Abstract
Background and Purpose
Spice/K2 herbal mixtures, containing synthetic cannabinoids such as JWH‐018, have been marketed as marijuana surrogates since 2004. JWH‐018 has cannabinoid CB1 receptor‐dependent reinforcing properties and acutely increases dopaminergic transmission selectively in the NAc shell. Here, we tested the hypothesis that repeated administration of JWH‐018 (i) modulates behaviour, (ii) affects dopaminergic transmission and its responsiveness to motivational stimuli, and (iii) is associated with a neuroinflammatory phenotype.
Experimental Approach
Rats were administered with JWH‐018 once a day for 14 consecutive days. We then performed behavioural, electrophysiological, and neurochemical evaluation at multiple time points after drug discontinuation.
Key Results
Repeated JWH‐018 exposure (i) induced anxious and aversive behaviours, transitory attentional deficits, and withdrawal signs; (ii) decreased spontaneous activity and number of dopamine neurons in the VTA; and (iii) reduced stimulation of dopaminergic transmission in the NAc shell while potentiating that in the NAc core, in response to acute JWH‐018 challenge. Moreover, (iv) we observed a decreased dopamine sensitivity in the NAc shell and core, but not in the mPFC, to a first chocolate exposure; conversely, after a second exposure, dialysate dopamine fully increased in the NAc shell and core but not in the mPFC. Finally, selected dopamine brain areas showed (v) astrogliosis (mPFC, NAc shell and core, VTA), microgliosis (NAc shell and core), and downregulation of CB1 receptors (mPFC, NAc shell and core).
Conclusion and Implications
Repeated exposure to JWH‐018 may provide a useful model to clarify the detrimental effects of recurring use of Spice/K2 drugs.
Keywords: addiction, dopamine, glial cells, habituation, novel psychoactive substances, synthetic cannabinoids, taste
Abbreviations
- CPu
caudate putamen
- eCB
endocannabinoid
- EPM
elevated plus maze
- GFAP
glial fibrillary acidic protein
- HPLC
high performance liquid chromatography
- IBA‐1
ionized calcium‐binding adapter molecule‐1
- IR
immunoreactivity
- JWH‐018
1‐pentyl‐3‐(1‐naphthoyl) indole
- MB
marble burying
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- NPS
new psychoactive substances
- PPI
prepulse inhibition
- PSB
pontamine sky blue
- SCRA
synthetic cannabinoid receptor agonist
- SD
Sprague–Dawley
- TH
tyrosine hydroxylase
- THC
Δ9‐tetrahydrocannabinol
- VTA
ventral tegmental area
What is already known?
JWH‐018 is a synthetic cannabinoid receptor agonist in “Spice/K2 drugs” marketed as legal marijuana surrogates.
The central effects induced by repeated exposure to synthetic cannabinoid receptor agonists are underestimated.
What does this study adds?
Repeated JWH‐018 exposure induced anxious and aversive behaviours, transitory attentional deficits, and withdrawal signs.
Repeated JWH‐018 exposure modified dopaminergic transmission (basal and stimulated), and glial and CB1 receptor expressions.
What is the clinical significance?
Our data are useful to elucidate the severe consequences of recurring use of Spice/K2 drugs.
Our data might help clinicians to manage synthetic cannabinoid intoxications and related use disorders.
1. INTRODUCTION
Novel psychoactive substances (NPS) are a broad variety of drugs not controlled by the United Nations drug conventions. The advent of NPS has contributed to the appearance and growth of a new “drug scenario” characterised by an increased number of drug users among youth and consumption of drugs with unknown effects and safety profiles. The first wave of NPS came to Europe in the early 2000s when herbal mixtures containing synthetic cannabinoids (SC), broadly known as Spice/K2 drugs, have been marketed as legal marijuana surrogates. JWH‐018 (1‐pentyl‐3‐(1‐naphthoyl) indole) is a synthetic cannabinoid receptor agonist (SCRA), highly potent and efficacious at CB1 and CB2 receptors, that has been found in several Spice/K2 drugs (Wiley et al., 2012). Initially developed for therapeutic purposes, JWH‐018 is considered the prototypical compound of the so‐called “first‐generation” class of synthetic cannabinoids. Thereafter, newer generations of SCRAs arose from slight modifications to the chemical structure of JWH‐018, generating legal drugs with higher potency and efficacy at CB1 and CB2 receptors and increased abuse liability (De Luca & Fattore, 2018). Compared to Δ9‐tetrahydrocannabinol (THC), SCRAs induce more severe adverse reactions and psychiatric consequences (Papanti et al., 2014; Schifano et al., 2015), likely due to their action as full agonists at CB1 receptors (De Luca et al., 2016; Pintori et al., 2017). Previously, we demonstrated that JWH‐018 elicits CB1 receptor‐dependent reinforcing properties and dopamine stimulant actions, preferentially on the nucleus accumbens (NAc) shell at doses fourfold lower (0.25 mg kg‐1, i.p.) than THC (De Luca et al., 2015).
The role of mesolimbic and mesocortical dopaminergic transmission in salient information and reward processing is well known (Di Chiara et al., 2004). In particular, drugs of abuse and natural rewarding stimuli, as highly appetitive taste stimuli, increase dopaminergic transmission in the NAc shell (Bassareo et al., 2002; Di Chiara, 1990; Volkow et al., 2003). However, the neurochemical effect of taste stimuli undergoes habituation, that is, adaptive regulation, following repeated exposure (Bassareo et al., 2002; Di Chiara, 1990). Importantly, we previously showed that this phenomenon requires an intact dopaminergic function in the medial prefrontal cortex (mPFC) (i.e., infralimbic and prelimbic cortices), as bilateral lesions of these regions abolished habituation of dopaminergic transmission in the NAc shell to repeated chocolate exposure (Bimpisidis et al., 2013). These and other data (De Luca et al., 2011) support the notion of top‐down control of mPFC on NAc dopaminergic transmission and its putative role in the loss of control of the motivational value of stimuli (De Luca, 2014; Goldstein & Volkow, 2011). The endocannabinoid (eCB) system and dopaminergic transmission strictly interact in the evaluation of salience information and reward (Manzanares et al., 2018; Sagheddu et al., 2015; Tan et al., 2014; Volkow et al., 2017). Indeed, dysfunctions of eCB signalling may lead to the dysregulation of mesolimbic and mesocortical dopaminergic transmission, which is associated with several neuropsychiatric disorders, including addiction (Martín‐Santos et al., 2010; Tan et al., 2014; Volkow et al., 2003, 2017; Volkow & Morales, 2015). Besides neurons, accumulating evidence strongly support an important contribution of glial cells in brain plasticity and in affective, motivational, and cognitive processes (Marin & Kipnis, 2013; Yirmiya & Goshen, 2011). Important glial alterations produced by different addictive drugs, including THC, have been observed (Lacagnina et al., 2017; Melis et al., 2017; Scofield & Kalivas, 2014; Secci et al., 2019).
Despite the widespread and growing use of Spice/K2 drugs, limited information is available on the effects induced by repeated exposure to SCRAs on mesolimbic and mesocortical dopaminergic transmission and on glial cells. To investigate these aspects in more depth, we evaluated the neurochemical and behavioural modifications occurring in adult male Sprague–Dawley rats repeatedly administered with JWH‐018. The dose able to selectively increase dopaminergic transmission in the NAc shell (0.25 mg kg‐1, i.p.) was selected on the basis of our previous studies, lower and higher doses being ineffective on dopamine release (De Luca et al., 2015). At separate time‐points after JWH‐018 discontinuation (1 h, 24 h, or 7 days), we performed a battery of behavioural tests to evaluate the occurrence of anxiety‐, aversive‐, compulsive‐like behaviours, attentional dysfunction, and spontaneous somatic withdrawal signs. In addition, changes in the activity of ventral tegmental area (VTA) dopaminergic neurons, during JWH‐018 withdrawal were recorded and compared to the tyrosine hydroxylase (TH)‐immunoreactivity (IR) in the same area. Furthermore, in order to explore whether or not a loss of control of the motivational value of stimuli can be induced by repeated exposure to JWH‐018, the pattern of dopaminergic responses to a natural rewarding stimulus (i.e., intraoral chocolate) was estimated by in vivo microdialysis in the NAc shell and core and in the mPFC. Moreover, the levels of markers of glia activation, such as glial fibrillary acidic protein (GFAP) and ionised calcium‐binding adapter molecule‐1 (IBA‐1), were measured in the mPFC, NAc, and VTA. Finally, changes in CB1 receptor expression were evaluated in selected dopaminergic terminals (i.e., mPFC and NAc). Taken together, these preclinical studies are useful in elucidating the severe consequences of the recurring use of Spice/K2 drugs with the ultimate goal of helping clinicians to manage synthetic cannabinoid intoxications and related use disorders.
2. METHODS
2.1. Animals
All animal care and experimental procedures were carried out in accordance with European Council directives (609/86 and 63/2010) and in compliance with the animal policies issued by the Italian Ministry of Health and the Ethical Committee for Animal Experiments (CESA, University of Cagliari). We made all efforts to minimise pain and suffering and to reduce the number of animals used. Animal studies are reported in compliance with the ARRIVE guidelines (Percie du Sert et al., 2020) and with the recommendations made by the British Journal of Pharmacology (Lilley et al., 2020). The total number of animals as well as their suffering was minimised, according to the 3Rs principles. Adult male Sprague–Dawley (SD, 275–300 g) rats (Envigo, Italy) were housed in groups of six at temperature of 22°C ± 2°C and 60% humidity, under a 12 h light/dark cycle (lights on at 7.00 am) and with ad libitum access to water and food (Mucedola, Italy).
2.2. Experimental design timeline
Rats were injected once a day with either JWH‐018 (0.25 mg kg‐1) or vehicle, i.p., for 14 consecutive days. Animals were assigned randomly to different experimental groups for behavioural, electrophysiological, neurochemical, and molecular evaluation at different time points (1 h, 24 h, or 7 days) after drug discontinuation (Figure 1). The experimental group sizes (n ≥ 5) were chosen based on our previous experimental protocols (Castelli et al., 2014; Sagheddu et al., 2020; Zanda et al., 2017) and are shown in the figure legends. Due to technical issues (e.g., catheter or dialysis probe obstruction), some animals were excluded from statistical analysis, thus reducing the group size in few cases. Data evaluation and analysis were performed by blinded experimenters.
FIGURE 1.

Time schedule of JWH‐018 exposure and experimental design. DA, dopamine; CB, cannabinoid; EPM, elevated plus maze; GFAP, glial fibrillary acidic protein; Glu, glutamate; IBA‐1, ionized calcium binding adaptor molecule‐1; MB, marble burying test; PPI, Prepulse inhibition; TH, tyrosine hydroxylase; VTA, ventral tegmental area
2.3. Behavioural tests
Separate groups of animals were used at different time‐points for each of the following behavioural tests.
2.3.1. Elevated plus maze
The elevated plus maze (EPM) is a simple method for assessing spatial anxiety in rodents (Morales et al., 2010). In the present study, the EPM paradigm was used to evaluate possible anxiogenic effects in rats induced by repeated JWH‐018 administration and JWH‐018 withdrawal syndrome (Morales et al., 2010). On each evaluation day, rats were placed for a 2 h period of acclimatisation into the experimental room. The EPM was made of white PVC and consisted of two opposite open arms (length 50 cm, width 10 cm) and two opposite closed arms (length 50 cm, width 10 cm), the latter enclosed by 40‐cm high walls along their length. The four arms converged to a central square (10 × 10 cm), thus reproducing the shape of a plus sign. The apparatus was elevated 50 cm from the floor. Rats having no prior experience of the EPM were placed in the central square and left free to explore the whole apparatus for a single 5‐min test session. The experiments were performed under illumination of 40 lux, which was uniform in both the open and closed arms of the apparatus. Rats' performance was videotaped and later evaluated to calculate the percentages of arm entries and of time spent in open and closed with respect to the total number of entries and to the total amount of time spent in the arms. A rat was considered inside a specific arm when it had all four paws inside that arm.
2.3.2. Marble burying test
The marble burying (MB) is used to evaluate compulsive activity/repetitive like‐behaviours in rodents (Zanda et al., 2017). Before starting the evaluation, rats were placed for a 2 h period of acclimatisation into the experimental room. The MB was conducted into an open transparent plastic cage (54 × 34.5 × 20 cm) with 5 cm of fresh hardwood chip bedding as previously described (Satta et al., 2016). Twenty‐four standard glass marbles (1.5 cm in diameter, arranged in six rows of four marbles each) were placed uniformly over the bedding surface. Individual rats were placed in the test cage, and the activity was monitored for 30 min by a video camera placed above the cage. At the end of the session, animals were gently removed from the cages, and the number of marbles partially (≥67%) and totally (>95%) buried was counted as previously described (Zanda et al., 2017). New bedding was used for each animal, and marbles were cleaned with soap and tap water between each evaluation.
2.3.3. Prepulse inhibition (PPI)
The prepulse inhibition (PPI) was performed to evaluate attentional deficits induced by repeated JWH‐018 exposure or drug withdrawal. On the day of the experiment, rats were placed for a 2 h period of acclimatisation into the experimental room. The startle reflex system consisted of four standard cages each placed inside a sound‐attenuated and ventilated chamber (Med Associated, USA). Startle cages were non‐restrictive Plexiglas cylinders (diameter 9 cm) mounted on a piezoelectric accelerometer platform connected to an analogue–digital converter. Background noise and acoustic bursts were conveyed through two speakers placed in proximity to the startle cage, to produce a variation in sound intensity within 1 dB. On the test day, each rat was placed in the experimental cage for a 5‐min acclimation period with a 70‐dB white noise background; this was continued for the remainder of the session. Animals were then tested on three consecutive trial blocks. The first and the third blocks consisted of five pulse‐alone trials of 40 ms at 115 dB, while the second block (test block) was a pseudorandom sequence of 50 trials including 12 pulse‐alone trials, 30 pulse trials preceded by 73, 76, or 82 dB prepulses (10 for each level of prepulse loudness), and eight no‐stimulus trials (where the only background noise was delivered). The percentage of PPI was calculated based only on the values relative to the second block and using the following formula: 100 − [(mean startle amplitude for prepulse + pulse trials/mean startle amplitude for pulse‐alone trials) × 100] (Spano et al., 2010).
2.3.4. Spontaneous somatic signs of withdrawal
Rats were individually placed in plastic cages (30 × 25 × 45 cm) with standard rat bedding. Cages were located in a sound‐proof room for behavioural observation. Point‐scoring was performed by an observer (placed behind a one‐way window), blind to the treatment. The spontaneous cannabinoid withdrawal signs were scored by counting the total number of events such as scratching, wet dog shakes, facial rubbing and licking, over a 30‐min test period (Diana et al., 1998).
2.3.5. Taste reactivity test
During the 5‐min intraoral chocolate infusion, animals were monitored and two classes of taste reactivity patterns were scored, that is, positive hedonic (appetitive) and negative hedonic (aversive). Positive hedonic reactions were paw licks, lateral tongue protrusions, and rhythmic tongue protrusion; aversive reactions were face washing, forelimb flails, gapes, chin rubs, paw tread, and locomotion (Bassareo et al., 2002; De Luca et al., 2012). Each reaction was scored and assigned one point if it lasted 1–5 s and two points if it lasted more than 5 s.
2.4. In vivo electrophysiology: single unit recordings from dopaminergic neurons in the VTA
Twenty‐four hours or seven days after JWH‐018 discontinuation, rats were anaesthetised with urethane (1.3 g kg‐1, i.p.) and placed in a stereotaxic apparatus (Kopf, Tujunga, USA) with their cibody temperature maintained at 37°C ± 1°C by a heating pad. The scalp was retracted, and one small hole was drilled above the parabrachial pigmented nucleus (PBP) of the posterior VTA according to the Rat Brain Atlas (Paxinos & Watson, 2007) coordinates (A: −5.8 to −6.2 from bregma, L: +0.4 to +0.6 from midline, V: −7.0 to ‐ 8.0 from the cortical surface). Extracellular single‐unit activity was recorded with glass micropipettes filled with 2% pontamine sky blue (PSB) dissolved in 0.5‐M sodium acetate (impedance 2.5–5 MΩ). The spontaneous population activity was determined by lowering the electrode within the area in six to nine predetermined tracks separated by 200 μm each other. Putative VTA dopaminergic neurons were selected when all criteria for identification were fulfilled: firing rate <10 Hz and duration of action potential >2.5 ms as measured from start to end (Grace & Bunney, 1984). Bursts were defined as the occurrence of two spikes at interspike interval <80 ms and terminated when the interspike interval exceeded 160 ms (Grace & Bunney, 1984). The electrical activity for each neuron was recorded for 2–3 min and filtered (bandpass 0.1–10,000 Hz). Individual action potentials were isolated and amplified (Neurolog System, Digitimer, UK) and displayed on a digital storage oscilloscope (TDS 3012, Tektronics, UK). Experiments were sampled on line with Spike2 software (Cambridge Electronic Design, UK) by a computer connected to CED 1401 interface (Cambridge Electronic Design, UK). At the end of recording sessions, DC current (15 mA for 1 min) was passed through the recording micropipette in order to eject PSB for marking the recording site. Brains were then rapidly removed and frozen in isopentane cooled to −40°C. The position of the electrodes was microscopically identified on serial 60‐μm sections stained with neutral red (Sagheddu et al., 2019).
2.5. Preparation of microdialysis probe and oral catheters
Vertical microdialysis probes, with an active dialysing portion of 1.5 mm for NAc and 3 mm for mPFC, were prepared with AN69 fibers (Hospal Dasco, Italy) as previously described (De Luca et al., 2015). The oral catheters were made of a 22‐G stainless steel needle and polyethylene (PE) tubing (Portex Ltd, Hythe, England) (ID 0.58 mm, OD 0.96 mm) as previously described (Bimpisidis et al., 2013). The needle was cut at one side (total length of 2 cm from the tip); the cut part was blunted and inserted in the PE tubing, which ended with a perforated circular disc.
2.6. Chocolate exposure
2.6.1. Surgery
In the same surgery session for the insertion of microdialysis probes, an oral catheter was inserted at the level of the first molar, passed along the space between the temporalis muscle and the skull by the tip of the 22‐G needle, and fixed on the top of the head of the rat with a small plastic tip filled with cyanoacrylate glue.
2.6.2. Infusion of chocolate solution
The oral catheter was connected to an infusion pump and the chocolate solution was pumped at a constant rate of 0.2 ml min‐1, to a total of 1 ml per 5 min (Bimpisidis et al., 2013; De Luca et al., 2011).
2.7. In vivo microdialysis
2.7.1. Surgery
Rats were anaesthetised with isoflurane and implanted with vertical dialysis probes in the NAc shell (A: +2.2, L: +1.0 from bregma, V: −7.8 from dura) or core (A: +1.4; L: +1.6 from bregma; V: −7.6 from dura) or in the mPFC (A: +3.7, L: +0.8 from bregma, V: −5.0 from dura), according to the Rat Brain Atlas coordinates (Paxinos & Watson, 2007).
2.7.2. Dopamine assessment
On the day after surgery, probes were perfused with Ringer's solution (composition in mM: 147 NaCl, 4 KCl, 2.2 CaCl2) at a constant rate of 1 μl min‐1. Dialysate samples (10 μl) were injected into an HPLC equipped with a reverse phase column (C8 3.5 μm, Waters, USA) and a coulometric detector (ESA, Coulochem II) to quantify dopamine, as described previously (De Luca et al., 2015). The first electrode of the detector was set at +130 mV (oxidation) and the second at −175 mV (reduction). The composition of the mobile phase was (in mM: 50 NaH2PO4, 0.1 Na2‐EDTA, 0.5 n‐octyl sodium sulphate and 15% (v/v) methanol, pH 5.5). The sensitivity of the assay for dopamine was 5 fmoles per sample. After 2 h washing, basal levels of dopamine were evaluated, estimated as the mean of three consecutive samples whose values did not differ more than 10%.
2.7.3. Glutamate and GABA assessment
Two days after surgery, probes were perfused with Ringer's solution at a constant rate (2.2 μl min‐1). Glutamate and GABA were analysed by HPLC coupled with laser‐induced fluorescence detection. Dialysate samples were stored at −80°C until processed when each sample (20 μl) was added to 500‐mM borate buffer (pH 8.7, 20 μl), 10‐mM KCN in borate buffer (120 μl), and 5‐mM naphtalene dicarboxaldehyde (NDA) (Fluka, Germany) in CH3OH (20 μl); the solution was vortex‐mixed and left 4 min at room temperature for derivatisation. Then, 15 μl were injected into the HPLC that consisted of a Waters 515 pump (Waters, Italy), an Agilent 1200 series autosampler (Agilent Technologies, Germany), and a Zetalif detector (Picometrics S.A. France) coupled with a He–Cd laser (Melles‐Griot, USA) working at 442 nm. Mobile phase A was 50‐mM sodium acetate (pH 5.5 with glacial acetic acid) and CH3CN (J.T. Baker, The Netherlands), 77% and 23% respectively; mobile phase B was 50‐mM sodium acetate/CH3CN (40:60). Separation was performed with a 3.0 × 150 mm, C18 (3.5 μm) Symmetry column (Waters, Italy) at 100% mobile phase A for 25 min, 100% B for 8 min and 100% A for the last 10 min. Before use, mobile phase was filtered through a 0.45‐μm MF Millipore filter (Millipore, USA) and degassed under vacuum in an ultrasonic bath. Flow rate was set at 1 ml min‐1 and column temperature was maintained at 28°C by a Series 1100 thermostat (Agilent Technologies, Germany). Fluorescence was measured setting detector range at 20 relative fluorescence units (RFU). After standard curve calibration, the area of the peaks was quantified by means of a Waters Millennium computer program. After 2 h of washing, basal levels of glutamate and GABA were evaluated as the mean of three consecutive samples whose values did not differ more than 10%.
2.7.4. Histology
At the end of the experiment, animals were sacrificed with an overdose of Equithesin (i.p.) and their brains removed and stored in formalin (8%) for histological examination to verify the correct placement of the microdialysis probe.
2.8. Immunohistochemical assays
Twenty‐four hours or 7 days after the last JWH‐018 injection, rats were deeply anaesthetised with Equithesin (5ml kg‐1, i.p.) and then transcardially perfused with 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1‐M phosphate‐buffered solution, pH 7.4. Brains were rapidly removed and postfixed in the same fixative overnight. After repeated washing in 0.1‐M PBS, brains were cryoprotected in 30% sucrose in PBS for 48 h. Immunostaining was performed on free‐floating coronal sections (40 μm) which were obtained using a cryostat at levels comprising the brain areas selected for this study according to the Rat Brain Atlas coordinates (Paxinos & Watson, 2007). In particular, sections containing mPFC (PrL and IL cortex; AP: +3.70 to +2.70), NAc (NAc shell and core; AP: +1.20 to +0.70), and VTA (AP: −4.80 to −6.30) were processed for GFAP and IBA‐1 immunoreactivity (IR). CB1 receptor‐IR analysis was performed in the sections containing mPFC and NAc while TH‐IR analysis only in the sections containing VTA. All immunohistochemical procedures and analyses comply with the recommendations detailed in the BJP editorial and adhere to the BJP checklist for immunohistochemistry (Alexander, Roberts, et al., 2018).
2.8.1. GFAP immunofluorescence
Tissue sections were incubated at 4C° for 24 h with a mouse monoclonal IgG anti‐GFAP antibody (1:5000; Millipore Cat# MAB360, RRID:AB_11212597) in PBS containing 0.2% Triton X‐100, 0.1% BSA, and 1% NGS. Then, sections were washed in PBS containing 0.2% Triton X‐100 and incubated with Alexa Fluor 594‐labelled goat anti‐mouse IgG (1:500; cat no. A‐11005, Molecular Probes, USA) for 1 h in the dark at room temperature (Castelli et al., 2014).
2.8.2. IBA‐1 immunofluorescence
Tissue sections were incubated at 4°C for 24 h with a rabbit polyclonal IgG anti‐IBA‐1 antibody directed against the synthetic peptide corresponding to C‐terminus of Iba‐1 (1:2000; FUJIFILM Wako Shibayagi Cat# 016‐20001, RRID:AB_839506) in PBS containing 0.2% Triton X‐100, 0.1% BSA, and 1% NGS. Then, sections were washed in PBS containing 0.2% Triton X‐100 and incubated with Alexa Fluor 488‐labelled goat anti‐rabbit IgG (1:500; cat no. A‐11012 Molecular Probes, USA) for 1 h in the dark at room temperature.
2.8.3. TH immunofluorescence
Tissue sections were incubated at 4°C for 24 h with a mouse monoclonal IgG anti‐TH antibody directed against an epitope on the outside of the regulatory N‐terminus (1:1000; Millipore Cat# MAB318, RRID:AB_2201528) in PBS containing 0.2% Triton X‐100, 0.1% BSA, and 1% NGS. Then, sections were washed in PBS containing 0.2% Triton X‐100 and incubated with Alexa Fluor 488‐labelled goat anti‐mouse IgG (1:500; cat no. A‐28175 Molecular Probes, USA) for 1 h in the dark at room temperature. Finally, all sections were rinsed and mounted on slides using VectaShield anti‐fade mounting media (Vector Inc.).
2.8.4. CB1 receptor immunofluorescence
Tissue sections were incubated at 4°C for 48 h with rabbit anti‐CB1 receptor polyclonal antibody directed against the last 15 amino acids of rat CB1 receptor (1:2000) (Bodor et al., 2005) in PBS containing 0.2% Triton X‐100, 2.5% BSA, and 10% NGS, kindly supplied by Dr. K. Mackie (Indiana State University, USA). Then, sections were washed in PBS containing 0.2% Triton X‐100 and incubated with biotinylated goat anti‐rabbit IgG (1:200, cat no. BA‐1000, Vector Laboratories, Burlingame, CA, USA) for 1 h in the dark at room temperature. Subsequently, sections were incubated with Avidin Alexa Fluor 488 (1:1000, cat no. A‐21370, Molecular Probes, USA) for 1 h in the dark at room temperature and then rinsed and mounted on slides using VectaShield anti‐fade mounting media (Vector Inc.).
Standard control experiments were performed by omitting either the primary or secondary antibody and yielded no cellular labelling (data not shown). All the diluting buffers and the final antibody dilutions were used only once.
2.9. Imaging and quantitative analysis of IBA‐1, TH, GFAP, and CB1 receptor immunofluorescent staining
An Olympus IX 61 microscope and an Olympus 12‐bit cooled F View II camera (Hamburg, Germany) were used for observations and for capturing the images, respectively. For each animal, analysis of IBA‐1‐ IR and GFAP‐IR were performed on one tissue section out of every three successive sections, for a total of 8, 6, and 12 sections containing the mPFC, the NAc shell and core, and the VTA, respectively. CB1 receptor‐IR analysis was performed in the sections containing the mPFC and the NAc shell and core while TH‐IR analysis only in sections containing the VTA.
2.9.1. IBA‐1 and TH analysis
The total size of the examined area in which IBA‐1 and TH neurons were counted was chosen according to the extension of the region under analysis, in order to include almost the whole area (either mPFC, NAc, or VTA). The number of both IBA‐1 and TH positive cells was counted bilaterally in the different sections per animal. In these sections, nonoverlapping randomly selected region of interest (ROIs) of 0.15 mm2, 6 and 4 ROIs, respectively, for IBA‐1 and TH, were examined with a 20× objective by two trained observers blind to drug treatment. Limits of the ROI were defined based on structural details within the tissue sections to ensure the ROIs did not overlap. The distance among the 6/4 ROIs was superior to 40 μm to avoid overlapping. IBA‐1 and TH positive cells touching the inferior or the right sides of the ROI were excluded from counting. The numbers of IBA‐1 and TH cells were expressed per mm2 and shown as means ± SEM.
2.9.2. GFAP and CB1 receptor analysis
The semiquantitative analysis of GFAP‐IR and CB1 receptor‐IR was carried out on three nonoverlapping areas (ROIs, roughly 140,000 and 16,000 μm2 for GFAP and CB1 receptors, respectively) from tissue slices of each brain region using the 20× and 60× objective for GFAP‐ and CB1 receptor‐IR, respectively. The focus depth was extended by summing the maximum intensity of several images taken at focus steps of 0.25‐μm depth intervals to a total of 2‐μm thicknesses using the Z‐stack module (Olympus soft Imaging solution, GNHB, Germany). Images were analysed using the Cell P AnalySIS software module. Positively stained fibers were detected by means of density thresholding applied to the single‐channel greyscale images. Average values of GFAP and CB1 receptor‐IR were calculated from images per brain region of each animal and were expressed as average values of percentage of area occupied by fibers.
2.10. Data and statistical analysis
Data and statistical analysis complies with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). Normality tests for data were carried out using Shapiro–Wilk's test. If data were found to be normally distributed, the effect of treatment was analysed using Student's t test (EPM, basal extracellular levels of dopamine, cells/track index, firing rate, TH‐IR experiments). If data were found to not be normally distributed and/or there was significant variance inhomogeneity, the effect of treatment was analysed using Mann–Whitney test (MB test, spontaneous somatic withdrawal signs, PPI, basal glutamate and GABA extracellular levels, % of spikes/bursts, aversive taste reactions).
The effect of treatment on dopamine responses to JWH‐018 challenge and chocolate exposures was analysed by repeated measures (RM) two‐way ANOVA (treatment × time) followed by Bonferroni's multiple comparisons. For RM tests, whenever we could not assume sphericity, a Geisser–Greenhouse correction was carried out by GraphPad Prism 8 software (GraphPad Prism, RRID:SCR_002798). The time course of dopamine response in each group (data not normally distributed) was analysed by non‐parametric RM one‐way ANOVA (Friedman's test) followed by Dunn's multiple comparisons. The effect of JWH‐018 on GFAP, IBA‐1, and CB1 receptor‐IR was analysed by two‐way ANOVA (treatment × brain area) followed by Bonferroni's multiple comparisons. Then, the effect of treatment on GFAP, IBA‐1, and CB1 receptor‐IR within each brain areas was analysed by Student's t test. Post hoc tests were conducted only if F in ANOVA achieved P < 0.05 and there was no significant variance inhomogeneity (Brown–Forsythe's test). In this study, P values < 0.05 were considered as statistically significant. Statistical analysis was performed with Statistica (StatSoft, Tulsa, OK, USA) or GraphPad Prism 8 (GraphPad Prism) software. Numerical data are given as mean ± SEM for parametric analysis or as median with 95% Cl for non‐parametric analysis.
2.11. Drugs and solutions
JWH‐018 was purchased from Tocris (Bristol, UK) and solubilised in 0.5% EtOH, 0.5% Tween 80, and 99% saline. A solution containing chocolate syrup (Nesquik Squeeze©, Nestle, Switzerland) and tap water (1:1) was used as a gustatory taste stimulus. A chocolate solution was administered twice in order to evaluate rats' taste responses to either a single or repeated exposure to a salient stimulus. Equithesin was prepared in our laboratory and contained 0.97 g pentobarbital, 4.25 g chloral hydrate, 2.1 g MgSO4, 42.8ml propylene glycol and 11.5ml 90% ethanol in a final volume of 100ml; the components of this anaesthetic mixture were supplied by Carlo Erba Reagents S.A.S. (Val de Reuil, France).
2.12. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY (http://www.guidetopharmacology.org), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2019; Alexander, Fabbro et al., 2019; Alexander, Kelly et al., 2019).
3. RESULTS
3.1. Effect of JWH‐018 repeated exposure in behavioural tests at different times after drug discontinuation
In order to disclose whether JWH‐018 repeated exposure induced behavioural abnormalities, such as anxiety‐like state, compulsive‐like behavioural activity, attentional deficits, and spontaneous signs of withdrawal, we performed a battery of behavioural tests (EPM, MB, PPI) at different times after JWH‐018 discontinuation (1 h, 24 h, or 7 days). Behavioural observation of spontaneous somatic withdrawal signs was performed at 24 h or 7 days after JWH‐018 discontinuation.
3.1.1. Elevated plus maze
One hour and 24 h after drug discontinuation, rats treated with JWH‐018 spent less time in the open arms of the EPM with respect to the Veh‐treated group (Figure 2a,e); no significant differences between groups were observed 7 days after drug discontinuation (Figure 2i) as well as in the total number of arm entries (data not shown).
FIGURE 2.

Repeated JWH‐018 exposure induces time‐dependent abnormalities in EPM, MB, PPI. Data shown are individual values with means ± SEM of the percentage of time spent in open arms during the EPM test (Veh n = 8, JWH‐018 n = 8, unpaired Student's t tests, panels a,e,i) or median ± 95% CI of the total number of marbles covered with bedding during the MB test (Veh n = 7, JWH‐018 n = 7, Mann–Whitney U test, panels b,f,j), mean ± SEM of startle amplitude (panels c,g,k), and median ± 95% CI of average percentage of PPI (1 h, Veh: n = 9, JWH‐018: n = 8, panel d; 24 h, Veh: n = 8, JWH‐018: n = 11, h; 7 days, Veh n = 8, JWH‐018 n = 8, Mann–Whitney U test, panel l) in vehicle‐ and JWH‐018‐treated rats at 1 h, 24 h, and 7 days after drug discontinuation. *P < 0.05, significantly different from Veh
3.1.2. Marble burying test
One hour, 24 h, and 7 days after drug discontinuation, rats treated with JWH‐018 buried a higher number of marbles with respect to the Veh‐treated group (Figure 2b,f,j).
3.1.3. Prepulse inhibition
Rats treated with JWH‐018 did not display significant differences in startle amplitude with respect to the Veh‐treated group at any time point. However, rats treated with JWH‐018 displayed a significant reduction of average % PPI levels 1 h after drug discontinuation (Figure 2d). No significant differences were observed at both 24 h and 7 days after the last JWH‐018 administration (Figure 2h,l).
3.1.4. Spontaneous somatic signs of withdrawal
Twenty‐four hours after drug discontinuation, JWH‐018‐treated rats exhibited a significant increase in signs of withdrawal such as licking, headshakes, teeth chattering, biting, chewing and tongue rolling, compared with the corresponding values from the Veh‐treated group (Table 1). At 7 days after drug discontinuation, JWH‐018‐treated rats exhibited a significant increase only in biting, with respect to the Veh‐treated group (Table 1).
TABLE 1.
Spontaneous somatic signs of withdrawal
| Signs of withdrawal (30‐min observation) | 24 h | 7 days | ||
|---|---|---|---|---|
| Veh | JWH‐018 | Veh | JWH‐018 | |
| Facial rubbing | 2 (0–5) | 2 (1–3) | 1 (0–3) | 2 (1–3) |
| Licking | 1 (0–4) | 4 (2–6)* | 2 (0–5) | 3 (1–7) |
| Wet dog shakes | 0 | 0 (0–1) | 0 | 0 |
| Arched back | 0 | 0 (0–1) | 0 (0–4) | 0 |
| Biting | 0 | 1 (0–4)* | 0 | 1 (0–2)* |
| Head shakes | 0 | 0 (0–1)* | 0 (0–2) | 0 (0–3) |
| Chewing | 0 (0–5) | 5 (2–14)* | 5 (1–9) | 5 (1–19) |
| Tongue rolling | 0 | 1 (0–4)* | 0 (0–1) | 0 (0–2) |
| Paw treading | 0 | 0 (0–1) | 0 | 0 |
| Forepaw fluttering | 0 (0–1) | 1 (0–1) | 1 (0–4) | 2 (0–3) |
| Teeth chattering | 0 | 1 (0–3)* | 1 (0–4) | 1 (0–1) |
| Scratching | 0.5 (0–1) | 1 (0–2) | 0 (0–1) | 0 (0–1) |
Note: Data are expressed as median ± 95% CI of behavioural withdrawal scores after a 30‐min observation 24 h (Veh: n = 12; JWH‐018: n = 13) and 7 days (Veh: n = 9; JWH‐018: n = 9) after JWH‐018 discontinuation. *P < 0.05, significantly different from Veh; Mann–Whitney U test.
3.2. Effect of JWH‐018 repeated exposure on dopaminergic neuronal activity and TH‐IR in the VTA at 24 h and 7 days after drug discontinuation
In order to disclose whether JWH‐018 repeated exposure altered the basal function of dopaminergic neurons, we evaluated at 24 h and 7 days after JWH‐018 discontinuation the spontaneous dopaminergic neuronal activity as well TH‐IR in the VTA.
As shown in Figure 3, repeated JWH‐018 exposure induced adaptive changes of VTA dopaminergic neuronal activity and of TH‐IR in the same area. Twenty‐four hours after JWH‐018 discontinuation, Student's t test showed a significant decrease of the number of spontaneously active dopaminergic cells in the JWH‐018‐treated rats, compared with those in the Veh‐treated group (Figure 3a). No significant difference in the average firing rate or percentage of spikes/bursts was observed between groups (Figure 3b,c). Seven days after JWH‐018 discontinuation, Student's t test showed a significant decrease in the number of spontaneously active dopaminergic cells in the JWH‐018‐treated group, compared with the Veh‐treated group (Figure 3e). The average firing rate was significantly lower in the JWH‐018‐treated than in the Veh‐treated group (Figure 3f), whereas no significant differences in the percentage of spikes in bursts were observed between groups (Figure 3g). Moreover, the number of TH‐positive cells in the VTA both at 24 h (Figure 3d) and at 7 days (Figure 3h) was significantly reduced after JWH‐018 discontinuation.
FIGURE 3.

Repeated JWH‐018 exposure induces persistent modifications in the activity of VTA dopaminergic (DA) neurons. Data shown are individual values with mean ± SEM of the number of spontaneously active VTA dopaminergic neurons (24 h, Veh: n = 10, JWH: n = 11, panel a; 7 days, Veh: n = 8, JWH: n = 8, unpaired Student's t tests, panel e) and firing rate of VTA dopaminergic neurons (24 h, Veh: n = 144 from 10 rats, JWH: n = 132 from 11 rats, panel b; 7 days, Veh: n = 125 from 8 rats, JWH: n = 96 from 8 rats, unpaired Student's t tests, panel f) recorded from Veh‐exposed (blue circles) and JWH‐018‐exposed (red circles) rats at 24 h and 7 days after drug discontinuation. The horizontal lines represent average values that are significantly different between the two groups. Bars represent median ± 95% CI of the percentage of spikes in bursts (24 h, Veh: n = 144 from 10 rats, JWH: n = 132 from 11 rats, panel c; 7 days, Veh: n = 125 from 8 rats, JWH: n = 96 from 8 rats, Mann–Whitney U test, panel g), and mean ± SEM of number of TH positive cells in the VTA expressed per mm2 in Veh‐exposed (blue bars) and JWH‐018‐exposed (red bars) rats at 24 h and 7 days after drug discontinuation (Veh n = 5, JWH‐018 n = 5, unpaired Student's t tests, panels d,h). Representative images of TH expression in vehicle‐ (top) and JWH‐018‐treated rats (bottom). *P < 0.05, significantly different from Veh
3.3. Effect of JWH‐018 repeated exposure on extracellular basal dopamine, GABA and glutamate levels in the NAc and mPFC at 24 h and 7 days after drug discontinuation
In order to disclose whether JWH‐018 repeated exposure induced neurochemical alterations in vivo, we evaluated 24 h and 7 days after JWH‐018 discontinuation, the basal extracellular levels of dopamine (mean of three consecutive samples whose values did not differ more than 10%) in the NAc and in the mPFC. Meantime, we evaluated also extracellular basal glutamate and GABA levels in the mPFC, since cortical glutamate and GABAergic transmission, both directly and indirectly, regulate mesolimbic and mesocortical dopaminergic pathways (Murase et al., 1993; Renard, Szkudlarek, et al., 2017).
3.3.1. Dopamine
Twenty‐four hours after JWH‐018 discontinuation, basal extracellular levels of DA, expressed as fmoles per 10‐μl sample (mean ± SEM), were reduced in the mPFC of the JWH‐018‐treated group, compared with the Veh‐treated group, while no significant differences were observed in the NAc (Student's t test; Figure 4a). Seven days after JWH‐018 discontinuation, no significant differences in basal DA levels between groups were observed (Figure 4b).
FIGURE 4.

Effect of JWH‐018 repeated exposure on extracellular basal dopamine (DA), glutamate (Glu) and GABA levels in the NAc and mPFC, at 24 h and 7 days after drug discontinuation. Bars represent mean ± SEM of basal extracellular levels of dopamine expressed as fmoles per 10 μl (panels a,b), and median ± 95% CI of glutamate (panels c,d) and GABA (panels e,f) expressed as μmoles per 10‐μl and nmoles per 10‐μl sample, respectively. The bars represent the basal dopamine (NAc shell: Veh n = 5, JWH‐018 n = 5; NAc core: Veh n = 5, JWH‐018 n = 5; mPFC: Veh n = 5, JWH‐018 n = 8, unpaired Student's t test), basal glutamate (Veh n = 5, JWH‐018 n = 6, Mann–Whitney U test) and basal GABA (Veh n = 5, JWH‐018 n = 5, Mann–Whitney U test) levels 24 h and 7 days after JWH‐018 discontinuation. *P < 0.05, significantly different from Veh
3.3.2. Glutamate
Twenty‐four hours after JWH‐018 discontinuation, basal extracellular levels of Glu, expressed as μmoles per 10‐μl sample (mean ± SEM) were decreased in the mPFC of the JWH‐018‐treated rats, compared with the Veh‐treated group (Mann–Whitney U test; Figure 4c). Seven days after JWH‐018 discontinuation, no significant differences in basal Glu levels in the mPFC between groups were observed (Figure 4d).
3.3.3. GABA
Twenty‐four hours after JWH‐018 discontinuation, basal extracellular levels of GABA, expressed as nmoles per 10‐μl sample (mean ± SEM), were decreased
in the mPFC of the JWH‐018‐treated group, compared with the Veh‐treated group (Mann–Whitney U test; Figure 4e). Seven days after JWH‐018 discontinuation, no significant differences in basal eGABA levels in the mPFC between groups were observed (Figure 4f).
3.4. Effect of JWH‐018 repeated exposure on dopamine in the NAc shell, NAc core, and mPFC during the last day of JWH‐018 treatment
Afterwards, in order to disclose whether JWH‐018 repeated exposure induced further neurochemical alterations, we evaluated the responsiveness of mesolimbic and mesocortical dopaminergic transmission to an acute JWH‐018 challenge. As shown in Figure 5a, the last administration of JWH‐018 (0.25 mg kg‐1, i.p.) elicited an increase of extracellular dopamine in the NAc shell of rats pretreated with vehicle. On the contrary, only rats pretreated with JWH‐018 showed a long‐lasting increase of dopamine release in the NAc core after the last JWH‐018 administration (Figure 5b). No significant effects were observed on dopamine responses in the mPFC (Figure 5c). Two‐way ANOVA analysis of NAc shell group showed a main effect of treatment, of time and a significant treatment × time interaction. Bonferroni's post hoc test revealed an increase of dialysate dopamine in the Veh‐treated group after JWH‐018 administration, with respect to basal values (30′; Figure 5a). To better evaluate the effect of each treatment, data were analysed separately by Friedman's test that revealed significant differences in the NAc shell of the Veh‐treated group. Dunn's test revealed an increase of dialysate dopamine after JWH‐018 administration with respect to basal values (20′–40′; Figure 5a). For the NAc core group, two‐way ANOVA showed a main effect of treatment, of time and a significant treatment × time interaction. Bonferroni's post hoc tests revealed a long‐lasting increase of dialysate dopamine in the NAc core of the JWH‐018‐treated group after JWH‐018 administration. compared to basal values and to the Veh‐treated group (20′–120′; Figure 5b). On the contrary, no significant differences in the levels of dialysate dopamine in the mPFC between groups were observed (Figure 5c).
FIGURE 5.
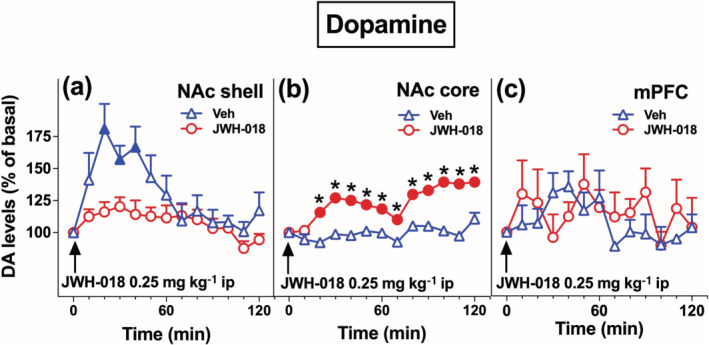
Repeated JWH‐018 exposure induces differential adaptive changes on the responsiveness of NAc shell and core dopamine (DA), but not of mPFC dopamine to JWH‐018 injection during the last day of treatment. Data are presented as mean ± SEM of change in extracellular dopamine expressed as the percentage of basal values. The arrow indicates the start of JWH‐018 ip injection (0.25 mg/kg) in Veh‐exposed and JWH‐018‐exposed rats implanted in the NAc shell (Veh: n = 7, JWH‐018: n = 6, panel a), NAc core (Veh: n = 6, JWH‐018: n = 6, panel b), and in the mPFC (Veh: n = 5, JWH‐018: n = 5, panel c). Solid symbols: P < 0.05 with respect to basal values; *P < 0.05, significantly different from Veh; RM two‐way ANOVA followed by Bonferroni's post hoc; Friedman's test followed by Dunn's post hoc test
3.5. Effect of JWH‐018 repeated exposure on the responsiveness of dopaminergic transmission to chocolate in the NAc shell, NAc core, and mPFC at 7 days after drug discontinuation
Furthermore, in order to disclose whether JWH‐018 repeated exposure was able or not to induce a loss of control of the motivational value of stimuli, we evaluated, 7 days after JWH‐018 discontinuation, the pattern of dopamine responses to repeated exposure to a natural rewarding stimulus (i.e., chocolate solution) in the NAc shell and core and in the mPFC. As shown in Figure 6, withdrawal from JWH‐018 repeated exposure increased dialysate dopamine in the mPFC but not in the NAc shell and core after the first exposure to chocolate. Conversely, dialysate dopamine increased in the NAc shell and core but not in the mPFC after the second exposure to chocolate.
FIGURE 6.

Repeated JWH‐018 exposure induces differential adaptive changes on the responsiveness of NAc shell, NAc core, and mPFC dopamine (DA) and changes in taste reactions to repeated chocolate exposure evaluated 7 days after JWH‐018 discontinuation. Data are presented as mean ± SEM of change in extracellular levels of dopamine, expressed as the percentage of basal values and as median ± 95% CI of behavioural appetitive and aversive score to chocolate. The arrow indicates the start of chocolate intraoral infusion (1 ml/5 min) in Veh‐exposed and JWH‐018‐exposed rats implanted in the NAc shell (Veh: n = 6, JWH‐018: n = 6, panel a,d), NAc core (Veh: n = 6, JWH‐018: n = 6, panel b,e), and in the mPFC (Veh: n = 6, JWH‐018: n = 6, panel c,f). Solid symbols: P < 0.05, significantly different from basal values; *P < 0.05, Veh significantly different from JWH‐018 within the first or second chocolate exposure group; RM two‐way ANOVA followed by Bonferroni's post hoc; Friedman's test followed by Dunn's post hoc. The bars represent appetitive (first exp, Veh: n = 11, JWH‐018: n = 11, panel g; second exp, Veh: n = 6, JWH‐018: n = 10, panel i) and aversive (first exp, Veh: n = 11, JWH‐018: n = 11, panel h; second exp, Veh: n = 6, JWH‐018: n = 10, panel j) taste reactions to the first and the second chocolate exposures. *P < 0.05, significantly different from Veh; Mann–Whitney U test
In the NAc shell, two‐way ANOVA analysis of the first chocolate exposure showed a main effect of time, treatment and a significant time × treatment interaction. Bonferroni's post hoc tests revealed an increase of dialysate dopamine in the NAc shell of the Veh‐treated group after the first chocolate exposure as compared to basal values (20′–40′) and to the JWH‐018‐treated group (30′) (Figure 6a). Considering the second chocolate exposure, two‐way ANOVA analysis showed a main effect of time, treatment, and a significant time × treatment interaction. Bonferroni's post hoc test revealed an increase of dialysate dopamine in the NAc shell of the JWH‐018‐treated group as compared to the Veh‐treated group (30′). To better evaluate the time course of the dopamine response in each group, data were analysed separately by Friedman's test that revealed significant differences in the Veh‐treated group after the first chocolate exposure and in the JWH‐018‐treated group after the second chocolate exposure. Dunn's test revealed an increase of dialysate dopamine with respect to basal values in the NAc shell of both the Veh‐treated (20′, first chocolate exposure) and JWH‐018‐treated groups (20′–40′, second chocolate exposure) (Figure 6a,d).
In the NAc core, two‐way ANOVA analysis of the first chocolate exposure showed no significant difference in the levels of dialysate dopamine between groups (Figure 6b). In contrast, two‐way ANOVA analysis of the second chocolate exposure showed a main effect of treatment and a significant time × treatment interaction. Bonferroni's post hoc tests revealed an increase of dialysate dopamine in the NAc core of the JWH‐018‐treated group after the second chocolate exposure as compared to basal values (10′) and to the Veh‐treated group (90′) (Figure 6e). Then, data were analysed separately by Friedman's test that revealed significant differences in the JWH‐018‐treated groups after the second chocolate exposure. Dunn's test revealed an increase of dialysate dopamine with respect to basal values (30′, 40′, 80′, 90′) (Figure 6e).
Finally, in the mPFC, two‐way ANOVA analysis of the first chocolate exposure showed a main effect of time and a significant time × treatment interaction. Bonferroni's post hoc test revealed an increase of dialysate dopamine in the mPFC of the JWH‐018‐treated group after the first chocolate exposure as compared to basal values (30′) (Figure 6c). Two‐way ANOVA of the second chocolate exposure showed a main effect of time, of treatment and a significant time × treatment interaction. Bonferroni's post hoc tests revealed an increase of dialysate dopamine in the mPFC of the Veh‐treated group as compared to basal values and to the JWH‐018‐treated group (20′–30′) (Figure 6f). Then, data were analysed separately by Friedman's test that revealed significant differences in the Veh‐treated group after the first chocolate exposure and the second chocolate exposure, and in the JWH‐018‐treated groups after the first chocolate exposure. Dunn's test revealed an increase of dialysate dopamine with respect to basal values in the mPFC of both the Veh‐treated (10′, first chocolate exposure; 10′–30′, second chocolate exposure) and JWH‐018‐treated groups (20′–30′, second chocolate exposure) (Figure 6c,f). During microdialysis experiments, taste reactions to chocolate exposures were also scored. Mann–Whitney U test showed a significant increase of aversive score to the first chocolate exposure in the JWH‐018‐treated rats, compared to the Veh‐treated group (Figure 6h), while no significant differences were observed during the second chocolate exposure (Figure 6j). No differences in appetitive score during both chocolate exposures were observed (Figure 6g,i).
3.6. Effect of JWH‐018 repeated exposure on GFAP and IBA‐1 IR in mesocortical and mesolimbic dopaminergic areas either 24 h or 7 days after JWH‐018 discontinuation
In order to disclose whether JWH‐018 repeated exposure was able to induce changes in glial cells (astrocytes and microglia), we evaluated 24 h and 7 days after JWH‐018 discontinuation the expression of GFAP and IBA‐1 IR in mesocortical and mesolimbic dopaminergic areas.
Repeated JWH‐018 exposure induced changes in GFAP and IBA‐1 IR in the mPFC, NAc shell and core, and VTA both 24 h and 7 days after JWH‐018 discontinuation. Twenty‐four hours after JWH‐018 discontinuation, two‐way ANOVA of GFAP‐IR showed a main effect of treatment and of brain area, but not of brain area × treatment significant interaction. To better evaluate the effect in each brain area, data were analysed separately. Student's t test showed a significant increase of GFAP‐IR levels in the mPFC (+24%), in the NAc shell (+42%) and core (+44%), and in the VTA (+24%) of the JWH‐018‐treated group, compared with the Veh‐treated group (Figure 7a). Considering IBA‐1 expression, two‐way ANOVA showed a main effect of treatment and brain area but not a brain area × treatment significant interaction. Subsequent analysis by Student's t tests showed a significant increase of IBA‐1 positive cells in the NAc shell (+17%) and core (+28%), and in the VTA (+20%) of JWH‐018‐treated rats, compared with the Veh‐treated group (Figure 7b). Similarly, 7 days after JWH‐018 discontinuation, two‐way ANOVA of GFAP‐IR showed a main effect of treatment and brain area but not a brain area × treatment significant interaction. Student's t tests revealed a significant increase of GFAP‐IR levels in the mPFC (+37%), NAc shell (+42%) and core (+32%), and in the VTA (+27%) of the JWH‐018‐treated rats, compared with the Veh‐treated group (Figure 8a). Considering IBA‐1 IR, two‐way ANOVA showed a main effect of treatment and of brain area but not of brain area × treatment significant interaction. Student's t tests revealed a significant increase of IBA‐1 positive cells in the NAc shell (+30%) and core (+31%) of the JWH‐018‐treated as compared to the Veh‐treated group (Figure 8b).
FIGURE 7.

Repeated JWH‐018 exposure induces changes in GFAP and IBA‐1 immunoreactivity (IR) in the mPFC, NAc shell and core and VTA 24 h after JWH‐018 discontinuation. Data are presented as mean ± SEM of density reading expressed as a percentage of the area covered by GFAP‐IR and of the number of IBA‐1 positive cells, expressed per mm2. The bars represent the percentage of area covered in different dopaminergic brain regions by GFAP‐IR (Veh: n = 5, JWH‐018: n = 5, panel a) and the number of IBA‐1 positive cells (Veh: n = 5, JWH‐018: n = 5, panel b). Representative images of GFAP and IBA‐1 expression in Veh‐ (top) and JWH‐018‐treated rats (bottom), in the mPFC, NAc (shell and core), and VTA, respectively. *P < 0.05, significantly different from Veh; unpaired Student's t test
FIGURE 8.
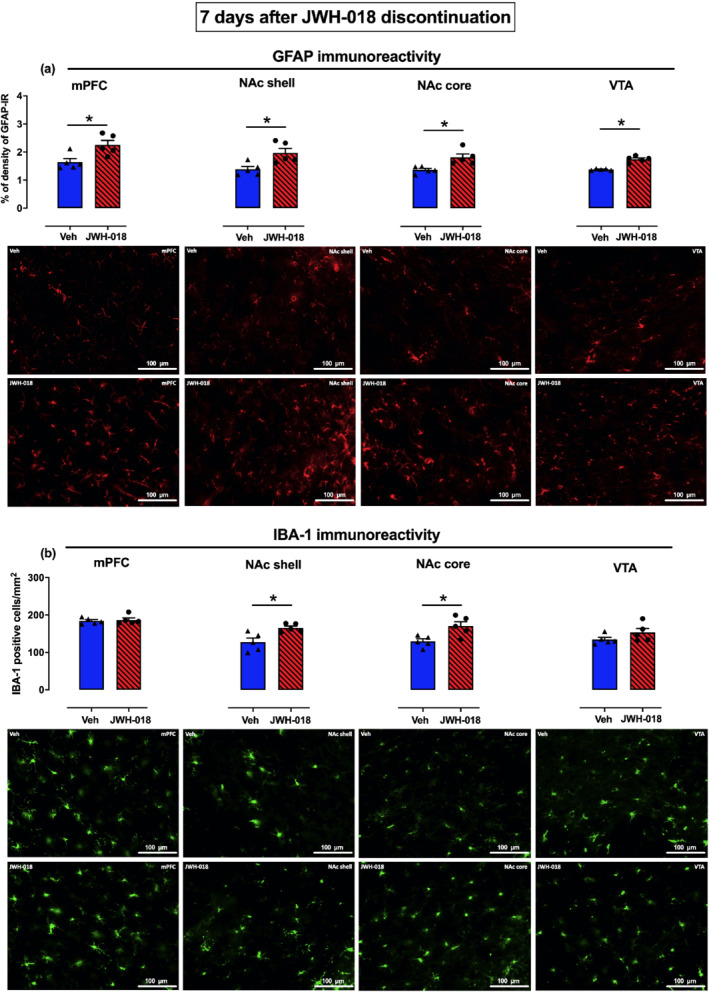
Repeated JWH‐018 exposure induces changes in GFAP and IBA‐1 immunoreactivity (IR) in the mPFC, NAc shell and core, and VTA 7 days after JWH‐018 discontinuation. Data are presented as mean ± SEM of density reading expressed as a percentage of the area covered by GFAP‐IR and of the number of IBA‐1 positive cells, expressed per mm2. The bars represent the percentage of area covered in different dopaminergic brain regions by GFAP‐IR (Veh: n = 5, JWH‐018: n = 5, panel a) and the number of IBA‐1 positive cells (Veh: n = 5, JWH‐018: n = 5, panel b). Representative images of GFAP and IBA‐1 expression in Veh‐ (top) and JWH‐018‐treated rats (bottom), in the mPFC, NAc (shell and core), and VTA, respectively. *P < 0.05, significantly different from Veh; unpaired Student's t test
3.7. Effect of JWH‐018 repeated exposure on the expression of CB1 receptors in the mPFC, NAc shell, and NAc core 7 days after JWH‐018 discontinuation
Finally, to assess whether JWH‐018 repeated exposure was able to induce persistent changes in CB1 receptors, we evaluated the expression of these receptors in mPFC and NAc shell and core, 7 days after JWH‐018 discontinuation. Two‐way ANOVA showed a main effect of treatment and of brain area but not of brain area × treatment significant interaction. To better evaluate the effect in each brain area, data were analysed separately. Student's t tests revealed a significant decrease of CB1 receptor‐IR in the mPFC (−23%), NAc shell (−29%), and NAc core (−42%) of the JWH‐018‐treated rats, compared with the Veh‐treated group (Figure 9).
FIGURE 9.

Repeated JWH‐018 exposure induces changes in the expression of CB1 receptors (CB1R) in the mPFC, NAc shell, and NAc core 7 days after JWH‐018 discontinuation. Data are presented as mean ± SEM of density reading expressed as a percentage of the area covered by CB1 receptor immunoreactivity (IR). The bars represent the percentage of area covered by CB1 receptor‐IR in the mPFC and in NAc shell and core (Veh: n = 5, JWH‐018: n = 5). Representative images of CB1 receptor expression in Veh‐ (top) and JWH‐018‐treated rats (bottom) in the mPFC and NAc (shell and core). *P < 0.05, significantly different from Veh; unpaired Student's t test
For all immunostainings (TH‐IR, GFAP‐IR, IBA‐1 IR, CB1 receptor‐IR), standard control experiments were performed by omitting either the primary or secondary antibodies and yielded no cellular labelling (data not shown).
4. DISCUSSION
The main findings of this study are threefold. First, repeated exposure to JWH‐018 and subsequent discontinuation induces behavioural, electrophysiological, and neurochemical changes related to withdrawal. Second, this treatment induces differential adaptive changes on dialysate dopamine in the NAc shell and core, and in the mPFC in response to a single or recurrent presentation of an appetitive salient stimulus. Finally, these changes are associated with astrogliosis, microgliosis, and downregulation of CB1 receptors in selected mesolimbic and mesocortical dopaminergic brain areas.
Repeated JWH‐018 exposure induced an anxiety‐like phenotype, as revealed by the reduction of time spent in the open arms of the EPM, at 1 and 24 h, but not at 7 days, after drug discontinuation. Notably, JWH‐018‐ and vehicle‐treated rats displayed no differences in the total number of entries in the arms of the EPM, which supports the view that reduction of the time spent in the open arms by JWH‐018‐treated rats stems from the presence of an anxiety‐like state rather than from non‐specific drug effects on arm exploration (i.e., alteration of locomotor activity). Notably, JWH‐018‐treated rats displayed a similar reduction in the time spent in the open arms 24 h after JWH‐018 administration, thus demonstrating that the modifications in the anxiety‐like states induced by repeated JWH‐018 begin during treatment and persist after drug discontinuation. Results of the MB test provide additional support to the possibility that repeated exposure to JWH‐018 has anxiogenic effects. Indeed, JWH‐018‐treated rats showed increased burying scores, which are compatible with the presence of an anxiety‐like state (Jimenez‐Gomez et al., 2011) and/or compulsive‐repetitive behaviours (Zanda et al., 2017). Taken together, these behavioural abnormalities might be due to a dysregulation of the eCB system, which is known to modulate anxiety‐related responses (Macrì et al., 2013; Parolaro et al., 2010).
Consistent with these data, altered anxiety‐related responses have been observed in humans after regular marijuana use (Sideli et al., 2020; Volkow et al., 2017) and in animals after repeated administration of SCRAs (Macrì et al., 2013). Moreover, use of SCRAs has been associated with acute (Papanti et al., 2014) and persistent psychosis (Sideli et al., 2020). Importantly, we found that in the PPI test repeated exposure to JWH‐018 induced transitory reductions of sensorimotor gating at 1 h after the last JWH‐018 administration. Few clinical and preclinical studies have investigated the effects of cannabis consumption on PPI and have reported mixed results (Quednow et al., 2004; Tournier & Ginovart, 2014). Our findings are in line with the notion that synthetic cannabinoids may possess psychotomimetic potential (Deng et al., 2018; Fattore, 2016).
Finally, in an attempt to get a better picture of behavioural anomalies relevant to hedonic/aversive state, we evaluated potential modifications of taste reactions to an intraoral sweet chocolate solution. We found that the first (but not the second) exposure to chocolate elicited significantly higher aversive taste reactions in JWH‐018‐treated than Veh‐treated rats, which suggests taste neophobia and confirms an aversive state in rats treated with JWH‐018. Moreover, similarly to the findings described in mice after subcutaneous THC (1–50 mg kg‐1) or JWH‐018 (1 mg kg‐1) treatment (Trexler et al., 2018), we observed marked spontaneous somatic signs of withdrawal in JWH‐018‐treated rats at 24 h and, to a lesser extent, 7 days after JWH‐018 discontinuation.
Electrophysiological recording of dopaminergic neurons located in the parabrachial pigmented nuclei of the posterior VTA, a subregion densely populated with dopaminergic cells projecting to the NAc (Yamaguchi et al., 2011), showed that repeated JWH‐018 exposure induces changes in basal dopaminergic neuron activity. In particular, we observed a decreased number of both spontaneously active VTA dopamine‐ and TH‐positive cells both 24 h and 7 days after JWH‐018 discontinuation and a decrease in firing rate at 7 days. These observations indicate a hypodopaminergic state (Melis et al., 2005) during withdrawal from JWH‐018, consistent with previous observations reported after chronic exposure to other drugs of abuse (Cannizzaro et al., 2019; Diana et al., 1998, 1999, 2003). The mechanism responsible for changes in dopaminergic cell activity in the VTA is not clear yet. One possibility is that repeated JWH‐018 administration induces a profound and long‐lasting downregulation of CB1 receptors expressed on GABA or glutamate afferents to dopaminergic neurons, as consistently shown in other brain regions such as the hippocampus (Dudok et al., 2015). This change in CB1 receptor activity might disrupt the balance between excitatory and inhibitory afferents impinging on dopaminergic cells, leading to a decrease in spontaneous activity. Consistent with this proposal, we observed a decrease of CB1 receptor expression in the mPFC (also in the NAc shell and core), which exerts an important regulatory action on dopaminergic signalling through its projection (Carr & Sesack, 2000; Taber et al., 1995). Nevertheless, our findings warrant further studies in order to recognise the projections of the single neurons recorded, as well as to evaluate the expression of CB1 receptors in specific cell types (i.e., neurons, astrocytes). Notably, these changes do not reflect differences between groups in the extracellular basal levels of dopamine in NAc shell or core, either at 24 h or at 7 days after drug discontinuation. Yet, reduced basal levels of dopamine have been detected at 24 h in the mPFC of JWH‐018‐treated rats, as compared to Veh‐treated animals, suggesting an alteration of mPFC dopaminergic transmission during acute withdrawal that may account for lack of its inhibitory control on the subcortical areas examined (i.e., NAc shell and core). This specific aspect has been studied in more depth by using a salient stimulus (i.e., intraoral chocolate) with a putative motivational value.
The microdialysis experiments showed that repeated JWH‐018 exposure reduces and potentiates the stimulation of dopaminergic transmission in the NAc shell and core, respectively, in response to acute JWH‐018 challenge. This differential adaptation of NAc shell and core dopamine resembles previous observations of reciprocal changes in the responsiveness of NAc shell/core dopamine to a THC challenge in rats sensitised to increasing doses of THC (Cadoni et al., 2008). After 7 days of withdrawal from JWH‐018, we also observed differential changes in the response of NAc shell and core and mPFC dopamine to an unfamiliar appetitive taste stimulus. On the first chocolate exposure, Veh‐treated rats showed an increase of dialysate dopamine in the NAc shell and in the mPFC but not in the NAc core. In rats withdrawing from chronic JWH‐018, however, dialysate dopamine still increased in the mPFC but not in the NAc shell and core. On the second chocolate exposure, dialysate dopamine increased in the mPFC, but not in the NAc shell and core, of Veh‐treated rats. In contrast, in rats withdrawing from JWH‐018, dialysate dopamine fully increased in the NAc shell and core but not in the mPFC. Thus, after pre‐exposure to chocolate, rats repeatedly treated with JWH‐018, in contrast to Veh‐treated animals (present findings) and naive animals (Bassareo et al., 2002), showed downregulation of dopamine responsiveness in the mPFC and a stimulatory dopamine response in the NAc shell and core. This reciprocal effect of dopamine responsiveness in the mPFC and in the NAc subdivisions could be due to the relief of dopaminergic transmission in the NAc from the inhibitory influence exerted by mPFC dopamine, although further experiments would be necessary to confirm this possibility. These findings would imply that repeated JWH‐018 exposure, at variance with morphine sensitisation (De Luca et al., 2011) and ablation of dopamine in the mPFC (Bimpisidis et al., 2013), decreases the sensitivity of the NAc shell to the first exposure to a natural rewarding stimulus like chocolate. This could be due to the influence of the aversive state associated to JWH‐018 withdrawal, as shown by the behavioural results. This interpretation is in line with the hypothesis that dopamine codes for novelty and motivational valence of aversive and hedonic stimuli (Bassareo et al., 2002).
Changes in neural–glial interactions play a role in the development and maintenance of drug dependence (Lacagnina et al., 2017) and chronic use of addictive drugs leads to glial alterations (Cutando et al., 2013; Kim et al., 2018). In this study, 24 h after the last JWH‐018 administration, we observed astrogliosis and microgliosis in different dopamine brain areas, which persisted up to 7 days after JWH‐018 discontinuation. In particular, increased expression of GFAP has been observed in all brain areas examined within the mesocorticolimbic dopaminergic pathways (mPFC, NAc shell/core, VTA), together with an increased number of IBA‐1 positive cells in the NAc shell and core, and in the VTA. Although we did not perform a stereological cell counting, the total size of the examined area in which IBA‐1 and TH neurons as well GFAP fibers were counted was chosen according to the Rat Brain Atlas coordinates (Paxinos & Watson, 2007) in order to include almost the whole area of each region analysed. Modifications in astrocyte structure and function have been associated to neuropsychiatric diseases, including addiction (Kim et al., 2018), resulting in either astrogliosis or astrocytopathy depending on drug class, dose, route of administration, length of withdrawal, and brain areas examined (Castelli et al., 2014; Fattore et al., 2002; Miguel‐Hidalgo, 2009).
Besides astrogliosis, our results revealed an increased number of microglia cells in the NAc shell and core, and in the VTA of JWH‐018‐treated rats, confirming the involvement of both types of glial cells in the effects of drugs of abuse (Lacagnina et al., 2017; Melis et al., 2017). In addition to changes in glial cells, we observed a concomitant downregulation of CB1 receptors, as previously observed in mice after repeated THC exposure (Cutando et al., 2013; Zamberletti et al., 2015), supporting the relationship between the eCB system function in glial cell physiology and neuroinflammation. Notably, the persistence of these glial alterations up to 7 days after JWH‐018 discontinuation is in line with observations with other drugs of abuse, that is, cocaine and amphetamines (Bowers & Kalivas, 2003; Granado et al., 2011). Interestingly, several parallel studies reported a reduction in astrocyte glutamate transporter‐1 (GLT‐1) function during cocaine, ethanol, or morphine withdrawal (Das et al., 2016; Fischer‐Smith et al., 2012; Kim et al., 2018), supporting the role of astrocytes in glutamate homeostasis at neuronal synapses (Bjornsen et al., 2014), in line with the concept of the “tripartite” (Hammond et al., 2015), now evolved in “quadripartite,” synapse (Fouyssac & Belin, 2019). Consistently, we observed that the extracellular levels of glutamate and GABA were markedly reduced in the mPFC 24 h after the last JWH‐018 administration, most likely due to a modified expression and/or activity of GABA and glutamate glial transporters. In light of these results, repeated JWH‐018 exposure could induce changes in eCB signalling (e.g., CB1 receptor downregulation in dopaminergic terminal areas), which through modifications in glial cell expression and activity might induce a perturbation of glutamate signalling and alter the balance between GABA and glutamate systems. This, in turn, might partially contribute to behavioural anomalies and dysregulation of the mesolimbic and mesocortical dopaminergic systems. Indeed, recent findings have demonstrated profound disturbances of the mesocorticolimbic circuitry following adolescent (but not adult) THC exposure, characterised by long‐lasting GABA hypoactivity and glutamate hyperactivity in the PFC, a subcortical hyper‐dopaminergic neuronal phenotype in the VTA, impairments in short‐term memory, social motivation and cognition, increased anxiety levels, and decreased motivation (Renard, Rosen, et al., 2017; Renard, Szkudlarek, et al., 2017). These discrepancies with our results may be due to differences in the drug tested (natural vs. synthetic cannabinoid), in the evaluation time intervals (7 vs. 30 days of drug withdrawal), and/or in the lifetime period of drug exposure (adolescence vs. adulthood). In particular, the latter point may represent a discriminative factor in the different consequences observed, as adolescence is a critical neurodevelopmental period characterised by increased neural plasticity and vulnerability to chemical insults (such as exposure to cannabinoids), particularly in cortical and limbic regions (Hurd et al., 2014; Spear, 2000).
Taken together, the results obtained in rats repeatedly exposed to JWH‐018 replicate some typical aspects of drug dependence, including the induction of an aversive state upon withdrawal and loss of responsiveness of NAc shell and core dopamine to a novel palatable reinforcer. Reinstatement of NAc dopamine response by a second exposure to the palatable taste suggests that a taste reward can overcome the inhibitory influence of JWH‐018 withdrawal on NAc dopaminergic transmission. Finally, the changes of glial cells in dopaminergic brain areas support the hypothesis of a role of neuroinflammation in cannabinoid dependence. In light of evidence showing sex differences in cannabinoid addiction (Fattore et al., 2007), dopaminergic neurotransmission (Becker, 2016), and neuroinflammation (Liu et al., 2019), further studies will assess the effects of JWH‐018 in male and female rats, using the self‐administration paradigm, to better mimic the exposure pattern of drug intake in humans.
AUTHOR CONTRIBUTIONS
M.A.D.L. and G.D.C. designed the study. N.P., M.P.C., G.D.C., and M.A.D.L. wrote the manuscript. N.P., C.M., and G.F. planned, performed, and analysed microdyalisis experiments, C.S., M.D.F., and M.P. planned, performed, and analysed electrophysiological experiments, N.S., N.P., M.S., L.F., and P.F. planned, performed, and analysed behavioural experiments; N.P., M.P.C., and M.G.E. planned, performed, and analysed immunohistochemical experiments. All authors have read and agreed to the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design and Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers, and other organisations engaged with supporting research.
ACKNOWLEDGEMENTS
This research has been funded by the Drug Policies Department, Presidency of the Council of Ministers, Italy, projects: “INSIDE‐018” (PI: Prof. De Luca, University of Cagliari) and “Effects of NPS: development of a multicentre research for the information enhancement of the Early Warning System” (PI: Prof. Marti, University of Ferrara) to M.A.D.L., and RAS‐FSC 2018 (Codice intervento: RC_CRP_034; CUP RASSR03071; project: “Multidisciplinary preclinical study on NPS and evaluation of their behavioral and neurophysiological effects related to age and sex”) to M.A.D.L., N.S., and L.F. Prof. De Luca would gratefully like to thank the Dipartimento Salute Mentale e Dipendenze (DSMD)‐zona Sud‐ATS Sardegna within the Convenzione sanitaria in materia di studio e ricerca tossicologica con il DiSB (UniCa) in oggetto al “PROGRAMMA REGIONALE PER L'ASSISTENZA SANITARIA DELLE PERSONE TOSSICODIPENDENTI NEGLI ISTITUTI PENITENZIARI DELLA SARDEGNA” (Resolution of the Special Commissioner ATS n. 121 of 21‐02‐2020).
Pintori N, Castelli MP, Miliano C, et al. Repeated exposure to JWH‐018 induces adaptive changes in the mesolimbic and mesocortical dopaminergic pathways, glial cells alterations, and behavioural correlates. Br J Pharmacol. 2021;178:3476–3497. 10.1111/bph.15494
Nicholas Pintori and Maria Paola Castelli contributed equally to this work.
Funding information RAS‐FSC 2018, Grant/Award Number: RC_CRP_034; CUP RASSR03071; Drug Policies Department, Presidency of the Council of Ministers, Italy, Grant/Award Numbers: "INSIDE‐018", Effects of NPS
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
REFERENCES
- Alexander, S. P. H., Christopoulos, A., Davenport, A. P., Kelly, E., Mathie, A., Peters, J. A., Veale, E. L., Armstrong, J. F., Faccenda, E., Harding, S. D., Pawson, A. J., Sharman, J. L., Southan, C., Davies, J. A., & CGTP Collaborators . (2019). The concise guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(S1), S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H., Fabbro, D., Kelly, E., Mathie, A., Peters, J. A., Veale, E. L., Armstrong, J. F., Faccenda, E., Harding, S. D., Pawson, A. J., Sharman, J. L., Southan, C., Davies, J. A., & CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H., Kelly, E., Mathie, A., Peters, J. A., Veale, E. L., Armstrong, J. F., Faccenda, E., Harding, S. D., Pawson, A. J., Sharman, J. L., Southan, C., Davies, J. A., & CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Transporters. British Journal of Pharmacology, 176, S397–S493. 10.1111/bph.14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H., Roberts, R. E., Broughton, B. R. S., Sobey, C. G., George, C. H., Stanford, S. C., Cirino, G., Docherty, J. R., Giembycz, M. A., Hoyer, D., Insel, P. A., Izzo, A. A., Ji, Y., MacEwan, D. J., Mangum, J., Wonnacott, S., & Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175(3), 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo, V., De Luca, M. A., & Di Chiara, G. (2002). Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. The Journal of Neuroscience, 22(11), 4709–4719. 10.1523/JNEUROSCI.22-11-04709.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, J. B. (2016). Sex differences in addiction. Dialogues in Clinical Neuroscience, 18(4), 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimpisidis, Z., De Luca, M. A., Pisanu, A., & Di Chiara, G. (2013). Lesion of medial prefrontal dopamine terminals abolishes habituation of accumbens shell dopamine responsiveness to taste stimuli. The European Journal of Neuroscience, 37(4), 613–622. 10.1111/ejn.12068 [DOI] [PubMed] [Google Scholar]
- Bjornsen, L. P., Hadera, M. G., Zhou, Y., Danbolt, N. C., & Sonnewald, U. (2014). The GLT‐1 (EAAT2; slc1a2) glutamate transporter is essential for glutamate homeostasis in the neocortex of the mouse. Journal of Neurochemistry, 128(5), 641–649. 10.1111/jnc.12509 [DOI] [PubMed] [Google Scholar]
- Bodor, A. L., Katona, I., Nyíri, G., Mackie, K., Ledent, C., Hájos, N., & Freund, T. F. (2005). Endocannabinoid signaling in rat somatosensory cortex: Laminar differences and involvement of specific interneuron types. The Journal of Neuroscience, 25(29), 6845–6856. 10.1523/jneurosci.0442-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers, M. S., & Kalivas, P. W. (2003). Forebrain astroglial plasticity is induced following withdrawal from repeated cocaine administration. The European Journal of Neuroscience, 17(6), 1273–1278. 10.1046/j.1460-9568.2003.02537.x [DOI] [PubMed] [Google Scholar]
- Cadoni, C., Valentini, V., & Di Chiara, G. (2008). Behavioral sensitization to Δ9‐tetrahydrocannabinol and cross‐sensitization with morphine: Differential changes in accumbal shell and core dopamine transmission. Journal of Neurochemistry, 106(4), 1586–1593. 10.1111/j.1471-4159.2008.05503.x [DOI] [PubMed] [Google Scholar]
- Cannizzaro, C., Talani, G., Brancato, A., Mulas, G., Spiga, S., De Luca, M. A., Sanna, A., Marino, R. A. M., Biggio, G., Sanna, E., & Diana, M. (2019). Dopamine restores limbic memory loss, dendritic spine structure, and NMDAR‐dependent LTD in the nucleus accumbens of alcohol‐withdrawn rats. The Journal of Neuroscience, 39(5), 929–943. 10.1523/jneurosci.1377-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, D. B., & Sesack, S. R. (2000). Projections from the rat prefrontal cortex to the ventral tegmental area: Target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. The Journal of Neuroscience, 20(10), 3864–3873. 10.1523/JNEUROSCI.20-10-03864.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli, M. P., Madeddu, C., Casti, A., Casu, A., Casti, P., Scherma, M., Fattore, L., Fadda, P., & Ennas, M. G. (2014). Δ9‐tetrahydrocannabinol prevents methamphetamine‐induced neurotoxicity. PLoS ONE, 9(5), e98079. 10.1371/journal.pone.0098079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J., Alexander, S., Cirino, G., Docherty, J. R., George, C. H., Giembycz, M. A., Hoyer, D., Insel, P. A., Izzo, A. A., Ji, Y., MacEwan, D. J., Sobey, C. G., Stanford, S. C., Teixeira, M. M., Wonnacott, S., & Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutando, L., Busquets‐Garcia, A., Puighermanal, E., Gomis‐González, M., Delgado‐García, J. M., Gruart, A., Maldonado, R., & Ozaita, A. (2013). Microglial activation underlies cerebellar deficits produced by repeated cannabis exposure. The Journal of Clinical Investigation, 123(7), 2816–2831. 10.1172/jci67569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, S. C., Althobaiti, Y. S., Alshehri, F. S., & Sari, Y. (2016). Binge ethanol withdrawal: Effects on post‐withdrawal ethanol intake, glutamate‐glutamine cycle and monoamine tissue content in P rat model. Behavioural Brain Research, 303, 120–125. 10.1016/j.bbr.2016.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca, M. A. (2014). Habituation of the responsiveness of mesolimbic and mesocortical dopamine transmission to taste stimuli. Frontiers in Integrative Neuroscience, 8, 21. 10.3389/fnint.2014.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca, M. A., Bimpisidis, Z., Bassareo, V., & Di Chiara, G. (2011). Influence of morphine sensitization on the responsiveness of mesolimbic and mesocortical dopamine transmission to appetitive and aversive gustatory stimuli. Psychopharmacology, 216(3), 345–353. 10.1007/s00213-011-2220-9 [DOI] [PubMed] [Google Scholar]
- De Luca, M. A., Bimpisidis, Z., Melis, M., Marti, M., Caboni, P., Valentini, V., Margiani, G., Pintori, N., Polis, I., Marsicano, G., Parsons, L. H., & Di Chiara, G. (2015). Stimulation of in vivo dopamine transmission and intravenous self‐administration in rats and mice by JWH‐018, a Spice cannabinoid. Neuropharmacology, 99, 705–714. 10.1016/j.neuropharm.2015.08.041 [DOI] [PubMed] [Google Scholar]
- De Luca, M. A., Castelli, M. P., Loi, B., Porcu, A., Martorelli, M., Miliano, C., Kellett, K., Davidson, C., Stair, J. L., Schifano, F., & Di Chiara, G. (2016). Native CB1 receptor affinity, intrinsic activity and accumbens shell dopamine stimulant properties of third generation SPICE/K2 cannabinoids: BB‐22, 5F‐PB‐22, 5F‐AKB‐48 and STS‐135. Neuropharmacology, 105, 630–638. 10.1016/j.neuropharm.2015.11.017 [DOI] [PubMed] [Google Scholar]
- De Luca, M. A., & Fattore, L. (2018). Therapeutic use of synthetic cannabinoids: Still an open issue? Clinical Therapeutics, 40, 1457–1466. 10.1016/j.clinthera.2018.08.002 [DOI] [PubMed] [Google Scholar]
- De Luca, M. A., Solinas, M., Bimpisidis, Z., Goldberg, S. R., & Di Chiara, G. (2012). Cannabinoid facilitation of behavioral and biochemical hedonic taste responses. Neuropharmacology, 63(1), 161–168. 10.1016/j.neuropharm.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, H., Verrico, C. D., Kosten, T. R., & Nielsen, D. A. (2018). Psychosis and synthetic cannabinoids. Psychiatry Research, 268, 400–412. 10.1016/j.psychres.2018.08.012 [DOI] [PubMed] [Google Scholar]
- Di Chiara, G. (1990). In‐vivo brain dialysis of neurotransmitters. Trends in Pharmacological Sciences, 11(3), 116–121. 10.1016/0165-6147(90)90197-G [DOI] [PubMed] [Google Scholar]
- Di Chiara, G., Bassareo, V., Fenu, S., De Luca, M. A., Spina, L., Cadoni, C., Acquas, E., Carboni, E., Valentini, V., & Lecca, D. (2004). Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology, 47(Suppl 1), 227–241. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15464140 [DOI] [PubMed] [Google Scholar]
- Diana, M., Brodie, M., Muntoni, A., Puddu, M. C., Pillolla, G., Steffensen, S., Spiga, S., & Little, H. J. (2003). Enduring effects of chronic ethanol in the CNS: Basis for alcoholism. Alcoholism, Clinical and Experimental Research, 27(2), 354–361. 10.1097/01.alc.0000057121.36127.19 [DOI] [PubMed] [Google Scholar]
- Diana, M., Melis, M., Muntoni, A. L., & Gessa, G. L. (1998). Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proceedings of the National Academy of Sciences of the United States of America, 95(17), 10269–10273. 10.1073/pnas.95.17.10269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana, M., Muntoni, A. L., Pistis, M., Melis, M., & Gessa, G. L. (1999). Lasting reduction in mesolimbic dopamine neuronal activity after morphine withdrawal. The European Journal of Neuroscience, 11(3), 1037–1041. 10.1046/j.1460-9568.1999.00488.x [DOI] [PubMed] [Google Scholar]
- Dudok, B., Barna, L., Ledri, M., Szabó, S. I., Szabadits, E., Pintér, B., Woodhams, S. G., Henstridge, C. M., Balla, G. Y., Nyilas, R., Varga, C., Lee, S. H., Matolcsi, M., Cervenak, J., Kacskovics, I., Watanabe, M., Sagheddu, C., Melis, M., Pistis, M., … Katona, I. (2015). Cell‐specific STORM super‐resolution imaging reveals nanoscale organization of cannabinoid signaling. Nature Neuroscience, 18(1), 75–86. 10.1038/nn.3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore, L. (2016). Synthetic cannabinoids‐further evidence supporting the relationship between cannabinoids and psychosis. Biological Psychiatry, 79(7), 539–548. 10.1016/j.biopsych.2016.02.001 [DOI] [PubMed] [Google Scholar]
- Fattore, L., Puddu, M. C., Picciau, S., Cappai, A., Fratta, W., Serra, G. P., & Spiga, S. (2002). Astroglial in vivo response to cocaine in mouse dentate gyrus: A quantitative and qualitative analysis by confocal microscopy. Neuroscience, 110(1), 1–6. 10.1016/S0306-4522(01)00598-X [DOI] [PubMed] [Google Scholar]
- Fattore, L., Spano, M. S., Altea, S., Angius, F., Fadda, P., & Fratta, W. (2007). Cannabinoid self‐administration in rats: Sex differences and the influence of ovarian function. British Journal of Pharmacology, 152(5), 795–804. 10.1038/sj.bjp.0707465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer‐Smith, K. D., Houston, A. C., & Rebec, G. V. (2012). Differential effects of cocaine access and withdrawal on glutamate type 1 transporter expression in rat nucleus accumbens core and shell. Neuroscience, 210, 333–339. 10.1016/j.neuroscience.2012.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouyssac, M., & Belin, D. (2019). Beyond drug‐induced alteration of glutamate homeostasis, astrocytes may contribute to dopamine‐dependent intrastriatal functional shifts that underlie the development of drug addiction: A working hypothesis. The European Journal of Neuroscience, 50(6), 3014–3027. 10.1111/ejn.14416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, R. Z., & Volkow, N. D. (2011). Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nature Reviews. Neuroscience, 12(11), 652–669. 10.1038/nrn3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace, A. A., & Bunney, B. S. (1984). The control of firing pattern in nigral dopamine neurons: Single spike firing. The Journal of Neuroscience, 4(11), 2866–2876. 10.1523/JNEUROSCI.04-11-02866.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granado, N., Ares‐Santos, S., Oliva, I., O'Shea, E., Martin, E. D., Colado, M. I., & Moratalla, R. (2011). Dopamine D2‐receptor knockout mice are protected against dopaminergic neurotoxicity induced by methamphetamine or MDMA. Neurobiology of Disease, 42(3), 391–403. 10.1016/j.nbd.2011.01.033 [DOI] [PubMed] [Google Scholar]
- Hammond, C., Cayre, M., Panatier, A., & Avignone, E. (2015). Chapter 2—Neuron–glial cell cooperation. In Hammond C. (Ed.), Cellular and molecular neurophysiology (Fourth ed.) (pp. 25–37). Boston: Academic Press. [Google Scholar]
- Hurd, Y. L., Michaelides, M., Miller, M. L., & Jutras‐Aswad, D. (2014). Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology, 76(Pt B(0 0)), 416–424. 10.1016/j.neuropharm.2013.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez‐Gomez, C., Osentoski, A., & Woods, J. H. (2011). Pharmacological evaluation of the adequacy of marble burying as an animal model of compulsion and/or anxiety. Behavioural Pharmacology, 22(7), 711–713. 10.1097/FBP.0b013e32834afebe [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, R., Healey, K. L., Sepulveda‐Orengo, M. T., & Reissner, K. J. (2018). Astroglial correlates of neuropsychiatric disease: From astrocytopathy to astrogliosis. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 87(Pt A), 126–146. 10.1016/j.pnpbp.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina, M. J., Rivera, P. D., & Bilbo, S. D. (2017). Glial and neuroimmune mechanisms as critical modulators of drug use and abuse. Neuropsychopharmacology, 42(1), 156–177. 10.1038/npp.2016.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley, E., Stanford, S. C., Kendall, D. E., Alexander, S. P. H., Cirino, G., Docherty, J. R., George, C. H., Insel, P. A., Izzo, A. A., Ji, Y., Panettieri, R. A., Sobey, C. G., Stefanska, B., Stephens, G., Teixeira, M. M., & Ahluwalia, A. (2020). ARRIVE 2.0 and the British Journal of Pharmacology: Updated guidance for 2020. British Journal of Pharmacology, 177(16), 3611–3616. https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.15178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. L., Li, J. M., Su, W. J., Wang, B., & Jiang, C. L. (2019). Sex differences in depressive‐like behaviour may relate to imbalance of microglia activation in the hippocampus. Brain, Behavior, and Immunity, 81, 188–197. 10.1016/j.bbi.2019.06.012 [DOI] [PubMed] [Google Scholar]
- Macrì, S., Lanuzza, L., Merola, G., Ceci, C., Gentili, S., Valli, A., Macchia, T., & Laviola, G. (2013). Behavioral responses to acute and sub‐chronic administration of the synthetic cannabinoid JWH‐018 in adult mice prenatally exposed to corticosterone. Neurotoxicity Research, 24(1), 15–28. 10.1007/s12640-012-9371-2 [DOI] [PubMed] [Google Scholar]
- Manzanares, J., Cabañero, D., Puente, N., García‐Gutiérrez, M. S., Grandes, P., & Maldonado, R. (2018). Role of the endocannabinoid system in drug addiction. Biochemical Pharmacology, 157, 108–121. 10.1016/j.bcp.2018.09.013 [DOI] [PubMed] [Google Scholar]
- Marin, I., & Kipnis, J. (2013). Learning and memory … and the immune system. Learning & Memory, 20(10), 601–606. 10.1101/lm.028357.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín‐Santos, R., Fagundo, A. B., Crippa, J. A., Atakan, Z., Bhattacharyya, S., Allen, P., Fusar‐Poli, P., Borgwardt, S., Seal, M., Busatto, G. F., & McGuire, P. (2010). Neuroimaging in cannabis use: A systematic review of the literature. Psychological Medicine, 40(3), 383–398. 10.1017/s0033291709990729 [DOI] [PubMed] [Google Scholar]
- Melis, M., Frau, R., Kalivas, P. W., Spencer, S., Chioma, V., Zamberletti, E., Rubino, T., & Parolaro, D. (2017). New vistas on cannabis use disorder. Neuropharmacology, 124, 62–72. 10.1016/j.neuropharm.2017.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis, M., Spiga, S., & Diana, M. (2005). The dopamine hypothesis of drug addiction: Hypodopaminergic state. International Review of Neurobiology, 63, 101–154. 10.1016/s0074-7742(05)63005-x [DOI] [PubMed] [Google Scholar]
- Miguel‐Hidalgo, J. J. (2009). The role of glial cells in drug abuse. Current Drug Abuse Reviews, 2(1), 72–82. [PubMed] [Google Scholar]
- Morales, P., Simola, N., Bustamante, D., Lisboa, F., Fiedler, J., Gebicke‐Haerter, P. J., Morelli, M., Tasker, R. A., & Herrera‐Marschitz, M. (2010). Nicotinamide prevents the long‐term effects of perinatal asphyxia on apoptosis, non‐spatial working memory and anxiety in rats. Experimental Brain Research, 202(1), 1–14. 10.1007/s00221-009-2103-z [DOI] [PubMed] [Google Scholar]
- Murase, S., Grenhoff, J., Chouvet, G., Gonon, F. G., & Svensson, T. H. (1993). Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neuroscience Letters, 157(1), 53–56. 10.1016/0304-3940(93)90641-w [DOI] [PubMed] [Google Scholar]
- Papanti, D., Orsolini, L., Francesconi, G., & Schifano, F. (2014). Noids in a nutshell: Everything you (don't) want to know about synthetic cannabimimetics. Advances in Dual Diagnosis, 7, 137–148. 10.1108/ADD-02-2014-0006 [DOI] [Google Scholar]
- Parolaro, D., Realini, N., Vigano, D., Guidali, C., & Rubino, T. (2010). The endocannabinoid system and psychiatric disorders. Experimental Neurology, 224(1), 3–14. 10.1016/j.expneurol.2010.03.018 [DOI] [PubMed] [Google Scholar]
- Paxinos, G., & Watson, C. (2007). The rat brain in stereotaxic coordinates. Academic Press/Elsevier. [Google Scholar]
- Percie du Sert, N., Hurst, V., Ahluwalia, A., Alam, S., Avey, M. T., Baker, M., Browne, W. J., Clark, A., Cuthill, I. C., Dirnagl, U., Emerson, M., Garner, P., Holgate, S. T., Howells, D. W., Karp, N. A., Lazic, S. E., Lidster, K., MacCallum, C. J., Macleod, M., … Würbel, H. (2020). The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol, 18, e3000410. 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintori, N., Loi, B., & Mereu, M. (2017). Synthetic cannabinoids: The hidden side of Spice drugs. Behavioural Pharmacology, 28(6), 409–419. 10.1097/fbp.0000000000000323 [DOI] [PubMed] [Google Scholar]
- Quednow, B. B., Kuhn, K. U., Hoenig, K., Maier, W., & Wagner, M. (2004). Prepulse inhibition and habituation of acoustic startle response in male MDMA (‘ecstasy’) users, cannabis users, and healthy controls. Neuropsychopharmacology, 29(5), 982–990. 10.1038/sj.npp.1300396 [DOI] [PubMed] [Google Scholar]
- Renard, J., Rosen, L. G., Loureiro, M., De Oliveira, C., Schmid, S., Rushlow, W. J., & Laviolette, S. R. (2017). Adolescent cannabinoid exposure induces a persistent sub‐cortical hyper‐dopaminergic state and associated molecular adaptations in the prefrontal cortex. Cerebral Cortex, 27(2), 1297–1310. 10.1093/cercor/bhv335 [DOI] [PubMed] [Google Scholar]
- Renard, J., Szkudlarek, H. J., Kramar, C. P., Jobson, C. E. L., Moura, K., Rushlow, W. J., & Laviolette, S. R. (2017). Adolescent THC exposure causes enduring prefrontal cortical disruption of GABAergic inhibition and dysregulation of sub‐cortical dopamine function. Scientific Reports, 7(1), 11420. 10.1038/s41598-017-11645-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagheddu, C., Muntoni, A. L., Pistis, M., & Melis, M. (2015). Endocannabinoid signaling in motivation, reward, and addiction: Influences on mesocorticolimbic dopamine function. International Review of Neurobiology, 125, 257–302. 10.1016/bs.irn.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Sagheddu, C., Pintori, N., Kalaba, P., Dragačević, V., Piras, G., Lubec, J., Simola, N., De Luca, M. A., Lubec, G., & Pistis, M. (2020). Neurophysiological and neurochemical effects of the putative cognitive enhancer (S)‐CE‐123 on mesocorticolimbic dopamine system. Biomolecules, 10(5), 779. 10.3390/biom10050779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagheddu, C., Scherma, M., Congiu, M., Fadda, P., Carta, G., Banni, S., Wood, J. A. T., Makriyannis, A., Malamas, M. S., & Pistis, M. (2019). Inhibition of N‐acylethanolamine acid amidase reduces nicotine‐induced dopamine activation and reward. Neuropharmacology, 144, 327–336. 10.1016/j.neuropharm.2018.11.013 [DOI] [PubMed] [Google Scholar]
- Satta, V., Scherma, M., Giunti, E., Collu, R., Fattore, L., Fratta, W., & Fadda, P. (2016). Emotional profile of female rats showing binge eating behavior. Physiology & Behavior, 163, 136–143. 10.1016/j.physbeh.2016.05.013 [DOI] [PubMed] [Google Scholar]
- Schifano, F., Orsolini, L., Duccio Papanti, G., & Corkery, J. M. (2015). Novel psychoactive substances of interest for psychiatry. World Psychiatry, 14(1), 15–26. 10.1002/wps.20174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield, M. D., & Kalivas, P. W. (2014). Astrocytic dysfunction and addiction: Consequences of impaired glutamate homeostasis. The Neuroscientist, 20(6), 610–622. 10.1177/1073858413520347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secci, M. E., Mascia, P., Sagheddu, C., Beggiato, S., Melis, M., Borelli, A. C., Tomasini, M. C., Panlilio, L. V., Schindler, C. W., Tanda, G., Ferré, S., Bradberry, C. W., Ferraro, L., Pistis, M., Goldberg, S. R., Schwarcz, R., & Justinova, Z. (2019). Astrocytic mechanisms involving kynurenic acid control Δ9‐tetrahydrocannabinol‐induced increases in glutamate release in brain reward‐processing areas. Molecular Neurobiology, 56(5), 3563–3575. 10.1007/s12035-018-1319-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideli, L., Quigley, H., La Cascia, C., & Murray, R. M. (2020). Cannabis use and the risk for psychosis and affective disorders. Journal of Dual Diagnosis, 16(1), 22–42. 10.1080/15504263.2019.1674991 [DOI] [PubMed] [Google Scholar]
- Spano, M. S., Fadda, P., Frau, R., Fattore, L., & Fratta, W. (2010). Cannabinoid self‐administration attenuates PCP‐induced schizophrenia‐like symptoms in adult rats. European Neuropsychopharmacology, 20(1), 25–36. 10.1016/j.euroneuro.2009.09.004 [DOI] [PubMed] [Google Scholar]
- Spear, L. P. (2000). The adolescent brain and age‐related behavioral manifestations. Neuroscience and Biobehavioral Reviews, 24(4), 417–463. 10.1016/s0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Taber, M. T., Das, S., & Fibiger, H. C. (1995). Cortical regulation of subcortical dopamine release: Mediation via the ventral tegmental area. Journal of Neurochemistry, 65(3), 1407–1410. 10.1046/j.1471-4159.1995.65031407.x [DOI] [PubMed] [Google Scholar]
- Tan, H., Ahmad, T., Loureiro, M., Zunder, J., & Laviolette, S. R. (2014). The role of cannabinoid transmission in emotional memory formation: Implications for addiction and schizophrenia. Frontiers in Psychiatry, 5, 73. 10.3389/fpsyt.2014.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier, B. B., & Ginovart, N. (2014). Repeated but not acute treatment with (9)‐tetrahydrocannabinol disrupts prepulse inhibition of the acoustic startle: Reversal by the dopamine D(2)/(3) receptor antagonist haloperidol. European Neuropsychopharmacology, 24(8), 1415–1423. 10.1016/j.euroneuro.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Trexler, K. R., Nass, S. R., Crowe, M. S., Gross, J. D., Jones, M. S., McKitrick, A. W., Siderovski, D. P., & Kinsey, S. G. (2018). Novel behavioral assays of spontaneous and precipitated THC withdrawal in mice. Drug and Alcohol Dependence, 191, 14–24. 10.1016/j.drugalcdep.2018.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow, N. D., Fowler, J. S., & Wang, G. J. (2003). The addicted human brain: Insights from imaging studies. The Journal of Clinical Investigation, 111(10), 1444–1451. 10.1172/jci18533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow, N. D., Hampson, A. J., & Baler, R. D. (2017). Don't worry, be happy: Endocannabinoids and cannabis at the intersection of stress and reward. Annual Review of Pharmacology and Toxicology, 57, 285–308. 10.1146/annurev-pharmtox-010716-104615 [DOI] [PubMed] [Google Scholar]
- Volkow, N. D., & Morales, M. (2015). The brain on drugs: From reward to addiction. Cell, 162(4), 712–725. 10.1016/j.cell.2015.07.046 [DOI] [PubMed] [Google Scholar]
- Wiley, J. L., Marusich, J. A., Martin, B. R., & Huffman, J. W. (2012). 1‐Pentyl‐3‐phenylacetylindoles and JWH‐018 share in vivo cannabinoid profiles in mice. Drug and Alcohol Dependence, 123(1–3), 148–153. 10.1016/j.drugalcdep.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, T., Wang, H. L., Li, X., Ng, T. H., & Morales, M. (2011). Mesocorticolimbic glutamatergic pathway. Journal of Neuroscience, 31(23), 8476–8490. 10.1523/JNEUROSCI.1598-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya, R., & Goshen, I. (2011). Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behavior, and Immunity, 25(2), 181–213. 10.1016/j.bbi.2010.10.015 [DOI] [PubMed] [Google Scholar]
- Zamberletti, E., Gabaglio, M., Prini, P., Rubino, T., & Parolaro, D. (2015). Cortical neuroinflammation contributes to long‐term cognitive dysfunctions following adolescent delta‐9‐tetrahydrocannabinol treatment in female rats. European Neuropsychopharmacology, 25(12), 2404–2415. 10.1016/j.euroneuro.2015.09.021 [DOI] [PubMed] [Google Scholar]
- Zanda, M. T., Fadda, P., Antinori, S., Di Chio, M., Fratta, W., Chiamulera, C., & Fattore, L. (2017). Methoxetamine affects brain processing involved in emotional response in rats. British Journal of Pharmacology, 174(19), 3333–3345. 10.1111/bph.13952 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.


