Abstract
Periodontitis has been associated with many systemic diseases and conditions, including metabolic syndrome. Metabolic syndrome is a cluster of conditions that occur concomitantly and together they increase the risk of cardiovascular disease and double the risk of type 2 diabetes. In this review, we focus on the association between metabolic syndrome and periodontitis; however, we also include information on diabetes mellitus and cardiovascular disease, since these two conditions are significantly intertwined with metabolic syndrome. With regard to periodontitis and metabolic syndrome, to date, the vast majority of studies point to an association between these two conditions and also demonstrate that periodontitis can contribute to the development of, or can worsen, metabolic syndrome. Evaluating the effect of metabolic syndrome on the salivary microbiome, data presented herein support the hypothesis that the salivary bacterial profile is altered in metabolic syndrome patients compared with healthy patients. Considering periodontitis and these three conditions, the vast majority of human and animal studies point to an association between periodontitis and metabolic syndrome, diabetes, and cardiovascular disease. Moreover, there is evidence to suggest that metabolic syndrome and diabetes can alter the oral microbiome. However, more studies are needed to fully understand the influence these conditions have on each other.
Keywords: cardiovascular disease, diabetes, metabolic syndrome, microbiome and inflammation, periodontitis
1. INTRODUCTION
Periodontitis is a “chronic inflammatory disease associated with dysbiotic plaque biofilms and characterized by a progressive destruction of the tooth supporting apparatus”.1 Periodontitis affects 42.2% of the US population aged older than 30 years and 59.8% of those aged older than 65 years.2 According to the World Health Organization, periodontitis is the major cause of tooth loss in adults.3 Periodontitis pathogenesis is multifactorial with environmental, microbial, and host involvement affecting disease outcomes. Many systemic conditions have been associated with periodontitis, including diabetes mellitus, cardiovascular disease, and metabolic syndrome.4, 5, 6, 7, 8, 9, 10, 11
Metabolic syndrome is a cluster of conditions that occur concomitantly and together they increase the risk of cardiovascular disease and double the risk of type 2 diabetes.12, 13, 14, 15 Metabolic syndrome affects approximately 34% of the US population16 and 10% of US adolescents.17 The prevalence of metabolic syndrome also increases with age and varies with ethnicity and gender.18 Several definitions of metabolic syndrome exist and differ slightly depending on the issuing agency. The most commonly utilized definition is provided by the National Cholesterol Education Program Adult Treatment Panel III. This definition requires that the individual has at least three of the following risk factors: (a) increased abdominal circumference, (b) low plasma levels of high‐density lipoprotein cholesterol, (c) increased values for plasma triglycerides, (d) elevated blood pressure, and (e) elevated glucose levels.19 Prediabetes is also accepted as part of metabolic syndrome because it is associated with insulin resistance and is highly predictive of new‐onset type 2 diabetes.20
The predominant underlying risk factors for metabolic syndrome appear to be abdominal obesity and insulin resistance. Other associated conditions are physical inactivity, aging, and hormonal imbalance.21 Among the risk factors, visceral adiposity appears to be a primary trigger for most of the pathways involved in metabolic syndrome.22 The exact mechanisms behind this systemic response remain unclear, but there is evidence to suggest that the inflammatory state caused by metabolic syndrome is associated with endothelial dysfunction, which might contribute to the increased risk of cardiovascular disease and type 2 diabetes.23, 24, 25
Herein, we will focus on the association between metabolic syndrome and periodontitis; however, we will also include information on diabetes and cardiovascular disease since these two conditions are significantly intertwined with metabolic syndrome.
2. METABOLIC SYNDROME AND PERIODONTITIS
2.1. Association between metabolic syndrome and periodontitis
Considerable interest has been focused on the connection between periodontitis and metabolic syndrome because both of these conditions are associated with systemic inflammation and insulin resistance26, 27 and they can potentially influence one another.
To date, there have been several longitudinal and cross‐sectional studies as well as a few meta‐analyses evaluating the relationship between these two conditions. The vast majority of the data point to an association between metabolic syndrome and periodontitis10, 11, 54 (Table 1). The three meta‐analyses31, 32, 55 were performed utilizing different parameters, but each of them found an association between metabolic syndrome and periodontitis, with an odds ratio ranging from 1.38 to 1.99. The meta‐analysis by Gobin et al55 included 39 studies and demonstrated an association between periodontitis and metabolic syndrome with a crude odds ratio of 1.99 (95% confidence interval: 1.75‐2.25) and an adjusted odds ratio of 1.46 (95% confidence interval: 1.31‐1.61). The authors of this study also performed a subgroup analysis of different countries. The pooled odds ratio was 1.68 (95% confidence interval: 1.41‐2) for Japan, 1.75 (95% confidence interval: 1.31‐2.34) for the USA, 1.81 (95% confidence interval: 1.35‐2.42) for Korea, and 2.29 (95% confidence interval: 1.53‐3.41) for China.55 The meta‐analysis conducted by Daudt et al,32 which included 26 studies with radiographic and clinical examination, also found an association between metabolic syndrome and periodontitis with an odds ratio of 1.38 (95% confidence interval: 1.26‐1.51). The authors went on to suggest that patients with metabolic syndrome are 38% more likely to have periodontitis.32 The systematic review/meta‐analysis by Nibali et al31 included 20 studies (one longitudinal study) and a total of 36 337 subjects. The authors concluded that there is a positive association between metabolic syndrome and periodontitis with an odds ratio of 1.71 (95% confidence interval: 1.42‐2.03). A critical review by Watanabe and Cho56 also concluded that there is a positive association between metabolic syndrome and periodontitis. In addition, several animal studies utilizing different periodontitis models demonstrated that rodents with metabolic syndrome or obesity, as a result of being fed a high‐fat diet, also exhibited exacerbated periodontal bone loss.57, 58, 59
TABLE 1.
The role of metabolic syndrome in periodontitis
| Longitudinal studies | ||||||
|---|---|---|---|---|---|---|
| Authors | Duration (y) | Age (y) | Sample size |
Metabolic syndrome parameters |
Periodontitis parameters |
Results |
| Morita et al40 | 4 | 20 to 56 | 1023 | BP, TG, HDL, TC, FPG, and BMI | PD (CPI) | Periodontitis was associated with a positive conversion of metabolic syndrome components |
| Bullon et al41 | 3 | 20 to 44 | 188 | Pregnancy, weight, BMI, BP, HbA1c, CRP, FPG, TG, TC, LDL, and HDL | Plaque, BOP, PD, recession | There is an association between periodontitis and metabolic syndrome |
| Iwasaki et al35 | 3 | ≥70 | 125 | Abd obesity, BP, TG, HDL, and FPG | CAL | Metabolic syndrome may be a risk factor for periodontitis in older Japanese individuals |
| Kaye et al42 | 33 | 760 | FPG, BP, TG, WC, and HDL | PD, CAL, ABL, tooth mobility | Metabolic syndrome may play a role in the development or worsening of periodontitis | |
| Nascimento et al34 | 8 or 16 | 539 | FPG, HDL, TG, WC, and BP | BOP, PD, CAL | Positive association between metabolic syndrome and periodontitis, when the multiple dimensions of both diseases were accounted in latent variables. When metabolic syndrome and periodontitis were treated as observed variables, no association was detected | |
| Sakurai et al43 | 2 | ≥30 | 390 | TG, HDL, BP, FPG, and WC | CPI | The prevalence of individuals with more positive metabolic syndrome components was higher in those with persistent/progressive periodontitis than in those with no/improved periodontitis |
| Tegelberg et al44 | 15 | 1964 | WC, TG, HDL, BP, and FPG | PD and ABL | Metabolic syndrome was associated in an exposure‐dependent manner with periodontitis | |
| Adachi et al45 | 1 | ≥35 | 136 |
WC, TG, HDL, BP, and FPG |
CPI |
There were no associations between periodontitis and the development of metabolic syndrome |
| Cross‐sectional/case‐control studies | |||||
|---|---|---|---|---|---|
| Authors | Age of patients (y) | Number of patients | Metabolic syndrome parameters | Perio Parameters | Results |
| Borges et al65 | 30 to 92 | 1315 |
BMI, dyslipidemia, BP and FPG |
CPI |
There were no associations between periodontitis and metabolic syndrome |
| Shimazaki et al28 | 40 to 79 | 584 | Abd obesity, TG, HDL, BP, and FPG | PD and CAL | Metabolic syndrome increases risk of periodontitis |
| D’Aiuto et al11 | ≥17 | 13 677 | WC, TG, HDL, BP, and insulin resistance | BOP, PD | Severe periodontitis is associated with metabolic syndrome in middle‐aged individuals |
| Khader et al37 | ≥25 or above | 156 | WC, TG, HDL, TC, BP, and FPG | PI, GI, PD, and CAL | Patients with metabolic syndrome displayed more severe and extensive periodontitis compared with subjects without metabolic syndrome |
| Li et al46 | 37 to 78 | 208 | Abd obesity, TG, HDL, BP, and FPG/or T2DM | CAL, PD, BOP, and PI | Patients with metabolic syndrome had poor periodontal conditions, and periodontitis was associated with metabolic syndrome, independent of other risk factors |
| Morita et al29 | 24 to 60 | 2478 | WC, TG, HDL, TC, BP and FPG, HbA1c, and BMI | CPI | BMI, BP, TG, FPG, and HbA1c were significantly elevated in patients with PD of ≥ 4 mm. The adjusted odds ratio of the presence of periodontitis was 1.8 when the subjects with 2 positive components and without positive component were compared. And the odds ratio was 2.4 when the subjects with 3 or 4 positive components and without positive components were compared |
| Kushiyama et al47 | 40 to 70 | 1070 | Obesity, BP, HDL, TG, and FPG | CPI | The higher the number of metabolic syndrome components the higher the odds ratio of having more severe periodontitis |
| Andriankaja et al30 |
20 to >90 |
7431 | Abd obesity, BP/or Med, TG, HDL and FPG/or Med | PD | The association between metabolic syndrome and periodontitis was significant in women. Abdominal obesity appeared to be the contributing metabolic factor for both genders |
| Benguigui et al48 | 35 to 74 | 276 | WC, TG, HDL, BP, and FPG | PI, GI, PD, and CAL | There is a relationship between metabolic disturbances and periodontitis, with insulin resistance playing a central role |
| Han et al49 | ≥18 | 1046 | Abd obesity, TG, HDL, BP, and FPG | BOP, PD, and calculus | Metabolic syndrome might be associated with periodontitis. The association was confounded by age, gender, and smoking. Metabolic syndrome with high glucose and hypertension showed higher impact on this association |
| Nesbitt et al10 | mean: 56.8 ± 12 | 190 | BP, WC, TG, and FPG | ABL | Patients with severe periodontitis were approximately 2.5% times more likely to have metabolic syndrome |
|
Timonen et al63 |
30 to 64 |
2050 |
Abd obesity Insulin resistance, BP, and dyslipidemia |
PD |
Metabolic syndrome was associated with PD ≥ 4 mm (adjusted risk ratio 1.19), and with pockets ≥ 6 mm (adjusted risk ratio 1.5) |
| Chen et al36 | >18 | 253 | WC, TG, HDL, TC, BP and FPG, or T2DM |
PI, GI, and PDI |
Moderate‐severe periodontitis is associated with metabolic syndrome in patients undergoing hemodialysis |
| Kwon et al297 | ≥19 | 7178 | WC, TG, HDL, BP, and FPG | PD | Periodontitis is significantly associated with metabolic syndrome with an odds ratio of 1.55 |
| Fukui et al298 | 34 to 77 | 6,421 | TG, HDL, BP, FPG, HDL, BP, and obesity |
PD and CAL |
Periodontal status, particularly in individuals suspected to have untreated periodontal infection, is significantly associated with metabolic syndrome |
| Furuta et al60 | 40 to 79 | 2370 |
WC, TG, BP HDL, and FPG |
PD, CAL, and BOP | Gender differences appear to exist in the association between periodontitis and metabolic syndrome. Metabolic syndrome might have a stronger association with periodontitis in females compared with males |
| Sora et al39 | 26 to 87 | 283 | Abd obesity, BP, HDL, TG, and FPG (OGTT) |
Plaque, PD, CAL, and BOP |
Metabolic syndrome is associated with the extent of severe periodontitis in this Gullah population with type 2 diabetes |
|
LaMonte et al64 |
50 to 79 |
657 |
Abd obesity, BP or Med, TG, FPG or Med and HDL |
ABL, PD, and CAL |
A consistent association between metabolic syndrome and measures of periodontitis was not seen in this cohort of postmenopausal women |
| Thanakun et al33 | 35 to 76 | 125 | WC, TG, HDL, BP, and FPG |
BOP, PD, and CAL |
Severe periodontitis was associated with metabolic syndrome (odds ratio 3.6) when 4‐5 metabolic syndrome components were analyzed the odds ratio increased to 5.49 in this Thai population |
| Minagawa et al9 | ≥80 | 234 |
WC, FPG, BP, and dyslipidemia |
PD and CAL | Metabolic syndrome was associated with the presence and severity of periodontitis (crude odds ratio 2.24) |
|
Chen et al299 |
23 to 58 | 303 | Abd obesity, BP, TG, HDL, and FPG | CPI | The prevalence of metabolic syndrome was sufficiently high to be a medical concern, and was associated with periodontitis |
| Gomes‐Filho et al50 | 24 to 89 | 419 | WC, TG, HDL, BP, and FPG | PD, CAL, and BOP | Periodontitis is associated with metabolic syndrome |
|
Musskopf et al51 |
18 to 81 |
363 | WC, TG, HDL, BP, and FPG | PI, GI, PD, CAL, and BOP | There is a weak association among metabolic syndrome and periodontitis. The association is observed in the age group of 41‐60 y |
| Jaramillo et al52 | 651 | TG, HDL, BP, BMI, and glucose tolerance | GI, PI, PD, CAL, and BOP | There is a positive association between metabolic syndrome and periodontitis. The adjusted odds ratio is 2.72. Glucose sensitivity is a strongly associated component | |
| Kikui et al53 | mean: 66.4 | 1856 | BP and/or Med, HDL, TG and/or Med, FPG/and Abd obesity | CPI | Metabolic syndrome and lower HDL cholesterol are associated with periodontitis. Subjects with 2 or more metabolic syndrome components had a significantly higher prevalence of periodontitis |
| Kim et al300 | 50 to 94 | 5078 | BMI, WC, BP, FPG, HDL, and TG |
PD and CAL |
Increasing the severity of periodontitis was associated with the risk of prevalent metabolic syndrome in Korean adults |
| Pham et al38 | mean: 57.8 ± 5.7 | 412 | BMI, WC, HDL, BP and FPG | PI, GI, PD, CAL, and BOP | More severe and extensive periodontitis was found in metabolic syndrome participants and increased with number of metabolic syndrome components. Participants with higher periodontal parameters had a higher risk of metabolic syndrome |
| Campos et al54 | 122 with metabolic syndrome and 366 controls | BP, TGs and LDL and/or WC | PI, BOP, PD, and CAL | There is an association between metabolic syndrome and periodontitis | |
Abbreviations: Abd, abdominal; ABL, alveolar bone level; BMI, body mass index (kg/m2); BOP, bleeding on probing; BP, blood pressure; CAL, clinical attachment level; CPI, community periodontal index; CRP, C‐reactive protein; FPG, fasting plasma glucose; GI, gingival bleeding index; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; Med, medication; OGTT, oral glucose tolerance test; PD, probing depth; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglycerides; WC, waist circumference.
Evaluating the role of metabolic syndrome in periodontitis development and progression, Kaye et al35, 42 and Iwasaki et al35, 42 performed longitudinal studies and concluded that metabolic syndrome increases the risk of development and progression of periodontitis (Table 1). In fact, Iwasaki et al35, 42 concluded that patients with metabolic syndrome were 2.6 times more likely to develop periodontitis. Likewise, the more components of metabolic syndrome an individual exhibited, the more prevalent and extensive the presentation of the periodontitis.35, 38
Until now, very little has been known about the potential gender predilection in the association between metabolic syndrome and periodontitis, and definitive conclusions cannot be made. Nonetheless, among the three relevant studies published, two concluded that there is a stronger association between metabolic syndrome and periodontitis in women,30, 60 while the other study did not find a relationship between gender, metabolic syndrome, and periodontitis; however, Kushiyama et al47 did comment that their small sample size could have influenced their results. As it relates to age, Minagawa et al9 suggested that metabolic syndrome and periodontitis are linked in the elderly population, which is consistent with the prevalence of metabolic syndrome and periodontitis increasing with age.61, 62
Although the vast majority of studies concluded that there is an association between metabolic syndrome and periodontitis, several studies found weak or no associations between these two conditions34, 45, 51, 63, 64, 65 (Table 1). It is worth noting that most of these studies were cross‐sectional in nature, with the longitudinal study spanning a period of 1 year; the study by Nascimento et al34 was performed on a relatively young population (31 years of age), whose age bracket has a comparatively low prevalence of periodontitis and metabolic syndrome. Additionally, in a 3‐year longitudinal study, Kobayashi et al66 concluded that toothbrushing frequency is inversely related to the incidence of metabolic syndrome.
2.2. Influence of periodontitis on metabolic syndrome
Some studies have suggested that periodontitis can affect systemic conditions.67, 68 For example, periodontitis elevates the levels of several inflammatory mediators, such as C‐reactive protein and interleukin‐6.69, 70 Moreover, periodontal treatment can decrease circulating levels of inflammatory mediators.71, 72 Given this information, researchers have sought to evaluate the potential of periodontitis to affect metabolic syndrome.
The majority of studies concluded that periodontitis may contribute to the development or exacerbation of metabolic syndrome10, 40, 73 (Table 2). Nesbitt et al10 performed a cross‐sectional study in 190 individuals evaluating periodontitis based on periodontal bone loss and concluded that periodontitis may contribute to the development of metabolic syndrome. Morita et al40 conducted a longitudinal study on 1023 adults and concluded that deeper periodontal pockets are associated with a positive conversion of one or more metabolic components during a 4‐year period (odds ratio: 1.6; 95% confidence interval: 1.1‐2.2). Moreover, Lopez et al73 suggested that reduction of periodontal inflammation reduces C‐reactive protein levels in patients with metabolic syndrome.
TABLE 2.
Effect of periodontitis on metabolic syndrome
| Authors | Cross‐sectional (CS) or longitudinal (L) | Duration | Age of patients (y) | Number of patients |
Metabolic syndrome parameters |
Periodontitis parameters |
Results |
|---|---|---|---|---|---|---|---|
| Kushiyama et al47 | CS | 40 to 70 | 1070 | Obesity, BP, HDL, TG, and FPG |
CPI |
The higher the number of metabolic syndrome components, the higher the odds ratio of having more severe periodontitis | |
| Morita et al40 | L | 4 y | 20 to 56, mean: 37.3 | 1023 |
BP, TG, HDL, TC, FPG, and BMI |
CPI |
Periodontal pockets were associated with a positive conversion of metabolic‐syndrome components |
| Nesbitt et al10 | CS | mean: 56.8 ± 12.7 | 190 |
BP, WC, TG, and FPG |
ABL | Alveolar bone loss is associated with metabolic syndrome | |
| Lopez et al73 | L | 1 y | 35 to 65 | 165 | Abd obesity, TG, HDL, BP, and FPG |
≥4 teeth with ≥ 4mm and CAL of ≥ 3mm |
Reduction of periodontal inflammation either with scaling and root planing and systemic antibiotics or with plaque control and subgingival scaling reduces CRP levels after 9 mo in patients with metabolic syndrome |
| Kim et al300 | CS | 50 to 94 | 5078 | BMI, WC, BP, FPG, HDL, and TG |
PD and CAL |
Increasing the severity of periodontitis was associated with the risk of prevalent metabolic syndrome in Korean adults |
Abbreviations: Abd, abdominal; ABL, alveolar bone level; BMI, body mass index (kg/m2); BP, blood pressure; CAL, clinical attachment level; CPI, community periodontal index; CRP, C‐reactive protein; FPG, fasting plasma glucose; HDL, high‐density lipoprotein; PD, probing depth; TC, total cholesterol; TG, triglycerides; WC, waist circumference.
2.3. Periodontal microbiome changes in patients with metabolic syndrome
In recent years, considerable attention has been given to the microbiome. Metabolic diseases alter the gut microbiome (reviewed by Karlsson et al74), and it is well known that the oral microbiome varies significantly between healthy and periodontitis patients.75 Additionally, alterations in the gut microbiome have been linked to obesity and metabolic syndrome.76 Furthermore, obesity can alter the oral microbiome of individuals with type 2 diabetes77 and it can reduce microbial diversity in the distal gut.78, 79 More specifically, individuals with lower microbiome diversity have marked overall adiposity, insulin resistance, and dyslipidemia compared with those with high bacterial richness.79 Tam et al77 evaluated 17 individuals with severe periodontitis and concluded that oral microbial composition varies significantly between obese (body mass index ≥ 30) and nonobese individuals with type 2 diabetes. This study implied that obesity was associated with a reduction in species diversity in the oral cavity.
2.4. We also hypothesized that the oral microbiome is altered in metabolic syndrome patients compared with healthy patients
To test this hypothesis, we performed microbial 16S rDNA profiling of unstimulated saliva from healthy individuals and individuals with metabolic syndrome, with and without periodontitis. The primary objective was to make comparisons between two groups, categorized as metabolic syndrome and systemically healthy (two‐group analysis: healthy vs metabolic syndrome). The secondary objective was to stratify the metabolic syndrome patients by periodontal health status (four‐group analysis: healthy vs healthy* vs metabolic syndrome healthy periodontium vs metabolic syndrome periodontitis). Note: three healthy subjects presented with an elevated systolic and/or diastolic blood pressure reading (stage I hypertension values, according to the American Heart Association) and thus were stratified into a healthy* group.
More specifically, this study consisted of a total of 22 subjects (12 metabolic syndrome and 10 healthy individuals). The 12 metabolic syndrome patients also had diabetes. Metabolic syndrome subjects were further stratified by periodontal status. Downstream analyses included alpha diversity, linear discriminant analysis effect size, and beta diversity using principal coordinate analysis. Kruskal‐Wallis and linear discriminant analysis were used for evaluating statistical significance between healthy and metabolic syndrome microbial communities.
2.5. Results
The saliva samples yielded 2 270 978 assigned reads, including 1 155 315 single species, 1 088 960 multispecies, and 24 866 novel species reads. At the species level, 573 total species were represented as operational taxonomic units: 330 single species, 145 multispecies, and 98 novel species. Detailed data can be found at http://www.homd.org/ftp/publication_data/20170412/qiime_results/cd_mc10/taxa_plots/taxa_summary_plots/bar_charts.html.
Evaluating the alpha diversity, the rarefaction plot for the two‐group analysis of metabolic syndrome vs healthy subjects (Figure 1A), and for the four‐group analysis of healthy vs healthy* vs metabolic syndrome healthy periodontium vs metabolic syndrome periodontitis using observed operational taxonomic units (Figure 1B), exhibits curves plateauing with the increased sequences sampled, indicative of adequate alpha diversity in terms of species richness. It is clear that the healthy salivary microbiome is more diverse compared with that found in the metabolic syndrome subjects.
FIGURE 1.
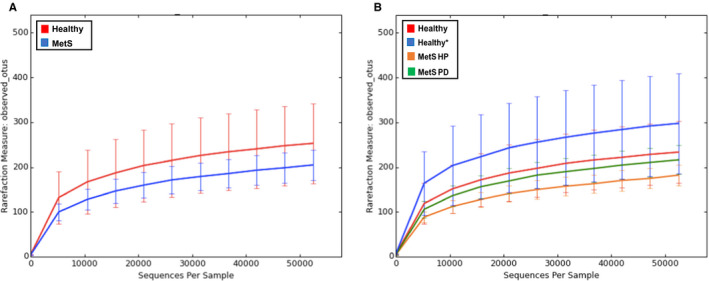
Rarefaction plots show distinct levels of alpha diversity between healthy and metabolic syndrome subjects. The two‐group analysis rarefaction plot (A) represents the rarefaction curves of the healthy and metabolic syndrome subjects. Both curves show similar species richness and the curves plateau as the number of sequences rise per sample. The four‐group analysis rarefaction plot (B) shows similar alpha diversity in terms of species richness and both begin to plateau and the curves of all four groups begin to plateau as the number of sequences rises per sample. MetS, metabolic syndrome; MetS HP, metabolic syndrome with healthy periodontium; MetS PD, metabolic syndrome with periodontitis; OTUs, operational taxonomic units
Evaluating beta diversity, three‐dimensional principal coordinate analysis plots were generated (Figure 2). The two‐group analysis (Figure 2A) suggests that subjects within each group show more relatedness to one another than to a subject of the opposite group. A less organized pattern is demonstrated in the four‐group analysis plot and no conclusive result regarding beta diversity can be observed from this plot (Figure 2B).
FIGURE 2.
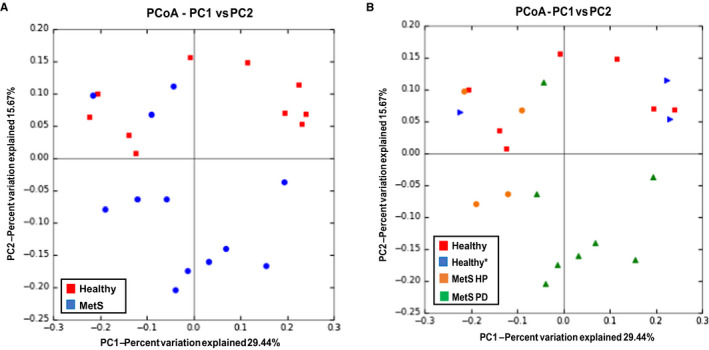
Principal coordinate analysis (PCoA) for all groups show the relative relatedness within groups. A two‐dimensional view of the three‐dimensional PCoA plot, derived from UniFrac as a distance metric, is shown in A, representing beta diversity for the two‐group analysis. It can be appreciated that the two groups appear to be more related within group than between group as evidenced by clusters. A two‐dimensional view of the three‐dimensional PCoA plot, derived from UniFrac as a distance metric, is shown in B, representing beta diversity for the four‐group analysis. No conclusive result regarding beta diversity can be observed from these plots. MetS, metabolic syndrome; MetS HP, metabolic syndrome with healthy periodontium; MetS PD, metabolic syndrome with periodontitis
Next, we evaluated the phylogenetic relationship of taxa among the groups. A cladogram was generated representing the phylogenetic relationship of taxa associated with the healthy and metabolic syndrome groups (Figure 3). At the species level, taxa significantly associated with healthy patients showed relatedness. The cladogram in Figure 3B represents the taxonomic relationship between taxa significantly associated with the four groups analyzed. Phylogenetically related taxa at the species level are significantly associated with health (specifically, the taxa stemming from the class Betaproteobacteria from the phylum Proteobacteria), healthy* (including taxa stemming from the phylum SR1, class Flavobacteria, and order Corynebacteriales), and metabolic syndrome with periodontitis (including taxa stemming from the phylum Tenericutes and phylum Spirochaetes, as well as the order Coriobacteriales).
FIGURE 3.
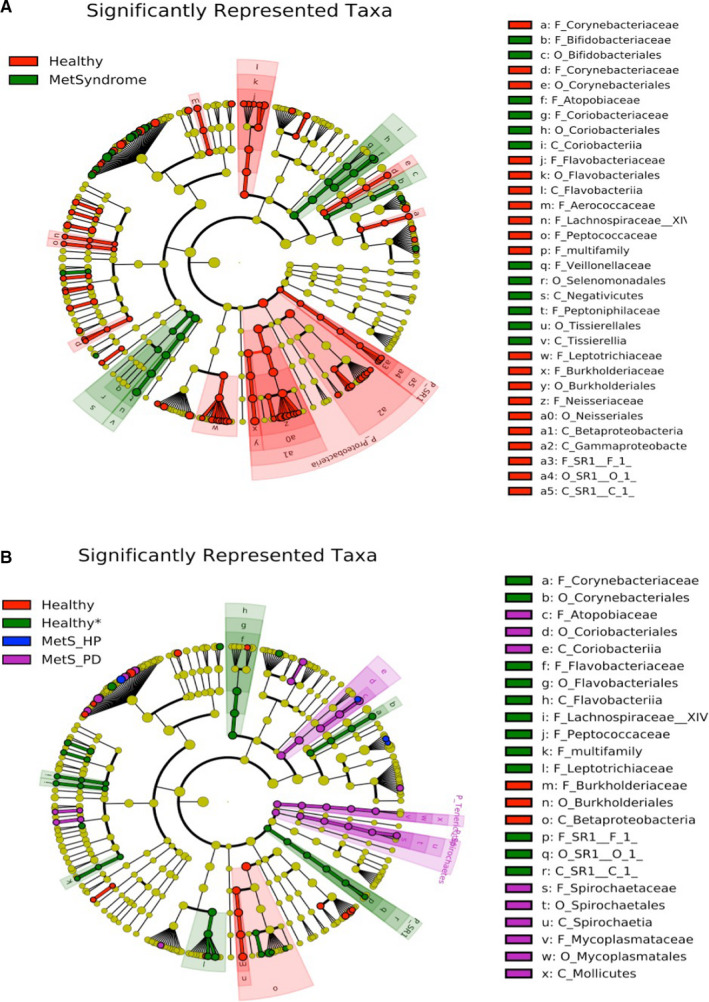
The cladograms show the phylogenetic relationship and taxonomic groupings of the taxa significantly associated with different subject groups.A, The phylogenetic relationship and taxonomic groupings of the taxa significantly associated with healthy and metabolic syndrome (MetSyndrome) subjects. Dots of red (health) and green (metabolic syndrome) represent significantly associated taxa to the labeled group and the size of the dot corresponds to relative abundance. At the outer edge of the cladogram, a letter is listed to represent this taxon and corresponds with the legend on the right. A strong association between taxa stemming from the phylum Proteobacteria and phylum SR1 and health is shown. Taxonomic relationships are shown in clusters of green for taxa significantly associated with the metabolic syndrome group. B, The phylogenetic relationship and taxonomic groupings of the taxa significantly associated with healthy (red), healthy* (green), metabolic syndrome with healthy periodontium (MetS_HP; blue), and metabolic syndrome with periodontitis (MetS_PD; purple). Colored dots represent significant taxa for the denoted group and the size of the dot corresponds to relative abundance. At the outer edge of the cladogram, letters are used to represent the significant taxa, listed in the legend on the right side of the figure. A strong association is shown between taxa stemming from the phyla Tenericutes and Spirochaetes and order Coriobacteriia for metabolic syndrome with periodontitis subjects; a strong association is shown between taxa stemming from the phylum SR1 and healthy* subjects, and taxa associated with the phylum Proteobacteria and healthy subjects
In summary, the 16S rDNA sequence analyses support the hypothesis that the salivary bacterial profile is altered in metabolic syndrome patients compared with healthy patients. Despite a small sample size, the healthy group was more diverse than the metabolic syndrome group (Figures 1 and 2). When further stratified, the metabolic syndrome healthy periodontium and metabolic syndrome periodontitis subject groups displayed comparatively different microbial profiles with both one another and with healthy subjects (Figure 3). Additionally, the metabolic syndrome periodontitis group displayed a large effect size difference and a greater abundance of two of the three classic periodontal “red complex” pathogens,80 namely, Tannerella forsythia, the phylum Spirochaetes, and genus Treponema. However, a significant effect size difference was not detected between the groups for Porphyromonas gingivalis. Based on our study, additional research with an increased subject population is warranted to further advance these novel findings.
2.6. Lessons from animal models: the role of dyslipidemia
To date, a few in vivo and in vitro studies have highlighted the role of dyslipidemia (in a high‐fat diet model) in the compounding effect of metabolic syndrome on periodontitis57, 58, 59, 81 (Table 3) and several studies have evaluated the role of impaired glucose in periodontitis; the latter are discussed in the Diabetes section of this review.
TABLE 3.
Metabolic syndrome: animal data
| Authors | Duration of the study (wk) | Number of samples |
Metabolic syndrome parameters |
Periodontitis parameters |
Results |
|---|---|---|---|---|---|
| Amar et al57 | 16 | N/A | Not evaluated | ABL | Mice with P. gingivalis‐induced periodontitis and diet‐induced obesity had a significantly higher level of alveolar bone loss compared with the lean controls |
| Watanabe et al185 | 13 | 28 | FPG and fasting insulin levels | ABL |
High‐fat/periodontitis rats developed more severe insulin resistance compared with high‐fat/control, low‐fat/ periodontitis or low‐fat/control rats as measured by fasting insulin levels and homeostasis model assessment analysis |
| Ohnishi et al301 | 20 | insulin resistance | ABL | Oxidative stress causes alveolar bone loss in a metabolic syndrome mouse model with type 2 diabetes | |
| Jin et al58 | 4 | 44 (22/ group) | FPG, TG, FFA, and TC | ABL | Simvastatin inhibited LPS‐induced bone loss and periodontal inflammation in rats with metabolic syndrome |
| Li et al59 | 16 | 14 | TC, TG, and FFA | ABL | Saturated fatty acid may play a role in metabolic syndrome‐associated periodontitis by enhancing LPS‐induced inflammatory cytokine expression |
| Lu et al81 | 16 | 28 (14/group) | FPG, TG, FFA, TC, and insulin | ABL | CD36 expression is upregulated in mice with periodontitis and metabolic syndrome and is involved in gene expression in macrophages stimulated by palmitate and LPS |
Abbreviations: ABL, alveolar bone level; CD36, cluster of differentiation; CPI, community periodontal index; FFA, free fatty acid; FPG, fasting plasma glucose; LPS, lipopolysaccharide; N/A, not applicable; TC, total cholesterol; TG, triglycerides.
Amar et al57 demonstrated that mice fed a high‐fat diet, but not presenting with diabetes, had 40% more periodontal bone loss and higher titers of Po. gingivalis compared with the control mice in a Po. gingivalis bacterial colonization model (silk ligature and Po. gingivalis oral inoculation). Although this study did not evaluate metabolic syndrome markers, these mice were obese and the study utilized a well‐known metabolic syndrome model.57 Subsequently, utilizing the same metabolic syndrome model, Li et al59 demonstrated that mice fed a high‐fat diet developed metabolic syndrome, as determined by obesity, hyperinsulinemia, insulin resistance, and dyslipidemia. In this study, metabolic syndrome led to a significant increase in osteoclastogenesis and periodontal bone loss. Moreover, lipopolysaccharide‐induced periodontitis exacerbated inflammatory cytokine expression (interleukin‐6, monocyte chemoattractant protein1, RANKL, and macrophage colony‐stimulating factor), osteoclastogenesis, and periodontal bone loss.59 In vitro studies utilizing osteoblasts derived from obese New Zealand mice demonstrated a decrease in cell proliferation and an increase in osteoblast apoptosis after Po. gingivalis exposure compared with control mice.82 Additionally, obese Zucker rats with metabolic syndrome had a statistically significant increase in Aggregatibacter actinomycetemcomitans‐lipopolysaccharide–induced periodontal bone loss compared with the nonobese, non‐metabolic syndrome group. Moreover, statin, a cholesterol‐lowering drug often prescribed to individuals with metabolic syndrome,83 alleviated periodontal bone loss in both groups, also pointing to dyslipidemia as a potential exacerbator of periodontal inflammation.84
To further establish the role of lipids in periodontitis, Li et al59 demonstrated that fatty acids (eg, palmitic acid) amplified the lipopolysaccharide‐mediated expression of markers involved in periodontitis, such as interleukin‐1‐alpha, interleukin‐1‐beta, C‐X‐C motif chemokine ligand 10, cluster of differentiation 86, colony stimulating factor 2, monocyte chemoattractant protein 1, toll‐like receptor, tumor necrosis factor‐alpha, and cluster of differentiation 14 in vitro.59 In addition, in vitro studies performed in macrophages showed a statistically significant upregulation of cluster of differentiation 36, a major fatty acid receptor, upon treatment with lipopolysaccharide plus palmitate in comparison with those macrophages treated with lipopolysaccharide or palmitate alone.81 Periodontitis and metabolic syndrome independently increased cluster of differentiation 36 levels significantly, and when metabolic syndrome and periodontitis were developed concurrently, there was an additive effect. Cluster of differentiation 36 expression in periodontal tissues was also positively correlated with osteoclastogenesis.
In summary, human studies and animal models demonstrate an association between metabolic syndrome and periodontitis, and metabolic syndrome can alter the oral microbiome and potentiate the deleterious effects of periodontitis.
3. DIABETES
Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. Approximately 415 million people in the world live with diabetes and this number is expected to increase to 642 million by 2040.85 In the USA, diabetes is present in 13% of adults. Diabetes increases with age, affecting 26.8% of individuals aged 65 years or older.86 Of all diabetes cases, type 2 diabetes accounts for approximately 90%‐95% of those with diabetes and encompasses individuals who have insulin resistance and usually have relative (rather than absolute) insulin deficiency.87 Type 2 diabetes affects more than 380 million people worldwide, representing 8.8% of individuals aged 20‐79 years.88
The chronic hyperglycemia caused by type 2 diabetes is associated with long‐term damage and disabling and life‐threatening health complications, such as cardiovascular disease, neuropathy, and nephropathy.89, 90, 91, 92 Periodontitis is highly likely to develop in individuals with diabetes and constitutes the sixth most frequent complication of diabetes.93 The reverse is also true, as individuals with periodontitis are more likely to develop diabetes, thus establishing a “two‐way” relationship between the two conditions.94, 95 Since much is known about the two‐way relationship between these two conditions, this review will emphasize the role of diabetes on the periodontal microbiome.
3.1. Role of type 2 diabetes in periodontitis
The association between diabetes and periodontitis has long been established, with most studies showing that poorly controlled diabetes affects periodontitis development and progression.96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116 The odds ratio for patients with diabetes having periodontitis varies; Emrich et al100, 105 reported an odds ratio of 2.81 and Tsai et al100, 105 reported that the odds ratio in individuals with better controlled diabetes is 1.56. Nelson et al,112 having evaluated subjects with type 2 diabetes, concluded that the rate of periodontitis is 2.6 times higher in subjects presenting with type 2 diabetes compared with those without it.112 Along those lines, several studies have shown that uncontrolled type 2 diabetes is associated with periodontitis progression; however, controlled type 2 diabetes or altered glycemic levels without diabetes are not associated with periodontitis.98, 109, 117, 118, 119 In addition, the longer duration of diabetes appears to correlate with periodontitis severity.120
Patients with diabetic retinopathy appear to have more severe periodontitis compared with those without retinopathy.121 Interestingly, despite the higher prevalence and severity of periodontitis in subjects with diabetes, Newton et al,122 evaluating 46 132 electronic charts of patients with periodontitis with and without diabetes, concluded that individuals with diabetes had significantly more periodontitis treatment compared with normoglycemic individuals.
The mechanisms by which diabetes affects periodontitis have been studied in animals and are described below. However, analyzing periodontal tissues and/or gingival crevicular fluid, it has been noted that interleukin‐1‐beta is increased and that diabetes also increases advanced glycation end products and oxidative stress.123, 124, 125
3.2. Role of periodontitis in diabetes
Chronic subclinical inflammation plays a role in the pathogenesis of type 2 diabetes.126, 127 Given that periodontitis leads to subclinical inflammation,128 many studies have evaluated the role of periodontitis on glycemic levels, as well as on the incidence of type 2 diabetes and diabetes complications.129, 130, 131, 132, 133, 134, 135, 136 Periodontitis is associated with hemoglobin A1c progression in individuals with diabetes.137 Additionally, deeper probing depths are more closely associated with increased hemoglobin A1c levels.68, 132, 138, 139 Even in healthy individuals, periodontitis can worsen glycemic control.140, 141, 142, 143, 144
Regarding the development of type 2 diabetes, most studies have linked periodontitis to a higher probability of developing diabetes.133, 145, 146 For instance, Winning et al147 concluded that the hazard ratio of developing type 2 diabetes in individuals with moderate‐severe periodontitis is 1.69. Another study, which evaluated 22 299 patient charts with a 5.47‐year mean follow‐up, concluded that patients with periodontitis requiring surgery are at a higher risk of developing type 2 diabetes.133 Another 5‐year study, utilizing tooth loosening as a proxy for periodontitis, also identified a correlation between incident diabetes and tooth loosening.148 On the other hand, some studies were unable to find an association between type 2 diabetes incidence and periodontitis.129, 149 For instance, Kebede et al129 followed 2047 subjects for a period of 11.1 years; although individuals with incident cases of diabetes tended to have poorer periodontal status, once the data were adjusted for age, gender, and central adiposity, the correlation no longer existed. This contradictory result could have been in part influenced by the population evaluated (Caucasian), a partial periodontal evaluation, examination of hemoglobin A1c at only two data points, and 21% of subjects reporting having had periodontal treatment during the study period.
Severe periodontitis is associated with a higher presence of diabetes‐related complications, including retinopathy, foot ulcerations, and renal and cardiovascular complications.134, 157 For example, Saremi et al134 evaluated Pima Indians in a long‐term study with a median follow‐up of 11 years, and concluded that periodontitis is a strong predictor of mortality from ischemic heart disease and diabetic nephropathy.
3.3. Role of periodontal treatment on glycemic control
Given the role of periodontitis in diabetes, studies have sought to understand whether periodontal treatment would ameliorate glycemic control. Unfortunately, to date, the results are conflicting in this regard.136, 158, 159, 160, 161, 162, 163, 164, 165 These contradictory results could be, at least in part, attributed to differences in the treatment rendered, the duration of the study (1‐12 months), and because a proxy for periodontitis (dental insurance data) was utilized.
Among the studies concluding that periodontal treatment lowered hemoglobin A1c levels158, 159, 160, 161, 162, 163 is a large study conducted by D’Aiuto et al158 presenting a 12‐month follow‐up. In agreement with the results of that study, Spangler et al163 evaluated medical records and dental insurance data as a proxy for periodontitis over a period of 5 years, and also concluded that patients who received periodontal treatment had slightly lower hemoglobin A1c levels compared with those who did not receive treatment. However, periodontal parameters were not considered in this study, only insurance data.
Conversely, several studies did not find a relationship between periodontitis treatment and hemoglobin A1c levels.136, 161, 164, 165 These findings were probably attributable to the short‐term (3‐month) follow‐up, which may not have been sufficient to demonstrate a change in hemoglobin A1c levels.
Given the differences in periodontal treatment in various studies, the inability to restrict patients from obtaining periodontal treatment for an extended period,34, 43 and the short‐term follow‐up, more studies are needed to determine if periodontal treatment affects glycemic control.
3.4. Lessons from animal models: interactions between diabetes and periodontitis
To date, different animal models of type 1 diabetes and type 2 diabetes have been utilized with the aim of understanding the mechanisms by which diabetes and periodontitis affect one another. Herein, we briefly discuss some of these findings.
When evaluating the role of diabetes in periodontitis, studies have shown that diabetes increases periodontal bone loss,166, 167, 168, 169, 170 but also that the severity of periodontal breakdown corresponds to the severity of diabetes.171 Moreover, diabetes without periodontitis leads to periodontal alterations, such as a decrease in bone crest height and an increase in inflammatory cells and osteoclast numbers.172, 173 The exact mechanisms by which diabetes affects periodontitis are not fully understood; however, it is known that diabetes worsens periodontitis in part by (a) increasing the inflammatory response,166, 170 (b) enhancing cell apoptosis,174, 175, 176 (c) increasing osteoclast formation, (d) increasing bone resorption,168, 174 and (e) suppressing bone formation.177 These changes can be partially explained because diabetes (a) increases advanced glycation end products and their respective receptors (receptor for advanced glycation end products),4, 178 (b) increases toll‐like receptor 2 and toll‐like receptor 4 expression,179, 180 (c) alters the RANKL ratio,181 and (d) leads to mitochondrial dysfunction,182, 183 which in turn enhances local and systemic oxidative damage.175
The reverse is also true: most animal studies have also concluded that periodontitis affects diabetes. For instance, rodents with induced periodontitis are more glucose intolerant,184 have more severe fasting insulin levels,185 and have increased pancreatic beta‐cell failure.186 Periodontitis even alters glucose metabolism in rodents with prediabetes.187 The mechanisms by which periodontitis affects diabetes are not entirely clear but include increased circulating levels of different cytokines, such as interleukin‐1‐beta, tumor necrosis factor‐alpha, and adiponectin, as well as increased adipose tissue inflammation.184, 185, 188
Taken together, animal studies also point to the bidirectional role of diabetes and periodontitis and shed some light on the mechanism by which these conditions lead to a pro‐inflammatory response. However, further studies are needed for a more comprehensive understanding of this relationship.
3.5. Type 2 diabetes and the microbiome
Considerable attention has been given to the ability of chronic conditions to alter the microbiome. For instance, individuals with type 2 diabetes or obesity have a modified gut microbiome.74, 189, 190, 191 Conversely, mice treated with Enterobacter cloacae B29, which was isolated from the intestines of obese patients with diabetes, also developed obesity and insulin resistance,192 showing that microbes can also directly induce diabetes‐related symptoms. Furthermore, intervening with the flora or modulating bacterial‐mucosal immunity‐inflammation may alleviate type 2 diabetes.193 Given the association between type 2 diabetes, periodontitis, and the changes that have been observed in the gut microbiome in individuals with type 2 diabetes, additional attention has been given to microbiome changes in the periodontium of individuals with type 2 diabetes.
3.6. Diabetes affecting the subgingival microbiome
Type 2 diabetes alters the subgingival and salivary bacterial profile of individuals (Table 4) by decreasing diversity and richness.194, 195, 196, 197, 198, 199 This is consistent with data observed within the gut microbiome.74, 189, 190, 191, 200 When evaluating the subgingival microbiome, not only is the diversity decreased in individuals with type 2 diabetes, but when these individuals are further divided by adequate or inadequate glycemic control, there is a notable further decrease in the microbiome diversity in those with inadequate glycemic control (hemoglobin A1c ≥ 8%).201 By contrast, a study performed by Tam et al77 evaluated the salivary microbiome of 17 individuals with periodontitis and type 2 diabetes, but the authors were unable to determine statistically if glycemic control could also change the oral microbiota. However, it was noted that, in individuals with type 2 diabetes, the microbial composition varied significantly between obese (body mass index ≥ 30kg/m2) and nonobese individuals with type 2 diabetes, with obesity reducing the diversity of species in the oral cavity.77
TABLE 4.
Diabetes mellitus, periodontitis, and the microbiome
| Authors |
Cross‐sectional or Longitudinal |
Age of patients (y) | Number of patients |
Diabetes parameters |
Periodontitis parameters | Results |
|---|---|---|---|---|---|---|
| Supragingival microbiome | ||||||
| Sbordone et al302 | Longitudinal (3 y) | 9 to 17 | 32 | HbA1c | PD, CAL, and BOP | There is no significant differences in clinical parameters between type 1 diabetes mellitus and non‐diabetes mellitus siblings |
| Silva‐Boghossian et al210 | Longitudinal (3 mo) | 40 | FPG and HbA1c | PD and CAL, BOP, S, and marginal bleeding | After scaling and root planing healthy individuals demonstrated improved periodontal status and reduced levels of putative periodontal pathogens at 3 mo compared with those with inadequate metabolic control | |
| Tam et al77 | Longitudinal (3 mo) | 18 to 80 | 18 | HbA1c | PD, CAL, BOP, and PI | Differences in microbial composition and diversity between obese and nonobese groups were statistically significant |
| Shi et al202 | Longitudinal (4‐7 wk) | 31 | HbA1c | PD, CAL, BOP, and GI | In individuals with periodontitis, the shift in the subgingival microbiome from the healthy state was less prominent in type 2 diabetes compared with healthy subjects | |
| Longo et al201 | Cross‐sectional | Adequate GC: 57.9 ± 8.39, Inadequate GC: 52.55 ± 5.32 | 21 | HbA1c | PD, CAL, BOP, marginal bleeding | The microbiome of individuals with adequate glycemic control had higher diversity than individuals with inadequate glycemic control. Inadequate glycemic control favored fermenting species. Higher abundances of anginosus group and Streptococcus agalactiae in diabetes may suggest that subgingival sites can be reservoir of potentially invasive pathogens |
| Rodríguez‐Hernández, et al205 | Cross‐sectional | ≥ 18 (non‐T2DM) and ≥ 35 (T2DM) | 178 | HbA1c | PD, CAL, BOP, S, and gingival inflammation | The microbial profile of individuals with type 2 diabetes was different from non‐type 2 individuals’ microbiota |
| Farina et al195 | Cross‐sectional | ≥ 40 | 12 | HbA1c/ Med | PD and CAL | The presence of type 2 diabetes and/or periodontitis was associated with a subgingival microbiome decrease in richness and diversity. The presence of type 2 diabetes was not associated with significant differences in the relative abundance of 1 or more species in patients either with or without periodontitis |
| Salivary microbiome | ||||||
| Sabharwal et al198 | Cross‐sectional | 18 to 65 | 146 | HbA1c | PD, BOP, and GI | Oral microbial diversity decreased in diabetes and increased with progression of periodontitis compared with periodontally healthy controls |
| Yang et al196 | Cross‐sectional | FPG | PD, GI, recession, and mobility | Salivary microbes were related to drug treatment and certain pathologic changes | ||
| Matsha et al197 | Cross‐sectional | mean: 47.0 ± 13.0 | 128 | FPG and HbA1c | PD, BOP, and CPI | Actinobacteria were significantly more abundant in subjects with diabetes, while Proteobacteria were less abundant. In the presence of gingival bleeding and diabetes, as compared with diabetes without gingival bleeding, Actinobacteria were markedly reduced while Bacteroidetes were more abundant. By contrast, no differences in Actinobacteria or Bacteroidetes abundance were observed between diabetes with and without PD ≥ 4 mm |
Abbreviations: BOP, bleeding on probing; CAL, clinical attachment level; CPI, community periodontal index; FPG, fasting plasma glucose; GI, gingival bleeding index; GC, glycemic control; HbA1c, hemoglobin A1c; Med, medication; PD, probing depth; PI, plaque index; S, suppuration; T2DM, type 2 diabetes mellitus.
Although the diversity of the subgingival and supragingival microbiome decreases when subjects with type 2 diabetes are compared with normoglycemic individuals, the bacterial shift in individuals with periodontitis is less prominent in type 2 diabetes subjects than in normoglycemic individuals.195, 196, 202
There is not much consensus regarding the specific differences in the microbiome (Table 4). However, individuals with diabetes had a higher total taxa of Saccharibacteria (TM7), Aggregatibacter, Neisseria, Gemella, and Eikenella.194 Matsha et al197 noted that Fusobacterium and Actinobacteria are more abundant in subjects with diabetes. Furthermore, in subjects with type 2 diabetes and bleeding on probing, there was an increase in the abundance of Bacteroidetes (Po. gingivalis belongs to this phylum).197
Targeted studies utilizing DNA‐DNA hybridization technology or PCR assays evaluated the presence of specific bacterial taxa or groups of bacteria but no consensus was reached. Aemaimanan et al203, 204 and Babaev et al203, 204 reported that individuals with poorly controlled type 2 diabetes have higher levels of red complex bacteria (Po. gingivalis, Treponema denticola, and Ta. forsythia).203, 204 On the other hand, Rodriguez‐Hernandez et al205 found a decrease in red complex bacteria in Mexican individuals with type 2 diabetes compared with normoglycemic individuals with periodontitis. However, individuals with type 2 diabetes and periodontitis had higher levels of the yellow and orange complexes.205
There are also some contradictions among different studies when analyzing individual bacterial species. For instance, Po. gingivalis was increased in individuals with periodontitis and type 2 diabetes,203, 204, 206, 207 but decreased in other individuals.194, 208, 209 Some studies found that the levels of Tr. denticola were higher in patients with type 2 diabetes compared with normoglycemic controls,203, 204, 210 while no difference was observed in other studies.206, 208
When type 2 diabetes and periodontitis were evaluated in individuals who smoke, the subgingival microbiome of smokers with type 2 diabetes had lower diversity, higher levels of gram‐negative facultative anaerobes, and lower levels of gram‐negative obligate anaerobes. In addition, the combination of smoking and type 2 diabetes led to synergistic changes in the microbiome.211
3.7. Periodontal treatment and the microbiome
Shi et al202 evaluated the subgingival microbiome through metagenomic shotgun sequencing of normoglycemic and type 2 diabetes individuals with periodontitis before and after scaling and root planing. Both groups showed clinical improvement and improvements in the levels of Prevotella intermedia, Po. gingivalis, and Ta. forsythia. However, individuals with poor glycemic control showed a reduced shift in the microbiome; and a reduced shift towards a healthy state.202 Silva‐Boghossian et al210 used a targeted approach method (ie, DNA‐DNA hybridization) to evaluate 45 species, and observed similar results. Scaling and root planing led to improvement in clinical parameters and a reduction in the pathogenic bacteria in both groups, but the reduction in the type 2 diabetes group was not as significant as that observed in the normoglycemic group. Regarding the findings of Silva‐Boghossian et al,210 it is worth noting that the patients with type 2 diabetes had more severe periodontitis compared with the normoglycemic individuals. Therefore, based on the data presented above, it appears that periodontitis treatment leads to a less pathogenic bacterial profile; however, the shift is not as prominent in type 2 diabetes and is even less significant in patients with poorer glycemic control.
3.8. Type 2 diabetes and the salivary microbiome
Goodson et al212 suggested that increased levels of glucose in the saliva may affect the salivary microbiome. To test this hypothesis, several groups evaluated the role of type 2 diabetes on the salivary microbiome. Salivary and subgingival microbial diversity were decreased in individuals with type 2 diabetes.198 However, treatment with metformin or metformin in combination with other medications did not rescue the flora. Nonetheless, the supragingival microbiome is different in patients with type 2 diabetes without medications and those being treated with metformin or with a combination of medications.196 Another study by the same group196 evaluated the salivary microbiome of normal weight, obese, and obese/type 2 diabetes children (aged 10‐19 years), noting that there was minimal difference in the alpha diversity among these groups. This study supported a modest link between periodontal inflammation and type 2 diabetes in the pediatric population. The gingival index was higher in individuals with type 2 diabetes but periodontitis was similar among the groups. At the genus level, there was a difference in abundance in eight operational taxonomic units and, after adjusting for the gingival index, there were still five significantly different genus‐level operational taxonomic units.213 While the rate of missing, decayed, and filled teeth was similar among groups, the gingival index was higher in type 2 diabetes. There was no difference in periodontitis, which is not surprising, given that periodontitis is more common in older populations.
3.9. Summary of type 2 diabetes, periodontitis, and the microbiome
In summary, type 2 diabetes affects the subgingival and salivary microbiome profile by decreasing diversity and richness. When glycemic control is added to the equation, there is a further decrease in diversity in individuals with inadequate glycemic control. Interestingly, the bacterial shift observed in individuals with periodontitis is less prominent in subjects with type 2 diabetes compared with normoglycemic controls. Moreover, in smokers, the diversity of the microbiome is further reduced. In future studies, it will be interesting to examine the potential for a bidirectional relationship between the periodontal microbiome and diabetes; that is, the potential for periodontal microbes to directly induce diabetes‐related pathology, since evidence suggests that pathogenic microbes from the gut of obese diabetic patients can directly induce diabetes‐related symptoms in rodent models.
4. CARDIOVASCULAR DISEASE
Cardiovascular disease is a group of disorders of the heart and blood vessels that includes coronary artery disease, cerebrovascular disease, congestive heart failure, and peripheral vascular disease.214, 215 In the USA, among individuals older than 20 years of age, 37.4% of men and 35.9% of women have some form of cardiovascular disease.214 These conditions can lead to myocardial infarction and stroke and they account for one third of all deaths worldwide.216 Of these conditions, atherosclerotic cardiovascular disease is the leading cause of vascular disease worldwide.217 Although genetics plays a role in cardiovascular disease,218 the key risk factors stem from lifestyle, such as dyslipidemia, hypertension, tobacco smoking, and altered glucose metabolism.219 Unfortunately, these key lifestyle factors are quite common and it is believed that 47% of Americans have at least one of them.220
Given the prevalence of cardiovascular disease and the need to prevent and treat it, Matilla et al221 sought to investigate early on whether dental disease could be correlated with the prevalence of cardiovascular disease, or more specifically with myocardial infarction. The authors concluded that dental health (periapical lesions, caries, vertical bone loss, and radiolucency in the furcation) was worse in patients with acute myocardial infarction compared with healthy individuals.221 In addition, significant interest has been focused on the potential role of periodontitis in cardiovascular disease. Our review of cardiovascular disease and periodontitis will be succinct, given that, in 2020, a consensus report was published on this topic by the European Federation of Periodontology and the World Heart Federation.222
4.1. Periodontitis and cardiovascular disease markers
Many inflammatory and oxidative stress markers are shared by cardiovascular disease and periodontitis. Therefore, the premise that periodontitis could affect cardiovascular disease is based on the elevated inflammatory serum marker levels in patients with periodontitis compared with periodontally healthy individuals, or patients who have been treated for periodontitis.223, 224, 225, 226, 227, 228, 229
4.2. Role of periodontitis in cardiovascular disease
Most clinical studies have shown an association between periodontitis and cardiovascular disease.230, 231, 232, 233, 234, 235, 236, 237, 238, 239, 240, 241 In 2012, a scientific statement was released by the American Heart Association confirming that there was an association between cardiovascular disease and periodontitis, but that a causal relationship could not be determined.242 Around the same time, a systematic review performed by Dietrich et al243 evaluated 12 studies and also concluded that there is an association between periodontitis and atherosclerotic cardiovascular disease. However, the authors cautioned that their findings may apply only to certain populations.243 A large Swedish case‐controlled study later evaluated periodontitis and its relation to coronary disease in 805 individuals and concluded that the risk of myocardial infarction significantly increases in patients with periodontitis (odds ratio: 1.28, confidence interval: 1.03‐1.6), even after adjusting for variables such as smoking habits, diabetes, years of education, and marital status.232 A more recent study by Sen et al244 also concluded that patients with periodontitis have more than double the risk of cardioembolic and thrombotic stroke compared with periodontally healthy individuals. Recently, a review was performed by Herrera et al245 with the aim of evaluating the association between periodontitis and cardiovascular disease, to assist in the 2020 consensus report published by the European Federation of Periodontology and the World Heart Federation. The authors concluded that individuals with periodontitis have a higher prevalence of coronary artery disease and an increased risk of myocardial infarction. However, when evaluating the role of periodontitis in a secondary atherosclerotic cardiovascular disease event, there was no consensus.222, 245
4.3. Effect of periodontal treatment on cardiovascular disease
Based on several observational studies and a Cochrane review that evaluated (a) self‐reported toothbrushing frequency, (b) improved oral hygiene, (c) dental visit frequency, (d) periodontal treatment, and (e) periodontal treatment outcomes, and correlated them to cardiovascular events,246, 247, 248, 249, 250, 251, 252 the 2020 consensus report by the World Heart Federation and the European Federation of Periodontology concluded that the progression of atherosclerotic cardiovascular disease may be influenced by successful periodontal treatment, including oral health instructions and more frequent dental visits, independent of traditional cardiovascular disease risk factor management. However, the consensus report found insufficient evidence to support or refute the potential benefit of periodontitis treatment in preventing or delaying atherosclerotic cardiovascular disease events.222
Interventional studies have sought to evaluate the effect of periodontal treatment on surrogate markers of cardiovascular disease, such as C‐reactive protein, fibrinogen, lipid profiles, white blood cells, and blood pressure.236, 253, 254, 255, 256, 257 In 2012, Bokhari et al253 evaluated patients with coronary heart disease and periodontitis and compared the effects of scaling and root planing with no periodontal treatment. The authors concluded that scaling and root planing decrease C‐reactive protein, fibrinogen, and white blood cell counts.253 Caula et al255 evaluated patients who underwent periodontal treatment and were then followed for a period of 6 months. The authors concluded that periodontal treatment leads to a reduction in C‐reactive protein, in addition to a reduction in triglycerides and erythrocyte sedimentation rates.255 Houcken et al257 recorded a decrease in systolic blood pressure after periodontal treatment. Moreover, data from a systematic review and meta‐analysis concluded that periodontal treatment, in addition to reducing serum levels of atherosclerotic cardiovascular disease biomarkers, improves endothelial function.258
To assess whether the timing of periodontal treatment has systemic effects, Graziani et al256 compared scaling and root planing performed within 24 hours vs scaling and root planing performed over a 4‐week period and evaluated inflammatory markers. The results indicated that there is an increased acute phase response, demonstrated by increased C‐reactive protein and interleukin‐6, when full mouth scaling and root planing are performed in a 24‐hour period. However, these results were transient and both treatment modalities ultimately offered similar results. Nonetheless, further studies are needed to determine if the elevated acute phase response has any impact on cardiovascular disease risk.256
The data presented here are in agreement with the 2020 World Heart Federation and European Federation of Periodontology consensus report, which points to evidence that periodontal treatment may reduce low‐grade systemic inflammation.222 It is important to note that a review by Herrera et al245 also pointed out that there have been a limited number of studies evaluating the role of periodontal treatment on cardiovascular disease outcomes.
4.4. Periodontal microbiome and cardiovascular disease
Considerable attention has been given recently to the microbiome because of its ability to modulate chronic conditions, including cardiovascular disease.259, 260 Given that periodontitis is triggered by a dysbiosis of pathogenic bacteria, that certain dental procedures, including toothbrushing, scaling, and root planing cause a transient bacteremia, and that periodontitis has been correlated with cardiovascular disease,261, 262, 263, 264, 265, 266, 267 researchers sought to determine if periodontopathogens could be found in the cardiovascular system. Indeed, several groups have identified periodontal pathogens, such as Tr. denticola, Aggregatibacter actinomycetemcomitans, Po. gingivalis, and Ta. forsythia, in cardiovascular tissue specimens (including atherosclerotic plaque, aortic valve, mitral valve, and aortic aneurysm).268, 269, 270, 271, 272 Although the mechanism by which bacteria enter into circulation is not fully understood, one study suggests that dendritic cells phagocytose and disseminate surviving periodontopathogens to atherosclerotic plaques and that these “primed” dendritic cells may provide key signals for atherogenic conversion.273
Moreover, while most studies were able to identify pathogenic periodontal bacteria in cardiovascular tissues, the exact role of the bacteria in cardiovascular disease is not fully understood. In an in vitro study, Lonn et al274 suggested that Po. gingivalis can modify vascular low‐density lipoprotein, very low‐density lipoprotein, and high‐density lipoprotein to an atherogenic form. A clinical study suggested that microRNA‐146, a regulator of innate immune responses, was a key molecule in associating periodontitis with coronary artery syndrome because of its dysregulation by periodontal pathogens.275, 276 Moreover, a review by Reyes et al277 effectively categorized the available research indicating that periodontal bacteria (a) disseminate and reach systemic vascular tissue, (b) are found in the affected tissues, (c) live within the affected site, (d) invade affected cell types in vitro, and (e) induce atherosclerosis in animal models of disease; (f) correct that noninvasive mutants of periodontal bacteria cause significantly reduced pathology in vitro and in vivo, and (g) that periodontal isolates from human atheromas can cause disease in animal models of infection.
To summarize the human and animal studies regarding the role of periodontal bacteria in cardiovascular disease, there are convincing data showing that periodontal bacteria can be found in cardiovascular tissues, but the role that bacteria play in these tissues still needs to be further elucidated.
4.5. The role of cardiovascular disease in periodontitis
While much emphasis has been placed on determining the potential role of periodontitis in cardiovascular disease, little has been done to evaluate the role of cardiovascular disease in periodontitis. As a result, the European Federation of Periodontology and World Heart Federation consensus report concluded that, to date, there is little scientific evidence that cardiovascular disease is a risk factor for periodontitis.222
In conclusion, much is known about the role of periodontitis in cardiovascular disease. However, it is important to note that these two conditions share risk factors, such as smoking, age, socioeconomic conditions, and obesity, which may lead to a possible common pathophysiology for periodontitis and cardiovascular disease.278, 279, 280
4.6. Lessons from animal models: periodontitis and cardiovascular disease
In addition to the role of periodontal bacteria in cardiovascular disease, as discussed above, animal studies have shed some light on the role of periodontitis in cardiovascular disease. Animal models have demonstrated that periodontitis increases systemic inflammation and that cardiovascular disease markers, such as C‐reactive protein, interleukin‐1‐beta, interleukin‐6, and vascular superoxide production, worsen lipid profile levels (total cholesterol, low‐density lipoprotein, and triglycerides).281, 282, 283 Evaluating endothelial changes in a ligature‐induced periodontitis model in rats, Brito et al283 observed a reduction in endothelium‐dependent vasodilatation, which is the hallmark of endothelial dysfunction. Moreover, Kose et al281 performed histologic analysis of the left ventricular heart tissues of rats and demonstrated that, at an early stage, periodontitis causes degenerative and hypotrophic changes in the heart tissue, and that prolonged systemic inflammatory stress caused be periodontitis may enhance the risk of hypertrophic changes.
In attempting to understand the role of periodontitis in cardiovascular disease, most studies have focused on the role of periodontopathogens in atherosclerosis because of their ability to induce severe oxidative stress, induce an inflammatory response, invade, colonize, and escape immune detection.284, 285, 286, 287 For instance, in response to Po. gingivalis, endothelial cells undergo oxidative stress and secrete various cytokines, such as tumor necrosis factor‐alpha, interleukin‐1‐beta, interleukin‐6, and interferon gamma.285, 288 Part of the mechanism by which Po. gingivalis accelerates atherosclerosis involves the nuclear factor kappa light chain enhancer of activated B cells signaling loop.289, 290 Moreover, periodontal pathogens are able to modulate lipid influx via fatty acid binding protein 4 in macrophages, strongly supporting another mechanism by which periodontitis may affect atherosclerotic progression.291
5. CONCLUSIONS
Metabolic syndrome, diabetes, and cardiovascular diseases are associated with periodontitis. Moreover, there is evidence to suggest that metabolic syndrome and diabetes can alter the oral microbiome. However, more studies are needed to fully understand the influence these conditions have on each other.
6. METHODS
This study was approved by the Institutional Review Board of the University of Michigan Medical School (#HUM000068346.) Twenty‐two subjects older than 18 years of age with a minimum of 10 teeth were recruited, and informed consent was obtained. Pregnant and lactating women were excluded from the study.
6.1. Subject diagnoses
A single examiner collected clinical data including the subjects’ weight, height, waist and hip circumference, blood pressure, and blood glucose levels. Subjects were diagnosed as healthy or metabolic syndrome. A diagnosis of metabolic syndrome was given to subjects who exhibited three or more of the following parameters: abdominal obesity (waist circumference of > 102 cm in men or > 88 cm in women), hyperlipidemia (self‐reported), hypertension (> 120 diastolic or > 80 systolic readings or use of antihypertensive medication), or diabetes (self‐reported or elevated blood glucose > 120 mg/dl).292 Patients who did not meet these criteria were considered systemically healthy and were assigned to the healthy group rather than the metabolic syndrome group.
Periodontal screening and recording examinations were completed for each subject. Subjects with no evident signs of gingivitis or periodontitis, who had probing depths of 1‐3 mm, bleeding on probing less than 30%, and no evidence of radiographic bone loss, were diagnosed as healthy periodontium. Subjects were diagnosed with periodontitis if they displayed severe gingival inflammation, erythema, possible edema, bleeding on probing greater than 30%, radiographic bone loss greater than 15%, and probing depths greater than 5 mm in more than one quadrant.
6.2. Data analysis
6.2.1. Two‐group analysis: healthy vs metabolic syndrome
A two‐group analysis was completed as follows: healthy subjects were compared with subjects with metabolic syndrome. The metabolic syndrome group included those with a healthy periodontium as well as those with periodontitis. The 12 metabolic syndrome patients had diabetes mellitus. No tobacco smokers were included in the healthy group, but of the metabolic syndrome population, five were current smokers and three were former smokers. Healthy subjects were aged 20‐46 (mean 28.1) years while metabolic syndrome subjects were aged 47‐78 (mean 64.5) years.
6.2.2. Four‐group analysis: healthy vs healthy* vs metabolic syndrome healthy periodontium vs metabolic syndrome periodontitis
A four‐group analysis was conducted on the following groups: (1) healthy (medically and dentally), (2) healthy* (subjects who presented with elevated blood pressure readings despite the subject having no self‐reported history of hypertension), (3) metabolic syndrome with healthy periodontium (metabolic syndrome healthy periodontium), and (4) metabolic syndrome with periodontitis (metabolic syndrome periodontitis). The healthy group consisted of seven subjects. Three healthy subjects presented with an elevated systolic and/or diastolic blood pressure reading (stage I hypertension values, according to the American Heart Association) and thus were stratified into a healthy* group. When questioned, all three subjects confirmed that they sought regular, routine medical care with a physician and had not been diagnosed as prehypertensive or hypertensive. Metabolic syndrome subjects were stratified into two groups, four metabolic syndrome healthy periodontium and eight metabolic syndrome periodontitis. Of the metabolic syndrome periodontitis group, seven (out of eight) subjects had a history of tobacco use (six current smokers and one former smoker), whereas the metabolic syndrome healthy periodontium group had only one former tobacco user.
6.3. Salivary analysis
Passive saliva was collected from each subject for 5 minutes. Samples were subsequently stored in a −80°C freezer until they were prepared for analysis. Prior to analysis, samples were thawed and centrifuged. Whole genomic bacterial DNA was extracted from salivary pellets and purified using MasterPure DNA purification kit (Epicentre, Madison, WI, USA). DNA was adjusted to a concentration of 20 ng/μl using a NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA).
The 16S rDNA next generation sequencing was performed using the Human Oral Microbiome Identification system (The Forsyth Institute, Cambridge, MA, USA).293, 294 The laboratory procedures of the Human Oral Microbiome Identification system follow a method modified from a previously published protocol.295 PCR amplification of DNA (10‐50 ng) was performed using universal primers targeting the V3‐V4 region of 16S genes (F341, R806). The products were purified using AMPure purification. Amplicons were pooled in libraries (100 ng) that were gel‐purified and quantified by quantitative PCR before being sequenced (MiSeq, Illumina, San Diego, CA, USA). In this study, reads were typically more than 50 000 per sample. The sequence read pairs were merged to single reads with a script (join_paired_ends.py) provided by quantitative insights into microbial ecology package version 1.91 with default settings. The merged reads were then taxonomically assigned to the species level based on a published algorithm296 with additional steps to further identify potential novel species. A complete description and the results of the taxonomy assignment are available online at http://www.homd.org/ftp/publication_data/20170412/ Basic local alignment search tool nucleotide was used to compare merged sequence reads with a panel of full‐length 16S ribosomal RNA sequences, consisting of 889 sequences from human oral microbiome database reference sequence V14.5, 495 from human oral microbiome database reference sequence extended V1.1, 3940 from GreenGeneGold, and 18 166 from the National Center for Biotechnology Information 16S rRNA reference. This combined reference set has a total of 23 490 sequences and represents 13 640 oral and non‐oral microbial species. After the taxonomy assignment, species‐level operational taxonomic units with at least 10 reads were subjected to several downstream bioinformatic analyses, including alpha and beta diversity assessments, provided in quantitative insights into microbial ecology. Samples with less than 500 read counts were excluded from the quantitative insights into microbial ecology analysis. Species‐level taxonomic plots were generated. Alpha and beta diversity measures were calculated for two‐ and four‐group analysis. Alpha diversity and species richness were evaluated with rarefaction plots using the operational taxonomic units as the metric. Beta diversity was evaluated with principal coordinate analysis plots, created using generalized UniFrac as the distance measurement based upon the R statistic generated using analysis of similarities.
Additionally, quantitative insights into microbial ecology data were used to employ galaxy for linear discriminant analysis effect size for metagenomic analysis. A histogram was generated to visualize the effect size (linear discriminant analysis) difference for two‐ and four‐group analysis. A cladogram taxonomic tree was generated to evaluate the phylogenetic comparisons for the two‐ and four‐group analyses at the species level.
Pirih FQ, Monajemzadeh S, Singh N, et al. Association between metabolic syndrome and periodontitis: The role of lipids, inflammatory cytokines, altered host response, and the microbiome. Periodontol 2000. 2021;87:50–75. 10.1111/prd.12379
Funding information
These studies were supported by funding from NIH/NIDCR (grant R01 DE025225) to Yvonne L. Kapila and J. Christopher Fenno. This funding source played no role in the design of the study and collection, analysis, and interpretation of data, or in the writing of the manuscript.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed by the current study are available online at http://www.homd.org/ftp/publication_data/20170412/qiime_results/cd_mc10/taxa_plots/taxa_summary_plots/bar_charts.html.
REFERENCES
- 1.Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. J Clin Periodontol. 2018;45(Suppl 20):S162‐S170. [DOI] [PubMed] [Google Scholar]
- 2.Eke PI, Thornton‐Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009–2014. J Am Dent Assoc. 2018;149(7):576‐588 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century–the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2003;31(Suppl 1):3‐23. [DOI] [PubMed] [Google Scholar]
- 4.Lalla E, Lamster IB, Drury S, Fu C, Schmidt AN. Hyperglycemia, glycoxidation and receptor for advanced glycation endproducts: potential mechanisms underlying diabetic complications, including diabetes‐associated periodontitis. Periodontology. 2000;23(1):50–62. [DOI] [PubMed] [Google Scholar]
- 5.Genco R, Offenbacher S, Beck J. Periodontal disease and cardiovascular disease: epidemiology and possible mechanisms. J Am Dent Assoc. 2002;133(Suppl):14S‐22S. [DOI] [PubMed] [Google Scholar]
- 6.Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TAPeriodontal disease and coronary heart disease risk. JAMA. 2000;284(11):1406‐1410. [DOI] [PubMed] [Google Scholar]
- 7.Stewart R, West M. Increasing Evidence for an Association Between Periodontitis and Cardiovascular Disease. Circulation. 2016;133(6):549‐551. [DOI] [PubMed] [Google Scholar]
- 8.Mealey BL, Oates TW, American Academy of Periodontology . Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77(8):1289‐1303. [DOI] [PubMed] [Google Scholar]
- 9.Minagawa K, Iwasaki M, Ogawa H, Yoshihara A, Miyazaki H. Relationship between metabolic syndrome and periodontitis in 80‐year‐old Japanese subjects. J Periodontal Res. 2015;50(2):173–179. 10.1111/jre.12190 [DOI] [PubMed] [Google Scholar]
- 10.Nesbitt M, Reynolds M, Shiau H, Choe K, Simonsick EM, Ferrucci L. Association of periodontitis and metabolic syndrome in the Baltimore Longitudinal Study of Aging. Aging Clinical and Experimental Research. 2010;22(3):238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Aiuto F, Sabbah W, Netuveli G, et al. Association of the metabolic syndrome with severe periodontitis in a large U.S. population‐based survey. J Clin Endocrinol Metab. 2008;93(10):3989‐3994. [DOI] [PubMed] [Google Scholar]
- 12.Simmons RK, Alberti KG, Gale KA, et al. The metabolic syndrome: useful concept or clinical tool? Report of a WHO Expert Consultation. Diabetologia. 2010;53(4):600‐605. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28(4):629‐636. [DOI] [PubMed] [Google Scholar]
- 14.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta‐analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403‐414. [DOI] [PubMed] [Google Scholar]
- 15.Lorenzo C, Williams K, Hunt KJ, Haffner SM. The National Cholesterol Education Program ‐ Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30(1):8‐13. [DOI] [PubMed] [Google Scholar]
- 16.Moore JX, Chaudhary N, Akinyemiju T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis. 2017;14:E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller JM, Kaylor MB, Johannsson M, Bay C, Churilla JR. Prevalence of metabolic syndrome and individual criterion in US adolescents: 2001–2010 National Health and Nutrition Examination Survey. Metab Syndr Relat Disord. 2014;12(10):527‐532. [DOI] [PubMed] [Google Scholar]
- 18.Day C. Metabolic syndrome, or What you will: definitions and epidemiology. Diab Vasc Dis Res. 2007;4(1):32‐38. [DOI] [PubMed] [Google Scholar]
- 19.Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III update: adjustments and options. Am J Cardiol. 2005;96(4A):53E‐59E. [DOI] [PubMed] [Google Scholar]
- 20.Expert Panel on Detection, E. and A . Treatment of High Blood Cholesterol in, Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) . JAMA. 2001;285(19):2486‐2497. [DOI] [PubMed] [Google Scholar]
- 21.American Heart A, National Heart L, Blood I, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13(6):322‐327. [PubMed] [Google Scholar]
- 22.Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb. 2011;18(8):629‐639. [DOI] [PubMed] [Google Scholar]
- 23.Arcaro G, Cretti A, Balzano S, et al. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation. 2002;105(5):576‐582. [DOI] [PubMed] [Google Scholar]
- 24.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113(15):1888‐1904. [DOI] [PubMed] [Google Scholar]
- 25.Sjoholm A, Nystrom T. Endothelial inflammation in insulin resistance. Lancet. 2005;365(9459):610‐612. [DOI] [PubMed] [Google Scholar]
- 26.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415‐1428. [DOI] [PubMed] [Google Scholar]
- 27.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640‐1645. [DOI] [PubMed] [Google Scholar]
- 28.Shimazaki Y, Saito T, Yonemoto K, Kiyohara Y, Iida M, Yamashita Y. Relationship of Metabolic Syndrome to Periodontal Disease in Japanese Women: The Hisayama Study. J Dental Res. 2007;86(3):271–275. 10.1177/154405910708600314 [DOI] [PubMed] [Google Scholar]
- 29.Morita T, Ogawa Y, Takada K, et al. Association between periodontal disease and metabolic syndrome. J Public Health Dent. 2009;69(4):248‐253. [DOI] [PubMed] [Google Scholar]
- 30.Andriankaja OM, Sreenivasa S, Dunford R, DeNardin E. Association between metabolic syndrome and periodontal disease. Aust Dent J. 2010;55(3):252‐259. [DOI] [PubMed] [Google Scholar]
- 31.Nibali L, Tatarakis N, Needleman I, et al. Clinical review: Association between metabolic syndrome and periodontitis: a systematic review and meta‐analysis. J Clin Endocrinol Metab. 2013;98(3):913‐920. [DOI] [PubMed] [Google Scholar]
- 32.Daudt LD, Musskopf ML, Mendez M, et al. Association between metabolic syndrome and periodontitis: a systematic review and meta‐analysis. Braz Oral Res. 2018;32:e35. [DOI] [PubMed] [Google Scholar]
- 33.Thanakun S, Watanabe H, Thaweboon Sroisiri, Izumi Y. Comparison of salivary and plasma adiponectin and leptin in patients with metabolic syndrome. Diabetol & Metab Syndr. 2014;6(1): 10.1186/1758-5996-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nascimento GG, Leite FRM, Peres KG, Demarco FF, Correa MB, Peres MA. Metabolic syndrome and periodontitis: A structural equation modeling approach. J Periodontol. 2019;90(6):655‐662. [DOI] [PubMed] [Google Scholar]
- 35.Iwasaki M, Sato M, Minagawa K, Manz MC, Yoshihara A, Miyazaki H. Longitudinal relationship between metabolic syndrome and periodontal disease among Japanese adults aged >/=70 years: the Niigata Study. J Periodontol. 2015;86(4):491‐498. [DOI] [PubMed] [Google Scholar]
- 36.Chen LP, Hsu SP, Peng YS, Chiang CK, Hung KY. Periodontal disease is associated with metabolic syndrome in hemodialysis patients. Nephrol Dial Transplantation. 2011;26(12):4068–4073. 10.1093/ndt/gfr209 [DOI] [PubMed] [Google Scholar]
- 37.Khader Y, Khassawneh B, Obeidat B, et al. Periodontal status of patients with metabolic syndrome compared to those without metabolic syndrome. J Periodontol. 2008;79(11):2048‐2053. [DOI] [PubMed] [Google Scholar]
- 38.Pham T. The association between periodontal disease severity and metabolic syndrome in Vietnamese patients. Int J Dent Hyg. 2018;16(4):484‐491. [DOI] [PubMed] [Google Scholar]
- 39.Sora ND, Marlow NM, Bandyopadhyay D, Leite RS, Slate EH, Fernandes JK. Metabolic syndrome and periodontitis in Gullah African Americans with type 2 diabetes mellitus. J Clin Periodontol. 2013;40(6):599‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morita T, Yamazaki Y, Mita A, et al. A cohort study on the association between periodontal disease and the development of metabolic syndrome. J Periodontol. 2010;81(4):512‐519. [DOI] [PubMed] [Google Scholar]
- 41.Bullon P, Jaramillo R, Santos‐Garcia R, et al. Relation of periodontitis and metabolic syndrome with gestational glucose metabolism disorder. J Periodontol. 2014;85(2):e1‐8. [DOI] [PubMed] [Google Scholar]
- 42.Kaye EK, Chen N, Cabral HJ, Vokonas P, Garcia RI. Metabolic Syndrome and Periodontal Disease Progression in Men. J Dent Res. 2016;95(7):822‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakurai SI, Yamada SI, Karasawa I, Sakurai A, Kurita H. A longitudinal study on the relationship between dental health and metabolic syndrome in Japan. J Periodontol. 2019;90(7):728‐746. [DOI] [PubMed] [Google Scholar]
- 44.Tegelberg P, Tervonen T, Knuuttila M, et al. Long‐term metabolic syndrome is associated with periodontal pockets and alveolar bone loss. J Clin Periodontol. 2019;46(8):799‐808. [DOI] [PubMed] [Google Scholar]
- 45.Adachi N, Kobayashi Y. One‐year follow‐up study on associations between dental caries, periodontitis, and metabolic syndrome. J Oral Sci. 2020;62(1):52‐56. [DOI] [PubMed] [Google Scholar]
- 46.Li P, He L, Sha YQ, Luan QX. Relationship of metabolic syndrome to chronic periodontitis. J Periodontol. 2009;80(4):541‐549. [DOI] [PubMed] [Google Scholar]
- 47.Kushiyama M, Shimazaki Y, Yamashita Y. Relationship between metabolic syndrome and periodontal disease in Japanese adults. J Periodontol. 2009;80(10):1610‐1615. [DOI] [PubMed] [Google Scholar]
- 48.Benguigui C, Bongard V, Ruidavets JB, et al. Metabolic syndrome, insulin resistance, and periodontitis: a cross‐sectional study in a middle‐aged French population. J Clin Periodontol. 2010;37(7):601‐608. [DOI] [PubMed] [Google Scholar]
- 49.Han DH, Lim SY, Sun BC, Paek D, Kim HD. The association of metabolic syndrome with periodontal disease is confounded by age and smoking in a Korean population: the Shiwha‐Banwol Environmental Health Study. J Clin Periodontol. 2010;37(7):609‐616. [DOI] [PubMed] [Google Scholar]
- 50.Gomes‐Filho IS, das Merces MC, de Santana Passos‐Soares J, et al. Severity of Periodontitis and Metabolic Syndrome: Is There an Association? J Periodontol. 2016;87(4):357‐366. [DOI] [PubMed] [Google Scholar]
- 51.Musskopf ML, Daudt LD, Weidlich P, Gerchman F, Gross JL, Oppermann RV. Metabolic syndrome as a risk indicator for periodontal disease and tooth loss. Clinl Oral Investig. 2017;21(2):675–683. 10.1007/s00784-016-1935-8 [DOI] [PubMed] [Google Scholar]
- 52.Jaramillo A, Contreras A, Lafaurie GI, et al. Association of metabolic syndrome and chronic periodontitis in Colombians. Clin Oral Investig. 2017;21(5):1537‐1544. [DOI] [PubMed] [Google Scholar]
- 53.Kikui M, Ono T, Kokubo Y, et al. Relationship between Metabolic Syndrome Components and Periodontal Disease in a Japanese General Population: the Suita Study. J Atheroscler Thromb. 2017;24(5):495‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campos JR, Costa FO, Cota LOM. Association between periodontitis and metabolic syndrome: A case‐control study. J Periodontol. 2020;91(6):784‐791. [DOI] [PubMed] [Google Scholar]
- 55.Gobin R, Tian D, Liu Q, Wang J. Periodontal Diseases and the Risk of Metabolic Syndrome: An Updated Systematic Review and Meta‐Analysis. Front in Endocrinol. 2020:11. 10.3389/fendo.2020.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe K, Cho YD. Periodontal disease and metabolic syndrome: a qualitative critical review of their association. Arch Oral Biol. 2014;59(8):855‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amar S, Zhou Q, Shaik‐Dasthagirisaheb Y, Leeman S. Diet‐induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc Natl Acad Sci USA. 2007;104(51):20466–20471. 10.1073/pnas.0710335105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin J, Machado ER, Yu H, et al. Simvastatin inhibits LPS‐induced alveolar bone loss during metabolic syndrome. J Dent Res. 2014;93(3):294‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Lu Z, Zhang X, et al. Metabolic syndrome exacerbates inflammation and bone loss in periodontitis. J Dent Res. 2015;94(2):362‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Furuta M, Shimazaki Y, Takeshita T, et al. Gender differences in the association between metabolic syndrome and periodontal disease: the Hisayama Study. J Clin Periodontol. 2013;40(8):743‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hildrum B, Mykletun A, Hole T, Midthjell K, Dahl A. Age‐specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: the Norwegian HUNT 2 study. BMC Public Health. 2007;7(1): 10.1186/1471-2458-7-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Velden U. The onset age of periodontal destruction. J Clin Periodontol. 1991;18(6):380‐383. [DOI] [PubMed] [Google Scholar]
- 63.Timonen P, Niskanen M, Suominen‐Taipale L, Jula A, Knuuttila M, Ylöstalo P. Metabolic Syndrome, Periodontal Infection, and Dental Caries. J Dent Res. 2010;89(10):1068–1073. 10.1177/0022034510376542 [DOI] [PubMed] [Google Scholar]
- 64.LaMonte MJ, Williams AM, Genco RJ, et al. Association between metabolic syndrome and periodontal disease measures in postmenopausal women: the Buffalo OsteoPerio study. J Periodontol. 2014;85(11):1489‐1501. [DOI] [PubMed] [Google Scholar]
- 65.Borges PKO, Gimeno SGA, Tomita NE, Ferreira SR. Prevalência e características associadas à síndrome metabólica em nipo‐brasileiros com e sem doença periodontal. Cad Saúde Pública. 2007;23(3):657–668. 10.1590/s0102-311x2007000300024 [DOI] [PubMed] [Google Scholar]
- 66.Kobayashi Y, Niu K, Guan L, et al. Oral health behavior and metabolic syndrome and its components in adults. J Dent Res. 2012;91(5):479‐484. [DOI] [PubMed] [Google Scholar]
- 67.Nishimura F, Soga Y, Iwamoto Y, Kudo C, Murayama Y. Periodontal disease as part of the insulin resistance syndrome in diabetic patients. J Int Acad Periodontol. 2005;7(1):16‐20. [PubMed] [Google Scholar]
- 68.Saito T, Shimazaki Y, Kiyohara Y, et al. The severity of periodontal disease is associated with the development of glucose intolerance in non‐diabetics: the Hisayama study. J Dent Res. 2004;83(6):485‐490. [DOI] [PubMed] [Google Scholar]
- 69.Saito T, Murakami M, Shimazaki Y, Matsumoto S, Yamashita Y. The Extent of Alveolar Bone Loss Is Associated With Impaired Glucose Tolerance in Japanese Men. J Periodontol. 2006;77(3):392–397. 10.1902/jop.2006.050061 [DOI] [PubMed] [Google Scholar]
- 70.Saito T, Murakami M, Shimazaki Y, Oobayashi K, Matsumoto S, Koga T. Association Between Alveolar Bone Loss and Elevated Serum C‐Reactive Protein in Japanese Men. J Periodontol. 2003;74(12):1741–1746. 10.1902/jop.2003.74.12.1741 [DOI] [PubMed] [Google Scholar]
- 71.D'Aiuto F, Parkar M, Nibali L, Suvan J, Lessem J, Tonetti MS. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: results from a randomized controlled clinical trial. Am Heart J. 2006;151(5):977‐984. [DOI] [PubMed] [Google Scholar]
- 72.D'Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83(2):156‐160. [DOI] [PubMed] [Google Scholar]
- 73.Lopez NJ, Quintero A, Casanova PA, Ibieta CI, Baelum V, Lopez R. Effects of periodontal therapy on systemic markers of inflammation in patients with metabolic syndrome: a controlled clinical trial. J Periodontol. 2012;83(3):267‐278. [DOI] [PubMed] [Google Scholar]
- 74.Karlsson F, Tremaroli V, Nielsen J, Backhed F. Assessing the Human Gut Microbiota in Metabolic Diseases. Diabetes. 2013;62(10):3341–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marchesan J, Jiao Y, Schaff RA, et al. TLR4, NOD1 and NOD2 mediate immune recognition of putative newly identified periodontal pathogens. Mol Oral Microbiol. 2016;31(3):243‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parekh PJ, Balart LA, Johnson DA. The Influence of the Gut Microbiome on Obesity, Metabolic Syndrome and Gastrointestinal Disease. Clin Transl Gastroenterol. 2015;6:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tam J, Hoffmann T, Fischer S, Bornstein S, Gräßler J, Noack B. Obesity alters composition and diversity of the oral microbiota in patients with type 2 diabetes mellitus independently of glycemic control. PLOS ONE. 2018;13(10):e0204724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turnbaugh PJ, Hamady M, Yatsunenko, et al. T. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541‐546. [DOI] [PubMed] [Google Scholar]
- 80.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL, Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134‐144. [DOI] [PubMed] [Google Scholar]
- 81.Lu Z, Li Y, Brinson CW, Kirkwood KL, Lopes‐Virella MF, Huang Y. CD36 is upregulated in mice with periodontitis and metabolic syndrome and involved in macrophage gene upregulation by palmitate. Oral Diseases. 2017;23(2):210–218. 10.1111/odi.12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dittmann C, Doueiri S, Kluge R, Dommisch H, Gaber T, Pischon N. Porphyromonas gingivalisSuppresses Differentiation and Increases Apoptosis of Osteoblasts From New Zealand Obese Mice. Journal of Periodontology. 2015;86(9):1095–1102. 10.1902/jop.2015.150032 [DOI] [PubMed] [Google Scholar]
- 83.Gotto AM Jr, Moon JE. Management of cardiovascular risk: the importance of meeting lipid targets. Am J Cardiol. 2012;110(1 Suppl):3A‐14A. [DOI] [PubMed] [Google Scholar]
- 84.Lu SY, Qi SD, Zhao Y, et al. Type 2 diabetes mellitus non‐genetic Rhesus monkey model induced by high fat and high sucrose diet. Exp Clin Endocrinol Diabetes. 2015;123(1):19‐26. [DOI] [PubMed] [Google Scholar]
- 85.Federation ID. IDF Diabetes Atlas, 8th edn. Brussels: Belgium; 2017. [Google Scholar]
- 86.National Diabetes Statitics Report 2020. Estimates of Diabetes and Its Burden in the United States . 2020.
- 87.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81‐90. [DOI] [PubMed] [Google Scholar]
- 88.Grp IDA. Update of mortality attributable to diabetes for the IDF Diabetes Atlas: Estimates for the year 2013. Diabetes Res Clin Pract. 2015;109(3):461‐465. [DOI] [PubMed] [Google Scholar]
- 89.Breyer MD, Tchekneva E, Qi Z, Takahashi T, Fogo AB, Harris RC. Examining diabetic nephropathy through the lens of mouse genetics. Curr Diab Rep. 2007;7(6):459‐466. [DOI] [PubMed] [Google Scholar]
- 90.Emerging Risk Factors, C . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet. 2010;375(9733):2215‐2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):e362‐425. [DOI] [PubMed] [Google Scholar]
- 92.Papatheodorou K, Banach M, Bekiari E, Rizzo M, Edmonds M. Complications of Diabetes 2017. Journal of Diabetes Research. 2018;20181–4. 10.1155/2018/3086167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16(1):329‐334. [PubMed] [Google Scholar]
- 94.Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol. 2001;6(1):99‐112. [DOI] [PubMed] [Google Scholar]
- 95.Borgnakke WS, Yl€ostalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. Journal of Periodontology. 2013;84(4‐s):S135–S152. 10.1902/jop.2013.1340013 [DOI] [PubMed] [Google Scholar]
- 96.Preferansow E, Golebiewska M, Kulikowska‐Bielaczyc E, Gorska M. The assessment of periodontium in patients with uncontrolled diabetes. Adv Med Sci. 2006;51(Suppl 1):170‐172. [PubMed] [Google Scholar]
- 97.Rajhans NS, Chaudhari VG, Kohad RM, Mhaske NH. A clinical study of the relationship between diabetes mellitus and periodontal disease. J of Indian Soc of Periodontol. 2011;15(4):388. 10.4103/0972-124x.92576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Apoorva SM, Sridhar N, Suchetha A. Prevalence and severity of periodontal disease in type 2 diabetes mellitus (non‐insulin‐dependent diabetes mellitus) patients in Bangalore city: An epidemiological study. J Indian Soc Periodontol. 2013;17(1):25‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jimenez M, Hu FB, Marino M, Li Y, Joshipura K J. Type 2 diabetes mellitus and 20 year incidence of periodontitis and tooth loss. Diabetes Res and Clin Pract. 2012;98(3):494–500. 10.1016/j.diabres.2012.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30(3):182‐192. [DOI] [PubMed] [Google Scholar]
- 101.Firatli E. The relationship between clinical periodontal status and insulin‐dependent diabetes mellitus. Results after 5 years. J Periodontol. 1997;68(2):136‐140. [DOI] [PubMed] [Google Scholar]
- 102.Dhir S, Wangnoo S, Kumar V. Impact of Glycemic Levels in Type 2 Diabetes on Periodontitis. Indian J Endocrinol Metab. 2018;22(5):672‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee CY, Kuan YH, Tsai YF, Tai CJ, Tsai TH, Huang KH. Correlation between diabetes mellitus and periodontitis in Taiwan: A nationwide cohort study. Diabetes Res and Clin Pract. 2019;150:245–252. 10.1016/j.diabres.2019.03.019 [DOI] [PubMed] [Google Scholar]
- 104.Haseeb M, Khawaja KI, Ataullah K, Munir MB, Fatima A. Periodontal disease in type 2 diabetes mellitus. J Coll Physicians Surg Pak. 2012;22(8):514‐518. [PubMed] [Google Scholar]
- 105.Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non‐insulin‐dependent diabetes mellitus. J Periodontol. 1991;62(2):123‐131. [DOI] [PubMed] [Google Scholar]
- 106.Shlossman M, Knowler WC, Pettitt D J, Genco RJ. Type 2 Diabetes Mellitus and Periodontal Disease. J Am Dent Assoc. 1990;121(4):532–536. 10.14219/jada.archive.1990.0211 [DOI] [PubMed] [Google Scholar]
- 107.Novak MJ, Potter RM, Blodgett J, Ebersole JL. Periodontal Disease in Hispanic Americans With Type 2 Diabetes. J Periodontol. 2008;79(4):629–636. 10.1902/jop.2008.070442 [DOI] [PubMed] [Google Scholar]
- 108.Lalla E, Cheng B, Lal S, et al. Diabetes mellitus promotes periodontal destruction in children. J Clin Periodontol. 2007;34(4):294‐298. [DOI] [PubMed] [Google Scholar]
- 109.Kowall B, Holtfreter B, Volzke H, et al. Pre‐diabetes and well‐controlled diabetes are not associated with periodontal disease: the SHIP Trend Study. J Clin Periodontol. 2015;42(5):422‐430. [DOI] [PubMed] [Google Scholar]
- 110.Firatli E, Yilmaz O, Onan U. The relationship between clinical attachment loss and the duration of insulin‐dependent diabetes mellitus (IDDM) in children and adolescents. J Clin Periodontol. 1996;23(4):362‐366. [DOI] [PubMed] [Google Scholar]
- 111.Taylor GW. Glycemic control and alveolar bone loss progression in type 2 diabetes. Ann Periodontol. 1998;3(1):30‐39. [DOI] [PubMed] [Google Scholar]
- 112.Nelson RG, Shlossman M, Budding LM, et al. Periodontal disease and NIDDM in Pima Indians. Diabetes Care. 1990;13(8):836‐840. [DOI] [PubMed] [Google Scholar]
- 113.Takeda M, Ojima M, Yoshioka H, et al. Relationship of serum advanced glycation end products with deterioration of periodontitis in type 2 diabetes patients. J Periodontol. 2006;77(1):15‐20. [DOI] [PubMed] [Google Scholar]
- 114.Dakovic D, Pavlovic MD. Periodontal disease in children and adolescents with type 1 diabetes in Serbia. J Periodontol. 2008;79(6):987‐992. [DOI] [PubMed] [Google Scholar]
- 115.Susanto H, Nesse W, Dijkstra PU, Agustina D, Vissink A, Abbas F. Periodontitis Prevalence and Severity in Indonesians With Type 2 Diabetes. Journal of Periodontology. 2011;82(4):550–557. 10.1902/jop.2010.100285 [DOI] [PubMed] [Google Scholar]
- 116.Plessas A, Robertson DP, Hodge PJ. Radiographic bone loss in a Scottish non‐smoking type 1 diabetes mellitus population: A bitewing radiographic study. J Periodontol. 2018;89(9):1043‐1051. [DOI] [PubMed] [Google Scholar]
- 117.Fernandes JK, Wiegand RE, Salinas CF, et al. Periodontal disease status in gullah african americans with type 2 diabetes living in South Carolina. J Periodontol. 2009;80(7):1062‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Costa FO, Miranda Cota LO, Pereira Lages EJ, et al. Progression of periodontitis and tooth loss associated with glycemic control in individuals undergoing periodontal maintenance therapy: a 5‐year follow‐up study. J Periodontol. 2013;84(5):595‐605. [DOI] [PubMed] [Google Scholar]
- 119.Noack B, Jachmann I, Roscher S, et al. Metabolic diseases and their possible link to risk indicators of periodontitis. J Periodontol. 2000;71(6):898‐903. [DOI] [PubMed] [Google Scholar]
- 120.Hugoson A, Thorstensson H, Faltt H, Kuylenstierna J. Periodontal conditions in insulin‐dependent diabetics. Journal of Clinical Periodontology. 1989;16(4):215–223. 10.1111/j.1600-051x.1989.tb01644.x [DOI] [PubMed] [Google Scholar]
- 121.Amiri AA, Maboudi A, Bahar A, et al. Relationship between Type 2 Diabetic Retinopathy and Periodontal Disease in Iranian Adults. N Am J Med Sci. 2014;6(3):139‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Newton KM, Chaudhari M, Barlow WE, et al. A population‐based study of periodontal care among those with and without diabetes. J Periodontol. 2011;82(12):1650‐1656. [DOI] [PubMed] [Google Scholar]
- 123.Akram Z, Alqahtani F, Alqahtani M, Al‐Kheraif AA, Javed F. Levels of advanced glycation end products in gingival crevicular fluid of chronic periodontitis patients with and without type‐2 diabetes mellitus. J Periodontol. 2020;91(3):396–402. 10.1002/jper.19-0209 [DOI] [PubMed] [Google Scholar]
- 124.Engebretson SP, Hey‐Hadavi J, Ehrhardt FJ, et al. Gingival crevicular fluid levels of interleukin‐1beta and glycemic control in patients with chronic periodontitis and type 2 diabetes. J Periodontol. 2004;75(9):1203‐1208. [DOI] [PubMed] [Google Scholar]
- 125.Monea A, Mezei T, Popsor S, Monea M. Oxidative Stress: A Link between Diabetes Mellitus and Periodontal Disease. Int J of Endocrinol. 2014;20141–4. 10.1155/2014/917631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Festa A, D’Agostino R, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic Subclinical Inflammation as Part of the Insulin Resistance Syndrome. Circulation. 2000;102(1):42–47. 10.1161/01.cir.102.1.42 [DOI] [PubMed] [Google Scholar]
- 127.Pradhan AD, Manson JE, Rifai N, Buring JE, Ricker PM. C‐reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327‐334. [DOI] [PubMed] [Google Scholar]
- 128.Garrett S, Adams DF, Bogle G, et al. The effect of locally delivered controlled‐release doxycycline or scaling and root planing on periodontal maintenance patients over 9 months. J Periodontol. 2000;71(1):22‐30. [DOI] [PubMed] [Google Scholar]
- 129.Kebede TG, Pink C, Rathmann W, et al. Does periodontitis affect diabetes incidence and haemoglobin A1c change? An 11‐year follow‐up study. Diabetes Metab. 2018;44(3):243‐249. [DOI] [PubMed] [Google Scholar]
- 130.Allen EM, Matthews JB, O' Halloran DJ, Griffiths HR, Chapple IL. Oxidative and inflammatory status in Type 2 diabetes patients with periodontitis. Journal of Clinical Periodontology. 2011;38(10):894–901. 10.1111/j.1600-051x.2011.01764.x [DOI] [PubMed] [Google Scholar]
- 131.Agrawal P, Srinivasa TS, Goyal P, Farista S, Sowmya NK, Deonani S. Comparative clinical evaluation of glycosylated haemoglobin level in healthy and chronic periodontitis patients: A chairside diagnostic method. Indian Journal of Dental Research. 2015;26(5):504. 10.4103/0970-9290.172049 [DOI] [PubMed] [Google Scholar]
- 132.Costa KL, Taboza ZA, Angelino GB, et al. Influence of Periodontal Disease on Changes of Glycated Hemoglobin Levels in Patients With Type 2 Diabetes Mellitus: A Retrospective Cohort Study. J Periodontol. 2017;88(1):17‐25. [DOI] [PubMed] [Google Scholar]
- 133.Lin SY, Lin CL, Liu JH. Association between periodontitis needing surgical treatment and subsequent diabetes risk: a population‐based cohort study. J Periodontol. 2014;85(6):779‐786. [DOI] [PubMed] [Google Scholar]
- 134.Saremi A, Nelson RG, Tulloch‐Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 135.Rosenthal IM, Abrams H, Kopczyk RA. The Relationship of Inflammatory Periodontal‐Disease to Diabetic Status in Insulin‐Dependent Diabetes‐Mellitus Patients. J Clin Periodontol. 1988;15(7):425‐429. [DOI] [PubMed] [Google Scholar]
- 136.Llambés F, Silvestre FJ, Hernández‐Mijares A, Guiha R, Caffesse R. The effect of periodontal treatment on metabolic control of type 1 diabetes mellitus. Clin Oral Investig. 2008;12(4):337–343. 10.1007/s00784-008-0201-0 [DOI] [PubMed] [Google Scholar]
- 137.Demmer RT, Desvarieux M, Holtfreter B, et al. Periodontal status and A1C change: longitudinal results from the study of health in Pomerania (SHIP). Diabetes Care. 2010;33(5):1037‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Izuora Ke, Ezeanolue E, Schlauch K, Neubauer M, Gewelber C, Umpierrez G. Impact of periodontal disease on outcomes in diabetes. Contemp Clin Trials. 2015;41:93–99. 10.1016/j.cct.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen L, Wei B, Li J, et al. Association of periodontal parameters with metabolic level and systemic inflammatory markers in patients with type 2 diabetes. J Periodontol. 2010;81(3):364‐371. [DOI] [PubMed] [Google Scholar]
- 140.Longo PL, Artese HP, Rabelo MS, et al. Serum levels of inflammatory markers in type 2 diabetes patients with chronic periodontitis. J Appl Oral Sci. 2014;22(2):103‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gokhale NH, Acharya AB, Patil VS, Trivedi DJ, Setty S, Thakur SL. Resistin Levels in Gingival Crevicular Fluid of Patients With Chronic Periodontitis and Type 2 Diabetes Mellitus. J Periodontol. 2014;85(4):610–617. 10.1902/jop.2013.130092 [DOI] [PubMed] [Google Scholar]
- 142.Islam SK, Seo M, Lee YS, Moon SS. Association of periodontitis with insulin resistance, β‐cell function, and impaired fasting glucose before onset of diabetes. Endocrine Journal. 2015;62(11):981–989. 10.1507/endocrj.ej15-0350 [DOI] [PubMed] [Google Scholar]
- 143.Perayil J, Suresh N, Fenol A, Vyloppillil R, Bhaskar A, Menon S. Comparison of Glycated Hemoglobin Levels in Individuals Without Diabetes and With and Without Periodontitis Before and After Non‐Surgical Periodontal Therapy. J Periodontol. 2014;85(12):1658–1666. 10.1902/jop.2014.130661 [DOI] [PubMed] [Google Scholar]
- 144.Rao Deepika PC, Saxena RM. Comparison of glycosylated hemoglobin levels in severe periodontitis patients and healthy controls: a study in an Indian population. Quintessence Int. 2013;44(4):319‐325. [DOI] [PubMed] [Google Scholar]
- 145.Ide R, Hoshuyama T, Wilson D, Takahashi K, Higashi T. Periodontal Disease and Incident Diabetes. J Dent Res. 2011;90(1):41–46. 10.1177/0022034510381902 [DOI] [PubMed] [Google Scholar]
- 146.Demmer RT, Jacobs DR Jr, Desvarieux M. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow‐up study. Diabetes Care. 2008;31(7):1373‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Winning L, Patterson CC, Neville CE, Kee F, Linden GJ. Periodontitis and incident type 2 diabetes: a prospective cohort study. J Clin Periodontol. 2017;44(3):266–274. 10.1111/jcpe.12691 [DOI] [PubMed] [Google Scholar]
- 148.Miyawaki A, Toyokawa S, Inoue K, Miyoshi Y, Kobayashi Y. Self‐Reported Periodontitis and Incident Type 2 Diabetes among Male Workers from a 5‐Year Follow‐Up to MY Health Up Study. PLOS ONE. 2016;11(4):e0153464. 10.1371/journal.pone.0153464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ide R, Hoshuyama T, Wilson D, Takahashi K, Higashi T. Periodontal Disease and Incident Diabetes. Journal of Dental Research. 2011;90(1):41–46. 10.1177/0022034510381902 [DOI] [PubMed] [Google Scholar]
- 150.Taylor GW, Burt BA, Becker MP, et al. Severe Periodontitis and Risk for Poor Glycemic Control in Patients with Non‐Insulin‐Dependent Diabetes Mellitus. J Periodontol. 1996;67(Suppl 10S):1085‐1093. [DOI] [PubMed] [Google Scholar]
- 151.Chang JF, Yeh JC, Chiu YL, Liou JC, Hsiung JR, Tung TH. Periodontal Pocket Depth, Hyperglycemia, and Progression of Chronic Kidney Disease: A Population‐Based Longitudinal Study. Am J Med. 2017;130(1):61–69.e1. 10.1016/j.amjmed.2016.08.024 [DOI] [PubMed] [Google Scholar]
- 152.Boillot A, Bouchard P, Moss K, Offenbacher S, Czernichow S. Periodontitis and retinal microcirculation in the Atherosclerosis Risk in Communities study. J Clin Periodontol. 2015;42(4):342–349. 10.1111/jcpe.12388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Amiri AA, Maboudi A, Bahar A, et al. Relationship between type 2 diabetic retinopathy and periodontal disease in Iranian adults. N Am J Med Sci. 2014;6(4):190. [PMC free article] [PubMed] [Google Scholar]
- 154.Chang JS, Tsai CR, Chen LT, Shan YS. Investigating the Association Between Periodontal Disease and Risk of Pancreatic Cancer. Pancreas. 2016;45(1):134–141. 10.1097/mpa.0000000000000419 [DOI] [PubMed] [Google Scholar]
- 155.Sharma P, Dietrich T, Ferro CJ, Cockwell P, Chapple ILC. Association between periodontitis and mortality in stages 3–5 chronic kidney disease: NHANES III and linked mortality study. J of Clin Periodontol. 2016;43(2):104–113. 10.1111/jcpe.12502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shultis WA, Weil EJ, Looker HC, et al. Effect of periodontitis on overt nephropathy and end‐stage renal disease in type 2 diabetes. Diabetes Care. 2007;30(2):306‐311. [DOI] [PubMed] [Google Scholar]
- 157.Abrao L, Chagas JK, Schmid H. Periodontal disease and risk for neuropathic foot ulceration in type 2 diabetes. Diabetes Res Clin Pract. 2010;90(1):34‐39. [DOI] [PubMed] [Google Scholar]
- 158.D'Aiuto F, Gkranias N, Bhowruth D, et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single‐centre, investigator‐masked, randomised trial. Lancet Diabetes Endocrino. 2018;6(12):954‐965. [DOI] [PubMed] [Google Scholar]
- 159.Iwamoto Y, Nishimura F, Nakagawa M, et al. The effect of antimicrobial periodontal treatment on circulating tumor necrosis factor‐alpha and glycated hemoglobin level in patients with type 2 diabetes. J Periodontol. 2001;72(6):774‐778. [DOI] [PubMed] [Google Scholar]
- 160.O'Connell PA, Taba M, Nomizo A, et al. Effects of periodontal therapy on glycemic control and inflammatory markers. J Periodontol. 2008;79(5):774‐783. [DOI] [PubMed] [Google Scholar]
- 161.Engebretson SP, Hey‐Hadavi J. Sub‐antimicrobial doxycycline for periodontitis reduces hemoglobin A1c in subjects with type 2 diabetes: A pilot study. Pharmacol Res. 2011;64(6):624‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mauri‐Obradors E, Merlos A, Estrugo‐Devesa A, Jané‐Salas E, López‐López J, Viñas M. Benefits of non‐surgical periodontal treatment in patients with type 2 diabetes mellitus and chronic periodontitis: A randomized controlled trial. J Clin Periodontol. 2018;45(3):345–353. 10.1111/jcpe.12858 [DOI] [PubMed] [Google Scholar]
- 163.Spangler L, Reid RJ, Inge R, et al. Cross‐Sectional Study of Periodontal Care and Glycosylated Hemoglobin in an Insured Population. Diabetes Care. 2010;33(8):1753‐1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vergnes JN, Canceill T, Vinel A, et al. The effects of periodontal treatment on diabetic patients: The DIAPERIO randomized controlled trial. J Clin Periodontol. 2018;45(10):1150‐1163. [DOI] [PubMed] [Google Scholar]
- 165.Aldridge JP, Lester V, Watts TLP, Collins A, Viberti G, Wilson RF. Single‐blind studies of the effects of improved periodontal health on metabolic control in Type 1 diabetes mellitus. J Clin Periodontol. 1995;22(4):271–275. 10.1111/j.1600-051x.1995.tb00147.x [DOI] [PubMed] [Google Scholar]
- 166.Lalla E, Lamster IB, Feit M, et al. Blockade of RAGE suppresses periodontitis‐associated bone loss in diabetic mice. J Clin Invest. 2000;105(8):1117‐1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Holzhausen M, Garcia DF, Pepato MT, Marcantonio E. The influence of short‐term diabetes mellitus and insulin therapy on alveolar bone loss in rats. J Periodontal Res. 2004;39(3):188–193. 10.1111/j.1600-0765.2004.00723.x [DOI] [PubMed] [Google Scholar]
- 168.Liu R, Bal HS, Desta T, et al. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J Dent Res. 2006;85(6):510‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang Q, Zhang P, Aprecio R, et al. Comparison of Experimental Diabetic Periodontitis Induced by Porphyromonas gingivalis in Mice. J Diabetes Res. 2016;2016:4840203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jiang ZL, Cui YQ, Gao R, et al. Study of TNF‐alpha, IL‐1beta and LPS levels in the gingival crevicular fluid of a rat model of diabetes mellitus and periodontitis. Dis Markers. 2013;34(5):295‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kim JH, Lee DE, Gunawardhana KS, et al. Effect of the interaction between periodontitis and type 1 diabetes mellitus on alveolar bone, mandibular condyle and tibia. Acta Odontol Scand. 2014;72(4):265‐273. [DOI] [PubMed] [Google Scholar]
- 172.Claudino M, Ceolin DS, Alberti S, et al. Alloxan‐induced diabetes triggers the development of periodontal disease in rats. PLoS One. 2007;2(12):e1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Takai N, Shinohara M, Yoshida Y, Ohura K, Mori M, Kakudo Y. Effect of Streptozotocin Diabetes on Gingivitis in Plaque‐susceptible Rats. J Dent Res. 1986;65(1):49–52. 10.1177/00220345860650010801 [DOI] [PubMed] [Google Scholar]
- 174.Fu YW, He HB. Apoptosis of periodontium cells in streptozototocin‐ and ligature‐induced experimental diabetic periodontitis in rats. Acta Odontol Scand. 2013;71(5):1206‐1215. [DOI] [PubMed] [Google Scholar]
- 175.Li X, Sun X, Zhang X, et al. Enhanced Oxidative Damage and Nrf2 Downregulation Contribute to the Aggravation of Periodontitis by Diabetes Mellitus. Oxid Med Cell Longev. 2018;2018:9421019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tunali M, Ataoglu T, Celik I. Apoptosis: an underlying factor for accelerated periodontal disease associated with diabetes in rats. Clin Oral Investig. 2014;18(7):1825‐1833. [DOI] [PubMed] [Google Scholar]
- 177.Kim JH, Lee DE, Woo GH, Cha JH, Bak EJ, Yoo YJ. Osteocytic Sclerostin Expression in Alveolar Bone in Rats With Diabetes Mellitus and Ligature‐Induced Periodontitis. J Periodontol. 2015;86(8):1005–1011. 10.1902/jop.2015.150083 [DOI] [PubMed] [Google Scholar]
- 178.Nishikawa T, Naruse K, Kobayashi Y, et al. Involvement of nitrosative stress in experimental periodontitis in diabetic rats. J Clin Periodontol. 2012;39(4):342‐349. [DOI] [PubMed] [Google Scholar]
- 179.Yang X, Wang B, Wang T, et al. Toll‐like receptor 4‐mediated hyper‐responsiveness of gingival epithelial cells to lipopolysaccharide in high‐glucose environments. J Periodontol. 2014;85(11):1620‐1628. [DOI] [PubMed] [Google Scholar]
- 180.Jiang SY, Wei CC, Shang TT, Lian Q, Wu CX, Deng JY. High glucose induces inflammatory cytokine through protein kinase C‐induced toll‐like receptor 2 pathway in gingival fibroblasts. Biochem Biophys Res Commun. 2012;427(3):666–670. 10.1016/j.bbrc.2012.09.118 [DOI] [PubMed] [Google Scholar]
- 181.Catalfamo DL, Britten TM, Storch DI, Calderon NL, Sorenson HL, Wallet SM. Hyperglycemia induced and intrinsic alterations in type 2 diabetes‐derived osteoclast function. Oral Dis. 2013;19(3):303–312. 10.1111/odi.12002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sun X, Mao Y, Dai P, et al. Mitochondrial dysfunction is involved in the aggravation of periodontitis by diabetes. J Clin Periodontol. 2017;44(5):463‐471. [DOI] [PubMed] [Google Scholar]
- 183.Sun S, Zhang D, Wu Y, et al. The expression of inducible nitric oxide synthase in the gingiva of rats with periodontitis and diabetes mellitus. Arch Oral Biol. 2020;112:104652. [DOI] [PubMed] [Google Scholar]
- 184.Pontes Andersen CC, Buschard K, Flyvbjerg A, Stoltze K, Holmstrup P. Periodontitis Deteriorates Metabolic Control in Type 2 Diabetic Goto‐Kakizaki Rats. J Periodontol. 2006;77(3):350–356. 10.1902/jop.2006.050184 [DOI] [PubMed] [Google Scholar]
- 185.Watanabe K, Petro BJ, Shlimon AE, Unterman TG. Effect of Periodontitis on Insulin Resistance and the Onset of Type 2 Diabetes Mellitus in Zucker Diabetic Fatty Rats. J Periodontol. 2008;79(7):1208–1216. 10.1902/jop.2008.070605 [DOI] [PubMed] [Google Scholar]
- 186.Liu Y, Zhang Q. Periodontitis aggravated pancreatic beta‐cell dysfunction in diabetic mice through interleukin‐12 regulation on Klotho. J Diabetes Investig. 2016;7(3):303‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pontes Andersen CC, Flyvbjerg A, Buschard K, Holmstrup P. Periodontitis Is Associated With Aggravation of Prediabetes in Zucker Fatty Rats. J Periodontol. 2007;78(3):559–565. 10.1902/jop.2007.060358 [DOI] [PubMed] [Google Scholar]
- 188.Xynogala I, Pepelassi E, Perrea D, et al. Adiponectin and interleukin‐6 levels in insulin‐treated diabetic rats with experimental periodontitis. Braz Oral Res. 2012;26(1):71‐76. [DOI] [PubMed] [Google Scholar]
- 189.Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut. 2014;63(9):1513‐1521. [DOI] [PubMed] [Google Scholar]
- 190.Tai N, Wong FS, Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord. 2015;16(1):55‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99‐103. [DOI] [PubMed] [Google Scholar]
- 192.Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7(4):880‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151‐1156. [DOI] [PubMed] [Google Scholar]
- 194.Casarin RC, Barbagallo A, Meulman T, et al. Subgingival biodiversity in subjects with uncontrolled type‐2 diabetes and chronic periodontitis. J Periodontal Res. 2013;48(1):30‐36. [DOI] [PubMed] [Google Scholar]
- 195.Farina R, Severi M, Carrieri A, et al. Whole metagenomic shotgun sequencing of the subgingival microbiome of diabetics and non‐diabetics with different periodontal conditions. Arch Oral Biol. 2019;104:13‐23. [DOI] [PubMed] [Google Scholar]
- 196.Yang Y, Liu S, Wang Y, et al. Changes of saliva microbiota in the onset and after the treatment of diabetes in patients with periodontitis. Aging (Albany NY). 2020;12(13):13090‐13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Matsha TE, Prince Y, Davids S, et al. Oral Microbiome Signatures in Diabetes Mellitus and Periodontal Disease. J Dent Res. 2020;99(6):658‐665. [DOI] [PubMed] [Google Scholar]
- 198.Sabharwal A, Ganley K, Miecznikowski JC, Haase EM, Barnes V, Scannapieco FA. The salivary microbiome of diabetic and non‐diabetic adults with periodontal disease. J Periodontol. 2019;90(1):26–34. 10.1002/jper.18-0167 [DOI] [PubMed] [Google Scholar]
- 199.Ogawa T, Honda‐Ogawa M, Ikebe K, et al. Characterizations of oral microbiota in elderly nursing home residents with diabetes. J Oral Sci. 2017;59(4):549‐555. [DOI] [PubMed] [Google Scholar]
- 200.Lambeth SM, Carson T, Lowe J, et al. Composition, Diversity and Abundance of Gut Microbiome in Prediabetes and Type 2 Diabetes. J Diabetes Obes. 2015;2(3):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Longo PL, Dabdoub S, Kumar P, et al. Glycaemic status affects the subgingival microbiome of diabetic patients. J Clin Periodontol. 2018;45(8):932‐940. [DOI] [PubMed] [Google Scholar]
- 202.Shi B, Lux R, Klokkevold P, et al. The subgingival microbiome associated with periodontitis in type 2 diabetes mellitus. ISME J. 2020;14(2):519‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Aemaimanan P, Amimanan P, Taweechaisupapong S. Quantification of key periodontal pathogens in insulin‐dependent type 2 diabetic and non‐diabetic patients with generalized chronic periodontitis, et al. Anaerobe. 2013;22:64–68. 10.1016/j.anaerobe.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 204.Babaev EA, Balmasova IP, Mkrtumyan AM, et al. Metagenomic Analysis of Gingival Sulcus Microbiota and Pathogenesis of Periodontitis Associated with Type 2 Diabetes Mellitus. Bull Exp Biol Med. 2017;163(6):718‐721. [DOI] [PubMed] [Google Scholar]
- 205.Rodríguez‐Hernández AP, Márquez‐Corona ML, Pontigo‐Loyola AP, Medina‐Solís CE, Ximenez‐Fyvie LA. Subgingival Microbiota of Mexicans with Type 2 Diabetes with Different Periodontal and Metabolic Conditions. Int J Environ Res Public Health. 2019;16(17):3184. 10.3390/ijerph16173184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhou M, Rong R, Munro D, et al. Investigation of the effect of type 2 diabetes mellitus on subgingival plaque microbiota by high‐throughput 16S rDNA pyrosequencing. PLoS One. 2013;8(4):e61516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li C, Liu J, Tan L, et al. The sociodemographic characteristics, periodontal health status, and subgingival microbiota of patients with chronic periodontitis and type 2 diabetes mellitus: a case‐control study in a Chinese population. J Periodontol. 2013;84(8):1058‐1066. [DOI] [PubMed] [Google Scholar]
- 208.Sardi GL, Maluenda G, Torguson R, et al. Impact of diabetes mellitus on long‐term clinical outcomes of patients on chronic hemodialysis after percutaneous coronary intervention, et al. J Interv Cardiol. 2012;25(2):147‐155. [DOI] [PubMed] [Google Scholar]
- 209.Campus G, Salem A, Uzzau S, Baldoni E, Tonolo G. Diabetes and Periodontal Disease: A Case‐Control Study. J Periodontol. 2005;76(3):418–425. 10.1902/jop.2005.76.3.418 [DOI] [PubMed] [Google Scholar]
- 210.Silva‐Boghossian CM, Orrico SRP, Gonçalves D, Correa FOB, Colombo APV. Microbiological changes after periodontal therapy in diabetic patients with inadequate metabolic control. Brazilian Oral Research. 2014;28(1):1–9. 10.1590/1807-3107bor-2014.vol28.0007 [DOI] [PubMed] [Google Scholar]
- 211.Ganesan SM, Joshi V, Fellows M, et al. A tale of two risks: smoking, diabetes and the subgingival microbiome. ISME J. 2017;11(9):2075‐2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Goodson JM, Hartman ML, Shi P, et al. The salivary microbiome is altered in the presence of a high salivary glucose concentration. PLoS One. 2017;12(3):e0170437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Janem WF, Scannapieco FA, Sabharwal A, et al. Salivary inflammatory markers and microbiome in normoglycemic lean and obese children compared to obese children with type 2 diabetes. PLoS One. 2017;12(3):e0172647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics‐2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146‐e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Organization WH. Cardiovascular Diseases (CVDs). Fact Sheet. 2017. [Google Scholar]
- 216.Mortality GBD, C. Causes of Death . Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Libby P, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5(1):56. [DOI] [PubMed] [Google Scholar]
- 218.Holdt LM, Teupser D. From genotype to phenotype in human atherosclerosis–recent findings. Curr Opin Lipidol. 2013;24(5):410‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Joseph P, Leong D, McKee M, et al. Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ Res. 2017;121(6):677‐694. [DOI] [PubMed] [Google Scholar]
- 220.Fryar CD, Chen TC, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999–2010. NCHS Data Brief. 2012;103:1‐8. [PubMed] [Google Scholar]
- 221.Mattila KJ, Nieminen MS, Valtonen VV, et al. Association between dental health and acute myocardial infarction. BMJ. 1989;298(6676):779‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sanz M, Marco Del Castillo A, Jepsen S, et al. Periodontitis and cardiovascular diseases: Consensus report. J Clin Periodontol. 2020;47(3):268‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Delange N, Lindsay S, Lemus H, Finlayson TL, Kelley ST, Gottlieb RA. Periodontal disease and its connection to systemic biomarkers of cardiovascular disease in young American Indian/Alaskan natives. Journal of Periodontology. 2018;89(2):219–227. 10.1002/jper.17-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ramirez JH, Parra B, Gutierrez S, et al. Biomarkers of cardiovascular disease are increased in untreated chronic periodontitis: a case control study. Aust Dent J. 2014;59(1):29‐36. [DOI] [PubMed] [Google Scholar]
- 225.Koppolu P, Durvasula S, Palaparthy R, et al. Estimate of CRP and TNF‐alpha level before and after periodontal therapy in cardiovascular disease patients. Pan Afr Med J. 2013;15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chopra R, Patil SR, Kalburgi NB, Mathur S. Association between alveolar bone loss and serum C‐reactive protein levels in aggressive and chronic periodontitis patients. J Indian So Periodontol. 2012;16(1):28. 10.4103/0972-124x.94600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hosomi N, Aoki S, Matsuo K, et al. Association of serum anti‐periodontal pathogen antibody with ischemic stroke. Cerebrovasc Dis. 2012;34(5–6):385‐392. [DOI] [PubMed] [Google Scholar]
- 228.Winning L, Patterson CC, Cullen KM, et al. The association between subgingival periodontal pathogens and systemic inflammation. J Clin Periodontol. 2015;42(9):799‐806. [DOI] [PubMed] [Google Scholar]
- 229.Diaz CM, Bullon B, Ruiz‐Salmeron RJ, et al. Molecular inflammation and oxidative stress are shared mechanisms involved in both myocardial infarction and periodontitis. J Periodontal Res. 2020;55(4):519‐528. [DOI] [PubMed] [Google Scholar]
- 230.Gomes‐Filho IS, Coelho JMF, Miranda SS, et al. Severe and moderate periodontitis are associated with acute myocardial infarction. J Periodontol. 2020. [DOI] [PubMed] [Google Scholar]
- 231.Renvert S, Ohlsson O, Pettersson T, Persson GR. Periodontitis: A Future Risk of Acute Coronary Syndrome? A Follow‐Up Study Over 3 Years. Journal of Periodontology. 2010;81(7):992–1000. 10.1902/jop.2010.090105 [DOI] [PubMed] [Google Scholar]
- 232.Ryden L, Buhlin K, Ekstrand E, et al. Periodontitis Increases the Risk of a First Myocardial Infarction: A Report From the PAROKRANK Study. Circulation. 2016;133(6):576‐583. [DOI] [PubMed] [Google Scholar]
- 233.Reichert S, Schulz S, Benten AC, et al. Periodontal conditions and incidence of new cardiovascular events among patients with coronary vascular disease. J Clin Periodontol. 2016;43(11):918‐925. [DOI] [PubMed] [Google Scholar]
- 234.Dorn JM, Genco RJ, Grossi SG, et al. Periodontal disease and recurrent cardiovascular events in survivors of myocardial infarction (MI): the Western New York Acute MI Study. J Periodontol. 2010;81(4):502‐511. [DOI] [PubMed] [Google Scholar]
- 235.Zanella SM, Pereira SS, Barbisan JN, et al. Periodontal disease, tooth loss and coronary heart disease assessed by coronary angiography: a cross‐sectional observational study. J Periodontal Res. 2016;51(2):221‐227. [DOI] [PubMed] [Google Scholar]
- 236.D'Aiuto F, Ready D, Tonetti MS. Periodontal disease and C‐reactive protein‐associated cardiovascular risk. J Periodontal Res. 2004;39(4):236‐241. [DOI] [PubMed] [Google Scholar]
- 237.Monteiro AM, Jardini MA, Alves S, et al. Cardiovascular disease parameters in periodontitis. J Periodontol. 2009;80(3):378‐388. [DOI] [PubMed] [Google Scholar]
- 238.Nicolosi LN, Lewin PG, Rudzinski JJ, et al. Relation between periodontal disease and arterial stiffness. J Periodontal Res. 2017;52(1):122‐126. [DOI] [PubMed] [Google Scholar]
- 239.Moura MF, Navarro TP, Silva TA, Cota LOM, Soares Dutra Oliveira AM, Costa FO. Periodontitis and Endothelial Dysfunction: Periodontal Clinical Parameters and Levels of Salivary Markers Interleukin‐1β, Tumor Necrosis Factor‐α, Matrix Metalloproteinase‐2, Tissue Inhibitor of Metalloproteinases‐2 Complex, and Nitric Oxide. Journal of Periodontology. 2017;88(8):778–787. 10.1902/jop.2017.170023 [DOI] [PubMed] [Google Scholar]
- 240.Kure K, Sato H, Aoyama N, Izumi Y, et al. Accelerated inflammation in peripheral artery disease patients with periodontitis. Journal of Periodontal & Implant Science. 2018;48(6):337. 10.5051/jpis.2018.48.6.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Aoyama N, Suzuki JI, Kobayashi N, et al. Periodontitis deteriorates peripheral arterial disease in Japanese population via enhanced systemic inflammation. Heart Vessels. 2017;32(11):1314‐1319. [DOI] [PubMed] [Google Scholar]
- 242.Lockhart PB, Bolger AF, Papapanou PN, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012;125(20):2520‐2544. [DOI] [PubMed] [Google Scholar]
- 243.Dietrich T, Sharma P, Walter C, Weston P, Beck J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. Journal of Clinical Periodontology. 2013;40S70–S84. 10.1111/jcpe.12062 [DOI] [PubMed] [Google Scholar]
- 244.Sen S, Giamberardino LD, Moss K. Periodontal Disease, Regular Dental Care Use, and Incident Ischemic Stroke, et al. Stroke. 2018;49(2):355‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Herrera D, Molina A, Buhlin K, Klinge B. Periodontal diseases and association with atherosclerotic disease. Periodontol 2000. 2020;83(1):66–89. 10.1111/prd.12302 [DOI] [PubMed] [Google Scholar]
- 246.de Oliveira C, Watt R, Hamer M. Toothbrushing, inflammation, and risk of cardiovascular disease: results from Scottish Health Survey. BMJ. 2010;340(may27 1):c2451–c2451. 10.1136/bmj.c2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chu D, Lee YL, Hu HY, Chou P. Dental prophylaxis decreases the risk of acute myocardial infarction: a nationwide population‐based study in Taiwan. Clinical Interventions in Aging. 2015;175. 10.2147/cia.s67854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Holmlund A, Lampa E, Lind L. Poor Response to Periodontal Treatment May Predict Future Cardiovascular Disease. J Dent Res. 2017;96(7):768‐773. [DOI] [PubMed] [Google Scholar]
- 249.Park SY, Kim SH, Kang SH, et al. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: a population‐based study from Korea. Eur Heart J. 2019;40(14):1138‐1145. [DOI] [PubMed] [Google Scholar]
- 250.Offenbacher S, Beck JD, Moss K, et al. Results from the Periodontitis and Vascular Events (PAVE) Study: a pilot multicentered, randomized, controlled trial to study effects of periodontal therapy in a secondary prevention model of cardiovascular disease. J Periodontol. 2009;80(2):190‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Couper DJ, Beck JD, Falkner KL, et al. The Periodontitis and Vascular Events (PAVE) pilot study: recruitment, retention, and community care controls. J Periodontol. 2008;79(1):80‐89. [DOI] [PubMed] [Google Scholar]
- 252.Li C, Lv Z, Shi Z, et al. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database Syst Rev. 2017;11:p. CD009197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bokhari SA, Khan AA, Butt AK, et al. Non‐surgical periodontal therapy reduces coronary heart disease risk markers: a randomized controlled trial. J Clin Periodontol. 2012;39(11):1065‐1074. [DOI] [PubMed] [Google Scholar]
- 254.Bresolin AC, Pronsatti MM, Pasqualotto LN, et al. Lipid profiles and inflammatory markers after periodontal treatment in children with congenital heart disease and at risk for atherosclerosis. Vasc Health Risk Manag. 2013;9:703‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Caúla AL, Lira‐Junior R, Tinoco EMB, Fischer RG. The effect of periodontal therapy on cardiovascular risk markers: a 6‐month randomized clinical trial. J Clin Periodontol. 2014;41(9):875–882. 10.1111/jcpe.12290 [DOI] [PubMed] [Google Scholar]
- 256.Graziani F, Cei S, Orlandi M. Acute‐phase response following full‐mouth versus quadrant non‐surgical periodontal treatment: A randomized clinical trial, et al. J Clin Periodontol. 2015;42(9):843‐852. [DOI] [PubMed] [Google Scholar]
- 257.Houcken W, Teeuw WJ, Bizzarro S, et al. Arterial stiffness in periodontitis patients and controls. A case‐control and pilot intervention study. J Hum Hypertens. 2016;30(1):24‐29. [DOI] [PubMed] [Google Scholar]
- 258.Teeuw WJ, Slot DE, Susanto H, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta‐analysis. J Clin Periodontol. 2014;41(1):70‐79. [DOI] [PubMed] [Google Scholar]
- 259.Clemente JC, Ursell LK, Parfrey LW, Knight R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell. 2012;148(6):1258–1270. 10.1016/j.cell.2012.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lau K, Srivatsav V, Rizwan A, et al. Bridging the Gap between Gut Microbial Dysbiosis and Cardiovascular Diseases. Nutrients. 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33(6):401–407. 10.1111/j.1600-051x.2006.00924.x [DOI] [PubMed] [Google Scholar]
- 262.Pérez‐Chaparro PJ, Gracieux P, Lafaurie GI, Donnio PY, Bonnaure‐Mallet M. Genotypic characterization ofPorphyromonas gingivalisisolated from subgingival plaque and blood sample in positive bacteremia subjects with periodontitis. Journal of Clinical Periodontology. 2008;35(9):748–753. 10.1111/j.1600-051x.2008.01296.x [DOI] [PubMed] [Google Scholar]
- 263.Grau AJ, Becher H, Ziegler CM. Periodontal disease as a risk factor for ischemic stroke. Stroke. 2004;35(2):496‐501. [DOI] [PubMed] [Google Scholar]
- 264.Desvarieux M, Demmer RT, Rundek T. Relationship between periodontal disease, tooth loss, and carotid artery plaque: the Oral Infections and Vascular Disease Epidemiology Study (INVEST), et al. Stroke. 2003;34(9):2120‐2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lofthus JE, Waki MY, Jolkovsky DL, et al. Bacteremia following subgingival irrigation and scaling and root planing. J Periodontol. 1991;62(10):602‐607. [DOI] [PubMed] [Google Scholar]
- 266.Pussinen PJ, Alfthan G, Jousilahti P, Paju S, Tuomilehto J. Systemic exposure to Porphyromonas gingivalis predicts incident stroke. Atherosclerosis. 2007;193(1):222–228. 10.1016/j.atherosclerosis.2006.06.027 [DOI] [PubMed] [Google Scholar]
- 267.Desvarieux M, Demmer RT, Jacobs DR Jr, et al. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J Hypertens. 2010;28(7):1413‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Nakano K, Nemoto H, Nomura R, et al. Detection of oral bacteria in cardiovascular specimens. Oral Microbiol Immunol. 2009;24(1):64‐68. [DOI] [PubMed] [Google Scholar]
- 269.Nakano K, Inaba H, Nomura R. Detection and serotype distribution of Actinobacillus actinomycetemcomitans in cardiovascular specimens from Japanese patients, et al. Oral Microbiol Immunol. 2007;22(2):136‐139. [DOI] [PubMed] [Google Scholar]
- 270.Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of Periodontal Pathogens in Atheromatous Plaques. J Periodontol. 2000;71(10):1554–1560. 10.1902/jop.2000.71.10.1554 [DOI] [PubMed] [Google Scholar]
- 271.Kozarov EV, Dorn BR, Shelburne CE, Dunn WA, Progulske‐Fox A. Human Atherosclerotic Plaque Contains Viable InvasiveActinobacillus actinomycetemcomitansandPorphyromonas gingivalis. Arterioscler, Thromb Vasc Biol. 2005;25(3):e17‐e18. 10.1161/01.atv.0000155018.67835.1a [DOI] [PubMed] [Google Scholar]
- 272.Armingohar Z, Jørgensen JJ, Kristoffersen AK, Abesha‐Belay E, Olsen I. Bacteria and bacterial DNA in atherosclerotic plaque and aneurysmal wall biopsies from patients with and without periodontitis. J Oral Microbiol. 2014;6(1):23408. 10.3402/jom.v6.23408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Carrion J, Scisci E, Miles B, et al. Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. J Immunol. 2012;189(6):3178‐3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lönn J, Ljunggren S, Klarström‐Engström K, Demirel I, Bengtsson T, Karlsson H. Lipoprotein modifications by gingipains ofPorphyromonas gingivalis. J Periodontal Res. 2018;53(3):403–413. 10.1111/jre.12527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bagavad Gita J, George AV, Pavithra N, Chandrasekaran SC, Latchumanadhas K, Gnanamani A. Dysregulation of miR‐146a by periodontal pathogens: A risk for acute coronary syndrome. J Periodontol. 2019;90(7):756–765. 10.1002/jper.18-0466 [DOI] [PubMed] [Google Scholar]
- 276.Nahid MA, Pauley KM, Satoh M, Chan EKL. miR‐146a Is Critical for Endotoxin‐induced Tolerance. J Biol Chem. 2009;284(50):34590–34599. 10.1074/jbc.m109.056317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Reyes L, Herrera D, Kozarov E, Roldá S, Progulske‐Fox A. Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. J Periodontol. 2013;84(4‐s):S30–S50. 10.1902/jop.2013.1340012 [DOI] [PubMed] [Google Scholar]
- 278.Naderi S, Merchant AT. The association between periodontitis and cardiovascular disease: an update. Curr Atheroscler Rep. 2020;22(10):52. [DOI] [PubMed] [Google Scholar]
- 279.Vedin O, Hagstrom E, Gallup D, et al. Periodontal disease in patients with chronic coronary heart disease: Prevalence and association with cardiovascular risk factors. Eur J Prev Cardiol. 2015;22(6):771‐778. [DOI] [PubMed] [Google Scholar]
- 280.Orlandi M, Graziani F, D'Aiuto F. Periodontal therapy and cardiovascular risk. Periodontol. 2020;83(1):107‐124. [DOI] [PubMed] [Google Scholar]
- 281.Kose O, Arabaci T, Gedikli S, et al. Biochemical and histopathologic analysis of the effects of periodontitis on left ventricular heart tissues of rats. J Periodontal Res. 2017;52(2):176‐185. [DOI] [PubMed] [Google Scholar]
- 282.Leira Y, Iglesias‐Rey R, Gomez‐Lado N, et al. Periodontitis and vascular inflammatory biomarkers: an experimental in vivo study in rats. Odontology. 2020;108(2):202‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Brito LC, DalBo S, Striechen TM, et al. Experimental periodontitis promotes transient vascular inflammation and endothelial dysfunction. Arch Oral Biol. 2013;58(9):1187‐1198. [DOI] [PubMed] [Google Scholar]
- 284.Lalla E, Kaplan S, Yang J, Roth GA, Papapanou PN, Greenberg S. Effects of periodontal therapy on serum C‐reactive protein, sE‐selectin, and tumor necrosis factor‐a? secretion by peripheral blood‐derived macrophages in diabetes. A pilot study. J Periodontal Res. 2007;42(3):274–282. 10.1111/j.1600-0765.2006.00945.x [DOI] [PubMed] [Google Scholar]
- 285.Di Pietro M, Filardo S, Falasca F, Turriziani O, Sessa R. Infectious Agents in Atherosclerotic Cardiovascular Diseases through Oxidative Stress. Int J Mol Sci. 2017;18(11):2459. 10.3390/ijms18112459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kadowaki T, Nakayama K, Okamoto K, et al. Porphyromonas gingivalis proteinases as virulence determinants in progression of periodontal diseases. J Biochem. 2000;128(2):153‐159. [DOI] [PubMed] [Google Scholar]
- 287.Inaba H, Kuboniwa M, Sugita H, Lamont RJ, Amano A. Identification of Signaling Pathways Mediating Cell Cycle Arrest and Apoptosis Induced by Porphyromonas gingivalis in Human Trophoblasts. Infect Immun. 2012;80(8):2847–2857. 10.1128/iai.00258-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Le Sage F, Meilhac O, Gonthier MP. Porphyromonas gingivalis lipopolysaccharide induces pro‐inflammatory adipokine secretion and oxidative stress by regulating Toll‐like receptor‐mediated signaling pathways and redox enzymes in adipocytes. Mol Cell Endocrinol. 2017;446:102‐110. [DOI] [PubMed] [Google Scholar]
- 289.Xie M, Tang Q, Yu S, et al. Porphyromonas gingivalis disrupts vascular endothelial homeostasis in a TLR‐NF‐kappaB axis dependent manner. Int J Oral Sci. 2020;12(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Xie M, Tang Q, Nie J, et al. BMAL1‐downregulation aggravates porphyromonas gingivalis‐induced atherosclerosis by encouraging oxidative Stress. Circ Res. 2020;126(6):e15‐e29. [DOI] [PubMed] [Google Scholar]
- 291.Kim DJ, Rho JH, Woo BH, et al. Periodontal pathogens modulate lipid flux via fatty acid binding protein 4. J Dent Res. 2019;98(13):1511‐1520. [DOI] [PubMed] [Google Scholar]
- 292.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Report. 2009;13:1‐7. [PubMed] [Google Scholar]
- 293.Gomes BP, Berber VB, Kokaras AS, Chen T, Paster BJ. Microbiomes of Endodontic‐Periodontal Lesions before and after Chemomechanical Preparation. J Endod. 2015;41(12):1975–1984. 10.1016/j.joen.2015.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Belstrøm D, Holmstrup P, Bardow A, Kokaras A, Fiehn NE, Paster BJ. Temporal Stability of the Salivary Microbiota in Oral Health. PLOS ONE. 2016;11(1):e0147472. 10.1371/journal.pone.0147472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4516‐4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Al‐Hebshi NN, Nasher AT, Idris AM, Chen T. Robust species taxonomy assignment algorithm for 16S rRNA NGS reads: application to oral carcinoma samples. J Oral Microbiol. 2015;7(1):28934. 10.3402/jom.v7.28934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kwon YE, Ha JE, Paik DI, Jin BH, Bae KH. The relationship between periodontitis and metabolic syndrome among a Korean nationally representative sample of adults. J Clin Periodontol. 2011;38(9):781–786. 10.1111/j.1600-051x.2011.01756.x [DOI] [PubMed] [Google Scholar]
- 298.Fukui N, Shimazaki Y, Shinagawa T, Yamashita Y. Periodontal Status and Metabolic Syndrome in Middle‐Aged Japanese. J Periodontol. 2012;83(11):1363–1371. 10.1902/jop.2012.110605 [DOI] [PubMed] [Google Scholar]
- 299.Chen X, Xie L, Liu Y, et al. Metabolic syndrome and periodontal disease among civilian pilots. Aerosp Med Hum Perform. 2016;87(12):1016‐1020. [DOI] [PubMed] [Google Scholar]
- 300.Kim OS, Shin MH, Kweon SS, et al. The severity of periodontitis and metabolic syndrome in Korean population: The Dong‐gu study. J Periodontal Res. 2018;53(3):362‐368. [DOI] [PubMed] [Google Scholar]
- 301.Ohnishi T, Bandow K, Kakimoto K, Machigashira M, Matsuyama T, Matsuguchi T. Oxidative stress causes alveolar bone loss in metabolic syndrome model mice with type 2 diabetes. J Periodontal Res. 2009;44(1):43–51. 10.1111/j.1600-0765.2007.01060.x [DOI] [PubMed] [Google Scholar]
- 302.Sbordone L, Ramaglia L, Barone A, Ciaglia RN, Iacono VJ. Periodontal Status and Subgingival Microbiota of Insulin‐Dependent Juvenile Diabetics: A 3‐Year Longitudinal Study. J Periodontol. 1998;69(2):120–128. 10.1902/jop.1998.69.2.120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed by the current study are available online at http://www.homd.org/ftp/publication_data/20170412/qiime_results/cd_mc10/taxa_plots/taxa_summary_plots/bar_charts.html.


