Abstract
A bulk of data suggest that the gut microbiota plays a role in a broad range of diseases, including those affecting the central nervous system. Recently, significant differences in the intestinal microbiota of patients with epilepsy, compared to healthy volunteers, have been reported in an observational study. However, an active role of the intestinal microbiota in the pathogenesis of epilepsy, through the so‐called “gut–brain axis,” has yet to be demonstrated. In this study, we evaluated the direct impact of microbiota transplanted from epileptic animals to healthy recipient animals, to clarify whether the microbiota from animals with epilepsy can affect the excitability of the recipients’ brain by lowering seizure thresholds. Our results provide the first evidence that mice who received microbiota from epileptic animals are more prone to develop status epilepticus, compared to recipients of “healthy” microbiota, after a subclinical dose of pilocarpine, indicating a higher susceptibility to seizures. The lower thresholds for seizure activity found in this study support the hypothesis that the microbiota, through the gut–brain axis, is able to affect neuronal excitability in the brain.
Keywords: brain excitability, epilepsy, fecal microbiota transplantation, microbiome, pilocarpine model
1. INTRODUCTION
The past decade of neuroscience research has seen a growing interest in the role of the gut microbiota in central nervous system (CNS) health and disease.1 The link between intestinal microbiota and CNS has led to the definition of a so‐called “gut–brain axis,” a bidirectional communication network between the digestive tract and the brain.2 This interaction is maintained through complex mechanisms, only partially understood, including the roles of the vagus nerve and the enteric nervous system, hormones, immune signaling molecules such as cytokines and chemokines, and neurotransmitters.3 Accumulating evidence links microbiota alterations, usually referred to as dysbiosis, to CNS disorders including multiple sclerosis,4 Parkinson disease,5 and Alzheimer disease.6
In this context, the exploration of the connection between gut microbiota and epilepsy is sparse, mainly focusing on the microbiota change induced by the ketogenic diet.7 Recently, significant differences in the intestinal microbiota have been reported in patients with epilepsy compared to healthy volunteers,8 as well as in WAG/Rij rats, a genetic model of absence epilepsy.9 Moreover, the transplant of the intestinal microbiota from chronically stressed to naive rats has been demonstrated to facilitate kindling epileptogenesis.10 However, to our knowledge, a direct proepileptogenic effect of gut microbiota collected from epileptic donors has yet to be demonstrated. In particular, it is not well established whether the intestinal microbiota alterations induced by epilepsy could, by themselves, modify brain activity and increase seizure susceptibility, possibly facilitating epileptogenesis.
We investigated whether the transplantation of microbiota derived from epileptic mice might play a role in the etiopathogenesis of epilepsy by increasing brain excitability of naive/healthy mice. In particular, such microbiota samples were collected and transplanted into healthy recipient pups with immature gut microbiota. Recipient mice were then submitted to a pro‐brain‐excitability challenge with a subclinical dose of pilocarpine, to fully explore whether this “epileptic” microbiota could increase susceptibility to seizures.
2. MATERIALS AND METHODS
Animal care and experimental procedures were conducted in accordance with the guidelines of the European Union directive 2010/63/EU. All protocols were approved by the local ethical committee (C.I.R.S.A.L., University of Verona) and Italian Ministry of Health (authorization 1107/2015‐PR). NMRI mice acquired from Charles River were housed in individually ventilated cages (IVCs), one animal per cage, with autoclaved food and water ad libitum, and kept in a sound‐attenuated room at constant temperature (22 ± 1.0℃) and humidity (60 ± 5%), with an inverted 12/12‐h light–dark cycle with lights on at 7:00 p.m., corresponding to Zeitgeber time (ZT) 0. The IVC system allows maintenance of mice with semidefined microbiota for up to 5 months and performance of gut colonization equally efficiently as with those obtained in isolator systems (details in Supporting Information).11
2.1. Donor group
A cohort of male, 7‐week‐old donor (D) mice were randomly assigned to control (D‐CTL) or pilocarpine (D‐EPI) groups (Figure 1A). A dose of 300 mg/kg of pilocarpine was intraperitoneally injected in D‐EPI animals to induce status epilepticus (SE) according to the pilocarpine model of temporal lobe epilepsy.12 Methyl‐scopolamine (1 mg/kg ip) was administered 30 min prior to pilocarpine to minimize peripheral cholinergic effects. Animals of the D‐CTL group intraperitoneally received an equal volume of phosphate‐buffered saline (PBS; pH = 7.4). All animals were observed for 2 h after either pilocarpine or PBS injection, and the behavior was monitored and scored using a revised version of the Racine scale for mice.13
FIGURE 1.
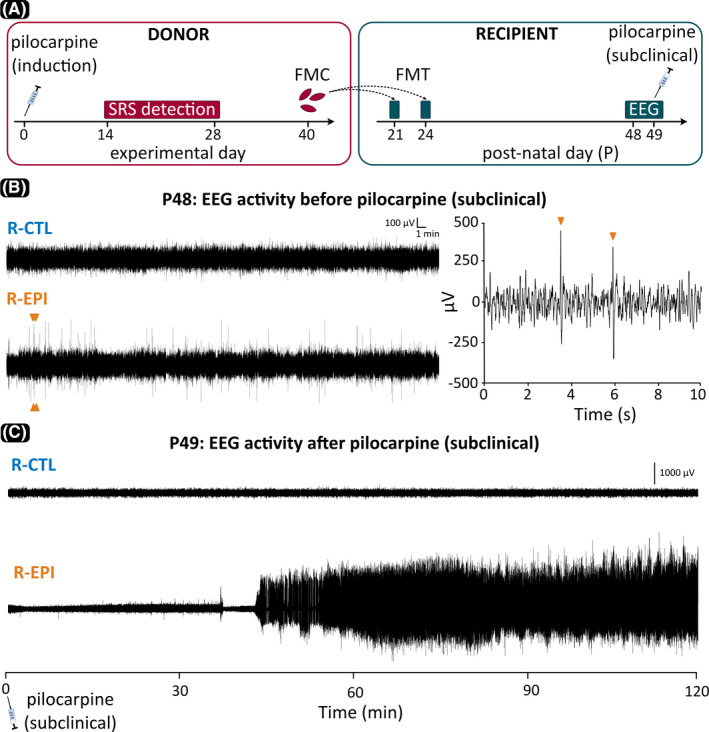
(A) Experimental design and timeline. Donor mice at experimental Day 0 were 7 weeks old. EEG, electroencephalography; FMC, fecal microbiota collection; FMT, fecal microbiota transplantation; SRS, spontaneous recurrent seizures. (B) Representative samples of EEG activity recorded 24 days after FMT and 1 day prior to subclinical pilocarpine challenge. Mice that received microbiota from epileptic donors (R‐EPI) showed frequent spikes, two of which, indicated by ocher arrowheads, are also shown in the expanded segment to the right. (C) Paroxysmal EEG activity following subclinical pilocarpine administration in an R‐EPI mouse versus a normal EEG trace in a subject that received microbiota from control donors (R‐CTL)
2.1.1. Detection of spontaneous recurrent seizures
Following the injections, all animals were visually monitored for 2 weeks (8 h per day) to detect convulsive seizures, that is, the spontaneous recurrent seizures (SRSs) typical of pilocarpine‐treated mice, as well as to rule out any other gross behavioral anomalies in both groups. D‐EPI mice showing overt SRSs were selected as microbiota donors together with normal D‐CTL animals.
2.1.2. Fecal sample collection
Fecal samples were obtained from 21 D‐EPI and 13 D‐CTL mice 40 days after pilocarpine/PBS administration. All sampling procedures were performed during the dark phase (ZT14–ZT16). Several stools were collected for each animal and placed in sterile tubes (1.5 ml) prefilled with 30% glycerol in PBS, then frozen and stored at −80℃ (details in Supporting Information).
2.2. Recipient group
Two weeks after arrival at the local animal facility, mice were mated and dams were treated with antibiotics (ampicillin 1 g/L, vancomycin .5 g/L, neomycin 1 g/L added to the autoclaved drinking water) from the 12th day of pregnancy until delivery, to limit the transfer of maternal microbes to pups.14 Newborn mice were kept with the mother until weaning at postnatal day 21 (P21).
2.2.1. Fecal transplantation
Upon weaning, male mice from eight different litters were randomly assigned to one of two recipient (R) groups. Animals in the R‐EPI group received microbiota from a single D‐EPI, epileptic mouse, whereas the R‐CTL group received microbiota from single D‐CTL mice (Figure 1A). For each R mouse, 50 mg of stool were resuspended in 1.25 ml of sterile saline solution. The suspension was centrifuged at 3000 × g for 15 min, and the precipitate was dissolved in saline (400 mg/ml) and used for transplantation.15
Mice were inoculated at ZT14–ZT16 via oral gavage (50 µl per animal) in sterile conditions at P21 and again at P24. Following fecal microbiota transplantation (FMT), mice were visually monitored for 14 days (4 h per day) to detect behavioral abnormalities, including convulsions.
2.2.2. Surgery and electroencephalographic recording
Two weeks after FMT, both R‐EPI and R‐CTL animals were surgically implanted with epidural recording electrodes (right ipsilateral frontoparietal bipolar derivation) for chronic electroencephalographic (EEG) monitoring. Ten days after surgery, all recipient animals were connected to the EEG acquisition setup and signals were amplified and digitized at a sampling frequency of 1 kHz (details in Supporting Information). Twenty baseline hours of EEG signals were collected for each animal and saved for offline analysis.
2.2.3. Analysis of EEG baseline recording
EEG signals obtained during the 20‐h‐long baseline recordings were digitally filtered (high‐pass at .5 Hz, low‐pass at 70 Hz, 50‐Hz notch filter) and examined for the presence of “spikes.” EEG spikes were defined as high‐voltage (>4 SD above background) positive or negative single deflections that lasted <50 ms. Each putative spike detected in a 2‐h period (ZT18–ZT20) was confirmed by an expert observer by visual inspection (Figure 1B).
2.2.4. Pilocarpine subclinical challenge
One day after baseline recordings, a subclinical dose of pilocarpine (260 mg/kg) was intraperitoneally injected in all recipient mice, and the clinical SE was EEG‐recorded and visually monitored for 120 min. Methyl‐scopolamine (1 mg/kg) was injected 30 min before the challenge to prevent peripheral cholinergic effects of pilocarpine. The onset of SE was established by behavioral observation and confirmed by EEG inspection (Figure 1C).
2.3. Statistical analysis
The Mann–Whitney U test was used to compare the number of single spikes between the two groups of recipient mice. The log‐rank test was used to compare the survival curve of SE between R‐EPI and R‐CTL groups. An alpha level of p < .05 was used to indicate statistically significant differences.
3. RESULTS
In baseline conditions, the visual inspection of 2 h of artifact‐free EEG revealed a higher number of single spikes in R‐EPI compared to R‐CTL (Mann–Whitney U = 79, p = .039; Figures 1B, 2A), potentially suggesting increased susceptibility to seizures in animals inoculated with microbiota derived from epileptic mice. On the other hand, no seizures were observed in either group during baseline recordings, and no significant differences were found in the signals’ power spectral density (data not shown). Following the administration of a subclinical dose of pilocarpine (260 mg/kg ip), R‐EPI mice were more prone to develop SE compared to R‐CTL mice (Figures 1C, 2B). In detail, 120 min after pilocarpine injection, ~50% of the R‐EPI mice entered SE (10/21), whereas only one mouse in the R‐CTL group showed the typical signs of SE (1/13) and only at a time when all but one R‐EPI mouse had already entered SE. In accordance with these observations, the log‐rank test showed a significant difference between the two recipient groups (χ2 = 5.78, p = .017), suggesting that microbiota derived from epileptic mice may lower the seizure threshold in healthy recipients (Figure 2B).
FIGURE 2.
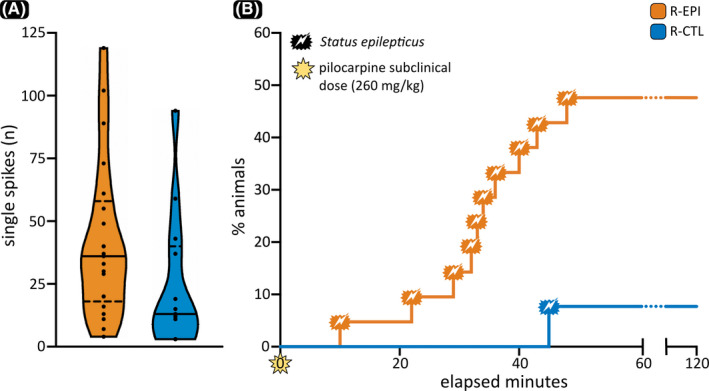
(A) Distribution of the number of single spike episodes detected over the 2‐h electroencephalographic recording in both recipient‐pilocarpine (R‐EPI) and recipient‐control (R‐CTL) mice. In each violin plot, the continuous horizontal line is the median, and the dashed lines represent the 25th and 75th percentiles. (B) Survival curves for R‐EPI and R‐CTL mice, showing the percentage of animals that entered status epilepticus during the 120 min following a subclinical dose of pilocarpine
4. DISCUSSION
A growing body of evidence underlines the importance of gut microbiota in the development and progression or improvement of CNS pathologies, including multiple sclerosis,4 Parkinson disease,5 and Alzheimer disease.6 Neuronal excitability is a cornerstone of brain function, and microbiota obtained from stressed animals have been shown to facilitate kindling‐induced seizures.10 Here we report an increased susceptibility to seizures in healthy mice after FMT from epileptic donors.
The so‐called “double‐hit” hypothesis states that an insult that in itself is not sufficient to lead to epilepsy could trigger a cascade of epileptogenic events once combined over time with a second subclinical stimulus. As previously shown, the microbiota is altered in both human8 and experimental epilepsy.9 We propose that the specific alterations caused by epilepsy in donors represent the first “hit” in recipient mice. In our experimental design, the transplantation of microbiota altered by chronic epilepsy was not sufficient to trigger seizures in the 3 weeks following FMT; however, the same animals were significantly more likely to develop SE upon the subsequent administration of a subclinical dose of pilocarpine (the second “hit”). In this respect, the pilocarpine challenge, previously shown to act on inflammatory pathways,16 could be regarded as similar to inflammatory conditions often associated with the onset of convulsive seizures, such as fever and influenza, in subjects who may have been previously sensitized.
The notion that brain excitability can be modulated via gastroenteric signaling is not new. The ketogenic diet has long been regarded as beneficial in drug‐resistant epilepsy in some children and adults.17 Only recently, however, the intestinal microbiota was recognized as an active contributor to this effect.7 Evidence from both experimental and clinical research suggests that probiotics, prebiotics, FMT, and nutrition‐based therapies targeting the gut–brain axis may provide safe and effective support in patients with several forms of neurological diseases,18 including epilepsy.19
The functional data provided in the present study, together with previously published observational data, highlight the necessity to identify alterations in the intestinal microbiota composition and to understand the molecular mechanisms of microbiota–gut–brain interactions in models of epilepsy. Ultimately, a better understanding of the role of microbiota in the gut–brain axis in epilepsy could lead to the identification of integrative therapeutic approaches.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
Supporting information
Supplementary Material
ACKNOWLEDGMENT
None.
Mengoni F, Salari V, Kosenkova I, Tsenov G, Donadelli M, Malerba G, et al. Gut microbiota modulates seizure susceptibility. Epilepsia. 2021;62:e153–e157. 10.1111/epi.17009
Francesca Mengoni and Valentina Salari contributed equally to this work.
Federico Del Gallo and Paolo Francesco Fabene are joint senior authors.
REFERENCES
- 1.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20(2):145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster JA, McVey Neufeld K‐A. Gut‐brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–12. [DOI] [PubMed] [Google Scholar]
- 3.Morais LH, Schreiber HL 4th, Mazmanian SK. The gut microbiota‐brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241–55. [DOI] [PubMed] [Google Scholar]
- 4.Freedman SN, Shahi SK, Mangalam AK. The “gut feeling”: breaking down the role of gut microbiome in multiple sclerosis. Neurotherapeutics. 2018;15(1):109–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheperjans F, Aho V, Pereira PAB, Koskinen K, Paulin L, Pekkonen E, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30(3):350–8. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y, Jaber V, Lukiw WJ. Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Front Cell Infect Microbiol. 2017;7:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti‐seizure effects of the ketogenic diet. Cell. 2018;173(7):1728–41.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong X, Liu XU, Chen C, Lin J, Li A, Guo K, et al. Alteration of gut microbiota in patients with epilepsy and the potential index as a biomarker. Front Microbiol. 2020;11:517797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Citraro R, Lembo F, De Caro C, Tallarico M, Coretti L, Iannone LF, et al. First evidence of altered microbiota and intestinal damage and their link to absence epilepsy in a genetic animal model, the WAG/Rij rat. Epilepsia. 2021;62(2):529–41. [DOI] [PubMed] [Google Scholar]
- 10.Medel‐Matus J‐S, Shin D, Dorfman E, Sankar R, Mazarati A. Facilitation of kindling epileptogenesis by chronic stress may be mediated by intestinal microbiome. Epilepsia Open. 2018;3(2):290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundberg R, Bahl MI, Licht TR, Toft MF, Hansen AK. Microbiota composition of simultaneously colonized mice housed under either a gnotobiotic isolator or individually ventilated cage regime. Sci Rep. 2017;7:42245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venø MT, Reschke CR, Morris G, Connolly NMC, Su J, Yan Y, et al. A systems approach delivers a functional microRNA catalog and expanded targets for seizure suppression in temporal lobe epilepsy. Proc Natl Acad Sci U S A. 2020;117(27):15977–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlócai MR, Tóth K, Watanabe M, Ledent C, Juhász G, Freund TF, et al. Redistribution of CB1 cannabinoid receptors in the acute and chronic phases of pilocarpine‐induced epilepsy. PLoS One. 2011;6(11):e27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy EA, King KY, Baldridge MT. Mouse microbiota models: comparing germ‐free mice and antibiotics treatment as tools for modifying gut bacteria. Front Physiol. 2018;9:1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Y‐L, Chen Y‐Q, Gong H, Li N, Wu K‐Q, Hu W, et al. Fecal microbiota transplantation ameliorates experimentally induced colitis in mice by upregulating AhR. Front Microbiol. 2018;9:1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabene PF, Mora GN, Martinello M, Rossi B, Merigo F, Ottoboni L, et al. A role for leukocyte‐endothelial adhesion mechanisms in epilepsy. Nat Med. 2008;14(12):1377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Yang YI, Wang Y, Tang H, Zhang F, Zhang Y, et al. Ketogenic diet for treatment of intractable epilepsy in adults: a meta‐analysis of observational studies. Epilepsia Open. 2018;3(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW, van Hilten JJ, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. 2020;10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iannone LF, Gómez‐Eguílaz M, Citaro R, Russo E. The potential role of interventions impacting on gut‐microbiota in epilepsy. Expert Rev Clin Pharmacol. 2020;13(4):423–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


