Abstract
Background
The present study aimed to evaluate the prognostic factors in human papillomavirus (HPV)‐positive and HPV‐negative oropharyngeal cancer (OPC) treated with definitive radiotherapy.
Methods
We retrospectively evaluated 101 patients with OPC who underwent definitive radiotherapy between 2008 and 2018.
Results
The median follow‐up period of the surviving patients was 68 months (range, 8–164 months). The 5‐year overall survival rate was 69.8%. Univariate analyses revealed that poor survival was associated with male sex, smoking ≥30 pack‐years, Eastern Cooperative Oncology Group performance status ≥1, tumor‐node‐metastasis (TNM) stage III‐IV (8th edition), HPV‐negativity, serum lactate dehydrogenase (LDH) ≥202, C‐reactive protein/albumin ratio ≥0.15, and lymphocyte‐to‐monocyte ratio <2.90. In multivariate analyses, poor survival was independently correlated with smoking ≥30 pack‐years (p < 0.01) and LDH ≥202 (p = 0.02).
Conclusions
The present study suggested that high LDH levels predicted poor survival after definitive radiotherapy for patients with both HPV‐positive and HPV‐negative OPC.
Keywords: head and neck squamous cell carcinoma, human papillomavirus, oropharyngeal cancer, radiotherapy, serum lactate dehydrogenase
1. INTRODUCTION
Over the last two decades, there has been a significant increase in the incidence of oropharyngeal cancer (OPC) due to human papillomavirus (HPV) infection in the United States and Europe.1, 2 Among high‐risk variants, HPV16 accounts for almost 85% of OPC cases in Western countries.1 Multiple studies have established that patients with HPV‐positive OPC have a better prognosis than patients with HPV‐negative.3, 4 Therefore, HPV‐positive OPC shows distinct epidemiological, clinical, and molecular features when compared to HPV‐negative OPC, and tumor HPV status is recognized as the strongest independent prognostic factor for radiotherapy (RT) outcomes.1, 4, 5, 6, 7, 8 Although the clinical and molecular‐genetic characteristics of HPV‐positive and HPV‐negative OPC clearly differ, whether the prognostic factors for these cancers are also distinct remains unclear.9
Currently, the eighth edition of the International Union Against Cancer (UICC)/American Joint Committee on Cancer (AJCC) tumor‐node‐metastasis (TNM) classification system for OPC considers the HPV status.10 The system accurately reflected the superior survival outcomes of HPV‐positive OPC. Additionally, it is well known that tobacco smoking history is a strong prognostic factor for survival in patients with OPC.11 Similarly, serum lactate dehydrogenase (LDH),12, 13, 14 neutrophil‐to‐lymphocyte ratio (NLR),15, 16 lymphocyte‐to‐monocyte ratio (LMR),17, 18 and C‐reactive protein (CRP)/albumin ratio (CRP/Alb)19 have been identified as prognostic factors in several types of cancers, including nasopharyngeal cancer (NPC), lung cancer, and hepatocellular carcinoma. These markers are more readily available and more cost‐effective to assess than others. Nevertheless, the prognostic significance of these hematological markers in OPCs has not been fully elucidated. Therefore, in this study, the medical records of patients with OPC treated with definitive RT were retrospectively analyzed to identify distinct prognostic subsets of OPC.
2. MATERIALS AND METHODS
2.1. Patients
This retrospective study was conducted in full accordance with the World Medical Association Declaration of Helsinki and approved by our institutional review board (R02‐112), and all patients provided informed consent for definitive RT. Between August 2008 and December 2018, we identified 150 consecutive patients with OPC who underwent RT alone or chemoradiotherapy (CRT) at our institution. We excluded patients who had follow‐up durations of less than 6 months without any specific events. In addition, two patients with distant metastasis at the time of diagnosis were excluded. Immunohistochemistry (IHC) for HPV/p16 expression was performed as previously described.8 In this study, 44 patients with unknown HPV status were excluded. Blood test data within 4 weeks before the first day of RT treatment were available for all but three patients who were excluded from the analyses. Therefore, 101 patients were eligible for the analysis. The characteristics of the study patients are summarized in Table 1. Pretreatment evaluations included a complete history, physical examination, routine blood tests, and laryngoscopy. All patients were examined by gastrointestinal fibroscopy and whole‐body computed tomography (CT), with or without head and neck magnetic resonance imaging (MRI). Staging was performed according to the UICC TNM classification system (7th and 8th editions).10 Based on the recommendations from the multidisciplinary tumor board, patients were treated with either RT alone or CRT. Forty‐nine patients (48.5%) underwent ipsilateral or bilateral neck dissection before definitive RT. Additionally, volume reduction surgery of the primary tumor was performed in 24 patients (23.8%) before RT.
TABLE 1.
Clinicopathological characteristics of patients with oropharyngeal cancer (no. of patients = 101) according to HPV status
| HPV‐positive (N = 50) | HPV‐negative (N = 51) | p‐value | |
|---|---|---|---|
| Median age at diagnosis (range) | 63 (43–85) | 65 (38–85) | 0.34 |
| Sex | 0.46 | ||
| Female | 13 (26.0%) | 10 (19.6%) | |
| Male | 37 (74.0%) | 41 (80.4%) | |
| Smoking (pack‐years) [median (range)] | 20 (0–105) | 40 (0–150) | <0.01 |
| ECOG‐PS | <0.01 | ||
| 0 | 43 (86.0%) | 33 (64.7%) | |
| ≥1 | 7 (14.0%) | 18 (35.3%) | |
| Treatment | 0.32 | ||
| CRT | 26 (52.0%) | 27 (52.9%) | |
| BRT | 11 (22.0%) | 6 (11.8%) | |
| RT alone | 13 (26.0%) | 18 (35.3%) | |
| T classification | 0.17 | ||
| 1–2 | 36 (72.0%) | 30 (58.8%) | |
| 3–4 | 14 (28.0%) | 21 (41.2%) | |
| N classification | 0.09 | ||
| 1–2 | 40 (80.0%) | 18 (35.3%) | |
| 3–4 | 10 (20.0%) | 33 (64.7%) | |
| TNM stage (7th edition) | 0.17 | ||
| I | 2 (4.0%) | 4 (7.8%) | |
| II | 3 (6.0%) | 7 (13.7%) | |
| III | 6 (12.0%) | 18 (35.3%) | |
| IV | 39 (78.0%) | 22 (43.2%) | |
| TNM stage (8th edition) | <0.01 | ||
| I | 32 (64.0%) | 4 (7.8%) | |
| II | 6 (12.0%) | 7 (13.7%) | |
| III | 12 (24.0%) | 17 (33.3%) | |
| IV | 0 (0%) | 23 (45.2%) |
Abbreviations: BRT, bioradiotherapy; CRT, chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; HPV, human papillomavirus; PS, performance status; RT, radiotherapy; TNM, tumor‐node‐metastasis.
2.2. Treatment
All 101 patients were treated using a two‐step intensity‐modulated RT (IMRT) technique. At our institution, a two‐step method is used for head and neck cancers instead of the simultaneous integrated boost method.20 The procedure of two‐step IMRT has been previously described in detail.8 In short, whole neck RT of 44.0–50.0 Gy/22–25 fractions was delivered, followed by boost doses to the high‐risk clinical target volume (CTV) up to a total dose of 70.0 Gy/35 fractions. The daily prescribed dose to the planning target volume (PTV) was 2 Gy in all patients. The prescribed dose was normalized to 95% of the PTV.
Between 2008 and 2018, 31 patients (30.7%) with TNM stage I or II disease, major comorbidity, or poor performance status were treated with RT alone. Concurrent chemotherapy was administered to 70 patients (69.3%). Between 2005 and 2018, 49 patients (48.5%) were treated with RT and three cycles of concomitant cisplatin (80.0–100 mg/m2 every 3 weeks). Four patients (4.0%) with poor renal function were treated with weekly carboplatin (five areas under the curve). Between 2013 and 2018, 17 elderly patients (16.8%) with poor renal function were treated with weekly cetuximab (250.0 mg/m2).
2.3. Statistical analyses
Comparisons between the HPV‐positive and HPV‐negative OPC groups were performed using t tests for continuous variables and Fisher's exact tests for categorical variables. Time‐to‐event analyses were performed from the start of definitive RT to the occurrence of the event. Receiver‐operating characteristic (ROC) curve analysis was used to determine the most appropriate cut‐off points for hematological biomarkers, including LDH, NLR, LMR, and CRP/Alb. The Kaplan–Meier method and log‐rank test were used to compare the overall survival (OS) curves. OS was defined as the time to death from any cause. In addition, potential prognostic factors were evaluated using Cox proportional hazards models, and the results were reported as hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). Significant factors identified in the univariate analyses were included in the multivariate model by backward elimination of the insignificant explanatory variables. All analyses were performed using JMP software (version 14.0.0; SAS Institute, Cary, NC), and differences were considered statistically significant at p < 0.05.
3. RESULTS
The median follow‐up period of the surviving patients was 68 months (range, 8–164 months). Figure 1 shows the OS after RT for patients with HPV‐positive and HPV‐negative OPC. The 5‐year OS was 69.8% (95% CI: 0.59–0.79). The cut‐off values obtained by ROC for LDH, CRP/Alb, NLR, and LMR were 202, 0.15, 6.10, and 2.90, respectively.
FIGURE 1.
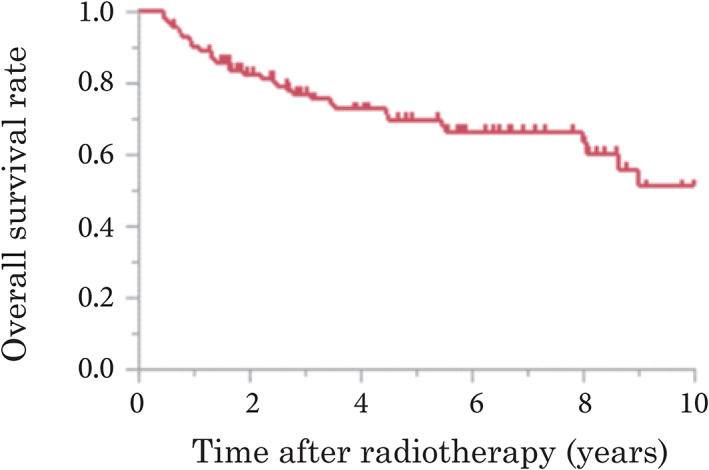
Overall survival (OS) after definitive radiotherapy in patients with human papillomavirus‐positive and ‐negative oropharyngeal cancer. The 5‐year OS was 69.8% (95% confidence interval: 0.59–0.79) [Color figure can be viewed at wileyonlinelibrary.com]
The results of the univariate and multivariate analyses of prognostic factors are shown in Table 2. Univariate analyses revealed that poor survival was associated with male sex, smoking ≥30 pack‐years, Eastern Cooperative Oncology Group performance status (ECOG‐PS) ≥1, TNM stage III‐IV (8th edition), HPV‐negativity, LDH ≥202, CRP/Alb ≥0.15, and LMR <2.90. In multivariate analyses, poor survival was independently correlated with smoking ≥30 pack‐years and LDH ≥202 (Figure 2) but not HPV status. To eliminate confounding bias between HPV status and TNM stage (8th edition), the TNM stage was excluded from the multivariate analysis, despite showing a significant result in the univariate analysis.
TABLE 2.
Univariate and multivariate analysis results of patients with oropharyngeal cancer
| Parameter | Patients (N = 101) | 5‐year survival (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p‐value | HR (95% CI) | p‐value | |||
| Age (years) | ||||||
| <65 | 51 | 75.3 | 1 | 0.23 | ||
| ≥65 | 50 | 64.6 | 1.52 (0.77–3.11) | |||
| Sex | ||||||
| Female | 23 | 89.8 | 1 | <0.01 | 1 | 0.48 |
| Male | 78 | 63.7 | 4.36 (1.53–18.4) | 1.61 (0.45–7.78) | ||
| Smoking (pack‐years) | ||||||
| <30 | 49 | 88.8 | 1 | <0.01 | 1 | <0.01 |
| ≥30 | 52 | 53.5 | 5.78 (2.55–15.5) | 4.20 (1.54–13.6) | ||
| ECOG‐PS | ||||||
| 0 | 76 | 77.1 | 1 | 0.01 | 1 | 0.28 |
| ≥1 | 25 | 47.3 | 2.59 (1.25–5.20) | 1.53 (0.70–3.27) | ||
| Treatment | ||||||
| CRT or BRT | 70 | 62.8 | 1 | 0.22 | ||
| RT alone | 31 | 82.9 | 0.62 (0.28–1.31) | |||
| TNM stage (7th edition) | ||||||
| I‐III | 40 | 74.9 | 1 | 0.32 | ||
| IV | 61 | 66.9 | 1.44 (0.71–3.07) | |||
| TNM stage (8th edition) | ||||||
| I‐II | 49 | 80.8 | 1 | <0.01 | ||
| III | 52 | 59.7 | 2.74 (1.35–6.03) | |||
| HPV | ||||||
| Positive | 50 | 79.4 | 1 | 0.02 | 1 | 0.58 |
| Negative | 51 | 61.3 | 2.37 (1.17–5.09) | 1.25 (0.58–2.81) | ||
| LDH | ||||||
| <202 | 60 | 80.9 | 1 | <0.01 | 1 | 0.02 |
| ≥202 | 41 | 52.9 | 3.48 (1.75–7.27) | 2.38 (1.13–5.27) | ||
| CRP/Alb | ||||||
| <0.15 | 35 | 75.6 | 1 | <0.01 | 1 | 0.67 |
| ≥0.15 | 66 | 59.6 | 2.57 (1.30–5.22) | 1.20 (0.52–2.78) | ||
| NLR | ||||||
| <6.10 | 82 | 72.7 | 1 | 0.16 | ||
| ≥6.10 | 19 | 56.5 | 1.77 (0.79–3.66) | |||
| LMR | ||||||
| <2.90 | 40 | 54.9 | 1 | <0.01 | 1 | 0.13 |
| ≥2.90 | 61 | 87.6 | 0.32 (0.15–0.63) | 0.51 (0.21–1.21) | ||
Abbreviations: Alb, albumin; BRT, bioradiotherapy; CI, confidence interval; CRP, C‐reactive protein; CRT, chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; HPV, human papillomavirus; HR, hazard ratio; LDH, serum lactate dehydrogenase; LMR, lymphocyte‐to‐monocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; PS, performance status; RT, radiotherapy; TNM, tumor‐node‐metastasis.
FIGURE 2.
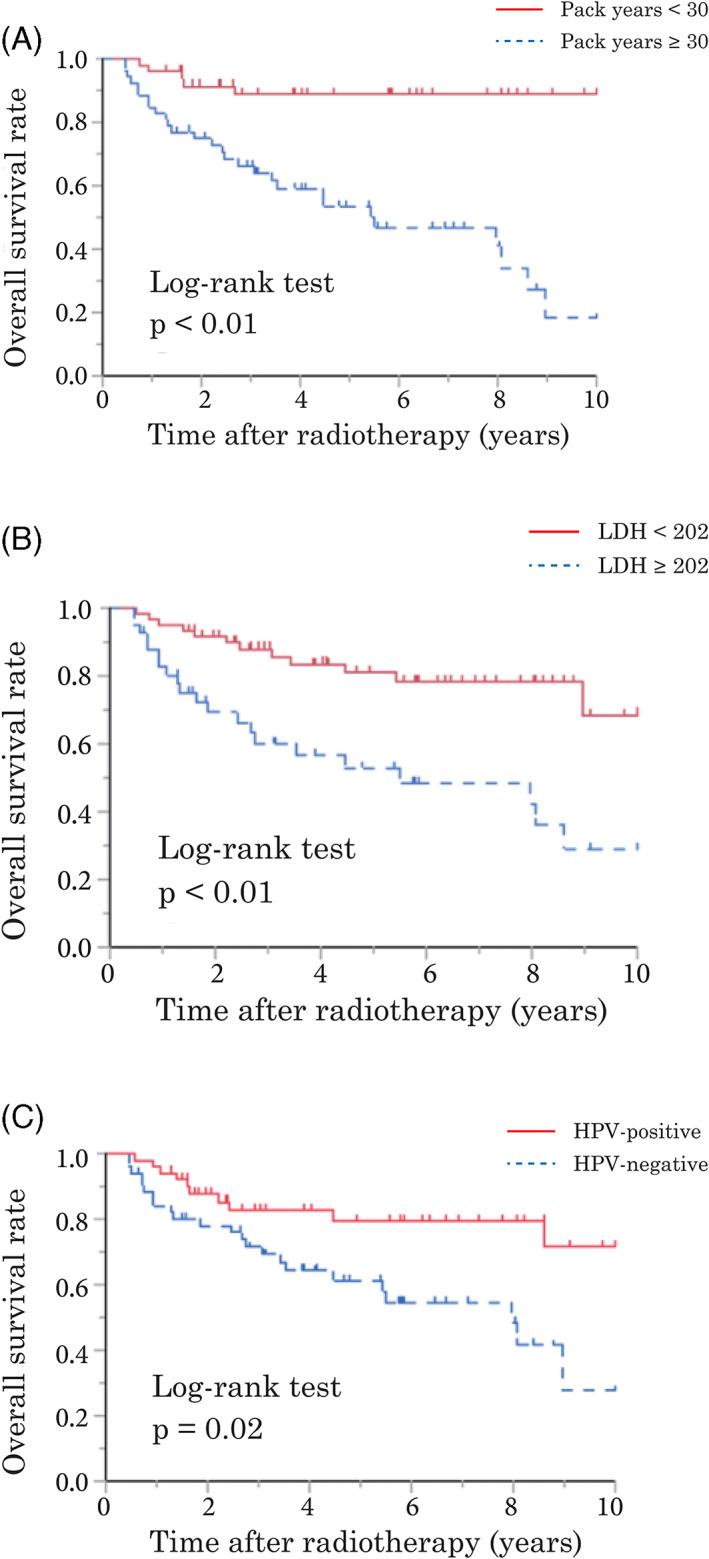
Kaplan–Meier's analysis of overall survival (OS) rates in patients with human papillomavirus (HPV)‐positive and ‐negative oropharyngeal cancer. OS according to smoking (A), serum lactate dehydrogenase (LDH) (B), and HPV status (C) [Color figure can be viewed at wileyonlinelibrary.com]
Patients were divided into HPV‐positive and HPV‐negative OPC groups and potential prognostic factors were subsequently assessed in univariate analyses. The results for the HPV‐positive and HPV‐negative OPC groups are shown in Tables 3 and 4, respectively. In patients with HPV‐positive OPC, poor survival was associated with smoking ≥30 pack‐years, LDH ≥202, and LMR <2.90. In patients with HPV‐negative OPC, univariate analyses identified male sex, smoking ≥30 pack‐years, TNM stage IV (7th edition), LDH ≥202, and CRP/Alb ≥0.15 as significant predictors of poor survival (Figure 3).
TABLE 3.
Univariate analysis results of patients with HPV‐positive oropharyngeal cancer
| Parameter | Patients (N = 50) | 5‐year survival (%) | HR (95% CI) | p‐value |
|---|---|---|---|---|
| Age (years) | ||||
| <65 | 27 | 87.4 | 1 | 0.14 |
| ≥65 | 23 | 70.9 | 12.7 (0.74–12.3) | |
| Sex | ||||
| Female | 13 | 92.3 | 1 | 0.17 |
| Male | 37 | 74.3 | 3.47 (0.65–64.1) | |
| Smoking (pack‐years) | ||||
| <30 | 30 | 89.5 | 1 | 0.03 |
| ≥30 | 20 | 63.8 | 4.21 (1.15–19.7) | |
| ECOG‐PS | ||||
| 0 | 43 | 83.5 | 1 | 0.13 |
| ≥1 | 7 | 53.6 | 3.13 (0.67–11.3) | |
| Treatment | ||||
| CRT or BRT | 37 | 75.6 | 1 | 0.85 |
| RT alone | 13 | 84.6 | 0.87 (0.18–3.25) | |
| TNM stage (7th edition) | ||||
| I‐III | 11 | 80.8 | 1 | 0.90 |
| IV | 39 | 78.8 | 1.11 (0.27–7.37) | |
| TNM stage (8th edition) | ||||
| I‐II | 38 | 81.4 | 1 | 0.22 |
| III | 12 | 74.1 | 2.31 (0.58–8.23) | |
| LDH | ||||
| <202 | 34 | 85.7 | 1 | 0.04 |
| ≥202 | 16 | 66.2 | 3.59 (1.01–14.2) | |
| CRP/Alb | ||||
| <0.15 | 34 | 84.1 | 1 | 0.29 |
| ≥0.15 | 16 | 70.7 | 1.98 (0.55–7.15) | |
| NLR | ||||
| <6.10 | 42 | 79.7 | 1 | 0.33 |
| ≥6.10 | 8 | 75.0 | 2.07 (0.43–7.68) | |
| LMR | ||||
| <2.90 | 17 | 60.5 | 1 | <0.01 |
| ≥2.90 | 33 | 88.6 | 0.18 (0.04–0.66) | |
Abbreviations: Alb, albumin; BRT, bioradiotherapy; CI, confidence interval; CRP, C‐reactive protein; CRT, chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; HPV, human papillomavirus; HR, hazard ratio; LDH, serum lactate dehydrogenase; LMR, lymphocyte‐to‐monocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; PS, performance status; RT, radiotherapy; TNM, tumor‐node‐metastasis.
TABLE 4.
Univariate analysis results of patients with HPV‐negative oropharyngeal cancer
| Parameter | Patients (N = 51) | 5‐year survival (%) | HR (95% CI) | p‐value |
|---|---|---|---|---|
| Age (years) | ||||
| <65 | 24 | 63.9 | 1 | 0.62 |
| ≥65 | 27 | 58.9 | 1.24 (0.53–2.96) | |
| Sex | ||||
| Female | 10 | 87.5 | 1 | <0.01 |
| Male | 41 | 55.2 | 7.51 (1.57–134) | |
| Smoking (pack‐years) | ||||
| <30 | 19 | 88.2 | 1 | <0.01 |
| ≥30 | 32 | 47.9 | 7.02 (2.05–43.9) | |
| ECOG‐PS | ||||
| 0 | 33 | 69.7 | 1 | 0.13 |
| ≥1 | 18 | 44.3 | 1.81 (0.76–4.19) | |
| Treatment | ||||
| CRT or BRT | 33 | 50.1 | 1 | 0.06 |
| RT alone | 18 | 81.4 | 0.41 (0.14–1.04) | |
| TNM stage (7th edition) | ||||
| I‐III | 29 | 72.2 | 1 | 0.03 |
| IV | 22 | 48.2 | 2.51 (1.09–6.06) | |
| TNM stage (8th edition) | ||||
| I‐II | 11 | 79.6 | 1 | 0.13 |
| III | 40 | 56.7 | 2.34 (0.80–9.98) | |
| LDH | ||||
| <202 | 26 | 75.7 | 1 | <0.01 |
| ≥202 | 25 | 45.5 | 3.14 (1.32–8.26) | |
| CRP/Alb | ||||
| <0.15 | 31 | 69.9 | 1 | 0.01 |
| ≥0.15 | 20 | 47.9 | 2.98 (1.28–7.47) | |
| NLR | ||||
| <6.10 | 40 | 66.1 | 1 | 0.37 |
| ≥6.10 | 11 | 42.4 | 1.56 (0.57–3.80) | |
| LMR | ||||
| <2.90 | 23 | 50.6 | 1 | 0.11 |
| ≥2.90 | 28 | 68.7 | 0.51 (0.22–1.16) | |
Abbreviations: Alb, albumin; BRT, bioradiotherapy; CI, confidence interval; CRP, C‐reactive protein; CRT, chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; HPV, human papillomavirus; HR, hazard ratio; LDH, serum lactate dehydrogenase; LMR, lymphocyte‐to‐monocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; PS, performance status; RT, radiotherapy; TNM, tumor‐node‐metastasis.
FIGURE 3.
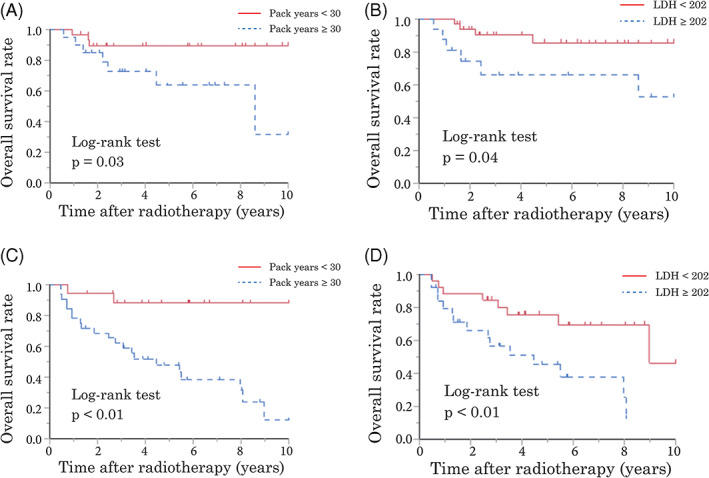
Kaplan–Meier analysis of overall survival (OS) rates. OS according to smoking (A) and serum lactate dehydrogenase (LDH) (B) in patients with human papillomavirus (HPV)‐positive oropharyngeal cancer (OPC). OS according to smoking (C) and LDH (D) in patients with HPV‐negative OPC [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In this study, the prognostic factors for patients with OPC were evaluated. Our study suggested that poor survival might be independently associated with high LDH levels in patients with OPC, regardless of HPV status. It is noteworthy that high LDH levels may be a stronger poor prognostic factor than HPV status and TNM stage (both 7th and 8th editions) in this study. Moreover, as is well‐known from previous studies, tobacco smoking affects treatment response and negatively affects patients with OPC.4, 11, 21
Elevated LDH levels have been identified as a negative prognostic indicator for many solid tumors, including non‐Hodgkin's lymphoma,12 germ cell tumors,13 and small‐cell lung cancer.14 The prognostic significance of serum LDH levels in NPC has been investigated in several retrospective studies.22, 23, 24 High levels of serum LDH were found to be correlated with a larger tumor burden in NPC.25 In addition, a high LDH level was associated with the extent of locoregional control and/or distant metastasis events and could predict a poor prognosis.25 However, there are limited data regarding the use of pretreatment serum LDH to predict the prognosis of patients with OPC. In both HPV‐positive and HPV‐negative OPC, OS was considerably worse in patients with higher LDH levels than in those with lower levels. To the best of our knowledge, this is the first study to indicate that LDH levels are associated with poor prognosis and that they can be an independent prognostic marker for patients with OPC regardless of HPV status. In addition, the results would imply that potentially LDH could be a biomarker for nonoropharyngeal head and neck cancers.
Although the prognostic value of LDH has been studied extensively, the underlying mechanism linking LDH to poor survival remains unknown. It has been hypothesized that serum LDH levels may reflect the extent of hypoxia in tumor cells, since it catalyzes the transformation of pyruvate to lactate under hypoxic conditions.26, 27 Due to rapid cancer cell proliferation, high metabolic demands, and functional angiogenesis, hypoxia is a characteristic feature of locally advanced solid tumors.28 In addition, it is well known that hypoxic tumor cells are radioresistant and/or chemoresistant.29 These findings indicate that high LDH levels related to tumor hypoxia might lead to poor survival after RT.
Based on their better outcomes, several ongoing clinical studies are evaluating the applicability of de‐escalating treatment for patients with HPV‐positive OPC.30, 31, 32, 33, 34 Recently, two clinical trials failed to show noninferior tumor control and reduced toxicity.33, 34 Mehanna et al. performed an open‐label randomized controlled phase 3 trial, De‐ESCALaTE HPV, to investigate the effect of substituting cisplatin with cetuximab on RT in patients with advanced HPV‐positive OPC with no or little tobacco smoking history.33 The results showed no difference in overall severe toxicity between the two groups. Surprisingly, they demonstrated that the cetuximab group showed significantly worse 2‐year OS and disease recurrence rates compared with the cisplatin group. In their study, LDH levels were not used as stratification factors. On the other hand, de‐escalation of dose and target volume in RT has been tested in phase 2 clinical trials, yet the results are currently immature.35, 36 Foster et al.35 and Seiwert et al.36 also stratified patients for de‐escalated treatment according to smoking history but not LDH levels. Although tobacco smoking history, a strong prognostic factor, was often used in the stratification for de‐escalated treatment as mentioned above, the results of these trials were not successful. Therefore, based on the present data, LDH might be useful for risk stratification when considering de‐escalated strategies for HPV‐positive OPC.
Additionally, in this study, high LDH levels were a stronger poor prognostic factor than HPV status and TNM stage (both 7th and 8th editions). However, the relatively small sample size and heterogeneous patient demographics of the present study might be associated with the insignificant differences in survival according to HPV status and TNM stage. With regards to TNM stage specifically, the univariate analysis demonstrated a significant difference in OS according to the 8th edition TNM stage but not the 7th edition. Whereas the 7th edition staging system considered only the anatomical extent of disease,10 the 8th edition considered HPV status. Our present data suggest that the 8th edition is suitable for staging patients with OPC, as stated in a previous study.37
The present study has several limitations, including its retrospective design, relatively small sample size, and heterogeneous patient characteristics. However, our multivariate analysis incorporating patients' background factors and treatment modalities addressed this heterogeneity to a certain extent. In addition, LDH was confirmed as a reproducible prognostic marker in patients with both HPV‐positive and HPV‐negative OPC. Moreover, we also assessed prognostic factors with respect to HPV status; however, the insufficient sample size of the subgroups precluded multivariate analyses. Furthermore, the heterogeneous patient characteristics might have been due to the time background between 2008 and 2018. However, the standard RT treatment for OPC did not change during this period.36, 37 In addition, we excluded patients with unknown HPV status from this study in order to increase the cohort's homogeneity. Further, the follow‐up period was sufficiently long (68 months) to assess survival outcomes. Therefore, we believe that the present study provides valuable data that can be reliably adopted in daily clinical practice and act as the foundation for future clinical trials.
In conclusion, the present study suggested that high LDH levels might be a predictive factor for poor survival after definitive RT for patients with HPV‐positive and HPV‐negative OPC. High LDH levels may be a stronger prognostic factor than HPV status and TNM stage. As this is a small sample size with wide confidence intervals and retrospective, the findings are really hypothesis that require validation in a larger well designed prospective trial.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTIONS
Takuya Uehara, Hiroshi Doi, Masahiro Inada, Saori Tatsuno, Yutaro Wada, Yasuo Oguma, Kazuki Ishikawa, Kiyoshi Nakamatsu, Makoto Hosono, and Yasumasa Nishimura performed radiotherapy. Takuya Uehara, Hiroshi Doi, and Masahiro Inada conceived this study, analyzed the data, and drafted the manuscript. Yasumasa Nishimura supervised the project. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This study was partially supported by Grant‐in‐Aid for Scientific Research (C), JSPS KAKENHI (20K08009). The authors are grateful to Yasutaka Chiba, PhD for advice on statistical analysis. The authors would like to acknowledge Editage (www.editage.jp) for English language editing.
Uehara T, Doi H, Ishikawa K, et al. Serum lactate dehydrogenase is a predictive biomarker in patients with oropharyngeal cancer undergoing radiotherapy: Retrospective study on predictive factors. Head & Neck. 2021;43(10):3132‐3141. 10.1002/hed.26814
Section Editor: James Rocco
Funding information Grant‐in‐Aid for Scientific Research (C), JSPS KAKENHI, Grant/Award Number: 20K08009
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294‐4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Dyne EA, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Bernald VB. Trends in human papillomavirus‐associated cancers—United States, 1999–2015. MMWR Morb Mortal Wkly Rep. 2018;67:918‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Posner MR, Lorch JH, Goloubeva O, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset. Ann Oncol. 2011;22:1071‐1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammarstedt L, Dahlstrand H, Lindquist D, et al. The incidence of tonsillar cancer in Sweden is increasing. Acta Otolaryngol. 2007;127:988‐992. [DOI] [PubMed] [Google Scholar]
- 6.Marur S, D'Souza G, Westra WH, Forastiere AA. HPV‐associated head and neck cancer: a virus‐related cancer epidemic. Lancet Oncol. 2010;11:781‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in humanpapillomavirus‐related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543‐550. [DOI] [PubMed] [Google Scholar]
- 8.Tatebe H, Doi H, Ishikawa K, et al. Two‐step intensity‐modulated radiation therapy for oropharyngeal cancer: initial clinical experience and validation of clinical staging. Anticancer Res. 2018;38:979‐986. [DOI] [PubMed] [Google Scholar]
- 9.Gillison ML, D'souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16‐positive and human papillomavirus type 16‐negative head and neck cancers. J Natl Cancer Inst. 2008;100:407‐420. [DOI] [PubMed] [Google Scholar]
- 10.O'Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV‐related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON‐S): a multicentre cohort study. Lancet Oncol. 2016;17:440‐451. [DOI] [PubMed] [Google Scholar]
- 11.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16‐positive and p16‐negative oropharyngeal cancer. J Clin Oncol. 2012;10(30):2102‐2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Non‐Hodgkin's Lymphoma Prognostic Factors Project . A predictive model for aggressive non‐Hodgkin's lymphoma. N Engl J Med. 1993;329:987‐994. [DOI] [PubMed] [Google Scholar]
- 13.Shamash J, Oliver RT, Gallagher CJ, et al. Pre‐induction LDH as a prognostic factor for outcome of high dose chemotherapy (HDCT) for germ cell tumors relapsing or refractory to conventional chemotherapy. Br J Cancer. 2000;82:2022‐2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byhardt RW, Hartz A, Libnoch JA, Hansen R, Cox JD. Prognostic influence of TNM staging and LDH levels in small cell carcinoma of the lung (SCCL). Int J Radiat Oncol Biol Phys. 1986;12:771‐777. [DOI] [PubMed] [Google Scholar]
- 15.Takenaka Y, Kitamura T, Oya R, et al. Prognostic role of neutrophil‐lymphocyte ratio in nasopharyngeal carcinoma: a meta‐analysis. PLoS One. 2017;12:e0181478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doi H, Nakamatsu K, Anami S, et al. Neutrophil‐to‐lymphocyte ratio predicts survival after whole‐brain radiotherapy in non‐small cell lung cancer. In Vivo. 2019;33:195‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte‐to‐monocyte ratio in patients with solid tumors: a systematic review and meta‐analysis. Cancer Treat Rev. 2015;41:971‐978. [DOI] [PubMed] [Google Scholar]
- 18.Gu L, Li H, Chen L, et al. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta‐analysis. Oncotarget. 2016;7:31926‐31942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinoshita A, Onoda H, Imai N, et al. The C‐reactive protein/albumin ratio, a novel inflammation‐based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22:803‐810. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura Y, Ishikura S, Shibata T, et al. A phase II study of adaptive two‐step intensity‐modulated radiation therapy (IMRT) with chemotherapy for loco‐regionally advanced nasopharyngeal cancer (JCOG1015). Int J Clin Oncol. 2020;25:1250‐1259. [DOI] [PubMed] [Google Scholar]
- 21.Hafkamp HC, Manni JJ, Haesevoets A, et al. Marked differences in survival rate between smokers and nonsmokers with HPV 16‐associated tonsillar carcinomas. Int J Cancer. 2008;122:2656‐2664. [DOI] [PubMed] [Google Scholar]
- 22.Cheng SH, Tsai SY, Horng CF, et al. A prognostic scoring system for locoregional control in nasopharyngeal carcinoma following conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:992‐1003. [DOI] [PubMed] [Google Scholar]
- 23.Turen S, Ozyar E, Altundag K, Gullu I, Atahan IL. Serum lactate dehydrogenase level is a prognostic factor in patients with locoregionally advanced nasopharyngeal carcinoma treated with chemoradiotherapy. Cancer Invest. 2007;25:315‐321. [DOI] [PubMed] [Google Scholar]
- 24.Cheng SH, Yen KL, Jian JJ, et al. Examining prognostic factors and patterns of failure in nasopharyngeal carcinoma following concomitant radiotherapy and chemotherapy: impact on future clinical trials. Int J Radiat Oncol Biol Phys. 2001;50:717‐726. [DOI] [PubMed] [Google Scholar]
- 25.Zhou GQ, Tang LL, Mao YP, et al. Baseline serum lactate dehydrogenase levels for patients treated with intensity‐modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. Int J Radiat Oncol Biol Phys. 2011;82:359‐365. [DOI] [PubMed] [Google Scholar]
- 26.Suguro M, Kanda Y, Yamamoto R, et al. High serum lactate dehydrogenase level predicts short survival after vincristine‐doxorubicin‐dexamethasone (VAD) salvage for refractory multiple myeloma. Am J Hematol. 2000;65:132‐135. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong AJ, George DJ, Halabi S. Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J Clin Oncol. 2012;30:3402‐3407. [DOI] [PubMed] [Google Scholar]
- 28.Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266‐276. [DOI] [PubMed] [Google Scholar]
- 29.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). 2015;3:83‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masterson L, Moualed D, Masood A, et al. De‐escalation treatment protocols for human papillomavirus‐associated oropharyngeal squamous cell carcinoma. Cochrane Database Syst Rev. 2014;2:CD010271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taberna M, Mena M, Pavón MA, Alemany L, Gillison ML, Mesía R. Human papillomavirus‐related oropharyngeal cancer. Ann Oncol. 2017;28:2386‐2398. [DOI] [PubMed] [Google Scholar]
- 32.Mirghani H, Blanchard P. Treatment de‐escalation for HPV‐driven oropharyngeal cancer: Where do we stand? Clin Transl Radiat Oncol. 2017;8:4‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low‐risk human papillomavirus‐positive oropharyngeal cancer (De‐ESCALaTE HPV): an open‐label randomised controlled phase 3 trial. Lancet. 2019;393:51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus‐positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non‐inferiority trial. Lancet. 2019;393:40‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster CC, Seiwert TY, MacCracken E, et al. Dose and volume de‐escalation for human papillomavirus‐positive oropharyngeal cancer is associated with favorable posttreatment functional outcomes. Int J Radiat Oncol Biol Phys. 2020;107:662‐671. [DOI] [PubMed] [Google Scholar]
- 36.Seiwert TY, Foster CC, Blair EA, et al. OPTIMA: a phase II dose and volume de‐escalation trial for human papillomavirus‐positive oropharyngeal cancer. Ann Oncol. 2019;30:297‐302. [DOI] [PubMed] [Google Scholar]
- 37.van Gysen K, Stevens M, Guo L, et al. Validation of the 8th edition UICC/AJCC TNM staging system for HPV associated oropharyngeal cancer patients managed with contemporary chemo‐radiotherapy. BMC Cancer. 2019;19:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


