Abstract
We introduce a new and highly efficient synthetic protocol towards multifunctional fluorescent cyclopeptides by solid‐phase peptide macrocyclization via dipyrrin construction, with full scope of proteinogenic amino acids and different ring sizes. Various bicyclic peptides can be created by dipyrrin‐based crosslinking and double dipyrrin‐ring formation. The embedded dipyrrin can be either transformed to fluorescent BODIPY and then utilized as cancer‐selective targeted protein imaging probe in vitro, or directly employed as a selective metal sensor in aqueous media. This work provides a valuable addition to the peptide macrocyclization toolbox, and a blueprint for the development of multifunctional dipyrrin linkers in cyclopeptides for a wide range of potential bioapplications.
Keywords: dipyrrin, fluorescent cyclic peptides, solid-phase peptide synthesis, zinc(II) sensing, αvβ3 imaging
A facile and highly efficient synthetic protocol towards multifunctional fluorescent cyclopeptides by solid‐phase peptide macrocyclization via dipyrrin staple construction is introduced, where the dipyrrin moiety serves as a novel multifunctional staple linker for targeted protein imaging and selective metal sensing.
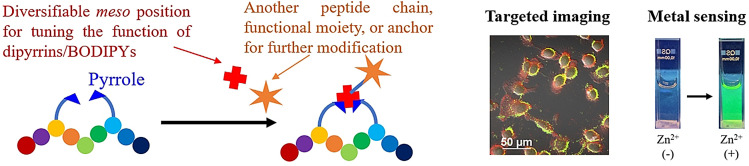
Conformationally constrained macrocyclic peptides[1] possessing larger target‐selective binding surfaces of higher affinity,[2] ameliorated cell permeability and stability,[3, 4] and versatile and remarkable pharmacological properties[5] compared with their linear counterparts have emerged as novel and promising molecular platforms for efficaciously modulating disease‐relevant protein–protein interactions (PPIs)—known therapeutic targets or those previously thought to be “undruggable”.[6] To this end, a repertoire of peptide macrocyclization and stapling approaches has been developed and diversified over the past decade.[7] Cross‐couplings,[8] click reactions,[9] C−H activations/functionalizations,[10] ring‐closing olefin metathesis reactions,[11] Diels–Alder cycloadditions,[12] multicomponent Ugi/Petasis‐type reactions,[13] and ligation‐mediated cyclizations,[14] among others,[15] have been established in site‐specific fashions employing a variety of anchoring residues/terminal groups.[16] New structural–functional moieties can be introduced into the backbones of peptide macrocycles via certain above‐mentioned approaches as the staple linkers, which can be manipulated to optimize the cyclopeptide ring size/rigidity, and furnish new handling sites for small‐molecule drug and fluorescent dye conjugations to access unprecedented chemotypes and become powerful tools to probe PPIs and orchestrate therapeutic applications.[17]
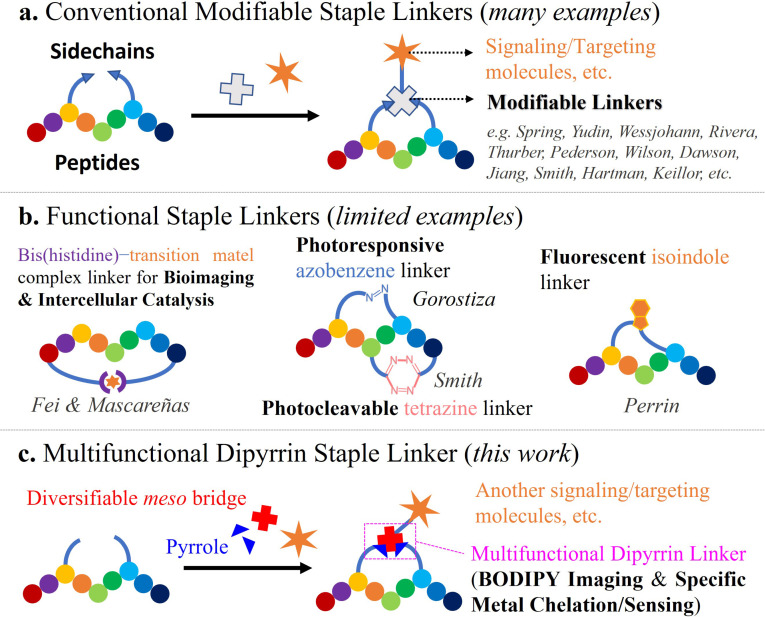
Although monofunctional modifiable staple linkers as “tritopic connectors” have been well‐reported, (Figure 1 a) other strategic functional designs and applications still remain underexplored. For luminescent staple linkers, Fei et al. have reported a luminogenic peptide stapling strategy utilizing bis(histidine)–iridium(III) complex coordination chemistry with RGD and oligoarginine peptides for cancer cell targeting, imaging and killing.[18, 19] Perrin et al. have realized a mild, metal‐free, and late‐stage fluorescent isoindole crosslink strategy by employing phthalaldehyde‐mediated “isoindole intramolecular stapling” chemoselectively with peptides’ amine‐thiol groups, where the isoindole can serve as a fluorophore.[20] On the other hand, through installing photoresponsive azobenzene,[21] by Gorostiza et al., or photocleavable s‐tetrazine staple linkers,[22] by Smith et al., precision optical modulations of stapled peptides for “off–on” inhibition and unstapling have also been feasible (Figure 1 b). For a catalytic linker, Mascareñas et al. realized the first “stapled pallado‐miniprotein”‐promoted depropargylation reaction in live cells.[23] To develop new multifunctional staple linkers for de novo macrocyclic/stapled peptide design for both medicinal and bioanalytical chemistry purposes, more advances in cyclization/coupling reactions and their translational applications into cyclopeptide syntheses, functionalizations and modifications are urgently needed.
Figure 1.
Contemporary examples of functional staple linkers in cyclic peptides. a) Structurally modifiable staple linkers for further ligations of fluorescent dyes and targeting vectors; b) functional staple linkers as the luminophore, the catalytic center, and the photoswitch/photocontrol; c) our new multifunctional dipyrrin staple linker for targeted BODIPY imaging and selective zinc sensing.
Very recently, our group has disclosed a facile, efficient, and purification‐economical Fmoc‐based solid‐phase synthetic method for fluorescent boron‐dipyrromethene (BODIPY)‐peptide conjugates via in situ dipyrrin construction.[24] We then speculated that such solid‐phase dipyrrin assembly reaction can also be compatibly utilized for peptide macrocyclization and stapling, i.e., the dipyrrin as a new multifunctional staple linker, to obtain highly sought‐after either fluorescent BODIPY‐containing, or metal‐chelating thus light‐emissive macrocyclic peptides. Herein, we unleash the potential of a new multi‐functional dipyrrin staple linker and introduce our solid‐phase peptide macrocyclization and multifunctionalization strategy via dipyrrin coupling and manipulation (Figure 1 c). Broad substrate scopes (i.e., amino acids and the meso position of dipyrrin) of the reaction are substantiated, while enhanced stabilities and higher selective target binding affinities of the resulting dipyrrin‐cyclopeptides than their linear counterparts are corroborated. Various bicyclic peptides can also be formed by dipyrrin‐based crosslinking and bicyclization. With the cyclic RGD and GHK peptide backbones, the dipyrrin staple linker can be either transformed to fluorescent BODIPY and then utilized for bladder cancer cell (T24)‐selective targeted αvβ3 integrin probe, or directly employed as a selective zinc(II) sensor in aqueous media (detection limit=4.37 nm). This work provides a practical addition to the reaction toolbox available for accessing peptide macrocycles, with dipyrrins being multifunctional staple linkers.
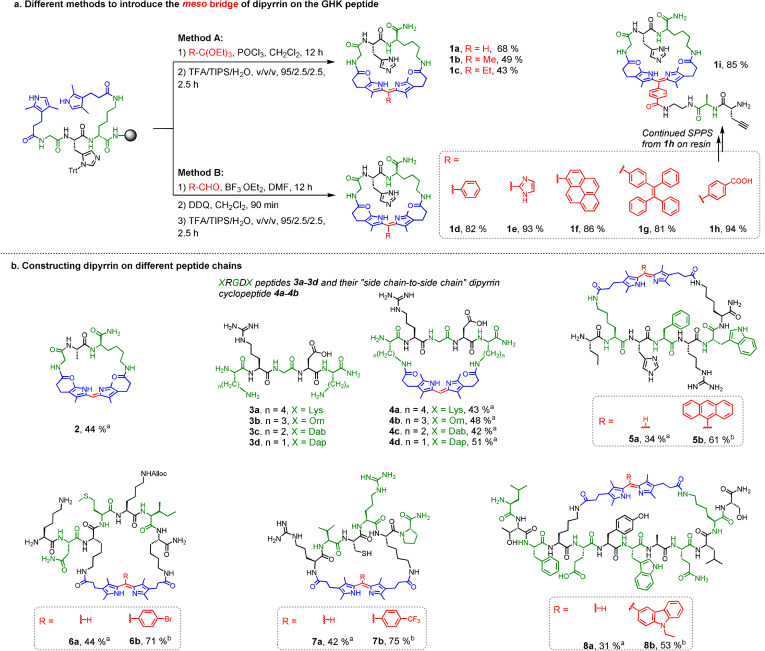
To construct our proposed cyclopeptides cyclized by the dipyrrin moiety, we initiated the study of dipyrrin‐directed peptide macrocyclization methodology with the GHK peptide sequence, based on the FDA‐approved drug prezatide, as the first trial. As shown in Figure 2 a, the commercially available COOH‐containing pyrrole building block, 3‐(2,4‐dimethyl‐1H‐pyrrol‐3‐yl)propanoic acid, was efficiently coupled onto two amine groups (N‐terminus and/or side chain of lysine) on the resin‐bound GHK under solid‐phase conditions. Upon systematic screening, Methods A and B were discovered and strategically optimized for introducing various alkyl and aromatic moieties at the meso position of the formed dipyrrins. The commercially available orthoesters were used for constructing dipyrrins with hydrogen, methyl or ethyl groups at the meso position effectively (Method A, 1 a–1 c) with the aid of POCl3 in CH2Cl2. With our previously established protocol,[24] the two pyrroles on‐resin can be condensed with various commercially available aldehydes (e.g., polarity‐sensitive pyrene and AIEgen tetraphenylethene) under the catalysis of BF3⋅OEt2 in DMF, and the resulting dipyrrin‐cyclopeptides could be obtained with high percent conversions (all >80 %) after DDQ oxidation and global cleavage and deprotection (Method B, 1 d–1 h). In essence, when 4‐carboxybenzaldehyde was used, COOH‐containing dipyrrin cyclopeptide could be obtained (1 h) with very high percent conversion (94 %), and continued SPPS with it afforded 1 i to have an additional peptide chain, which may accomplish other functions or serve as anchors for further manipulations.
Figure 2.
Formation of dipyrrin cyclopeptides. Percent conversions were reported and determined by HPLC. a) The synthetic routes for dipyrrin‐GHK cyclopeptides, where the dipyrrin linker can be constructed by Methods A/B for the scope of meso bridge. b) The synthetic routes for constructing dipyrrin on different peptide chains containing two pyrrolyl groups, incorporating all proteinogenic amino acids of different ring sizes by Method Aa and/or Method Bb.
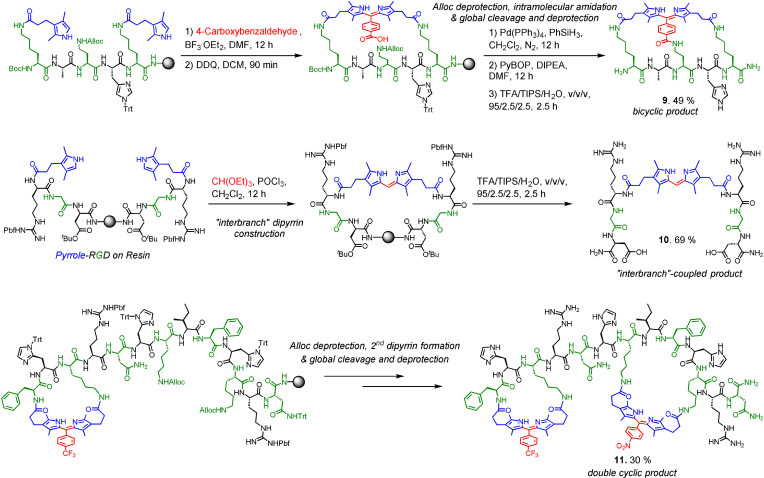
We then adopted these methods on a series of other peptide chains (Figure 2 b) to yield 2 (GAK peptide), 4 a–4 d (XRGDX peptides, where X are amino acid residues with amines on their side chain in different lengths for screening a suitable αvβ3‐targeting RGD cyclic peptide), 5 (derived from FDA‐approved cyclic peptide drug Bremelanotide), 6, 7, and 8 (derived from ATSP‐6935, a P53‐targeting cyclic peptide under clinical trial), all in reasonable conversions (≈30–70 %). Both head‐to‐side chain and side chain‐to‐side chain cyclizations could be performed with different ring sizes (containing 3–8 residues) and 20 standard proteinogenic amino acids had been proved to be compatible with both Methods A and B. We further strove for some special cases with our methods (Figure 3). The bridged bicyclic product 9 (Figure S5, S6) was obtainable by Method B with 4‐carboxybenzaldehyde followed by intramolecular amidation (49 % percent conversion). When Method A was carried out on a RGD peptide with only one amine group, “interbranch” coupling happened to create the dipyrrin‐bis(RGD)‐peptide conjugate 10 (Figure S7) in ≈70 % percent conversion. In particular, we achieved a head‐to‐side chain and a side chain‐to‐side chain dipyrrin cyclic linker on the same peptide chain to afford double‐cyclic 11 (Figure S8, S9) in a stepwise manner in 30 % conversion.
Figure 3.
Special cases: bicyclic product 9, “interbranch”‐coupled product 10, and double‐dipyrrin cyclic peptide 11. Percent conversions were reported and determined by HPLC.
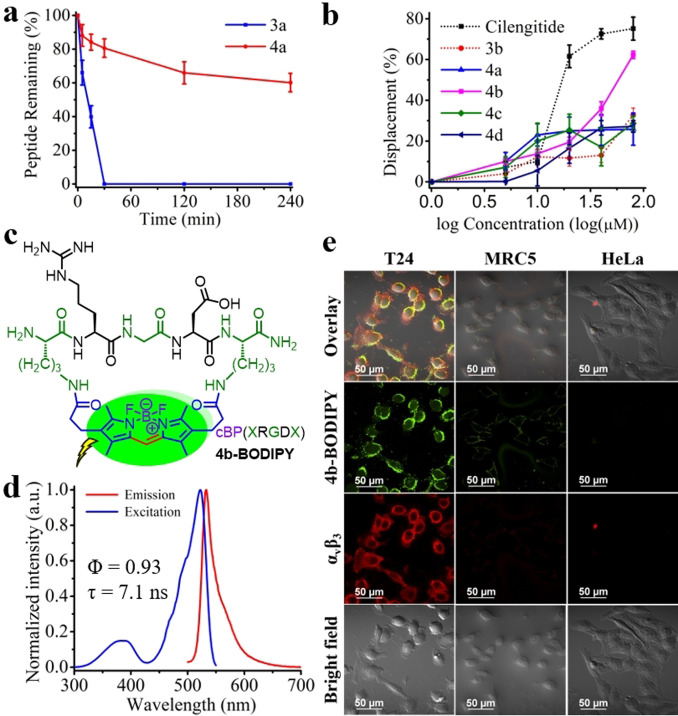
RGD peptides are known for their preferential binding to the αvβ3 integrin,[25] which is overexpressed in bladder cancer cells.[26] With the new structures of dipyrrin‐embedded cyclic RGD (cRGD) peptides (4 a–4 d) secured by our new synthetic methodology, we next evaluated their protease resistance, analyzed their conformation, examined their selective αvβ3 binding affinity, and measured their photophysics upon further BODIPY transformation in order to develop them as new fluorescent cyclopeptide‐based targeted αvβ3 probes as a model case study. A protease stability assay, with trypsin at 37 °C, was conducted for both 3 a (linear KRGDK peptide as the control, Figure S3) and 4 a (cyclic dipyrrin‐embedded KRGDK peptide). The assay results showed that 3 a was decomposed completely within 30 min, while around 60 % of 4 a still survived after 240 min (Figure 4 a), thereby proving the far higher protease resistance of cyclic 4 a than the linear 3 a. Circular dichroism (CD) measurements were performed, at 25 °C, to analyze if there would be any conformational differences between the linear 3 a–3 d (Figure S3 and S4) and cyclic 4 a–4 d, and random coiled structures of all of them were demonstrated, with the shape of peak signals of the cyclic samples being more obvious (Figure S19). The αvβ3 binding competitive displacement assay[27] was carried out with cyclic 4 a–4 d, linear 3 a, and the positive control cilengitide (a commercially available cyclic RGDfV peptide selectively targeting αvβ3) and the result validated that 4 b showed a higher binding affinity than 4 a, 4 c–4 d, as well as its linear version 3 b (Figure 4 b). Molecular docking was also conducted for 3 a–3 d and 4 a–4 d; all of them overlapped with the RGD motif of fibronectin (Figure S21–S23, the native ligand in the crystal structure of αvβ3) with the estimated binding energies in the order of 4 b>4 a/4 c>4 d>3 a–3 d that accorded well with the result of a binding affinity assay (Table S6). Therefore, boron complexation was implemented on 4 b to convert it into the corresponding BODIPY‐embedded cyclo‐XRGDX‐peptide 4 b‐BODIPY (X=Orn, Figure 4 c) with bright fluorescence (quantum yield (Φ)=0.93; lifetime (τ)=7.1 ns, Figure 4 d). We performed confocal in vitro imaging for 4 b‐BODIPY with αvβ3‐overexpressed bladder cancer T24 cell lines, as well as the negative control normal MRC5 and cervical cancer HeLa cell lines (Figure 4 e). Both 4 b‐BODIPY (green) and the fluorescent αvβ3 specific antibody (red) displayed good preferential cellular uptake and excellent signal overlapping in T24 cell lines, but not in the others. This indicated the great potential of 4 b‐BODIPY as a fluorescent cyclopeptide‐based targeted αvβ3 probe[28] whose fluorescence comes from the BODIPY staple linker.
Figure 4.
Dipyrrin/BODIPY‐embedded cRGD peptides as potential αvβ3 probe. a) In vitro trypsin resistance assays for cyclic 4 a versus linear 3 a at 37 °C. b) αvβ3 binding assay of cyclic 4 a–d, linear 3 a, and the positive control cilengitide. c) The structures of BODIPY‐embedded cyclopeptides 4 b‐BODIPY. d) The normalized excitation/emission spectra of 4 b‐BODIPY in HEPES buffer, with the quantum yield (Φ)=0.93 and the lifetime (τ)=7.1 ns. e) Confocal imaging of 10 μm 4 b‐BODIPY (green) and fluorescent αvβ3‐specific antibody (red) in T24, MRC5, and HeLa cell lines. Error bars represent the standard deviation of the mean.
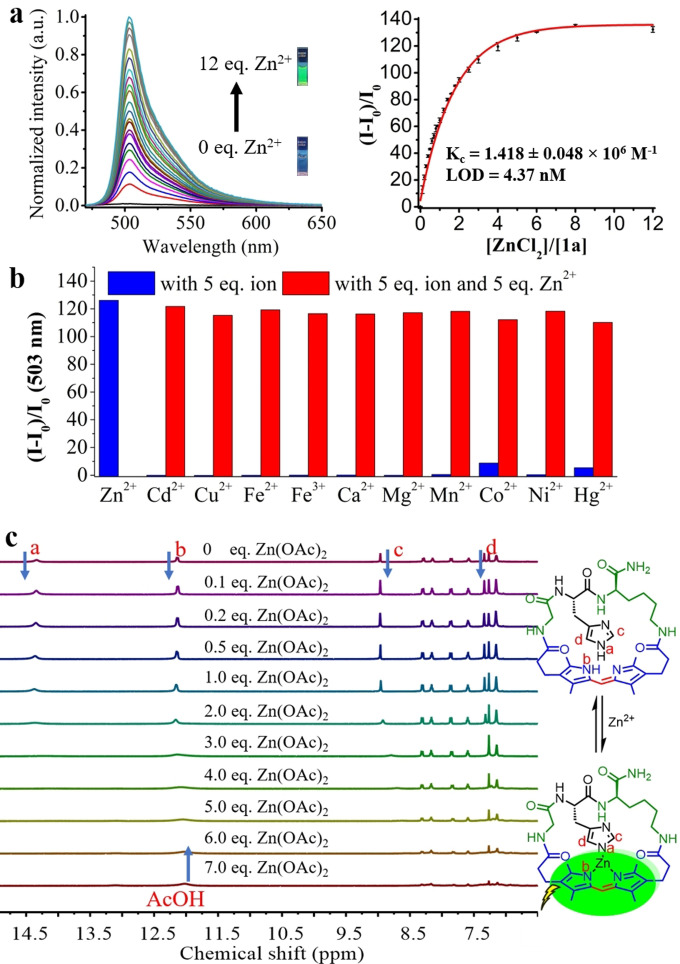
In addition, GHK peptide and dipyrrin are well‐known moieties to chelate with zinc(II) ions;[29] we were then interested to see if 1 a (of cyclic GHK peptide, cGHK) could be employed as a new potential fluorescent Zn2+ sensor. Impressively, 1 a exhibited a highly sensitive and selective response toward Zn2+ over a wide range of dications in aqueous media as fluorescence intensity increased over 130‐fold upon the addition of Zn2+ (Figure 5 a,b, Video S1). The stoichiometry between 1 a and Zn2+ was determined as 1:1 by Job's plot (Figure S13). The detection limit of 1 μm 1 a toward Zn2+ was calculated to be 4.37 nm (Figure S12), while the quantum yield of 1 a with saturated Zn2+ was recorded to be 0.31. No significant temperature effect (at 0 °C, 25 °C and 37 °C) was found towards 1 a’s emission intensity (Figure S14); 1 a functioned pretty well under basic conditions (pH 7–11) but not under acidic conditions (pH<6.5, Figure S15). To better understand 1 a’s zinc binding ability and sensing mechanism, we measured the binding constant of 1 a with Zn2+ in HEPES buffer, which was found to be 1.418×106 m −1, a value of not so strong binding. Therefore, the fluorescence could be quenched by addition of DTPA, a strong metal chelator. On the other hand, fluorescence Zn2+ titrations were also performed for meso‐substituted 1 b and 1 d, GAK‐based variant 2, as well as the linear analogue 8, and none of them manifested a performance as good as 1 a’s. For 1 b and 1 d, with the methyl/phenyl substitution on the meso position, they showed very faint fluorescence over the titration,[30] despite showing similar binding constants with Zn2+ as 1 a (Figure S16). The dipyrrin‐embedded GAK cyclopeptide 2 missing the imidazole side chain also illustrated over 100‐fold enhancement as the addition of excess Zn2+; however, its sensitivity was proved far poorer than 1 a’s, with its binding constant being ≈1/50 of that of 1 a (Figure S17). For the linear analogue of dipyrrin‐attached GHK peptide conjugate S6 (Figure S10), its binding constant was found to lie in between that of 1 a and 2 (Figure S18). All these findings substantiated that the unique scaffold of dipyrrin‐embedded cGHK peptide and the presence of imidazole group were crucial for the serendipitous Zn2+ chelating and sensing properties of 1 a. The binding between 1 a and Zn2+ was then monitored by NMR titration (Figure 5 c). Upon the addition of Zn2+ salt, both the signal of imidazole‐NH and dipyrrin‐NH were decreased/broadened simultaneously, which indicated the zinc atom had bound to imidazole‐NH and dipyrrin‐NH at the same time. The two imidazole‐CH were also found shifting during the titration, while other signals remained unchanged.
Figure 5.
Dipyrrin‐embedded cGHK peptide 1 a as a selective Zn2+ sensor in aqueous buffer HEPES. a) The fluorescence titration of 1 a with Zn2+. b) The fluorescent responses of 1 a toward various metal ions. c) The changes in NMR spectrum of 1 a upon the addition of Zn(OAc)2.
In summary, we report a new and highly efficient solid‐phase synthetic methodology towards multifunctional fluorescent cyclic peptides of broad amino acid scope and varying ring size via dipyrrin coupling‐driven head‐to‐side chain and side chain‐to‐side chain peptide macrocyclization, where the resulting dipyrrin serves as a new multifunctional staple linker. Various complex bicyclic peptide structures can be obtained; in two of our models, with the cyclic RGD and GHK peptide backbones, the embedded dipyrrin can be, respectively, either transformed to fluorescent BODIPY (4 b‐BODIPY) and then utilized as a bladder cancer cell (T24)‐selective targeted αvβ3 integrin probe in vitro, or directly employed as a selective zinc(II) sensor (1 a) in aqueous media. From these first‐generation multifunctional fluorescent cyclopeptides (4 b‐BODIPY and 1 a), insights have been gained for the importance of the linker length of the cyclic peptide scaffold as well as the presence of donor side chain as multidentate ligand. This work provides a valuable addition to the peptide macrocyclization–multifunctionalization toolbox and empowers the further development of multifunctional dipyrrin‐cyclopeptides for multifarious bioapplications.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Acknowledgements
This work was supported by grants from the Hong Kong Research Grant Council (HKBU 12300320), CAS‐Croucher Funding Scheme for Joint Laboratories (CAS 18204), UGC Research Matching Grant Scheme (NLMT and HKBU Joint Lab for Combating Prostate Cancer), and the Hong Kong Polytechnic University (Start‐up Fund for RAPs under the Strategic Hiring Scheme P0035714). N.J.L. is grateful for a Royal Society Wolfson Merit Award and for the Dr Kennedy Wong Distinguished Visiting Professorship at Hong Kong Baptist University. K.L.W. acknowledges Dr. Mok Man Hung Endowed Professorship in Chemistry at Hong Kong Baptist University. The University Research Facility in Life Sciences (ULS) at the Hong Kong Polytechnic University is also gratefully acknowledged.
Y. Wu, H.-F. Chau, W. Thor, K. H. Y. Chan, X. Ma, W.-L. Chan, N. J. Long, K.-L. Wong, Angew. Chem. Int. Ed. 2021, 60, 20301.
Contributor Information
Dr. Wai‐Lun Chan, Email: wai-lun-kulice.chan@polyu.edu.hk.
Prof. Dr. Nicholas J. Long, Email: n.long@imperial.ac.uk.
Prof. Dr. Ka‐Leung Wong, Email: klwong@hkbu.edu.hk.
References
- 1.For selected recent reviews, see:
- 1a.Bozovičar K., Bratkovič T., Int. J. Mol. Sci. 2021, 22, 1611; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1b.González-Muñiz R., Bonache M. Á., Pérez de Vega M. J., Molecules 2021, 26, 445; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1c.Bluntzer M. T. J., O'Connell J., Baker T. S., Michel J., Pept. Sci. 2020, 113, e24191; [Google Scholar]
- 1d.Vinogradov A. V., Yin Y., Suga H., J. Am. Chem. Soc. 2019, 141, 4167–4181; [DOI] [PubMed] [Google Scholar]
- 1e.Ali A. M., Atmaj J., Oosterwijk N. V., Groves M. R., Dömling A., Comput. Struct. Biotechnol. J. 2019, 17, 263–281; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1f.Hill T. A., Shepherd N. E., Diness F., Fairlie D. P., Angew. Chem. Int. Ed. 2014, 53, 13020–13041; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 13234–13257. [Google Scholar]
- 2.For selected representative works, see:
- 2a.Speltz T. E., Fanning S. W., Mayne C. G., Fowler C., Tajkhorshid E., Greene G. L., Moore T. W., Angew. Chem. Int. Ed. 2016, 55, 4252–4255; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 4324–4327; [Google Scholar]
- 2b.Maltsev O. V., Marelli U. K., Kapp T. G., Di Leva F. S., Di Maro S., Nieberler M., Reuning U., Schwaiger M., Novellino E., Marinelli L., Kessler H., Angew. Chem. Int. Ed. 2016, 55, 1535–1539; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 1559–1563. [Google Scholar]
- 3.For a recent review, see:
- 3a.Dougherty P. G., Sahni A., Pei D., Chem. Rev. 2019, 119, 10241–10287; For a selected work, see: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3b.Peraro L., Zou Z., Makwana K. M., Cummings A. E., Ball H. L., Yu H., Lin Y.-S., Levine B., Kritzer J. A., J. Am. Chem. Soc. 2017, 139, 7792–7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For a selected work, see: Bird G. H., Madani N., Perry A. F., Princiotto A. M., Supko J. G., He X., Gavathiotis E., Sodroski J. G., Walensky L. D., Proc. Natl. Acad. Sci. USA 2010, 107, 14093–14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For selected recent works on cyclopeptides with remarkable bioactivities, see:
- 5a.Wu S., He Y., Qiu X., Yang W., Liu W., Li X., Li Y., Shen H.-M., Wang R., Yue Z., Zhao Y., Proc. Natl. Acad. Sci. USA 2018, 115, E5669–E5678; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5b.Fadzen C. M., Wolfe J. M., Cho C.-F., Chiocca A., Lawler S. E., Pentelute B. L., J. Am. Chem. Soc. 2017, 139, 15628–15631; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5c.Kuster A., Mozaffari N. L., Wilkinson O. J., Wojtaszek J. L., Zurfluh C., Przetocka S., Zyla D., von Aesch C., Dillingham M. S., Williams R. S., Sartori A. A., Sci. Adv. 2021, 7, eabc6381; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5d.Chen X., Fu J., Zhou F., Yang Q., Wang J., Feng H., Jiang W., Jin L., Tang X., Jiang N., Yin J., Han J., J. Med. Chem. 2020, 63, 12595–12613; [DOI] [PubMed] [Google Scholar]
- 5e.Mitra S., Montgomery J. E., Kolar M. J., Li G., Jeong K. J., Peng B., Verdine G. L., Mills G. B., Moellering R. E., Nat. Commun. 2017, 8, 660; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5f.Chang Y. S., Graves B., Guerlavais V., Tovar C., Packman K., To K.-H., Olson K. A., Kesavan K., Gangurde P., Mukherjee A., Baker T., Darlak K., Elkin C., Filipovic Z., Qureshi F. Z., Cai H., Berry P., Feyfant E., Shi X. E., Horstick J., Allen Annis D., Manning A. M., Fotouhi N., Nash H., Vassilev L. T., Sawyer T. K., Proc. Natl. Acad. Sci. USA 2013, 110, E3445–E3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For selected recent reviews, see:
- 6a.Nevola L., Giralt E., Chem. Commun. 2015, 51, 3302–3315; [DOI] [PubMed] [Google Scholar]
- 6b.Tsomaia N., Eur. J. Med. Chem. 2015, 94, 459–470. [DOI] [PubMed] [Google Scholar]
- 7.For selected reviews, see:
- 7a.White C. J., Yudin A. K., Nat. Chem. 2011, 3, 509–524; [DOI] [PubMed] [Google Scholar]
- 7b.Lau Y. H., de Andrade P., Wu Y., Spring D. R., Chem. Soc. Rev. 2015, 44, 91–102. [DOI] [PubMed] [Google Scholar]
- 8.For selected recent works, see:
- 8a.Kubota K., Dai P., Pentelute B. L., Buchwald S. L., J. Am. Chem. Soc. 2018, 140, 3128–3133; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8b.Ben-Lulu M., Gaster E., Libman A., Pappo D., Angew. Chem. Int. Ed. 2020, 59, 4835–4839; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 4865–4869. [Google Scholar]
- 9.For a selected recent work, see: Lau Y. H., Wu Y., Rossmann M., Tan B. X., de Andrade P., Tan Y. S., Verma C., McKenzie G. J., Venkitaraman A. R., Hyvçnen M., Spring D. R., Angew. Chem. Int. Ed. 2015, 54, 15410–15413; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 15630–15633. [Google Scholar]
- 10.For selected recent works, see:
- 10a.Mendive-Tapia L., Preciado S., García J., Ramón R., Kielland N., Albericio F., Lavilla R., Nat. Commun. 2015, 6, 7160; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b.Noisier A. F. M., García J., Ionuţ I. A., Albericio F., Angew. Chem. Int. Ed. 2017, 56, 314–318; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 320–324; [Google Scholar]
- 10c.Lorion M. M., Kaplaneris N., Son J., Kuniyil R., Ackermann L., Angew. Chem. Int. Ed. 2019, 58, 1684–1688; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 1698–1702; [Google Scholar]
- 10d.Kaplaneris N., Rogge T., Yin R., Wang H., Sirvinskaite G., Ackermann L., Angew. Chem. Int. Ed. 2019, 58, 3476–3480; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 3514–3518; [Google Scholar]
- 10e.Zhang X., Lu G., Sun M., Mahankali M., Ma Y., Zhang M., Hua W., Hu Y., Wang Q., Chen J., He G., Qi X., Shen W., Liu P., Chen G. A., Nat. Chem. 2018, 10, 540–548; [DOI] [PubMed] [Google Scholar]
- 10f.Li B., Li X., Han B., Chen Z., Zhang X., He G., Chen G., J. Am. Chem. Soc. 2019, 141, 9401–9407; [DOI] [PubMed] [Google Scholar]
- 10g.Bai Z., Cai C., Sheng W., Ren Y., Wang H., Angew. Chem. Int. Ed. 2020, 59, 14686–14692; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 14794–14800. [Google Scholar]
- 11.For selected recent works, see:
- 11a.Hilinski G. J., Kim Y.-W., Hong J., Kutchukian P. S., Crenshaw C. M., Berkovitch S. S., Chang A., Ham S., Verdine G. L., J. Am. Chem. Soc. 2014, 136, 12314–12322; [DOI] [PubMed] [Google Scholar]
- 11b.Mangold S. L., O'Leary D. J., Grubbs R. H., J. Am. Chem. Soc. 2014, 136, 12469–12478; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11c.Xu C., Shen X., Hoveyda A. H., J. Am. Chem. Soc. 2017, 139, 10919–10928. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery J. E., Donnelly J. A., Fanning S. W., Speltz T. E., Shangguan X., Coukos J. S., Greene G. L., Moellering R. E., J. Am. Chem. Soc. 2019, 141, 16374–16381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For a selected recent review, see:
- 13a.Reguera L., Rivera D. G., Chem. Rev. 2019, 119, 9836–9860; for selected representative works, see: [DOI] [PubMed] [Google Scholar]
- 13b.Ricardo M. G., Ali A. M., Plewka J., Surmiak E., Labuzek B., Neochoritis C. G., Atmaj J., Skalniak L., Zhang R., Holak T. A., Groves M., Rivera D. G., Dömling A., Angew. Chem. Int. Ed. 2020, 59, 5235–5241; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 5273–5279; [Google Scholar]
- 13c.Ricardo M. G., Llanes D., Wessjohann L. A., Rivera D. G., Angew. Chem. Int. Ed. 2019, 58, 2700–2704; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 2726–2730; [Google Scholar]
- 13d.Yamaguchi A., Kaldas S. J., Appavoo S. D., Diaz D. B., Yudin A. K., Chem. Commun. 2019, 55, 10567–10570; [DOI] [PubMed] [Google Scholar]
- 13e.Morejón M. C., Laub A., Westermann B., Rivera D. G., Wessjohann L. A., Org. Lett. 2016, 18, 4096–4099; [DOI] [PubMed] [Google Scholar]
- 13f.Vasco A. V., Pérez C. S., Morales F. E., Garay H. E., Vasilev D., Gavín J. A., Wessjohann L. A., Rivera D. G., J. Org. Chem. 2015, 80, 6697–6707; [DOI] [PubMed] [Google Scholar]
- 13g.Ricardo M. G., Morales F. E., Garay H., Reyes O., Vasilev D., Wessjohann L. A., Rivera D. G., Org. Biomol. Chem. 2015, 13, 438–446. [DOI] [PubMed] [Google Scholar]
- 14.For a selected recent review, see: Chow H. Y., Zhang Y., Matheson E., Li X., Chem. Rev. 2019, 119, 9971–10001. [DOI] [PubMed] [Google Scholar]
- 15.For a selected recent review, see:
- 15a.Rivera D. G., Ojeda-Carralero G. M., Reguera L., Van der Eycken E. V., Chem. Soc. Rev. 2020, 49, 2039–2059; for selected representative works, see: [DOI] [PubMed] [Google Scholar]
- 15b.Zhang Y., Zhang Q., Wong C. T. T., Li X., J. Am. Chem. Soc. 2019, 141, 12274–12279; [DOI] [PubMed] [Google Scholar]
- 15c.Malins L. R., de Gruyter J. N., Robbins K. J., Scola P. M., Eastgate M. D., Reza Ghadiri M., Baran P. S., J. Am. Chem. Soc. 2017, 139, 5233–5241; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15d.Lautrette G., Touti F., Lee H. G., Dai P., Pentelute B. L., J. Am. Chem. Soc. 2016, 138, 8340–8343; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15e.Bandyopadhyay A., Gao J., J. Am. Chem. Soc. 2016, 138, 2098–2101; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15f.Li B., Tang H., Turlik A., Wan Z., Xue X.-S., Li L., Yang X., Li J., He G., Houk K. N., Chen G., Angew. Chem. Int. Ed. 2021, 60, 6646–6652; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 6720–6726; [Google Scholar]
- 15g.Ceballos J., Grinhagena E., Sangouard G., Heinis C., Waser J., Angew. Chem. Int. Ed. 2021, 60, 9022–9031; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 9104–9113; [Google Scholar]
- 15h.Silva M. J. S. A., Faustino H., Coelho J. A. S., Pinto M. V., Fernandes A., Compañón I., Corzana F., Gasser G., Gois P. M. P., Angew. Chem. Int. Ed. 2021, 60, 10850–10857; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 10945–10952; [Google Scholar]
- 15i.Adebomi V., Cohen R. D., Wills R., Chavers H. A. H., Martin G. E., Raj M., Angew. Chem. Int. Ed. 2019, 58, 19073–19080; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 19249–19256; [Google Scholar]
- 15j.Raynal L., Rose N. C., Donald J. R., Spicer C. D., Chem. Eur. J. 2021, 27, 69–88; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15k.Wang Y., Chou D. H.-C., Angew. Chem. Int. Ed. 2015, 54, 10931–10934; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 11081–11084; [Google Scholar]
- 15l.Morewood R., Nitsche C., Chem. Sci. 2021, 12, 669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X., Chen S., Zhang W.-D., Hu H.-G., Chem. Rev. 2020, 120, 10079–10144. [DOI] [PubMed] [Google Scholar]
- 17.For selected representative works, see:
- 17a.Legre J., Gaynord J. S., Robertson N. S., Sore H. F., Hyvönen M., Spring D. R., Adv. Ther. 2018, 1, 1800052; [Google Scholar]
- 17b.Hili R., Rai V., Yudin A. K., J. Am. Chem. Soc. 2010, 132, 2889–2891; [DOI] [PubMed] [Google Scholar]
- 17c.Roxin Á., Chen J., Scully C. C. G., Rotstein B. H., Yudin A. K., Zheng G., Bioconjugate Chem. 2012, 23, 1387–1395; [DOI] [PubMed] [Google Scholar]
- 17d.Vasco A. V., Méndez Y., Porzel A., Balbach J., Wessjohann L. A., Rivera D. G., Bioconjugate Chem. 2019, 30, 253–259; [DOI] [PubMed] [Google Scholar]
- 17e.Grison C. M., Burslem G. M., Miles J. A., Pilsl L. K. A., Yeo D. J., Imani Z., Warriner S. L., Webb M. E., Wilson A. J., Chem. Sci. 2017, 8, 5166–5171; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17f.Wu Y., Kaur A., Fowler E., Wiedmann M. M., Young R., Galloway W. R. J. D., Olsen L., Sore H. F., Chattopadhyay A., Kwan T. T.-L., Xu X., Walsh S. J., de Andrade P., Janecek M., Arumugam S., Itzhaki L. S., Lau Y. H., Spring D. R., ACS Chem. Biol. 2019, 14, 526–533. [DOI] [PubMed] [Google Scholar]
- 18.Ma X., Kia J., Cao R., Wang X., Fei H., J. Am. Chem. Soc. 2014, 136, 17734–17737. [DOI] [PubMed] [Google Scholar]
- 19.Ji S., Yang X., Chen X., Li A., Yan D., Xu H., Fei H., Chem. Sci. 2020, 11, 9126–9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todorovic M., Schwab K. D., Zeisler J., Zhang C.-C., Bénard F., Perrin D. M., Angew. Chem. Int. Ed. 2019, 58, 14120–14124; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 14258–14262. [Google Scholar]
- 21.Nevola L., Martín-Quirós A., Eckelt K., Camarero N., Tosi S., Llobet A., Giralt E., Gorostiza P., Angew. Chem. Int. Ed. 2013, 52, 7704–7708; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 7858–7862. [Google Scholar]
- 22.Brown S. P., A. B.Smith III , J. Am. Chem. Soc. 2015, 137, 4034–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Learte-Aymamí S., Vidal C., Gutiérrez-González A., Mascareñas J. L., Angew. Chem. Int. Ed. 2020, 59, 9149–9154; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 9234–9239. [Google Scholar]
- 24.Wu Y., Tam W.-S., Chau H.-F., Kaur S., Thor W., Aik W. S., Chan W.-L., Zweckstetter M., Wong K.-L., Chem. Sci. 2020, 11, 11266–11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.For a selected review, see: Danhier F., Le Breton A., Préat V., Mol. Pharm. 2012, 9, 2961–2973. [DOI] [PubMed] [Google Scholar]
- 26.Sachs M. D., Rauen K. A., Ramamurthy M., Dodson J. L., De Marzo A. M., Putzi M. J., Schoenberg M. P., Rodriguez R., Urology 2002, 60, 531–536. [DOI] [PubMed] [Google Scholar]
- 27.Šimeček J., Notni J., Kapp T. G., Kessler H., Wester H.-J., Mol. Pharm. 2014, 11, 1687–1695. [DOI] [PubMed] [Google Scholar]
- 28.Mendive-Tapia L., Wang J., Vendrell M., Pept. Sci. 2021, 113, e24181. [Google Scholar]
- 29.For a selected work, see:
- 29a.Schirer A., El Khoury Y., Faller P., Hellwig P., JBIC J. Biol. Inorg. Chem. 2017, 22, 581–589; [DOI] [PubMed] [Google Scholar]
- 29b.Wang P., Wang S., Chen L., Wang W., Wang B., Liao Y. A., Spectrochim. Acta Part A 2020, 240, 118549; for a selected recent review, see: [DOI] [PubMed] [Google Scholar]
- 29c.Singh R. S., Paitandi R. P., Gupta R. K., Pandey D. S., Coord. Chem. Rev. 2020, 414, 213269. [Google Scholar]
- 30.
- 30a.Berezin M. B., Antina E. V., Guseva G. B., Kritskaya A. Y., Semeikin A. S., Inorg. Chem. Commun. 2020, 111, 107611; [Google Scholar]
- 30b.Al-Sheikh Ali A., Cipot-Wechsler J., Cameron T. S., Thompson A., J. Org. Chem. 2009, 74, 2866–2869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information