Abstract
Objective
To evaluate the effect of withdrawing ixekizumab in patients with psoriatic arthritis (PsA) in whom minimal disease activity (MDA) has been achieved after open‐label ixekizumab treatment.
Methods
SPIRIT‐P3 was a multicenter, randomized, double‐blind withdrawal study of biologic treatment–naive adult patients with PsA who were treated with open‐label ixekizumab for 36 weeks (160 mg at week 0, then 80 mg every 2 weeks). Patients in whom MDA was sustained for >3 consecutive months were randomized 1:1, between weeks 36 and 64, to undergo blinded withdrawal of ixekizumab treatment (placebo) or to continue ixekizumab treatment every 2 weeks up to week 104. The primary efficacy end point was time to relapse (loss of MDA) for randomized patients. Patients who experienced a relapse were re‐treated with ixekizumab every 2 weeks up to week 104.
Results
A total of 394 patients were enrolled and received open‐label ixekizumab every 2 weeks. Of those patients, 158 (40%) achieved sustained MDA and were randomized to undergo withdrawal of ixekizumab treatment (placebo every 2 weeks; n = 79) or to continue ixekizumab treatment every 2 weeks (n = 79). Disease relapse occurred more rapidly with treatment withdrawal (median 22.3 weeks [95% confidence interval (95% CI) 16.1–28.3]) compared to those who continued treatment with ixekizumab (median not estimable; P < 0.0001). Sixty‐seven patients (85%) compared to 30 patients (38%) experienced relapse in the placebo group and the continued treatment group, respectively. Median time to achieving MDA again with re‐treatment was 4.1 weeks (95% CI 4.1–4.3); in 64 of 67 patients (96%) who experienced relapse with treatment withdrawal, MDA was achieved again with re‐treatment. Safety was consistent with the known safety profile for ixekizumab.
Conclusion
Continued ixekizumab therapy is superior to ixekizumab withdrawal in maintaining low disease activity in biologic treatment–naive patients with PsA. Re‐treatment with ixekizumab following a relapse may restore disease control in cases of treatment interruption.
INTRODUCTION
Psoriatic arthritis (PsA) is a chronic, heterogeneous, inflammatory disease that may lead to serious disability if not appropriately treated (1, 2, 3). There are a number of disease‐modifying antirheumatic drugs (DMARDs) available to patients with PsA, including conventional synthetic DMARDs (csDMARDs) and biologic DMARDs (bDMARDs) (4, 5). These treatments can help patients achieve low disease activity or remission across the manifestations of PsA; however, it is unclear whether patients with long‐term low disease activity or remission need to continue treatment to maintain this outcome. Dose tapering or treatment discontinuation may potentially be cost effective and could limit potential side effects associated with PsA treatments. Data on treatment withdrawal in PsA are limited and inconsistent, with a few small observational, uncontrolled studies available (6, 7, 8, 9, 10). These studies used various outcome measures to evaluate the effect of bDMARD treatment withdrawal (8, 10) or to compare csDMARD and bDMARD withdrawal (6, 7, 9), or analyzed patients from a PsA registry (10), indicating that further assessment in a large, controlled withdrawal trial is warranted.
Ixekizumab, a high‐affinity monoclonal antibody that selectively targets interleukin‐17A (IL‐17A) (11), has been demonstrated to improve the signs and symptoms of active PsA in 2 phase III trials with long‐term extensions (SPIRIT‐P1 [12, 13, 14] and SPIRIT‐P2 [15, 16, 17]). The present study, SPIRIT‐P3, is the first large, multicenter, randomized, double‐blind, placebo‐controlled withdrawal study in patients with PsA. The study evaluated the efficacy and safety of withdrawing ixekizumab treatment versus continued ixekizumab treatment in patients who had achieved stable minimal disease activity (MDA) (18) while receiving ixekizumab therapy, and the impact of re‐treatment after relapse.
PATIENTS AND METHODS
Trial design
SPIRIT‐P3 (ClinicalTrials.gov identifier NCT02584855; European Union Clinical Trials Register identifier 2015‐002433‐22) was a phase III, multicenter study with an initial open‐label treatment period, followed by a randomized double‐blind withdrawal period (Figure 1). The study was conducted at 86 sites in 12 countries (for a list of investigators, see Appendix A). During the initial 36‐week open‐label treatment period, all patients received a starting dose of 160 mg ixekizumab at week 0, followed by 80 mg ixekizumab every 2 weeks to week 36. Between weeks 36 and 64, patients who exhibited sustained MDA for ≥4 visits over 3 consecutive months were eligible for 1:1 blinded randomization to continue receiving ixekizumab every 2 weeks or to undergo ixekizumab withdrawal every 2 weeks (placebo) up to week 104. Patients whose disease relapsed following ixekizumab withdrawal (i.e., no longer meeting MDA criteria) (see below) received ixekizumab every 2 weeks in a blinded manner until week 104. Patients who did not meet randomization criteria by week 64 continued receiving open‐label ixekizumab every 2 weeks uninterrupted up to week 104. Patients were discontinued from the study if ≥20% improvement in tender joint counts (TJCs) and swollen joint counts (SJCs) had not been achieved at week 24 or at any subsequent visit through week 104, except from the point of randomization until the visit following relapse in patients during the randomized withdrawal period.
Figure 1.
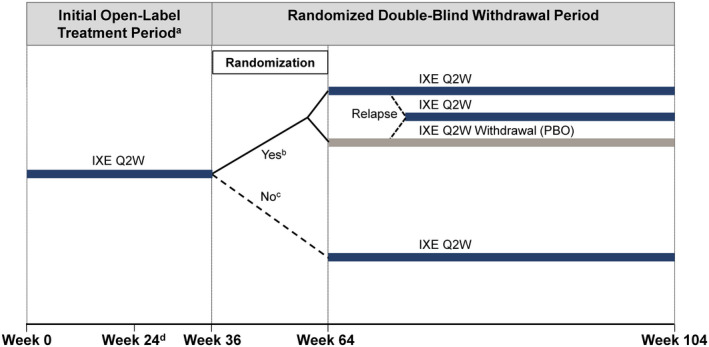
SPIRIT‐P3 study design. a Encompassed week 0 (study baseline) up to week 36. b Between weeks 36 and 64 (inclusive), patients treated with ixekizumab (IXE) every 2 weeks (Q2W) in whom minimal disease activity (MDA) was achieved for 4 consecutive visits for at least 36 weeks were eligible for randomization at the visit at which these criteria were met. Patients were randomized 1:1 to the ixekizumab every 2 weeks group or the ixekizumab withdrawal group. Patients remained in their treatment groups up to week 104 or until relapse (no longer met MDA), at which point they received ixekizumab every 2 weeks up to week 104. c Patients who did not meet the randomization eligibility criteria by week 64 continued to receive ixekizumab every 2 weeks uninterrupted up to week 104. d Patients in whom ≥20% improvement in tender joint count and swollen joint count at week 24 or at any subsequent visit through week 104, except from the point of randomization until the visit after relapse for patients in the randomized double‐blind withdrawal period, were discontinued from the study. PBO = placebo.
All patients provided written informed consent before any study assessments, examinations, or procedures were performed. The study was approved by the ethical or institutional review boards at each participating study site and was conducted in accordance with the Declaration of Helsinki, the Council for International Organizations of Medical Sciences, the International Conference on Harmonisation Guidelines for Good Clinical Practice, and applicable laws and regulations.
Trial participants
SPIRIT‐P3 enrolled adults age ≥18 years with a confirmed diagnosis of active PsA for ≥6 months and who fulfilled the criteria of the Classification of Psoriatic Arthritis Study Group (19). Active PsA was defined as the presence of ≥3 of 68 tender joints and ≥3 of 66 swollen joints at screening and baseline. Patients were required to have documented inadequate response or intolerance to ≥1 csDMARDs and active psoriatic skin lesions or a documented history of plaque psoriasis. Exclusion criteria included current use of >1 csDMARD, current or prior use of bDMARDs or small‐molecule agents for treatment of psoriasis or PsA, active Crohn’s disease or ulcerative colitis, or active uveitis.
During the initial open‐label treatment period, alteration of csDMARD dosage and/or introduction of a new csDMARD were permitted. During the randomized withdrawal period, alteration of the csDMARD dosage and/or introduction of a new csDMARD in patients who were randomized was not permitted until the point of relapse. Patients who were not randomized could continue alteration of csDMARD dosage and/or introduction of a new csDMARD throughout the randomized withdrawal period.
Randomization and blinding
During the randomized withdrawal period, randomized treatment was assigned to eligible patients using an interactive web‐response system. Patients who met the criteria for randomized withdrawal were assigned in a 1:1 ratio (stratified by geographic region and csDMARD use at the time of randomization) to receive blinded ixekizumab treatment every 2 weeks (1 80‐mg subcutaneous injection or matching placebo every 2 weeks) from week 36 to week 104. Patients, study site personnel, and investigators remained blinded with regard to treatment assignment and dosage adjustments throughout the randomized withdrawal period from week 36 to week 104.
Procedures
Following open‐label treatment with ixekizumab every 2 weeks, MDA was used to establish eligibility for entry into the randomized withdrawal period. Patients were assessed for MDA at each post‐baseline visit, starting at week 2. MDA was considered to have been achieved if at least 5 of the following 7 disease activity measures were met: TJC ≤1, SJC ≤1, Psoriasis Area and Severity Index (PASI) (20) total score ≤1 or body surface area (BSA) affected by psoriasis ≤3%, patient pain visual analog scale (VAS) score ≤15 (of a maximum possible 100), patient global assessment of disease activity (PtGA) VAS score ≤20 (of a maximum possible 100), Health Assessment Questionnaire (HAQ) disability index (DI) (21) score ≤0.5, and number of tender entheseal points ≤1 (18). Patients in whom sustained MDA was exhibited at ≥4 visits over 3 consecutive months qualified for randomization to either continue ixekizumab treatment every 2 weeks or undergo withdrawal of ixekizumab treatment (placebo). The first opportunity for randomization at week 36 was based on 3 months of sustained MDA from week 24. Patients were considered to have experienced a relapse if their status could no longer be classified as MDA (i.e., <5 of 7 of the above criteria were met) at any point in the randomized withdrawal period up to week 104.
Efficacy and safety assessments
The primary efficacy end point was time to relapse (loss of MDA) during the randomized withdrawal period. Secondary efficacy end points included the cumulative relapse rate and time to loss of response for each individual MDA component. Median time to regain MDA and sustained MDA was evaluated in patients who experienced a relapse and were re‐treated with ixekizumab every 2 weeks during the randomized withdrawal period. Post hoc efficacy analyses were performed for patients who experienced a relapse during the randomized withdrawal period, and were conducted to assess the number of MDA components lost at the time of relapse and to evaluate relapse rates in patients in whom very low disease activity (VLDA) was achieved (7 of 7 disease activity measures met) (22) and in patients in whom MDA was achieved but VLDA was not achieved. Safety assessments included treatment‐emergent adverse events, serious adverse events, and adverse events of special interest.
Statistical analysis
Sample size was determined using the assumption that of ~400 patients who entered the initial open‐label treatment period, 136 patients (34%) would meet the criteria for sustained MDA and qualify for entry into the randomized withdrawal period (68 per treatment group). It was further assumed that ~60% and ~20% of patients in the ixekizumab withdrawal group and the continued ixekizumab treatment every 2 weeks group, respectively, would experience a relapse by no longer meeting the MDA criteria. According to these assumptions, 39 patients were needed to meet relapse criteria in the combined treatment groups in order to achieve 95% power to test the superiority of ixekizumab treatment compared to withdrawal of ixekizumab treatment (placebo) for time to relapse with a 2‐sided α significance level of 0.05.
The open‐label population was defined as all patients who received at least 1 dose of open‐label ixekizumab every 2 weeks during the open‐label treatment period. The randomized withdrawal intent‐to‐treat (ITT) population included all randomized patients (those who achieved sustained MDA and 3 patients who did not achieve sustained MDA who were inadvertently randomized). Patients in the randomized withdrawal ITT population were analyzed according to their treatment assignment (withdrawal of ixekizumab treatment [placebo] or continued ixekizumab treatment every 2 weeks). The relapse population was defined as all randomized patients who experienced relapse (i.e., no longer meeting MDA criteria) after randomization and received at least 1 dose of ixekizumab every 2 weeks after experiencing relapse.
The primary efficacy end point was time to relapse (loss of MDA) during the randomized withdrawal period for the randomized withdrawal ITT population. The Kaplan‐Meier product limit method was used to estimate survival curves for time to variables. Treatment comparisons were performed using a log‐rank test, with adjustment for geographic region and csDMARD use at the time of randomization. P values less than 0.05 were considered statistically significant. Time to relapse in weeks was defined as follows: ([date of relapse − date of first injection of randomized dose of study treatment in the randomized double‐blind withdrawal period] + 1)/7. Patients completing the withdrawal period without meeting relapse criteria were censored at the date of completion (the date of the last scheduled visit in the withdrawal period). Patients without a date of completion or discontinuation were censored at the latest nonmissing date from the following dates: date of last injection of study treatment in the withdrawal period and date of last attended visit in the withdrawal period.
Cumulative proportion of relapse was analyzed using a logistic regression model, with treatment, geographic region, and csDMARD use at the time of randomization as factors. Since the first opportunity for randomization at week 36 was based on 3 months of sustained MDA from week 24 in a 104‐week study, the cumulative proportion of relapse was analyzed up to the first 40 weeks of the randomized withdrawal period.
Safety data are presented for the randomized withdrawal ITT population and for the all‐ixekizumab combined safety population, which comprised all patients who received at least 1 dose of ixekizumab during the study.
RESULTS
Patient disposition and baseline characteristics
Between November 18, 2015 and October 30, 2018, 511 patients were screened, of whom 100 (20%) did not pass the screening (see Supplementary Figure 1, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41716/abstract). Three hundred ninety‐four patients were enrolled in the study and treated with open‐label ixekizumab every 2 weeks. By week 36, 291 patients (74%) completed the open‐label treatment period, and 103 patients (26%) discontinued the study. The main reason for discontinuation was lack of efficacy. Sustained MDA was achieved in a total of 158 of 394 patients (40%), and they qualified for double‐blind randomization (79 patients were randomized to the withdrawal of ixekizumab group [placebo] and 79 were randomized to the continued ixekizumab treatment every 2 weeks group). Sustained MDA was not achieved in a total of 133 of the 394 patients (34%). These patients were not randomized and continued receiving open‐label ixekizumab treatment every 2 weeks (Supplementary Figure 1, http://onlinelibrary.wiley.com/doi/10.1002/art.41716/abstract).
Patients enrolled in the open‐label study had symptoms of PsA for an average of 8 years. The mean age of these patients was 47 years, and 54% were women. The majority of patients were taking concomitant csDMARDs (most commonly methotrexate) and had high disease activity at baseline, with a mean TJC of 21 and a mean SJC of 10; 70% and 84% had enthesitis according to the Leeds Enthesitis Index (23) and Spondyloarthritis Research Consortium of Canada index, respectively (24) (Table 1). In the randomized withdrawal ITT population, a higher proportion of the patients were male, and disease activity was numerically lower, compared to the open‐label population. Within the randomized withdrawal ITT population, baseline demographic and disease characteristics were generally well balanced between the withdrawal of ixekizumab treatment group and the continued ixekizumab treatment every 2 weeks group (Table 1).
Table 1.
Demographic and baseline clinical characteristics of patients in the SPIRIT‐P3 study*
|
Open‐label population (ixekizumab every 2 weeks) (n = 394) |
Randomized withdrawal ITT population | ||
|---|---|---|---|
|
Ixekizumab withdrawal (n = 79) |
Ixekizumab every 2 weeks (n = 79) |
||
| Age, years | 47 ± 11.4 | 43 ± 10.5 | 44 ± 10.8 |
| Male, no. (%) | 182 (46) | 40 (51) | 47 (60) |
| BMI, kg/m2 | 29 ± 6.3 | 29 ± 7.2 | 28 ± 5.0 |
| Time since PsA onset, years | 7.9 ± 7.1 | 7.5 ± 7.5 | 7.1 ± 6.3 |
| Current csDMARD use, no. (%)† | 291 (74) | 60 (76) | 59 (75) |
| TJC, 68 joints | 21 ± 14.3 | 16 ± 12.3 | 17 ± 11.5 |
| SJC, 66 joints | 10 ± 8.1 | 9.0 ± 5.6 | 9.4 ± 7.4 |
| HAQ DI total score | 1.2 ± 0.6 | 1.0 ± 0.5 | 1.1 ± 0.6 |
| Pain VAS score (maximum possible 100) | 61 ± 18.0 | 59 ± 18.9 | 60 ± 19.4 |
| PtGA score (maximum possible 100) | 62 ± 18.9 | 61 ± 19.5 | 59 ± 18.3 |
| PASI total score‡ | 7.1 ± 9.5 | 7.6 ± 10.2 | 8.4 ± 8.2 |
| BSA, %§ | 14 ± 17.6 | 14 ± 17.8 | 17 ± 18.2 |
| LEI score >0, no. (%) | 276 (70.1) | 47 (59.5) | 48 (60.8) |
| LEI total score¶ | 2.6 ± 1.5 | 2.5 ± 1.3 | 2.4 ± 1.3 |
| Enthesitis SPARCC score >0, no. (%) | 330 (83.8) | 57 (72.2) | 62 (78.5) |
| Enthesitis SPARCC score# | 5.3 ± 3.7 | 4.4 ± 3.3 | 4.6 ± 3.1 |
Except where indicated otherwise, values are the mean ± SD. ITT = intent‐to‐treat; BMI = body mass index; PsA = psoriatic arthritis; TJC = tender joint count; SJC = swollen joint count; HAQ DI = Health Assessment Questionnaire disability index; VAS = visual analog scale; PtGA = patient global assessment of disease activity.
Current use of conventional synthetic disease‐modifying antirheumatic drugs (csDMARDs) (methotrexate, sulfasalazine, leflunomide, hydroxychloroquine, or cyclosporine) reported in open‐label population and at time of randomization.
In patients with a baseline Psoriasis Area and Severity Index (PASI) >0.
In patients whose percentage of body surface area (BSA) affected by psoriasis was >0 at baseline.
In patients with a baseline Leeds Enthesitis Index (LEI) >0.
Based on 16‐point entheseal point assessment in patients with a baseline Spondyloarthritis Research Consortium of Canada (SPARCC) enthesitis score >0.
Clinical end points
Patients experienced relapse (lost MDA) more rapidly with treatment withdrawal compared to continued ixekizumab treatment every 2 weeks (P < 0.0001) (Figure 2). The median time to relapse for patients in the withdrawal of ixekizumab treatment group was 22.3 weeks (95% confidence interval [95% CI] 16.1–28.3), while the median time to relapse for patients in the continued ixekizumab treatment group was not estimable, as <50% of patients experienced relapse by the end of the study period. The cumulative relapse rate in the first 40 weeks of the randomized withdrawal period was significantly higher in the treatment withdrawal group (58 of 79 patients [73%]) compared to the continued ixekizumab treatment every 2 weeks group (27 of 79 patients [34%]) (P < 0.0001). The cumulative relapse rate from week 24 to week 104 was also significantly higher in the treatment withdrawal group (67 of 79 patients [85%]) compared to the continued ixekizumab treatment every 2 weeks group (30 of 79 patients [38%]) (P < 0.0001).
Figure 2.
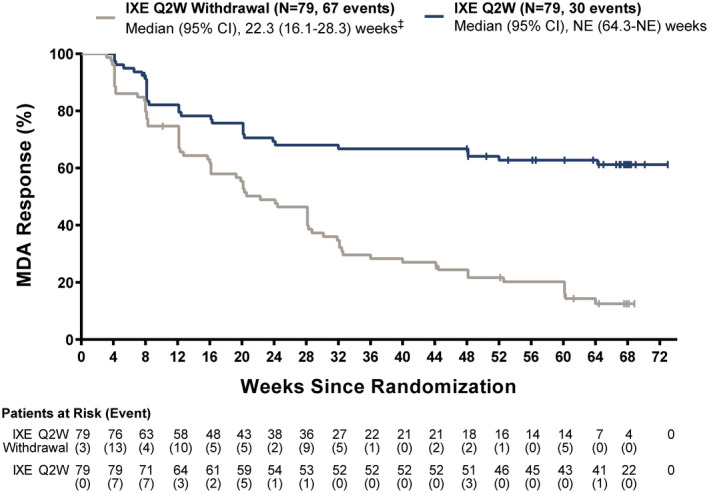
Time to relapse (loss of MDA) in the randomized withdrawal intent‐to‐treat population. ‡ P < 0.0001 versus ixekizumab withdrawal. 95% CI = 95% confidence interval; NE = not estimable (see Figure 1 for other definitions).
For individual components of MDA, relapse occurred more frequently and time to relapse was significantly shorter in patients in the treatment withdrawal group compared to those in the continued ixekizumab treatment every 2 weeks group among patients who met the MDA component at randomization. A total of 72% of patients lost TJC ≤1 (at a median of 22.3 weeks) in the treatment withdrawal group compared to 48% (at a median of 64.3 weeks) in the continued ixekizumab treatment group (P = 0.0022); 45% of patients lost SJC ≤1 (at a median of 28.7 weeks) in the treatment withdrawal group compared to 15% in the continued ixekizumab treatment group (median not estimable) (P < 0.0001); 44% of patients lost PASI total score ≤1 (at a median of 36.0 weeks) in the treatment withdrawal group compared to 12% in the continued ixekizumab treatment group (median not estimable) (P < 0.0001); 24% of patients lost BSA ≤3% in the treatment withdrawal group compared to 4% in the continued ixekizumab treatment group (medians not estimable) (P = 0.0001); 90% of patients lost patient pain VAS score ≤15 (at a median of 16.1 weeks) in the treatment withdrawal group compared to 42% in the continued ixekizumab treatment group (median not estimable) (P < 0.0001); and 76% of patients lost PtGA VAS score ≤20 (at a median of 20.6 weeks) in the treatment withdrawal group compared to 26% in the continued ixekizumab treatment group (median not estimable) (P < 0.0001). For loss of the HAQ DI and enthesitis components of the MDA criteria, the differences between treatment groups were not significant (Figure 3). Kaplan‐Meier curves of time to loss of all individual components are shown in Supplementary Figures 2–9, on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41716/abstract.
Figure 3.
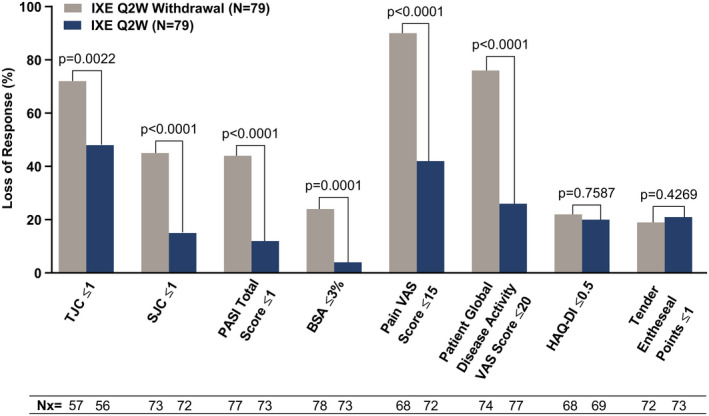
Loss of response for individual components of MDA in the randomized withdrawal intent‐to‐treat population. P values were determined by adjusted log rank test after stratification by geographic region and use of conventional synthetic disease‐modifying antirheumatic drugs. TJC = tender joint count; SJC = swollen joint count; PASI = Psoriasis Area and Severity Index; BSA = body surface area affected by psoriasis; VAS = visual analog scale; HAQ DI = Health Assessment Questionnaire disability index; Nx = the number of patients who met the MDA component at randomization and subsequently lost the response (see Figure 1 for other definitions).
Median time to reachievement of MDA on re‐treatment following relapse was 4.1 weeks (95% CI 4.1–4.3) in the ixekizumab withdrawal/ixekizumab re‐treatment group and 4.7 weeks (95% CI 4.1–8.3) in the continued ixekizumab/ixekizumab re‐treatment group, with week 0 representing the start of re‐treatment (Figure 4A). MDA was reachieved during the re‐treatment period in 64 of 67 patients (95.5%) in the ixekizumab withdrawal/ixekizumab re‐treatment group and 27 of 30 patients (90.0%) in the continued ixekizumab/ixekizumab re‐treatment group.
Figure 4.
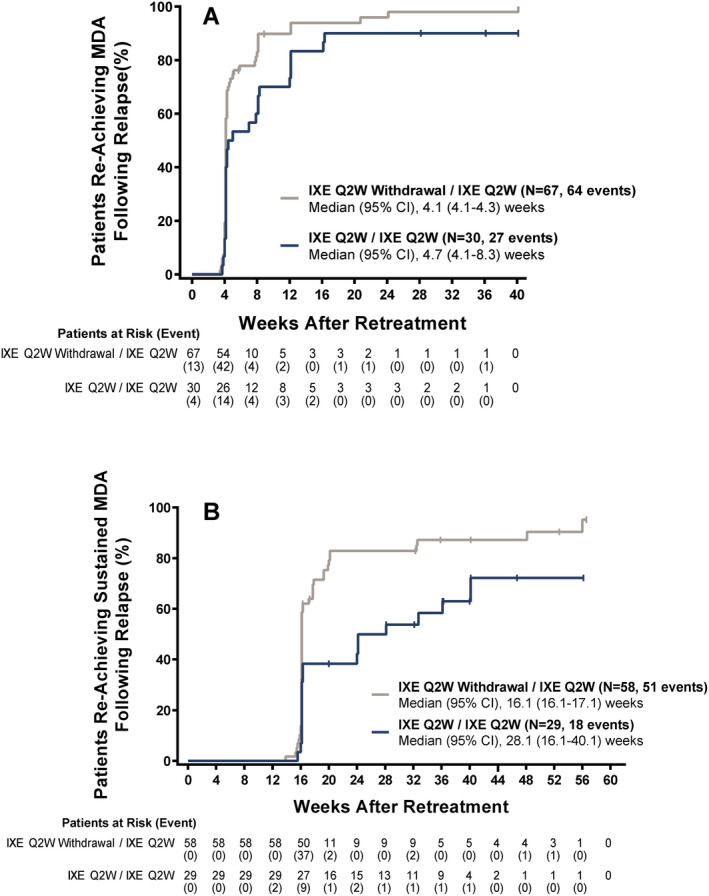
Time to reachievement of MDA following relapse (A) or reachievement of sustained MDA (≥4 visits over 3 consecutive months) following relapse (B). Week 0 represents re‐treatment. Data were available through week 40 in A and through week 60 in B. 95% CI = 95% confidence interval (see Figure 1 for other definitions).
Median time to reachievement of sustained MDA (≥4 visits over 3 consecutive months) on re‐treatment following relapse was 16.1 weeks (95% CI 16.1–17.1) in the ixekizumab withdrawal/ixekizumab re‐treatment group and 28.1 weeks (95% CI 16.1–40.1) in the continued ixekizumab/ixekizumab re‐treatment group, with week 0 representing the start of re‐treatment (Figure 4B). Sustained MDA was reachieved during the re‐treatment period in 51 of 58 patients (87.9%) in the ixekizumab withdrawal/ixekizumab re‐treatment group and 18 of 29 patients (62.1%) in the continued ixekizumab/ixekizumab re‐treatment group.
In the post hoc analysis of the randomized withdrawal ITT population, the proportion of patients in whom VLDA was achieved (7 of 7 disease activity measures met) was similar in the 2 treatment groups at the time of randomization (see Supplementary Figure 10, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41716/abstract). Of the 37 patients in whom VLDA had been achieved at randomization, 30 (81%) experienced a relapse (lost MDA) during the randomized withdrawal period; MDA was maintained in the other 7 patients. Of the 40 patients in the continued ixekizumab treatment every 2 weeks group in whom VLDA had been achieved at randomization, 10 (25%) experienced a relapse (lost MDA) during the randomized withdrawal period, and MDA was maintained in 30 patients.
In the same post hoc analysis, of the 40 patients in the treatment withdrawal group in whom MDA had been achieved but VLDA had not been achieved at randomization, 36 patients (90%) experienced a relapse during the randomized withdrawal period, and MDA was maintained in 4 patients (Supplementary Figure 10, http://onlinelibrary.wiley.com/doi/10.1002/art.41716/abstract). Of the 38 patients in the continued ixekizumab treatment group in whom MDA had been achieved but VLDA had not been achieved at randomization, 19 patients (50%) experienced a relapse during the randomized withdrawal period, and MDA was maintained in 19 patients.
Safety
Overall, safety data were consistent with the data obtained in previous studies regarding PsA treated with ixekizumab, with no unexpected safety signals (Table 2). Two deaths (0.5%) occurred during the open‐label treatment period. One patient died due to an accidental drowning, which was not considered to be related to the study drug. The other patient died of pneumonia, which the investigator considered to be related to the study drug. One case of inflammatory bowel disease (0.3%) (adjudicated as Crohn’s disease) was reported during the open‐label treatment period. The patient had a previous history of irritable bowel syndrome, and the event resulted in study discontinuation.
Table 2.
Safety results in patients in the SPIRIT‐P3 study*
|
All‐ixekizumab combined (every 2 weeks) (n = 394)† |
Randomized withdrawal ITT population‡ | ||
|---|---|---|---|
|
Ixekizumab withdrawal (n = 79) |
Ixekizumab every 2 weeks (n = 79) |
||
| Exposure, no. of person‐years | 631.1 | 42.1 | 71.0 |
| TEAEs | 325 (82.5) | 40 (50.6) | 40 (50.6) |
| Mild | 156 (39.6) | 30 (38.0) | 24 (30.4) |
| Moderate | 144 (36.5) | 9 (11.4) | 14 (17.7) |
| Severe | 25 (6.3) | 1 (1.3) | 2 (2.5) |
| Serious AE | 28 (7.1) | 2 (2.5) | 1 (1.3) |
| Discontinuations due to AE | 21 (5.3) | 1 (1.3) | 0 (0) |
| Deaths | 2 (0.5) | 0 (0) | 0 (0) |
| Most frequent TEAEs§ | |||
| Nasopharyngitis | 70 (17.8) | 4 (5.1) | 11 (13.9) |
| Upper respiratory tract infection | 65 (16.5) | 4 (5.1) | 9 (11.4) |
| Injection site reaction | 62 (15.7) | 0 (0) | 1 (1.3) |
| Bronchitis | 34 (8.6) | 1 (1.3) | 4 (5.1) |
| Urinary tract infection | 21 (5.3) | 3 (3.8) | 1 (1.3) |
| Sinusitis | 20 (5.1) | 1 (1.3) | 0 (0) |
| AEs of special interest¶ | |||
| Infections | 243 (61.7) | 20 (25.3) | 29 (36.7) |
| Serious infections | 5 (1.3) | 1 (1.3) | 0 (0) |
| Injection site reactions | 80 (20.3) | 0 (0) | 2 (2.5) |
| Hepatic events | 37 (9.4) | 3 (3.8) | 6 (7.6) |
| Allergic reactions/hypersensitivity events# | 25 (6.3) | 0 (0) | 3 (3.8) |
| Cytopenias | 21 (5.3) | 1 (1.3) | 5 (6.3) |
| Depression | 13 (3.3) | 0 (0) | 0 (0) |
| Cerebrocardiovascular events** | 3 (0.8) | 0 (0) | 0 (0) |
| Malignancies | 2 (0.5) | 0 (0) | 0 (0) |
| Inflammatory bowel disease** | 1 (0.3)†† | 0 (0) | 0 (0) |
Except where indicated otherwise, values are the number (%). ITT = intent‐to‐treat; TEAEs = treatment‐emergent adverse events.
Patients who had at least 1 dose of ixekizumab.
Randomization to relapse or week 104.
Defined as >5% of TEAEs reported in the all‐ixekizumab combined group.
Reported as AEs according to the high‐level term in Medical Dictionary for Regulatory Activities, v.21.1. Groups of AEs of special interest are shown. No events of interstitial lung disease were reported in any group.
No allergic reactions/hypersensitivity events were anaphylaxis events.
Adjudicated event.
Crohn’s disease.
DISCUSSION
In the SPIRIT‐P3 study of biologic treatment–naive patients with active PsA in whom sustained MDA was achieved with open‐label ixekizumab treatment every 2 weeks, continued ixekizumab therapy was superior to withdrawal in maintaining MDA. Ixekizumab withdrawal resulted in significantly earlier relapse and a higher proportion of patients experiencing a relapse compared to continued treatment. Further, ixekizumab withdrawal, compared to continued treatment, was associated with more and earlier relapses in the majority of individual components of MDA. Importantly, re‐treatment with ixekizumab resulted in a rapid return to MDA for the vast majority of patients who experienced a relapse following ixekizumab withdrawal. Overall safety findings were consistent with those observed in previous ixekizumab PsA studies (25).
The attainment of remission or, alternatively, a low disease activity status is a treatment goal in chronic inflammatory diseases, including PsA. MDA is a recommended and clinically relevant treat‐to‐target outcome in PsA (26), and is also increasingly being used as an end point in clinical trials due to its capacity to discriminate between different treatments (27). We used sustained achievement of MDA as a strict criterion to randomize patients and loss of MDA as the criterion for relapse.
In SPIRIT‐P3, 73% of the patients in whom sustained MDA was achieved experienced a relapse in the first 40 weeks when ixekizumab treatment was withdrawn, while only 34% of the patients in the continued treatment group experienced a relapse. Relapse started as early as 4 weeks after ixekizumab withdrawal, which was the first time point of assessment after randomization. Treatment withdrawal impacted multiple components of MDA in PsA. TJC, PtGA, and pain scores were the most frequently lost components with ixekizumab withdrawal. In a smaller randomized withdrawal study in patients with PsA who experienced a relapse following discontinuation of tumor necrosis factor inhibitor (TNFi) therapy (6), PtGA and pain scores similarly worsened (10 of 12 treated). These observations imply that patient‐reported outcomes are important indicators to assess fluctuations in disease activity, along with objective measures of disease activity such as SJC or skin scores.
Of note in the SPIRIT‐P3 study, significantly more patients experienced a reemergence of psoriasis with treatment withdrawal compared to those who continued ixekizumab treatment. When re‐treatment with ixekizumab every 2 weeks was instituted after a relapse, MDA was regained in 96% of patients in the ixekizumab withdrawal group. In many patients, MDA was regained as early as 4 weeks, which was the first time point of assessment after re‐treatment. Of the 30 patients assigned to the continued treatment group who lost MDA and continued to receive ixekizumab every 2 weeks, 27 patients (90%) regained MDA, and the median time to regain MDA was 4.7 weeks. The loss of MDA with continued ixekizumab treatment may partially be due to a nocebo effect, or reflective of temporal fluctuation in the signs and symptoms of the disease, which is supported by the rapid restoration of MDA even though the actual treatment was not changed. A small proportion of patients in the ixekizumab withdrawal group (12 of 79 [15%]) did not experience a relapse during the randomized withdrawal period. These patients represent drug‐free remission, and elucidation of the characteristics of those patients in whom long‐term remission was achieved with drug withdrawal will be of much value in clinical practice; however, the number of these patients was small, and the duration of follow‐up in this study up to 104 weeks may not be long enough to reliably determine true long‐term, drug‐free remission status.
To date, SPIRIT‐P3 is the first large, multicenter, randomized, double‐blind withdrawal trial in PsA. There have been a few previous uncontrolled observational and open‐label studies investigating the possibility of continued PsA remission/low disease activity following csDMARD and/or bDMARD withdrawal (6, 7, 8, 9, 10). These studies differed in patient population and in definition of remission/low disease activity and flare, and yielded conflicting results.
Two small studies (n = 26 and n = 17) (6, 7) showed that disease control was quickly lost in the vast majority of patients with PsA after discontinuation of csDMARD or bDMARD treatment, while 2 somewhat larger studies (n = 47 and n = 236) (8, 9) showed that up to 24% of patients may be able to maintain drug‐free remission for up to 18–56 weeks. Finally, in an analysis of TNFi withdrawal in a cohort of 325 patients from the Corrona registry, low disease activity was lost in 45% of patients in a median of 29 months, indicating that in some patients with PsA, clinical benefit was maintained after TNFi discontinuation (10). In addition to several other important methodologic differences, the mean duration of prior TNF therapy in patients in the Corrona registry was 1.5 years, which is significantly longer than the maximum 36‐week duration of open‐label ixekizumab treatment prior to withdrawal in our study. While the duration of prior low disease activity among patients with PsA in the Corrona study was not reported, the duration of prior remission/low disease activity was found to be a positive predictor for maintaining drug‐free disease control in rheumatoid arthritis (28, 29). Similar to our findings in PsA, withdrawal of biologic treatment (TNFi) has generally been shown to result in rapid flare in patients with axial spondyloarthritis, another subset of the spondyloarthritis group of diseases (30, 31, 32, 33). It remains to be evaluated which patient and disease characteristics, including some potential biomarkers, may predict the outcome of treatment discontinuation in patients with PsA.
The SPIRIT‐P3 study has limitations that should be considered. The approved dosage regimen for PsA treatment in the US and Europe is ixekizumab every 4 weeks, while the dosage used in this study was ixekizumab every 2 weeks. This study was started when the pivotal phase III studies in PsA (SPIRIT‐P1 and SPIRIT‐P2) were still ongoing, evaluating the safety and efficacy of ixekizumab in 2 dosage regimens: 80 mg every 2 weeks or every 4 weeks. The efficacy and safety of ixekizumab in the 2 dosage regimens are similar (12, 15); thus, the results from this study are scientifically and clinically relevant. The study was designed to assess complete treatment discontinuation and did not assess dosage reduction. Approaches in clinical practice may differ, where treatment may be tapered or discontinued after longer periods of sustained remission/low disease activity than assessed in this study.
In conclusion, continued ixekizumab therapy was superior to withdrawal in maintaining MDA in biologic treatment–naive patients with PsA in whom sustained MDA was achieved via treatment with ixekizumab every 2 weeks. Among patients who experienced a relapse after ixekizumab withdrawal, the vast majority regained MDA after re‐treatment with ixekizumab every 2 weeks. These results indicate that continuous ixekizumab treatment is optimal for maintaining good disease control in PsA. However, disease control can be regained after re‐treatment with ixekizumab in cases of treatment interruption.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Coates had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Coates, Kerr, Adams.
Acquisition of data
Pillai, Tahir, Valter, Alves, Adams.
Analysis and interpretation of data
Coates, Pillai, Tahir, Valter, Chandran, Kameda, Okada, Kerr, Alves, Park, Adams, Gallo, Hufford, Hojnik, Mease, Kavanaugh.
ROLE OF THE STUDY SPONSOR
Eli Lilly and Company provided funding for the study. Eli Lilly and Company contributed to the study design, data collection, data analysis, data interpretation, preparation of the manuscript, and the decision to submit the paper for publication. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication. All authors critically revised the manuscript. Medical writing support was provided by Melody Pupols, PhD of Syneos Health (support for this assistance was funded by Eli Lilly and Company) and Emily K. Blue, PhD (an employee of Eli Lilly and Company).
Supporting information
Fig S1‐S10
APPENDIX A. MEMBERS OF THE SPIRIT‐P3 STUDY GROUP
Members of the SPIRIT‐P3 Study Group are as follows: Khalid Ahmed, Nicholas Barkham, Linda Belhorn, Daniela Bichovska, Michaela Blahova, Ladislav Bortlik, Johannes Breedt, Michael Brooks, Paul Caldron, Silvia Ciernik, Stefan Daniluk, Roger Diegel, Pavol Dobrovodsky, Eva Dokoupilova, Leobardo Teran Estrada, Francisco Javier Blanco Garcia, Olena Garmish, Iurii Gasanov, Mariela Geneva‐Popova, David Goddard, Oleksandr Golovchenko, Katarzyna Gruszecka, Tomas Hala, Jolana Hejlova, Mary Howell, Elena Ilivanova, Ramina Jajoo, Ewa Kaliszuk‐Kaminska, Nadezhda Kapandjieva, Steven Klein, Mariusz Korkosz, Milan Krpciar, Dolores Alonso Martinez, Nomawethu Matsiliza, Marcin Mazurek, Małgorzata Miakisz, Eric Mueller, Raili Müller, Leysan Myasoutova, Oleh Nadashkevych, Antonio Fernandez Nebro, Petr Nemec, Clark Neuwelt, Meera Oza, Margus Pail, Iris Colunga Pedraza, Dimitar Penev, Lucie Podrazilova, Jennifer‐Anne Potts, Grazyna Pulka, Eve‐Kai Raussi, Riteesha Reddy, Dmytro Rekalov, Maria Rell‐Bakalarska, Juan Cruz Rizo Rodriguez, Euthalia Roussou, Anna Rychlewska‐Hanczewska, Federico Navarro Sarabia, Liliana Sedova, Shadi Shahouri, Sergii Shevchuk, David Sikes, Lubomira Simova, Andrea Skublova, Małgorzata Socik‐Pojawa, Sheldon Solomon, Catherine Spargo, Mykola Stanislavchuk, Helena Stehlikova, Zuzana Stejfova, Maria Stopinska‐Polaszewska, Katarzyna Swierkocka, Hasan Tahir, Sandra Tälli, Gareth Tarr, Cesar Francisco Pacheco Tena, Erika Timanikova, Vira Tseluyko, Zuzana Urbanova, Ivo Valter, Edgar Hernandez Vargas, Viktoriia Vasylets, Federico Galvan Villegas, Petr Vitek, Monika Wronisz, and Hana Zelenkova.
ClinicalTrials.gov identifier: NCT02584855.
Supported by Eli Lilly and Company.
Dr. Coates has received consulting fees, speaking fees, and/or honoraria from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Celgene, Gilead, Janssen, Eli Lilly and Company, Medac, Pfizer, and UCB (less than $10,000 each) and from Novartis (more than $10,000). Dr. Pillai, Ms Kerr, Ms Alves, and Drs. Park, Adams, Gallo, Hufford, and Hojnik are employees of Eli Lilly and Company and own stock or stock options in Eli Lilly and Company. Dr. Tahir has received speaking fees and contributed to advisory boards for Novartis, AbbVie, Eli Lilly and Company, and Gilead (less than $10,000 each). Dr. Chandran has received consulting fees, speaking fees, and/or honoraria from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Janssen, Novartis, Pfizer, and UCB (less than $10,000 each) and has a spouse who is an employee of Eli Lilly and Company. Dr. Kameda has received consulting fees, speaking fees, and/or honoraria from AbbVie, Astellas, Bristol Myers Squibb, Chugai, Eisai, Gilead, Janssen, Novartis, Pfizer, and UCB (less than $10,000 each) and from Asahi Kasei, Eli Lilly and Company, and Mitsubishi Tanabe (more than $10,000 each) and has received research support from AbbVie, Asahi Kasei, Astellas, Chugai, Eisai, Mitsubishi Tanabe, and Novartis. Dr. Okada has received consulting fees, speaking fees, and/or honoraria from Eli Lilly and Company (less than $10,000). Dr. Mease has received consulting fees, speaking fees, and/or honoraria from Amgen, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Galápagos, Genentech, Gilead, GlaxoSmithKline, Sun, and UCB (less than $10,000 each) and from AbbVie, Janssen, Eli Lilly and Company, Novartis, and Pfizer (more than $10,000 each). Dr. Kavanaugh has received consulting fees, speaking fees, and/or honoraria from Eli Lilly and Company, Novartis, Pfizer, Amgen, and AbbVie (less than $10,000 each). No other disclosures relevant to this article were reported.
Contributor Information
Laura C. Coates, Email: laura.coates@ndorms.ox.ac.uk.
for the SPIRIT‐P3 Study Group:
Khalid Ahmed, Nicholas Barkham, Linda Belhorn, Daniela Bichovska, Michaela Blahova, Ladislav Bortlik, Johannes Breedt, Michael Brooks, Paul Caldron, Silvia Ciernik, Stefan Daniluk, Roger Diegel, Pavol Dobrovodsky, Eva Dokoupilova, Leobardo Teran Estrada, Francisco Javier Blanco Garcia, Olena Garmish, Iurii Gasanov, Mariela Geneva‐Popova, David Goddard, Oleksandr Golovchenko, Katarzyna Gruszecka, Tomas Hala, Jolana Hejlova, Mary Howell, Elena Ilivanova, Ramina Jajoo, Ewa Kaliszuk‐Kaminska, Nadezhda Kapandjieva, Steven Klein, Mariusz Korkosz, Milan Krpciar, Dolores Alonso Martinez, Nomawethu Matsiliza, Marcin Mazurek, Małgorzata Miakisz, Eric Mueller, Raili Müller, Leysan Myasoutova, Oleh Nadashkevych, Antonio Fernandez Nebro, Petr Nemec, Clark Neuwelt, Meera Oza, Margus Pail, Iris Colunga Pedraza, Dimitar Penev, Lucie Podrazilova, Jennifer‐Anne Potts, Grazyna Pulka, Eve‐Kai Raussi, Riteesha Reddy, Dmytro Rekalov, Maria Rell‐Bakalarska, Juan Cruz Rizo Rodriguez, Euthalia Roussou, Anna Rychlewska‐Hanczewska, Federico Navarro Sarabia, Liliana Sedova, Shadi Shahouri, Sergii Shevchuk, David Sikes, Lubomira Simova, Andrea Skublova, Małgorzata Socik‐Pojawa, Sheldon Solomon, Catherine Spargo, Mykola Stanislavchuk, Helena Stehlikova, Zuzana Stejfova, Maria Stopinska‐Polaszewska, Katarzyna Swierkocka, Hasan Tahir, Sandra Tälli, Gareth Tarr, Cesar Francisco Pacheco Tena, Erika Timanikova, Vira Tseluyko, Zuzana Urbanova, Ivo Valter, Edgar Hernandez Vargas, Viktoriia Vasylets, Federico Galvan Villegas, Petr Vitek, Monika Wronisz, and Hana Zelenkova
References
- 1.Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis [review]. Drugs 2014;74:423–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zachariae H, Zachariae R, Blomqvist K, Davidsson S, Molin L, Mørk C, et al. Quality of life and prevalence of arthritis reported by 5,795 members of the Nordic Psoriasis Associations: data from the Nordic Quality of Life Study. Acta Derm Venereol 2002;82:108–13. [DOI] [PubMed] [Google Scholar]
- 3.Boehncke WH, Menter A. Burden of disease: psoriasis and psoriatic arthritis [review]. Am J Clin Dermatol 2013;14:377–88. [DOI] [PubMed] [Google Scholar]
- 4.Gossec L, Smolen JS, Ramiro S, de Wit M , Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- 5.Lee MP, Lii J, Jin Y, Desai RJ, Solomon DH, Merola JF, et al. Patterns of systemic treatment for psoriatic arthritis in the US: 2004–2015. Arthritis Care Res (Hoboken) 2018;70:791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araujo EG, Finzel S, Englbrecht M, Schreiber DA, Faustini F, Hueber A, et al. High incidence of disease recurrence after discontinuation of disease‐modifying antirheumatic drug treatment in patients with psoriatic arthritis in remission. Ann Rheum Dis 2015;74:655–60. [DOI] [PubMed] [Google Scholar]
- 7.Moverley A, Coates L, Marzo‐Ortega H, Waxman R, Torgerson D, Cocks K, et al. A feasibility study for a randomised controlled trial of treatment withdrawal in psoriatic arthritis (REmoval of treatment for patients in REmission in psoriatic ArThritis (RETREAT (F)). Clin Rheumatol 2015;34:1407–12. [DOI] [PubMed] [Google Scholar]
- 8.Chimenti MS, Esposito M, Giunta A, Graceffa D, Bobino G, Teoli M, et al. Remission of psoriatic arthritis after etanercept discontinuation: analysis of patients' clinical characteristics leading to disease relapse. Int J Immunopathol Pharmacol 2013;26:833–8. [DOI] [PubMed] [Google Scholar]
- 9.Cantini F, Niccoli L, Nannini C, Cassarà E, Pasquetti P, Olivieri I, et al. Frequency and duration of clinical remission in patients with peripheral psoriatic arthritis requiring second‐line drugs. Rheumatology (Oxford) 2008;47:872–6. [DOI] [PubMed] [Google Scholar]
- 10.Huynh DH, Boyd TA, Etzel CJ, Cox V, Kremer J, Mease P, et al. Persistence of low disease activity after tumour necrosis factor inhibitor (TNFi) discontinuation in patients with psoriatic arthritis. RMD Open 2017;3:e000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Lu J, Allan BW, Tang Y, Tetreault J, Chow C, et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin‐17A. J Inflamm Res 2016;9:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mease PJ, van der Heijde D , Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin‐17A specific monoclonal antibody, for the treatment of biologic‐naive patients with active psoriatic arthritis: results from the 24‐week randomised, double‐blind, placebo‐controlled and active (adalimumab)‐controlled period of the phase III trial SPIRIT‐P1. Ann Rheum Dis 2017;76:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van der Heijde D, Gladman DD, Kishimoto M, Okada M, Rathmann SS, Moriarty SR, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52‐week results from a phase III study (SPIRIT‐P1). J Rheumatol 2018;45:367–77. [DOI] [PubMed] [Google Scholar]
- 14.Chandran V, van der Heijde D , Fleischmann RM, Lespessailles E, Helliwell PS, Kameda H, et al. Ixekizumab treatment of biologic‐naive patients with active psoriatic arthritis: 3‐year results from a phase III clinical trial (SPIRIT‐P1). Rheumatology (Oxford) 2020;59:2774–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nash P, Kirkham B, Okada M, Rahman P, Combe B, Burmester G, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24‐week randomised, double‐blind, placebo‐controlled period of the SPIRIT‐P2 phase 3 trial. Lancet 2017;389:2317–27. [DOI] [PubMed] [Google Scholar]
- 16.Genovese MC, Combe B, Kremer JM, Tsai T, Behrens F, Adams DH, et al. Safety and efficacy of ixekizumab in patients with PsA and previous inadequate response to TNF inhibitors: week 52 results from SPIRIT‐P2. Rheumatology (Oxford) 2018;57:2001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orbai AM, Gellett AM, Kerr L, Constantin A. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis and previous inadequate response to TNF inhibitors: two‐year follow‐up from a phase 3 study [abstract]. Arthritis Rheumatol 2018;70Suppl 10. URL: https://acrabstracts.org/abstract/efficacy‐and‐safety‐of‐ixekizumab‐in‐patients‐with‐active‐psoriatic‐arthritis‐and‐previous‐inadequate‐response‐to‐tnf‐inhibitors‐two‐year‐follow‐up‐from‐a‐phase‐3‐study/. [Google Scholar]
- 18.Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 19.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, and the CASPAR Study Group . Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. [DOI] [PubMed] [Google Scholar]
- 20.Fredriksson T, Pettersson U. Severe psoriasis—oral therapy with a new retinoid. Dermatologica 1978;157:238–44. [DOI] [PubMed] [Google Scholar]
- 21.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 22.Coates LC, Helliwell PS. Defining low disease activity states in psoriatic arthritis using novel composite disease instruments [letter]. J Rheumatol 2016;43:371–5. [DOI] [PubMed] [Google Scholar]
- 23.Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008;59:686–91. [DOI] [PubMed] [Google Scholar]
- 24.Maksymowych WP, Mallon C, Morrow S, Shojania K, Olszynski WP, Wong RL, et al. Development and validation of the Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis Index. Ann Rheum Dis 2009;68:948–53. [DOI] [PubMed] [Google Scholar]
- 25.Mease P, Roussou E, Burmester GR, Goupille P, Gottlieb A, Moriarty S, et al. Safety of ixekizumab in patients with psoriatic arthritis: results from a pooled analysis of three clinical trials. Arthritis Care Res (Hoboken) 2019;71:367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coates LC, Helliwell PS. Treat to target in psoriatic arthritis: evidence, target, research agenda. Curr Rheumatol Rep 2015;17:517. [DOI] [PubMed] [Google Scholar]
- 27.Coates LC, Strand V, Wilson H, Revicki D, Stolshek B, Samad A, et al. Measurement properties of the minimal disease activity criteria for psoriatic arthritis. RMD Open 2019;5:e001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ajeganova S, Huizinga T. Sustained remission in rheumatoid arthritis: latest evidence and clinical considerations. Ther Adv Musculoskelet Dis 2017;9:249–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka Y, Takeuchi T, Mimori T, Saito K, Nawata M, Kameda H, et al. Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by Remicade in RA) study. Ann Rheum Dis 2010;69:1286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baraliakos X, Listing J, Brandt J, Zink A, Alten R, Burmester G, et al. Clinical response to discontinuation of anti‐TNF therapy in patients with ankylosing spondylitis after 3 years of continuous treatment with infliximab. Arthritis Res Ther 2005;7:R439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandt J, Listing J, Haibel H, Sörensen H, Schwebig A, Rudwaleit M, et al. Long‐term efficacy and safety of etanercept after readministration in patients with active ankylosing spondylitis. Rheumatology (Oxford) 2005;44:342–8. [DOI] [PubMed] [Google Scholar]
- 32.Sebastian A, Wojtala P, Lubinski L, Mimier M, Chlebicki A, Wiland P. Disease activity in axial spondyloarthritis after discontinuation of TNF inhibitors therapy. Reumatologia 2017;55:157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song IH, Althoff CE, Haibel H, Hermann KG, Poddubnyy D, Listing J, et al. Frequency and duration of drug‐free remission after 1 year of treatment with etanercept versus sulfasalazine in early axial spondyloarthritis: 2 year data of the ESTHER trial. Ann Rheum Dis 2012;71:1212–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S10


