (Supporting Fig. S5B), but CC pretreatment abrogated the cordycepin‐mediated reductions in LW and the LW/BW ratio (Fig. 8A). However, the white adipose weight and the white adipose weight/body weight ratio did not differ between the cordycepin and CC treatment groups after 24 weeks of HFD feeding (Supporting Fig. S5C). The reductions in hepatic and serum lipid (TG and TC) levels mediated by cordycepin were also abrogated by CC in mice fed the HFD for 24 weeks (Fig. 8B; Supporting Fig. S5D). H&E and oil red O staining showed that CC pretreatment abrogated the alleviation of hepatocyte damage, inflammatory infiltration, and lipid accumulation in the livers of mice fed the HFD (Fig. 8C). We next examined lipid metabolism–related and inflammatory genes in the livers of HFD‐fed mice by quantitative real‐time PCR assays. The regulatory effects of cordycepin on the expression of lipid metabolism–related genes (Cd36, Fasn, Scd1, and Pparα) and inflammatory genes (Ccl2, Il1b, and Il6) were abolished by CC pretreatment (Fig. 8D,E). Consistently, the reductions in the serum levels of ALT and AST caused by cordycepin in HFD‐fed mice were also abrogated by CC pretreatment (Fig. 8F). CC significantly repressed the cordycepin‐induced phosphorylation of hepatic AMPKα and ACC in HFD‐fed mice (Fig. 8G). Collectively, these data indicate that CC completely eliminates the protective effects of cordycepin in mice with NASH, suggesting that cordycepin exerts its anti‐NASH effects primarily through AMPK‐mediated hypolipidemia and anti‐inflammatory mechanisms.
FIG. 8.
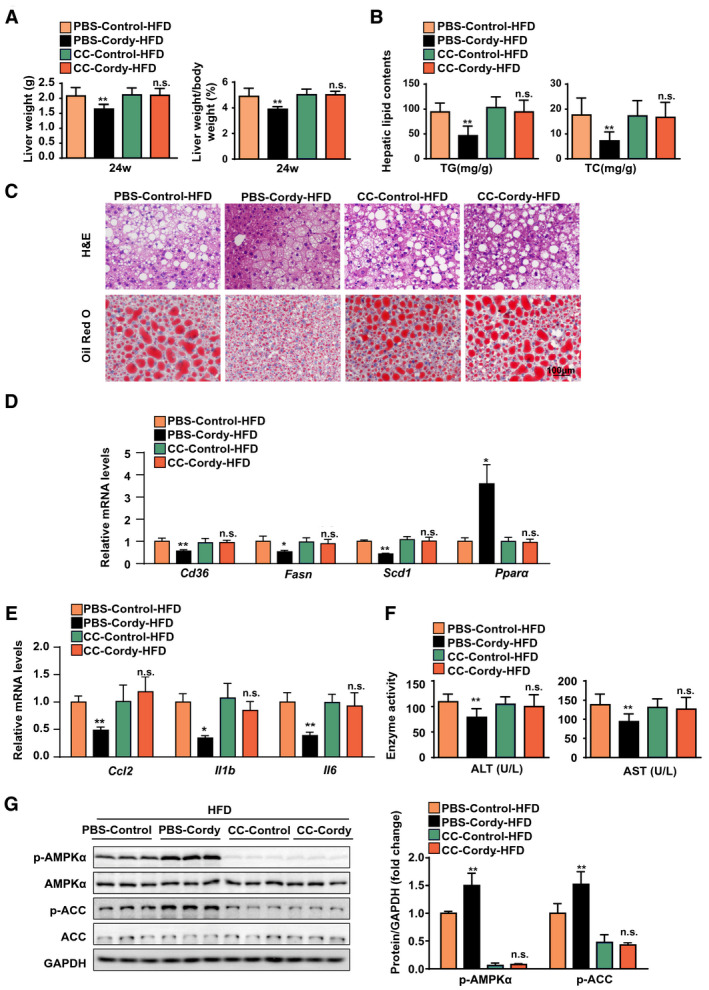
The AMPK pathway is required for cordycepin to improve HFD‐induced liver inflammation and steatosis in mice. (A) LWs and LW/BW ratios of the indicated groups. n = 10 per group. (B) Hepatic lipid levels in the indicated groups. n = 10 per group. (C) Representative H&E‐stained and oil red O–stained liver sections from the indicated HFD‐fed mice. n = 6 mice per group. Scale bar, 100 μm. (D,E) Quantitative real‐time PCR analysis of the expression of lipid‐related genes (Cd36, Fasn, Scd1, and Pparα) and inflammation‐related factors (Ccl2, Il1b, and Il6) in the indicated groups. The relative mRNA expression was normalized to that of Actb. n = 4 per group. (F) Serum ALT and AST concentrations in the indicated groups. n = 10 per group. (G) Western blot analysis of the total and phosphorylated protein levels of AMPKα and ACC in the indicated groups. n = 3 per group. GAPDH served as a loading control. The data are presented as the mean ± SD. Significant difference between the phosphate‐buffered saline‐control‐HFD group and the phosphate‐buffered saline‐cordycepin‐HFD group: *P < 0.05, **P < 0.01. Abbreviation: Cordy, cordycepin; n.s., no significant difference between the CC‐control‐HFD group and the CC‐cordycepin‐HFD group; PBS, phosphate‐buffered saline.
Discussion
NASH, which is characterized by hepatic steatosis, inflammation, liver fibrosis, and liver damage, has become a leading cause of liver transplantation and liver‐associated death.( 2 ) In the present study, we have shown that obese mice fed the HFD and the HFHC diet are prone to rapid development of severe obesity, NAFLD, and NASH. We have also demonstrated that cordycepin is able to ameliorate hepatic steatosis, inflammation, and fibrosis through activation of the AMPK signaling pathway.
Lifestyle modification is beneficial for treating NAFLD and should be considered the cornerstone of long‐term management.( 27 ) However, poor adherence to lifestyle modification makes NAFLD management a difficult task. Pharmaceutical treatment is an essential component of the treatment of NAFLD and NASH, yet no drugs to date have been specifically approved.( 28 ) The expanding knowledge regarding the beneficial effects of traditional Chinese medicine on metabolic diseases (especially NAFLD and NASH), coupled with the extremely long duration of modern drug discovery processes, has driven researchers to pursue potential efficacious and safe therapies using traditional Chinese medicine. Cordyceps militaris, especially cordycepin, has been widely used as a kind of crude drug and folk tonic food for the treatment of diseases associated with inflammation and oxidative injury, such as hepatotoxicity and liver injury.( 20, 29 ) Cordycepin is useful for the treatment of metabolic disorders such as hyperlipidemia( 30 ) and hyperglycemia.( 31 ) The lipid‐decreasing effects of cordycepin might be due to activation of AMPK and inhibition of white adipose tissue differentiation. Cordycepin is a promising agent for future potential clinical applications. In the present study, we have demonstrated that cordycepin mitigates metabolic stress–induced NASH by preventing steatosis, inflammation, and fibrosis.
Hepatocytes are responsible for the major physiological and metabolic functions of the liver.( 32 ) To fuel these metabolic and nonmetabolic functions, hepatocytes take up and use large amounts of lipids such as free fatty acids and lipoprotein remnants. Hepatocytes are the main cells in the liver that store TGs and the cells in which lipids accumulate in the context of NAFLD. In obesity, the uptake and synthesis of lipids exceed the flux through use or secretion pathways, leading to a net increase in intrahepatic lipids.( 33 ) In line with previous studies,( 18 ) our current study demonstrates that cordycepin has excellent antihyperlipidemic effects in rodent hepatocytes and livers under conditions of metabolic stress.
As a hallmark of the pathogenesis of NASH, the inflammatory response involves two critical events: damage to hepatocytes and the release of numerous types of proinflammatory cytokines. The hepatic and serum levels of proinflammatory cytokines including IL‐6, TNFα, IL‐1β, CCL5, and CXCL10 are associated with the severity of NASH. Inflammatory cells and cytokines are both implicated in NASH pathogenesis. In addition, both the innate and adaptive immune systems are involved in the pathogenesis of NASH; however, most attention has focused on the innate immune system. The proinflammatory pathways involving apoptosis signaling kinase‐1–c‐Jun N‐terminal kinase, mitogen‐activated protein kinases, extracellular signal–regulated kinase, and NF‐κB are potent mediators of inflammation and thus potential targets for therapy.( 34 ) Previous studies have demonstrated that cordycepin suppresses the production of proinflammatory cytokines in LPS‐stimulated macrophages.( 35 ) Moreover, cordycepin modulates macrophage polarization by down‐regulating M1 cytokines and up‐regulating M2 cytokines.( 36 ) Recent studies have shown that the anti‐inflammatory effects of cordycepin may be due to suppression of NF‐κB and NLR family pyrin domain containing 3 (NLRP3) inflammasome activation; the NLRP3 inflammasome is the most important regulator of the inflammatory process in macrophages.( 35, 37 ) In the current study, cordycepin inhibited NF‐κB signaling pathway activation and attenuated the secretion of proinflammatory cytokines in hepatocytes under metabolic stress.
Changes in metabolism can cause physiological dysfunction, which in turn may lead to diseases such as diabetes, NASH, and cardiovascular diseases. AMPK is a key metabolic regulator that senses energy status and controls energy expenditure and storage.( 38 ) AMPK acts as a central regulator of fatty acid, cholesterol, and glucose homeostasis through phosphorylation of metabolism‐regulating enzymes, including ACC, glycogen synthase, glucose transporter 4, HMG‐CoA reductase, hormone‐sensitive lipase, and mammalian target of rapamycin. AMPK is a heterotrimer of a catalytic α‐chain and the regulatory β‐chain and γ‐chain.( 39 ) AMPK down‐regulation is associated with NAFLD. Consistent with this association, mice on NASH‐inducing diets had reduced AMPK activity in the current study. Furthermore, activation of hepatic AMPK attenuates HFD‐induced NAFLD.( 40 ) Studies on liver‐specific AMPK knockout mice have demonstrated that loss of AMPK exaggerates diet‐induced NASH pathology,( 41 ) suggesting AMPK signaling activation as a therapeutic target for NASH treatment. Furthermore, a recent study showed that low‐dose sorafenib safely suppresses NASH progression in mice and monkeys and that AMPK activation is required for the therapeutic effects of sorafenib in NASH.( 42 ) AMPK has been reported to be an anti‐inflammatory protein that is involved in the regulation of energy homeostasis,( 43 ) and cordycepin has been revealed to activate AMPK by binding with the α1‐subunit and γ1‐subunit near the autoinhibitory domain of AMPK.( 44 ) In our current study, cordycepin significantly increased AMPK phosphorylation and inhibited NF‐κB activation and inflammation in the context of NASH in vivo and in vitro. ACC, as a downstream target of AMPK, is a key enzyme in the process of lipid metabolism. AMPK inactivates the ACCs by phosphorylating specific serine residues in the N terminus in vivo.( 45 ) In contrast, CC, an AMPK inhibitor, abolished the protective effects of cordycepin against NASH in the current study. In a recent study, cordycepin was identified to activate AMPK through interaction with the γ‐subunit only in the context of lipid regulation in HepG2 cancer cells.( 46 ) In our study, we further identified that cordycepin can activate AMPK through interaction with the α‐subunit of AMPK. However, the mechanisms by which cordycepin activates AMPK remain unknown.
Adenosine, an endogenous purine nucleoside, mediates a wide range of physiological and pathological functions by interacting with four cell surface receptor subtypes: A1, A2a, A2b and A3.( 47 ) In the liver, adenosine‐mediated protection relies upon stimulation of A2a receptors, which protects hepatocytes against saturated fatty acid–induced lipotoxicity and the development of NASH.( 48, 49 ) Adenosine can activate brown adipose tissue and recruit beige adipocytes through A2a receptors.( 50 ) Furthermore, A2a receptor–mediated FGF21 expression is most likely mediated through promotion of AMPK phosphorylation and up‐regulation of downstream PPAR‐γ coactivator 1α in brown adipocytes.( 51 ) Cordycepin is an analogue of adenosine, and previous studies have demonstrated that it may exert its pharmacological effects through the A2a receptor.( 52 ) In our current study, cordycepin promoted AMPK phosphorylation and activated the AMPK downstream pathway. However, future studies investigating whether cordycepin stimulates AMPK phosphorylation through the A2a receptor are warranted.
In conclusion, the current study identifies cordycepin as a natural activator of AMPK that mitigates NASH by preventing hepatic steatosis, inflammation, and fibrosis. Therefore, cordycepin may be an agent for the improvement of therapeutic outcomes in patients with NASH.
Supporting information
Supplementary Material
Supported by grants from the National Key R&D Plan of China’s "Research on Modernization of Traditional Chinese Medicine" program (2018YFC1704200), the National Natural Science Foundation of China (81530102, 81830113, 81870420, and 82070590), and the Major Basic and Applied Basic Research Projects in Guangdong Province of China (2019B030302005).
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1.Rinella ME, Tacke F, Sanyal AJ, Anstee QM; Participants of the AASLD/EASL Workshop. Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. J Hepatol 2019;71:823‐833. [DOI] [PubMed] [Google Scholar]
- 2.Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA 2020;323:1175‐1183. [DOI] [PubMed] [Google Scholar]
- 3.Cai J, Xu M, Zhang X, Li H. Innate immune signaling in nonalcoholic fatty liver disease and cardiovascular diseases. Annu Rev Pathol 2019;14:153‐184. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 5.Younossi ZM. Patient‐reported outcomes and the economic effects of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: the value proposition. Hepatology 2018;68:2405‐2412. [DOI] [PubMed] [Google Scholar]
- 6.Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non‐alcoholic fatty liver disease. Metabolism 2016;65:1017‐1025. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Zhou F, Wang W, Zhang X‐J, Ji Y‐X, Zhang P, et al. Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology 2020;71:1851‐1864. [DOI] [PubMed] [Google Scholar]
- 8.Zhou F, Zhou J, Wang W, Zhang X‐J, Ji Y‐X, Zhang P, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta‐analysis. Hepatology 2019;70:1119‐1133. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy S, Syed BA. Nonalcoholic steatohepatitis (NASH) drugs market. Nat Rev Drug Discov 2016;15:745‐746. [DOI] [PubMed] [Google Scholar]
- 10.Vilar‐Gomez E, Martinez‐Perez Y, Calzadilla‐Bertot L, Torres‐Gonzalez A, Gra‐Oramas B, Gonzalez‐Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367‐378.e5; quiz e14‐15. [DOI] [PubMed] [Google Scholar]
- 11.Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology 2015;149:379‐388; quiz e15‐16. [DOI] [PubMed] [Google Scholar]
- 12.Tuli HS, Sharma AK, Sandhu SS, Kashyap D. Cordycepin: a bioactive metabolite with therapeutic potential. Life Sci 2013;93:863‐869. [DOI] [PubMed] [Google Scholar]
- 13.Qing R, Huang Z, Tang Y, Xiang Q, Yang F. Cordycepin negatively modulates lipopolysaccharide‐induced cytokine production by up‐regulation of heme oxygenase‐1. Int Immunopharmacol 2017;47:20‐27. [DOI] [PubMed] [Google Scholar]
- 14.Won K‐J, Lee S‐C, Lee C‐K, Lee HM, Lee SH, Fang Z, et al. Cordycepin attenuates neointimal formation by inhibiting reactive oxygen species–mediated responses in vascular smooth muscle cells in rats. J Pharmacol Sci 2009;109:403‐412. [DOI] [PubMed] [Google Scholar]
- 15.Cao T, Xu R, Xu Y, Liu Y, Qi D, Wan Q. The protective effect of cordycepin on diabetic nephropathy through autophagy induction in vivo and in vitro. Int Urol Nephrol 2019;51:1883‐1892. [DOI] [PubMed] [Google Scholar]
- 16.An Y, Li Y, Wang X, Chen Z, Xu H, Wu L, et al. Cordycepin reduces weight through regulating gut microbiota in high‐fat diet–induced obese rats. Lipids Health Dis 2018;17:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon SY, Park SJ, Park YJ. The anticancer properties of cordycepin and their underlying mechanisms. Int J Mol Sci 2018;19:3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo P, Kai QU, Gao J, Lian Z‐Q, Wu C‐M, Wu C‐A, et al. Cordycepin prevents hyperlipidemia in hamsters fed a high‐fat diet via activation of AMP‐activated protein kinase. J Pharmacol Sci 2010;113:395‐403. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, Wu B, Wang X, Ma F, Li Y, An Y, et al. Cordycepin regulates body weight by inhibiting lipid droplet formation, promoting lipolysis and recruiting beige adipocytes. J Pharm Pharmacol 2019;71:1429‐1439. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Zhong L, Zhu H, Wang F. The protective effect of cordycepin on D‐galactosamine/lipopolysaccharide‐induced acute liver injury. Mediators Inflamm 2017;2017:3946706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cha JY, Ahn HY, Cho YS, Je JY. Protective effect of cordycepin‐enriched Cordyceps militaris on alcoholic hepatotoxicity in Sprague‐Dawley rats. Food Chem Toxicol 2013;60:52‐57. [DOI] [PubMed] [Google Scholar]
- 22.Guo Z, Chen W, Dai G, Huang Y. Cordycepin suppresses the migration and invasion of human liver cancer cells by downregulating the expression of CXCR4. Int J Mol Med 2020;45:141‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343‐1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gautheron J, Gores GJ, Rodrigues CMP. Lytic cell death in metabolic liver disease. J Hepatol 2020;73:394‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S, Verma AK, Rani R, Sharma A, Wang J, Shah SA, et al. Hepatic deficiency of augmenter of liver regeneration predisposes to nonalcoholic steatohepatitis and fibrosis. Hepatology 2020;72:1586‐1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawley SA, Ross FA, Russell FM, Atrih A, Lamont DJ, Hardie DG. Mechanism of activation of AMPK by cordycepin. Cell Chem Biol 2020;27:e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen MM, Cai JJ, Yu Y, She ZG, Li H. Current and emerging approaches for nonalcoholic steatohepatitis treatment. Gene Expr 2019;19:175‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konerman MA, Jones JC, Harrison SA. Pharmacotherapy for NASH: current and emerging. J Hepatol 2018;68:362‐375. [DOI] [PubMed] [Google Scholar]
- 29.Zhang T, Guo J, Gu J, Chen K, Li H, Wang J. Protective role of mTOR in liver ischemia/reperfusion injury: involvement of inflammation and autophagy. Oxid Med Cell Longev 2019;2019:7861290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo P, Kai Q, Gao J, Lian ZQ, Wu CM, Wu CA, et al. Cordycepin prevents hyperlipidemia in hamsters fed a high‐fat diet via activation of AMP‐activated protein kinase. J Pharmacol Sci 2010;113:395‐403. [DOI] [PubMed] [Google Scholar]
- 31.Ma L, Zhang S, Du M. Cordycepin from Cordyceps militaris prevents hyperglycemia in alloxan‐induced diabetic mice. Nutr Res 2015;35:431‐439. [DOI] [PubMed] [Google Scholar]
- 32.Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol 2017;27:R1147‐R1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finck BN. Targeting metabolism, insulin resistance, and diabetes to treat nonalcoholic steatohepatitis. Diabetes 2018;67:2485‐2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman SL, Neuschwander‐Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J, Li YZ, Hylemon PB, Zhang LY, Zhou HP. Cordycepin inhibits LPS‐induced inflammatory responses by modulating NOD‐like receptor protein 3 inflammasome activation. Biomed Pharmacother 2017;95:1777‐1788. [DOI] [PubMed] [Google Scholar]
- 36.Shin S, Moon S, Park Y, Kwon J, Lee S, Lee C‐K, et al. Role of cordycepin and adenosine on the phenotypic switch of macrophages via induced anti‐inflammatory cytokines. Immune Netw 2009;9:255‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HG, Shrestha B, Lim SY, Yoon DH, Chang WC, Shin DJ, et al. Cordycepin inhibits lipopolysaccharide‐induced inflammation by the suppression of NF‐kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol 2006;545:192‐199. [DOI] [PubMed] [Google Scholar]
- 38.Hardie DG. AMP‐activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev 2011;25:1895‐1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weng SY, Schuppan D. AMPK regulates macrophage polarization in adipose tissue inflammation and NASH. J Hepatol 2013;58:619‐621. [DOI] [PubMed] [Google Scholar]
- 40.Boudaba N, Marion A, Huet C, Pierre R, Viollet B, Foretz M. AMPK re‐activation suppresses hepatic steatosis but its downregulation does not promote fatty liver development. EBioMedicine 2018;28:194‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao P, Sun X, Chaggan C, Liao Z, Wong K, He F, et al. An AMPK‐caspase‐6 axis controls liver damage in nonalcoholic steatohepatitis. Science 2020;367:652‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jian C, Fu J, Cheng XU, Shen L‐J, Ji Y‐X, Wang X, et al. Low‐dose sorafenib acts as a mitochondrial uncoupler and ameliorates nonalcoholic steatohepatitis. Cell Metab 2020;31:e811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carling D. AMPK signalling in health and disease. Curr Opin Cell Biol 2017;45:31‐37. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Chen Z, Jiang Z, Luo P, Liu L, Huang YU, et al. Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents. Nat Commun 2019;10:2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP‐activated protein kinase. Biochem Soc Trans 2002;30:1064‐1070. [DOI] [PubMed] [Google Scholar]
- 46.Wu C, Guo Y, Su Y, Zhang X, Luan H, Zhang X, et al. Cordycepin activates AMP‐activated protein kinase (AMPK) via interaction with the gamma1 subunit. J Cell Mol Med 2014;18:293‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borea PA, Gessi S, Merighi S, Varani K. Adenosine as a multi‐signalling guardian angel in human diseases: when, where and how does it exert its protective effects? Trends Pharmacol Sci 2016;37:419‐434. [DOI] [PubMed] [Google Scholar]
- 48.Imarisio C, Alchera E, Sutti S, Valente G, Boccafoschi F, Albano E, et al. Adenosine A(2a) receptor stimulation prevents hepatocyte lipotoxicity and non‐alcoholic steatohepatitis (NASH) in rats. Clin Sci (Lond) 2012;123:323‐332. [DOI] [PubMed] [Google Scholar]
- 49.Alchera E, Rolla S, Imarisio C, Bardina V, Valente G, Novelli F, et al. Adenosine A2a receptor stimulation blocks development of nonalcoholic steatohepatitis in mice by multilevel inhibition of signals that cause immunolipotoxicity. Transl Res 2017;182:75‐87. [DOI] [PubMed] [Google Scholar]
- 50.Gnad T, Scheibler S, von Kügelgen I, Scheele C, Kilić A, Glöde A, et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 2014;516:395‐399. [DOI] [PubMed] [Google Scholar]
- 51.Ruan CC, Kong LR, Chen XH, Ma Y, Pan XX, Zhang ZB, et al. A2A receptor activation attenuates hypertensive cardiac remodeling via promoting brown adipose tissue‐derived FGF21. Cell Metab 2018;28:476‐489.e5. [DOI] [PubMed] [Google Scholar]
- 52.Cao Z‐P, Dai D, Wei P‐J, Han Y‐Y, Guan Y‐Q, Li H‐H, et al. Effects of cordycepin on spontaneous alternation behavior and adenosine receptors expression in hippocampus. Physiol Behav 2018;184:135‐142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


