Abstract
Gold(I) catalysts are ideal for the activation of alkynes under very mild conditions. However, unlike allenes or alkenes, the triple bond of alkynes cannot be prochiral. In addition, the linear coordination displayed by gold(I) complexes places the chiral ligand far away from the substrate resulting in an inefficient transfer of chiral information. This poses a significant challenge for the achievement of high enantiocontrol in gold(I)‐catalyzed reactions of alkynes. Although considerable progress on enantioselective gold(I)‐catalyzed transformations has recently been achieved, the asymmetric activation of non‐prochiral alkyne‐containing small molecules still represents a great challenge. Herein we summarize recent advances in intra‐ and intermolecular enantioselective gold(I)‐catalyzed reactions involving alkynes, discussing new chiral ligand designs that lie at the basis of these developments. We also focus on the mode of action of these catalysts, their possible limitations towards a next‐generation of more efficient ligand designs. Finally, square planar chiral gold(III) complexes, which offer an alternative to chiral gold(I) complexes, are also discussed.
Keywords: alkynes, asymmetric catalysis, chiral ligands, cyclization, gold
Striking gold! Several asymmetric gold(I)‐catalyzed transformations have been discovered the last few years. Despite this major progress, the development of enantioselective reactions involving alkynes is still challenging. This minireview summarizes the most recent advances in new‐generation chiral ligand designs, focused on their mode of enantioinduction and limitations. Alternative chiral gold(III)‐catalyzed methodologies are also discussed.
1. Introduction
Gold(I) catalysts are the most efficient for the selective activation of alkynes and other C−C multiple bonds under exceptionally mild conditions.[1] Upon π‐activation, a broad range of nucleophiles can engage inter‐ or intramolecularly in numerous cyclizations and cycloadditions providing access to molecular complexity from simple starting materials in one single transformation.[2] By means of gold(I) catalysis, diverse total syntheses of naturally occurring compounds have been completed constructing complex carbon skeletons that would be otherwise difficult to access.[3] In contrast to the fast evolution of this research field, the development of broad scope enantioselective gold(I)‐catalyzed transformations has represented a more difficult task.[4] The linear binding geometry adopted by gold(I) complexes places the ancillary chiral ligand on the direct opposite side of the substrate resulting in an inefficient transfer of chiral information during the stereodetermining outer‐sphere nucleophilic addition. Moreover, the relatively free rotation around the L*‐Au and the Au–substrate bonds makes the fixation of the substrate in a chiral environment more challenging (Figure 1a).
Figure 1.
a) Limitations associated with enantioselective gold(I) catalysis. b) Three main approaches to enantioselective gold(I) catalysis.
Three main conceptual designs have shown successful in enantioselective gold(I) catalysis. The most studied system involves the use of axially chiral binuclear gold(I) complexes with either bisphosphines or diaminocarbene ligands (Figure 1b).[5] Thus, enantioselective intra‐ and intermolecular cyclopropanations,[6] cycloisomerizations of 1,n‐enynes,[7] hydrofunctionalizations of alkenes[8] and allenes[9] and other annulations[10] have been developed. On the other hand, highly modular, and readily available one‐point binding phosphoramidite ligands based on BINOL,[11] TADDOL[12] or SPINOL backbones have been particularly effective in the cyclization of allenes.[13] Analysis of the X‐ray crystal structures of these last complexes reveals the importance of the chiral amine moiety to provide a chiral environment around the reactive center.[14]
Equally successful for the activation of allenes[15] has been the synergistic use of chiral or non‐chiral gold(I) complexes with chiral counterions such as phosphates.[16] In this case the chiral environment is held next to the reactive center by the formation of tight ion pairs with the cationic gold complexes. However, this approach cannot be extended to the activation of terminal alkynes[17] as phosphates are basic enough to deprotonate the alkynyl proton, leading to the formation of unreactive gold(I) acetylides.[18] Although efficient for several processes, none of these approaches show sufficient broad scope. In addition, strategies for the enantioselective activation of alkynes, which are not prochiral, present an additional level of difficulty and thus, remain limited.
1.1. Scope and organization
In this review we summarize recent advances towards the development of new enantioselective gold(I)‐catalyzed systems with focus on the activation of alkynes (ca. 2014–). For the sake of completeness, selected earlier reactions will be discussed briefly. This review has been mainly divided into intra‐ and intermolecular transformations. In particular, we center the discussion on new ligand designs that have emerged spanning from highly π‐acidic α‐cationic phosphonites and chiral monophosphines to bifunctional ligands. In addition, the specific working mode of the new chiral ligands will be addressed. Finally, we will discuss important progress on gold(III)‐catalyzed enantioselective transformations.
The asymmetric activation of allenes,[4a, 4b, 4c, 4d, 4e, 4f] alkenes[4e, 4f] and the use of chiral counterions[16] have been reviewed elsewhere and will not be discussed herein.
2. Intramolecular Gold(I)‐Catalyzed Transformations
2.1. α‐Cationic phosphonites: enantioselective hydroarylation reactions
Intrigued by the physical and chiroptical properties of ortho‐fused polyaromatic molecules and their broad application in various fields of chemistry including asymmetric catalysis,[19] molecular machines,[20] and liquid crystal technologies[21] the group of Alcarazo recently reported the enantioselective synthesis of carbo[6]helicenes 2 via sequential gold(I)‐catalyzed hydroarylation of diynes 1 introducing α‐cationic[22] phosphonite ligands L1–4 (Scheme 1).[23]
Scheme 1.
Gold(I)‐catalyzed synthesis of chiral carbohelicenes. The counterion SbF6 − has been omitted for clarity.
In this ligand design, the modular TADDOL backbone, previously shown to be effective in enantioselective gold(I) catalysis,[12, 13, 14] is merged with an imidazolium unit to increase the π‐acceptor character of the ligands, and ultimately the π‐acidity of the corresponding gold complexes. Accordingly, analogous neutral phosphoramidite gold complexes did not display catalytic activity. Shortly after, improved reactivities and enantioselectivities were obtained replacing the imidazolium unit by more electron withdrawing 1,3‐dimesityl‐1,2,3‐triazolium and 1,4‐dimesityl‐1,2,4‐triazolium moieties in ligands L3 and L4.[24] Similarly, the synthesis of otherwise configurationally unstable chiral lower order carbo[4]helicenes 4 was also achieved via double gold(I)‐catalyzed hydroarylation reaction introducing appropriate substituents at the 1‐ and 12‐position to prevent racemization.[25] Finally, the Alcarazo group also developed the enantioselective gold(I)‐catalyzed hydroarylation of carbo[4]helicenes 5 containing a pendant substituted alkyne giving chiral carbo[5]helicenes 6.[26] However, in this transformation, the TADDOL‐based α‐cationic ligands L1–4 did not give satisfactory level of enantioselectivity. Hence, ligands of type L5 containing a 3,3’‐disubstituted binaphthol moiety were developed, maintaining the strong π‐acceptor character of the ligand.
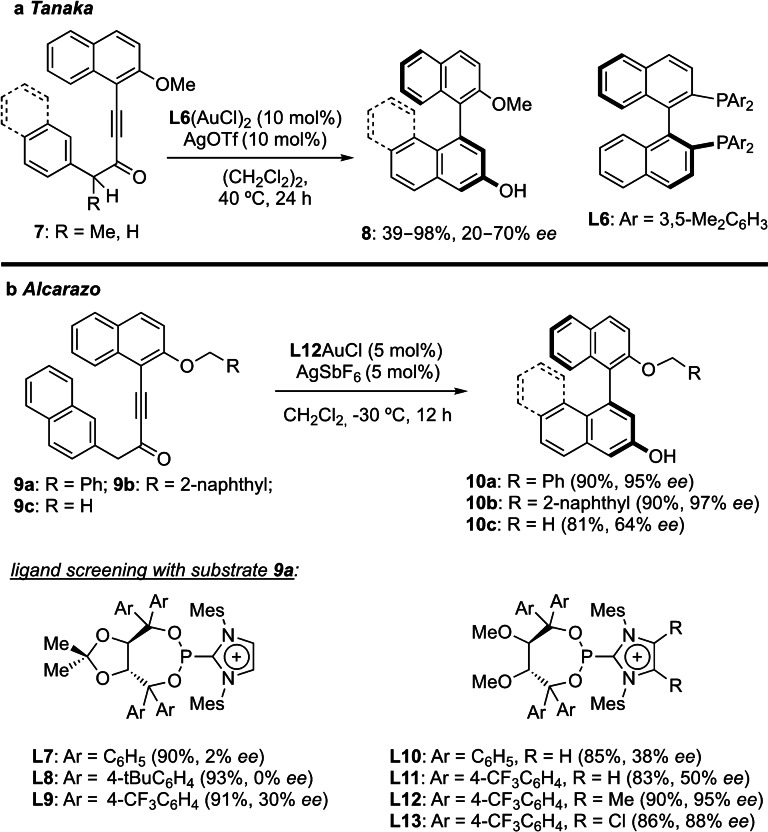
In the context of enantioselective gold(I)‐catalyzed hydroarylation reactions, Tanaka and his coworkers reported the synthesis of axially chiral 1,1’‐naphthyl‐2,3’‐diols 8 from alkynones 7 using xylyl‐binap‐supported digold(I) complexes, albeit with modest enantioselectivities (Scheme 2a).[27] In view of the general importance of atropoisomeric biaryls, found in several natural products,[27] organocatalysts[28] and in the backbone of chiral ligands for transition metal catalysis[29] the Alcarazo group applied the pronounced π‐acidity of their α‐cationic phosphinite gold(I) complexes on the synthesis of this type of biaryls (Scheme 2b).[30] Among the surveyed ligands, it was found that flexible methyl ethers on the TADDOL backbone were superior to cyclic acetonides (L7–9 vs L10–13). Moreover, additional substituents on the imidazolium ring narrow the chiral pocket and in turn lead to an increase in enantioselectivity. Finally, attractive non‐covalent π‐π interactions between substrate and ligand were found to play a crucial role in this transformation as product 10 b with an extended 2‐naphthyl substituent gave slightly higher enantioselectivities compared to 10 a, whereas 10 c containing a methyl group was obtained with modest levels of enantioselectivity.
Scheme 2.
Gold(I)‐catalyzed atroposelective hydroarylations.
2.2. Biaryl ligands
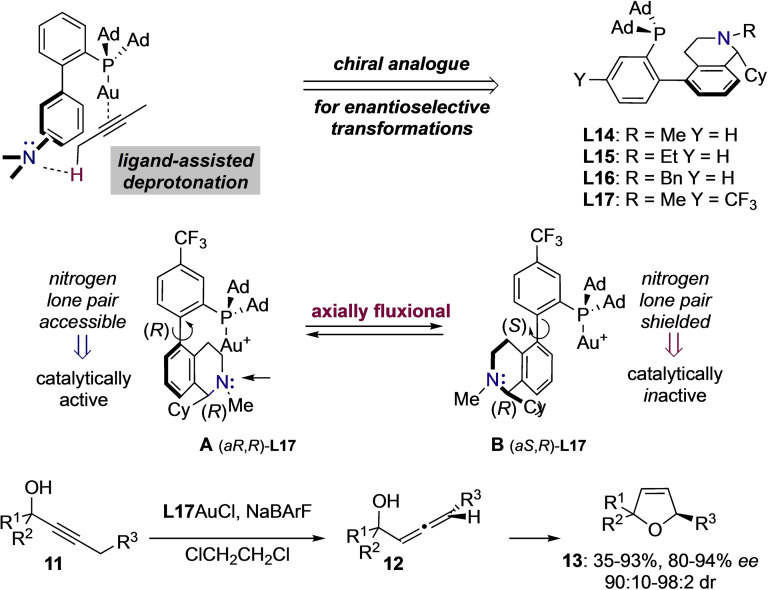
Bulky dialkyl biarylphosphine ligands have occupied a central role in the development and discovery of gold(I)‐catalyzed transformations.[31] Due to their electronic and steric properties this family of ligands is able to stabilize key carbo cationic‐ and carbene‐like intermediates giving rise to unprecedented reactions. Recently, the group of Liming Zhang showed that the particular geometry of biarylphosphine ligands represents an attractive platform to introduce remote basic units on the bottom aryl ring of the biphenyl scaffold allowing for a bifunctional activation mode and in turn, for the discovery of novel selectivities and reactivities, including the propargylic deprotonation of gold(I)‐activated alkynes.[32] This concept of ligand‐assisted activation has been analogously exploited in the development of asymmetric reactions as the substrate is predisposed in a chiral environment. As such, axially fluxional ligands L14–L17 containing a remote chiral 1,2,3,4‐tetrahydroisoquinoline have been designed by the same group for the sequential isomerization of propargylic alcohols 11 to allenylmethanol derivatives 12 and in situ stereospecific cyclization to form chiral 2,5‐disubstituted dihydrofuranes 13 (Scheme 3).[33]
Scheme 3.
Axially fluxional biarylphosphine ligands with remote basic chiral element.
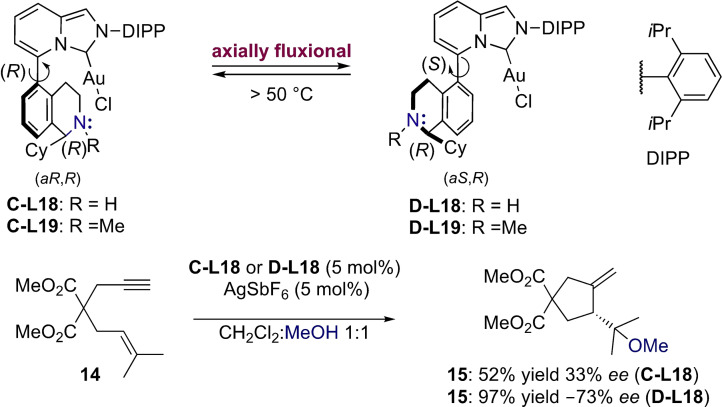
Due to the free rotation around the biaryl axis, the corresponding cationic complex of ligand L17 would exist as a mixture of isomers A and B. In the latter however, the cyclohexyl substituent points towards the catalytic site shielding the basic nitrogen and preventing the interaction with substrates of type 11. Thus, isomer B is catalytically inactive and the enantioselectivities in products 13 are attributed to reaction with isomer A. Shortly after, the strongly σ‐donating axially fluxional NHC‐analogues were introduced replacing the bulky PAd2 moiety by the rigid imidazo[1,5‐a]pyridine framework L18–L19.[34] Interestingly, the analogous atropoisomers C and D only interconvert at elevated temperatures (>50 °C) and were therefore used separately in various asymmetric transformations including the alkoxycyclization of 1,6‐enyne 14. Although corresponding cycloadducts 15 were obtained with good yields, the level of enantioselectivity was modest (Scheme 4).
Scheme 4.
Axially fluxional NHC‐gold(I) complexes with remote basic chiral element.
Very recently, the same group reported the asymmetric intramolecular net addition of propargylic C−H bonds to aliphatic aldehydes 16 for the formation of synthetically useful 5‐ and 6‐membered‐fused homopropargylic alcohols 17.[35] Remarkably, cooperative activation of the triple bond allowed for the preferential deprotonation of the propargylic C−H bond (pK a >30) by the remotely positioned basic unit (pK a ∼10) in the presence of much more acidic aldehyde α‐hydrogens (pK a ∼17) (Scheme 5).
Scheme 5.
a) Asymmetric gold(I)‐catalyzed construction of chiral homopropargylic alcohols 17. b) Ligand tetrahydroisoquinoline moiety conformations and relative energies.
To ensure the high efficiency of these transformations, including reaction yields, enantio‐ and diastereoselectivites, a DFT‐guided ligand design was performed (Scheme 5b). In a previously reported design (see Scheme 3), catalytically active, because nitrogen‐exposed, conformer A‐L17 was found to be less stable than the nitrogen‐buried conformer A’ due to the gauche interactions between the cyclohexyl and the N−Me substituents in the tetrahydroisoquinoline unit. This leads to an overall lower reactivity of L17AuCl. To minimize the destabilizing gauche interactions, the 1‐cyclohexyl substituent was replaced by a 1‐methyl, and a 8‐methyl moiety was introduced to maintain the pseudo axial orientation of the 1‐methyl group. DFT calculations confirmed that conformers E and E’ of the 1,8,N‐trimethyltetrahydroquinoline unit in L20 have comparable energies.
Our laboratory recently introduced a family of chiral modified JohnPhos‐type ligands bearing a C 2‐symmetric diaryl pyrrolidine at the 4’ position of the biphenyl scaffold 18–23 (Scheme 6).[36] The latter is employed as a geometrical holder positioning the chiral information directly next to the reactive center and thus, circumventing the inherent difficulty posed by the linear coordination in gold(I) catalysts. Moreover, the C 2‐symmetric chiral element prevents the formation of rotamers and the bulky dialkyl phosphine component of the ligand design blocks rotation around the C−P bond directing the P−Au−Cl axis towards the chiral source for efficient enantioinduction. These complexes found application in three different enantioselective cyclizations of 1,6‐arylenynes 24, 26 and 28 to give cyclopenta[b]naphthalenes 25, azabicyclo[4.1.0]hept‐4‐enes 27 and 1,2‐dihydronaphthalenes 29 in good yields and enantioselectivities. Additionally, products 29 were successfully employed in the first enantioselective total synthesis of three members of the carexane family of natural products 30 a‐c.
Scheme 6.
Dialkyl pyrrolidinylbiphenylphosphine ligands.
Surprisingly, although 1,6‐arylenynes 24 a and 28 a are structurally related, opposite enantioselectivities were observed in the corresponding products 25 a and 29 a, arising from the preferential reaction of the respective Si and Re faces of the alkenes (Scheme 7).
Scheme 7.
Enantioselective folding of enynes 24 a and 28 a. Two most relevant binding orientations (F and G) are shown. Energy values are given in kcal/mol relative to the most stable binding orientation.
We elucidated the mode of enantioinduction of the catalysts both experimentally and computationally as arising from attractive non‐covalent π‐π‐interactions between stereodirecting aromatic moieties of the substrates and the aromatic substituents of the chiral pyrrolidine in the ligand backbone. The absolute configuration in products 25 a and 29 a can originate from the combination of two binding orientations (F and G) of the substrate coordinated to gold through the alkyne and the reaction of the Re or Si face of the alkene. In agreement with our experimental results our computational work predicts that enyne 25 a reacts preferentially via binding orientation G through the Si face of the alkene leading to the (R) enantiomer of 29 a whereas the (S) absolute configuration in 29 a arises from reaction at the Re prochiral face of the alkene via binding orientation F. Thus, upon substrate recognition, the ligand induces a specific binding orientation that dictates the enantioselective folding inside the chiral pocket and ultimately, the absolute configuration in products 25 a and 29 a. Additional stabilization of the transition state was provided by interactions with the biphenyl scaffold of the ligand and in case of enyne 24 a aryl‐OMe interactions were also found to be stereocontrolling.
2.3. Chiral cavitand‐type ligands
The groups of Sollogoub/Fensterbank and Armspach, have developed different gold catalysts confined inside of cyclodextrins.[37, 38] Thus, using NHC‐capped β‐cyclodextrin gold(I) catalyst 31, the Sollogoub group achieved excellent enantioselectivities (up to 94–98 % ee) in the hydroxy‐ and methoxycyclization of 1,6‐enynes 14 (Scheme 8).[37c, 37d]
Scheme 8.
Enantioselective gold(I)‐catalyzed alkoxycyclization of E‐1,6‐enynes using NHC‐capped β‐cyclodextrin gold(I) catalysts.
Recently, a new family of chiral gold(I) complexes of type 33 have been designed in our laboratories based on cavitands.[39] These new complexes were easily prepared modularly from resorcin[4]arenes and commercially available chiral secondary amines, and proved to be active for the enantioselective alkoxycyclization of 1,6‐enynes, giving excellent yields and high enantiomeric ratios (Scheme 9). This protocol has been applied for the first total synthesis of carbazole alkaloid (+)‐mafaicheenamine C, and its enantiomer, assigning its absolute configuration as R, via 37. Computational studies and NCI plots identified attractive non‐covalent interactions in the reaction cavity as key factor to achieve high enantioselectivities.
Scheme 9.
Enantioselective gold(I)‐catalyzed alkoxycyclization of E‐1,6‐dienyne 34 using cavitand complex 33. Application in the total synthesis of (+)‐mafaicheenamine C, and its enantiomer.
2.4. Helically chiral monophosphines
The groups of Marinetti and Voiturez introduced helically chiral monodentate ligands with embedded meta‐fused phospholes.[40] The corresponding gold(I) complex 41 has been found to be catalytically active in the cyclization of N‐tethered 1,6‐enynes of type 38 to give fused tricyclic compounds 39 and azabicyclo[4.1.0]‐hept‐4‐enes 40 (Scheme 10).[41]
Scheme 10.
Helically chiral monophosphines in enantioselective gold(I) catalysis.
While modification of the ligand's helix have been beneficial in the enantioselective activation of allenes,[42] phosphathiohelicene‐supported gold(I) complexes 42–45 were applied in the formal [4+2] cycloaddition of 1,6‐arylenyne 47. Importantly, the thiopehene‐containing scaffold can be selectively brominated, allowing for the modular access to a broader family of complexes via Suzuki coupling.[43]
2.5. Chiral sulfinamide monophosphine ligands
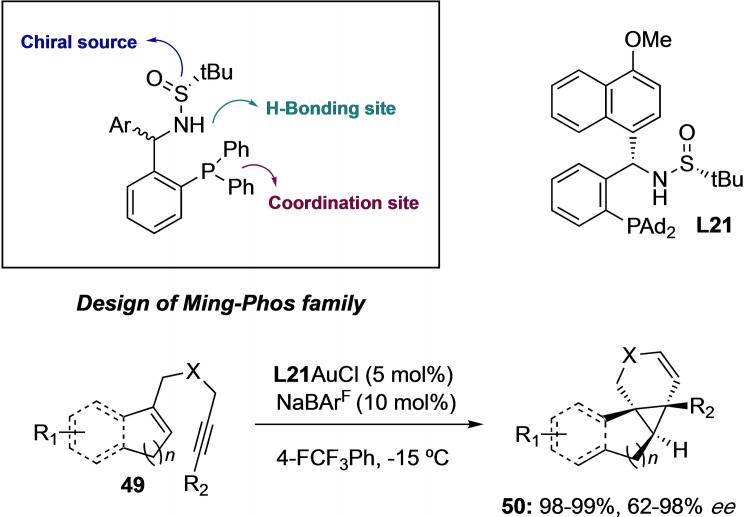
Recent progress relies on the design of chiral ligands such as sulfinamide monophosphines. The group of Juliang Zang has contributed to the development of the so‐called Ming‐Phos family of ligands from commercially available sources.[44] These bifunctional monophosphines proved to be efficient for various enantioselective inter‐ and intramolecular cycloadditions fixing the substrates via non‐covalent interactions (Scheme 11).[45] The first intramolecular gold(I)‐catalyzed enantioselective cyclopropanation of indenes and trisubstituted alkenes allowed for the efficient formation of [5‐3‐6] fused‐ring systems in products 50 (containing two vicinal all‐carbon quaternary stereogenic centers) by using Xiang‐Phos ligand L21.[46] A broad range of N‐tethered enynes 49 gave rise to the diastereo and enantioselective construction of fused tetracyclic compounds 50 in good to excellent enantioselectivities.
Scheme 11.
Gold(I)‐catalyzed Intermolecular cycloaddition of indenes and trisubstituted alkenes.
3. Intermolecular Gold(I) Catalyzed Reactions of Alkynes
Although significant progress has been made in the intramolecular gold(I)‐catalyzed reactions of alkynes to obtain complex polycyclic structures, the development of intermolecular transformations is still challenging.[47] In these transformations, two independent unsaturated substrates (alkynes and alkenes) can compete for the binding to the gold center having similar association constants.[48] Furthermore, inherently acidic gold catalysts can also promote side reactions such as the polymerization of alkenes.[49] The complexity of inducing enantioselectivity in these reactions increases when linear alkynes react in an intermolecular fashion.[50] Nonetheless, a wide range of gold‐catalyzed intermolecular transformations have been developed recently, opening the door to the construction of complex molecular frameworks from simple starting materials.[51]
3.1. Binuclear gold(I) complexes
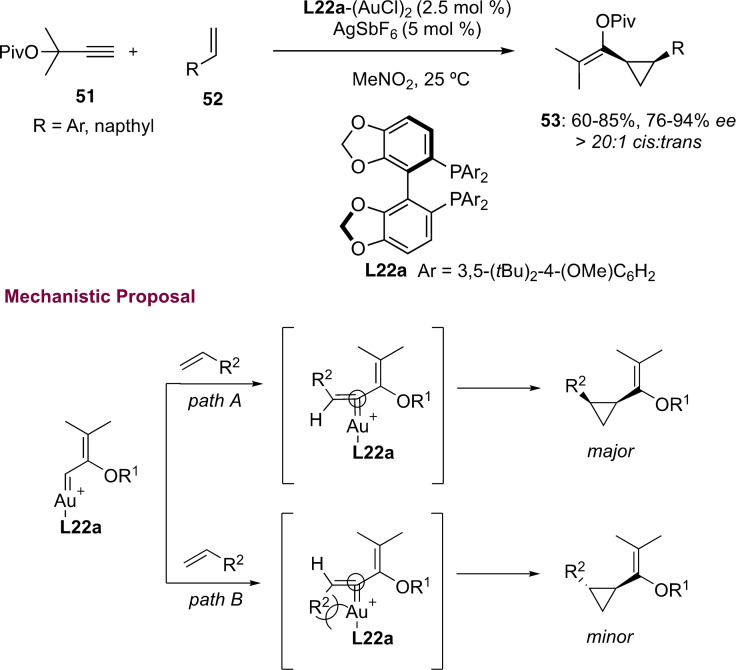
The use of DTBM‐SEGPHOS ligand (L22) in gold(I) catalysis was disclosed by the group of Toste in the intermolecular cyclopropanation of sterically hindered propargylic pivalate 51 and styrenes of type 52 (Scheme 12).[52] The steric hindrance of the aryl substituent at the alkene in path B was found to be key for high enantiodiscrimination. Moreover, the proposed transition state showed an outer sphere attack of the vinyl group in a 90° angle with respect to the gold(I) carbene in path A, which facilitates the enantiodiscrimination giving rise to cis‐cyclopropanes 53 in good to excellent enantioselectivies.
Scheme 12.
Enantioselective intermolecular cyclopropanation of alkenes using pivalate ester 51.
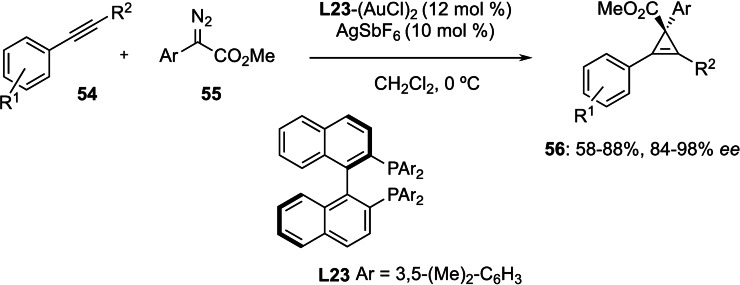
Later, as an extension of previous work using achiral silver catalysts,[53] the group of Davies developed the asymmetric cyclopropenation of internal alkynes 54 with aryldiazoacetates 55 using binuclear gold(I) catalyst based on the (S)‐xylylBINAP ligand (L23) (Scheme 13).[54] The resulting cyclopropenes 56, which are important synthons,[55] were obtained in moderate to excellent (up to 98 % ee) enantiomeric ratios.
Scheme 13.
Enantioselective cyclopropenation of internal alkynes 54.
Propiolic acid and some of its derivatives can also take part in a gold(I) catalyzed intermolecular reaction with 1,1‐di‐ or trisubstituted alkenes leading to α,β‐unsaturated δ‐lactones 59 by a formal [4+2] annulation, as demonstrated by group of Shin (Scheme 14).[56] Thus, upon the formation of the corresponding cyclopropyl gold carbene Int1, the more substituted carbon of the alkene is attacked intramolecularly by the propiolate moiety. Initially, when ligand (R)‐L22b was used α,β‐unsaturated δ‐lactones were obtained with a maximum of 65 % ee. Later, under slightly different conditions and using sodium dodecyl sulphate (SDS) as an anionic surfactant, lactones 59 were obtained with better enantioselectivities.[57]
Scheme 14.
Asymmetric gold(I)‐catalyzed intermolecular [4+2] annulation.
Cyclobutenes of type 64 are important structural motifs occurring in many natural products and in biologically relevant compounds.[58] Therefore, these carbocycles have been used as valuable synthons to access a diversity of scaffolds,[59] such as cyclobutanes and cyclopropanes.[60] Our group reported the gold(I)‐catalyzed regioselective formation of cyclobutenes 64 from terminal alkynes 60 with alkenes 61 (Scheme 15).[50a] The reaction proceeds satisfactorily using cationic gold(I) complexes 62 with sterically hindered tBuXPhos ligand. Formation of the unreactive σ,π‐(alkyne)digold(I) species was found as a secondary process in this transformation. Later, we found that cationic gold complexes 63 containing bulky and less acidic BAr4 F− as counterion, instead of SbF6 − (complex 62), are superior catalysts for these reactions due to the anion effects.[61] Aryl and cyclopropyl alkynes are required to ensure high reactivities. Electron‐rich alkenes, preferentially being bi‐ and tri‐substituted, are the best reaction counterparts for the most efficient [2+2] cycloadditions.
Scheme 15.
Gold(I)‐catalyzed [2+2] cycloaddition of terminal alkynes with alkenes.
Several strategies have been designed for the efficient preparation of cyclobutenes using Lewis acids,[62] transition metals,[63] and photochemical processes.[64] Among these strategies, the gold(I)‐catalyzed [2+2] cycloaddition of alkynes with alkenes is one of the most straightforward methodologies to afford enantioenriched 4‐membered carbocycles.
The first example of gold(I)‐catalyzed enantioselective intermolecular [2+2] cycloaddition was reported by the group of Lassaletta and Fernández using an axially chiral imidazoisoquinolin‐2‐ylidene as ligand.[65] However, cyclobutenes were obtained with low enantiomeric ratios (65 : 35 er).
In this context, our research group discovered that non‐C2 chiral Josiphos digold(I) precatalysts were optimal for the enantioselective intermolecular [2+2] cycloaddition of alkynes with alkenes (Scheme 16).[66] Enantioenriched cyclobutenes of type 64 were generally obtained in moderate to good enantiomeric ratios by cycloaddition of aryl alkynes 60 and α‐methyl styrenes catalyzed by catalyst 66, whereas Josiphos complex 67 allowed for the cycloaddition of trisubstituted alkenes. It was demonstrated that only one of the gold centers is directly involved in the activation of the alkyne, the second one is required to induce high enantioinduction. The rate‐determining step of this transformation was highly influenced by the electronic properties of the alkene. When less electron rich alkenes were used as the cycloaddition partners, as expected, the slowest step was found to be the C−C bond formation by an electrophilic addition of the alkyne‐gold(I) complex, while for more electron rich alkenes, the rate determining step is the associative ligand exchange reaction. The origin of the enantioselectivity is attributed to a combination of stabilizing π‐stacking interactions and unfavorable steric effects in the most favorable transition state. This intermolecular [2+2] cycloaddition was applied in the second generation asymmetric total synthesis of rumphellaone A from 65 a.[67]
Scheme 16.
Gold(I)‐catalyzed [2+2] cycloaddition of alkynes with alkenes. Catalytic cycle for the reaction of phenylacetylene with α‐methyl‐styrene and catalyst 66 and computed two possible transition states (TSR and TSS ). Application to the enantioselective total synthesis of rumphellaone A.
Binuclear Josiphos digold(I) complex 67 (Scheme 16) was found to be also effective for the enantioselective intermolecular thioallylation of propiolates with allyl sulfides (Scheme 17).[68] This intermolecular variant of the Claisen rearrangement led to the selective preparation of enantioenriched β‐thioacrylates 68 in moderated to excellent enantiomeric excesses. Mechanistic studies revealed that sulfonium‐induced asymmetric Claisen rearrangement minimizes the allyl dissociation giving rise to higher enantioselectivities. A Hammet analysis suggested the [3,3]‐sigmatropic rearrangement as turnover limiting step.
Scheme 17.
Gold(I)‐catalyzed enantioselective Thio‐Claisen rearrangement.
The group of Schmalz reported the enantioselective gold(I)‐catalyzed preparation of highly substituted furo[3,4‐d]tetrahydropyridazines 71 by using a chiral phosphine‐phosphite ligand L24,[69] previously developed by the same laboratory.[70] The tandem cyclization/intermolecular [3+3]‐cycloaddition process between 2‐(1‐alkynyl)‐2‐alken‐1‐ones 69 and azomethine imines 70 allows for the regio‐ and diastereoselective preparation of products 71 in mild conditions with high yields and excellent enantioselectivities (Scheme 18).
Scheme 18.
Enantioselective gold(I)‐catalyzed synthesis of furo[3,4‐d]tetrahydropyridazines 71.
3.2. Mononuclear gold(I) complexes
The gold(I)‐catalyzed oxidation of alkynes involves the formation of highly electrophilic α‐oxo gold(I) carbenes.[71] With the goal of stabilizing these intermediates, the group of Zhang introduced a bidentate P,N‐ligand which forms a tricoordinated gold(I) complex L25AuCl, providing additional electron density to the gold atom. Thus, stabilization of gold carbene intermediate H occurs via back donation and mesomeric cationic species H’ becomes less accessible (Scheme 19a).[72] On this basis, a chiral version of these P,N‐ligands (L25a–h) was developed for the intramolecular enantioselective oxidative cyclopropanation of 1,5‐enynes 72.[73] In this transformation, the activated alkyne reacts with 8‐methylquinoline 1‐oxide 73 to give the corresponding α‐oxo gold(I) carbene 74, which reacts with the pendant olefin to give bicyclo[3.1.0]hexan‐2‐ones 75 in good yields and enantioselectivities up to 93 %. However, product 75’ containing all‐carbon quaternary stereocenter at the cyclopropane was obtained with low enantiomeric excess (Scheme 19b).
Scheme 19.
Bidentate P,N‐ligands in enantioselective cyclopropanations of 1,5‐enynes.
The intermolecular [3+2] cycloaddition of 2‐(1‐alkynyl)‐2‐alken‐1‐ones 76 with 3‐alkenylindoles 77 was achieved in high enantiomeric ratios using Ming‐Phos ligand L26‐supported gold(I) catalysts, previously designed by the group of Juliang Zhang (Scheme 20).[74] A wide variety of enantioenriched substituted bicyclic furans 78 were obtained by the highly diastereoselective tandem heterocyclization/cycloaddition of readily available starting materials. The same chiral sulfinamide ligand L26 was later found to be efficient catalyst for the highly exo‐ and enantioselective synthesis of seven membered oxa‐bridged rings 80 in 80–98 % yield and with high exo selectivity (exo/endo up to 50 : 1) and up to 97 % ee.[75]
Scheme 20.
Enantioselective gold(I)‐catalyzed synthesis of indolyl‐substituted cyclopenta[c]furans 78 and oxa‐bridged benzocycloheptanes 80.
The asymmetric intermolecular tandem [3+3] cycloaddition of 2‐(1‐alkynyl)‐2‐alken‐1‐ones 76 with nitrones 81 was achieved using a gold(I) complex with Ming‐Phos ligand L26 (Scheme 21).[76] Both enantiomers of the furo[3,4‐d][1,2]oxazinesin product 82 a–b could be obtained in high enantioselectivities using either diastereomer of the chiral ligand. The proposed enantioinduction model relies on H‐bonding of the sulfinamide moiety with nitrones.
Scheme 21.
A gold(I)‐catalyzed asymmetric intermolecular tandem [3+3]‐cyclization reaction of 2‐(1‐alkynyl)‐2‐alken‐1‐ones 76 with nitrones 81.
3.3. Asymmetric counteranion‐directed catalysis
Recently, Marinetti and Guinchard reported a new strategy that uses a BINOL‐derived chiral phosphate counterion tethered to a monophosphine ligand L27 (Scheme 22)..[77] This design provides high enantioinduction in the tandem cycloisomerization/ nucleophilic addition of 2‐alkynylenones 83 at low catalyst loading. According to DFT calculations, the stereochemical control is driven by the geometrical constraints and molecular folding in the key intermediate that is stabilized by the phosphate anion group via ion‐pairing.
Scheme 22.
Gold(I)‐catalyzed asymmetric cycloisomerization/nucleophilic addition.
4. Enantioselective Gold(III)‐Catalyzed Transformations
Compared to homogeneous gold(I) catalysis, the use of high‐valent gold(III) catalysts remains underdeveloped.[78] This is mainly traced back to their high redox potential that facilitates reduction to Gold(I) or formation of gold(0) species.[79] The stabilization of gold(III) complexes is commonly achieved with weak π‐acceptor nitrogen‐containing ligands such as pyridines,[80] Schiff bases,[81] triazoles[82] or cyclometalated biphenyls.[83] The potential of chiral gold(III) catalysts lies in their square‐planar coordination geometry that brings the chiral information closer to the reactive center and therefore simplifies enantioinduction. The group of Toste reported the application of a chiral gold(III) catalyst 87 for the kinetic resolution of racemic 1,5‐enynes 86 affording chiral bicyclo[3.1.0]hexenes 88 and enantioenriched 1,5‐enynes 89 in the same transformation (Scheme 23a).[84] The potential of chiral gold(III) catalysts lies in their square‐planar coordination geometry that brings the chiral information closer to the reactive center and therefore simplifies enantioinduction. A similar chiral gold(III) complex 92 was used for the enantioselective gold(III)‐catalyzed Diels‐Alder reaction between 2,4‐dienals 90 and cyclopentadiene 91 (Scheme 23b).[85] DFT calculations suggest that the high regio‐ and enantioselectivities originate from attractive non‐covalent π‐π interactions between the 2‐chloro‐naphthyl substituent of the triazolium ligand and the proximal double bond of the substrate.
Scheme 23.
Enantioselective gold(III)‐catalyzed a) cyclization of 1,5‐enynes and b) Diels‐Alder reaction.
The group of Wong recently reported the synthesis and catalytic activity of new gold(III) complexes formed by reaction of cyclometalated oxazoline gold(III) dichloride complexes 95 with BINOL derivatives 94 (Scheme 24).[86] The BINOL moiety underwent an interesting axial‐to‐central chirality transfer[87] giving the C,O‐chelated gold(III) complexes 96. Although ca. 50 new 3,3’‐ and 6,6’‐substituted gold(III) complexes were synthesized, the carboalkoxylation of ortho‐alkynylbenzenaldehydes 97 in the presence of MeOH and catalytic amounts of D‐(+)‐10‐camphorsulfonic acid afforded indanone 98 with only modest 52 % yield and 41 % ee. Later, the same group found that replacing BINOL (complex 96) by simple biphenol, O,O’‐chelated 4,4’‐biphenol cyclometalated oxazoline gold(III) complexes 100 were obtained which catalyzed the interconversion of compound 99 to analogous indanone products 101 with good to excellent yields and enantioselectivities.[88]
Scheme 24.
O,O’‐cyclometalated Gold(III) complexes in enantioselective catalysis.
5. Conclusion
The stringencies imposed by the linear coordination usually adopted by gold(I) complexes have led to the development of new imaginative ligand designs to achieve good to excellent enantioselectivity levels in reactions involving alkynes as substrates. However, often limitations exist with terminally unsubstituted alkynes in reactions in which the alkyne‐Au(I) complex becomes the electrophile. Thus, even under the optimized conditions, intermolecular reactions of terminal alkynes leading to α,β‐unsaturated δ‐lactones or cyclobutenes developed by the group of Shin[55] and our own group,[64] respectively, proceed with 90 % or higher enantiomeric excess in only a few cases. A new generation of gold(I) catalysts should still be developed to achieve consistently high enantioselectivities and broad scope in cyclization and cycloaddition reactions. Finally, the examples shown in section 4 clearly highlight that there is much room for the development of new families of chiral gold(III)‐catalysts for the enantioselective activation of alkynes.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Giuseppe Zuccarello was born in Basel (Switzerland). He completed his BSc in Chemistry at the University of Basel and obtained his master's degree in Chemistry at the Swiss Federal Institute of Technology in Zürich (ETHZ) working on stereodivergent total synthesis of Δ9‐tetrahydrocannabinols under the supervision of Prof. Erick M. Carreira. In 2020 he completed his doctoral studies at the Institute of Chemical Research of Catalonia (ICIQ) in Tarragona (Spain) under the supervision of Prof. Antonio M. Echavarren working on the design and synthesis of new chiral gold(I) complexes and their application in catalysis. In the same year he joined the research group of Prof. Gregory C. Fu at the California Institute of Technology (Caltech) in Pasadena (USA) as a SNSF postdoctoral fellow. His research interests currently focus on copper‐ and nickel‐catalyzed enantioconvergent cross couplings.

Biographical Information
Imma Escofet was born in La Granada, in the Alt Penedès region of Catalonia (Barcelona, Spain). She studied Chemistry at the University of Barcelona (UB) and did her Bachelor Thesis at the University of Aberdeen (Scotland, UK) under the supervision of Prof. Laurent Trembleau (2010). Then, she joined the research group of Prof. Antonio M. Echavarren at the Institute of Chemical Research of Catalonia (ICIQ) in Tarragona, as a laboratory engineer where she also completed her PhD studies (2020) working on computational mechanistic studies of gold(I) catalysis and design of new chiral ligands.

Biographical Information
Ulysse Caniparoli received his dual Engineering degree in Chemistry and MSc. in Biochemistry from the Ecole Nationale Supérieure de Chimie de Montpellier, France. He is currently a Ph.D. candidate in ICIQ, Spain, under the supervision of Prof. Antonio M. Echavarren. His research is focused on development of new ligand designs for gold(I) asymmetric catalysis.

Biographical Information
Prof. Dr Antonio M. Echavarren was born in Bilbao (Spain). He received his PhD at the Universidad Autónoma de Madrid (UAM, 1982). After a postdoctoral stay in Boston College and Colorado State University, he joined the Institute of Organic Chemistry of the CSIC in Madrid. In 1992 he returned to the UAM as a Professor of Organic Chemistry and in 2004 he moved to Tarragona as a Group Leader at the Institute of Chemical Research of Catalonia (ICIQ). In 2013 he got an ERC Adv. Grant to develop gold catalysis and in 2019 a second ERC Adv. Grant to develop new catalysts for the biomimetic cyclization of unsaturated substrates. He received the 2004 Janssen‐Cylag Award in Organic Chemistry and the 2010 Medal of the Royal Spanish Chemical Society and an Arthur C. Cope Scholar Award from the ACS. He is the President of the Spanish Royal Society of Chemistry (RSEQ).

Acknowledgements
We acknowledge the European Research Council (Advanced Grant No. 835080), Ministerio de Ciencia e Innovación (PID2019‐104815GB−I00), Severo Ochoa Excellence Accreditation2020‐2023 (CEX2019‐000925‐S), the AGAUR (2017 SGR 1257), and CERCA Program/Generalitat de Catalunya for financial support.
G. Zuccarello, I. Escofet, U. Caniparoli, A. M. Echavarren, ChemPlusChem 2021, 86, 1283.
References
- 1.
- 1a.Hashmi A. S. K., Gold Bull. 2003, 36, 3–9; [Google Scholar]
- 1b.Hashmi A. S. K., Gold Bull. 2004, 37, 51–65; [Google Scholar]
- 1c.Hashmi A. S. K., Chem. Rev. 2007, 107, 3180–3211; [DOI] [PubMed] [Google Scholar]
- 1d.Fürstner A., Davies P. W., Angew. Chem. Int. Ed. 2007, 46, 3410–3449; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 3478–3519; [Google Scholar]
- 1e.Jiménez-Núñez E., Echavarren A. M., Chem. Rev. 2008, 108, 3326–3350; [DOI] [PubMed] [Google Scholar]
- 1f.Fürstner A., Chem. Soc. Rev. 2009, 38, 3208–3221; [DOI] [PubMed] [Google Scholar]
- 1g.Shapiro N. D., Toste F. D., Synlett 2010, 5, 675–691; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1h.Obradors C., Echavarren A. M., Acc. Chem. Res. 2014, 47, 902–912; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1i.Fensterbank L., Malacria M., Acc. Chem. Res. 2014, 47, 953–965; [DOI] [PubMed] [Google Scholar]
- 1j.Dorel R., Echavarren A. M., Chem. Rev. 2015, 115, 9028–9072; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1k.Pflästerer D., Hashmi A. S. K., Chem. Soc. Rev. 2016, 45, 1331–1367; [DOI] [PubMed] [Google Scholar]
- 1l.Boyle J. W., Zhao Y., Chan P. W. H., Synthesis 2018, 50, 1402–1416; [Google Scholar]
- 1m.Zuccarello G., Zanini M., Echavarren A. M., Isr. J. Chem. 2020, 60, 360–372; [Google Scholar]
- 1n.Mato M., Franchino A., Garcĺa-Morales C., Echavarren A. M., Chem. Rev. 2020, 121, 8613–8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.
- 2a.Lee Y. T., Kang Y. K., Chung Y. K., J. Org. Chem. 2009, 74, 7922–7934; [DOI] [PubMed] [Google Scholar]
- 2b.Obradors C., Leboeuf D., Aydin J., Echavarren A. M., Org. Lett. 2013, 15, 1576–1579; [DOI] [PubMed] [Google Scholar]
- 2c.Escribano-Cuesta A., Pérez-Galán P., Herrero-Gómez E., Sekine M., Braga A. A. C., Maseras F., Echavarren A. M., Org. Biomol. Chem. 2012, 10, 6105–6111. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a.Day D. P., Chan P. W. H., Adv. Synth. Catal. 2016, 358, 1368–1384; [Google Scholar]
- 3b.Mayans J. G., Armengol-Relats H., Calleja P., Echavarren A. M., Isr. J. Chem. 2018, 58, 639–658. [Google Scholar]
- 4.
- 4a.Widenhoefer R. A., Chem. Eur. J. 2008, 14, 5382–5391; [DOI] [PubMed] [Google Scholar]
- 4b.Sengupta S., Shi X., ChemCatChem 2010, 2, 609–619; [Google Scholar]
- 4c.Pradal A., Toullec P. Y., Michelet V., Synthesis 2011, 10, 1501–1514; [Google Scholar]
- 4d.Wang Y. M., Lackner A. D., Toste F. D., Acc. Chem. Res. 2014, 47, 889–901; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4e.Zi W., Toste F. D., Chem. Soc. Rev. 2016, 45,4567–4589; [DOI] [PubMed] [Google Scholar]
- 4f.Li Y., Li W., Zhang J., Chem. Eur. J. 2017, 23, 467–512; [DOI] [PubMed] [Google Scholar]
- 4g.Jiang J. J., Wong M. K., Chem. Asian J. 2021, 16, 364–377. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a.Bartolomé C., García-Cuadrado D., Ramiro Z., Espinet P., Inorg. Chem. 2010, 49, 9758–9764; [DOI] [PubMed] [Google Scholar]
- 5b.Wang Y. M., Kuzniewski C. N., Rauniyar V., Hoong C., Toste F. D., J. Am. Chem. Soc. 2011, 133, 12972–12975; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5c.Niemeyer Z. L., Pindi S., Khrakovsky D. A., Kuzniewski C. N., Hong C. M., Joyce L. A., Sigman M. S., Toste F. D., J. Am. Chem. Soc. 2017, 139, 12943–12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.
- 6a.Johansson M. J., Gorin D. J., Staben S. T., Toste F. D., J. Am. Chem. Soc. 2005, 127, 18002–18003; [DOI] [PubMed] [Google Scholar]
- 6b.Watson I. D. G., Ritter S., Toste F. D., J. Am. Chem. Soc. 2009, 131, 2056–2057; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6c.Briones J. F., Davies H. M. L., J. Am. Chem. Soc. 2012, 134, 11916–11919. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a.Muñoz M. P., Adrio J., Carretero J. C., Echavarren A. M., Organometallics 2005, 24, 1293–1300; [Google Scholar]
- 7b.Chao C. M., Beltrami D., Toullec P. Y., Michelet V., Chem. Commun. 2009, 45, 6988–6990; [DOI] [PubMed] [Google Scholar]
- 7c.Chao C. M., Vitale M. R., Toullec P. Y., Genêt J. P., Michelet V., Chem. Eur. J. 2009, 15, 1319–1323; [DOI] [PubMed] [Google Scholar]
- 7d.Gawade S. A., Bhunia S., Liu R.-S., Angew. Chem. Int. Ed. 2012, 51, 7835–7838; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 7955–7958. [Google Scholar]
- 8.
- 8a.Zhang Z., Du Lee S., Widenhoefer R. A., J. Am. Chem. Soc. 2009, 131, 5372–5373; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8b.Mukherjee P., Widenhoefer R. A., Angew. Chem. Int. Ed. 2012, 51, 1405–1407.; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 1434–1436; [Google Scholar]
- 8c.Du Lee S., Timmerman J. C., Widenhoefer R. A., Adv. Synth. Catal. 2014, 356, 3187–3192; [Google Scholar]
- 8d.Bandini M., Bottoni A., Chiarucci M., Cera G., Pietro Miscione G., J. Am. Chem. Soc. 2012, 134, 20690–20700. [DOI] [PubMed] [Google Scholar]
- 9.Miles D. H., Veguillas M., Toste F. D., Chem. Sci. 2013, 4, 3427–3431. [Google Scholar]
- 10.
- 10a.Zhou G., Liu F., Zhang J., Chem. Eur. J. 2011, 17, 3101–3104; [DOI] [PubMed] [Google Scholar]
- 10b.Huang L., Yang H. B., Zhang D. H., Zhang Z., Tang X. Y., Xu Q., Shi M., Angew. Chem. Int. Ed. 2013, 52, 6767–6771; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 6899–6903; [Google Scholar]
- 10c.Guo R., Li K. N., Liu B., Zhu H. J., Fan Y. M., Gong L. Z., Chem. Commun. 2014, 50, 5451–5454; [DOI] [PubMed] [Google Scholar]
- 10d.Sota Y., Yamamoto M., Murai M., Uenishi J., Uemura M., Chem. Eur. J. 2015, 21, 4398–4404; [DOI] [PubMed] [Google Scholar]
- 10e.Zi W., Wu H., Toste F. D., J. Am. Chem. Soc. 2015, 137, 3225–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso I., Trillo B., López F., Montserrat S., Ujaque G., Castedo L., Lledós A., Mascareñas J. L., J. Am. Chem. Soc. 2009, 131, 13020–13030. [DOI] [PubMed] [Google Scholar]
- 12.Teller H., Corbet M., Mantilli L., Gopakumar G., Goddard R., Thiel W., Fürstner A., J. Am. Chem. Soc. 2012, 134, 15331–15342. [DOI] [PubMed] [Google Scholar]
- 13.
- 13a.Luzung M. R., Mauleo P., Toste F. D., J. Am. Chem. Soc. 2007, 129, 12402–12403; [DOI] [PubMed] [Google Scholar]
- 13b.Suárez-Pantiga S., Hernández-Díaz C., Rubio E., González J. M., Angew. Chem. Int. Ed. 2012, 51, 11552–11555; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 11720–11723; [Google Scholar]
- 13c.Wang Y., Zhang P., Liu Y., Xia F., Zhang J., Chem. Sci. 2015, 6, 5564–5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teller H., Flügge S., Goddard R., Fürstner A., Angew. Chem. Int. Ed. 2010, 49, 1949–1953; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 1993–1997. [Google Scholar]
- 15.Hamilton G. L., Kang E. J., Mba M., Toste F. D., Science 2007, 317, 496–499. [DOI] [PubMed] [Google Scholar]
- 16.Inamdar S. M., Konala A., Patil N. T., Chem. Commun. 2014, 50, 15124–15135. [DOI] [PubMed] [Google Scholar]
- 17.Raducan M., Moreno M., Bour C., Echavarren A. M., Chem. Commun. 2012, 48, 52–54. [DOI] [PubMed] [Google Scholar]
- 18.Ferrer S., Echavarren A. M., Organometallics 2018, 37, 781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.
- 19a.Takenaka N., Chen J., Captain B., Sarangthem R. S., Chandrakumar A., J. Am. Chem. Soc. 2010, 132, 4536–4537; [DOI] [PubMed] [Google Scholar]
- 19b.Saleh N., Shen C., Crassous J., Chem. Sci. 2014, 5, 3680–3694. [Google Scholar]
- 20.Chen W. C., Lee Y. W., Chen C. T., Org. Lett. 2010, 12, 1472–1475. [DOI] [PubMed] [Google Scholar]
- 21.Saito N., Kanie K., Matsubara M., Muramatsu A., Yamaguchi M., J. Am. Chem. Soc. 2015, 137, 6594–6601. [DOI] [PubMed] [Google Scholar]
- 22.
- 22a.Alcarazo M., Chem. Eur. J. 2014, 20, 7868–7877; [DOI] [PubMed] [Google Scholar]
- 22b.Alcarazo M., Acc. Chem. Res. 2016, 49, 1797–1805. [DOI] [PubMed] [Google Scholar]
- 23.González-Fernández E., Nicholls L. D. M., Schaaf L. D., Farès C., Lehmann C. W., Alcarazo M., J. Am. Chem. Soc. 2017, 139, 1428–1431. [DOI] [PubMed] [Google Scholar]
- 24.Nicholls L. D. M., Marx M., Hartung T., González-Fernández E., Golz C., Alcarazo M., ACS Catal. 2018, 8, 6079–6085. [Google Scholar]
- 25.Alcarazo M., Hartung T., Machleid R., Simon M., Golz C., Angew. Chem. Int. Ed. 2020, 59, 5660–5664; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 5709–5713. [Google Scholar]
- 26.Redero P., Hartung T., Zhang J., Nicholls L. D. M., Zichen G., Simon M., Golz C., Alcarazo M., Angew. Chem. Int. Ed. 2020, 59, 23527–23531; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 23733–23737. [Google Scholar]
- 27.Satoh M., Shibata Y., Kimura Y., Tanaka K., Eur. J. Org. Chem. 2016, 4465–4469. [Google Scholar]
- 28.Akiyama T., Chem. Rev. 2007, 107, 5744–5758. [DOI] [PubMed] [Google Scholar]
- 29.
- 29a.Teichert J. F., Feringa B. L., Angew. Chem. Int. Ed. 2010, 49, 2486–2528; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 2538–2582; [Google Scholar]
- 29b.Berthod M., Mignani G., Woodward G., Lemaire M., Chem. Rev. 2005, 105, 1801–1836. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J., Simon M., Golz C., Alcarazo M., Angew. Chem. Int. Ed. 2020, 59, 5647–5650; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 5696–5699. [Google Scholar]
- 31.Zuccarello G., Zanini M., Echavarren A. M., Isr. J. Chem. 2020, 60, 360–372. [Google Scholar]
- 32.
- 32a.Wang Y., Wang Z., Li Y., Wu G., Cao Z., Zhang L., Nat. Commun. 2014, 5, 3470; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32b.Wang Z., Wang Y., Zhang L., J. Am. Chem. Soc. 2014, 136, 8887–8890; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32c.Li X., Wang Z., Ma X., Liu P. N., Zhang L., Org. Lett. 2017, 19, 5744–5747; [DOI] [PubMed] [Google Scholar]
- 32d.Wang Z., Ying A., Fan Z., Hervieu C., Zhang L., ACS Catal. 2017, 7, 3676–3680; [Google Scholar]
- 32e.Li T., Zhang L., J. Am. Chem. Soc. 2018, 140, 17439–17443; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32f.Liao S., Porta A., Cheng X., Ma X., Zanoni G., Zhang L., Angew. Chem. Int. Ed. 2018, 57, 8250–8254; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 8382–8386; [Google Scholar]
- 32g.Wang H., Li T., Zheng Z., Zhang L., ACS Catal. 2019, 9, 10339–10342; [Google Scholar]
- 32h.Li T., Yang Y., Li B., Bao X., Zhang L., Org. Lett. 2019, 21, 7791–7794. [DOI] [PubMed] [Google Scholar]
- 33.Cheng X., Wang Z., Quintanilla C. D., Zhang L., J. Am. Chem. Soc. 2019, 141, 3787–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J. Q., Liu Y., Wang X. W., Zhang L., Organometallics 2019, 38, 3931–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li T., Cheng X., Qian P., Zhang L., Nat. Catal. 2021, 4, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuccarello G., Mayans J. G., Escofet I., Scharnagel D., Kirillova M. S., Pérez-Jimeno A. H., Calleja P., Boothe J. R., Echavarren A. M., J. Am. Chem. Soc. 2019, 141, 11858–11863. [DOI] [PubMed] [Google Scholar]
- 37.
- 37a.Guitet M., Zhang P., Marcelo F., Tugny C., Jiménez-Barbero J., Buriez O., Amatore C., Mouriès-Mansuy V., Goddard J.-P., Fensterbank L., Zhang Y., Roland S., Ménand M., Sollogoub M., Angew. Chem. Int. Ed. 2013, 52, 7213–7218; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 7354–7359; [Google Scholar]
- 37b.Zhang P., Tugny C., Meijide Suárez J., Guitet M., Derat E., Vanthuyne N., Zhang Y., Bistri O., Mouriès-Mansuy V., Ménand M., Roland S., Fensterbank L., Sollogoub M., Chem 2017, 3, 174–191; [Google Scholar]
- 37c.Tugny C., del Rio N., Koohgard M., Vanthuyne N., Lesage D., Bijouard K., Zhang P., Meijide Suárez J., Roland S., Derat E., Bistri-Aslanoff O., Sollogoub M., Fensterbank L., Mouriès-Mansuy V., ACS Catal. 2020, 10, 5964–5972; [Google Scholar]
- 37d.Zhu X., Xu G., Chamoreau L. M., Zhang Y., Mouriès-Mansuy V., Fensterbank L., Bistri-Aslanoff O., Roland S., Sollogoub M., Chem. Eur. J. 2020, 26, 15901–15909. [DOI] [PubMed] [Google Scholar]
- 38.
- 38a.Kaya Z., Andna L., Matt D., Bentouhami E., Djukic J. P., Armspach D., Chem. Eur. J. 2018, 24, 17921–17926; [DOI] [PubMed] [Google Scholar]
- 38b.Kaya Z., Andna L., Matt D., Bentouhami E., Djukic J. P., Armspach D., Eur. J. Org. Chem. 2019, 4528–4537. [Google Scholar]
- 39.Martín-Torres I., Ogalla G., Yang J-M., Rinaldi A., Echavarren A. M., Angew. Chem. Int. Ed. 2021, 60, 9339—9344; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 9425–9430.. [Google Scholar]
- 40.
- 40a.Aillard P., Retailleau P., Voituriez A., Marinetti A., Chem. Commun. 2014, 50, 2199–2201; [DOI] [PubMed] [Google Scholar]
- 40b.Yavari K., Moussa S., Ben Hassine B., Retailleau P., Voituriez A., Marinetti A., Angew. Chem. Int. Ed. 2012, 51, 6748–6752; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 6852–6856; [Google Scholar]
- 40c.Yavari K., Retailleau P., Voituriez A., Marinetti A., Chem. Eur. J. 2013, 19, 9939–9947. [DOI] [PubMed] [Google Scholar]
- 41.Yavari K., Aillard P., Zhang Y., Nuter F., Retailleau P., Voituriez A., Marinetti A., Angew. Chem. Int. Ed. 2014, 53, 861–865; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014,126, 880–884. [Google Scholar]
- 42.
- 42a.Aillard P., Retailleau P., Voituriez A., Marinetti A., Chem. Eur. J. 2015, 21, 11989–11993; [DOI] [PubMed] [Google Scholar]
- 42b.Magné V., Sanogo Y., Demmer C. S., Retailleau P., Marinetti A., Guinchard X., Voituriez A., ACS Catal. 2020, 10, 8141–8148. [Google Scholar]
- 43.Aillard P., Dova D., Magné V., Retailleau P., Cauteruccio S., Licandro E., Voituriez A., Marinetti A., Chem. Commun. 2016, 52, 10984–10987. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z. M., Chen P., Li W., Niu Y., Zhao X. L., Zhang J., Angew. Chem. Int. Ed. 2014, 53, 4350–4354; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 4439–4443. [Google Scholar]
- 45.Wang Y., Zhang P., Di X., Dai Q., Zhang Z.-M., Zhang J., Angew. Chem. Int. Ed. 2017, 56, 15905–15909; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 16121–16125. [Google Scholar]
- 46.Zhang P.-C., Wang Y., Zhang Z.-M., Zhang J., Org. Lett. 2018, 20, 7049–7052. [DOI] [PubMed] [Google Scholar]
- 47.
- 47a.Muratore M. E., Homs A., Obradors C., Echavarren A. M., Chem. Asian J. 2014, 9, 3066–3082; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47b.García-Morales C., Echavarren A. M., Synlett 2018, 29, 2225–2237. [Google Scholar]
- 48.
- 48a.Brown T. J., Dickens M. G., Widenhoefer R. A., Chem. Commun. 2009, 6451–6453; [DOI] [PubMed] [Google Scholar]
- 48b.Brown T. J., Widenhoefer R. A., J. Organomet. Chem. 2011, 696, 1216–1220; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48c.Brooner R. E. M., Widenhoefer R. A., Angew. Chem. Int. Ed. 2013, 52, 11714–11724; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 11930–11941. [Google Scholar]
- 49.Urbano J., Hormigo A. J., de Frémont P., Nolan P. S., Díaz-Requejo M. M., Pérez P. J., Chem. Commun. 2008, 4, 759–761. [DOI] [PubMed] [Google Scholar]
- 50.
- 50a.Francos J., Grande-Carmona F., Faustino H., Iglesias-Sigüenza J., Díez E., Alonso I., Fernández R., Lassaletta J. M., López F., Ujaque G., Chem. Eur. J. 2013, 19, 15248–15260;24114995 [Google Scholar]
- 50b.Li G. H., Zhou W., Li W., Bi Q. W., Wang Z., Zhao G. Z., Hu W. X., Chen Z., Chem. Commun. 2013, 49, 4770–4772; [DOI] [PubMed] [Google Scholar]
- 50c.Varela I., Faustino H., Díez E., Iglesias-Sigüenza J., Grande-Carmona F., Fernández R., Lassaletta J. M., Mascareñas J. L., López F., ACS Catal. 2017, 7, 2397–2402. [Google Scholar]
- 51.
- 51a.López-Carrillo V., Echavarren A. M., J. Am. Chem. Soc. 2010, 132, 9292–9294; [DOI] [PubMed] [Google Scholar]
- 51b.De Orbe M. E., Ameno L., Kirillova M. S., Wang Y., Maseras F., Echavarren A. M., J. Am. Chem. Soc. 2017, 139, 10302–10311; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51c.De Orbe M. E., Echavarren A. M., J. Org. Chem. 2018, 22, 2740–2752; [Google Scholar]
- 51d.Frey W., Hashmi A. S. K., Blanco M. C., Kurpejovic E., Bats J. W., Adv. Synth. Catal. 2006, 348, 709–713; [Google Scholar]
- 51e.Huguet N., Lebœuf D., Echavarren A. M., Chem. Eur. J. 2013, 19, 6581–6585; [DOI] [PubMed] [Google Scholar]
- 51f.Obradors C., Echavarren A. M., Chem. Eur. J. 2013, 19, 3547–3551; [DOI] [PubMed] [Google Scholar]
- 51g.Li B., Zhao Y., Lai Y., Loh T., Angew. Chem. Int. Ed. 2012, 51, 8041–8045; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 8165–8169; [Google Scholar]
- 51h.Bai Y., Tao W., Ren J., Wang Z., Angew. Chem. Int. Ed. 2012, 51, 4112–4116; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 4188–4192; [Google Scholar]
- 51i.Dateer R. B., Shaibu B. S., Liu R., Angew. Chem. Int. Ed. 2012, 51, 113–117; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 117–121; [Google Scholar]
- 51j.Bai Y., Luo Z., Wang Y., Gao J., Zhang L., J. Am. Chem. Soc. 2018, 140, 5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johansson M. J., Gorin D. J., Staben S. T., Toste F. D., J. Am. Chem. Soc. 2005, 127, 18002–18003. [DOI] [PubMed] [Google Scholar]
- 53.Briones J. F., Davies H. M. L., Org. Lett. 2011, 13, 3984–3987. [DOI] [PubMed] [Google Scholar]
- 54.Briones J. F., Davies H. M. L., J. Am. Chem. Soc. 2012, 134, 11916–11919. [DOI] [PubMed] [Google Scholar]
- 55.
- 55a.Padwa A., Acc. Chem. Res. 1979, 12, 310–317; [Google Scholar]
- 55b.Baid M. S., Chem. Rev. 2003, 103, 1271–1294; [DOI] [PubMed] [Google Scholar]
- 55c.Rubin M., Rubina M., Gevorgyan V., Chem. Rev. 2007, 107, 3117–3179. [DOI] [PubMed] [Google Scholar]
- 56.Yeom, Koo J., Park H., Wang Y., Liang Y., Yu Z., Shin S., J. Am. Chem. Soc. 2012, 134, 208–211. [DOI] [PubMed] [Google Scholar]
- 57.Kim H., Choi S. Y., Shin S., Angew. Chem. Int. Ed. 2018, 57, 13130–13134; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 13314–13318. [Google Scholar]
- 58.
- 58a.Chapman L., Smith H. G., King R. W., J. Am. Chem. Soc. 1963, 85, 803–806; [Google Scholar]
- 58b.Fokialakis N., Magiatis P., Terzis A., Tillequin F., Skaltsounis A. L., Tetrahedron Lett. 2001, 42, 5323–5325; [Google Scholar]
- 58c.Sittiwong W., Zinniel D. K., Fenton R. J., Marshall D. D., Story C. B., Kim B., Lee J., Powers R., Barletta R. G., Dussault P. H., ChemMedChem. 2014, 9, 1838–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.
- 59a.Arichi N., Yamada K., Yamaoka Y., Takasu K., J. Am. Chem. Soc. 2015, 137, 9579–9582; [DOI] [PubMed] [Google Scholar]
- 59b.Misale A., Niyomchon S., Maulide N., Acc. Chem. Res. 2016, 49, 2444–2458. [DOI] [PubMed] [Google Scholar]
- 60.
- 60a.Namyslo J. C., Kaufmann D. E., Chem. Rev. 2003, 103, 1485–1537; [DOI] [PubMed] [Google Scholar]
- 60b.Lukin K., Kishore V., Gordon T., Org. Process Res. Dev. 2013, 17, 666–671; [Google Scholar]
- 60c.Kallemeyn M., Ku Y., Mulhern M. M., Bishop R., Pal A., Jacob L., Org. Process Res. Dev. 2014, 18, 191–197; [Google Scholar]
- 60d.Guisán-Ceinos M., Parra A., Martín-Heras V., Tortosa M., Angew. Chem. Int. Ed. 2016, 55, 6969–6972; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 7083–7086; [Google Scholar]
- 60e.Roy S. R., Eijsberg H., Bruffaerts J., Marek I., Chem. Sci. 2017, 8, 334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Homs A., Obradors C., Lebœuf D., Echavarren A. M., Adv. Synth. Catal. 2014, 356, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.
- 62a.Snider B. B., Acc. Chem. Res. 1980, 13, 426–432; [Google Scholar]
- 62b.Clark R. D., Untch K. G., J. Org. Chem. 1979, 44, 248–255; [Google Scholar]
- 62c.Fienemann H., Hoffmann H. M. R., J. Org. Chem. 1979, 44, 2802–2804; [Google Scholar]
- 62d.Snider B. B., Rodini D. J., Con R. S. E., Sealfon S., J. Am. Chem. Soc. 1979, 101, 5283–5293; [Google Scholar]
- 62e.Quendo A., Rousseau G., Tetrahedron Lett. 1988, 29, 6443–6446; [Google Scholar]
- 62f.Franck-Neumann M., Miesch M., Gross L., Tetrahedron Lett. 1992, 33, 3879–3882; [Google Scholar]
- 62g.Sweis R. F., Schramm M. P., Kozmin S. A., J. Am. Chem. Soc. 2004, 126, 7442–7443; [DOI] [PubMed] [Google Scholar]
- 62h.Okamoto K., Shimbayashi T., Tamura E., Ohe K., Org. Lett. 2015, 17, 5843–5845. [DOI] [PubMed] [Google Scholar]
- 63.
- 63a.Lautens M., Klute W., Tam W., Chem. Rev. 1996, 96, 49–92; [DOI] [PubMed] [Google Scholar]
- 63b.Sakari K., Kochi T., Kakiuchi F., Org. Lett. 2013, 15, 1024–1027; [DOI] [PubMed] [Google Scholar]
- 63c.Fructos M. R., Prieto A., Tetrahedron 2016, 72, 355–369. [Google Scholar]
- 64.
- 64a.Lee-Ruff E., Mladenova G., Chem. Rev. 2003, 103, 1449–1484; [DOI] [PubMed] [Google Scholar]
- 64b.Yang C., Inoue Y., Chem. Soc. Rev. 2014, 43, 4123–4143; [DOI] [PubMed] [Google Scholar]
- 64c.Xu Y., Conner M. L., Brown M. K., Angew. Chem. Int. Ed. 2015, 54, 11918–11928; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 12086–12097; [Google Scholar]
- 64d.Blum T. R., Miller Z. D., Bates D. M., Guzei I. A., Yoon T. P., Science 2016, 354, 1391–1395; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64e.Poplata S., Tröster A., Zou Y.Q, Bach T., Chem. Rev. 2016, 116, 9748–9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grande-Carmona F., Iglesias-Sigüenza J., Álvarez E., Díez E., Fernández R., Lassaletta J. M., Organometallics 2015, 34, 5073–5080. [Google Scholar]
- 66.García-Morales C., Ranieri B., Escofet I., López-Suarez L., Obradors C., Konovalov A. I., Echavarren A. M., J. Am. Chem. Soc. 2017, 139, 13628–13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ranieri B., Obradors C., Mato M., Echavarren A. M., Org. Lett. 2016, 18, 1614–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim H., Jang J., Shin S., J. Am. Chem. Soc. 2020, 142, 20788–20795. [DOI] [PubMed] [Google Scholar]
- 69.Du Q., Neudörfl J.-M., Schmalz H.-G., Chem. Eur. J. 2018, 24, 2379–2383. [DOI] [PubMed] [Google Scholar]
- 70.Velder J., Robert T., Weidner I., Neudörfl J.-M., Lex J., Schmalz H.-G., Adv. Synth. Catal. 2008, 350, 1309–1315. [Google Scholar]
- 71.
- 71a.Ye L., He W., Zhang L., J. Am. Chem. Soc. 2010, 132, 8550–8551; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71b.Ye L., Cui L., Zhang G., Zhang L., J. Am. Chem. Soc. 2010, 132, 3258–3259; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71c.Wang Y., Ji K., Lan S., Zhang L., Angew. Chem. Int. Ed. 2012, 51, 1915–1918; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 1951–1954; [Google Scholar]
- 71d.He W., Li C., Zhang L., J. Am. Chem. Soc. 2011, 132, 8482–8485. [DOI] [PubMed] [Google Scholar]
- 72.Luo Y., Ji K., Li Y., Zhang L., J. Am. Chem. Soc. 2012, 134, 17412–17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ji K., Zheng Z., Wang Z., Zhang L., Angew. Chem. Int. Ed. 2015, 54, 1245–1249; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 1261–1265. [Google Scholar]
- 74.Wang Y., Zhang Z.-M., Liu F., He Y., Zhang J., Org. Lett. 2018, 20, 6403–6406. [DOI] [PubMed] [Google Scholar]
- 75.Di X., Wang Y., Wu L., Zhang Z−M., Dai Q., Li W., Zhang J., Org. Lett. 2019, 21, 3018–3022. [DOI] [PubMed] [Google Scholar]
- 76.Zhou L., Xu B., Ji D., Zhang Z.-M., Zhang J., Chin. J. Chem. 2020, 38, 577–582. [Google Scholar]
- 77.Zhang Z., Smal V., Retailleau P., Voituriez A., Frison G., Marinetti A., Guinchard X., J. Am. Chem. Soc. 2020, 142, 3797–3805. [DOI] [PubMed] [Google Scholar]
- 78.
- 78a.Schmidbaur H., Schier A., Arabian J. Sci. Eng. 2012, 37, 1187–1225; [Google Scholar]
- 78b.Rodriguez J., Bourissou D., Angew. Chem. Int. Ed. 2018, 57, 386–388; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 392–394. [Google Scholar]
- 79.
- 79a.Casado R., Contel M., Laguna M., Romero P., Sanz S., J. Am. Chem. Soc. 2003, 125, 11925–11935; [DOI] [PubMed] [Google Scholar]
- 79b.Hashmi A. S. K., Blanco M. C., Fischer D., Bats J. W., Eur. J. Org. Chem. 2006, 1387–1389; [Google Scholar]
- 79c.De Frémont P., Singh R., Stevens E. D., Petersen J. L., Nolan S. P., Organometallics 2007, 26, 1376–1385; [Google Scholar]
- 79d.Wolf W. J., Winston M. S., Toste F. D., Nat. Chem. 2014, 6, 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.
- 80a.Hashmi A. S. K., Weyrauch J. P., Rudolph M., Kurpejovic E., Angew. Chem. Int. Ed. 2004, 43, 6545–6547; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2004, 116, 6707–6709; [Google Scholar]
- 80b.Langseth E., Görbitz C. H., Heyn R. H., Tilset M., Organometallics 2012, 31, 6567–6571. [Google Scholar]
- 81.González-Arellano C., Corma A., Iglesias M., Sánchez F., Chem. Commun. 2005, 1990–1992. [DOI] [PubMed] [Google Scholar]
- 82.
- 82a.Yang Y., Hu W., Ye X., Wang D., Shi X., Adv. Synth. Catal. 2016, 358, 2583–2588; [Google Scholar]
- 82b.Yang Y., Qin A., Zhao K., Wang D., Shi X., Adv. Synth. Catal. 2016, 358, 1433–1439. [Google Scholar]
- 83.Wu C. Y., Horibe T., Jacobsen C. B., Toste F. D., Nature 2015, 517, 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bohan P. T., Toste F. D., J. Am. Chem. Soc. 2017, 139, 11016–11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reid J. P., Hu M., Ito S., Huang B., Hong C. M., Xiang H., Sigman M. S., Toste F. D., Chem. Sci. 2020, 11, 6450–6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cui J.-F., Ko H.-M., Shing K.-P., Deng J.-R, Lai N. C.-H., Wong M.-K., Angew. Chem. Int. Ed. 2017, 56, 3074–3079; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 3120–3125. [Google Scholar]
- 87.Bergens S. H., Leung P., Bosnich B., Rheingold A. L., Organometallics 1990, 9, 2406–2408. [Google Scholar]
- 88.Jiang J.-J., Cui J.-F., Yang B., Ning Y., Lai N. C.-H., Wong M.-K., Org. Lett. 2019, 21, 6289–6294. [DOI] [PubMed] [Google Scholar]