Abstract
Psilocin (1) is the dephosphorylated and psychotropic metabolite of the mushroom natural product psilocybin. Oxidation of the phenolic hydroxy group at the C‐4 position of 1 results in formation of oligomeric indoloquinoid chromophores responsible for the iconic blueing of bruised psilocybin‐producing mushrooms. Based on previous NMR experiments, the hypothesis included that the 5,5’‐coupled quinone dimer of 1 was the primary product responsible for the blue color. To test this hypothesis, ring‐methylated 1 derivatives were synthesized to provide stable analogs of 1 dimers that could be completely characterized. The chemically oxidized derivatives were spectroscopically analyzed and compared to computationally derived absorbance spectra. Experimental evidence did not support the original hypothesis. Rather, the blue color was shown to stem from the quinoid 7,7’‐coupled dimer of 1.
Keywords: bufotenin, chromophores, oxidation, psilocybin, tryptamine
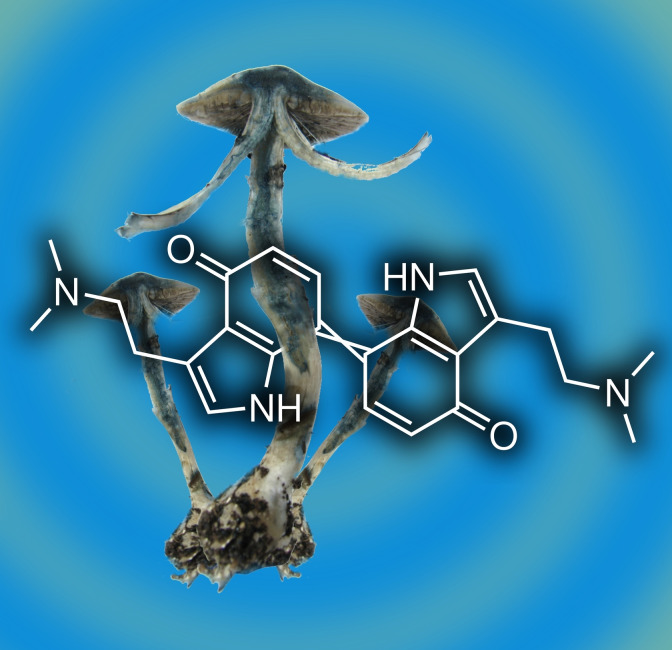
Self‐made color frenzy: Psilocin oxidation causes the iconic blueing of Psilocybe “magic” mushrooms. To prevent oligomerization, but allow for dimerization, 5‐ and 7‐methylated psilocin monomers were synthesized. Chemical oxidation enabled spectroscopic chromophore investigation of the dimeric gateway compounds and comparison to computationally derived absorption spectra. The 7,7’‐coupled quinoid psilocyl dimer was identified as major initial blue product, while the 5,5’‐coupled dimer contributes a greenish tint.
Introduction
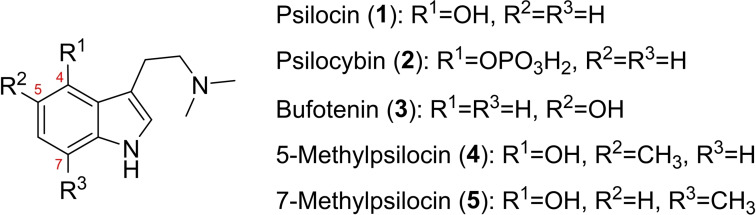
Tryptamines, in particular when 4‐ or 5‐hydroxylated, represent a group of potent psychotropic compounds of natural or synthetic origin. Most prominently, psilocin (1, 4‐hydroxy‐N,N‐dimethyltryptamine, Figure 1) causes profound changes in perception and consciousness.[1] It is the pharmacologically active dephosphorylated analog of psilocybin (2, Figure 1) and is formed, for example, upon ingestion of so‐called ‘magic’ mushrooms of the genus Psilocybe and other genera. The pharmaceutical relevance of 2 as a candidate drug against therapy‐refractory depression and anxiety has impressively been underlined by clinical studies.[2] Fungal tryptamines occur as oligomers as well. In bruised or injured Psilocybe mushrooms, 2 is dephosphorylated as well to 1, whose indolic 4‐OH group is subsequently oxidized. This short cascade, catalyzed by the phosphatase PsiP and the laccase PsiL, prepares 2 via 1 for instant oligomerization into a heterogeneous quinoid set of compounds that accounts for the distinctive blue hue of Psilocybe fruiting bodies.[3] Given their protein‐precipitating and radical oxidation properties, these oligomers may add a possible second layer of bioactivity – beyond the receptor‐binding nature of monomeric 1 – that perhaps protects the fungus from feeding invertebrates. Coupling between two monomers preferentially occurs on positions C‐5 and C‐7, which implies three possibilities for primary dimeric regioisomers (5,5’‐, 7,7’‐, or 5,7’‐coupled) that possibly exist within the product mixture in the initial phase of 1 oxidation.
Figure 1.
Chemical structures of natural tryptamines, and synthetic C‐5 and C‐7 methylated psilocin derivatives.
Despite numerous efforts to identify the main blue chromophore in 1 oligomerization,[4, 5] the structure(s) that confer the hue on bruised Psilocybe mushrooms had been elusive, as was the mechanism by which the colored matter is formed. From previous mass spectrometry and NMR‐based studies, the 5,5’‐coupled product was initially hypothesized to contribute the blue color, given that coupling at the C‐5 position was the major reaction in the investigated enzymatic oxidation of 1.[3] Furthermore, literature on analogous chromophores plausibly suggested a rather reddish color for the 7,7’‐coupled quinoid dimers.[6] However, the mixture was intractable and too reactive, and rapid oligomerization occurred. Therefore, a single defined dimer could not be isolated and only indirect conclusions could be drawn. Furthermore, the color and corresponding saturation of bruised mushrooms changes both temporally and even from specimen to specimen. This implies the presence of multiple chromophores, which complicates a detailed analysis of individual components of the hue.
We sought more profound insight into the oxidation chemistry of 1 by precise characterization and analysis of the individual chromophores produced by its initial dimeric products. A pivotal report by Laatsch describes research to reveal structure‐color correlations by analogously oxidizing specifically designed 4‐hydroxyindole model compounds which carried specific methylations to interrupt product oligomerization at the dimeric level.[4] Inspired by this report, and supporting the recently accomplished chemoenzymatic synthesis of 5‐methylpsilocybin,[7] we synthesized strategically 5‐ and 7‐methylated 1 derivatives that were oxidatively dimerized into stable compounds that could be isolated and characterized. To discern differences in oxidation and reaction behavior of hydroxylated tryptamines, the 5‐hydroxy isomer of 1, bufotenin (3), was also chemically dimerized in a parallel approach and investigated for spectroscopic features.
Results
Chromophore analysis
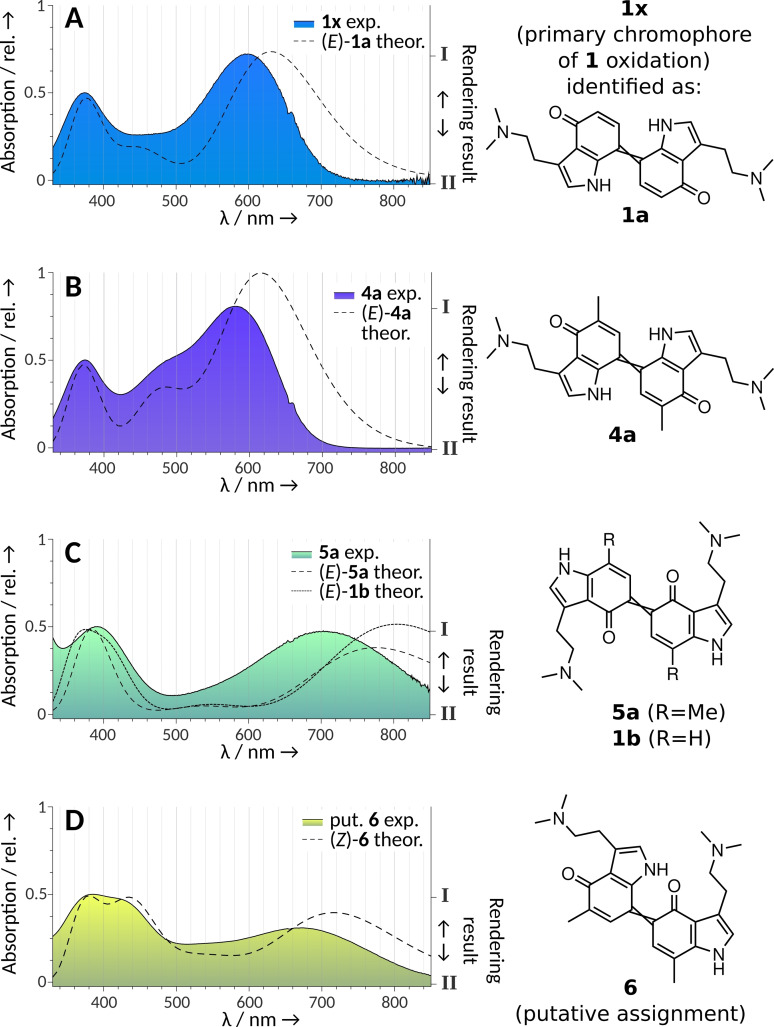
Our previous study on 1 oxidation used mass spectrometry, along with IR and in situ 13C NMR spectroscopy, and identified a heterogeneous mixture of psilocyl oligomers (up to 13‐mers) as products. We also recognized C‐5 as the primary coupling position and therefore proposed the 5,5’‐dimer being primarily responsible for the blue color.[3] During chromatographic and mass spectrometric analysis of the blue product mixture, an unstable compound (hereafter referred to as 1 x) was observed as well, whose ion mass was m/z 405 [M+H]+, which is compatible with a didehydrodimer of 1. However, the exact coupling position(s) remained unknown. The simultaneously recorded UV/Vis spectrum of 1 x suggested a potential contribution to the blue coloration, which prompted us to re‐investigate the structure of the chromophores. The longest absorption maximum appeared at λ=600 nm (Figure 2A). This wavelength corresponds to orange light absorption and, consequently, to a blue hue. Consistently, computational rendering of the full corresponding visible transmission spectrum returned azure RGB‐colors (Figure 2A and Figure S1 in the Supporting Information) for this species. Therefore, we hypothesized most of the blue tint during the initial 1 oxidation cascade is caused by 1 x, as other chromatographic peaks of the oxidation mixture with absorption at long wavelengths showed a much lower intensity.[3]
Figure 2.
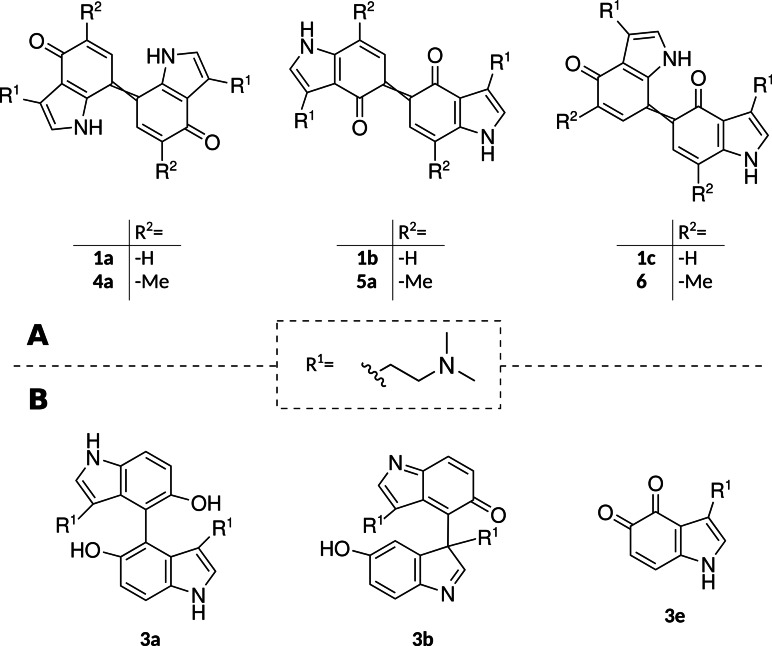
Visual‐range diode array absorption spectra of quinoid dimers of 1 and of their 5‐ and 7‐methylated congeners 4 and 5, as well as comparison with their theoretical spectra calculated with time‐dependent density functional theory (dashed lines). The shading under the curves represents the actual substance colors under the chromatographic conditions (acetonitrile/H2O+0.1 % formic acid) depicted as color gradients between two rendered RGB values, obtained by two different computational methods (HDTV for I and Wα for II, see the Supporting Information). The right y‐axis refers to this gradient. A) The experimental spectrum represents the elusive putative dimer 1 x, which was later shown to be consistent with 1 a (=7,7’‐coupled unmethylated 1), i. e., the main dimeric blue compound of 1 oxidation. B) Dimer 4 a (=7,7’‐coupled, 5,5’‐dimethylated 4). C) Dimer 5 a (5,5’‐coupled, 7,7’‐dimethylated) and theoretical spectrum of 1 b (5,5’‐coupled unmethylated 1). D) Dimer 6 (5,7’‐coupled, 7,5’‐dimethylated 4 and 5).
We sought to clarify which of the three hypothetical 1‐didehydrodimers 1 a (7,7’‐coupled), 1 b (5,5’‐coupled) and 1 c (5,7’‐coupled) possessed absorption properties matching those of 1 x, the putative 1 didehydrodimer that initially attracted our attention during LC‐DAD‐MS analysis. The unstable dimers 1 a‐1 c appeared not readily accessible in pure form via synthesis. Thus, we designed an analogy model and used comparative calculations. The experimental design aimed at selectively interrupting 1 oxidation on the dimeric level before oligomerization proceeds, using 5‐ and 7‐methylated congeners of 1 to strategically block the reactive positions on the indole core. Due to the methyl‐blocked positions, the ring cannot further engage in oligomerization. Consequently, the dimers become amenable to experimental determination of spectroscopic features and subsequent comparison with in silico predicted spectra, calculated by time‐dependent density functional theory (TD‐DFT).
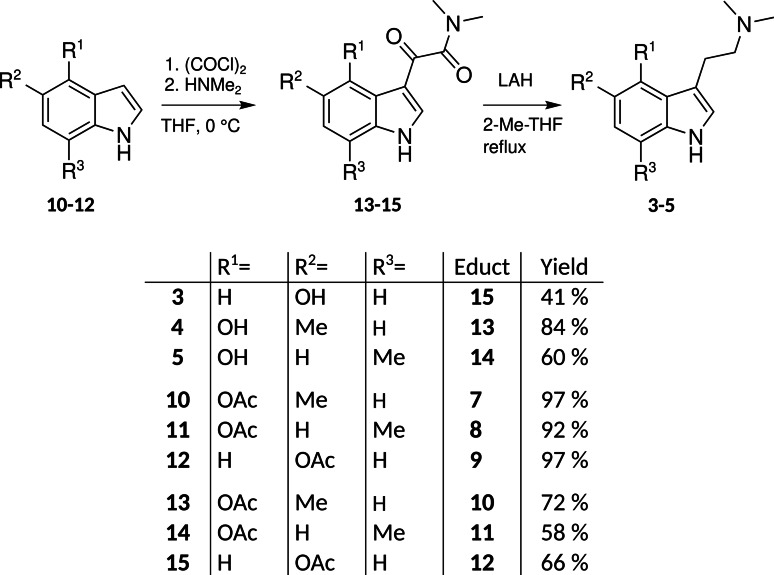
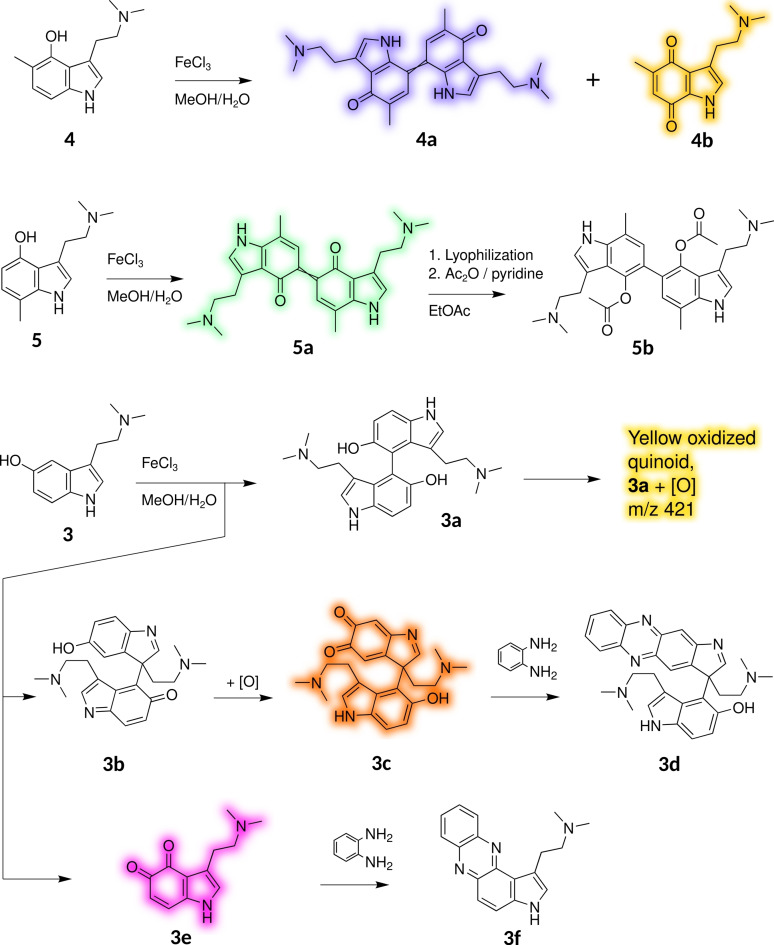
We synthesized 5‐methylpsilocin (4, Figures 1 and S2a‐b) and 7‐methylpsilocin (5, Figures and S3a‐b) from the respective methylated indoles by Speeter‐Anthony tryptamine synthesis (Scheme 1).[8] Upon oxidation of 4 by FeIII in MeOH/H2O, we obtained two stable products that were identified by NMR spectroscopy. The symmetric quinoid para dimer 4 a (Figures 2B and S4a‐e) was found, along with the less abundant monomeric para‐quinone 4 b (Figures 3 and S5a–e).
Scheme 1.
Hydroxytryptamines, intermediates, and analogs, synthesized by Speeter‐Anthony chemistry from 5‐methyl‐1H‐indol‐4‐ol 7, 7‐methyl‐1H‐indol‐4‐ol 8, and 1H‐indol‐5‐ol 9, respectively, as initial reactants.
Figure 3.
A) Hypothetical dimeric quinoids obtained upon 5‐ and/or 7‐coupling of 1 (1 a–c), as well as their respective methylated congeners used in this study. B) Identified primary oxidation products formed upon oxidation of 3 by FeIII in MeOH/H2O.
While the latter is yellow (λ max=432 nm), 4 a showed an intense violet color. Its absorption spectrum is similar to the one obtained from 1 oxidation, that is, 1 x, with a maximum at λ=583 nm (Figure 2B).
Next, we oxidized 5. From published observations with analogous oxidations of methylated naphthols,[4, 6] we hypothesized that a symmetric 5,5’‐coupled ortho‐dimer would absorb at longer wavelengths than the 7,7’‐coupled para dimer. In fact, in the initially obtained green solution, LC‐DAD‐MS identified a didehydrodimer (λ max=703 nm, m/z 433 [M+H]+) as the major product. Its absorption spectrum (Figure 2C), derived from diode array data, corresponded much less to the spectrum obtained from didehydrodimer 1 x, compared to the spectrum obtained from 7,7‐coupled didehydrodimer 4 a. Furthermore, it proved to be unstable and its color faded rapidly, leading to a near‐colorless solution within minutes. After acetylation of the lyophilized oxidation products, using acetic anhydride and pyridine in ethyl acetate, derivative 5 b (Scheme 2, Figures S6a–d) was isolated as the major product (Figure S7). From the characterization of derivative product 5 b, we concluded the ortho‐coupled quinoid 5 a to represent the initially formed dimeric green chromophore, which is easily reduced to its hydroquinoid form to enable acetylation.
Scheme 2.
Oxidation products of 5‐ or 7‐methylated 1 (compounds 4 and 5) and of 3, including derivatives used for structural characterization. Color shading represents actual substance colors as rendered by the described method (HDTV, see Figure 2 and the Supporting Information).
After combined oxidation of equimolar amounts of 4 and 5, we detected another didehydrodimer (m/z 433) that was not found following the separate oxidation of either substrate. The new didehydrodimer demonstrated a yellowish‐greenish color of low saturation and produced broad absorption bands with maximum absorption at λ max=385 and 673 nm (Figure 2D). We tentatively propose the 5,7’‐coupled dimer 6 (Figures 3A and S8) as its structure.
With the experimental absorption spectra obtained from compounds 1 x, 1 a, 4 a, 5 a, and 1 b (Figure 2), we revised our initial hypothesis and preliminarily ascribed the quinoid 7,7’‐dimeric structure 1 a (Figure 3B) to the principal didehydrodimer responsible for the blue chromophore formed upon 1 oxidation. This assignment contrasted our previous hypothesis that the 5,5’‐coupled dimer 1 b was primarily responsible for the blue chromophore. However, despite the similar relative profile in absorbance spectra for 1 a compared to 4 a, the observed overall shift of −17 nm in the longest local maximum from λ=600 nm for putative 1 a (Figure 2A) to 583 nm for methylated derivative 4 a (Figure 2B) was larger than expected. For example, in analogous para‐naphthylidenequinones, the respective 2,2’‐bismethylation accounted for only −3 nm shift.[6b] We therefore corroborated our structural assignments by the quantum chemical calculation of the excitation energies at the B3LYP/def2‐TZVP level of theory.
Quantum chemical calculation
This particular approach was chosen based on results of a benchmark study that compared computed absorption spectra of indigoid dyes with experimentally generated data,[9] but by using a more modern basis set in our study. We modeled the respective diprotonated dimers in aqueous medium (implicit solvent modeling), since the experimental spectra were recorded in a mainly aqueous acidified solvent mixture.
The structure of 4 a (i. e., the 7,7’‐coupled, 5,5’‐methylated dimeric compound) had been proven by NMR (Figures 3A and S4a–e). Using the TD‐DFT approach, a theoretical absorbance spectrum of its E isomer was calculated. The obtained first excitation energy deviated by −0.12 eV (+34 nm) from the experimental value, which is within the common error range of such calculations.[9] Furthermore, the shoulder around λ=490 nm in the spectrum of 4 a (Figure 2B), contributing to its violet appearance, was reproduced by the calculations. Furthermore, an analogous theoretical absorption spectrum for putative structure 1 x was obtained and compared to the experimental result (Figure 2A). The theoretical shift in the longest local maximum for 5,5’‐bismethylation of the para dimer (i. e., 4 a compared to 1 a) was −14 nm and therefore close to the experimentally observed value of −17 nm. The assigned structures for the 5,5’‐coupled dimer 5 a and the putative asymmetrical dimer 6 were also consistent with the calculated theoretical absorbance spectra (Figure 2C and D).
Considering the combined experimentally derived data and the quantum chemical calculations, we concluded that the prototypical blue chromophore of 1 oxidation products, and of bruised Psilocybe mushrooms alike, is represented by the quinoid 7,7’‐coupled dimer 1 a (Figure 3A). Hence, our experimental data does not support our previous hypothesis of the symmetric 5,5’‐coupled 1 b as main component of the blue color. Still, both 1 b and the asymmetric 5,7’‐dimer 1 c may contribute to occasionally occurring green tints and to general desaturation or darkening of the primary blue color.
Characterization of bufotenin oxidation products
To compare oxidation properties and propensity toward dimer formation with naturally occurring C‐4‐ versus C‐5‐hydroxytryptamines, we investigated the oxidation products of bufotenin (3, Figures 1 and S9a‐b), a regioisomer of 1. We used size exclusion chromatography[10] to separate the mixture obtained by 3 oxidation in aqueous MeOH using FeIII to provide four main fractions (A–D, in order of elution, Figure S10). Putative ortho‐quinones (3 c and 3 e, Scheme 2) were further derivatized with o‐phenylenediamine, and the products were characterized.
Fraction A was yellow and contained an intractable heterogeneous mixture of trimers and higher order products. Fraction B was also yellow and contained the major product of the reaction. LC–DAD‐MS analysis identified a colorless compound as primary component. Its mass (m/z 407 [M+H]+) pointed to a dehydrodimer. Furthermore, a yellow species (λ max=434 nm, m/z 421 [M+H]+), possibly derived from the former by further oxidation was present in trace quantities. After lyophilization of fraction B, the main dehydrodimer was isolated by semi‐preparative HPLC and identified by HRMS, 1H and 13C NMR as brachybotryne, that is, 4,4’‐bibufotenin (3 a, Figures 3B and S11a–d).[11] Fraction C was orange and contained a near‐colorless didehydrodimer (3 b, very weak visual absorption band at about λ=460 nm, m/z 405 [M+H]+, Figure S12) that autoxidatively produced a more strongly visible‐light‐absorbing compound (3 c, λ max=462 nm, m/z 421 [M+H]+, Scheme 2).
After treatment with o‐phenylenediamine, the phenazine compound 3 d (Scheme 2, Figures S13a–e) was isolated, suggestive of 3 b and 3 c to represent the respective initial oxidation products. Fraction D was purple and contained mainly the monomeric o‐quinone 3 e (λ max=540 nm, m/z 219 [M+H]+, Scheme 2) by direct treatment with o‐phenylenediamine and subsequent isolation of phenazine 3 f (Scheme 2, Figures S14a–d). Comparing oxidation products of 1 and 3, we infer that the main coupling sites are switched from C‐5 and C‐7 in 4‐HTs to C‐3 and C‐4 in 5‐HTs. In part, this can be viewed as a direct outcome of respective stabilization patterns of the initially formed hydroxytryptamine radicals. The obtained major oligomeric quinoids of oxidized 4‐HTs generally show lower first excitation energies than those of 5‐HTs, yielding blue to green colors in the former versus yellow to orange colors in the latter.
Discussion
Color changes or formation following oxidation of small molecule natural products are known from other mushroom species as well. Gyrocyanine is a l‐tyrosine‐derived diarylcyclopentenone pigment that was isolated from various boletes. Upon mycelial injury and concomitant exposure to air, an intensely blue anion is formed.[12] A similar chromophore change is observed with pulvinic acids, for example variegatic acid, which is a yellow lactone pigment that turns into a blue quinone methide anion chromophore when oxidized.[13] Contrasting 1, these natural products remain monomeric as the blue chromophore is formed, although oxidative dimerization is known to turn variegatic acid into badione A and norbadione A, which confer the brownish color on the cap of the producing mushrooms.
Recently, we presented an enzymatic cascade both for 2 biosynthesis[14] and for conversion of 2 to 1 and subsequent oligomerization along with evidence for a complex mixture of chromophoric structures.[3] We hypothesized that in higher oligomers of 1, more than one of the respective chromophoric systems is present, eventually leading to blueish polymers. Building on these previous results, we now identified the chromophore of 1 a, the symmetrical 7,7’‐didehydrodimer of 1, to contribute most of the blue color in the reaction. 5,5’‐couplings may contribute a green tint by forming ortho‐biindolylidenedione chromophores, while asymmetrical (e. g., 5,7’‐coupled) or higher order chromophores may cause color desaturation or browning. Purification of the methylated quinoid model reaction products revealed that the p‐quinoid structures were more stable than the o‐quinoids. The latter were prone to decolorization by follow‐up reactions and reduction to their hydroquinoid form, presumably driven by the close proximity of the electron withdrawing carbonyls and a more positive redox potential, as found for analogous biphenols.[15, 16] As the 5‐position of the indole scaffold appears kinetically preferred during coupling,[3] we assume these instabilities explain why the blue color predominates: the kinetically favored, yet unstable o‐coupled green chromophores fade rapidly while the more chemically stable para‐quinoids persist and confer the blue color.
The oxidation of 3 closely resembles that of serotonin.[10] Given the yellow hue of the oxidation products of 3, they may contribute to the yellowish appearance of basidiocarps of the false death cap (Amanita citrina), known to biosynthesize 3.[17] Research on the natural occurrence of 3 oxidation products is scarce, and their potential ecological functions have remained unknown. However, numerous reports exist on the bioactivity and ecological function of other mono‐ or dimeric indolic mushroom natural products. Indigo, indirubin and isatin (an indoline) were observed in the confrontation zone of two mushroom species.[18] Modified and extended indole systems, as found in the iminoquinone mycenarubin D of the mushroom Mycena haematopus [19] or in the β‐carboline infractopicrin of Cortinarius infractus,[20] were recognized as active against soil bacteria and conferring a highly bitter taste on the producer which deters feeding predators, respectively. In contrast, the ecological advantage for 2 production in Psilocybe mushrooms remains obscure. Given that 1 binds to neurotransmitter receptors with high affinity, monomeric 1, formed from 2, may protect the mushroom from mycophagous insects by interfering with their behavior.[21] An alternative hypothesis includes that 1 is produced from 2 to fulfill an ecological function as an oligomeric „defense‐on‐demand“ mixture.[2b] Its polyphenolic properties may generate reactive oxygen species and mediate binding to and precipitation of proteins. Therefore, the chromophoric psilocyl oligomers may exert an immediate deleterious effect on feeding insects and may represent the true ecologically relevant molecules for which 2 is accumulated as their chemically stable precursor.
Conclusion
The dimerization of 1 is a key step of the spectacular blueing reaction of Psilocybe magic mushrooms. Due to the dynamic progress and subsequent oligo‐/polymerization, the nature of the initial blue compound(s) had remained elusive. Experimental and computational evidence derived from 1 as well as from methylated derivatives as surrogates proved the quinoid chromophore of the 7,7’‐coupled dimer of 1 to confer the blue color on injured mushrooms. Our work sheds light on an iconic phenomenon that has intrigued natural product chemists, and amateurs alike, for decades.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
We thank A. Perner, H. Heinecke, and V. Hänsch (Leibniz Institute for Natural Product Research and Infection Biology, Hans‐Knöll‐Institute Jena) for recording HRMS and NMR spectra, and for support with chemical syntheses, respectively. We thank O. Lenz (Otto Group, Dresden) for adapting the specrend source code. C.L. acknowledges a doctoral fellowship by the International Leibniz Research School (ILRS) for Microbial Interactions. This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project ID 239748522‐SFB 1127 and by the Usona Institute (Madison, WI). Open access funding enabled and organized by Projekt DEAL.
C. Lenz, S. Dörner, A. Sherwood, D. Hoffmeister, Chem. Eur. J. 2021, 27, 12166.
References
- 1.
- 1a.Tyls F., Palenicek T., Horacek J., Europ. Neuropsychopharmacol. 2014, 24, 342–356; [DOI] [PubMed] [Google Scholar]
- 1b.Kargbo R. B., ACS Med. Chem. Lett. 2020, 11, 399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.
- 2a.Nichols D. E., J. Antibiot. 2020, 73, 679–686; [DOI] [PubMed] [Google Scholar]
- 2b.Lenz C., Sherwood A., Kargbo R., Hoffmeister D., ChemPlusChem 2021, 86, 28–35; [DOI] [PubMed] [Google Scholar]
- 2c.Fricke J., Lenz C., Wick J., Blei F., Hoffmeister D., Chem. Eur. J. 2019, 25, 897–903. [DOI] [PubMed] [Google Scholar]
- 3.Lenz C., Wick J., Braga D., García-Altares M., Lackner G., Hertweck C., Gressler M., Hoffmeister D., Angew. Chem. Int. Ed. 2020, 59, 1450–1454; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 1466–1470. [Google Scholar]
- 4.Laatsch H. in Yearbook of the European College for the Study of Consciousness 1997 (Eds.: Leuner H.-C., Schlichting M.), Verlag für Wissenschaft und Bildung, Berlin, 1998, pp. 241–256. [Google Scholar]
- 5.
- 5a.Blaschko H., Levine W. G., Biochem. Pharmacol. 1960, 3, 168–169; [Google Scholar]
- 5b.Blaschko H., Levine W. G., Br. J. Pharmacol. 1960, 15, 625–633; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5c.Levine W. G., Nature 1967, 215, 1292–1293. [DOI] [PubMed] [Google Scholar]
- 6.
- 6a.Göltner C., Laatsch H., Liebigs Ann. Chem. 1991, 1085–1089; [Google Scholar]
- 6b.Kral A., Laatsch H., Z. Naturforsch. B 2014, 48, 1401–1407. [Google Scholar]
- 7.Fricke J., Sherwood A. M., Halberstadt A. L., Kargbo R. B., Hoffmeister D., J. Nat. Prod. 2021, 84, 1403–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speeter M. E., Anthony W. C., J. Am. Chem. Soc. 1954, 76, 6208–6210. [Google Scholar]
- 9.Perpète E. A., Jacquemin D., J. Mol. Struct. 2009, 914, 100–105. [Google Scholar]
- 10.
- 10a.Wrona M. Z., Dryhurst G., J. Org. Chem. 1987, 52, 2817–2825; [Google Scholar]
- 10b.Wrona M. Z., Dryhurst G., J. Pharm. Sci. 1988, 77, 911–917. [DOI] [PubMed] [Google Scholar]
- 11.dos S. Ribeiro M. A., Gomes C. M. B., Formagio A. S. N., Pereira Z. V., Melo U. Z., Basso E. A., da Costa W. F., Baldoqui D. C., Sarragiotto M. H., Tetrahedron Lett. 2016, 57, 1331–1334. [Google Scholar]
- 12.
- 12a.Steglich W., Thilmann A., Besl H., Bresinsky A., Z. Naturforsch. 1977, 32c, 46–48; [Google Scholar]
- 12b.Gill M., Steglich W., Prog. Chem. Org. Nat. Prod. 1987, 51, 1–317. [DOI] [PubMed] [Google Scholar]
- 13.Steglich W., Furtner W., Prox A., Z. Naturforsch. 1968, 23b, 1044–1050. [PubMed] [Google Scholar]
- 14.
- 14a.Fricke J., Blei F., Hoffmeister D., Angew. Chem. Int. Ed. 2017, 56, 12352–12355; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 12524–12527; [Google Scholar]
- 14b.Blei F., Baldeweg F., Fricke J., Hoffmeister D., Chem. Eur. J. 2018, 24, 10028–10031. [DOI] [PubMed] [Google Scholar]
- 15.Guan B., Wan P., J. Chem. Soc. Chem. Commun. 1993, 409–410. [Google Scholar]
- 16.Jonsson M., Lind J., Merényi G., J. Phys. Chem. A 2002, 106, 4758–4762. [Google Scholar]
- 17.Beutler J. A., Der Marderosian A. H., J. Nat. Prod. 1981, 44, 422–431. [Google Scholar]
- 18.Krause K., Jung E.-M., Lindner J., Hardiman I., Poetschner J., Madhavan S., Matthäus C., Kai M., Menezes R. C., Popp J., Svatoš A., Kothe E., PLoS One 2020, 15, e0232145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohmann J. S., Wagner S., von Nussbaum M., Pulte A., Steglich W., Spiteller P., Chem. Eur. J. 2018, 24, 8609–8614. [DOI] [PubMed] [Google Scholar]
- 20.
- 20a.Steglich W., Kopanski L., Wolf M., Moser M., Tegtmeyer G., Tetrahedron Lett. 1984, 25, 2341–2344; [Google Scholar]
- 20b.Spiteller P., Chem. Eur. J. 2008, 14, 9100–9110. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds H. T., Vijayakumar V., Gluck-Thaler E., Korotkin H. B., Matheny P. B., Slot J. C., Evol. Lett. 2018, 2, 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information