Abstract
Background
Non‐severe hemophilia A patients have a life‐long inhibitor risk. Yet, no studies have analyzed risk factors for inhibitor development after 50 factor VIII (FVIII) exposure days (EDs).
Objectives
This case‐control study investigated treatment‐related risk factors for inhibitor development in non‐severe hemophilia A and assessed whether these risk factors were different for early versus late inhibitor development.
Patients/Methods
Non‐severe hemophilia A patients (FVIII:C 2%–40%) were selected from the INSIGHT study. Inhibitor‐positive patients were defined as early (<50 EDs) or late (>50EDs) cases and matched to 1–4 inhibitor‐negative controls by year of birth, cumulative number of EDs, and center/country. We investigated treatment intensity during the last 10 EDs prior to inhibitor development. Intensive treatment was defined as: surgery, peak treatment (10 consecutive EDs), and high mean FVIII dose (>45 IU/kg/ED). Odds ratios (OR) were calculated by logistic regression.
Results
Of 2709 patients, we analyzed 63 early and 26 late cases and 195 and 71 respectively matched controls. Peak treatment was associated with early and late inhibitor risk (crude OR 1.8, 95% confidence interval [CI] 1.0–3.4; 4.0, 95%CI 1.1–14.3). This association was slightly less pronounced after adjustment for mean FVIII dose. High mean FVIII dose was also associated with early and late inhibitor risk (crude OR 2.8, 95%CI 1.5–5.1; 4.5, 95%CI 1.2–16.6). Surgery increased inhibitor risk for early cases. This was less pronounced for late cases.
Conclusions
Our findings suggest that intensive FVIII treatment remains a risk factor for inhibitor development in non‐severe hemophilia A after more than 50 EDs. Therefore, persistent caution is required throughout the life‐time treatment course.
Keywords: anti‐drug antibody, factor VIII, hemophilia A, inhibitor, risk factors
Essentials.
Patients with non‐severe hemophilia A have a life‐long risk of inhibitor development.
We performed an international case‐control study in 355 non‐severe hemophilia A patients.
Intensive treatment increased inhibitor risk, independent of preceding number of factor VIII exposures.
Persistent clinical vigilance is required to mitigate inhibitor risk in non‐severe hemophilia A.
1. INTRODUCTION
Hemophilia A is an X‐linked hereditary bleeding disorder, caused by a decrease of functional clotting factor VIII (FVIII). The severity of disease depends on the residual FVIII activity.1, 2 Patients with severe disease (FVIII activity < 1 IU/dL) experience spontaneous joint and muscle bleeds and require prophylactic FVIII treatment.3, 4 Patients with non‐severe hemophilia A (FVIII activity 1–40 IU/dL) bleed less frequently and do not usually present with spontaneous bleeds. They receive on‐demand treatment to prevent or treat bleedings elicited by trauma or surgery.5
The development of neutralizing anti‐FVIII antibodies (inhibitors) is the most severe complication of treatment with FVIII concentrates, occurring in 25% to 40% of patients with severe hemophilia A and in 3% to 13% of patients with non‐severe hemophilia A.6, 7, 8, 9 Inhibitors neutralize the biological activity of therapeutic FVIII and render replacement therapy ineffective, thereby increasing morbidity and mortality.10, 11, 12
In severe hemophilia A, the risk of inhibitor development strongly depends on the cumulative number of exposure days (EDs) to FVIII concentrates. In inhibitor patients with severe hemophilia A, most patients develop inhibitors early in life during the first 10 to 15 exposures to FVIII concentrate. The inhibitor risk decreases to less than 1% after 50 cumulative EDs.8, 13, 14 In inhibitor patients with non‐severe hemophilia A, the median cumulative number of EDs until inhibitor development is 28 and 69% of patients develop inhibitors before the 50th ED. In contrast to severe hemophilia A, patients with non‐severe hemophilia A carry a life‐long risk of inhibitor development, as in these patients no reduction in inhibitor risk is seen during the lifetime treatment course.15
Although the exact etiology of inhibitor onset is not yet fully understood, multiple genetic and environmental risk factors have been identified.14, 15, 16, 17, 18 Knowledge of risk factors is of utmost importance, because it enables us to identify high‐risk patients and provides the opportunity for preventive measures. Based on previous research, intensive FVIII treatment is considered an important risk factor for inhibitor development, especially when tissue damage due to severe bleeding, infection, or surgery, is present.8, 14, 19 The interplay of intense FVIII treatment and tissue damage may be explained by the danger theory. This theory states that FVIII protein by itself is not enough to elicit inhibitor formation and that substances released by damaged tissue (danger signals) are required to elicit an effective anti‐FVIII immune response.20
This is in line with the results of a previous case‐control study that was conducted in the INSIGHT cohort–a large population including 2709 patients with non‐severe hemophilia A (FVIII activity 2–40 IU/dL) who received at least one exposure to FVIII concentrate between 1980 and 2011 in 1 of the 34 participating hemophilia treatment centers in Europe and Australia.
The case‐control study demonstrated that both surgical intervention (adjusted odds ratio [aOR] 4.2, 95% confidence interval [CI] 1.7–10.3) and a high mean dose (>45 IU/kg/ED) of FVIII concentrate (aOR 7.5, CI 1.6–35.6) were associated with inhibitor development in patients with non‐severe hemophilia A. However, this study exclusively included patients that developed inhibitors early during the treatment course (before 75 EDs): the median cumulative number of EDs prior to inhibitor development was 25 (interquartile range [IQR], 12–40 EDs).18 Therefore, the question remains whether intensive FVIII treatment also contributes to an increased inhibitor risk in patients who already received more than 50 exposures to FVIII concentrates.
In this case‐control study, we present the data of all inhibitor patients included in the INSIGHT cohort. We aimed to investigate the association between the intensity of FVIII treatment and inhibitor development, and to assess whether this association was different for early inhibitor development (during the first 50 EDs) and late inhibitor development (after 50 EDs).
2. METHODS
2.1. Patients
This case‐control study was conducted in the large non‐severe hemophilia A population (FVIII baseline activity 2–40 IU/dL) of the previously described INSIGHT cohort.6, 16 All patients had received at least one exposure to FVIII concentrate in 1 of the 34 participating centers in Europe and Australia, between 1 January 1980 and 1 January 2011. Approval was obtained from every center's institutional review board and the study was conducted in accordance with the Declaration of Helsinki.
2.2. Study outcome
The primary outcome of the study was clinically relevant inhibitor development, defined as at least two consecutive positive Bethesda inhibitor assay titers of ≥1.0 Bethesda units (BU) per ml. Patients with inhibitor titers between 0.6 and 1.0 BU/mL had to fulfill one of the following two criteria to be considered as having a clinically relevant inhibitor: (1) a decrease in endogenous FVIII plasma level to at least 50% of the baseline level or (2) a reduced half‐life of <6 h after FVIII concentrate administration.15 A high titer inhibitor was defined as a peak titer ≥5.0 BU/ml.
2.3. Cases and controls
Patients with inhibitor development were identified as cases. Patients who developed inhibitors during the first 50 exposures to FVIII were defined as early inhibitor patients. Patients who developed inhibitors after 50 EDs were defined as late inhibitor patients. Each case was matched to 1 to 4 inhibitor‐negative controls by year of birth (±10 years), cumulative number of EDs to FVIII concentrates, and center or country. Controls of cases received at least the same number of cumulative EDs as the case to which they were matched. For cases, the end of the observation period was defined as the last ED before inhibitor development. For controls, the end of the observation period was at a similar number of EDs as the case to which they were matched.
2.4. Data collection
Detailed demographic and clinical data were collected from medical records using standardized electronic case report forms. Patient data included: year of birth, ethnicity, F8 genotype, baseline FVIII activity level, and family history for inhibitor development. Treatment data were collected for the first FVIII exposure and the last 10 EDs until the end of the observation period and included: calendar date of each exposure day, reason for treatment, and FVIII dose. The reason for treatment was classified as: surgery, hemorrhage (traumatic or spontaneous), and other (i.e., prophylaxis).
2.5. Determinants
The intensity of FVIII treatment was analyzed as surgery, peak treatment, and mean dose of FVIII product in the last 10 cumulative EDs of the observation period.
2.5.1. Surgery
We evaluated whether patients received at least one ED for surgery in the last 10 EDs. Surgical procedures were classified as major or minor depending on whether the duration of FVIII treatment was more or less than 5 consecutive EDs.1
2.5.2. Peak treatment
We defined a peak treatment moment as 10 EDs to FVIII concentrate within a period of less than 15 calendar days.
2.5.3. Mean FVIII dose
We calculated the mean FVIII dose per kilogram (kg) bodyweight over the last 10 EDs. High FVIII dose was defined as a mean FVIII dose of more than 45 IU/kg/ED. Low dose was defined as a mean FVIII dose of 45 IU/kg/ED or less. The cut‐off for high versus low dose factor replacement was based on the results of the earlier case‐control study in the INSIGHT cohort, demonstrating that a mean dose higher than 45 IU/kg/ED of FVIII concentrate was associated with inhibitor development in patients with non‐severe hemophilia A that developed their inhibitor early (<75 EDs).18
2.6. Data analysis
Descriptive statistics are presented as medians and IQRs for continuous variables and frequencies and percentages for categorical variables. To investigate the association between determinants and inhibitor development, we used an unconditional logistic regression model and adjusted for the matching factors that may influence the risk of inhibitor development: year of birth in four categories (<1945; 1945–1964; 1965–1987; >1987) and cumulative number of EDs in four categories (1–19; 20–50; 51–99; >100).21, 22 The analysis was performed separately in two groups: (1) early inhibitor patients versus matched controls and (2) late inhibitor patients versus matched controls. Each determinant was adjusted for one predefined confounder: surgery was adjusted for age at last ED, peak treatment for mean FVIII dose, and mean FVIII dose for peak treatment. Results are presented as crude odds ratio (crude OR; adjusted for matching factors) and aOR (adjusted for matching factors and the predefined confounder).
2.7. Missing data
If the exact calendar date of an exposure day was missing, we unconditionally imputed the missing date with the median between the treatment date before and after the missing date (<1%). Missing data for the reason for treatment were replaced with the reason for treatment of the ED that appeared one calendar day before or after the missing item (2%). All other missing values for reason of treatment were unconditionally imputed with “traumatic bleed,” assuming that this is the most probable reason of treatment in patients with non‐severe hemophilia A (4%). If the FVIII dose was missing, we replaced the missing item with the average FVIII dose that was given for that specific treatment indication in that specific center (14%). To calculate the FVIII dose per kg, we imputed the weight of these patients on each ED, according to age‐weight statistics (for adults) and growth curves (for children; Centraal Bureau voor de Statistiek; Royal College of Paediatrics & Child Health; Australian Paediatric Endocrine Group; McDowell et al., 2008; Australian Bureau of Statistics, 2012; Destatis Statistisches Bundesamt, 2013; Food & Agriculture Organization of the United Nations, 2015).18
3. RESULTS
3.1. Patient characteristics
In this case‐control study, 355 patients were included, for whom detailed data of the last 10 cumulative EDs of the observation period were available. An overview of the patient inclusion is illustrated in Figure 1.
FIGURE 1.
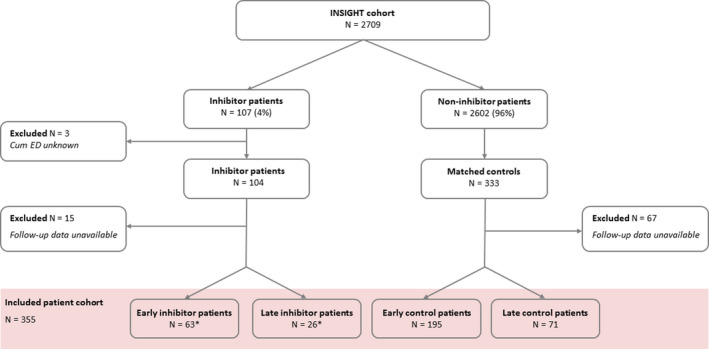
Flowchart of patient inclusion. ED, exposure day; Early inhibitor patients: ≤50 EDs prior to inhibitor development; Late inhibitor patients >50 EDs prior to inhibitor development. From the INSIGHT cohort (N = 2709), 104 inhibitor patients and 333 matched controls were selected. Of these patients, 15 inhibitor patients and 67 controls were excluded because only baseline data were available (no data on exposure days). In this study, 355 patients were included on whom detailed data on the last 10 cumulative EDs were available. *In the early inhibitor group (n = 63), 36 (57%) patients had a high‐titer inhibitor. In the late inhibitor group (n = 26), 13 (50%) patients had a high‐titer inhibitor
3.1.1. Baseline characteristics
Early inhibitor patients and controls had a comparable median year of birth (1961 vs. 1964). Late inhibitor patients appeared to be older (median year of birth: 1958) than controls (median year of birth: 1976; Table 1). Median baseline FVIII was largely similar in all groups. Yet, there were more patients with moderate hemophilia in the late control group than in the late inhibitor group (42% vs. 27%). In inhibitor patients, F8 gene mutations were predominantly located in the C1 domain while in controls, F8 gene mutations were predominantly located in the A2 domain (Figure 2).
TABLE 1.
Patient characteristics
| Characteristics | Early inhibitor patients (n = 63) | Early control patients (n = 195) | Late inhibitor patients (n = 26) | Late control patients (n = 71) |
|---|---|---|---|---|
| Year of birth (IQR) | 1961 (1940–1989) | 1964 (1943–1987) | 1958 (1943–1981) | 1976 (1950–1989) |
| FVIII:C baseline (IU/dl) | 8.0 (5.0–14.0) | 8.0 (4.0–14.0) | 7.0 (4.0–11.5) | 5.0 (3.0–12.0) |
| Moderate hemophilia (%) | 12 (19) | 50 (26) | 7 (27) | 30 (42) |
| Caucasian ethnicity (%) | 56 (89) | 187 (96) | 26 (100) | 70 (99) |
| Family history inhibitors (%) | 7 (11) | 3 (2) | 1 (4) | 1 (1) |
| Unknown (%) | 22 (35) | 57 (29) | 11 (42) | 20 (28) |
Values are presented as medians (IQR) or as numbers (%).
Abbreviations: FVIII, factor VIII; IQR, interquartile range.
FIGURE 2.
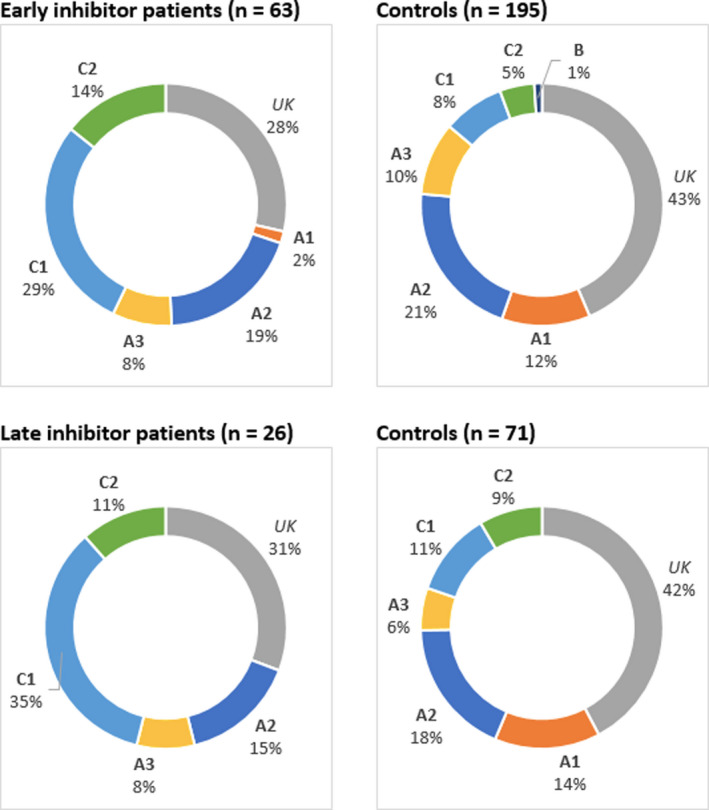
Distribution of F8 genotypes. The four graphs illustrate the distribution of F8 genotypes on the A1‐3, C1‐2, and B domains of the FVIII protein in cases and controls. Abbreviations: UK, unknown
3.1.2. Inhibitor characteristics
Early inhibitors developed after a median of 18 (IQR 11–30) cumulative EDs and late inhibitors developed after a median of 75 (IQR 68–97) cumulative EDs. The peak inhibitor titer was comparable in early and late inhibitor patients (median 7.0 [IQR 2.0–32.0]; median 5.2 [IQR 1.3–19.3] IU/dL, respectively). The clinical phenotype of the inhibitor was also comparable in early and late inhibitors—about half of the patients had a decrease of FVIII activity level and more than 90% had an increased bleeding tendency during the inhibitor period (Table 2).
TABLE 2.
Characteristics of inhibitor patients
| Characteristics | Early inhibitor (n = 63) | Late inhibitor (n = 26) |
|---|---|---|
| Year of birth (IQR) | 1961 (1940–1989) | 1958 (1943–1981) |
| Year of inhibitor development (IQR) | 2002 (1997–2006) | 2002 (1998–2007) |
| Age at inhibitor development, years (IQR) | 35.9 (11.3–61.3) | 45.9 (19.0–62.2) |
| Cum EDs until inhibitor development (IQR) | 18 (11–30) | 75 (68–97) |
| Cum EDs until inhibitor development in categories (%) | ||
| 1–9 | 14 (22) | — |
| 10–29 | 33 (52) | — |
| 30–49 | 16 (25) | — |
| 50–74 | — | 12 (46) |
| 75–100 | — | 8 (31) |
| >100 | — | 6 (23) |
| Peak inhibitor titer (BU/dl) | 7.0 (2.0–32.0) | 5.2 (1.3–19.3) |
| High‐titer inhibitor (%) | 36 (57) | 13 (50) |
| Increased bleeding tendency (%) | 60 (95) | 24 (92) |
| Decreased baseline FVIII activity level (%) | 39 (62) | 12 (46) |
| Unknown | 11 (18) | 5 (19) |
| Decrease of FVIII activity level <1% (%) | 21 (33) | 4 (15) |
| Unknown | 11 (18) | 5 (19) |
High‐titer inhibitor ≥5 BU/ml. Values are presented as median (IQR) or as number (%).
Abbreviations: BU, Bethesda unit; cum EDs, cumulative number of exposure days; FVIII, factor VIII; IQR, interquartile range.
3.2. Treatment characteristics
Overall, both early and late cases were treated more intensively than controls (Table 3).
TABLE 3.
Treatment characteristics
| Characteristics | Early inhibitor patients (n = 63) | Early control patients (n = 195) | Late inhibitor patients (n = 26) | Late control patients (n = 71) |
|---|---|---|---|---|
| First exposure day | ||||
| Year of first treatment (IQR) | 1996 (1980–2000) | 1988 (1979–1996) | 1982 (1972–1989) | 1983 (1976–1995) |
| Unknown (%) | 15 (24) | 38 (19) | 5 (19) | 19 (27) |
| Age at first treatment, years (IQR) | 23 (6–42) | 12 (4–34) | 14 (4–35) | 4 (3–13) |
| Unknown (%) | 15 (24) | 38 (19) | 5 (19) | 19 (27) |
| Reason for first treatment (%) | ||||
| Surgery | 27 (43) | 48 (25) | 7 (27) | 5 (7) |
| Hemorrhage (traumatic, spontaneous) | 30 (48) | 133 (68) | 7 (27) | 21 (30) |
| Other (prophylaxis, testing) | 4 (6) | 4 (2) | 0 | 3 (4) |
| Unknown | 2 (3) | 10 (5) | 12 (46) | 42 (59) |
| Last 10 cumulative exposure days | ||||
| Year of last ED (IQR) | 2000 (1997–2005) | 1999 (1994–2005) | 2002 (1998–2007) | 2002 (1995–2006) |
| Age at last ED, years (IQR) | 36 (11–61) | 31 (12–55) | 46 (19–62) | 24 (15–42) |
| Interval of last 10 cum EDs, days (IQR) | 51 (10–749) | 244 (16–1276) | 33 (9–377) | 125 (21–563) |
| Surgery (%) | 37 (59) | 86 (44) | 12 (46) | 18 (25) |
| Major surgery (%) | 17 (27) | 29 (15) | 4 (15) | 5 (7) |
| Peak treatment (%) | 23 (37) | 50 (24) | 8 (31) | 6 (8) |
| Mean FVIII dose (IU/kg) | 38.2 (27.6–57.4) | 31.5 (23.8–42.6) | 35.4 (19.9–46.8) | 25.7 (18.9–37.0) |
| Mean FVIII dose >45 IU/kg/ED (%) | 28 (44) | 45 (23) | 7 (27) | 8 (11) |
Values are presented as median (IQR) or as number (%).
Abbreviations: cum EDs, cumulative number of exposure days; FVIII, factor VIII; IQR, interquartile range.
3.2.1. First exposure day
Overall, inhibitor patients were older at first treatment than controls and were treated more often for surgery (Table 3).
3.2.2. Surgery in the last 10 exposure days
Inhibitor patients were treated more often for surgery than controls in the last 10 cumulative EDs: early inhibitor patients versus controls ([59% vs. 44%] and late inhibitor patients versus controls [46% vs. 25%]; Table 3). The aOR for surgery was increased for early inhibitor development (1.8, 95% CI 1.0–3.3). For late inhibitor development this was less pronounced (aOR 1.5, 95% CI 0.5–4.5; Table 4).
TABLE 4.
Intensive treatment in last 10 cumulative exposure days and inhibitor development
| Early inhibitor development | Late inhibitor development | |||||
|---|---|---|---|---|---|---|
| N | Crude OR (95% CI) | aOR (95% CI) | N | Crude OR (95% CI) | aOR (95% CI) | |
| Surgery | ||||||
| No surgery | 135 | 1 | 1 | 67 | 1 | 1 |
| Surgery | 123 | 1.8 (1.0–3.4) | 1.8 (1.0–3.3) | 30 | 1.5 (0.5–4.6) | 1.5 (0.5–4.5) |
| Peak treatment | ||||||
| No peak treatment | 185 | 1 | 1 | 83 | 1 | 1 |
| Peak treatment | 73 | 1.8 (1.0–3.4) | 1.6 (0.8–3.0) | 14 | 4.0 (1.1–14.3) | 3.3 (0.8–13.1) |
| Mean FVIII dose | ||||||
| Low dose (≤45 IU/kg/ED) | 185 | 1 | 1 | 82 | 1 | 1 |
| High dose (>45 IU/kg/ED) | 73 | 2.8 (1.5–5.1) | 2.7 (1.4–4.9) | 15 | 4.5 (1.2–16.6) | ‐a |
Crude OR: adjusted for matching factors (year of birth in categories [<1945; 1945–1964; 1965–1987; >1987] and cumulative number of EDs in categories [1–19; 20–49; 50–99; >100]). Adjusted OR: adjusted for matching factors and predefined confounding factor. Surgery was adjusted for age at last ED (continuous variable). Peak treatment was adjusted for mean FVIII dose (continuous variable). Mean FVIII dose was adjusted for peak treatment (dichotomous variable).
Abbreviations: CI, confidence interval; ED, exposure days; FVIII, factor VIII; OR, odds ratio.
aOR for high dose could not be calculated in the late inhibitor group, because there were no control patients that received a peak treatment and a high dose.
3.2.3. Peak treatment in the last 10 exposure days
Inhibitor patients received peak treatment more frequently than controls in the last 10 EDs (early inhibitor patients vs. controls [37% vs. 24%] and late inhibitor patients vs. controls [31% vs. 8%]; Table 3). In both early and late inhibitor development, peak treatment moments were associated with an increased risk of inhibitor development (crude OR 1.8, 95% CI 1.0–3.4 and 4.0, 95% CI 1.1–14.3). This association was slightly less pronounced after adjustment for mean FVIII dose (aOR 1.6, 95% CI 0.8–3.0 and 3.3, 95% CI 0.8–13.1; Table 4).
3.2.4. High mean FVIII dose in the last 10 exposure days
Inhibitor patients were treated more often with a high mean FVIII dose compared to controls in the last 10 EDs (early inhibitor patients vs. controls [44% vs. 23%] and late inhibitor patients vs. controls [27% vs. 11%]). A high mean FVIII dose was associated with both early and late inhibitor risk (crude OR 2.8, 95% CI 1.5–5.1 and 4.5, 95% CI 1.2–16.6). After adjustment for peak moments, the aOR for early inhibitor development was similar (2.7, 95% CI 1.4–4.9). FVIII dose could not be adjusted for peak treatment in the late inhibitor group, as all control patients that were treated with a high FVIII dose did not receive a peak treatment in the last 10 EDs (Table 4).
4. DISCUSSION
4.1. Summary of results
In this case‐control study, including 355 patients with non‐severe hemophilia A, we investigated treatment‐related risk factors for inhibitor development and assessed whether these risk factors were different for early inhibitor development (during first 50 EDs) and late inhibitor development (after 50 EDs). The most important finding of this study was that both early and late inhibitor patients were treated more intensively than matched controls prior to inhibitor development, with respect to surgery, peak treatment, and high mean FVIII dose.
4.2. Strengths and limitations
The unique, large, and unselected INSIGHT cohort provided the first opportunity to study the risk factors for late inhibitor development in non‐severe hemophilia A. Detailed patient data for each exposure to FVIII concentrates enabled matching and adjustment for the influence of predefined confounders.
As a result of the retrospective design of the study and the large follow‐up period, our analysis was limited to the last 10 EDs prior to inhibitor development. Therefore, the influence of earlier risk factors could not be investigated. However, it is likely that the characteristics of the last exposures prior to inhibitor development are more important than exposures much earlier in life, when investigating the risk of inhibitor development.
Due to the natural course of non‐severe hemophilia A and infrequent exposure to FVIII concentrates, only a small proportion of the INSIGHT cohort had received more than 50 FVIII exposures. Therefore, it was challenging to find well‐matched controls for the late inhibitor patients. Consequently, late inhibitor patients were older than their respective controls. To rule out confounding by calendar effect we adjusted for year of birth and still found a positive association between intensive treatment and late inhibitor development.
Our cohort also included patients born before FVIII treatment was available. Therefore, in patients born before the 1980s, the time between birth and first treatment with FVIII concentrate was longer than patients born after the 1980s. However, as mentioned above, this difference in year of birth did not influence the reported association between intensive treatment and late inhibitor development.
Due to the limited number of late inhibitor patients and controls, we performed unconditional logistic regression analysis, with correction for the matching variables year of birth and EDs in categories.21, 22 Another consequence of the limited number of patients was that we could not include all possible confounders in the logistic regression model. Therefore, residual confounding may have occurred.
Last, because of a large proportion of missing data on FVIII product type, we were not able to analyze the effect of this determinant on inhibitor risk in patients with early versus patients with late inhibitor development.
Yet, it is important to emphasize that, to our knowledge, this study included the largest cohort of late inhibitor patients and controls reported to date. Therefore, based on these data, we caution physicians to remain watchful for inhibitor development throughout the lifetime treatment course of patients with non‐severe hemophilia A, especially when treating these patients intensively with FVIII concentrates.
4.3. Intensity of treatment in the last 10 exposure days
4.3.1. Surgery, peak treatment, and high factor VIII dose
Our results suggest that surgery in the last 10 EDs increases the risk of early inhibitor development, but this was less clear for late inhibitor development. According to the immunological danger theory, it is conceivable that frequent and high‐dosed FVIII exposure combined with tissue damage due to surgery or major hemorrhage, may be associated with inhibitor development.20 In line with this theory, the analysis of our study confirmed that peak treatment and high FVIII dose in the last 10 EDs increases the risk of early and late inhibitor development. The observed association between inhibitor development and peak treatment and high FVIII dose could potentially have another explanation: frequent and high‐dose FVIII exposures may be a reflection of yet undetected inhibitor development rather than being its cause.14 It is known from the literature that inhibitor surveillance after FVIII exposure in non‐severe hemophilia A is poor.23 Consequently, a low titer inhibitor may be missed and years later, when a peak treatment moment occurs in the context of surgery, unexpectedly low FVIII levels may reveal that an inhibitor has occurred. We cannot rule out that this may partially explain the described association. However, the clear association between intensive treatment at the start of treatment and a higher inhibitor incidence reported in previous studies indicates that intensive treatment precedes inhibitor development.14, 18 To validate our results, it is important that in future studies inhibitor tests are performed regularly before and after intensive treatment.
In patients with severe hemophilia A the association between intensive treatment and inhibitor development has also been observed.8, 14 Furthermore, our findings are consistent with an earlier study investigating the impact of intensive FVIII treatment on inhibitor risk in 54 boys (range: 0–18 years) with mild hemophilia A.24 In all four inhibitor patients, inhibitor development was preceded by a peak treatment ranging from 8 to 27 consecutive exposures to FVIII concentrates. However, that study did not correct for the number of cumulative EDs, which is an important confounding factor in non‐severe patients that are treated very infrequently with FVIII concentrates. Moreover, all inhibitor patients developed inhibitors before the 50th exposure to FVIII (median: 22 EDs, range: 13–27 EDs), allowing no conclusions on late inhibitor development.
4.3.2. Age and the risk of inhibitor development
Previous studies in non‐severe hemophilia A have reported older age as a risk factor for inhibitor development in patients who received intensive FVIII treatment.25, 26 Furthermore, several murine and human studies have demonstrated an age‐related dysregulation of the immune system, leading to loss of immune tolerance.27, 28, 29, 30, 31 Based on these studies, it could be postulated that older patients may have a higher a priori risk of inhibitor development than younger patients and that intensive treatment with FVIII concentrates may trigger the immune system.
In the current study we were not able to investigate the association between age and inhibitor development, because patients were matched for year of birth.
4.4. Early versus late inhibitor development
Interestingly, we found that early and late inhibitor patients had comparable patient characteristics and eliciting risk factors (intensive treatment), suggesting common physiological mechanisms underlying inhibitor development. Early and late inhibitor patients also had a similar distribution of F8 genotypes, with a predominant clustering of mutations on the C1 domain. This is consistent with prior studies demonstrating that mutations in this domain convey an increased risk of inhibitor development in non‐severe hemophilia A.32, 33
A subset of included patients carrying a high‐risk F8 genotypic profile developed inhibitors after more than 50 exposures, some even after more than 100 exposures. We hypothesize that late inhibitor patients did not develop (high‐titer) inhibitors earlier during the treatment course because they did not yet receive an intensive FVIII treatment eliciting the required danger signals to provoke a clinically relevant anti‐FVIII immune response.
4.5. Recommendations for future research
As previously mentioned, our study included the largest cohort of patients who developed inhibitors late in the treatment course described to date. However, it is important to mention that our analysis covers a certain time era. Due to better FVIII treatment and monitoring, patients with hemophilia A undergo surgery more often than 30 years ago and are therefore more frequently exposed to FVIII concentrates. As a result, patients receive 50 EDs at a younger age. Whether this influences the risk for inhibitor development is still undefined.
To strengthen our results and recommendations, further research is required in the current hemophilia treatment era. However, setting up well‐designed studies on risk factors in non‐severe hemophilia A is challenging, especially due to the long follow‐up until inhibitor development.15 Therefore, further international collaboration is needed to set up a larger observational study, in which treatment data for every exposure day are collected for inhibitor patients and matched controls. To increase the generalizability of the results, future studies should include patients from all over the world, as race and ethnicity may influence the risk of inhibitor development.34 Moreover, there is a need for more translational immunological studies. If we increase our understanding of immunological mechanisms driving inhibitor development, it will provide us the opportunity to identify possible biomarkers for the early detection of evolving inhibitors in high‐risk patients, and potential targets for new preventive and therapeutic strategies.
Several studies suggest that the presence of non‐neutralizing FVIII antibodies (NNAs) may indicate the development of inhibitors.35, 36, 37 This was recently supported by the Hemophilia Inhibitor Previously Untreated Patient Study (HIPS), that prospectively evaluated the changes in the immune system of severe previously untreated patients during the first 50 exposures to FVIII concentrates.38 This study demonstrated that the development of inhibitors may be associated with distinct signatures of evolving NNAs consisting of IgG1, followed by IgG3 and subsequently the appearance of high‐affinity IgG4. If the presence of NNAs indeed predicts inhibitor development, this may allow for tailored inhibitor surveillance. However, before NNAs can be implemented in hemophilia management, more sufficiently powered clinical studies are needed.
5. CONCLUSION
In conclusion, our data suggest that intensive treatment with FVIII concentrates increases the risk of inhibitor development in non‐severe hemophilia A independent of cumulative number of EDs. Therefore, persistent vigilance is required throughout the lifetime treatment course of patients with non‐severe hemophilia A, to prevent or early detect inhibitor development.
CONFLICTS OF INTEREST
AA, CLE, ASV, CV, MGM, SM, and JGB have nothing to disclose. MC has received research grants from Bayer, CSL Behring, Daiichi Sankyo, Portola/Alexion, Roche, Sanquin Blood Supply, and UniQure, and consultancy and/or lecturing fees from Bayer, CSL Behring, Medcon International, MED talks, NovoNordisk, Pfizer, and Sobi. GC has received speaker, consultancy, and/or advisory fees from Ablynx, Bayer, CSL Behring, Kedrion, Novo Nordisk, Shire/Takeda, Sobi, Roche, UniQure, and Werfen, and research grants from CSL Behring, Pfizer, and Sobi. DPH has received research grants from Bayer, Octapharma, and Takeda, and advisory and/or lecturing fees from Bayer, Biomarin, Biotest, Grifols, Octapharma, Pfizer, Roche, Sanofi, Sobi, Takeda, and UniQure. CH has received consultancy and/or lecturing fees from Pfizer, Bayer, Shire/Takeda, Sobi, Biogen, CAF‐DCF, CSL Behring, LFB, Novo Nordisk, Roche, Octapharma, and Kedrion, and research funding from Pfizer, Bayer, Shire/Takeda, and Sobi. BL has received research grants from Baxter and CSL Behring. FWGL has received research support from CSL Behring, Shire/Takeda, UniQure, and Sobi and consultancy fees from UniQure, Novo Nordisk, Biomarin, and Shire/Takeda. He is a DSMB member for a study by Roche. MEM has received consultancy, advisory, and/or speaker fees from Bayer, CSL Behring, Catalyst, Biomarin, Kedrion, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, and Takeda. JO has received honoraria and consulting fees from Chugai, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, and Shire, and grants from CSL Behring, Novo Nordisk, Octapharma, Pfizer, and Shire. CM has received personal honoraria, travel support, fees to institution for study patients, and/or unrestricted grants from Bayer, Baxalta/Shire/Takeda, Biotest, CSL Behring, Grifols, Novo Nordisk, Roche, and SOBI. KF has received unrestricted research grants from CSL Behring, Novo Nordisk, and consultancy fees from Grifols, Takeda, and Novo Nordisk. SCG has received an unrestricted research grant from Sobi.
AUTHOR CONTRIBUTIONS
AA, SCG, and KF designed the study. AA interpreted and analyzed the data and wrote the manuscript. SCG, KF, and CLE redacted the manuscript. AA edited the final version of the manuscript. CLE, ASV, CV, MC, GC, DPH, CH, BL, FWGL, MEM, MGM, SM, JO, CM, JGB, and KF collected data or supervised data collection, and reviewed and approved the final version of the manuscript. A complete list of the members of the INSIGHT Study Group appears in the supporting information.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by an unrestricted research grant from CSL Behring (K. Fijnvandraat). The sponsors had no role in the choice of members of the steering committee and the participating centers nor the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Abdi A, Eckhardt CL, van Velzen AS, et al; the INSIGHT Study Group . Treatment‐related risk factors for inhibitor development in non‐severe hemophilia A after 50 cumulative exposure days: A case‐control study. J Thromb Haemost. 2021;19:2171–2181. 10.1111/jth.15419
Manuscript handled by: Jill Johnsen
Final decision: Jill Johnsen, 1 June 2021
REFERENCES
- 1.Blanchette VS, Key NS, Ljung LR, Manco‐Johnson MJ, van den Berg HM , Srivastava A. Definitions in hemophilia: Communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–1939. [DOI] [PubMed] [Google Scholar]
- 2.White GC, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J. Definitions in hemophilia: recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the international society on thrombosis and haemostasis. Thromb Haemost. 2001;85(03):560. [PubMed] [Google Scholar]
- 3.Donadel‐Claeyssens S, Aronis‐Vournas S, Auerswald G, et al. Current co‐ordinated activities of the PEDNET (European Paediatric Network for Haemophilia Management). Haemophilia. 2006;12(2):124–127. [DOI] [PubMed] [Google Scholar]
- 4.Manco‐Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava A, Brewer AK, Mauser‐Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1‐47. [DOI] [PubMed] [Google Scholar]
- 6.Wight J, Paisley S. The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia. 2003;9(4):418‐435. [DOI] [PubMed] [Google Scholar]
- 7.Rota M, Cortesi PA, Steinitz‐Trost KN, Reininger AJ, Gringeri A, Mantovani LG. Meta‐analysis on incidence of inhibitors in patients with haemophilia A treated with recombinant factor VIII products. Blood Coag Fibrinol. 2017;28(8):627–637. [DOI] [PubMed] [Google Scholar]
- 8.Gouw SC, Van Der Bom JG, Van Den Berg HM. Treatment‐related risk factors of inhibitor development in previously untreated patients with hemophilia A: The CANAL cohort study. Blood. 2007;109(11):4648‐4654. [DOI] [PubMed] [Google Scholar]
- 9.Peyvandi F, Mannucci PM, Garagiola I, et al. A Randomized Trial of Factor VIII and Neutralizing Antibodies in Hemophilia A. N Engl J Med. 2016;374(21):2054‐2064. [DOI] [PubMed] [Google Scholar]
- 10.Walsh CE, Soucie JM, Miller CH. Impact of inhibitors on hemophilia a mortality in the United States. Am J Hematol. 2015;90(5):400‐405. [DOI] [PubMed] [Google Scholar]
- 11.Leissinger C, Cooper DL, Solem CT. Assessing the impact of age, race, ethnicity and inhibitor status on functional limitations of patients with severe and moderately severe haemophilia A. Haemophilia. 2011;17(6):884‐889. [DOI] [PubMed] [Google Scholar]
- 12.Eckhardt CL, Mauser‐Bunschoten EP, Peters M, Leebeek FWG, van der Meer FJM , Fijnvandraat K. Inhibitor incidence after intensive FVIII replacement for surgery in mild and moderate haemophilia A: a prospective national study in the Netherlands. Br J Haematol. 2012;157(6):747‐752. [DOI] [PubMed] [Google Scholar]
- 13.Hill FGH. The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977–99. J Thromb Haemost. 2004;2(7):1047‐1054. [DOI] [PubMed] [Google Scholar]
- 14.Gouw SC, Van Den Berg HM, Fischer K, et al. Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: The RODIN study. Blood. 2013;121(20):4046‐4055. [DOI] [PubMed] [Google Scholar]
- 15.Eckhardt CL, Van Velzen AS, Peters M, et al. Factor VIII gene (F8) mutation and risk of inhibitor development in nonsevere hemophilia a. Blood. 2013;122(11):1954‐1962. [DOI] [PubMed] [Google Scholar]
- 16.Santagostino E, Mancuso ME, Rocino A, et al. Environmental risk factors for inhibitor development in children with haemophilia A: a case‐control study. Br J Haematol. 2005;130(3):422‐427. [DOI] [PubMed] [Google Scholar]
- 17.Maclean PS, Richards M, Williams M, et al. Treatment related factors and inhibitor development in children with severe haemophilia A. Haemophilia. 2011;17(2):282‐287. [DOI] [PubMed] [Google Scholar]
- 18.van Velzen AS , Eckhardt CL, Peters M, et al. Intensity of factor VIII treatment and the development of inhibitors in nonsevere hemophilia A patients: results of the INSIGHT case‐control study. J Thromb Haemost. 2017;15(7):1422‐1429. [DOI] [PubMed] [Google Scholar]
- 19.Marcucci M, Mancuso ME, Santagostino E, et al. Type and intensity of FVIII exposure on inhibitor development in PUPs with haemophilia A. A patient‐level meta‐analysis. Thromb Haemost. 2015;113(5):958‐967. [DOI] [PubMed] [Google Scholar]
- 20.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301‐305. [DOI] [PubMed] [Google Scholar]
- 21.Kuo C‐L, Duan Y, Grady J. Unconditional or conditional logistic regression model for age‐matched case‐control data? Front Public Heal. 2018;6:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearce N. Analysis of matched case‐control studies. BMJ. 2016;352:i969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batty P, Austin SK, Khair K, et al. Treatment burden, haemostatic strategies and real world inhibitor screening practice in non‐severe haemophilia A. Br J Haematol. 2017;176(5):796‐804. [DOI] [PubMed] [Google Scholar]
- 24.Sharathkumar A, Lillicrap D, Blanchette VS, et al. Intensive exposure to factor VIII is a risk factor for inhibitor development in mild hemophilia A. J Thromb Haemost. 2003;1(6):1228‐1236. [DOI] [PubMed] [Google Scholar]
- 25.Kempton CL, Allen G, Hord J, et al. Eradication of factor viii inhibitors in patients with mild and moderate hemophilia A. Am J Hematol. 2012;87:933‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauser‐Bunschoten EP, Den Uijl IEM, Schutgens REG, Roosendaal G, Fischer K. Risk of inhibitor development in mild haemophilia A increases with age. Haemophilia. 2012;18(2):263‐267. [DOI] [PubMed] [Google Scholar]
- 27.Arnold CR, Wolf J, Brunner S, Herndler‐Brandstetter D, Grubeck‐Loebenstein B. Gain and loss of T cell subsets in old age–age‐related reshaping of the T cell repertoire. J Clin Immunol. 2011;31(2):137‐146. [DOI] [PubMed] [Google Scholar]
- 28.Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing Res Rev. 2011;10(3):330‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolich‐Zugich J, Li G, Uhrlaub JL, Renkema KR, Smithey MJ. Age‐related changes in CD8 T cell homeostasis and immunity to infection. Semin Immunol. 2012;24(5):356‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haynes L, Lefebvre JS. Age‐related deficiencies in antigen‐specific CD4 T cell responses: lessons from mouse models. Aging Dis. 2011;2(5):374‐381. [PMC free article] [PubMed] [Google Scholar]
- 31.Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Semin Immunol. 2012;24:342‐349. [DOI] [PubMed] [Google Scholar]
- 32.Hay CRM, Ludlam CA, Colvin BT, et al. Factor VIII inhibitors in mild and moderate‐severity haemophilia A. Thromb Haemost. 1998;79(4):762‐766. [PubMed] [Google Scholar]
- 33.D’Oiron R, Pipe SW, Jacquemin M. Mild/moderate haemophilia A: new insights into molecular mechanisms and inhibitor development. Haemophilia. 2008;14(Suppl 3):138‐146. [DOI] [PubMed] [Google Scholar]
- 34.Peyvandi F, Ettingshausen CE, Goudemand J, Jiménez‐Yuste V, Santagostino E, Makris M. New findings on inhibitor development: from registries to clinical studies. Haemophilia. 2017;23:4‐13. [DOI] [PubMed] [Google Scholar]
- 35.Whelan SFJ, Hofbauer CJ, Horling FM, et al. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood. 2013;121(6):1039‐1048. [DOI] [PubMed] [Google Scholar]
- 36.Hofbauer CJ, Whelan SFJ, Hirschler M, et al. Affinity of FVIII‐specific antibodies reveals major differences between neutralizing and nonneutralizing antibodies in humans. Blood. 2015;125(7):1180‐1189. [DOI] [PubMed] [Google Scholar]
- 37.Cannavò A, Valsecchi C, Garagiola I, et al. Nonneutralizing antibodies against factor VIII and risk of inhibitor development in severe hemophilia A. Blood. 2017;129(10):1245‐1250. [DOI] [PubMed] [Google Scholar]
- 38.Reipert BM, Gangadharan B, Hofbauer CJ, et al. The prospective Hemophilia Inhibitor PUP Study reveals distinct antibody signatures prior to FVIII inhibitor development. Blood Adv. 2020;4(22):5785‐5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


