Abstract
Background :
Balloon-expandable pulmonary valves are usually not suitable for dilated native outflow tracts.
Methods :
Indian Venus P-valve registry was retrospectively analyzed for efficacy, complications, and midterm outcomes. Straight valve was used in prestented conduits in patients with right ventricular pressure above two-thirds systemic pressure and/or right ventricular dysfunction. Flared valve 1–4 mm larger than balloon waist was used in native outflow in symptomatic patients, large ventricular volumes, and ventricular dysfunction.
Objectives :
A self-expanding porcine pericardial Venus P-valve is available in straight and flared designs..
Results :
Twenty-nine patients were included. Straight valve was successful in all seven conduits, reducing gradients significantly, including one patient with left pulmonary artery (LPA) stent. Flared valve was successfully implanted in 20 out of 22 native outflow tracts. Sharp edges of the older design contributed to two failures. Complications included two migrations with one needing surgery, endocarditis in one, insignificant wire-frame fractures in three, and groin vascular complication in one patient. There were no deaths or valve-related reinterventions at a mean follow-up of 47.8 ± 24.5 months (1–85 months). Modifications of technique succeeded in three patients with narrow LPA. There was significant improvement in symptoms, right ventricular volume, and pulmonary regurgitant fraction.
Conclusion :
Straight and flared Venus P-valves are safe and effective in appropriate outflow tracts. Straight valve is an alternative to balloon-expandable valves in stenosed conduits. Flared valve is suitable for large outflows up to 34 mm, including patients with LPA stenosis. Recent design modifications may correct previous technical failures. Studies should focus on durability and late complications.
Keywords: Balloon sizing, infective endocarditis, left pulmonary artery stenosis, percutaneous pulmonary valve implantation, self-expanding valve, valve migration
INTRODUCTION
Surgical reconstruction of right ventricular outflow tracts (RVOTs) using patches or conduits is an integral part of management of various congenital heart diseases. In 85% of patients, reconstruction involves a transannular patch and in 15% of patients, may involve a conduit.[1] Percutaneous valves are an alternative to surgery for narrowing or regurgitation of the outflow tracts when these patients need a pulmonary valve replacement.[2,3] Melody valve (Medtronic Inc., Minneapolis, MN) has limited utility in native outflow tracts.[4] Outflows with transannular patches have varied morphology and need a different approach.[5] Various valve designs have been evaluated to address this unmet need.[6,7,8] The SAPIEN valve (Edwards Lifesciences, Irvine, CA) has the potential for use in native outflow tracts up to 29 mm.[9]
The Venus P-valve (Venus Medtech, Hangzhou, PRC) is available in two designs. The straight design is used in prestented conduits.[10] The flared design addresses the majority of native outflow tracts with transannular patches.[11,12,13] The initial experience has been encouraging for both designs.[10,11,12,13,14,15,16,17] Reports from China, South America, Thailand, and Europe describe patients implanted with Venus P-valve.[10,11,12,13,14,15,16,17] The device still awaits approval for clinical use from the regulatory bodies.
We present the procedural details and midterm follow-up of patients in the Indian registry, who underwent percutaneous pulmonary valve implantation (PPVI) with these two designs of Venus P-valve in the past 6 years.
METHODS
This multicenter retrospective observational study evaluated patients who underwent Venus P-valve implantation after obtaining approval for compassionate use from the Indian regulatory body. Indian registry, involving seven institutions, aimed to analyze the safety, efficacy of the procedure and to study the midterm outcome and complications. Ethical committees of all the participating institutions approved the study. Fully informed written consent was obtained from all the patients for the procedure and anonymized reporting of the results. Institutional review boards that included pediatric cardiac surgeons at all participating institutions reviewed each patient details before the procedure.
Initial evaluation
Echocardiography evaluated patients with significant RVOT regurgitation or stenosis. In patients with stenosis, a multislice computed tomography analyzed the diameter and length of the stenotic lumen, extent of circumferential calcification, and relationship to the coronary arteries. In regurgitant outflows, cardiac magnetic resonance imaging (CMR) analyzed the regurgitant fraction, ventricular volumes, and their function. The indication for PPVI was based on standard guidelines.[18,19,20]
Stenotic outflow tracts
Echocardiography identified features of systemic venous congestion, right ventricular dysfunction, secondary tricuspid regurgitation, outflow gradients, hilar pulmonary arteries, and other residual lesions. Patients with symptomatic worsening unexplained by other reasons, right ventricular systolic pressures more than two-thirds of systemic pressures, and right ventricular systolic dysfunction underwent stent angioplasty to relieve the obstruction before a straight Venus P-valve implantation.
Stent angioplasty for stenotic outflow
Proximity of conduits to coronary arteries was predicted by computed tomography. After excluding very dense circumferential calcification and patients at risk of coronary occlusion, the remaining patients underwent serial dilatations starting with Mustang Balloons (Boston Scientific, Marlborough, MA) up to 12 mm and later Atlas Gold Balloons (Bard Peripheral Vascular, Tempe, AZ) beyond 14 mm with 2 mm serial increments, under careful angiographic monitoring. After dilating the conduit to more than 80% of its original diameter, selective coronary angiograms were performed during full inflation of the final balloon to exclude interference with coronary flows. Circumferentially calcified conduits were stented with covered cheatham-platinum (CP) (Numed Inc., Hopkinton, NY) or covered AndraStent XXL (Andramed, Reutlingen, Germany) stents. If calcification was noncircumferential, stenting was performed using Palmaz XL (Cordis endovascular, Santa Clara, CA), LD Max (ev3 endovascular Inc., Plymouth, MN), CP or AndraStent XXL stents. Stent recoil was reinforced using one or two additional stents until pressure gradients dropped below 20 mmHg.
Regurgitant dilated outflows
Symptomatic patients and those who met CMR and electrocardiographic criteria for indication for PPVI underwent balloon interrogation before flared Venus P-valve implantation.[5] Outflow tracts larger than 34 mm and pyramidal-shaped Type I outflow tracts were excluded.[5] Lesions warranting corrective surgery, such as resection of RVOT aneurysms, closure of large residual defects, repair or replacement of severely regurgitant tricuspid valves were also excluded. Patients with severe unilateral pulmonary artery stenosis that precluded surgical or interventional rehabilitation were included, provided the outflow anatomy was suitable for Venus P-valve.
Balloon interrogation
The differences between the RVOT dimensions on CMR and balloon interrogation made the latter a mandatory step.[11,12,13,14,15,16,17] After placing a stiff Lunderquist guidewire (Cook medical, Bloomington, IN) to straighten the outflow tract, marker pigtail angiograms were performed in orthogonal projections to measure the RVOT dimensions. Once the hemodynamics was recorded, a long compliant AGA sizing balloon (Abbott, Plymouth, MN) was inflated across the outflow until a right ventriculogram confirmed complete occlusion. Selective coronary angiography during full balloon inflation assessed the risk of compression. The waist was measured after calibration with the marker pigtail placed alongside the sizing balloon. Venus P-valves were customized 2–4 mm larger than the interrogating balloon waist. Patients with balloon waist above 34 mm were excluded.
Venus P-valve
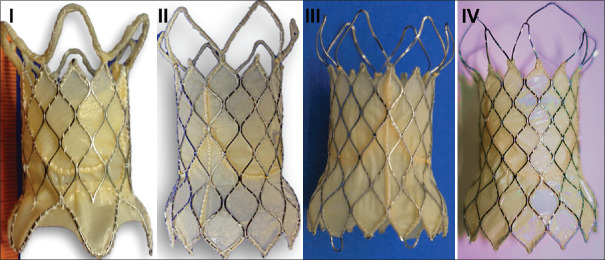
Venus P-valve comprised of a self-expandable nitinol framework with a trileaflet porcine pericardial valve, available in straight and flared designs.[10,17] Both the designs were available in diameters of 18–36 mm in 2 mm increments. The flared design of the valve went through three modifications from its first design. The first modular design with three sutured components was changed to a unibody with a thin pericardial lining for the struts of the distal flare. In the third design, this thin pericardial lining was removed from the six open cells in the distal flare.[11,12,13,14] The fourth design since 2018 had five open cells in distal flare that connected all the sharp edges to the outer wire frame [Figure 1]. The valve was rinsed to remove the glutaraldehyde preservative and crimped in ice-cold saline to reduce the nitinol memory, using a proprietary compression device and loaded on the delivery system.
Figure 1.
Venus P, a trileaflet porcine pericardial valve on a self-expanding nitinol frame, went through three modifications. The initial modular design (I) consisting of separate midvalve segment connected to proximal and distal flare changed to unibody design (II) on a single nitinol frame. Thin porcine pericardial strips that lined the six open cells of distal flare were removed in the third (III) design. The recent design (IV) connected the sharp edges through nitinol wires to the five open cells in the distal flare. The midvalve segment of the first-generation modular design without the flares on either side formed the straight design valve
Implantation procedure
The procedure was performed under general anesthesia. Two femoral venous and one arterial accesses were used. In patients with occluded iliac veins on one side, a right internal jugular venous access was used for the marker pigtail angiographic catheter. The valve delivery system was advanced toward the RVOT over a Lunderquist wire parked deep in left pulmonary artery (LPA). The distal flare was exposed in the proximal LPA. The remaining constrained valve was progressively exposed under guidance from repeated right ventriculograms in such a way that the proximal marker coincided with the proximal waist. If an initial attempt from LPA failed, the valve was deployed from right pulmonary artery. When the large profile rigid delivery system failed to advance through a sharp angulated RVOT, a 12–16 mm balloon was inflated in the distal outflow through the second venous access to displace the advancing valve away from the lateral walls of the outflow. A 65 cm long 26F braided sheath, when available, was placed across the outflow to facilitate passage of the delivery system to the desired site.[21] After deployment, right ventricular and pulmonary artery angiogram were performed to assess valve position, competence, and adequacy of flow through the branch pulmonary arteries. Hemostasis was achieved by figure-of-8 suture and manual compression.
Follow-up
Periprocedural antibiotics and low-molecular-weight heparin were continued for 3 days, and patients were discharged on dual antiplatelet therapy that was continued indefinitely. Antibiotic prophylaxis was administered for all major dental and urogenital surgical procedures. Clinical evaluation combined with electrocardiogram, fluoroscopy, and echocardiogram was performed at 1 and 3 months after the procedure and 6 monthly thereafter. In patients who did not receive any ferromagnetic stents, CMR was performed after 6 months.
Statistical analysis
Data were entered in Microsoft Excel and analyzed using SPSS version 23 (IBM Corp, Armonk, NY). The distribution of categorical data was expressed as frequency and percentage. The continuous variables were expressed as mean with standard deviation or median with range. The comparison of the continuous variables was carried out by independent Student's t-test or one-way analysis of variance whichever was appropriate based on the distribution of data and number of groups. All statistical analysis was carried out at 5% level of significance and P < 0.05 was considered as significant.
RESULTS
Patient population
A total of 35 patients were evaluated with balloon interrogation in the cardiac catheterization laboratory [Figure 2]. Twenty-nine patients selected after balloon interrogation from seven institutions were included for Venus P-valve implantation from March 2014 after excluding 6 patients [Table 1]. The measurements on CMR, catheter angiography, and balloon interrogation influenced the indication and selection of the valve [Table 2]. Seven patients with circumferential conduits, who received straight Venus P-valve, formed Group A Tables 3 and 4 and 22 patients with native outflow tracts reconstructed with transannular patches underwent implantation of flared Venus P-valve and formed Group B [Tables 5 and 6].
Figure 2.
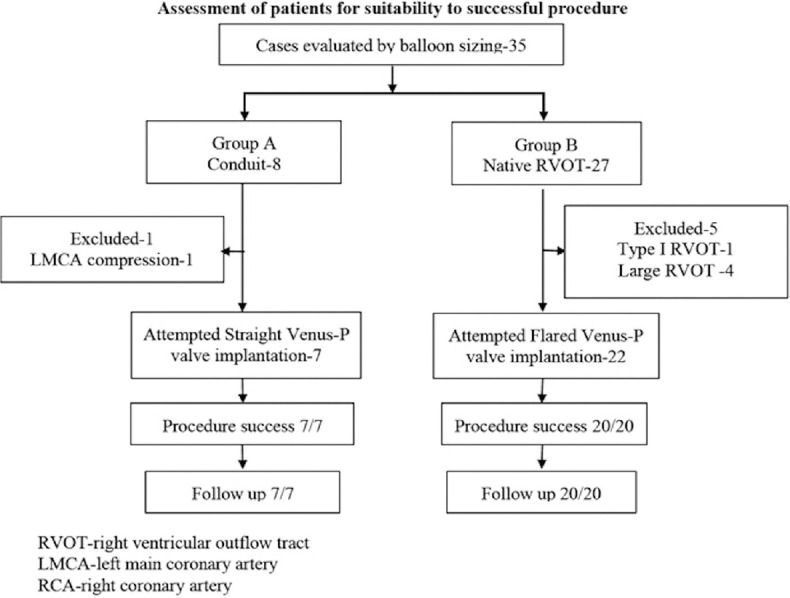
Flow chart of total patients assessed for percutaneous pulmonary valve implantation and procedural success
Table 1.
Patient clinical details
| Parameters | Values |
|---|---|
| Number of patients | 29 |
| Group A: Circumferential conduits | 7 |
| Group B: Native outflow with transannular patch | 22 |
| Age | 25.48±11.72 years (12-61 years) |
| Weight | 59.9±13.7 kg (39-86 kg) |
| Male:female | 20:09 |
| Age at primary corrective surgery (years), range | 8.82±8.5 (1-30) |
| Time from primary surgery to PPVI (years), median (range) | 16.3±7.8, 15 (3-45) |
| Number of surgeries before PPVI | |
| 1 | 19 |
| 2 | 8 |
| 3 | 1 |
| Group A - Primary diagnosis | 7 patients |
| TOF with PA | 4/7 |
| Ross surgery for aortic valve disease | 2/7 |
| Transposition of great arteries VSD PS | 1/7 |
| Group B - Primary diagnosis | 22 patients |
| TOF transannular patch | 18/22 |
| Double outlet RV transannular patch | 2/22 |
| Infundibular and valvar pulmonary stenosis | 1/22 |
| Pulmonary band for VSD | 1/22 |
| NYHA class | |
| I | 8 |
| II | 19 |
| III | 1 |
| IV | 1 |
| Arrhythmias | |
| None | 28 |
| Chronic atrial fibrillation | 1 |
| QRS duration on electrocardiogram | 152.14±28.2 ms (80-220) |
| QRS duration>140 (ms) | 18/29 |
| QRS duration>160 (ms) | 7/29 |
| Hemodynamic lesion | |
| Predominant pulmonary stenosis | 5/29 |
| Isolated pulmonary regurgitation | 21/29 |
| Combined stenosis and regurgitation | 3/29 |
PPVI: Percutaneous pulmonary valve implantation, VSD: Ventricular septal defect, PS: Pulmonary stenosis; NYHA: New York Heart Association, TOF: Tetralogy of Fallot, PA: Pulmonary atresia, RV: Right ventricle
Table 2.
Cardiac magnetic resonance imaging and angiography*
| Parameters | Mean values |
|---|---|
| Group A | |
| Right ventricular outflow dimension by magnetic resonance (mm) | 19.3±1.2 (18-20) |
| Right ventricular outflow dimension by angiography (mm) | 21.1±1.2 (19-23) |
| Right ventricular outflow dimension by balloon sizing (mm) | 20.9±1.7 (19-24) |
| Mean difference (angiography vs. balloon) (mm) | 0.3±0.8 (−1-+1) |
| Mean difference (magnetic resonance vs. balloon) (mm) | 0±1.7 (−2-1) |
| LPA stenosis or hypoplasia | 1/7 |
| Group B | |
| Pulmonary regurgitant fraction (%) | 45.6±11.8 (26-64) |
| Indexed right ventricular end-diastolic volume (ml) | 167.26±25.9 (120-230) |
| Right ventricular ejection fraction (%) | 46.9±9.6 (27-65) |
| Right ventricular outflow dimension by magnetic resonance (mm) | 25.1±4.2 (19-32) |
| Right ventricular outflow dimension by angiography (mm) | 26.1±3.3 (20-32) |
| Right ventricular outflow dimension by balloon sizing (mm) | 28.0±3.3 (23-33) |
| Mean difference (angiography vs. balloon) (mm) | 1.9±2.4 (−8-+2) |
| Mean difference (magnetic resonance vs. balloon) (mm) | 2.8±2.9 (−10-+1) |
| LPA stenosis or hypoplasia | 4/22 |
*Values expressed in mean±SD (range). SD: Standard deviation, LPA: Left pulmonary artery
Table 3.
Group A patients individual clinical details
| Number | Age (years)/sex | Weight (kg)/height (cm) | Diagnosis and surgery | Number of cardiac surgeries | Age at first surgery | Age at last surgery | Hemodynamics | PS grad echo/cath (mmHg) | Stent used | Poststent PS grad (mmHg) | RV EDV (ml) | PRF (%) | RV EF (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12/female | 42/161 | TOF PA xenograft conduit | 1 | 7 | 7 | Stenosis | 96/76 | LDMax 36 mm P4014 | 15 | |||
| 2 | 29/male | 64/184 | TOF PA homograft Conduit revision | 2 | 14 | 16 | Mixed | 40/38 | P5014 | 10 | 184 | 30 | 23 |
| 3 | 24/female | 77/163 | Ross procedure homograft | 1 | 12 | 12 | Stenosis | 106/100 | P4014 | 10 | |||
| 4 | 37/female | 48/163 | TOF PA homograft | 1 | 20 | 20 | Mixed | 96/79 | CP 26 mm LPA CP 16 mm | 6 | 66 | 39 | 73 |
| 5 | 24/female | 42/150 | TOF PA homograft conduit revision | 3 | 5 | 11 | Stenosis | 74/62 | CCP 45 mm Andra XXL 43 mm | 12 | 117 | 17 | 40 |
| 6 | 34/male | 79/171 | Ross procedure homograft | 1 | 21 | 21 | Stenosis | 76/74 | Andra XL 30 mm Andra XL 39 mm | 20 | |||
| 7 | 24/male | 86/176 | Rastelli DTGA VSD PS conduit revision | 2 | 6 | 11 | Stenosis | 84/95 | Andra XL 57 mm Andra XL 57 mm P4014 | 0 |
PS: Pulmonary stenosis, Echo: Echocardiography, Cath: Catheterization, Grad: Gradient, RVEDVI: Right ventricular end-diastolic volume index, PRF: Pulmonary regurgitant fraction, RVEF: Right ventricular ejection fraction, TOF: Tetralogy of Fallot, PA: Pulmonary atresia, CP: Cheatham platinum, LPA: Left pulmonary artery, CCP: Covered CP, DTGA: d-transposition of great arteries, VSD: Ventricular septal defect
Table 4.
Group A patients individual hemodynamics and follow-up
| Number | RA (mmHg) | RV (mmHg) | PA (mmHg) systolic/diastolic/mean | Waist CMR/angio/balloon (mm) | Valve size* | Additional procedural features | Immediate postvalve gradient (mmHg) | Years after last surgery | Weeks after stent | PR severity | Valve gradient (mmHg) | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | 35 | 30/11/18 | -/21/21 | 22-35 | Two stents | 6 | 5 | 6 | 0 | 23 | 85 |
| 2 | 11 | 66 | 46/11/26 | 21/21/20 | 22-35 | Noncircumferential calcification | 13 | 13 | 2 | 0 | 22 | 74 |
| 3 | 11 | 47 | 35/18/26 | -/21/20 | 22-35 | RPA deployment; balloon assistance | 18 | 12 | 208 | I | 35 | 74 |
| 4 | 12 | 68 | 40/14/22 | 18/21/20 | 22-35 | Post-LPA stent | 11 | 17 | 35 | 0 | 18 | 74 |
| 5 | 15 | 40 | 33/10/21 | 20/19/19 | 22-30 | Dense circumferential calcification | 20 | 13 | 3 | 0 | 22 | 67 |
| 6 | 19 | 66 | 44/14/29 | -/22/22 | 22-30 | Two stents | 1 | 14 | 11 | I | 30 | 57 |
| 7 | 10 | 41 | 30/6/18 | -/23/24 | 24-30 | Three stents | 17 | 13 | 22 | I | 18 | 42 |
*Diameter-length (mm). RA: Right atrium, RV: Right ventricle, PA: Pulmonary artery, CMR: Cardiac magnetic resonance imaging, PR: Pulmonary regurgitation, RPA: Right pulmonary artery, LPA: Left pulmonary artery
Table 5.
Group B patient clinical details
| Number | Age/sex | Weight (kg) | Diagnosis | Number of cardiac surgeries | Age at first surgery (years) | Predominant pathology | NYHA class | Branch PA | QRS duration (ms) | RVEDVI (ml) | PRF (%) | RVEF (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 19/male | 45 | TOF TAP | 2 | 4 | Regurgitation | II | 140 | 153 | 40 | 62 | |
| 2 | 15/male | 66 | DORV TAP | 1 | 1 | Regurgitation | II | LPA stenosis | 170 | 120 | 30 | 60 |
| 3 | 41/male | 71 | TOF TAP | 1 | 11 | Regurgitation | I | 220 | 190 | 63 | 27 | |
| 4 | 23/female | 53 | TOF TAP | 1 | 2 | Regurgitation | I | 160 | 161 | 53 | 65 | |
| 5 | 23/male | 69 | TOF TAP | 2 | 1 | Regurgitation | I | 160 | 156 | 49 | 36 | |
| 6 | 14/male | 46 | TOF TAP | 1 | 1 | Regurgitation | I | 120 | 189 | 64 | 48 | |
| 7 | 21/male | 76 | TOF TAP | 2 | 18 | Regurgitation | II | 160 | 144 | 53 | 43 | |
| 8 | 19/male | 53 | TOF TAP | 2 | 2 | Regurgitation | II | 200 | 139 | 26 | 43 | |
| 9 | 61/male | 75 | TOF TAP | 1 | 16 | Regurgitation | II | 160 | 171 | 42 | 41 | |
| 10 | 14/male | 64 | TOF TAP | 1 | 1 | Regurgitation | II | LPA stenosis | 140 | 150 | 35 | 56 |
| 11 | 44/male | 40 | TOF TAP | 2 | 22 | Regurgitation | IV | 170 | 230 | 36 | 50 | |
| 12 | 21/female | 59 | TOF TAP | 1 | 3 | Regurgitation | II | 120 | 159 | 52 | 46 | |
| 13 | 18/male | 39 | TOF TAP | 1 | 4 | Regurgitation | I | 170 | 151 | 46 | ||
| 14 | 17/male | 70 | PA band VSD | 2 | 2 | Regurgitation | I | 140 | 161 | 35 | ||
| 15 | 33/male | 75 | TOF TAP | 1 | 26 | Regurgitation | II | 160 | 150 | 30 | 53 | |
| 16 | 17/male | 75 | TOF TAP | 1 | 6 | Regurgitation | II | 160 | 195 | 54 | 40 | |
| 17 | 16/male | 40 | TOF TAP | 1 | 2 | Regurgitation | II | 150 | 172 | 60 | 43 | |
| 18 | 15/male | 58 | TOF TAP | 1 | 4 | Regurgitation | I | 160 | 196 | 40 | 47 | |
| 19 | 49/male | 60 | DORV TAP | 1 | 30 | Moderate PS+regurgitation | II | LPA hypoplasia | 160 | 166 | 39 | 46 |
| 20 | 20/female | 58 | TOF TAP | 1 | 1 | Regurgitation | II | 140 | 191 | 48 | 50 |
NYHA: New York Heart Association, PA: Pulmonary artery, RVEDVI: Right ventricular end-diastolic volume index, PRF: Pulmonary regurgitant fraction, RVEF: Right ventricular ejection fraction, TOF: Tetralogy of Fallot, TAP: Transannular patch, DORV: Double outlet right ventricle, LPA: Left pulmonary artery, VSD: Ventricular septal defect
Table 6.
Group B patients hemodynamics and follow-up
| Number | RA (mmHg) | RV (mmHg) | PA (mmHg) systolic/diastolic/mean | Waist CMR/angio/balloon (mm) | Valve size* | Years after surgery | Events | PR severity | Stent fracture | RV EDVI (ml) | PRF (%) | RVEF % | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 29 | 24/4/14 | 27/28/28 | 34-30 | 15 | Mild | Yes | 99 | 0 | 54 | 67 | |
| 2 | 7 | 45 | 35/2/14 | 22/26/26 | 28-30 | 15 | RPA deployment balloon assist | Mild | 88 | 1 | 50 | 56 | |
| 3 | 7 | 33 | 35/5/16 | 32/31/33 | 36-30 | 31 | No | 18 | |||||
| 4 | 8 | 22 | 22/5/15 | 19/25/23 | 32-25 | 21 | Long 65 cm sheath used | No | 107 | 4 | 49 | 18 | |
| 5 | 7 | 43 | 32/5/15 | 29/29/32 | 36-30 | 21 | No | 24 | |||||
| 6 | 5 | 40 | 30/5/14 | 30/30/30 | 36-30 | 14 | Trivial | Yes | 24 | ||||
| 7 | 4 | 40 | 28/5/10 | -/27/27 | 34-25 | 3 | Trivial | 89 | 8 | 49 | 24 | ||
| 8 | 6 | 40 | 30/5/15 | 22/23/24 | 30-25 | 17 | No | 131 | 3 | 41 | 30 | ||
| 9 | 18 | 60 | 57/15/32 | 23/23/23 | 30-30 | 45 | Trivial | 95 | 3 | 44 | 42 | ||
| 10 | 7 | 32 | 24/3/12 | 27/26/29 | 32-30 | 14 | Moderate TR medical follow-up | Trivial | 46 | ||||
| 11 | 14 | 50 | 45/10/22 | 28/25/27 | 30-30 | 22 | VSD device | No | 46 | ||||
| 12 | 8 | 40 | 40/10/20 | 32/32/32 | 36-30 | 17 | Surgery for femoral AV fistula | Trivial | Yes | 67 | |||
| 13 | 6 | 32 | 20/5/13 | 25/25/27 | 36-35 | 14 | Trivial | 57 | |||||
| 14 | 18 | 45 | 44/20/28 | 19/23/23 | 28-25 | 15 | Trivial | 25 | |||||
| 15 | 10 | 40 | 30/5/14 | 25/27/29 | 30-30 | 7 | Trivial | 110 | 5 | 51 | 84 | ||
| 16 | 7 | 40 | 34/4/12 | 23/28/33 | 32-30 | 12 | Proximal shift Surgery | No | 84 | ||||
| 17 | 5 | 40 | 38/10/18 | 20/20/26 | 30-30 | 14 | No | 155 | 8 | 50 | 62 | ||
| 18 | 5 | 60 | 40/10/17 | 27/25/28 | 34-30 | 11 | No | 24 | |||||
| 19 | 8 | 65 | 25/10/15 | 20/20/28 | 32-30 | 19 | Balloon dilatation for PS | No | 18 | ||||
| 20 | 3 | 32 | 31/1/13 | 27/29/32 | 36-25 | 19 | Trivial | 1 |
*Diameter-length (mm). RA: Right atrium, RV: Right ventricle, PA: Pulmonary artery, CMR: Cardiac magnetic resonance imaging, PR: Pulmonary regurgitation, RVEDVI: Right ventricular end-diastolic volume index, PRF: Pulmonary regurgitant fraction, RVEF: Right ventricular ejection fraction, RPA: Right pulmonary artery, TR: Tricuspid regurgitation, AV: Arteriovenous, PS: Pulmonary stenosis
Patient inclusion
The conduit stenosis was severe causing elevation of right ventricular pressures to more than two-thirds of systemic pressures in six patients in Group A. One patient in heart failure with right ventricular ejection fraction of 23% had a conduit gradient of 38 mmHg and was included. Two patients in Group B had indexed right ventricular end-diastolic volume <150 ml. While one of these had right ventricular ejection fraction <47%, the other was symptomatic without any other explanations. Among the four included patients with LPA stenosis, one was treated by stent angioplasty and the others had poor left lung arborization. One patient with a residual ventricular septal defect underwent device closure 3 months after valve implantation.
Prestenting of narrowed conduits
Prestenting in seven patients with narrowed conduits in Group A reduced the mean outflow gradient from 74.9 ± 20.7 mmHg (38–100 mmHg) to 12.29 ± 6.87 mmHg (0–20 mmHg). A single stent was used in three patients, two stents in three, and three stents in one patient [Table 3]. The covered stent was used in one patient with dense circumferential conduit calcification, while the others received bare stents. The median duration from prestenting to PPVI was 11 weeks, ranging from 2 to 208 weeks.
Procedural success
Valve implantation was successful in 27 (93%) patients. Seven cases (Group A) received straight Venus P-valve and 20 (Group B) received flared Venus P-valve. Two procedures with flared design failed as the sharp edges from the midsegment of the third generation valve pierced through the nylon sleeve capsule while manipulating the rigid delivery system through a sharply angulated RVOT. A safe uncomplicated withdrawal of the delivery system was followed later by elective surgery.
Procedural details
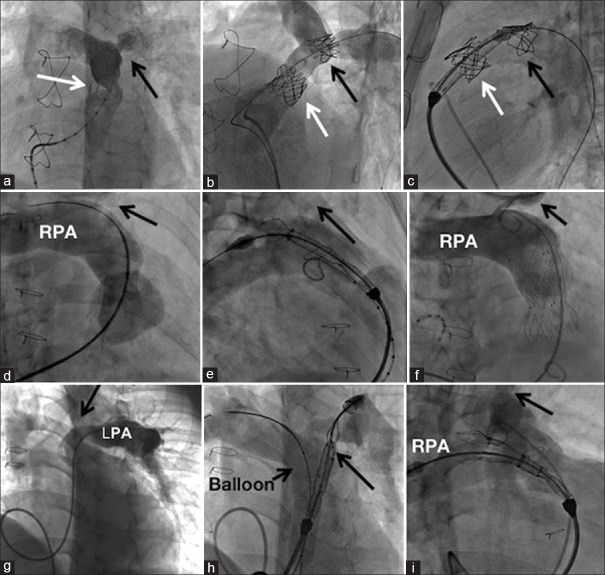
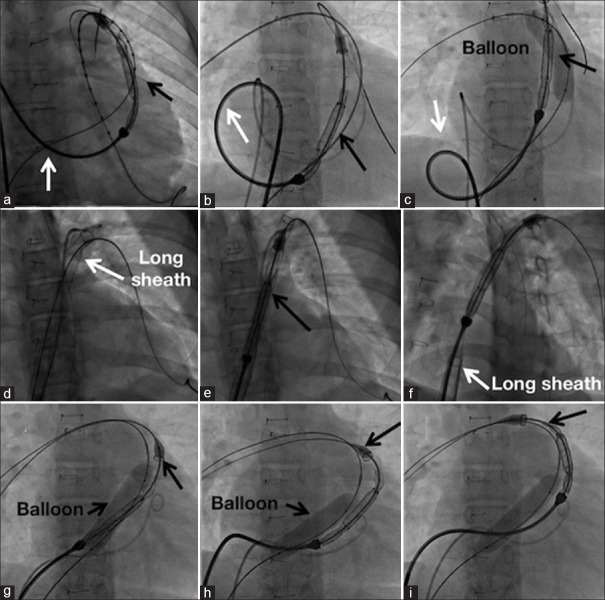
Three patients in Group B and one patient in Group A had LPA stenosis. The delivery system with nose cone could not be advanced into the LPA in one of these patients despite downsizing the valve from 30 mm to 28 mm. After inflating a balloon in distal outflow tract to reduce friction of the delivery system, the distal flare was opened in proximal LPA leading to successful deployment [Figure 3]. In one further patient with unobstructed LPA, similar difficulties were encountered needing deployment from the right pulmonary artery. Conduit stenosis associated with discrete LPA origin narrowing in a patient was stented using two separate bare CP stents. Five months later, a straight design Venus P-valve implantation was implanted uneventfully, as the nose cone of the short delivery system barely crossed the LPA stent. The recently introduced 65 cm long braided sheath placed across the RVOT to facilitate passage of the rigid valve delivery system was used in only one patient [Figure 4].
Figure 3.
Valve implantation in the presence of left pulmonary artery stenosis. Severe conduit stenosis (white arrow) and left pulmonary artery stenosis (black arrow) seen in left anterior oblique projection (a) were stented (b) before a straight Venus P-valve implantation (c). The left pulmonary artery stent did not hinder the valve delivery system. Extreme hypoplasia of left pulmonary artery in the second patient with native outflow tract (d) forced a right pulmonary artery (RPA) deployment (e) of a flared Venus P-valve. (f) A sharp kink in left pulmonary artery (LPA) origin shown in left anterior oblique projection (g) in third patient was crossed by the delivery system of a flared Venus P-valve aided by a balloon in the outflow tract (h). A similar angulation in fourth patient would not permit the delivery system (i) and managed by a RPA deployment
Figure 4.
Modification of techniques to advance the valve delivery system. The large profile delivery system of Venus P-valve could be advanced to the outflow tract only with some manipulations by creating a large right atrial loop (a) that was slowly unwound (b) resulting in advance of the valve assembly. An inflated balloon (c) reduced the friction in the distal outflow. When a long 26F braided sheath was available (d), it allowed unhindered passage of the delivery system (e) and valve deployment was preceded by withdrawing the long sheath (f). Extreme tortuous left pulmonary artery was managed by right pulmonary artery deployment (g) where an inflated balloon (h) reduced the friction and allowed progressive advance (i) of the delivery system
Procedural complications
A 32 mm flared Venus P-valve migrated proximally in a 17-year-old male causing severe tricuspid regurgitation. The valve was anchored surgically on cardiopulmonary bypass after opening the transannular patch and continued to function well after 6 years of follow-up. A similar less severe migration of another 32 mm flared valve in a 14-year-old male resulted in moderate tricuspid regurgitation that was conservatively followed for 3 years without any progression. A retrospective review of their CMR showed a mild pyramidal shape of the RVOT. Femoral arteriovenous fistula after removal of a 26F sheath used for deploying a 36 mm flared valve needed surgical repair in one patient.
Complications and reinterventions on follow-up
Followup data of all 27 patients were available. At a median follow-up of 46 months (1–85 months), there were no deaths or valve-related reinterventions. A residual ventricular septal defect was closed with a device 3 months after PPVI in one patient. There were no other reinterventions. Transient fever after procedure seen in early patients was prevented later by thorough prolonged rinsing of the valve to remove the preservative. One episode of culture-negative infective endocarditis at 4 years after implantation was successfully treated medically with no change in valve function. Valve wire-frame fracture was seen in 3 (11.4%) patients in Group B, noticed at 1, 12, and 24 months on fluoroscopy, and was confined to the proximal flare without compromising the valve function [Figure 5].
Figure 5.
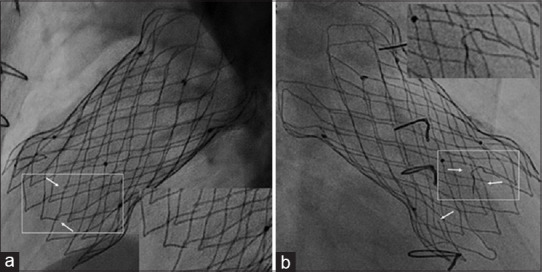
The thin nitinol frame in the proximal flare exposed to the contractile right ventricle showed wire-frame fractures on fluoroscopy in three patients. A magnified view in lateral view (a) and right anterior oblique view (b) shows three small fractures, magnified in the inserts. However, the function of the valve assembly that is present in the middle segment was not affected
Follow-up assessment
One-year and 2-years follow-up were completed by 26 (96.3%) and 23 (85.2%) patients, respectively. Seven patients had trivial to mild clinically inaudible pulmonary regurgitation at their last follow-up. There was no paravalvular leak in any of the patients. A repeat CMR in eight patients showed significant reduction in right ventricular volume and regurgitant fraction [Table 7]. All Group B patients had valve gradients <20 mmHg on the last follow-up. The reduction in outflow gradients in conduits persisted on follow-up. All patients had a subjective improvement of symptoms and effort tolerance, but none underwent any cardiopulmonary exercise tests. Antiplatelets were not discontinued in any patient.
Table 7.
Comparison of parameters before and after valve implantation
| Parameter | Pre | Post | P |
|---|---|---|---|
| CMR in Group B patients | |||
| RVEDVI (ml) | 147.38±18.57 | 107.11±22.56 | 0.001 |
| PRF (%) | 37.25±14.33 | 4.11±2.76 | <0.001 |
| RVEF (%) | 48.06±8.85 | 48.78±3.9 | 0.7 |
| Hemodynamic PS gradient in Group A patients (mmHg) | 74.86±20.74 | 12.29±6.87 | <0.001 |
CMR: Cardiac magnetic resonance imaging, RVEDVI: Right ventricular end-diastolic volume index, PRF: Pulmonary regurgitant fraction, RVEF: Right ventricular ejection fraction, PS: Pulmonary stenosis
DISCUSSION
Utility of a functioning pulmonary valve
Many patients with reconstructed RVOT need pulmonary valve replacement in adolescence or adulthood.[19] Patient selection is guided by worsening symptoms along with certain hemodynamic, CMR, and electrocardiographic indicators.[18] A consistent reduction in right ventricular volumes, improved left ventricular filling, and improved symptoms are observed after PPVI, although ejection fraction, exercise parameters, and arrhythmia burden are largely unchanged.[19] Stenosed conduits demonstrate reduction in gradients and improved symptoms after PPVI.[19] Chest adhesions, bleeding, cardiac ischemia, and multiorgan dysfunction favor percutaneous valves to surgery.[22] Lack of mortality and life-threatening complications in our group show safety of the percutaneous strategy.
Percutaneous options for dilated outflow tracts
Balloon-expandable valves cater for a minority with narrowed conduits or small outflow tracts. Despite the availability of SAPIEN valves up to 29 mm, two-thirds of RVOT are larger than 26–27 mm and may not be suitable for this valve.[4,9,23] The flared Venus P-valve is designed primarily to address these patients.[11,12,13,14,15,16,17,18] RVOT dimensions exceeded 28 mm in our group. None of the current valves except Venus P could have been considered for such large outflow tracts.[13]
Implantation success
PPVI was successful in 93% of cases. Two failures were related to the sharp edges of the older design that protruded through the nylon sleeve capsule of the delivery system while manipulating an angulated RVOT. They were removed uneventfully. The recent modification of design connected the sharp edges to the outer frame.[13] The present modification eliminated this problem despite using a large 36 mm valve in six subsequent patients. The Chinese Venus P study published the largest experience of 55 patients with 98.2% success, but the largest valve was 32 mm.[14] Procedural success was comparable to the United States Melody valve trial and Compassion trial.[24,25]
Comparison with other Venus P registries
Delayed referral and advanced hemodynamic derangements are common in developing countries due to social and logistic reasons.[26] The right ventricular volumes in our patients were larger than patients reported in large Chinese, European, South American, and multicenter Venus P registries.[12,14,16,17] The larger balloon waist dimensions in this cohort compared with the other registries mandated valves larger than 28 mm in all the patients with native RVOT. Six out of the twenty patients needed the largest 36 mm valve. Inadequate right ventricular remodeling after delayed interventions should force early recognition and intervention.[27]
Valve deployment
The Venus P-valve is usually deployed from the LPA, which accommodates the distal flare of the valve.[17] A stented or stenotic LPA hindered this deployment and was excluded in two large registries.[14,17] In our series, LPA stenosis in three patients in Group B was managed with right pulmonary artery deployment in two and balloon-assisted deployment in one [Figure 2]. A stent deployed in the LPA in one Group A patient did not pose any problem as the nose cone of the straight valve delivery system did not protrude beyond the stent. Therefore, a narrow LPA need not be considered as an absolute contraindication for Venus P-valve.
Straight Venus P-valve in stented conduits
Complications
Valve migration in two patients was managed with surgical anchoring in one and conservatively in the other. The reasons for proximal migration may be technical due to non-release of the ear-loop or anatomical due to pyramidal RVOT.[14] Repeated angiographic monitoring and a slow release are key steps to avoid a proximal deployment. There were no distal migrations in our study, which may lead to pulmonary artery occlusion.[17] The Compassion trial reports migration of SAPIEN valve in dilated RVOT.[25] Wireframe fracture in proximal flare in 3 (11%) patients did not affect the valve function. Unlike the Melody valve, in which competence was dependent on the stent-frame integrity, fracture of the proximal flare did not affect the function of the Venus P-valve located in the mid-zone.[17,28] Infective endocarditis in one patient after 4 years was managed without any impairment of valve function. Incidence of endocarditis in our cohort with a median follow-up of 46 months was relatively less compared to other reports. Early endocarditis after Venus P-valve recorded in 7% of patients in the Chinese study was comparable to an annual risk of 2% in the pooled analysis after Melody valve implantation.[12,29] In the Chinese study, one mortality and another patient needing surgery among four patients with infective endocarditis is of concern and needs meticulous patient education about dental hygiene and antibiotic prophylaxis.[14,30] Early withdrawal of aspirin after 6 months in the Chinese study was associated with pulmonary thromboembolism needing urokinase.[14] There were no thromboembolic episodes in our registry while using long-term dual antiplatelet therapy with 150 mg aspirin and 75 mg clopidogrel. Rapid discontinuation of aspirin after 6 months leading to thrombus formation on the valve leaflets is a known risk factor for endocarditis.[31]
Intermediate term valve function
The mean follow-up in our study was 47.8 ± 24.5 months with the longest follow-up of 85 months, without any need for reintervention. The follow-up in this registry was longer than in the previous reports.[14,16,17] There was no significant increase in RVOT gradient or occurrence of significant regurgitation, suggesting good durability of the valve. The straight Venus P-valve available in larger sizes is a good alternative to Melody and SAPIEN valve in stented conduits. Continued surveillance is warranted to look for late mechanical disturbances in the valve frame.[32]
Limitations
The study has inherent bias related to any retrospective study. The procedural and follow-up protocols vary among institutions affected the analysis of data. Logistic reasons prevented routine CMR in all the patients and formal cardiopulmonary exercise testing. The parameters used for analysis differed from Group A and Group B patients, but both were presented together as they formed part of the Indian registry.
CONCLUSIONS
The procedural and follow-up experience of patients in this Indian registry showed safety and efficacy of straight and flared Venus P-valves used in appropriate situations. The straight design could be a safe and durable alternative to the Melody valve in prestented conduits. The flared design expanded the scope of percutaneous treatment to larger outflow tracts up to 34 mm. LPA stenosis was not an absolute contraindication. Long-term antiplatelet therapy may be a good strategy against valve thrombosis and endocarditis. The recent modification in the design overcame some of the reasons for previous technical failures. Further studies should focus on long-term durability and late complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Alkashkari W, Alsubei A, Hijazi ZM. Transcatheter pulmonary valve replacement: Current state of art. Curr Cardiol Rep. 2018;20:27. doi: 10.1007/s11886-018-0966-y. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Xiong T, Bai P, Chu C, Dong N. Clinical outcomes of transcatheter versus surgical pulmonary valve replacement: A meta-analysis. J Thorac Dis. 2019;11:5343–51. doi: 10.21037/jtd.2019.11.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribeiro JM, Teixeira R, Lopes J, Costa M, Pires A, Gonçalves L. Transcatheter versus surgical pulmonary valve replacement: A systemic review and meta-analysis. Ann Thorac Surg. 2020;110:1751–61. doi: 10.1016/j.athoracsur.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Cheatham JP, Hellenbrand WE, Zahn EM, Jones TK, Berman DP, Vincent JA, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131:1960–70. doi: 10.1161/CIRCULATIONAHA.114.013588. [DOI] [PubMed] [Google Scholar]
- 5.Schievano S, Coats L, Migliavacca F, Norman W, Frigiola A, Deanfield J, et al. Variations in right ventricular outflow tract morphology following repair of congenital heart disease: Implications for percutaneous pulmonary valve implantation. J Cardiovasc Magn Reson. 2007;9:687–95. doi: 10.1080/10976640601187596. [DOI] [PubMed] [Google Scholar]
- 6.Huber CH, Hurni M, Tsang V, von Segesser LK. Valved stents for transapical pulmonary valve replacement. J Thorac Cardiovasc Surg. 2009;137:914–8. doi: 10.1016/j.jtcvs.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Bergersen L, Benson LN, Gillespie MJ, Cheatham SL, Crean AM, Hor KN, et al. Harmony feasibility trial: Acute and short-term outcomes with a self-expanding transcatheter pulmonary valve. JACC Cardiovasc Interv. 2017;10:1763–73. doi: 10.1016/j.jcin.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Amahzoune B, Szymansky C, Fabiani JN, Zegdi R. A new endovascular size reducer for large pulmonary outflow tract. Eur J Cardiothorac Surg. 2010;37:730–2. doi: 10.1016/j.ejcts.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 9.Zahn EM. The Edwards SAPIEN valve in the pulmonic position: The not-so-new kid on the block. J Am Coll Cardiol Intv. 2018;11:1917–9. doi: 10.1016/j.jcin.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Morgan GJ, Sivakumar K, Promphan W, Goreczny S, Prachasilchai P, Qureshi S. Early clinical experience with the straight design of Venus P-valve in dysfunctional right ventricular outflow tracts. Catheter Cardiovasc Interv. 2020;96:E653–9. doi: 10.1002/ccd.28819. [DOI] [PubMed] [Google Scholar]
- 11.Cao QL, Kenny D, Zhou D, Pan W, Guan L, Ge D, et al. Early clinical experience with a novel self-expanding percutaneous stent-valve in the native right ventricular outflow tract: Venus P Valve for rehabilitation of the RVOT in patients with chronic severe PR. Catheter Cardiovasc Interv. 2014;84:1131–7. doi: 10.1002/ccd.25544. [DOI] [PubMed] [Google Scholar]
- 12.Promphan W, Prachasilchai P, Siripornpitak S, Qureshi SA, Layangool T. Percutaneous pulmonary valve implantation with the Venus P-valve: Clinical experience and early results. Cardiol Young. 2016;26:698–710. doi: 10.1017/S1047951115001067. [DOI] [PubMed] [Google Scholar]
- 13.Alkashkari W, AlRahimi J, Albugami S, Cao Q, Hijazi ZM. Transcatheter pulmonary valve replacement: The Venus P valve-current status. Struct Heart Dis J. 2018;4:1–8. [Google Scholar]
- 14.Zhou D, Pan W, Jilaihawi H, Zhang G, Feng Y, Pan X, et al. A self-expanding percutaneous valve for patients with pulmonary regurgitation and an enlarged native right ventricular outflow tract: One-year results. EuroIntervention. 2019;14:1371–7. doi: 10.4244/EIJ-D-18-00715. [DOI] [PubMed] [Google Scholar]
- 15.Husain J, Praichasilchai P, Gilbert Y, Qureshi SA, Morgan GJ. Early European experience with the Venus P-valve®: Filling the gap in percutaneous pulmonary valve implantation. EuroIntervention. 2016;12:e643–51. doi: 10.4244/EIJV12I5A105. [DOI] [PubMed] [Google Scholar]
- 16.Garay F, Pan X, Zhang YJ, Wang C, Springmuller D. Early experience with the Venus p-valve for percutaneous pulmonary valve implantation in native outflow tract. Neth Heart J. 2017;25:76–81. doi: 10.1007/s12471-016-0932-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan G, Prachasilchai P, Promphan W, Rosenthal E, Sivakumar K, Kappanayil M, et al. Medium-term results of percutaneous pulmonary valve implantation using the Venus P-valve: International experience. EuroIntervention. 2019;14:1363–70. doi: 10.4244/EIJ-D-18-00299. [DOI] [PubMed] [Google Scholar]
- 18.Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e81–192. doi: 10.1016/j.jacc.2018.08.1029. [DOI] [PubMed] [Google Scholar]
- 19.Geva T. Indications for pulmonary valve replacement in repaired tetralogy of fallot: The quest continues. Circulation. 2013;128:1855–7. doi: 10.1161/CIRCULATIONAHA.113.005878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geva T, Gauvreau K, Powell AJ, Cecchin F, Rhodes J, Geva J, et al. Randomized trial of pulmonary valve replacement with and without right ventricular remodeling surgery. Circulation. 2010;122:S201–8. doi: 10.1161/CIRCULATIONAHA.110.951178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hascoet S, Karsenty C, Tortigue M, Watkins AC, Riou JY, Boet A, et al. A modified procedure for percutaneous pulmonary valve implantation of the Edwards SAPIEN 3 valve. EuroIntervention. 2019;14:1386–8. doi: 10.4244/EIJ-D-18-00530. [DOI] [PubMed] [Google Scholar]
- 22.Vida VL, Berggren H, Brawn WJ, Daenen W, Di Carlo D, Di Donato R, et al. Risk of surgery for congenital heart disease in the adult: A multicentered European study. Ann Thorac Surg. 2007;83:161–8. doi: 10.1016/j.athoracsur.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 23.Lurz P, Kister T. Why we need another percutaneous pulmonary valve: If size matters. EuroIntervention. 2019;14:1347–9. doi: 10.4244/EIJV14I13A243. [DOI] [PubMed] [Google Scholar]
- 24.Zahn EM, Hellenbrand WE, Lock JE, McElhinney DB. Implantation of the melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit early results from the US Clinical Trial. J Am Coll Cardiol. 2009;54:1722–9. doi: 10.1016/j.jacc.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 25.Kenny D, Hijazi ZM, Kar S, Rhodes J, Mullen M, Makkar R, et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: Early phase 1 results from an international multicenter clinical trial. J Am Coll Cardiol. 2011;58:2248–56. doi: 10.1016/j.jacc.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 26.Rashid U, Qureshi AU, Hyder SN, Sadiq M. Pattern of congenital heart disease in a developing country tertiary care centre. Factors associated with delayed diagnosis. Ann Pediatr Cardiol. 2016;9:210–5. doi: 10.4103/0974-2069.189125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tretter JT, Friedberg MK, Wald RM, McElhinney DB. Defining and refining indications for transcatheter pulmonary valve replacement in patients with repaired tetralogy of Fallot: Contributions from anatomical and functional imaging. Int J Cardiol. 2016;221:916–25. doi: 10.1016/j.ijcard.2016.07.120. [DOI] [PubMed] [Google Scholar]
- 28.McElhinney DB, Hellenbrand WE, Zahn EM, Jones TK, Cheatham JP, Lock JE, et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122:507–16. doi: 10.1161/CIRCULATIONAHA.109.921692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ran L, Wang W, Secchi F, Xiang Y, Shi W, Huang W. Percutaneous pulmonary valve implantation in patients with right ventricular outflow tract dysfunction: A systematic review and meta-analysis? Ther Adv Chronic Dis. 2019;10:2040622319857635. doi: 10.1177/2040622319857635. doi: 10.1177/2040622319857635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C, Li YJ, Ma L, Pan X. Infective endocarditis in a patient with transcatheter pulmonary valve implantation. Int Heart J. 2019;60:983–5. doi: 10.1536/ihj.18-497. [DOI] [PubMed] [Google Scholar]
- 31.Malekzadeh-Milani S, Ladouceur M, Patel M, Boughenou FM, Iserin L, Bonnet D, et al. Incidence and predictors of Melody® valve endocarditis: A prospective study. Arch Cardiovasc Dis. 2015;108:97–106. doi: 10.1016/j.acvd.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Riahi M, Ang HL, Jones M, Prachasilchai P, Baruteau AE, Promphan W, et al. Infolding of the Venus P-Valve after transcatheter pulmonary valve implantation. Circ Cardiovasc Interv. 2018;11:e005923. doi: 10.1161/CIRCINTERVENTIONS.117.005923. [DOI] [PubMed] [Google Scholar]