Abstract
The hypothalamus has been suggested to be important in the initiation cascade of migraine attacks based on clinical and biochemical observations. Previous imaging studies could not disentangle the changes due to the attack and those due to the trigger compound. With a novel approach, we assessed hypothalamic neuronal activity in early premonitory phases of glyceryl‐trinitrate (GTN)‐induced and spontaneous migraine attacks. We measured the hypothalamic blood oxygen level‐dependent (BOLD) response to oral glucose ingestion with 3T‐functional magnetic resonance imaging (MRI) in 27 women, 16 with migraine without aura and 11 controls group matched for age and body mass index (BMI), on 1 day without prior GTN administration and on a second day after GTN administration (to coincide with the premonitory phase of an induced attack). Interestingly, subgroups of patients with and without GTN‐triggered attacks could be compared. Additionally, five migraineurs were investigated in a spontaneous premonitory phase. Linear mixed models were used to study between‐ and within‐group effects. Without prior GTN infusion, the BOLD response to glucose was similar in migraine participants and controls (P = .41). After prior GTN infusion, recovery occurred steeper and faster in migraineurs (versus Day 1; P < .0001) and in those who developed an attack versus those who did not (P < .0001). Prior GTN infusion did not alter the glucose‐induced response in controls (versus baseline; P = .71). Just before spontaneous attacks, the BOLD‐response recovery was also faster (P < .0001). In this study, we found new and direct evidence of altered hypothalamic neuronal function in the immediate preclinical phase of both GTN‐provoked and spontaneous migraine attacks.
Keywords: attack onset, attack provocation, headache, neuroimaging
Recent data suggest that the hypothalamus is important in the initiation cascade of migraine attacks based on clinical and biochemical observations. Here, we found new and direct evidence of altered hypothalamic neuronal function in the immediate preclinical phase of both GTN‐provoked and spontaneous migraine attack. The hypothalamic blood oxygen level‐dependent (BOLD) response to oral glucose ingestion after overnight fasting was measured with 3T‐functional MRI in women with migraine and nonmigraine controls at three time points. At baseline, there was no difference in the response between patients and controls: a normal, persisting drop in BOLD signal reflecting reduced neuronal metabolic activity in the lateral hypothalamic area where glucosensitive neurons are located. Both at the beginning of GTN‐provoked migraine attacks and at the beginning of spontaneous attacks, this BOLD response was steeper (vs. baseline) in the patient group reflecting an unresponsiveness to the glucose ingestion. These findings imply a migraine‐attack related disturbance of normal hypothalamic functioning: a disinhibited hypothalamic satisfaction. This abnormal response seems attack‐specific, as it was not found in the control group nor in the migraine.
List of abbreviations
- BMI
body mass index
- BOLD
blood oxygen level‐dependent
- CBF
cerebral blood flow
- fMRI
functional magnetic resonance imaging
- GTN
glyceryl trinitrate
- HA
headache (only used in tables)
- ICHD
International Classification of Headache Disorders
- MRI
magnetic resonance imaging
- NRS
numeric rating scale
- PET
positron emission tomography
- TE
time to echo
- TR
repetition time
1. INTRODUCTION
Migraine is a common, multiphasic, paroxysmal neurovascular brain disorder with recurring disabling attacks of headache, associated autonomic features and, in one third of patients, aura (Headache Classification Committee of the International Headache Society (IHS), 2018; Ferrari, 1998). Up to 80% of patients experience 2–48 h before the headache so‐called premonitory symptoms such as yawning, craving for specific food, fluid retention, tiredness and mood changes (Blau, 1980; Cuvellier et al., 2009; Giffin et al., 2003; Kelman, 2004; Laurell et al., 2016; Quintela et al., 2006; Schoonman, Evers, et al., 2006). This premonitory phase is therefore considered as the true starting point of a migraine attack. From there, it is likely that a cascade of events follows in which separate pathophysiological mechanisms can explain the development of the headache, the aura and the associated symptoms. This makes migraine a complex disorder. Although mechanisms behind some of the later (secondary) events in the cascade are now relatively well‐understood, the mechanism of the primary initiating event behind a migraine attack is still largely unknown (Goadsby et al., 2017).
The episodic nature of attacks (Alstadhaug et al., 2005, 2007), the clinical characteristics of premonitory symptoms (Blau, 1980), the observation that attacks may be triggered by acute changes in sleep pattern (Cortelli & Pierangeli, 2007; Dodick et al., 2003; Kelman & Rains, 2005; Overeem et al., 2002; Rainero et al., 2008) and the finding that serum (Awaki et al., 1989; Elwan et al., 1991; Papakostas et al., 1987; Peres et al., 2001; Sarchielli et al., 2008; van Oosterhout et al., 2018) and cerebrospinal fluid (Elwan et al., 1991; Sarchielli et al., 2008) levels of hypothalamic hormones are abnormal during (Awaki et al., 1989; Elwan et al., 1991; Peres et al., 2001; van Oosterhout et al., 2018) and in‐between (Papakostas et al., 1987; Sarchielli et al., 2008; van Oosterhout et al., 2018) migraine attacks all suggest a role for the hypothalamus in the initiation cascade of migraine attacks (Goadsby et al., 2017).
Studies using blood oxygen level‐dependent functional magnetic resonance imaging (BOLD fMRI) (Schulte & May, 2016) or positron emission tomography (PET) (Denuelle et al., 2007; Maniyar et al., 2014) have indeed found hypothalamic cerebral blood flow (CBF) changes in glyceryl trinitrate (GTN)‐induced (Maniyar et al., 2014) and a few spontaneous (Denuelle et al., 2007; Schulte & May, 2016) migraine attacks. These studies, however, were all small, clinically heterogeneous or lacked nonmigraine control groups. Moreover, the fMRI studies mainly investigated connectivity rather than hypothalamic function, and no attempt was made to disentangle changes caused by GTN from changes due to the attack (Maniyar et al., 2014).
In the present study, we sought to better determine the role of the hypothalamus in the initiation of migraine attacks. As spontaneous migraine attacks are erratic and therefore complicate studying the initiation phase, we took advantage of the GTN migraine attack‐provocation model (Iversen, 2001). Most migraineurs (but not nonmigraineurs), develop premonitory symptoms approximately 90 min after infusion and a migraine‐like attack a few hours later (Afridi et al., 2004). We assessed and compared hypothalamic neuronal activity in women with (n = 19) or without (n = 14) migraine, with and without prior GTN infusion. Measurements with prior GTN infusion were done at 90 min after infusion to optimally coincide with possible premonitory symptoms. Five migraine patients could also be investigated during the premonitory symptoms phase of a spontaneous attack, enabling clinical validation of the findings in GTN‐provoked attacks. Hypothalamic neuronal activity was measured as the fMRI BOLD response to glucose ingestion, which specifically activates glucose‐sensitive neurons within the hypothalamus (Matsuda et al., 1999; Smeets et al., 2005). The normal persisting drop in BOLD signal in response to glucose ingestion reflects reduced neuronal metabolic activity and is considered a signal of glucose satisfaction (Oomura, 1980; Smeets et al., 2005). The design we used enabled disentangling the effects due to the attack from those due to GTN.
2. MATERIAL AND METHODS
2.1. Participants
We included 20 women with migraine without aura according to the International Classification of Headache Disorders‐2 (ICHD‐2) criteria (Olesen & Steiner, 2004) and 16 age‐ and body mass index (BMI) group‐matched control women without a personal and first degree family history of migraine or any other regularly occurring headaches. Migraineurs also fulfilled the new ICHD‐3 criteria (IHS, 2018). All participants were recruited from the Leiden University Medical Centre Migraine Neuro Analysis (LUMINA) programme including individuals with migraine and nonheadache controls from the Dutch population who all agreed to participate in migraine‐related scientific research. Individuals (both patients and control subjects) within the LUMINA cohort were recruited nationwide and were diagnosed using a validated algorithm (van Oosterhout et al., 2011). Participants with migraine were to have 1–6 migraine attacks and no more than 10 days of nonmigraine headache per month. In addition, they had to be free of migraine for at least 3 days before and 2 days after each study day, as was checked by a telephone call 7 days after each study day. Exclusion criteria for all participants included diabetes, premenstrual syndrome, hypertension, any psychiatric or neurologic disease, fever in the week prior and use of vasoactive, neuroactive or antibiotic medication in the 2 weeks prior to the measurement days.
2.2. Standard protocol approvals, registration and patient consents
The study was approved by the local medical ethics committee, and all subjects provided written informed consent prior to participation. The study was conducted in accordance to the Declaration of the World Medical Association and the Declaration of Helsinki (World Medical Association, 2013).
2.3. fMRI BOLD response to glucose ingestion
fMRI BOLD provides an indirect and non‐invasive method to assess changes in neuronal activity in the brain by measuring changes in the BOLD signal. These changes occur due to fluctuations in local concentrations of oxygenated and deoxygenated haemoglobin, local perfusion (blood flow and volume) and haematocrit that result from changes in neuronal activity (Attwell & Iadecola, 2002; Ogawa et al., 1992). Glucose‐sensitive neurons within the lateral hypothalamus respond to glucose triggering after fastening. Physiologically, the hypothalamic BOLD response to oral glucose administration follows a typical pattern with an initial, relatively rapid and steep decrease of the signal becoming noticeable after about four minutes, and reaching its nadir after another 4 min. This is then followed by a slow recovery towards baseline levels over the next ten to twelve minutes (Matsuda et al., 1999; Smeets et al., 2005). For glucose ingestion, a standard solution as used for the glucose tolerance test was made by mixing 300‐mL tap water with 75‐g glucose (Natufood, Natudis, Harderwijk, the Netherlands) (Smeets et al., 2005).
2.4. GTN migraine provocation model
After cannulating an antecubital vein, GTN (0.5 μg−1·kg−1·min−1) is administered over 20 min with the study participant in supine position (Iversen, 2001). Immediately after infusion, all study participants (migraineurs and nonmigraineurs alike) develop a brief, non‐specific mild headache without associated features. In approximately 80% of migraineurs, but in none of nonmigraineurs, this is followed, 3–6 h later, by a migraine‐like attack (Iversen, 2001; Juhasz et al., 2003; Schoonman, Bakker, et al., 2006; Thomsen et al., 1994, 1996). In many migraineurs, GTN‐provoked migraine‐like attacks are preceded by premonitory symptoms, which typically start at around 90 min after GTN infusion (Iversen, 2001; Juhasz et al., 2003; Schoonman, Bakker, et al., 2006; Thomsen et al., 1994, 1996).
2.5. Study design
All participants were scanned on two separate days after overnight fasting and abstention of coffee, tea and alcohol; water intake was allowed. Prior to scanning, all participants underwent a detailed standardized interview and full neurological examination. On the first (baseline) day, at around 9:00 AM, this was followed by an fMRI scan, which lasted for 21 min. All participants ingested a standard glucose solution via a perioral tube approximately 7 min into the fMRI sequence. They remained supine with the MR scanning continuously. On the second (=provocation) day, a 20‐min GTN infusion was begun at 08:30 AM. The post‐GTN fMRI scan, which involved ingestion of glucose at 7 min after onset, was performed 90 min (mean ± SD: 91 ± 20; range 30–133 min) after start of the GTN infusion. This time point was chosen to afford the highest likelihood of capturing possible premonitory symptoms of an ensuing GTN‐provoked attack (Iversen, 2001; Juhasz et al., 2003; Schoonman, Bakker, et al., 2006; Thomsen et al., 1994, 1996).
2.6. Spontaneous attacks
Participants with migraine were also asked to come to the MRI at the onset of any symptoms heralding a spontaneous migraine attack. They were then scanned during the premonitory phase of a spontaneous attack, using the same fMRI and glucose ingestion protocols.
2.7. Clinical parameters
We assessed premonitory symptoms, headache and migraine characteristics (according to the ICHD‐2 (Olesen & Steiner, 2004) (also fulfilling the new ICHD‐3 criteria, IHS, 2018) and pain severity (numeric rating scale [NRS], ranging from 0 [no headache] to 10 [most severe headache possible]) before, every 5 min during and every 30 min after GTN infusion in both migraine patients and controls. Socio‐demographic and clinical variables including migraine subtype, attack frequency and medication use were recorded during a structured interview before the baseline study day.
2.8. Data acquisition
MRI was performed on a 3.0 Tesla Achieva clinical scanner (Philips Healthcare, Best, the Netherlands) using a 32‐channel phased array head coil. The same scan protocol was used for all MR sessions. It composed of a whole brain high‐resolution 3‐D T1 sequence for imaging anatomical structures (TR 9.7 ms; TE 4.6 ms; flip angle 8°; FOV = 220 × 174 × 156 mm; 130 slices with a thickness of 1.2 mm and a voxel size of 0.86 × 0.86 mm), a structural hypothalamus scan (single slice scan; TR 550 ms; TE 10 ms; FOV = 208 × 208 mm; voxel size = 0.52 × 0.52 × 14 mm; scan time 1.14 min) and midbrain single slice fMRI scan (TR 120 ms; TE 30 ms; FOV 208 × 208 mm; voxel size = 0.81 × 0.81 × 14 mm; scan time 21.2 min; 500 dynamics). Anatomic images were screened for accidental findings by a neuroradiologist (MCK).
2.9. Data processing
Preprocessing and analysis of fMRI data were done using FSL version 5.03 (Jenkinson et al., 2012). Data were preprocessed as described in earlier studies (Teeuwisse et al., 2012). Data were averaged block‐wise for each set of four subsequent volumes, reducing the 500 dynamic scans to 125. The hypothalamus was segmented manually on the middle volume of the single slice MRI scan according to anatomical landmarks as previously described (Teeuwisse et al., 2012). To correct for scanner drift, all hypothalamic BOLD values were corrected for the BOLD signal obtained in an internal reference region of interest (ROI), drawn manually in grey matter, superior of the genu of the corpus callosum by an experienced imaging data analyst who was blinded for group and session (AvO). To establish the post‐ingestion hypothalamic BOLD response to intervention, the mean preglucose signal (first 7 min of the 21‐min fMRI scan) was used for contrast. All data points (n = 125) were divided by the mean baseline value and converted to percentages, yielding the percentage signal change relative to baseline, and this percentage signal change was then averaged per minute.
2.10. Statistical analysis
General characteristics were compared using Mann–Whitney U tests for continuous variables and Fisher exact tests for categorical data. Continuous data are presented as mean ± SD or as median with minimum–maximum. All fMRI results are reported as percentage BOLD change relative to the mean preglucose (reference) BOLD signal (0‐ to 7‐min predrinking). Data between Minutes 8 and 11 were omitted from the statistical analysis for artefacts in BOLD signal due to swallowing of the glucose solution. Data from Minute 11 and up (11–21) were considered the postglucose drinking BOLD response and were used for statistical analysis. Statistical analysis for comparison between groups (migraine; control), GTN (baseline; provocation) and/or migraine attack was performed by mixed model analysis as described earlier (Teeuwisse et al., 2012; van Opstal et al., 2018): group and GTN status were used as a fixed effect, time point as a variate and subject as a random factor. For within‐group comparisons, this model was applied to paired datasets.
Primary analysis was the difference in fMRI BOLD response to glucose at 90 min after GTN infusion versus the fMRI BOLD response to glucose without prior GTN infusion (i.e., baseline measurement) in participants with migraine, while focussing on those who developed an GTN‐induced migraine‐like attack. Secondary analyses included (i) baseline day differences between participants with migraine and controls; (ii) effects of GTN on the fMRI BOLD response to glucose in participants and controls; (iii) differences in fMRI BOLD response to glucose in GTN‐induced versus spontaneous attacks; and (iv) differences in post‐GTN fMRI BOLD signal response to glucose in migraine participants in whom GTN did not provoke an attack versus controls. For further exploratory analyses, the fMRI BOLD response after glucose (average derived from 11‐ to 21‐min postglucose) was correlated with clinical migraine and demographic parameters using Pearson correlation coefficients. Uncorrected P values of <.05 were deemed significant for all tests. All statistical analyses were performed with the Statistical Package of Social Sciences (SPSS, version 23.0; SPSS, Chicago, III).
3. RESULTS
3.1. Participants
The study flow is depicted in Figure 1 (reasons for exclusion are given in the legends). Of the 20 migraine participants with a baseline scan, two were excluded, leaving 18 participants eligible for the GTN scan. In two, post‐GTN scans could not be performed. One post‐GTN scan had to be excluded, leaving 15 post‐GTN scans in migraine participants with also a baseline scan. Of the 16 migraine participants with a post‐GTN scan, 13 (81%) developed a migraine‐like attack including the one of whom the scan had to be excluded due to movement artefacts. Paired data with both baseline and post‐GTN scans were available for 15 migraine participants, 12 with and 3 without a provoked attack. In five migraine participants, scans could also be performed in the premonitory phase of a spontaneous attack. Of the 16 controls with a baseline scan, two were excluded, and in four, no post‐GTN scan could be performed, leaving 11 controls with a post‐GTN scan. Paired data with both baseline and post‐GTN scan were available for 10 controls.
FIGURE 1.
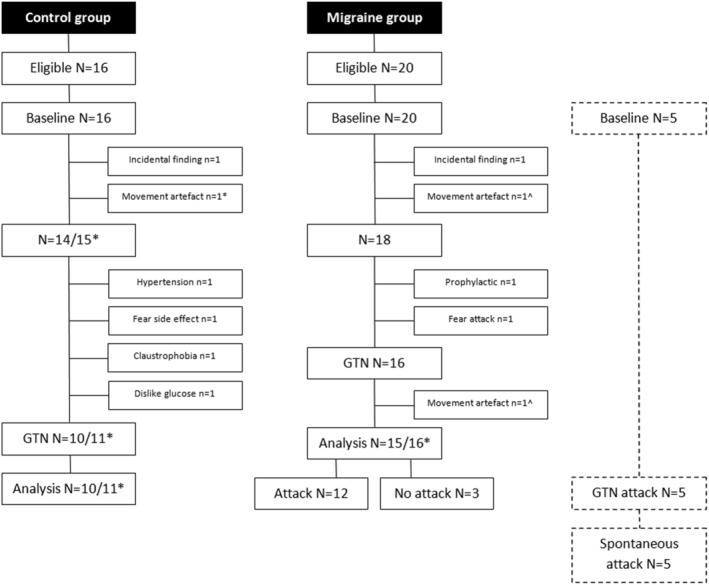
Flowchart of study participants. Flowcharts depicting eligibility and exclusion of study participants at different stages of the study. *Control participant in whom the baseline scan was excluded due to movement artefact, but glyceryl trinitrate (GTN) scan could be included in the unpaired analysis. ^Migraine participant in whom both baseline and GTN scan were excluded due to movement artefacts
3.2. Clinical and demographic characteristics
Clinical and demographic characteristics from all participants whose data were used in the analyses are summarized in Table 1. Demographic characteristics did not differ between the two groups. Participants with migraine had been free of migraine for 12.8 ± 8.7 (range 4–30) days before and >3 days after the attack‐free measurement and 10.3 ± 6.8 (range 4–30) days before and >3 days after the provocation day. Duration between baseline and GTN scan did not differ between migraine patients and controls (5.7 ± 6.9 months vs. 6.0 ± 5.6 months; P = .89). There were no major baseline differences between the 13 migraine participants who developed a migraine attack after GTN and the three who did not.
TABLE 1.
Baseline characteristics of study population
| Variable | Migraine without aura | Controls | P | |||
|---|---|---|---|---|---|---|
| n = 19 | n = 15 | |||||
| Socio‐demographic | ||||||
| Age, ya | 44 | 24–51 | 40 | 25–50 | .34 | |
| BMI, kg/m2 a | 23.8 | 20.1–26.2 | 23.1 | 20.5–27.2 | .85 | |
| Right‐handedness, n (%)b | 17 | (89.5%) | 14 | (93.3%) | .69 | |
| Migraine‐specific | ||||||
| Attack frequency/monthc | 2 | 1–6 | ||||
| Age at onset, yd | 18.5 | ± | 7.9 | |||
| Disease duration, yd | 21.3 | ± | 11.2 | |||
| Attack severity, NRSc | 8 | 5–10 | ||||
Abbreviations: BMI, body mass index; NRS, numeric rating scale; y, years.
Mann–Whitney U test, depicted as median and minimum–maximum.
Fisher exact test.
Depicted as median and minimum–maximum.
Depicted as mean ± SD.
3.3. Clinical effects of GTN infusion
GTN infusion caused an immediate transient mild non‐specific headache in all participants with migraine (mean NRS score = 3.8 ± 2.4) and controls (2.8 ± 2.0; P = .25). A delayed migraine‐like attack developed in 13/16 (81%) participants with migraine at a mean of 4:54 ± 2:06 h after start of the GTN infusion versus in 0/12 controls (0%; P = .007). All participants who developed a migraine‐like attack reported one or more premonitory symptoms from 60 ± 54 min (median 180; range 30–240 min) after onset of the GTN infusion and from 135 ± 116 min (median 112; range 30–460 min) before the headache started (Table 2). In contrast, none of those who did not develop a migraine‐like attack reported any premonitory symptom.
TABLE 2.
Description of clinical characteristics at baseline and after GTN provocation in study participants
| Group | Age | Baseline | GTN‐90 | GTN effect | Headache onset delay (h:min) | Headache characteristicsa | Headache intensityb | Associated symptomsc | Mimics usual migraine | Premonitory Symptoms |
|---|---|---|---|---|---|---|---|---|---|---|
| M | 44 | No HA | No HA | + | 4:30 | Left/throb/+ | 6 | −/−/+/+ | + | Yawning; nose feeling warm |
| M | 40 | No HA | ||||||||
| M | 44 | No HA | ||||||||
| M | 46 | No HA | No HA | − | n.a. | n.a. | n.a. | n.a. | n.a. | None |
| M | 35 | No HA | No HA | + | 4:30 | Right/throb/+ | 7 | +/+/+/− | + | Yawning; nausea |
| M | 50 | No HA | No HA | + | 5:20 | Right/throb/+ | 9 | +/+/+/+ | + | Stiff neck; fatigue; problems concentrating |
| M | 25 | No HA | No HA | − | n.a. | n.a. | n.a. | n.a. | n.a. | None |
| M | 50 | No HA | ||||||||
| M | 44 | No HA | No HA | − | n.a. | n.a. | n.a. | n.a. | n.a. | None |
| M | 48 | No HA | No HA | + | 3:35 | Bil/pres/+ | 5 | −/−/+/+ | + | Stiff neck; osmophobia |
| M | 50 | No HA | Premonitory | + | 2:50 | Left/pres/+ | 8 | +/+/+/+ | + | Yawning; fatigue; feeling cold; polyuria |
| M | 49 | No HA | No HA | + | 3:20 | Right/pres/+ | 10 | +/+/+/0 | + | Fatigue; dry mouth |
| M | 40 | No HA | No HA | + | 9:00 | Right/throb/+ | 3 | −/−/+/+ | + | Feeling warm; fatigue; nausea |
| M | 35 | No HA | No HA | + | 5:40 | Left/pres/+ | 8 | +/−/+/+ | + | Craving; osmophobia; yawning; restless |
| M | 51 | No HA | No HA | + | 6:20 | Left/throb/− | 5 | +/−/+/− | + | Nausea; yawning; |
| M | 28 | No HA | Premonitory | + | 3:40 | Bil/pres/+ | 6 | +/+/−/− | + | Fatigue; dry mouth; problems concentrating |
| M | 32 | No HA | No HA | + | 4:10 | Right/pres/+ | 6 | +/+/+/+ | + | Yawning; fatigue; heavy eyes; no appetite |
| M | 24 | No HA | No HA | + | 7:40 | Left/pres/+ | 6 | +/−/+/− | + | Stiff neck; feeling cold |
| M | 28 | No HA | No HA | + | 2:40 | Bil/pres/+ | 9 | +/−/+/+ | + | Pressure feeling; light dizziness |
| C | 27 | No HA | No HA | − | ||||||
| C | 25 | No HA | No HA | − | ||||||
| C | 40 | No HA | No HA | − | ||||||
| C | 46 | No HA | No HA | − | ||||||
| C | 50 | No HA | No HA | − | ||||||
| C | 44 | No HA | No HA | − | ||||||
| C | 39 | No HA | No HA | − | ||||||
| C | 44 | No HA | No HA | − | ||||||
| C | 30 | No HA | No HA | − | ||||||
| C | 45 | No HA | No HA | − | ||||||
| C | 43 | No HA | No HA | − | ||||||
| C | 46 | No HA | No HA | − | ||||||
| C | 28 | No HA | No HA | − | ||||||
| C | 29 | No HA | No HA | − | ||||||
| C | 25 | No HA | No HA | − |
Note: All participants who developed a migraine‐like attack reported one or more premonitory symptoms from 60 ± 54 min (median 180; range 30–240 min) after onset of the GTN infusion and from 135 ± 116 min (median 112; range 30–460 min) before the headache started. For assessing the effect of migraine attack phases, measurements were individually labelled as no headache, premonitory and headache. ‘Premonitory symptoms’ indicate symptoms that were reported by migraine patients >30 min after GTN infusion and that were recognized as their usual premonitory symptoms.
Abbreviations: −, no migraine attack provoked; +, provoked migraine attack after GTN infusion; C, healthy control; GTN‐90, 90 min after glyceryl trinitrate infusion; HA, headache; M, migraine patient.
Lateralization (left/right/bil = bilateral)/quality (throb = throbbing; pres = pressing)/aggravation.
Numeric rating scale (NRS) score for maximum headache severity from 0 (no pain at all) to 10 (worst pain ever).
Nausea/vomiting/photophobia/phonophobia.
3.4. BOLD response to glucose: With versus without prior GTN infusion
Without prior GTN infusion, the BOLD response to glucose was prototypical (steep decrease followed by a slow recovery) as reported previously (Matsuda et al., 1999; Smeets et al., 2005) and similar in the migraine and control group (Figure 2; P = .41). After prior GTN infusion, the BOLD response remained the same in the control group (P = .71; Figure 3a) but was clearly changed in the migraine group, with a much faster and steeper recovery phase after prior GTN infusion compared with the response without prior GTN infusion (total migraine group; intraindividual comparison: P < .0001; Figure 3b). A post hoc analysis revealed that this response (after GTN infusion) differed between patients with and without provoked migraine attack (between‐group comparison; P < .0001; Figure 4b). The fast recovery had only occurred in the 13 migraine participants who later developed a migraine attack (intraindividual comparison versus own baseline: P < .0001). In those three who did not get an attack, the recovery, in contrast, was slower (intraindividual comparison versus own baseline; P < .004).
FIGURE 2.
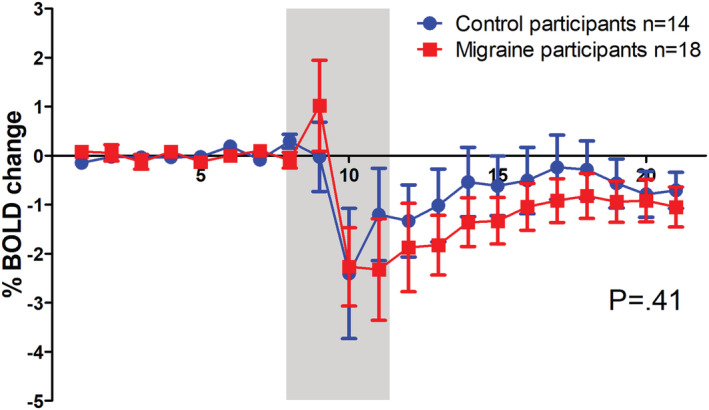
Comparison of blood oxygen level‐dependent (BOLD) responses to glucose ingestion at baseline day in migraine and control participants. The BOLD response to glucose at a baseline day did not differ between migraine and control participants. Grey box indicates drinking period of which data were omitted from the analysis because they are considered drinking artefacts. Error bars indicate 1 standard error
FIGURE 3.
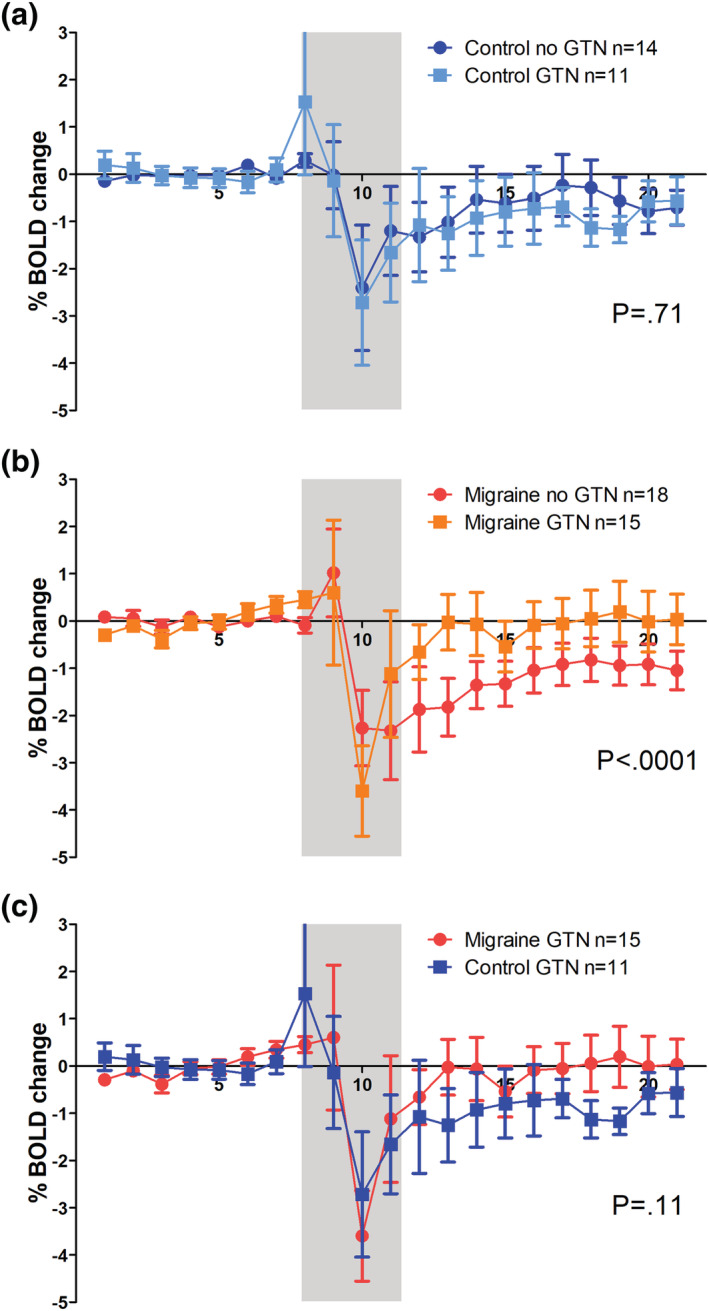
Comparison of blood oxygen level‐dependent (BOLD) responses to glucose ingestion after glyceryl trinitrate (GTN) provocation in migraine and control participants. The BOLD response to glucose after GTN did not differ from the BOLD response at baseline (without prior GTN infusion) in controls (a) but was higher in migraine participants (b). The BOLD response to glucose after GTN is higher in migraine participants then in control participants, although not significant (c). Grey box indicates drinking period of which data were omitted from the analysis because they are considered drinking artefacts. Error bars indicate 1 standard error
FIGURE 4.
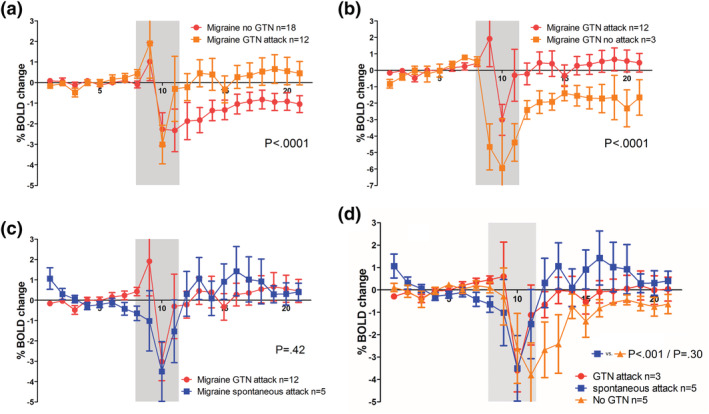
Comparison of blood oxygen level‐dependent (BOLD) responses to glucose ingestion in migraine participants in provoked and spontaneous attacks. In the preictal phase of a glyceryl trinitrate (GTN)‐induced migraine attack, the BOLD response to glucose is higher compared with the interictal response at baseline (paired analysis; a). The response pattern in spontaneous attack is similar to the pattern in GTN‐induced attacks (b). After GTN infusion, this abnormal response pattern was only seen in the subgroup of migraine patients who developed a migraine‐like attack, that is, who were in the premonitory early phase of the attack. In the five migraine participants scanned during a spontaneous attack, the response pattern in the spontaneous attack was similar to that in a GTN‐provoked attack (c). Visually, the BOLD responses in both spontaneous attacks and GTN‐provoked attacks differ from the response at baseline without GTN. The response pattern in spontaneous attacks reached statistical significance on between‐group level (P < .0001) but not on intraindividual level due to small sample sizes (P = .30) (d). Grey box indicates drinking period of which data were omitted from the analysis because they are considered drinking artefacts. Error bars indicate 1 standard error
Although the BOLD response to glucose after prior GTN infusion was visually different (faster and steeper recovery) in the total migraine group compared with controls, this difference did not reach statistical significance (between‐group comparison: P = .11; Figure 3c), also not when only the 13 migraine participants who got an attack were included (P = .12).
3.5. Similar BOLD response in spontaneous and GTN‐provoked attacks
Five migraine participants could also be studied in the premonitory phase of a spontaneous attack. The time from onset of spontaneous premonitory symptoms to glucose ingestion (during scan) was 288 ± 280 min (vs. 91 ± 20 min in the GTN provocation part; P = .03). In these patients, the BOLD responses to glucose in spontaneous and GTN‐provoked attacks were similar (intraindividual comparison; P = .42; Figure 4c). Both responses differed visually from the attack‐free measurement (Figure 4d), but the response pattern in spontaneous attacks reached statistical significance at between‐group level (versus baseline; P < .0001) but not at the intraindividual level likely due to small sample sizes (P = .30).
4. DISCUSSION
To assess the role of the hypothalamus in the initiation of migraine attacks, we intraindividually compared hypothalamic neuronal activity in the premonitory symptom phase of 13 GTN‐induced and five spontaneous migraine attacks with the activity outside an attack. Activity was also compared interindividually with measurements with and without prior GTN infusion in three migraine participants and 11 controls who did not develop premonitory symptoms or an attack after GTN infusion. Hypothalamic neuronal activity was measured as the hypothalamic fMRI BOLD response to glucose ingestion, reflecting hypothalamic glucose‐sensitive neuronal activity. Without prior GTN infusion, the hypothalamic BOLD response was similar in migraine participants and controls. However, while the response did not change after GTN infusion in controls, in the 13 migraine participants who developed a migraine attack, but not in the three who did not so, hypothalamic activity was different, with a faster and more abrupt recovery phase. Importantly, all hypothalamic responses and changes from baseline were similar in GTN‐induced and spontaneous attacks, validating the findings in GTN‐induced attacks as representative for what is occurring in spontaneous attacks.
In the early phases of provoked and spontaneous migraine attacks, migraine patients did not respond to glucose ingestion with a normal, persisting drop in the BOLD signal. Such a drop is considered to reflect reduced neuronal metabolic activity in the lateral hypothalamic area where glucosensitive neurons are located. This normal response is known across species (Smeets et al., 2005) and is seen as a signal of glucose satisfaction, that is, a normal ‘satisfied’ state after glucose ingestion (Oomura, 1980; Smeets et al., 2005). The migraine patients rather showed an unresponsiveness to the glucose trigger during the early attack phase, implying a migraine‐attack related disturbance of normal hypothalamic functioning, a disinhibited hypothalamic satisfaction. This abnormal response seems attack‐specific, as it was not found in the control group, nor in the migraine group when there was no forthcoming attack. Although it is tempting to link this to the common premonitory symptom of craving, it would be oversimplifying to do so as the hypothalamic control of different homeostatic mechanisms is rather complex.
GTN influences cardiovascular parameters (Verheyden et al., 2008), and the changes we observed could theoretically have been due to GTN rather than related to the initiation cascade of an attack. However, one would then have expected similar changes to occur in the control group. Moreover, we measured 90 min after GTN infusion, which significantly exceeds GTN τ1/2 (2.5–4 min).
Only two studies have previously investigated the role of the hypothalamus in the initiation phase of migraine attacks. The study by Maniyar et al. showed activations in the posterolateral hypothalamus in eight migraine with aura patients with premonitory symptoms after GTN infusion, using H2 15O PET CBF as a marker for neuronal activity (Maniyar et al., 2014). Although this suggests that the hypothalamus is pivotal in the early, premonitory phase of the migraine attack, a possible GTN effect cannot be excluded as there was no contemporaneous control group. Schulte et al. (Schulte & May, 2016) daily assessed the hypothalamic fMRI blood flow response to a trigeminal nociceptive stimulus (nasal administration of gaseous ammonia) for 30 consecutive days in a single migraine patient. They prospectively captured three migraine attacks and found an increased hypothalamic response in the 24 h prior to onset of the migraine headache (Schulte & May, 2016). Although they did not include a control group to correct for possible diurnal, weekly or menstrual effects, the findings nonetheless suggest an important role for the hypothalamus in the early phases of the migraine attack. A third study found hypothalamic activation in migraine headache but did not perform measurements during the premonitory phase. Collectively, these and our data suggest a pivotal role of the hypothalamus in the early phases of migraine attack initiation.
There is a growing interest in the hypothalamus as the site of initiation of a migraine attack initiator based on clinical and biochemical arguments (Akerman et al., 2011; Blau, 1984; Charbit et al., 2010). In this study, we have shown an increased hypothalamic BOLD response patterns to glucose after fasting in migraine patients. Normally, the ingestion of oral glucose induces transient silencing of the activity of glucosensitive neurons in the lateral hypothalamus. However, in the preictal phase of both spontaneous and GTN‐triggered attacks, the ingestion of glucose was followed by a much faster and steeper response than normally. In the triggered migraineurs, the suppression of the hypothalamic hyperactivity was apparently temporarily and shorter than in nonmigraine individuals and was suggestive of an impending migraine attack. We might draw an analogy between this ‘overdrive’ of ‘craving’ state of these neurons and the clinical symptom of craving experienced the hours before the migraine headache starts.
Our study has several strengths. The paired design in patients and controls enabled to disentangle the effects of GTN and the attack. Using a validated model to provoke premonitory symptoms and migraine‐like attacks enabled us to capture the preclinical initiation phase of attacks (Afridi et al., 2004), which is hardly possible for spontaneous attacks due to their erratic nature. Finally, we did manage to capture the presymptomatic phase of migraine attacks in five patients, which allowed for a clinical validation of the findings in GTN‐provoked attacks. We included only female migraine without aura patients, limiting the overall generalizability. The results, however, were a homogeneous study group. Although we are aware that changes in gonadal hormones could possibly have affected these findings, adjusting the main analysis for this effect was not possible in a robust way due to small numbers. An additional analysis however showed that the time since last menstruation during the GTN day did not differ between migraine and controls, thereby minimizing its possible effect. In addition, the time period between baseline and GTN scan session did not differ between groups.
Theoretically, a nitric oxide donor such as GTN could modify hypothalamic response to glucose ingestion, as nitric oxide is known to affect insulin regulation (Bahadoran et al., 2020). In obesity (Matsuda et al., 1999) or diabetes type 2 patients (Vidarsdottir et al., 2007) similar blunted inhibitory fMRI responses to glucose have been reported. In our study, this would not explain for the migraine‐control differences, because the GTN protocol was similar in both groups, and none of the participant had known diabetes. Technically, the small ROI made the data acquisition susceptible to, for example, drinking movement artefacts, leading to exclusion of three subjects from the analyses. We were not able to distinguish between the different hypothalamic nuclei. Possibly, different nuclei could be involved in different migraine attack phases, being hyperactive in one phase and hypoactive in the other. More sophisticated imaging techniques could perhaps be used in the future to disentangle these possible differential effects.
5. CONCLUSION
To conclude, this is the first study showing a disturbed function or reactivity of the hypothalamus during the earliest phases of both GTN‐provoked and spontaneous migraine attacks. This emphasizes the role of the hypothalamus in the early phase of migraine attacks.
CONFLICT OF INTEREST
The authors report no relevant conflict of interest.
AUTHOR CONTRIBUTIONS
Willebrordus P. J. van Oosterhout performed the design and conceptualization of study, data acquisition, data analysis, editing and interpretation and drafted and revised the manuscript for intellectual content. Anne Marie van Opstal performed the data acquisition, data analysis, figure editing, text editing and interpretation. Guus G. Schoonman, Gisela M. Terwindt, Michel D. Ferrari and Mark C. Kruit performed the design and conceptualization of study, data editing and interpretation and revised the manuscript for intellectual content. Jeroen van der Grond contributed to data collection, analysis and interpretation and revised the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15369.
ACKNOWLEDGEMENTS
The authors would like to thank W.M. Teeuwisse, Department of Radiology, Leiden University Medical Center, for his technical support during the study and Dr. E.W. van Zwet, consultant in medical statistics, for his advice in the data analysis. Furthermore, the authors would like to thank then medical students B.C. Nievaart MD, B.S. Uitbeijerse MD and W.C.G. van Gorp MD for their practical assistance during data collection. This work was supported by grants of the Netherlands Organization for Scientific Research (VIDI 917.11.319 to G.M.T. and Spinoza Premium 2009 to M.D.F.), the European Commission (FP7‐EUROHEADPAIN ‐ no. 602633 to M.D. F.) and Merel Foundation (to W.P.J.v.O.). They had no role in the design or conduct of the study.
van Oosterhout, W. P. J., van Opstal, A. M., Schoonman, G. G., van der Grond, J., Terwindt, G. M., Ferrari, M. D., & Kruit, M. C. (2021). Hypothalamic functional MRI activity in the initiation phase of spontaneous and glyceryl trinitrate‐induced migraine attacks. European Journal of Neuroscience, 54(3), 5189–5202. 10.1111/ejn.15369
Michel D. Ferrari and Mark C. Kruit contributed equally.
Edited by: Susan Rossell
Funding information FP7 (FP7‐EUROHEADPAIN), Grant/Award Number: 602633; Netherlands Organization for Scientific Research, Grant/Award Number: VIDI 917.11.319; Merel Foundation
DATA AVAILABILITY STATEMENT
All data, methods and materials used to conduct this research are mentioned in this article.
REFERENCES
- Afridi, S. K., Kaube, H., & Goadsby, P. J. (2004). Glyceryl trinitrate triggers premonitory symptoms in migraineurs. Pain, 110(3), 675–680. 10.1016/j.pain.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Akerman, S., Holland, P. R., & Goadsby, P. J. (2011). Diencephalic and brainstem mechanisms in migraine. NatRevNeurosci, 12(10), 570–584. [DOI] [PubMed] [Google Scholar]
- Alstadhaug, K. B., Bekkelund, S., & Salvesen, R. (2007). Circannual periodicity of migraine? European Journal of Neurology, 14(9), 983–988. 10.1111/j.1468-1331.2007.01828.x [DOI] [PubMed] [Google Scholar]
- Alstadhaug, K. B., Salvesen, R., & Bekkelund, S. I. (2005). Seasonal variation in migraine. Cephalalgia, 25(10), 811–816. 10.1111/j.1468-2982.2005.01018.x [DOI] [PubMed] [Google Scholar]
- Attwell, D., & Iadecola, C. (2002). The neural basis of functional brain imaging signals. Trends in Neurosciences, 25(12), 621–625. 10.1016/S0166-2236(02)02264-6 [DOI] [PubMed] [Google Scholar]
- Awaki, E., Takeshima, T., & Takahashi, K. (1989). A neuroendocrinological study in female migraineurs: Prolactin and thyroid stimulating hormone responses. Cephalalgia, 9(3), 187–193. 10.1046/j.1468-2982.1989.903187.x [DOI] [PubMed] [Google Scholar]
- Bahadoran, Z., Mirmiran, P., & Ghasemi, A. (2020). Role of nitric oxide in insulin secretion and glucose metabolism. Trends in Endocrinology and Metabolism, 31(2), 118–130. 10.1016/j.tem.2019.10.001 [DOI] [PubMed] [Google Scholar]
- Blau, J. N. (1980). Migraine prodromes separated from the aura: complete migraine. British Medical Journal, 281(6241), 658–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau, J. N. (1984). Migraine pathogenesis—The neural hypothesis reexamined. Journal of Neurology Neurosurgery and Psychiatry, 47(5), 437–442. 10.1136/jnnp.47.5.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit, A. R., Akerman, S., & Goadsby, P. J. (2010). Dopamine: What's new in migraine? Current Opinion in Neurology, 23(3), 275–281. [DOI] [PubMed] [Google Scholar]
- Cortelli, P., & Pierangeli, G. (2007). Hypothalamus and headaches. Neurological Sciences, 28, S198–S202. 10.1007/s10072-007-0776-2 [DOI] [PubMed] [Google Scholar]
- Cuvellier, J. C., Mars, A., & Vallee, L. (2009). The prevalence of premonitory symptoms in paediatric migraine: A questionnaire study in 103 children and adolescents. Cephalalgia, 29(11), 1197–1201. 10.1111/j.1468-2982.2009.01854.x [DOI] [PubMed] [Google Scholar]
- Denuelle, M., Fabre, N., Payoux, P., Chollet, F., & Geraud, G. (2007). Hypothalamic activation in spontaneous migraine attacks. Headache, 47(10), 1418–1426. 10.1111/j.1526-4610.2007.00776.x [DOI] [PubMed] [Google Scholar]
- Dodick, D. W., Eross, E. J., & Parish, J. M. (2003). Clinical, anatomical, and physiologic relationship between sleep and headache. Headache, 43(3), 282–292. 10.1046/j.1526-4610.2003.03055.x [DOI] [PubMed] [Google Scholar]
- Elwan, O., Abdella, M., el Bayad, A. B., & Hamdy, S. (1991). Hormonal changes in headache patients. Journal of the Neurological Sciences, 106(1), 75–81. [DOI] [PubMed] [Google Scholar]
- Ferrari, M. D. (1998). Migraine. Lancet, 351(9108), 1043–1051. 10.1016/S0140-6736(97)11370-8 [DOI] [PubMed] [Google Scholar]
- Giffin, N. J., Ruggiero, L., Lipton, R. B., Silberstein, S. D., Tvedskov, J. F., Olesen, J., Altman, J., Goadsby, P. J., & Macrae, A. (2003). Premonitory symptoms in migraine—An electronic diary study. Neurology, 60(6), 935–940. 10.1212/01.WNL.0000052998.58526.A9 [DOI] [PubMed] [Google Scholar]
- Goadsby, P. J., Holland, P. R., Martins‐Oliveira, M., Hoffmann, J., Schankin, C., & Akerman, S. (2017). Pathophysiology of migraine: A disorder of sensory processing. Physiological Reviews, 97(2), 553–622. 10.1152/physrev.00034.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society (IHS) . (2018). The International Classification of Headache Disorders, 3rd edition. Cephalalgia, 38(1), 1–211. [DOI] [PubMed] [Google Scholar]
- Iversen, H. K. (2001). Human migraine models. Cephalalgia, 21(7), 781–785. 10.1177/033310240102100710 [DOI] [PubMed] [Google Scholar]
- Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., & Smith, S. M. (2012). Fsl. Neuroimage, 62(2), 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Juhasz, G., Zsombok, T., Modos, E. A., Olajos, S., Jakab, B., Nemeth, J., Szolcsanyi, J., Vitrai, J., & Bagdy, G. (2003). NO‐induced migraine attack: Strong increase in plasma calcitonin gene‐related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain, 106(3), 461–470. 10.1016/j.pain.2003.09.008 [DOI] [PubMed] [Google Scholar]
- Kelman, L. (2004). The premonitory symptoms (prodrome): A tertiary care study of 893 migraineurs. Headache, 44(9), 865–872. 10.1111/j.1526-4610.2004.04168.x [DOI] [PubMed] [Google Scholar]
- Kelman, L., & Rains, J. C. (2005). Headache and sleep: Examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache, 45(7), 904–910. 10.1111/j.1526-4610.2005.05159.x [DOI] [PubMed] [Google Scholar]
- Laurell, K., Artto, V., Bendtsen, L., Hagen, K., Haggstrom, J., Linde, M., Soderstrom, L., Tronvik, E., Wessman, M., Zwart, J. A., & Kallela, M. (2016). Premonitory symptoms in migraine: A cross‐sectional study in 2714 persons. Cephalalgia, 36(10), 951–959. 10.1177/0333102415620251 [DOI] [PubMed] [Google Scholar]
- Maniyar, F. H., Sprenger, T., Monteith, T., Schankin, C., & Goadsby, P. J. (2014). Brain activations in the premonitory phase of nitroglycerin‐triggered migraine attacks. Brain, 137(Pt 1), 232–241. 10.1093/brain/awt320 [DOI] [PubMed] [Google Scholar]
- Matsuda, M., Liu, Y., Mahankali, S., Pu, Y., Mahankali, A., Wang, J., DeFronzo, R. A., Fox, P. T., & Gao, J. H. (1999). Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes, 48(9), 1801–1806. 10.2337/diabetes.48.9.1801 [DOI] [PubMed] [Google Scholar]
- Ogawa, S., Tank, D. W., Menon, R., Ellermann, J. M., Kim, S. G., Merkle, H., & Ugurbil, K. (1992). Intrinsic signal changes accompanying sensory stimulation—Functional brain mapping with magnetic‐resonance‐imaging. Proceedings of the National Academy of Sciences of the United States of America, 89(13), 5951–5955. 10.1073/pnas.89.13.5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen, J., & Steiner, T. J. (2004). The International Classification of Headache Disorders: 2nd edition. Cephalalgia, 24(Suppl 1), 9–160. [DOI] [PubMed] [Google Scholar]
- Oomura, Y. (1980). Input–Output Organization in the Hypothalamus Relating to Food Intake Behavior. Marcel Dekker. [Google Scholar]
- Overeem, S., van Vliet, J. A., Lammers, G. J., Zitman, F. G., Swaab, D. F., & Ferrari, M. D. (2002). The hypothalamus in episodic brain disorders. Lancet Neurology, 1(7), 437–444. 10.1016/S1474-4422(02)00191-6 [DOI] [PubMed] [Google Scholar]
- Papakostas, Y., Daras, M., Markianos, M., & Stefanis, C. (1987). Increased prolactin response to thyrotropin releasing hormone during migraine attacks. Journal of Neurology, Neurosurgery & Psychiatry, 50(7), 927–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres, M. F. P., del Rio, M. S., Seabra, M. L. V., Tufik, S., Abucham, J., Cipolla‐Neto, J., Silberstein, S. D., & Zukerman, E. (2001). Hypothalamic involvement in chronic migraine. Journal of Neurology Neurosurgery and Psychiatry, 71(6), 747–751. 10.1136/jnnp.71.6.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintela, E., Castillo, J., Munoz, P., & Pascual, J. (2006). Premonitory and resolution symptoms in migraine: A prospective study in 100 unselected patients. Cephalalgia, 26(9), 1051–1060. 10.1111/j.1468-2982.2006.01157.x [DOI] [PubMed] [Google Scholar]
- Rainero, I., De, M. P., & Pinessi, L. (2008). Hypocretins and primary headaches: neurobiology and clinical implications. Expert Review of Neurotherapeutics, 8(3), 409–416. [DOI] [PubMed] [Google Scholar]
- Sarchielli, P., Rainero, I., Coppola, F., Rossi, C., Mancini, M., Pinessi, L., & Calabresi, P. (2008). Involvement of corticotrophin‐releasing factor and orexin‐A in chronic migraine and medication‐overuse headache: findings from cerebrospinal fluid. Cephalalgia, 28(7), 714–722. 10.1111/j.1468-2982.2008.01566.x [DOI] [PubMed] [Google Scholar]
- Schoonman, G. G., Bakker, D., Schmitz, N., van der Geest, R. J., Van der Grond, J., Ferrari, M. D., & Van Buchem, M. A. (2006). Magnetic resonance angiography of the human middle meningeal artery: Implications for migraine. Journal of Magnetic Resonance Imaging, 24(4), 918–921. [DOI] [PubMed] [Google Scholar]
- Schoonman, G. G., Evers, D. J., Terwindt, G. M., van Dijk, J. G., & Ferrari, M. D. (2006). The prevalence of premonitory symptoms in migraine: a questionnaire study in 461 patients. Cephalalgia, 26(10), 1209–1213. 10.1111/j.1468-2982.2006.01195.x [DOI] [PubMed] [Google Scholar]
- Schulte, L. H., & May, A. (2016). The migraine generator revisited: Continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain, 139(Pt 7), 1987–1993. 10.1093/brain/aww097 [DOI] [PubMed] [Google Scholar]
- Smeets, P. A., de Graaf, C., Stafleu, A., van Osch, M. J., & van der Grond, J. (2005). Functional MRI of human hypothalamic responses following glucose ingestion. NeuroImage, 24(2), 363–368. 10.1016/j.neuroimage.2004.07.073 [DOI] [PubMed] [Google Scholar]
- Teeuwisse, W. M., Widya, R. L., Paulides, M., Lamb, H. J., Smit, J. W., de Roos, A., van Buchem, M. A., Pijl, H., & van der Grond, J. (2012). Short‐term caloric restriction normalizes hypothalamic neuronal responsiveness to glucose ingestion in patients with type 2 diabetes. Diabetes, 61(12), 3255–3259. 10.2337/db11-1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen, L. L., Brennum, J., Iversen, H. K., & Olesen, J. (1996). Effect of a nitric oxide donor (glyceryl trinitrate) on nociceptive thresholds in man. Cephalalgia, 16(3), 169–174. 10.1046/j.1468-2982.1996.1603169.x [DOI] [PubMed] [Google Scholar]
- Thomsen, L. L., Kruuse, C., Iversen, H. K., & Olesen, J. (1994). A nitric oxide donor (nitroglycerine) triggers genuine migraine attacks. European Journal of Neurology, 1, 73–80. 10.1111/j.1468-1331.1994.tb00053.x [DOI] [PubMed] [Google Scholar]
- van Oosterhout, W. P., Weller, C. M., Stam, A. H., Bakels, F., Stijnen, T., Ferrari, M. D., & Terwindt, G. M. (2011). Validation of the web‐based LUMINA questionnaire for recruiting large cohorts of migraineurs. Cephalalgia, 31(13), 1359–1367. [DOI] [PubMed] [Google Scholar]
- van Oosterhout, W. P. J., Schoonman, G. G., van Zwet, E. W., Dekkers, O. M., Terwindt, G. M., van den Brink, A. M., & Ferrari, M. D. (2018). Female sex hormones in men with migraine. Neurology, 91(4), e374–e381. [DOI] [PubMed] [Google Scholar]
- van Opstal, A. M., van den Berg‐Huysmans, A. A., Hoeksma, M., Blonk, C., Pijl, H., Rombouts, S., & van der Grond, J. (2018). The effect of consumption temperature on the homeostatic and hedonic responses to glucose ingestion in the hypothalamus and the reward system. The American Journal of Clinical Nutrition, 107(1), 20–25. 10.1093/ajcn/nqx023 [DOI] [PubMed] [Google Scholar]
- Verheyden, B., Liu, J., van Dijk, N., Westerhof, B. E., Reybrouck, T., Aubert, A. E., & Wieling, W. (2008). Steep fall in cardiac output is main determinant of hypotension during drug‐free and nitroglycerine‐induced orthostatic vasovagal syncope. Heart Rhythm, 5(12), 1695–1701. 10.1016/j.hrthm.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Vidarsdottir, S., Smeets, P. A., Eichelsheim, D. L., van Osch, M. J., Viergever, M. A., Romijn, J. A., van der Grond, J., & Pijl, H. (2007). Glucose ingestion fails to inhibit hypothalamic neuronal activity in patients with type 2 diabetes. Diabetes, 56(10), 2547–2550. 10.2337/db07-0193 [DOI] [PubMed] [Google Scholar]
- World Medical Association . (2013). World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA, 310(20), 2191–2194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data, methods and materials used to conduct this research are mentioned in this article.


