Abstract
Atropo‐enantioselective biaryl coupling through C−H bond functionalization is an emerging technology allowing direct construction of axially chiral molecules. This approach is largely limited to electrophilic coupling partners. We report a highly atropo‐enantioselective C−H arylation of tetralone derivatives paired with aryl boronic esters as nucleophilic components. The transformation is catalyzed by chiral cyclopentadienyl (Cpx) iridium(III) complexes and enabled by oxidatively enhanced reductive elimination from high‐valent cyclometalated Ir‐species.
Keywords: asymmetric catalysis, atropselectivity, C-H functionalization, chiral cyclopentadienyl, iridium
A highly atropo‐enantioselective C−H arylation of tetralone derivatives paired with aryl boronic esters as nucleophilic components is presented. The transformation is catalyzed by chiral cyclopentadienyl (Cpx) iridium(III) complexes and enabled by oxidatively enhanced reductive elimination from high‐valent cyclometalated Ir‐species.
Atropisomerism is a fundamental property of a variety of organic molecules having a profound effect on their properties and biological activity.[1] The chiral axis arising from restricted bond rotation is a key stereochemical element existing in many natural products[2] and drugs,[3] and is an exploited property in materials[4] and chiral ligands.[5] Notably, atropisomerism has an increasing impact on medicinal chemistry where atropisomers of drug candidates may significantly differ in biological activity.[6] Developments of efficient methods for the stereocontrolled assembly of biologically relevant atropchiral molecules are therefore highly desirable.[7] In this respect, atropo‐enantioselective C−H functionalization is a promising emerging technology enabling their direct and atom efficient construction.[8] Most current atropselective C−H functionalization methodologies base on de novo syntheses of an aromatic ring[9] or use biaryl substrates having a pre‐existing axis.[10] Functionalization of C−H bonds then locks the axis by (dynamic) kinetic resolution[11] or creates chirality via desymmetrization processes.[12] Less explored, but retrosynthetically straightforward, is the direct coupling of two aryl fragments. This strategy directly forges the chiral axis from simple precursors. However, the hefty steric requirements to form stable atropisomers is a challenge for the catalyst performance and requires a very high efficiency to accommodate such hindered substrates. We and others reported capable Pd‐,[13] Ir‐,[14] and Rh‐catalysts.[15] An important aspect of this disconnection strategy is the choice of a suitable coupling partners. Electrophilic partners like aryl halides used in Pd‐catalysis,[13a, 13b] and quinone diazides suitable for Rh‐[15] and Ir‐based[14] systems do not require external oxidant to close the catalytic cycle (Scheme 1 a).[16] The versatility and wide availability of aryl boronic esters and silanes would make their use as carbon nucleophiles under oxidative conditions[17] an attractive complementary choice for direct atroposelective couplings. Besides a single moderately enantioselective example under Pd‐catalysis,[13c, 13d] this approach is largely underdeveloped.
Scheme 1.
a) Coupling partners strategies in atropo‐enantioselective C−H arylations; b) Oxidative CpxIrIII‐catalyzed atropo‐enantioselective C−H arylation with aryl boronic esters.
We were intrigued by recent reports of Chang et al. detailing C−H arylations enabled by oxidatively induced reductive elimination from high‐valent metal centers.[18] Further oxidation of the iridium(III) center by an external oxidant increased the driving force for the C−C bond‐forming reductive elimination, enabling direct C−H arylations with nucleophilic donors like aryl silanes[18d] and aryl boronic esters.[18a, 18b, 18c] We hypothesized that this boosted reductive elimination from the high‐valent iridium complex I3 is advantageous for the conversion of sterically demanding substrates to axially‐chiral products 3 (Scheme 1 b). This would overcome the high stability of cyclometalated CpxIr(III) complexes. Complexes with o‐alkoxy groups similar to I1 are stable under acidic conditions and exploitable as powerful hydrogenation catalysts.[19] Herein, we demonstrate a highly enantioselective C−H arylation of tetralone derivatives 1 with boronic esters 2 giving access to atropchiral biaryls 3.
We investigated the feasibility of the transformation with oxime 1 a derived from 7‐benzyloxy tetralone and naphthalen‐1‐ylboronic boronic ester 2 a in the presence of iridium catalyst [Cp*IrCl2]2 under oxidative conditions. Silver triflimide was used as a halide abstractor of the starting Ir‐complex. Hydrated copper(II) trifluoroacetate was expected to facilitate transmetallation, and silver(I) fluoride was used as the oxidant (Table 1).[18c, 18d] The initial reaction performed at 40 °C in trifluorotoluene as solvent afforded densely substituted arylation product 3 aa albeit in 13 % yield (Entry 1). The racemization barrier (ΔG ≠ enant) of biaryl product 3 aa was determined to be 33 kcal mol−1 in toluene (see SI for details), indicating a high stability of the chiral axis locked by the three ortho substituents of the axis‐forming aromatic rings. A very slow erosion of enantiopurity (from 97:3 to 96:4 er) was observed after heating 3 aa at 110 °C for 2.5 h. We next focused on identifying a chiral Ir‐complex[20] that could not only infer high stereocontrol but as well improve the efficiency of the process. We hypothesized that Ir‐complexes bearing chiral disubstituted cyclopentadienyl (Cpx) ligands[21, 22] might be suitable candidates due to their less sterically congested nature in comparison to the pentasubstituted Cp* ligand. Indeed, Ir1 improved the yield of arylation product 3 aa to 49 % at room temperature. It provides as well as an excellent enantiomeric ratio of 95:5 er (entry 2). Increasing the temperature to 40 °C improved the yield to 77 %, however at the expense of the enantiomeric ratio which decreased to 92.5:7.5 er (entry 3), demonstrating its largely superior reactivity compared to [Cp*IrCl2]2. Additional CpxIr complexes[23] bearing different ether substituents on the binaphthyl backbone (Ir2–Ir5) were evaluated. In this respect, catalyst Ir2 with a MOM ether substituent[24] resulted in a comparable yield and enantioselectivity for 3 aa (entry 4). The use of Ir3 with a slightly larger cyclopentyl ether sidewall improved the enantiomeric ratio to 95:5, however at a moderate yield (entry 5). In contrast, complexes Ir4 and Ir5 having isopropoxy[20a] and pentan‐3‐yloxy ether substituents delivered oxime product 3 aa not only in better selectivity of 95.5:4.5 er (Ir4) and 97:3 er (Ir5), but as well higher yields of 76 %, respectively 72 % (entries 6 and 7). A slight increase of the temperature to 45 °C improved the yield to 84 % while the selectivity remained constant at 97:3 er (entry 8). The reduction of the amount of CuII to 0.5 equivalents reduced the yield to 71 % with no change in er.
Table 1.
Optimization of the Ir‐catalyzed atropo‐enantioselective C−H arylation of oxime 1 a.[a]

|
Entry |
[Ir] |
Temp. [°C] |
yield[b] [%] |
er[c] |
|---|---|---|---|---|
|
1 |
[Cp*IrCl2]2 |
40 |
13 |
– |
|
2 |
Ir1 |
23 |
49 |
95:5 |
|
3 |
Ir1 |
40 |
77 |
92.5:7.5 |
|
4 |
Ir2 |
40 |
78 |
91.5:9.5 |
|
5 |
Ir3 |
40 |
57 |
95:5 |
|
6 |
Ir4 |
40 |
76 |
95.5:4.5 |
|
7 |
Ir5 |
40 |
72 |
97:3 |
|
8 |
Ir5 |
45 |
84 (84)[d] |
97:3 |
|
9[e] |
Ir5 |
45 |
71 |
97:3 |
[a] Conditions: 50 μmol 1 a, 100 μmol 2 a, 2.5 μmol [Ir], 15 μmol AgNTf2, 50 μmol Cu(CO2CF3)2⋅H2O, 110 μmol AgF in PhCF3 (0.2 M) for 14 h; [b] yields determined by 1H‐NMR with an internal standard; [c] enantiomeric ratio determined by chiral HPLC; [d] isolated yield at 0.1 mmol scale; [e] 25 μmol Cu(CO2CF3)2⋅H2O; MBn=4‐methyl benzyl, Bn=benzyl.
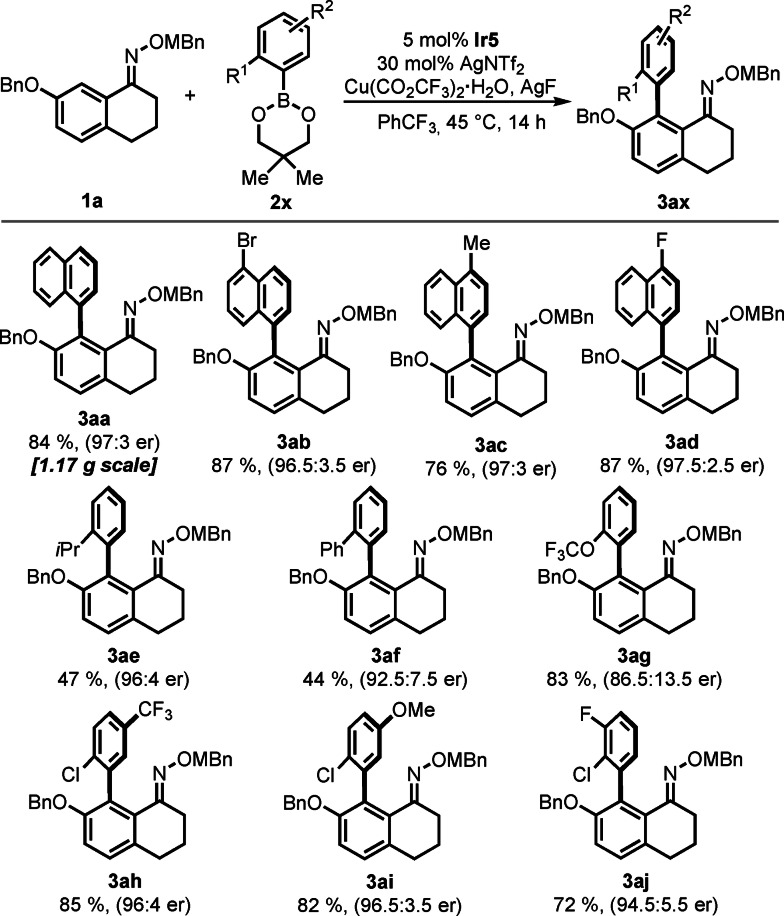
We next evaluated the scope of the atrop‐enantioselective biaryl formation with respect to diversely substituted aryl boronic esters (Scheme 2). A variety of naphthyl boronic esters 2 b–2 d were smoothly converted to their corresponding enantioenriched products 3 ab–3 ad in good yield and excellent selectivities up to 97.5:2.5 er. Besides naphthyl type‐substrates, we investigated ortho‐substituted aryl boronic esters. The yields for aryl substrates with axis‐locking substituents 2‐iPr (3 ae), 2‐Ph (3 af) was slightly lower whereas very high selectivity levels were maintained. Product 3 ag having a 2‐trifluoromethoxy group locking the chiral axis was formed in 83 % yield albeit slightly lower selectivity. However, aryl boronic esters with 2‐MeO or 2‐CF3 groups did not engage in a productive pathway, leaving oxime 1 a untouched (see SI for unreactive substrates). In contrast, trisubstituted aryl boronic esters with an ortho‐chlorine substituent and an additional functional group (CF3, OMe, F) reacted smoothly giving products 3 ah–3 aj in high yields and selectivities between 94.5:5.5 and 96.5.3.5 er. Notably, probing the atropo‐enantioselective C−H arylation at 2.8 mmol scale yielded 1.17 g of biaryl 3 aa in 84 % yield and 97:3 er, indicated neither an efficiency nor a selectivity erosion at 28‐fold scale‐up.
Scheme 2.
Scope for the Ir‐catalyzed atropo‐enantioselective C−H arylation with boronic esters 2 x. Conditions: 0.10 mmol 1 a, 0.20 mmol 2 x, 5 μmol Ir5, 30 μmol AgNTf2, 0.10 mmol Cu(CO2CF3)2⋅H2O, 0.22 mmol AgF in PhCF3 (0.2 M) at 45 °C for 14 h.
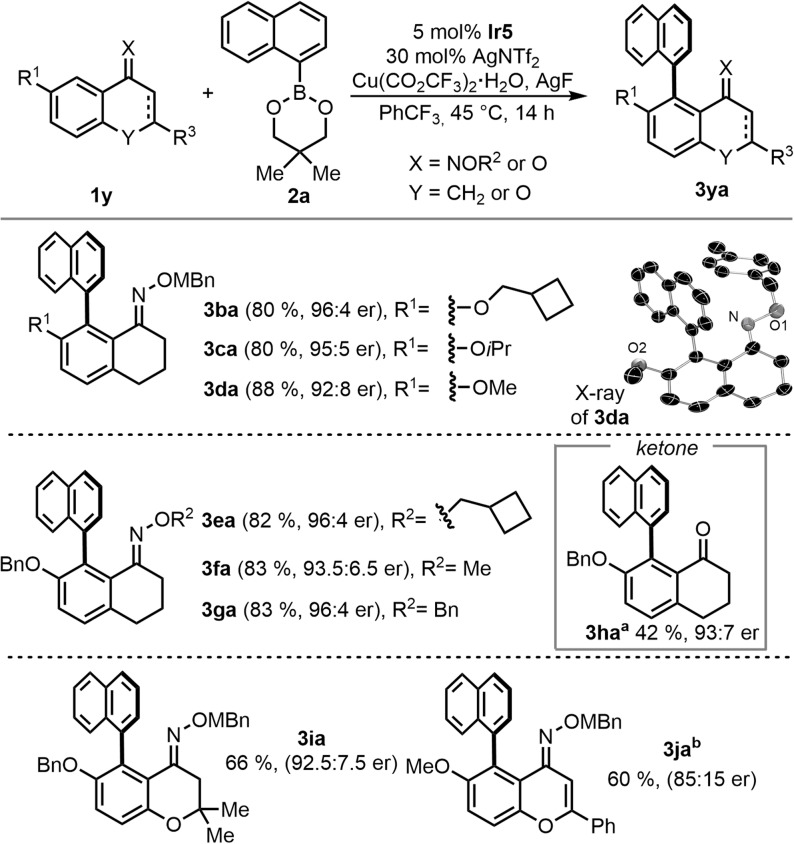
The scope with respect to the oxime substrates 1 was investigated using naphthalen‐1‐ylboronic boronic ester 2 a (Scheme 3). The transformation provided efficient access to biaryl products having different ether substituents R1 including methyl cyclobutyl (3 ba, 80 % yield, 96:4 er), isopropyl (3 ca, 80 %, 95:5 er), and methyl (3 da, 88 % yield, 92:8 er). Suitable crystals of 3 da unequivocally revealed the absolute configuration of the chiral axis to be (R) by single crystal X‐ray diffraction analysis. Substrates with different oxime substituents R2 reacted smoothly with boronic ester 2 a regardless of their size. For instance, methyl cyclobutyl oxime 3 ea was formed in 82 % yield and 96:4 er. Methyl and benzyl substituted oximes led to the products 3 fa and 3 ga in similar yields and selectivity. The C−H arylation of a chromanone‐derived oxime 1 i gave product 3 ia in 66 % yield and 92.5:7.5 er. In addition, flavone‐derived substrate 1 j was converted at 23 °C to its corresponding product 3 ja with 85:15 er using complex Ir1. The oxime‐directing group is not a prerequisite for the directed C−H functionalization. In this respect, ketone 1 h could be converted to the expected biaryl product, providing 3 ha in 93:7 er. In this case, the less sterically demanding Ir1 complex was superior to Ir5.
Scheme 3.
Scope for the Ir‐catalyzed atropo‐enantioselective C−H arylation of different oximes and tetralones 1 y. Conditions: 0.10 mmol 1 y, 0.20 mmol 2 a, 5 μmol Ir5, 30 μmol AgNTf2, 0.10 mmol Cu(CO2CF3)2⋅H2O, 0.22 mmol AgF in PhCF3 (0.2 M) at 45 °C for 14 h; a) with 5 μmol Ir1; b) with 5 μmol Ir1 at 23 °C in PhCF3 (0.125 M). Crystal structure of 3 da as ORTEP drawing with 50 % probability thermal ellipsoids;[25] Hydrogen atoms omitted for clarity.
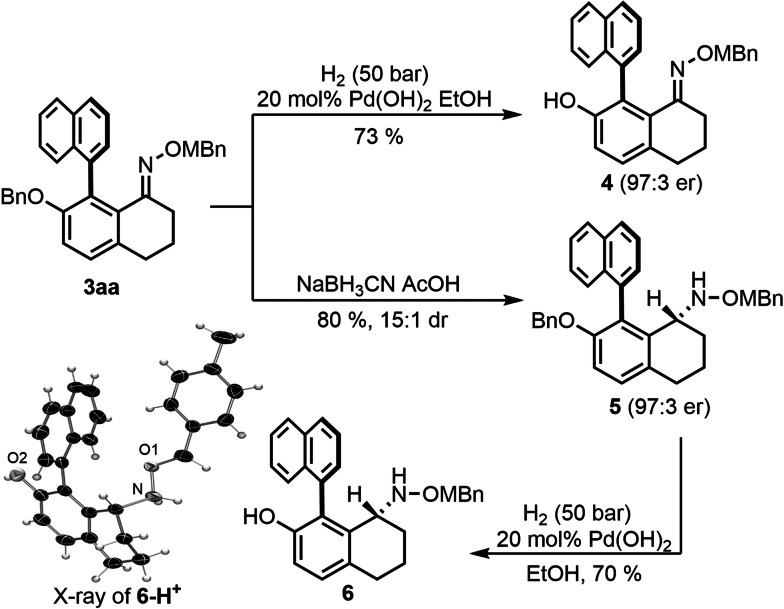
The reactivity profile of atropochiral oxime 3 aa, in particular with respect to its variety of reducible positions was investigated (Scheme 4). For instance, hydrogenation in the presence of Pearlman catalyst resulted in a selective cleavage of the phenolic benzyl group without a reduction of any other group. Phenol 4 displayed the same enantioenrichment as the starting material. In contrast, reduction of 3 aa with sodium cyanoborohydride selectively reduced the C=N bond without cleavage of the sensitive N−O bond, affording without epimerization protected alkoxy amine 5 in 80 % yield and with an excellent dr of 15:1. The chiral axis dictated the diastereoselectivity of the reduction resulting in a selective hydride delivery from the less hindered face giving diastereomer 5. A further reduction to phenol 6 and subsequent conversion to its 4‐nitrobenzenesulfonate salt 6‐H+ gave crystals suitable for X‐ray analysis and unequivocally confirmed the configurations of products 5 and 6.
Scheme 4.
Selective transformations of biaryl oxime 3 aa. Crystal structure of 6‐H+ as ORTEP drawing with 50 % probability thermal ellipsoids;[25] Anion omitted for clarity.
In summary, we reported a highly atropo‐enantioselective, CpxIr(III)‐catalyzed C−H arylation of α‐tetralone derivatives with boronic esters as nucleophilic coupling partners. Facile reductive elimination from high valent Ir‐complexes and the use of fine‐tuned disubstituted Cpx ligands enabled the stereoselective synthesis of highly substituted biaryls. The method provides an efficient access to unexplored atropochiral oxime‐derived α‐tetralones, as well as chromanone and flavone products. Moreover, this exemplifies that oxidation‐induced eliminations are suitable to improve catalytic performance of otherwise relatively stable chiral cyclometalated Cp‐iridium(III) complexes.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Acknowledgements
This work is supported by the Swiss National Science Foundation (no 157741). We thank Dr. R. Scopelliti and Dr. F. Fadaei Tirani for X‐ray crystallographic analysis of compounds 3 da, 6‐H+ .
Ł. Woźniak, N. Cramer, Angew. Chem. Int. Ed. 2021, 60, 18532.
Contributor Information
Dr. Łukasz Woźniak, https://www.epfl.ch/labs/lcsa/
Prof. Dr. Nicolai Cramer, Email: nicolai.cramer@epfl.ch.
References
- 1.
- 1a.Atropisomerism and Axial Chirality (Ed.: Lassaletta J. M.), World Scientific, Singapore, 2019; [Google Scholar]
- 1b.Siegel J. S., Synlett 2018, 29, 2120; [Google Scholar]
- 1c.Zask A., Murphy J., Ellestad G. A., Chirality 2013, 25, 265. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a.Smyth J. E., Butler N. M., Keller P. A., Nat. Prod. Rep. 2015, 32, 1562; [DOI] [PubMed] [Google Scholar]
- 2b.Bringmann G., Gulder T., Gulder T. A. M., Breuning M., Chem. Rev. 2011, 111, 563; [DOI] [PubMed] [Google Scholar]
- 2c.Bringmann G., Menche D., Acc. Chem. Res. 2001, 34, 615. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a.Glunz P. W., Bioorg. Med. Chem. Lett. 2018, 28, 53; [DOI] [PubMed] [Google Scholar]
- 3b.Toenjes S. T., Gustafson J. L., Future Med. Chem. 2018, 10, 409; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3c.Clayden J., Moran W. J., Edwards P. J., LaPlante S. R., Angew. Chem. Int. Ed. 2009, 48, 6398; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 6516. [Google Scholar]
- 4.
- 4a.Wu Y.-L., Ferroni F., Pieraccini S., Schweizer W. B., Frank B. B., Spada G. P., Diederich F., Org. Biomol. Chem. 2012, 10, 8016; [DOI] [PubMed] [Google Scholar]
- 4b.Wen K., Yu S., Huang Z., Chen L., Xiao M., Yu X., Pu L., J. Am. Chem. Soc. 2015, 137, 4517; [DOI] [PubMed] [Google Scholar]
- 4c.Hartley C. S., Lazar C., Wand M. D., Lemieux R., J. Am. Chem. Soc. 2002, 124, 13513. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a.Noyori R., Takaya H., Acc. Chem. Res. 1990, 23, 345; [Google Scholar]
- 5b.Chen Y., Yekta S., Yudin A. K., Chem. Rev. 2003, 103, 3155; [DOI] [PubMed] [Google Scholar]
- 5c.Kočovský P., Vyskočil Š., Smrčina M., Chem. Rev. 2003, 103, 3213; [DOI] [PubMed] [Google Scholar]
- 5d.Berthod M., Mignani G., Woodward G., Lemaire M., Chem. Rev. 2005, 105, 1801; [DOI] [PubMed] [Google Scholar]
- 5e.Pereira M. M., Calvete M. J. F., Carrilho R. M. B., Abreu A. R., Chem. Soc. Rev. 2013, 42, 6990; [DOI] [PubMed] [Google Scholar]
- 5f.Rokade B. V., Guiry P., ACS Catal. 2018, 8, 624. [Google Scholar]
- 6.LaPlante S. R., Fader L. D., Fandrick K. R., Fandrick D. R., Hucke O., Kemper R., Miller S. P. F., Edwards P. J., J. Med. Chem. 2011, 54, 7005. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a.Cheng J. K., Xiang S.-H., Li S., Ye L., Tan B., Chem. Rev. 2021, 121, 4805–4902; [DOI] [PubMed] [Google Scholar]
- 7b.Bao X., Rodriguez J., Bonne D., Angew. Chem. Int. Ed. 2020, 59, 12623; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 12723. [Google Scholar]
- 8.For reviews on atroposelective C−H functionalization see:
- 8a.Wencel-Delord J., Colobert F., SynOpen 2020, 4, 107; [Google Scholar]
- 8b.Liao G., Zhou T., Yao Q.-J., Shi B.-F., Chem. Commun. 2019, 55, 8514; For selected reviews on enantioselective C−H functionalization, see: [DOI] [PubMed] [Google Scholar]
- 8c.Achar T. K., Maiti S., Jana S., Maiti D., ACS Catal. 2020, 10, 13748; [Google Scholar]
- 8d.Woźniak Ł., Tan J.-F., Nguyen Q.-H., Madron du Vigné A., Smal V., Cao Y.-X., Cramer N., Chem. Rev. 2020, 120, 10516; [DOI] [PubMed] [Google Scholar]
- 8e.Woźniak Ł., Cramer N., Trends Chem. 2019, 1, 471; [Google Scholar]
- 8f.Loup J., Dhawa U., Pesciaioli F., Wencel-Delord J., Ackermann L., Angew. Chem. Int. Ed. 2019, 58, 12803; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 12934; [Google Scholar]
- 8g.Saint-Denis T. G., Zhu R.-Y., Chen G., Wu Q.-F., Yu J.-Q., Science 2018, 359, eaao4798; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8h.Newton C. G., Wang S.-G., Oliveira C. C., Cramer N., Chem. Rev. 2017, 117, 8908. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a.Sun L., Chen H., Liu B., Chang J., Kong L., Wang F., Lan Y., Li X., Angew. Chem. Int. Ed. 2021, 60, 8391; [DOI] [PubMed] [Google Scholar]
- 9b.Wang F., Qi Z., Zhao Y., Zhai S., Zheng G., Mi R., Huang Z., Zhu X., He X., Li X., Angew. Chem. Int. Ed. 2020, 59, 13288; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 13390; [Google Scholar]
- 9c.Li H., Yan X., Zhang J., Guo W., Jiang J., Wang J., Angew. Chem. Int. Ed. 2019, 58, 6732; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 6804; [DOI] [PubMed] [Google Scholar]
- 9d.Tian M., Bai D., Zheng G., Chang J., Li X., J. Am. Chem. Soc. 2019, 141, 9527; [DOI] [PubMed] [Google Scholar]
- 9e.Shan G., Flegel J., Li H., Merten C., Ziegler S., Antonchick A. P., Waldmann H., Angew. Chem. Int. Ed. 2018, 57, 14250; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 14446. [Google Scholar]
- 10.Selected examples:
- 10a.Wang Q., Zhang W.-W., Zheng C., Gu Q., You S.-L., J. Am. Chem. Soc. 2021, 143, 114; [DOI] [PubMed] [Google Scholar]
- 10b.Wang Q., Zhang W.-W., Song H., Wang J., Zheng C., Gu Q., You S.-L., J. Am. Chem. Soc. 2020, 142, 15678; [DOI] [PubMed] [Google Scholar]
- 10c.Romero-Arenas A., Hornillos V., Iglesias-Sigüenza J., Fernández R., López-Serrano J., Ros A., Lassaletta J. M., J. Am. Chem. Soc. 2020, 142, 2628; [DOI] [PubMed] [Google Scholar]
- 10d.Zhan B.-B., Wang L., Luo J., Lin X.-F., Shi B.-F., Angew. Chem. Int. Ed. 2020, 59, 3568; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 3596; [Google Scholar]
- 10e.Wang Q., Cai Z.-J., Liu C.-X., Gu Q., You S.-L., J. Am. Chem. Soc. 2019, 141, 9504; [DOI] [PubMed] [Google Scholar]
- 10f.Luo J., Zhang T., Wang L., Liao G., Yao Q.-J., Wu Y.-J., Zhan B.-B., Lan Y., Lin X.-F., Shi B.-F., Angew. Chem. Int. Ed. 2019, 58, 6708; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 6780; [Google Scholar]
- 10g.Zhang J., Xu Q., Wu J., Fan J., Xie M., Org. Lett. 2019, 21, 6361; [DOI] [PubMed] [Google Scholar]
- 10h.Zhang S., Yao Q.-J., Liao G., Li X., Li H., Chen H.-M., Hong X., Shi B.-F., ACS Catal. 2019, 9, 1956; [Google Scholar]
- 10i.Zheng J., Cui W.-J., Zheng C., You S.-L., J. Am. Chem. Soc. 2016, 138, 5242; [DOI] [PubMed] [Google Scholar]
- 10j.Zheng J., You S.-L., Angew. Chem. Int. Ed. 2014, 53, 13244; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 13460. [Google Scholar]
- 11.Selected examples:
- 11a.Yao Q.-J., Xie P.-P., Wu Y.-J., Feng Y.-L., Teng M.-Y., Hong X., Shi B.-F., J. Am. Chem. Soc. 2020, 142, 18266; [DOI] [PubMed] [Google Scholar]
- 11b.He C., Hou M., Zhu Z., Gu Z., ACS Catal. 2017, 7, 5316; [Google Scholar]
- 11c.Gao D.-W., Gu Q., You S.-L., ACS Catal. 2014, 4, 2741. [Google Scholar]
- 12.Liao G., Li B., Chen H.-M., Yao Q.-J., Xia Y.-N., Luo J., Shi B.-F., Angew. Chem. Int. Ed. 2018, 57, 17151; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 17397. [Google Scholar]
- 13.
- 13a.Nguyen Q.-H., Guo S.-M., Royal T., Baudoin O., Cramer N., J. Am. Chem. Soc. 2020, 142, 2161; [DOI] [PubMed] [Google Scholar]
- 13b.Newton C. G., Braconi E., Kuziola J., Wodrich M. D., Cramer N., Angew. Chem. Int. Ed. 2018, 57, 11040; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 11206; [Google Scholar]
- 13c.Yamaguchi K., Kondo H., Yamaguchi J., Itami K., Chem. Sci. 2013, 4, 3753; [Google Scholar]
- 13d.Yamaguchi K., Yamaguchi J., Studer A., Itami K., Chem. Sci. 2012, 3, 2165. [Google Scholar]
- 14.Jang Y.-S., Woźniak Ł., Pedroni J., Cramer N., Angew. Chem. Int. Ed. 2018, 57, 12901; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 13083. [Google Scholar]
- 15.
- 15a.Pan C., Yin S.-Y., Wang S.-B., Gu Q., You S.-L., Angew. Chem. Int. Ed. 2021, 10.1002/anie.202103638; [DOI] [Google Scholar]; Angew. Chem. 2021, 10.1002/ange.202103638; [DOI] [Google Scholar]
- 15b.Kong L., Han X., Liu S., Zou Y., Lan Y., Li X., Angew. Chem. Int. Ed. 2020, 59, 7188; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 7255; [Google Scholar]
- 15c.Shaaban S., Li H., Otte F., Strohmann C., Antonchick A. P., Waldmann H., Org. Lett. 2020, 22, 9199; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15d.Jia Z.-J., Merten C., Gontla R., Daniliuc C. G., Antonchick A. P., Waldmann H., Angew. Chem. Int. Ed. 2017, 56, 2429; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 2469. [Google Scholar]
- 16.To our knowledge, methods using oxidative C−H/C−H couplings have not been reported for the creation of a stable axis. However, it has been used to lock an existing axis, see ref. [10b].
- 17.For review see:
- 17a.Giri R., Thapa S., Kafle A., Adv. Synth. Catal. 2014, 356, 1395; For selected examples see: [Google Scholar]
- 17b.Reddy D. M., Wang S.-C., Du K., Lee C.-F., J. Org. Chem. 2017, 82, 10070; [DOI] [PubMed] [Google Scholar]
- 17c.Nareddy P., Jordan F., Brenner-Moyer S. E., Szostak M., ACS Catal. 2016, 6, 4755; [Google Scholar]
- 17d.Sun C.-L., Liu N., Li B.-J., Yu D.-G., Wang Y., Shi Z.-J., Org. Lett. 2010, 12, 184. [DOI] [PubMed] [Google Scholar]
- 18.
- 18a.Kim J., Kim D., Chang S., J. Am. Chem. Soc. 2020, 142, 19052; [DOI] [PubMed] [Google Scholar]
- 18b.Kim J., Kim S., Kim D., Chang S., J. Org. Chem. 2019, 84, 13150; [DOI] [PubMed] [Google Scholar]
- 18c.Kim J., Shin K., Jin S., Kim D., Chang S., J. Am. Chem. Soc. 2019, 141, 4137; [DOI] [PubMed] [Google Scholar]
- 18d.Shin K., Park Y., Baik M.-H., Chang S., Nat. Chem. 2018, 10, 218. [DOI] [PubMed] [Google Scholar]
- 19.
- 19a.Mas-Roselló J., Smejkal T., Cramer N., Science 2020, 368, 1098; [DOI] [PubMed] [Google Scholar]
- 19b.Mas-Rosello J., Cope C. J., Tan E., Pinson B., Robinson A., Smejkal T., Cramer N., Angew. Chem. Int. Ed. 2021, 10.1002/anie.202103806; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2021, 10.1002/ange.202103806. [DOI] [Google Scholar]
- 20.
- 20a.Jang Y.-S., Dieckmann M., Cramer N., Angew. Chem. Int. Ed. 2017, 56, 15088; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 15284; [Google Scholar]
- 20b.Dieckmann M., Jang Y.-S., Cramer N., Angew. Chem. Int. Ed. 2015, 54, 12149; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 12317. [Google Scholar]
- 21.Fore reviews, see:
- 21a.Mas-Roselló J., Herraiz A. G., Audic B., Laverny A., Cramer N., Angew. Chem. Int. Ed. 2021, 60, 13198; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 13306; [Google Scholar]
- 21b.Yoshino T., Satake S., Matsunaga S., Chem. Eur. J. 2020, 26, 7346; [DOI] [PubMed] [Google Scholar]
- 21c.Newton C. G., Kossler D., Cramer N., J. Am. Chem. Soc. 2016, 138, 3935; [DOI] [PubMed] [Google Scholar]
- 21d.Ye B., Cramer N., Acc. Chem. Res. 2015, 48, 1308. [DOI] [PubMed] [Google Scholar]
- 22.
- 22a.Ye B., Cramer N., J. Am. Chem. Soc. 2013, 135, 636; [DOI] [PubMed] [Google Scholar]
- 22b.Ye B., Cramer N., Science 2012, 338, 504. [DOI] [PubMed] [Google Scholar]
- 23.
- 23a.Smits G., Audic B., Wodrich M. D., Corminboeuf C., Cramer N., Chem. Sci. 2017, 8, 7174; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23b.Audic B., Wodrich M. D., Cramer N., Chem. Sci. 2019, 10, 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye B., Cramer N., Synlett 2015, 26, 1490. [Google Scholar]
- 25.Deposition numbers 2082962 (3da) and 2082963 (6-H +) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information