Abstract
Objectives
To assess the association between waking‐state oral behaviours and temporomandibular disorder (TMD) subgroups and to develop new scoring methods for the Oral Behavior Checklist (OBC).
Methods
Patients with any TMD diagnosis, according to the diagnostic criteria for TMD (DC/TMD), were divided into subgroups: ‘Dysfunctional‐TMD’ (n = 70), only mechanical dysfunction; ‘Painful‐TMD’ (n = 204), only myalgia, arthralgia or both; and ‘Painful‐Dysfunctional TMD’ (n = 95), combined pain and dysfunction. A group of individuals without TMD, ‘Non‐TMD’ (n = 374), was used for testing associations. Participants completed the OBC. An exploratory factor analysis, followed by a confirmatory factor analysis of the OBC responses, identified 2 major factors, named non‐functional activities (NFA) and functional activities (FA). Component total scores were computed. Differences among subgroups for OBC‐MS (mean score) and NFA and FA factor scores were estimated using one‐way ANOVA and Tukey post hoc tests. Significance was set at p < .05.
Results
The OBC‐MS in Non‐TMD, Painful‐TMD and Painful‐Dysfunctional TMD subgroups was higher than in the Dysfunctional‐TMD subgroup (p ≤ .001). NFA in Painful‐TMD and Painful‐Dysfunctional TMD subgroups were higher than in the Non‐TMD group (p < .05); NFA in the Dysfunctional‐TMD subgroup were lower than in the Painful‐TMD subgroup (p = .034). In contrast, FA in Painful‐TMD, Dysfunctional‐TMD and Painful‐Dysfunctional TMD subgroups were lower than in the Non‐TMD group (p < .0001).
Conclusions
A new scoring method for the OBC results in item reduction and creation of meaningful subscales for functional and non‐functional behaviours, which are differentially associated with painful and dysfunctional TMDs. This may help clinicians to better tailor treatment for the management of subtypes of TMD patients.
Keywords: checklist, joint disease, masticatory system, oral health, teeth grinding disorders, TMD subgroups
New subscales of the “Oral Behaviors Checklist” based on functional oral activities (FA) and nonfunctional activities (NFA) show different associations with temporomandibular disorders (TMD) subgroups. NFA are higher in subjects with only TMD pain or with both TMD pain and dysfunction. FA are lower in all TMD versus subjects free from TMD. This may help clinicians to better tailor treatment of subtypes of TMD patients.
1. INTRODUCTION
Temporomandibular disorders (TMDs) are conditions involving the temporomandibular joints (TMJs), the masticatory muscles or both and are characterised by musculoskeletal pain and limitations in functional movement.1 The aetiology is multifactorial, and oral behaviours (OB), including physiological and non‐physiological functions of the masticatory system, are considered contributing factors to the onset and persistence of painful TMDs.2, 3, 4, 5 Physiological OB include mastication, communication, swallowing and breathing.6, 7, 8 Non‐physiological OB include bruxism (i.e., masticatory muscle activities occurring during sleep and/or during wakefulness) and other waking‐state oral behaviours (waking OB) (e.g., excursive positioning, gum chewing, object biting and tongue pushing).9, 10, 11
Self‐reports (questionnaires or checklists) completed by patients are often used to detect OB.11, 12 Among them, the Oral Behavior Checklist (OBC)13 is a valid instrument quantifying the frequency of OB. OB have been suggested to cause overload and microtrauma to the masticatory system,14 although this remains poorly understood and investigated. For example, using similar instruments for OB assessment in cross‐sectional comparison between healthy controls and chronic painful TMD, both no association15 and strong associations14 are reported. Yet, OB have been clearly identified as a risk factor for the development of painful TMD.4
Some of the items in the OBC reflect behaviours, such as singing, readily considered as appropriate functions of the masticatory system, whereas other items reflect behaviours, such as routine bracing the jaw, also readily considered as non‐functional uses of the masticatory system. In addition, distinct waking OB may have different mechanisms of action on the masticatory muscles and TMJ.16, 17, 18, 19, 20, 21 Consequently, the items within the OBC should be questioned for their purpose in identifying clinically relevant behaviours. For example, what is a normal (or physiological) behaviour versus a parafunctional (non‐physiological) behaviour? Or, are all such behaviours ‘parafunctional’ when performed to a high extent? These distinctions might underlie an association between different kinds of waking OB and different TMD subgroups.
The aims of this study were as follows: (1) to assess the mean score of a 19‐item version of OBC for waking OB in Non‐TMD versus TMD, and among TMD subgroups, (2) to develop new scoring methods for the OBC based on item analysis and (3) to assess the association between subscales of waking OB and Non‐TMD, TMD, and TMD subgroups. In summary, our hypothesis is that the large range of waking OB identified by the OBC may be indicators of a few underlying types of waking OB. Their association with different TMD subgroups may improve our understanding of the potential causal contribution of OB to TMD onset and chronicity as well as better direct treatment.
2. MATERIALS AND METHODS
2.1. Study participants
In this cross‐sectional study, the sample was selected from among 785 consecutive patients referred to the Dental Clinic of the University of Naples ‘Federico II’ for a TMD consultation, from May 2015 to May 2017. The DC/TMD Axis I and Axis II assessment instruments were administered using the Italian translation22; for this study, the Symptom Questionnaire and the OBC were used. The Symptom Questionnaire assesses facial pain symptoms and TMJ symptoms over the prior 30 days. Patients ≥18 years old with a TMD diagnosis according to the Diagnostic Criteria for TMD (DC⁄TMD)1 were eligible as TMD cases; examiners calibrated for DC⁄TMD assessed clinical records in order to confirm inclusion.3 Patients with trigeminal neuralgia, fibromyalgia, burning mouth syndrome, atypical facial pain, atypical odontalgia, migraine, cervical pain and/or neuropathic pain were excluded. A Non‐TMD group as controls, age‐ and sex‐matched to a TMD case, was recruited from individuals accompanying dental clinic patients within a month after identifying each case, and recruitment was based on absence of a TMD. Potential controls were screened for signs and symptoms of TMD using the Symptom Questionnaire and were selected if they answered negatively to all items. The local ethics committee approved the study protocol (no. 48/18), and participants signed an informed consent.
2.2. TMD subgroups
Temporomandibular disorder cases were assigned to subgroups on the basis of pain (presence or absence; Symptom Questionnaire Q1) and TMJ‐dysfunction (presence or absence of clicking and locking; Symptom Questionnaire Q8‐Q14). Three subgroups were created: (1) participants without TMD‐pain and affected only by TMD‐dysfunction (Dysfunctional‐TMD); (2) participants with TMD‐pain (including myalgia, arthralgia or both) and without TMD‐dysfunction (Painful‐TMD); and (3) participants with both TMD‐pain and TMD‐dysfunction (Painful‐Dysfunctional TMD). The controls were negative for both pain and dysfunction problems.
2.3. Assessment of waking‐state oral behaviours
The OBC is a self‐reported 21‐item questionnaire that quantifies the frequency of OB performed during the preceding month. The instrument as a whole is considered reliable; moreover, it appears valid for evaluating waking OB.7, 13, 23, 24
For the purpose of this investigation, the first two questions, assessing sleep‐related OB, were excluded from the analysis. Response options of the remaining items assessing waking OB range from 0 (none of the time) to 4 (all the time); the mean score of OBC (OBC‐MS), obtained by summing the scores and dividing by the number of item in analysis (19 items), was an index of each participant's self‐assessment of the extent of waking‐state OB.25
2.4. Statistical analysis
Group sample sizes of 350 controls and 350 TMD cases have 80% power to detect a small effect size (0.2) in OBC‐MS, considering equal group standard deviation and a significance level (alpha) of 0.05. Continuous variables were reported as means and standard deviations or median and interquartile range (IQR), depending on the variable's distribution.
For a better interpretation of specific behaviours of the OBC‐MS, we used exploratory factor analysis (EFA) to reduce the data set and to identify latent factors that underlie observed variables. The data set was randomly split into a discovery data set for the EFA (two‐thirds of total; n = 495) and a validation data set for the confirmatory factor analysis (CFA) (one‐third of total; n = 248). Since the items in the OBC use ordinal responses, the polychoric covariance matrix and varimax rotation of factor loadings were used to estimate the presumed underlying latent trait. Items with factor loadings <0.45 were dropped. Factors with an eigenvalue greater than 1.0 and able to collectively explain more than 50% of the variability were retained and determined the number of factors in conjunction with reference to the scree plot.
To test whether the hypothesised factor structure represented a good fit to the data, the CFA provided the following statistical measures (and associated critical values): maximum likelihood chi‐square/degrees of freedom (χ2/df; p‐value > .05), root mean square error of approximation (RMSEA < 0.08), Tucker‐Lewis index (TLI > 0.9), comparative fit index (CFI ≥ 0.9) and standardised root mean square error (SRMR < 0.08). Furthermore, modification indices and any additional paths were assessed. Modification indices suggest links to change in the model structure, and that new parameters should be added to the model, usually to covary error terms that are part of the same factor.
The Shapiro‐Wilk test was used to evaluate for normal distribution of data. The t‐test was used to analyse differences in OBC‐MS between Non‐TMD and TMD participants (combining all three case groups). Differences among the Non‐TMD group and TMD subgroups (in OBC‐MS and in any OBC factor scores) were estimated using one‐way ANOVA, followed by Tukey post hoc tests. A p‐value < .05 was defined statistically significant. All statistical analyses were performed using Stata version 14 (Stata Corp.).
3. RESULTS
Out of 785 consecutive patients screened, 416 were excluded due to failure to meet selection criteria. The final TMD group included 369 patients who were assigned to 3 subgroups. The Non‐TMD group included 374 subjects (Figure 1).
FIGURE 1.
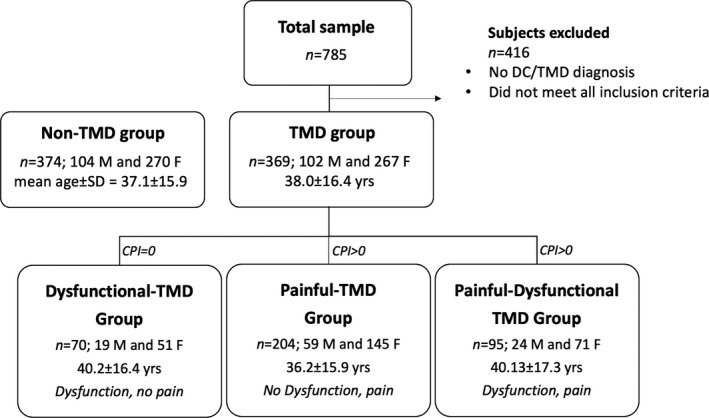
Sample description. Sample recruitment and division in groups: Non‐TMD group and TMD group. TMD group has subsequently been divided into 3 subgroups: Dysfunctional‐TMD, Painful‐TMD and Painful‐Dysfunctional TMD group
The OBC‐MS did not differ in the simple contrast between TMD (mean ± SD = 1.04 ± 0.6) and Non‐TMD groups (1.11 ± 0.5) (p = 0.13) (Figure 2A). Yet, the OBC‐MS differed when considering the three TMD subgroups and the Non‐TMD group (F(3,739) = 9.41; p < .0001). Post hoc pair‐wise comparisons indicated that the mean score in each of Non‐TMD (1.11 ± 0.5), Painful‐TMD (1.13 ± 0.6) and Painful‐Dysfunctional TMD participants (1.07 ± 0.6) was significantly higher as compared to the Dysfunctional‐TMD participants (0.76 ± 0.5) (p ≤ .001) (Figure 2B). The OBC‐MS did not differ between Non‐TMD, Painful‐TMD and Painful‐Dysfunctional TMD subgroups (p > .8) (Figure 2B). Post hoc pair‐wise comparisons indicated that the mean score in each of the Non‐TMD, Painful‐TMD and Painful‐Dysfunctional TMD participants was significantly higher as compared to the Dysfunctional‐TMD participants (p ≤ .001) (Figure 2B).
FIGURE 2.
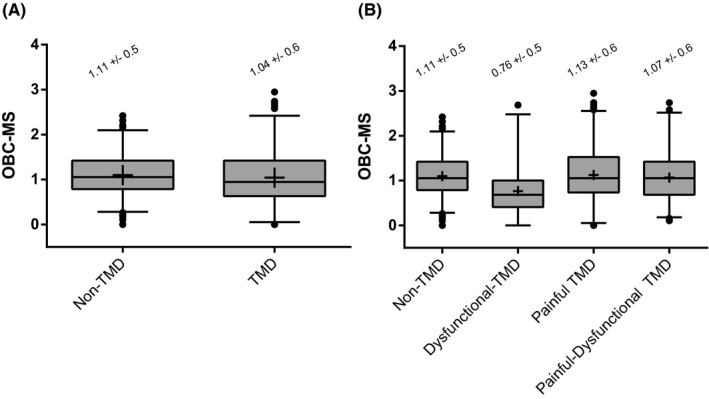
Descriptive comparison of OBC mean score. OBC‐MS (mean score) comparison between Non‐TMD and TMD group (Figure 2A) and among subgroups Non‐TMD, Dysfunctional‐TMD, Painful‐TMD and Painful‐Dysfunctional TMD (Figure 2B). Box and whisker plots were used in both panels. Box describes median and interquartile range, whiskers represent 2.5 and 97.5 percentiles of distribution, and points describe outliers. The mean value was indicated by the symbol ‘+'. The mean and standard deviation values for each group and subgroup were reported
Two factors were identified from the EFA (Figure 3; Table 1). The first factor, non‐functional activities (NFA), included 6 items (items 3, 4, 5, 6, 7 and 11) representing non‐functional oral activities, such as clenching, grinding or holding. The second factor, functional activities (FA), included 6 items (items 12, 13, 17, 18, 19 and 20) representing activities related to normal jaw function, such as chewing, talking and yawning. Items 14, 15, 16 and 21 had factor loadings <0.45 and were excluded (Table 1). Items 8, 9 and 10 exhibited ambiguous fit within the factor structure and were also excluded (Figure 3).
FIGURE 3.
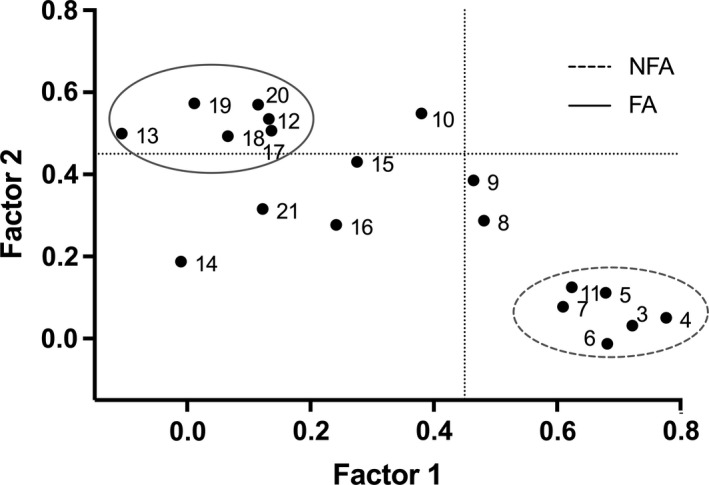
Scatterplot of EFA loadings. Scatterplot of exploratory factor analysis (EFA) loading of OBC items related to diurnal oral behaviours. On x‐axis and y‐axis, loadings are shown for factor 1 and factor 2. Numerals in the plot space refer to item numbers (see Table 1 for clarification). Circles with different line drawing represent OBC new scale (NFA and FA, see methods and results). Dotted line represents loading factor <0.45. The two identified factors represent a clean structure with, considering the exploratory nature of this analysis, an acceptable/good consistency (Cronbach's alpha: 0.68 for FA and 0.81 for NFA)
TABLE 1.
Exploratory factor analysis of OBC items
| Items | Description of items | Factor 1 | Factor 2 |
|---|---|---|---|
| OBC_3 | Grind teeth together during waking hours | 0.72 | |
| OBC_4 | Clench teeth together during waking hours | 0.78 | |
| OBC_5 | Press, touch or hold teeth together other than while eating (that is, contact between upper and lower teeth) | 0.68 | |
| OBC_6 | Hold, tighten or tense muscles without clenching or bringing teeth together | 0.68 | |
| OBC_7 | Hold or jut jaw forward or to the side | 0.61 | |
| OBC_8 | Press tongue forcibly against teeth | 0.48 | |
| OBC_9 | Place tongue between teeth | 0.46 | |
| OBC_10 | Bite, chew or play with your tongue, cheeks or lips | 0.55 | |
| OBC_11 | Hold jaw in rigid or tense position, such as to brace or protect the jaw. | 0.62 | |
| OBC_12 | Hold between the teeth or bite objects such as hair, pipe, pencil, pens, fingers and fingernails | 0.54 | |
| OBC_13 | Use chewing gum | 0.50 | |
| OBC_14 | Play musical instrument that involves use of mouth or jaw (e.g., woodwind, brass, string instruments) | ||
| OBC_15 | Lean with your hand on the jaw, such as cupping or resting the chin in the hand | ||
| OBC_16 | Chew food on one side only | ||
| OBC_17 | Eating between meals (i.e., food that requires chewing) | 0.51 | |
| OBC_18 | Sustained talking (e.g., teaching, sales, customer service). | 0.49 | |
| OBC_19 | Singing | 0.57 | |
| OBC_20 | Yawning | 0.57 | |
| OBC_21 | Hold telephone between your head and shoulders | ||
| Eigen | 4.4 | 1.8 | |
| Cumulative percentage | 47% | 82% |
OBC items in analysis are related to waking oral behaviours. The exploratory factor analysis (EFA) is based on polychoric correlation matrix and varimax rotation of factor loadings. Items with a loading factor lower than 0.45 were omitted and not listed. The last two rows represent eigenvalue, and cumulative percentage of factors 1 and 2 is reported at the bottom of the table.
The confirmatory factor analysis (Figure S1A) revealed an adequate fit to the model (Table S1). In addition, modification indices analysis with additional path specification (between error terms) definitely improved the model fit (Figure S1B; Table S1). The discovery and validation data sets used in EFA and CFA are described in Table S2.
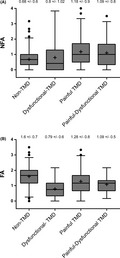
The NFA subscale was higher (p < .001) in the TMD group (mean ± SD = 1.09 ± 0.9) compared to the Non‐TMD group. In contrast, FA scores were higher (p < .001) in the Non‐TMD group compared to the TMD group (1.15 ± 0.7). Differences in NFA subscale scores were observed among the TMD subgroups and the Non‐TMD group (F(3, 491) = 15.1; p < 0.0001). In particular, the total NFA scores in the Painful‐TMD subgroup and the Painful‐Dysfunctional TMD subgroup were higher compared to the Non‐TMD group and the Dysfunctional‐TMD subgroup; these differences were significant for the Painful‐TMD subgroup and for the Painful‐Dysfunctional TMD versus Non‐TMD group comparison (both p < .001) as well as for the Painful‐TMD subgroup against the Dysfunctional‐TMD subgroup (p = .034). The total score of NFA did not differ between Painful‐TMD and Painful‐Dysfunctional TMD subgroups (p = .89) (Figure 4A).
FIGURE 4.
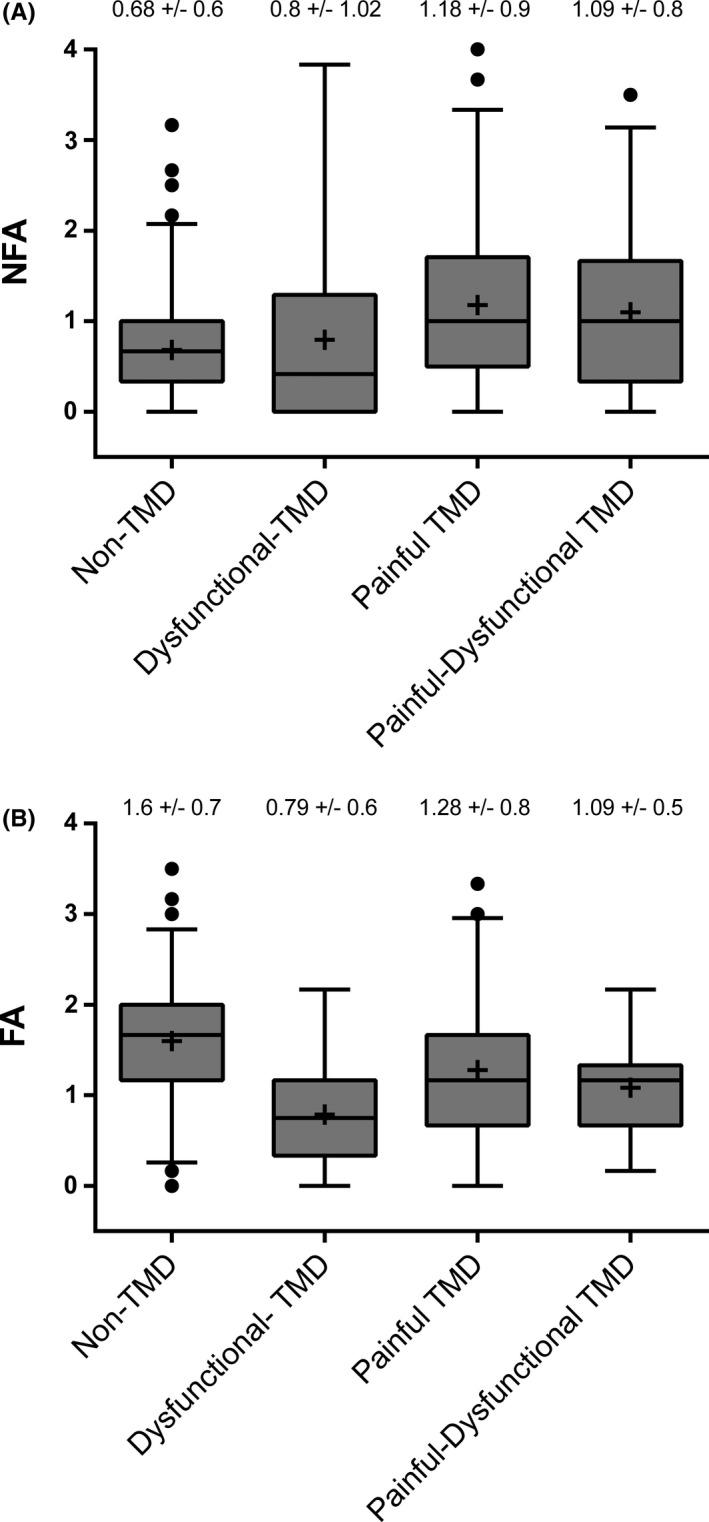
Descriptive comparison of OBC new scale. Comparison of NFA (non‐functional activities) (Figure 4A) and FA (functional activities) factors (Figure 4B) in Non‐TMD and in TMD subgroups (Dysfunctional‐TMD, Painful‐TMD and Painful‐Dysfunctional TMD). Box describes median and interquartile range, whiskers represent 2.5 and 97.5 percentiles of distribution, and points describe outliers. The mean value was indicated by the symbol ‘+'. The mean and standard deviation values for each group and subgroup were reported
The mean total score of FA was different among the TMD subgroups and the Non‐TMD group (F(3, 491) = 23.75; p < .0001). In particular, the total FA scores in the Painful‐TMD subgroup, the Dysfunctional‐TMD group and the Painful‐Dysfunctional TMD subgroup were lower than in the Non‐TMD group. The total FA score in the Painful‐TMD subgroup was higher compared to the Dysfunctional‐TMD subgroup (p < .001), but was not higher compared to the Painful‐Dysfunctional TMD subgroup (p = .22) (Figure 4B).
4. DISCUSSION
In summary, main findings of the present study were as follows: (1) OBC‐MS did not differ between TMDs of all types and non‐TMD participants, yet OBC‐MS was lower in Dysfunctional‐TMD participants compared to Non‐TMD, Painful‐TMD and Painful‐Dysfunctional TMD participants; (2) the OBC can be improved via careful partitioning of about half of the items into two contrasting constructs and discarding the remainder of the items for routine use; (3) OBC subscale scores representing non‐functional activity (NFA) were higher in Painful‐TMD and Painful‐Dysfunctional TMD groups; and (4) OBC subscale scores representing normal functional activities (FA) were lower in TMDs of all types versus the Non‐TMD group.
The lack of a significant difference of the OBC‐MS between the TMD and Non‐TMD groups is in contrast to a lot of studies, showing that extensive OB were more common among TMD patients than among non‐TMD individuals.14, 26, 27 In perhaps the largest study of this type and with potentially the strongest design with regard to assessing the distribution of OBC scores in non‐cases,4 non‐cases of TMD who later developed a painful TMD had higher OBC scores at enrolment. These discrepancies in the OBC mean score patterns, comparing TMD cases and non‐cases, can also be attributed to the methods used (e.g., electromyography, self‐reports), to the sampling variation and to the type of OB measured (e.g., waking‐state bruxism or other OB).
The finding that Dysfunctional‐TMD subgroup presented a significantly lower OBC‐MS than Non‐TMD, Painful‐TMD and Painful‐Dysfunctional TMD subgroups is consistent with one study where most non‐pain controls with TMJ‐dysfunction reported no clenching and less frequent biting of the lips or mouth than non‐pain controls with no joint dysfunction assessed using a non‐validated oral habits survey.28 Our result is consistent also with a more recent research in which individuals with non‐painful TMD diagnosis reported a significantly lower frequency of OB, according to the OBC, compared to individuals with painful TMD and/or TMD‐free individuals TMD.12
The lack of difference of OBC‐MS between Non‐TMD, Painful‐TMD and Painful‐Dysfunctional TMD subgroups is in contrast with a previous study, in which individuals with myofascial pain exhibited a greater frequency of daytime clenching episodes, as measured with sEMG, and reported higher OBC scores compared to TMD‐free individuals.29 However, the higher OB score can be explained by the non‐representative sample that only included younger females with higher pain intensity.
Due to these contradictory findings from prior studies, including our own, we investigated how the extent of OB was being computed; we used EFA/CFA to create a revised version of the OBC which included two factors: non‐functional and functional activities. The NFA (i.e., clenching/ grinding teeth, or holding teeth together/muscles/or jaw in a rigid position during waking hours) were significantly more frequent in patients affected from all types of TMD compared to TMD‐free subjects. On the contrary, FA (i.e., chewing, talking and yawning) were more frequent in controls compared to all types of TMD cases. This agrees with several studies where patients with TMD reported more detrimental OB, such as diurnal clenching/grinding compared to individuals free from TMD that, on the contrary, may execute mainly normal movements of the jaw.30, 31, 32
Four items had a loading factor lower than 0.45 and could not be circumscribed in a clearly defined clinical group. In particular, ‘play musical instruments’ is related to a restricted group of people; ‘hold telephone between your head and shoulders’ is rarely encountered nowadays given the wide diffusion of cell phones and computers; and ‘lean with your hand on the jaw’ and ‘chew food on one side only’ are common among people and may not be considered parafunctions. ‘Press tongue against teeth’, ‘place tongue between teeth’ and ‘bite, chew or play with your tongue, cheeks or lips’ were excluded because they did not fit either NFA or FA.
When considering TMD subgroups, the NFA score was higher in subjects with pain related to TMD, whereas the FA score was lower in subjects with TMD as compared to Non‐TMD subjects.
In particular, NFA were present more frequently in patients reporting only TMD‐pain or both TMD‐pain and TMD‐dysfunction than in individuals who were either without any TMD or who only reported TMJ‐dysfunction. Similarly, in a previous study, Painful‐TMD patients showed significantly more frequent and more intense teeth contact and tension in the jaw and face than controls and subjects presenting only disc displacement.33 In particular, OB, such as tooth clenching, biting objects and bracing the jaw, have been considered risk factors for the development of myofascial pain.26
The FA scores in the Painful‐TMD, Dysfunctional‐TMD and Painful‐Dysfunctional TMD subjects were lower than in Non‐TMD subjects, indicating that individuals affected by either TMD‐Pain and TMJ‐dysfunction performed functional oral activities at a significantly lower frequency. This finding may reflect the impact that pain has on normal function, as proposed in both pain adaptation model and models of kinesiophobia. The pain adaptation model proposes that the pain causes changes in muscle activity that limit movement and protect the motor system from further injury and thereby promote healing.34 Therefore, it might explain why Painful‐TMD patients reported a lower frequency of FA. On the other hand, dysfunctional patients could intentionally avoid certain movements provoking discomfort, because they might be afraid of worsening their health condition, a phenomenon that is called kinesiophobia.35 The strengths of the present investigation are that the assessment of waking OB frequency was conducted through a validated questionnaire (OBC), TMD diagnosis was obtained through a standardised clinical assessment (DC/TMD), and the sample was large, representative for heterogeneity and well‐defined. From a statistical perspective, the data set was large enough to allow us to split the sample and allow a CFA to fully confirm the model explored with EFA.
A limitation is that the present study was conducted on a sample of patients attending the local dental Clinic for a TMD consultation and may be not representative of the general population; the same must be said for the non‐cases serving as controls. The small difference in OBC‐MS between cases and non‐cases may reflect the sampling distribution as confirmed by a further analysis that we conducted by considering data reported by Ohrbach et al. 2011 (Table S3). The differences related to the sample distribution suggest an even stronger rationale to develop a more construct‐relevant scoring method given the heterogeneous nature of the OBC items; the use of NFA and FA scores could represent a more robust measure of the respective constructs of interest, relevant to the TMD population, and less influenced by potential sampling problems.
5. CONCLUSION
This report showed that specific waking‐state oral behaviours included in the OBC belong to two different latent constructs regarding type of behaviour (functional and non‐functional) and these types are differently associated with painful and dysfunctional TMDs. These two constructs represent a proposed new scoring method for the OBC and may yield insights into further distinctions among subtypes of TMDs, causal mechanisms and better tailoring treatment protocols for the management of these patients.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
VD contributed to conception and design, acquisition, analysis and interpretation of data, and drafting of the paper. RO contributed to analysis and interpretation of data, and drafting of the paper and critical revision. FL contributed to drafting of the paper and critical revision. VS contributed to design, statistical analysis, interpretation of data and critical revision. NP contributed to acquisition of data and drafting of the paper. AM contributed to conception and design, analysis and interpretation of data, and drafting of the paper. All authors approved the final version of the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/joor.13221.
Supporting information
Fig S1
Table S1‐S3
ACKNOWLEDGEMENTS
The authors thank Dr Iacopo Cioffi, Dr Roberto Rongo, Dr Sonia Sharma and Dr Maurits K A van Selms for their suggestions. This research was supported by the Division of Orthodontics, Department of Neurosciences, Reproductive Sciences and Oral Sciences, University of Naples Federico II. The statistician Dr Vittorio Simeon was supported by the Programma VALERE, University of Campania ‘Luigi Vanvitelli’.
Donnarumma V, Ohrbach R, Simeon V, Lobbezoo F, Piscicelli N, Michelotti A. Association between waking‐state oral behaviours, according to the oral behaviors checklist, and TMD subgroups. J Oral Rehabil. 2021;48:996–1003. 10.1111/joor.13221
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author [VD] upon reasonable request.
REFERENCES
- 1.Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortium network* and orofacial pain special interest group†. J Oral Facial Pain Headache. 2014;28(1):6‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manfredini D, Lobbezoo F. Relationship between bruxism and temporomandibular disorders: a systematic review of literature from 1998 to 2008. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(6):e26‐e50. [DOI] [PubMed] [Google Scholar]
- 3.Ohrbach R, Gonzalez Y, List T, Michelotti A, Schiffman E. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) Clinical Examination Protocol: Version 02 June 2013. Accessed date 19 May 2020. www.rdc‐tmdinternational.org
- 4.Ohrbach R, Bair E, Fillingim RB, et al. Clinical orofacial characteristics associated with risk of first‐onset TMD: the OPPERA prospective cohort study. J Pain. 2013;14(12):T33‐T50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohrbach R, Kreibig SD, Michelotti A. Psychosocial factors in sleep and awake bruxism and other oral parafunctions. In: Lavigne GJ, Cistulli PASM, eds. Sleep Medicine for Dentists: An Evidence‐Based Overview. 2nd ed. Batavia, IL: Quintessence Publishing USA; 2020:142‐145. [Google Scholar]
- 6.Ohrbach R, Beneduce C, Markiewicz MR, McCall WD Jr. Psychometric properties of the oral behaviors checklist: preliminary findings. J Dent Res. 2004;83(special issue A):1194. [Google Scholar]
- 7.Ohrbach R, Markiewicz MR, McCall WD Jr. Waking‐state oral parafunctional behaviors: specificity and validity as assessed by electromyography. Eur J Oral Sci. 2008;116(5):438‐444. [DOI] [PubMed] [Google Scholar]
- 8.van der Meulen MJ, Lobbezoo F, Aartman IHA, Naeije M. Validity of the Oral behaviours checklist: correlations between OBC scores and intensity of facial pain. J Oral Rehabil. 2014;41(2):115‐121. [DOI] [PubMed] [Google Scholar]
- 9.Lavigne GJ, Rompré PH, Poirier G, Huard H, Kato T, Montplaisir JY. Rhythmic masticatory muscle activity during sleep in humans. J Dent Res. 2001;80(2):443‐448. [DOI] [PubMed] [Google Scholar]
- 10.Molina OF, Dos Santos J, Mazzetto M, Nelson S, Nowlin T, Mainieri ÉT. Oral jaw behaviors in TMD and bruxism: a comparison study by severity of bruxism. Cranio. 2001;19(2):114‐121. [DOI] [PubMed] [Google Scholar]
- 11.Lobbezoo F, Ahlberg J, Raphael KG, et al. International consensus on the assessment of bruxism: report of a work in progress. J Oral Rehabil. 2018;45(11):837‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khawaja SN, Nickel JC, Iwasaki LR, Crow HC, Gonzalez Y. Association between waking‐state oral parafunctional behaviours and bio‐psychosocial characteristics. J Oral Rehabil. 2015;42(9):651‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markiewicz MR, Ohrbach R, McCall WD Jr. Oral behaviors checklist: reliability of performance in targeted waking‐state behaviors. J Orofac Pain. 2006;20(4):306‐316. [PubMed] [Google Scholar]
- 14.Ohrbach R, Fillingim RB, Mulkey F, et al. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case‐control study. J Pain. 2011;12(11):T27‐T45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Meulen MJ, Lobbezoo F, Aartman IH, Naeije M. Self‐reported oral parafunctions and pain intensity in temporomandibular disorder patients. J Orofac Pain. 2006;20(1):31‐35. [PubMed] [Google Scholar]
- 16.Takenami Y, Kuboki T, Acero COJ, Maekawa K, Yamashita A, Azuma Y. The effects of sustained incisal clenching on the temporomandibular joint space. Dentomaxillofac Radiol. 1999;28(4):214‐218. [DOI] [PubMed] [Google Scholar]
- 17.Svensson P, Burgaard A, Schlosser S. Fatigue and pain in human jaw muscles during a sustained, low‐intensity clenching task. Arch Oral Biol. 2001;46(8):773‐777. [DOI] [PubMed] [Google Scholar]
- 18.Huang GJ, LeResche L, Critchlow CW, Martin MD, Drangsholt MT. Risk factors for diagnostic subgroups of painful Temporomandibular Disorders (TMD). J Dent Res. 2002;81(4):284‐288. [DOI] [PubMed] [Google Scholar]
- 19.Glaros AG, Burton E. Parafunctional clenching, pain, and effort in temporomandibular disorders. J Behav Med. 2004;27(1):91‐100. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes G, Franco‐Micheloni L, De Siqueira J, Aparecida Godi Gonçalves D, Camparis M. Parafunctional habits are associated cumulatively to painful temporomandibular disorders in adolescents. Braz Oral Res. 2016;30(1). [DOI] [PubMed] [Google Scholar]
- 21.van Selms M, Visscher C, Knibbe W, Thymi M, Lobbezoo F. The association between self‐reported awake oral behaviors and orofacial pain depends on the belief of patients that these behaviors are harmful to the jaw. J Oral Facial Pain Headache. 2020;34(3):273‐280. [DOI] [PubMed] [Google Scholar]
- 22.Ohrbach R, editor. Diagnostic Criteria for Temporomandibular Disorders: Assessment Instruments. Version 15May2016. [Criteri diagnostici per i disordini temporomandibolari: Strumenti valutativi (DC/TMD) Version 17 Jan 2017] Michelotti A, Segù M, Wrenn C, Rongo R. Trans. Accessed date 20 May 2020. www.rdc‐tmdinternational.org
- 23.Kaplan S, Ohrbach R. Self‐report of waking‐state oral parafunctional behaviors in the natural environment. J Oral Facial Pain Headache. 2016;30(2):107‐119. [DOI] [PubMed] [Google Scholar]
- 24.Donnarumma V, Cioffi I, Michelotti A, Cimino R, Vollaro S, Amato M. Analysis of the reliability of the Italian version of the oral behaviours checklist and the relationship between oral behaviours and trait anxiety in healthy individuals. J Oral Rehabil. 2018;45(4):317‐322. [DOI] [PubMed] [Google Scholar]
- 25.Ohrbach R, Knibbe W. Diagnostic Criteria for Temporomandibular Disorders: Scoring Manual for Self‐Report Instruments. Version 29 May 2016. Accessed date 17 May 2020. www.rdc‐tmdinternational.org
- 26.Michelotti A, Cioffi I, Festa P, Scala G, Farella M. Oral parafunctions as risk factors for diagnostic TMD subgroups. J Oral Rehabil. 2010;37(3):157‐162. [DOI] [PubMed] [Google Scholar]
- 27.Leketas M, Šǎferis V, Kubilius R, Cervino G, Bramanti E, Cicciù M. Oral behaviors and parafunctions: comparison of temporomandibular dysfunction patients and controls. J Craniofac Surg. 2017;28(8):1933‐1938. [DOI] [PubMed] [Google Scholar]
- 28.Moss RA, Lombardo TW, Villarosa GA, Simkin L, Hodgson JM. Oral habits and TMJ dysfunction in facial pain and non‐pain subjects. J Oral Rehabil. 1995;22(1):79‐81. [DOI] [PubMed] [Google Scholar]
- 29.Cioffi I, Landino D, Donnarumma V, Castroflorio T, Lobbezoo F, Michelotti A. Frequency of daytime tooth clenching episodes in individuals affected by masticatory muscle pain and pain‐free controls during standardized ability tasks. Clin Oral Investig. 2017;21(4):1139‐1148. [DOI] [PubMed] [Google Scholar]
- 30.Franco‐Micheloni A, Fernandes G, Gonçalves D, Camparis C. Temporomandibular disorders in a young adolescent Brazilian population: epidemiologic characterization and associated factors. J Oral Facial Pain Headache. 2015;29(3):242‐249. [DOI] [PubMed] [Google Scholar]
- 31.Karibe H, Shimazu K, Okamoto A, Kawakami T, Kato Y, Warita‐Naoi S. Prevalence and association of self‐reported anxiety, pain, and oral parafunctional habits with temporomandibular disorders in Japanese children and adolescents: a cross‐sectional survey. BMC Oral Health. 2015;15(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sermet Elbay Ü, Demirturk Kocasarac H, Elbay M, Kaya C, Uğurluel C, Baydemir C. Temporomandibular disorders and oral parafunction in children living with their parents and children living in institutional protective care: a comparative study. Int Dent J. 2017;67(1):20‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glaros AG, Williams K, Lausten L, Friesen LR. Tooth contact in patients with temporomandibular disorders. Cranio. 2005;23(3):188‐193. [DOI] [PubMed] [Google Scholar]
- 34.Lund JP, Donga R, Widmer CG, Stohler CS. The pain‐adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69(5):683‐694. [DOI] [PubMed] [Google Scholar]
- 35.Visscher CM, Ohrbach R, van Wijk AJ, Wilkosz M, Naeije M. The tampa scale for kinesiophobia for temporomandibular disorders (TSK‐TMD). Pain. 2010;150(3):492‐500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐S3
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [VD] upon reasonable request.


