Abstract
Gangliosides are biologically important sialic acid-containing glycolipids found commonly in human and other vertebrates. Isolation of pure gangliosides from cells or tissues is difficult and chemical synthesis of gangliosides usually involves numerous steps with low synthetic yields. We report here a chemoenzymatic synthesis and purification protocol for GM3 and GD3, two ganglioside cancer antigens. One-pot multienzyme (OPME) glycosylation reactions are used to prepare GM3 sphingosine and GD3 sphingosine sequentially from chemically synthesized lactosyl sphingosine. A facile C18-cartridge purification procedure after each OPME reaction provides the desired pure glycosyl sphingosine product which is readily acylated to form the target ganglioside.
Keywords: Carbohydrate, chemoenzymatic synthesis, ganglioside, glycolipid, glycosphingolipid, sialic acid
INTRODUCTION:
Gangliosides are sialic acid-containing glycosphingolipids which consist of a glycan moiety β-linked to the primary hydroxyl group of a special type of lipid called ceramide (Martinez, Zhu, Han, & Fink, 2007). They are mainly presented in the outer leaflet of cell plasma membrane and play important roles in various biological processes. They interact with a diverse array of cell surface receptors and serve as receptors of bacterial toxins (Schnaar, 2019; Schnaar & Kinoshita, 2015). Overexpression of some gangliosides has been correlated to cancer progression, and these gangliosides can be considered as cancer biomarkers and are candidates for cancer vaccine development (Cheever et al., 2009; Daniotti, Vilcaes, Torres Demichelis, Ruggiero, & Rodriguez-Walker, 2013).
The potential applications of gangliosides in cancer diagnosis, cancer vaccine development, and as chemical biological probes make this class of glycolipids attractive synthetic targets. Chemical synthesis of gangliosides (Morales-Serna, Boutureira, Diaz, Matheu, & Castillon, 2007; Vankar & Schmidt, 2000), however, suffers from lengthy and challenging synthetic procedures and low yields. Glycosyltransferase-dependent enzymatic and chemoenzymatic methods are able to form glycosidic bonds with desired regio- and stereo-specificities in high efficiency (Koeller & Wong, 2000; Muthana, Cao, & Chen, 2009; Yu & Chen, 2016). They are well suited for synthesizing gangliosides.
Nevertheless, the in vivo biosynthesis of gangliosides from lactosyl ceramide (LacβCer) by vertebrate glycosyltransferases is not readily duplicated in vitro due to the poor water solubility of LacβCer and limited access to the corresponding vertebrate glycosyltransferases which are membrane-associated enzymes in the Golgi. We have developed an efficient chemoenzymatic strategy for total synthesis of pure gangliosides (Yu et al., 2018) and other glycosphingolipids (GSLs) (A. Santra et al., 2017) from commercially available relatively inexpensive starting materials. In this strategy, water soluble lactosyl sphingosine (LacβSph) is chemically synthesized and used as an acceptor substrate for glycosyltransferase-based one-pot multienzyme (OPME) systems to obtain a diverse array of glycosphingosines. Each OPME system contains a glycosyltransferase to form the desired glycosidic linkage in the glycosphingosine product in a regio- and stereo-specific manner, and the corresponding sugar nucleotide donor is generated in situ from a simple and inexpensive monosaccharide and one or two nucleoside 5’-triphosphates (NTPs). OPME system avoids the use of expensive sugar nucleotides as starting materials (Yu & Chen, 2016). The product is easily purified from the reaction mixtures containing starting materials and byproducts using a single C18-reverse phase cartridge-based purification process. A final acylation step installs a desired fatty acyl chain in high yields (98–99%) to obtain the target ganglioside which is also readily purified using a C18-reverse phase chromatography. Due to the easy synthesis and purification processes using this OPME chemoenzymatic strategy, the approach can be readily applied to generate different gangliosides and other glycosphingolipids.
Here, we describe a protocol for applying OPME systems to the efficient synthesis of structurally defined GM3 and GD3 gangliosides. GM3 and GD3 are among prioritized cancer antigens (Cheever et al., 2009; Groux-Degroote, Rodríguez-Walker, Dewald, Daniotti, & Delannoy, 2018) and are cancer vaccine candidates (Mazorra, Mesa, Fernández, & Fernández, 2008; Ragupathi et al., 2000). GM3 is an important intermediate for the biosynthesis of more complex ganglio-series gangliosides. It is the most abundant ganglioside in mammalian tissues other than the brain where more complex ganglio-series gangliosides predominate. GM3 has been found to interact and influence the activity of insulin receptor, epidermal growth factor (EGF) receptor, and other cell surface receptors (Inokuchi et al., 2018; Schnaar, 2019). GD3 is biosynthesized from GM3 with one more sialic acid α2–8-linked to the terminal sialic acid residue in GM3. It has been found to be the major ganglioside in neural stem cells (Itokazu, Wang, & Yu, 2018).
The protocols for the synthesis of GM3 and GD3 with a ceramide containing an eighteen-carbon sphingosine and a sixteen-carbon fatty acyl chain (Fig. 1) are described as examples. The synthetic and purification processes, however, can be applied to gangliosides containing different sialic acid forms and fatty acyl chains of different lengths or structures. Similar procedures can be developed for the production of other glycosphingolipids.
Figure 1.
Structures of gangliosides GM3 and GD3. The sphingosine component of the ceramide is shown in red.
STRATEGIC PLANNING:
As shown in Fig. 2, in this protocol lactosyl sphingosine (LacβSph) (Yu et al., 2018) is used as the acceptor substrate for Pasteurella multocida α2–3-sialyltransferase 3 (PmST3) (Thon, Li, Yu, Lau, & Chen, 2012) in a one-pot two-enzyme sialylation system to synthesize Neu5Acα2–3LacβSph (GM3βSph), and GM3βSph is used as the acceptor substrate for the α2–8-sialyltransferase activity of Campylobacter jejuni CstII (CjCstII) (Cheng et al., 2008) in another one-pot two-enzyme sialylation system to synthesize Neu5Acα2–8Neu5Acα2–3LacβSph (GD3βSph). In these systems, commercially available N-acetylneuraminic acid (Neu5Ac) is activated by Neisseria meningitidis CMP-sialic acid synthetase (NmCSS) (Yu, Yu, Karpel, & Chen, 2004) and transferred by a sialyltransferase to a suitable acceptor to produce the desired sialylated product. Depending on the specificity of the sialyltransferase, an α2–3- or α2–8-linked sialoglycan can be produced. GM3βSph and GD3βSph are readily purified from the starting materials and byproducts in the reaction mixtures by a single C18-reverse phase cartridge-based purification. A final acylation step installs a desired fatty acyl chain in high yields to obtain the target ganglioside, GM3βCer or GD3βCer, which is also purified using a single C18-reverse phase chromatography.
Figure 2.
Chemoenzymatic synthetic procedures described in this protocol for producing GM3 and GD3 gangliosides from lactosyl sphingosine (LacβSph).
Lactosyl sphingosine (LacβSph) is synthesized from lactose and phytosphingosine in 12 steps with 40% yield as described in the reported reference (Yu et al., 2018). It is also available from commercial supplies, such as MilliporeSigma (cat. no. 42137), Biosynth Carbosynth Limited (cat. no. OL16328) and Avanti Polar Lipid (cat. no. 860542P).
Basic Protocol 1:
Chemoenzymatic synthesis and purification of GM3 and GD3 gangliosides
Materials:
Tris base (Fisher Scientific, cat. no. BP152-10)
Lactosyl sphingosine (LacβSph, see description in the STRATEGY PLANNING section above)
N-Acetylneuraminic acid (Neu5Ac) (NingBo Hongxiang Bio-chem, Ningbo, China)
Cytidine 5’-triphosphate disodium salt (CTP) (Hangzhou Meiya Pharmacy, Hangzhou, China)
Magnesium chloride hexahydrate (MgCl2 .6H2O) (Fisher Scientific, cat. no. M33-500)
Isopropanol (i-PrOH) (MilliporeSigma, cat. no. 34863)
Ammonium hydroxide (NH4OH) (Fisher Scientific, cat. no. AC42330-5000)
p-Anisaldehyde (MilliporeSigma, cat. no. A0519-1L)
Sulfuric acid (H2SO4) (MilliporeSigma, cat. no. 258105-2.5L)
Sodium hydroxide (NaOH) (Fisher Scientific, cat. no. S318-500)
Hydrochloric acid fuming 37% (MilliporeSigma, cat. no. 258148-2.5L)
95% Ethanol (EtOH) (Fisher Scientific, cat. no. AC615110010)
Acetonitrile (MilliporeSigma, cat. no. 271004-2L)
Methanol (MeOH) (MilliporeSigma, cat. no. 34860-4L)
Methanol-d4 (MeOD) (Acros Organics, cat. no. A0407905))
Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, cat. no. S233-500)
Tetrahydrofuran (THF) (MilliporeSigma, cat. no. 186562-1L)
Palmitoyl chloride (MilliporeSigma, cat. no. 76152)
Millipore water system (Milli-Q Biocel, Academic A10)
Microcentrifuge tube (1.5 mL, Fisher Scientific, cat. no. 05-408-129)
Microcentrifuge tube (0.6 mL, Fisher Scientific, cat. no. 05-408-120)
Pipettes (Fisher Scientific Elite Pipette)
Pipet tips (Fisher Scientific: 0.1-10 mL, cat. no. 02-707-454; 1-200 mL, cat. no. 02-707-446; 100-1250 mL, cat. No. 02-707-400)
Shaker (Fisher Scientific, cat.no. 88-816-021)
Thin-layer silica gel plates (Sorbent Technologies, cat. no. 2115126)
Thin-layer silica gel plate cutter (Sorbent Technologies, cat. no. TLC-Cutter)
Pencil (Dixon, No.2)
Hair dryer (Revlon)
Hotplate and stirrer (Corning, cat. no. 6795 420)
Clear Straight Sided Round Bottle (TLC jar, 120 mL, Qorpak, cat. no. GLA00858)
Liquid Chromatography Mass Spectrometry (LCMS) (Shimadzu, cat. no. 228-45200-58)
Centrifuge tube (15 mL, Sterile, Fisher Scientific, cat. no. 07-200-886)
Incubator (Thermo Fisher, Heratherm IGS180)
Sorvall ST 16R centrifuge (Thermo Fisher, cat. no. 75004381)
PTFE Disposable stir bar (Diameter: 0.125 in, Length: 0.5 in, Fisher Scientific, cat. no. 14-513-93)
Discovery® DSC-18 SPE Tube (bed weight 5 g) (MilliporeSigma, cat. no. 52608-U)
SPE tube adapter (MilliporeSigma, cat. no. 57267)
Flask (25 mL round-bottom) (MilliporeSigma, cat. no. 31-501-102)
Glass pipet (Fisher Scientific, cat. no. 13-678-20C)
Lab pipeline air supply
Complete Fast-Freeze Flask (300 mL, Labconco, cat. no. 7540600)
Labconco Freezone 2.5 L Freeze dryer (Labconco, cat. no. 700201000)
Enzymes:
Neisseria meningitidis CMP-sialic acid synthetase (NmCSS) (Li, Yu, Cao, Muthana, & Chen, 2012; Yu et al., 2004)
Pasteurella multocida α2-3-sialyltransferase 3 (PmST3) (Thon et al., 2012)
Campylobacter jejuni α2-3/8-sialytransferase (CjCstII) (Cheng et al., 2008)
Note: The enzymes used in this protocol including NmCSS, PmST3, and CjCstII are lyophilized powders of the recombinant proteins expressed and purified in our laboratory. The detailed expression and purification information of these enzymes can be found from the corresponding references cited. NmCSS (MilliporeSigma cat. no. C1999-10UN, Chemily Glycoscience cat. no. EN01009) and CjCstII (Chemily Glycoscience cat. no. EN01003) can also be purchased from commercially available sources. PmST3 is not available commercially at this time. The potential users of PmST3 can contact us for purified enzymes before it becomes commercially available.
Protocol steps – Step annotations:
1. Enzymatic synthesis and purification of GM3βSph and GD3βSph
It is necessary to carry out small-scale enzymatic assays to make sure that the enzymes are active and to optimize reaction conditions before starting preparative-scale synthesis. This is because enzymes have different specific activities when different substrates are used and enzymes may have lost some of their activity during storage.
Small-scale enzymatic assays for testing the formation of GM3βSph from LacβSph
-
Prepare enzyme stock solutions by dissolving lyophilized enzyme powders completely in prechilled deionized water to a concentration of 1 mg/mL.
Note: The enzyme stock solutions can be kept at 4 °C in a refrigerator for up to two days without losing significant activity. To store for up to one week, add glycerol to the enzyme stock solution to reach 10% glycerol concentration and keep the enzyme stock solutions at 4 °C.
Prepare the following enzyme working stock solutions right before setting up the reactions by dilution using prechilled deionized water: NmCSS (0.1 mg/mL) working stock solution is prepared by adding 9 μL of prechilled dH2O to 1 μL of 1 mg/mL enzyme stock solution and PmST3 (0.2 mg/mL) working stock solution is prepared by adding 4 μL of dH2O to 1 μL of 1 mg/mL enzyme stock solution. Keep the enzyme working stock solutions on ice before using them for the assays below.
-
For a 10 μL volume reaction, add each of the following components from the corresponding stock solutions to a 0.6 mL microcentrifuge tube:
Note: Enzymes should be added the last (NmCSS followed by PmST3) right before the reaction begins. The order of adding other components is not critical.
| (Working) Stock solution | Volume needed | Final concentration |
|---|---|---|
| a) Tris-HCl buffer (1 M, pH 8.5) | 1 μL | 100 mM |
| b) MgCl2 (200 mM) | 1 μL | 20 mM |
| c) LacβSph (100 mM) | 1 μL | 10 mM |
| d) Neu5Ac (100 mM) | 1.5 μL | 15 mM |
| e) CTP (100 mM) | 1.5 μL | 15 mM |
| f) NmCSS (0.1 mg/mL) | 0.5 μL | 5 μg/mL |
| g) PmST3 (0.2 mg/mL) | 1 μL | 20 μg/mL |
| h) Add 2.5 μL of deionized water to the reaction mixture to make the total volume of 10 μL. Vortex and spin down the tube. | ||
-
4. Incubate the reaction mixture in the 0.6 mL microcentrifuge tube at 37 °C in an incubator.
Note: Shaking is not necessary for the small-scale reactions.
-
5. Pipet out 1 μL of reaction mixture at 1 h, 2 h, 4 h, 8 h, 12 h, and 24 h time points and monitor the product formation using thin-layer chromatography (TLC).
- Prepare a 5 cm (L) × 3 cm (W) TLC plate. Use a pencil to lightly draw a line parallel to and about 1 cm away from the bottom edge along the width of the plate. Mark the plate with the pencil that is at least 0.5 cm away from each other and from the edges.
- On the line, starting from left to right spot 0.6 μL of each of the following samples: LacβSph standard (using a 10 mM LacβSph solution), the reaction mixture, and a co-spot of LacβSph and the reaction mixture.
- Let the sample spots on the TLC plate dry by leaving it on a pre-heated hotplate for 10-60 seconds or blow drying the TLC plate using a hair dryer (Caution: Use medium heat to avoid overheating the sample spots. When white spots appear and disappear at sample spotting sites, remove the TLC plate from the heat source right away).
- Prepare 6.5 mL of a developing solvent consisting of isopropanol (i-PrOH): H2O: ammonium hydroxide (NH4OH) = 5:1:0.5 (by volume) and add the solvent mixture to a TLC jar (120 mL). (Caution: Wear safety goggles, lab coat, and gloves. Open solvent containers and prepare TLC solvent in a fume hood).
- Pick up the TLC plate at a top corner (away from the sample line) using a pair of forceps and put it in the jar with the side close to the sample line on the bottom and the plate standing in the jar at a slight angle (~15 °). Make sure that the pencil line of the TLC plate is above the surface level of the developing solvent. Cap the jar tightly without disturbing the solvent or the TLC plate in the container.
- Take out the TLC plate at a top corner using the forceps when the front line of the solvent reaches about 1 cm from the top edge of the TLC plate. Use a paper towel to wipe clean the glass side of the TLC plate. Dry the TLC plate by putting it on a pre-heated hotplate or blow drying the TLC plate using a hair dryer (Caution: Use medium heat to avoid overheating the TLC plate). Once the TLC plate is dry, remove the TLC plate from the heat source by picking it up at a corner using the forceps and leave it on the bench at room temperature to let it cool down completely.
- Pick up the TLC plate at a top corner using the forceps. Immerse the whole plate into the p-anisaldehyde sugar staining solution and let it soak for 5 seconds.
- Take out the TLC plate at a top corner using the forceps. Use a paper towel to wipe clean the glass side of the TLC plate. Heat the TLC plate by putting it on a pre-heated hotplate or blow drying the TLC plate using a hair dryer until sample spots appear on the TLC plate. Take the TLC plate away from the heat source and leave it on the bench at room temperature to let it cool down completely.
- Measure the distance of compound traveled (the starting point to the center of the spot on the TLC plate) and the distance of the solvent (from the starting point to the solvent front) traveled on the TLC plate. Retention factor (Rf) equals to the distance of compound traveled/the distance of solvent traveled. LacβSph, Rf = 0.42; GM3βSph, Rf = 0.54.
Note: It is expected that within 8 hours, more than 90% of LacβSph is converted to form the GM3βSph product. If a shorter reaction time is desired, the concentrations of enzymes can be increased proportionally. If low yields or no production formation are observed for a 24 h-reaction, reactions with 10-fold more enzymes can be tested. Adjust the amounts of the enzymes and the reaction times to obtain the desired yields in the desired reaction times.
Small-scale enzymatic assays for testing the formation of GD3βSph from GM3βSph
6. Prepare a stock solution of GM3βSph (100 mM).
-
7. Prepare enzyme stock solutions by dissolving lyophilized enzyme powders completely in prechilled deionized water to a concentration of 1 mg/mL.
Note: The enzyme stock solutions can be kept at 4 °C in a refrigerator for up to two days without losing significant activity. To store for up to one week, add glycerol to the enzyme stock solution to reach 10% glycerol concentration and keep the enzyme stock solutions at 4 °C.
8. Prepare the following enzyme working stock solutions right before setting up the reactions by dilution using prechilled deionized water: NmCSS (0.1 mg/mL) working stock solution is prepared by adding 9 μL of prechilled dH2O to 1 μL of 1 mg/mL enzyme stock solution and CjCstII (0.25 mg/mL) working stock solution is prepared by adding 3 μL of dH2O to 1 μL of 1 mg/mL enzyme stock solution. Keep the enzyme working stock solutions on ice before using them for the assays below.
-
9. For a 10 μL volume reaction, add each of the following components from the corresponding stock solutions to a 0.6 mL microcentrifuge tube:
Note: The enzyme (NmCSS) should be added the last right before the reaction begins. The order of adding other components is not critical.
| (Working) Stock solution | Volume needed | Final concentration |
|---|---|---|
| a) Tris-HCl buffer (1 M, pH 8.5) | 1 μL | 100 mM |
| b) MgCl2 (200 mM) | 1 μL | 20 mM |
| c) GM3βSph (100 mM) | 1 μL | 10 mM |
| d) Neu5Ac (100 mM) | 2 μL | 20 mM |
| e) CTP (100 mM) | 2 μL | 20 mM |
| f) NmCSS (0.1 mg/mL) | 0.5 μL | 5 μg/mL |
10. Vortex and spin down the tube.
11. Incubate the tube containing the reaction mixture in an incubator at 37 °C for 2 hours.
-
12. Monitor the reaction for the formation of CMP-Neu5Ac by Liquid Chromatography Mass Spectrometry (LC-MS) or TLC (Developing solvent consists of EtOH: 1 M ammonium acetate = 7:3., by volume. CMP-Neu5Ac, Rf = 0.58; Nue5Ac, Rf = 0.73)
Note: It is expected that most of Neu5Ac (80%) is converted to CMP-Neu5Ac in 2 hours. If a shorter reaction time is desired, the concentration of NmCSS can be increased proportionally. If low yields or no production formation are observed for a 4 h-reaction, reactions with 10-fold more enzymes can be tested. Adjust the amounts of the enzymes and the reaction times to obtain the desired yields in the desired reaction times.
13. When more than 80% of Neu5Ac is converted to CMP-Neu5Ac, add CjCstII (1 μL of 0.25 mg/mL working stock solution) to the reaction mixture in the tube. Add 1.5 μL of deionized water to make the total volume of 10 μL. Incubate the reaction mixture in the microcentrifuge tube at room temperature.
- 14. Pipet out 1 μL of reaction mixture at 1 h, 2 h, 4 h, 8 h, 12 h, and 24 h time points and monitor the product formation using thin-layer chromatography (TLC).
- Prepare a 5 cm (L) × 3 cm (W) TLC plate as described above in Step 5a.
- On the line, starting from left to right spot 0.6 μL of each of the following samples: GM3βSph standard (using a 10 mM GM3βSph solution), the reaction mixture, and a co-spot of GM3βSph and the reaction mixture.
- Dry, develop, and stain the TLC plate by following Steps 5c-5h as described above.
-
Measure the product Rf by following the procedures described above in Step 5i. As CjCstII can add multiple α2-8-linked Neu5Ac to GM3βSph, GT3βSph can also be formed as a minor product. GM3βSph, Rf = 0.54; GD3βSph, Rf = 0.31; GT3βSph, Rf = 0.17.Note: As CjCstII can catalyze the addition of one or more Neu5Ac to GM3βSph, it is important to monitor the reaction progress by TLC. Using the conditions described, it is expected that the optimal yield for the formation of GD3βSph is achieved in 12 hours. If a shorter reaction time is desired, the concentrations of enzymes can be increased proportionally. If GT3βSph with two α2-8-linked Neu5Ac is the predominant product, the amount of CjCstII or the reaction time needs to be decreased. If low yields or no production formation are observed for a 24 h-reaction, reactions with 10-fold more enzymes can be tested. Adjust the amounts of the enzymes and the reaction times to optimize yields for the formation of the target GD3βSph product in the desired reaction times.
Preparative-scale synthesis of GM3βSph from LacβSph
GM3βSph (13.8 mg) is obtained in an overall yield of 94%.
15. Transfer LacβSph (10 mg, 0.016 mmol), Neu5Ac (7.5 mg, 0.024 mmol), and CTP (13.4 mg, 0.024 mmol) into a 15 mL plastic centrifuge tube.
16. Add 0.5 mL of deionized water to the tube. Swirl or vortex the tube to dissolve the compounds completely.
17. Adjust the pH of the solution to 7.0 by adding a NaOH (4 M) solution.
18. Add 0.16 mL of Tris-HCl buffer (1 M, pH 8.5) and 0.032 mL of MgCl2 (1 M) into the tube.
19. Add 0.08 mg of NmCSS (80 μL) and 0.32 mg (320 μL) of PmST3 from the 1 mg/mL stock solution into the tube (Adjust the amounts of enzymes according to the optimized conditions obtained from small-scale assays).
20. Add deionized water into the plastic centrifuge tube to a final volume of 1.6 mL.
21. Cap the plastic centrifuge tube tightly and incubate it at 37 °C with agitation at a speed of 150 rpm in an incubator shaker for 18 h.
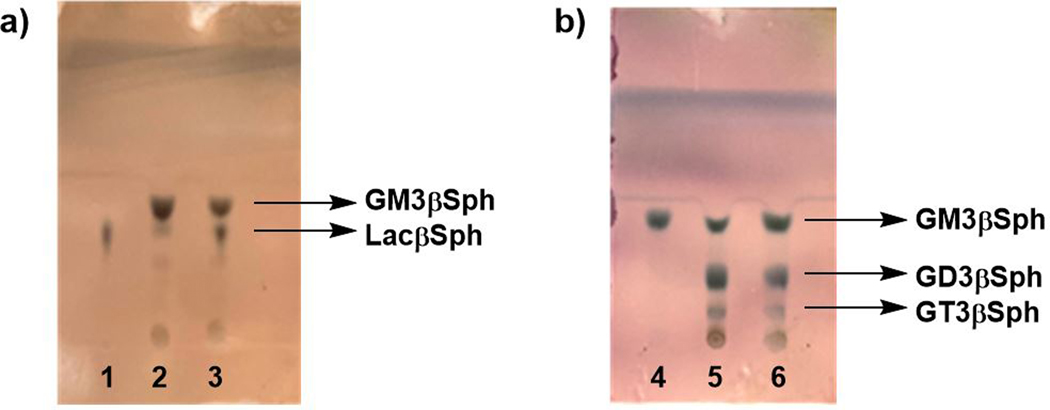
22. After 16 h, check the reaction progress by performing TLC following the procedures described above in Step 5. Fig. 3a shows the results of a sample reaction with LacβSph Rf = 0.42 and GM3βSph Rf = 0.54.
23. Once the reaction is completed (LacβSph is completely converted to the product or the reaction is no longer proceeding by TLC analysis), add the same volume (1.6 mL) of pre-chilled 95% ethanol (EtOH) into the tube to stop the reaction. Swirl the tube to mix and incubate the mixture at 4 °C refrigerator or on ice for 30 min.
24. Centrifuge the reaction mixture at a speed of 6902.5 × g for 30 min. Transfer the supernatant into a 25 mL round-bottom flask.
25. Add 2 mL of deionized water to the precipitate, resuspend, and centrifuge the tube at a speed of 6902.5 × g for 30 min. Repeat the washing procedure for two times. Combine the supernatant into the same 25 mL round-bottom flask.
26. Concentrate the supernatant to a final volume of 1–2 mL (about 4 hours) in a fume hood by gently blowing compressed air to the sample in the flask using a glass pipet connecting to the lab pipeline air supply. (Caution: Do not blow too hard or splash the solvent).
- 27. Purify the sample using a 5 g Discovery C18 cartridge.
- Precondition a C18 cartridge (5 g resin) by washing with 25 mL of MeOH followed by 25 mL of deionized water.
- Load the solution from Step 23 to the preconditioned C18 cartridge, wash the cartridge with 15 mL of deionized water.
- Elute the GM3βSph product with 20 mL of 60% acetonitrile in water (v/v) and then elute the unreacted LacβSph with 100% acetonitrile. The recovered LacβSph can be reused.
- Identify the fractions by TLC and collect the fractions containing the pure product.
- Remove the solvent in hood by gently blowing compressed air onto the sample solution using a glass pipet connecting to the lab pipeline air supply.
28. Dissolve the purified product with 1 mL of deionized water and lyophilize using the freeze-dryer to produce GM3Sph as a white powder (13.8 mg, 94% yield). The GM3βSph obtained is stable for at least one year if stored at −20 °C in a freezer.
29. Analyze the purified product by 1H and 13C NMR using MeOD as the solvent.
Figure 3.
TLC analysis results of one-pot two-enzyme (OP2E) synthesis of a) GM3βSph and b) GD3βSph. Lanes: 1, LacβSph standard; 2, GM3βSph synthesis reaction mixture; 3, co-spots of GM3βSph synthesis reaction mixture and LacβSph standard; 4, GM3βSph standard; 5, GD3βSph synthesis reaction mixture; 6, co-spots of GD3βSph synthesis reaction mixture and GM3βSph standard. Developing solvent i-PrOH: H2O: NH4OH = 5:1:0.5 (by volume).
Preparative-scale synthesis of GD3βSph from GM3βSph
GD3βSph (8.4 mg) is obtained with an overall yield of 62%
30. Transfer GM3βSph (10 mg, 0.011 mmol), Neu5Ac (6.8 mg, 0.022 mmol), and CTP (12.5 mg, 0.022 mmol) into a 15 mL plastic centrifuge tube.
31. Add 0.5 mL of deionized water to the tube. Swirl or vortex the tube to dissolve the compounds completely.
32. Adjust the pH of the solution to 7.0 by adding a NaOH (4 M) solution.
33. Add 0.11 mL of Tris-HCl buffer (1 M, pH 8.5) and 0.022 mL of MgCl2 (1 M) into the tube.
34. Add 0.09 mg of NmCSS (90 μL) from 1 mg/mL stock solution (Adjust the amounts of enzymes according to the optimized conditions obtained from small-scale assays).
35. Add deionized water into the tube to achieve a final volume of 1.1 mL.
36. Incubate the reaction mixture in an incubator shaker at 37 °C for 2 hours with agitation at 150 rpm. Monitor the formation of CMP-Neu5Ac by LC-MS or TLC (developing solvent consists of EtOH: 1 M ammonium acetate = 7:3 (by volume). CMP-Nue5Ac, Rf = 0.58; Nue5Ac, Rf = 0.73)
37. When around 80% of the Neu5Ac is converted to CMP-Neu5Ac, add 0.3 mg of CjCstII (300 μL) from 1 mg/mL stock solution into the reaction mixture in the tube (Adjust the amounts of enzymes according to the optimized conditions obtained from small-scale assays).
38. Cap the plastic centrifuge tube tightly and incubate it in an incubator shaker at room temperature with agitation at a speed of 150 rpm for overnight.
39. Monitor the product formation by performing TLC following the procedures described above in Step 14. Fig. 3b shows the results of a sample reaction with GM3βSph Rf = 0.54, GD3βSph Rf = 0.31, GT3βSph Rf = 0.17.
40. When the formation of GD3βSph is optimal, add the same volume (1.1 mL) of pre-chilled 95% ethanol (EtOH) into the tube to stop the reaction. Swirl the tube to mix and incubate the mixture at 4 °C refrigerator or on ice for 30 min.
41. Centrifuge the reaction mixture at a speed of 6902.5 × g for 30 min. Transfer the supernatant into a 25 mL round-bottom flask.
42. Add 2 mL of deionized water to the precipitate, resuspend, and centrifuge the tube at a speed of 6902.5 × g for 30 min. Repeat the washing procedure for two times. Combine the supernatant into the same 25 mL round-bottom flask.
43. Concentrate the supernatant to a final volume of 1–2 mL (about 4 hours) in a fume hood by gently blowing compressed air to the sample in the flask using a glass pipet connecting to the lab pipeline air supply. (Caution: Do not blow too hard or splash the solvent).
-
44. Purify the sample using a 5 g Discovery C18 cartridge.
Precondition the C18 Cartridge (5 g) as described above in Step 27a.
Load the solution from Step 43 to the preconditioned C18 cartridge, wash the cartridge with 15 mL of deionized water. Elute the byproduct GT3βSph with 5 mL of 25% acetonitrile in water (by volume) at a time. Monitor the fractions by TLC. Once all GT3βSph is eluted out, increase the concentration of acetonitrile in water to 35% (by volume) and use 5 mL at a time to elute the cartridge. Monitor the fractions by TLC. Once all GD3βSph product is eluted out, increase the concentration of acetonitrile in water to 60% (by volume) and use 5 mL at a time to elute the cartridge until all unreacted GM3βSph is eluted out as monitored by TLC.
Identify the fractions by TLC and collect the fractions containing the pure GD3βSph product and remove the solvent in a fume hood by gently blowing compressed air onto the sample solution using a glass pipet connecting to the lab pipeline air supply.
-
45. Dissolve the product with 1 mL of deionized water and lyophilize using the freeze-dryer to produce GD3βSph as a white powder (8.2 mg, 62% yield). The GD3βSph obtained is stable for at least one year if stored at −20 °C in a freezer.
Note: The relatively low yield (62%) for GD3βSph is due to the formation of the GT3βSph side product.
46. Analyze the purified product by 1H and 13C NMR using MeOD as the solvent.
2. Chemical synthesis and purification of GM3 and GD3 gangliosides
Synthesize GM3 ganglioside from GM3βSph
GM3 (24.8 mg) is obtained in an overall yield of 96%.
47. Transfer 20 mg of GM3Sph (0.021 mmol) into a 25 mL round-bottom flask and add a stir bar. Subsequently, add 2 mL of saturated NaHCO3 and 1 mL of THF (Caution: THF is a flammable solvent and is toxic. Wear safety goggles, lab coat, and gloves. Handle reagents and carry out reactions in a fume hood with care).
48. Dissolve palmitoyl chloride (7.8 mg, 1.5 eq) in 1 mL of THF and transfer the mixture to the reaction flask (Caution: THF is a flammable solvent and is toxic. Wear safety goggles, lab coat, and gloves. Handle reagents and carry out reactions in a fume hood with care).
49. Place the flask on a stirring plate and stir the reaction mixture vigorously without splashing at room temperature for 3 h.
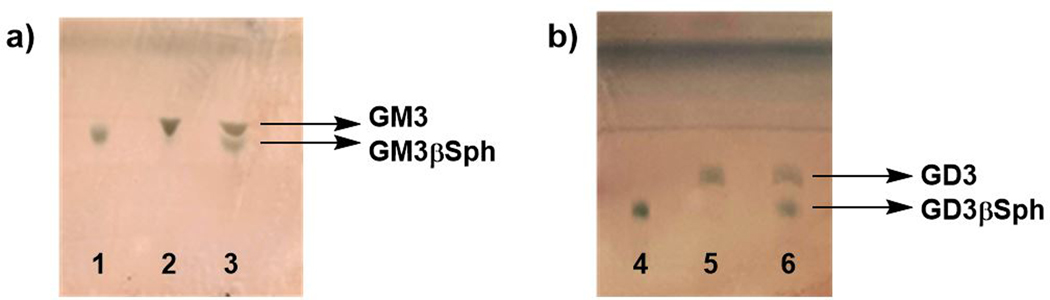
50. Monitor the reaction by TLC using a developing solvent consisting of i-PrOH: H2O: NH4OH = 5:1:0.5 (by volume) by following the procedures described above in Step 5. Fig. 4a shows the results of a sample reaction with GM3βSph Rf = 0.54 and GM3 Rf = 0.62.
51. Once the reaction is completed when GM3Sph is completely converted to form the product GM3, adjust the pH of the solution to pH 7.0 carefully using 2 M HCl.
52. Concentrate the solution in a fume hood by gently blowing compressed air to the sample in the flask using a glass pipet connecting to the lab pipeline air supply. (Caution: Do not blow too hard or splash the solvent).
53. Dissolve the residue with 2 mL of deionized water and load the sample to a preconditioned 5 g Discovery C18 cartridge (Precondition the C18 Cartridge as described above in Step 27a).
54. Wash the cartridge with water (10 mL), then elute GM3 using 5 mL of 80% acetonitrile in water (by volume) at a time. Collect fractions (2–3 mL per tube) and monitor the elution progress by TLC. Combine the fractions containing the pure GM3 product and remove solvent in a fume hood by gently blowing compressed air to the sample in the flask (using a glass pipet connecting to the lab pipeline air supply). (Caution: Do not blow too hard or splash the solvent).
55. Add 1 mL of deionized water to the product in a 15 mL plastic tube and lyophilize using the freeze-dryer to provide pure GM3 as a white powder (24.8 mg, 96% yield). The GM3 obtained is stable for at least one year if stored at −20 °C in a freezer.
56. Analyze the purified product by 1H and 13C NMR using MeOD as the solvent.
Figure 4.
TLC analysis results of the formation of gangliosides from a) GM3βSph and b) GD3βSph. Lanes: 1, GM3βSph standard; 2, GM3 synthesis reaction mixture; 3, Co-spots of GM3 synthesis reaction mixture and GM3βSph standard; 4, GD3βSph standard; 5, GD3 synthesis reaction mixture; 6, co-spots of GD3 synthesis reaction mixture and GD3βSph standard. Developing solvent: i-PrOH: H2O: NH4OH = 5:1:0.5 (by volume).
Synthesize GD3 ganglioside from GD3βSph
GD3 (11.6 mg) is obtained in an overall yield of 97%.
57. Transfer 10 mg of GD3βSph (0.0083 mmol) into a 25 mL flask and add a stir bar. Subsequently, add 2 mL of sat. NaHCO3 and 1 mL of THF (Caution: THF is a flammable solvent and is toxic. Handle with care. Wear safety goggles, lab coat, and gloves. Handle reagents and carry out reactions in a fume hood).
58. Dissolve palmitoyl chloride (3.1 mg, 1.5 eq) in 1 mL of THF and transfer the mixture to the reaction flask (Caution: THF is a flammable solvent and is toxic. Wear safety goggles, lab coat, and gloves. Handle reagents and carry out reactions in a fume hood with care).
59. Place the flask on a stirring plate and stir the reaction mixture vigorously without splashing at room temperature for 3 h.
60. Monitor the reaction by TLC using a developing solvent consisting of i-PrOH: H2O: NH4OH = 5:1:0.5 (by volume) by following the procedures described above in Step 5. Fig. 4b shows the results of a sample reaction with GD3βSph Rf = 0.29 and GD3 Rf = 0.42.
61. Once the reaction is completed when GD3Sph is completely converted to form the product GD3, adjust the pH of the solution to pH 7.0 using 2 M HCl.
62. Concentrate the solution in a fume hood by gently blowing compressed air to the sample in the flask using a glass pipet connecting to the lab pipeline air supply. (Caution: Do not blow too hard or splash the solvent).
63. Dissolve the residue with 2 mL of deionized water and load the sample to a preconditioned 5 g Discovery C18 cartridge (Precondition the C18 Cartridge as described above in Step 27a).
64. Wash the cartridge with water (10 mL), then elute GD3 with 5 mL of 50% acetonitrile in water (by volume) at a time. Collect fractions (2–3 mL per tube) and monitor the elution progress by TLC. Combine the fractions containing the pure GD3 product and remove solvent in a fume hood by gently blowing compressed air to the sample in the flask using a glass pipet connecting to the lab pipeline air supply. (Caution: Do not blow too hard or splash the solvent).
65. Add 1 mL of deionized water to the product in a 15 mL plastic tube and lyophilize to provide pure GD3 as a white powder (11.6 mg, 97% yield). The GD3 obtained is stable for at least one year if stored at −20 °C in a freezer.
66. Analyze the purified product by 1H and 13C NMR using MeOD as the solvent.
NMR data for GM3βSph:
1H NMR (800 MHz, CD3OD) δ 5.89 (dt, J = 14.4, 6.4 Hz, 1H), 5.53 (dd, J = 15.2, 7.2 Hz, 1H), 4.46 (d, J = 8.0 Hz, 1H), 4.39 (d, J = 8.0 Hz, 1H), 4.30 (t, J = 6.4 Hz, 1H), 4.10 (dd, J = 9.6, 3.2 Hz, 1H), 4.01–3.32 (m, 19H), 2.91 (dd, J = 12.0, 4.0 Hz, 1H), 2.15 (m, 2H), 2.06 (s, 3H, CH3), 1.76 (t, J = 12.0 Hz, 1H), 1.50–1.31 (m, 22 H, 11×CH2), 0.95 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (200 MHz, CD3OD) δ 174.06, 173.38, 135.05, 127.22, 103.62, 102.36, 99.57, 79.12, 76.24, 75.67, 75.10, 74.76, 73.48, 73.04, 71.49, 70.06, 69.34, 68.65, 67.82, 67.51, 66.50, 63.22, 61.27, 60.16, 55.20, 52.48, 40.66, 31.91, 31.59, 29.32, 29.31, 29.28, 29.27, 29.16, 29.00, 28.92, 28.73, 22.26, 21.09, 12.97.
NMR data for GD3βSph:
1H NMR (800 MHz, CD3OD) δ 5.81 (dt, J = 15.2, 7.2 Hz, 1H), 5.53 (dd, J = 15.2, 7.2 Hz, 1H), 4.53 (d, J = 8.0 Hz, 1H), 4.36 (d, J = 8.0 Hz, 1H), 4.14–3.47 (m, 26H), 3.27 (dd, J = 9.6, 8.0 Hz, 1H), 2.98 (m, 2H), 2.76 (dd, J = 12.0, 4.0 Hz, 1H), 2.12 (m, 2H), 2.07 (s, 3H, CH3), 2.05 (s, 3H, CH3), 1.74 (m, 2H), 1.47–1.28 (m, 22 H, 11xCH2), 0.93 (t, J = 7.2 Hz, 3H, CH3); 13C NMR (200 MHz, CD3OD) δ 174.01, 173.53, 173.30, 173.10, 134.29, 129.08, 103.32, 102.64, 100.83, 99.91, 79.19, 77.06, 75.33, 75.31, 74.96, 74.59, 74.09, 73.18, 73.13, 72.73, 71.51, 69.73, 69.35, 68.82, 68.09, 67.09, 63.13, 62.00, 61.33, 60.33, 54.79, 52.68, 52.45, 41.34, 40.84, 31.96, 31.59, 29.31, 29.28, 29.16, 29.00, 28.91, 28.88, 22.26, 21.57, 21.15, 12.98.
NMR data for GM3:
1H NMR (800 MHz, CD3OD) δ 5.72 (dt, J = 14.4, 7.2 Hz, 1H), 5.48 (dd, J = 15.2, 7.2 Hz, 1H), 4.46 (d, J = 8.0 Hz, 1H), 4.35 (d, J = 8.0 Hz, 1H), 4.10 (dd, J = 9.6, 4.0 Hz, 1H), 4.12–3.45 (m, 19H), 3.32 (t, J = 8.0 Hz, 1H), 2.90 (dd, J = 12.0, 4.0 Hz, 1H), 2.22 (t, J = 8.0 Hz, 2H), 2.09–2.04 (m, 2H), 2.05 (s, 3H, CH3), 1.79–1.75 (m, 1H), 1.63–1.60 (m, 2H), 1.44–1.25 (m, 46 H, 23×CH2), 0.94 (t, J = 7.2 Hz, 6H, 2×CH3); 13C NMR (200 MHz, CD3OD) δ 174.46, 174.00, 173.44, 133.54, 129.90, 103.59, 102.99, 99.60, 79.31, 76.16, 75.58, 74.97, 74.69, 73.44, 73.34, 71.48, 69.34, 68.60, 68.44, 67.88, 67.48, 63.10, 61.24, 60.29, 53.23, 52.45, 40.61, 35.90, 32.00, 31.63, 31.62, 29.41, 29.38, 29.36, 29.35, 29.34, 29.32, 29.30, 29.28, 29.24, 29.17, 29.04, 29.03, 29.00, 28.99, 28.96, 25.70, 22.28, 21.10, 12.99
NMR data for GD3
1H NMR (800 MHz, CD3OD) δ 5.73 (dt, J = 15.2, 7.2 Hz, 1H), 5.49 (dd, J = 15.2, 7.2 Hz, 1H), 4.54 (d, J = 8.0 Hz, 1H), 4.35 (d, J = 8.0 Hz, 1H), 4.26–3.47 (m, 26H), 3.33 (t, J = 8.0 Hz, 1H), 2.96 (dd, J = 12.0, 4.0 Hz, 1H), 2.76 (dd, J = 12.0, 4.0 Hz, 1H), 2.21 (t, J = 7.2 Hz, 2H), 2.08–2.05 (m, 2H), 2.07 (s, 3H, CH3), 2.05 (s, 3H, CH3), 1.76–1.71 (m, 2H), 1.64–1.60 (m, 2H), 1.45–1.26 (m, 46 H, 23×CH2), 0.94 (t, J = 7.2 Hz, 6H, 2×CH3); 13C NMR (200 MHz, CD3OD) δ 174.47, 174.02, 173.56, 173.33, 173.11, 133.62, 129.89, 103.35, 102.94, 100.73, 99.93, 79.13, 76.98, 75.29, 75.23, 74.92, 74.59, 74.15, 73.33, 73.11, 71.53, 71.48, 69.33, 68.84, 68.56, 68.05, 67.08, 63.10, 62.01, 61.32, 60.17, 53.19, 52.69, 52.45, 41.37, 40.77, 37.82, 35.92, 32.02, 31.63, 31.63, 31.60, 29.43, 29.41, 29.39, 29.38, 29.37, 29.35, 29.33, 29.32, 29.31, 29.29, 29.26, 29.24, 29.20, 29.05, 29.04, 29.03, 29.02, 29.00, 28.97, 25.72, 22.29, 22.26, 21.57, 21.16, 13.02, 12.98
Note: The NMR data (shown above) and spectra obtained match to those reported previously by us (Yu et al., 2018). The presence of the Neu5Ac moiety is evident from the chemical shifts of the Neu5Ac H-3eq (2.91), H-3ax (1.76), and C-3 (40.66) in GM3βSph and Neu5Ac H-3eq (2.90), H-3ax (1.77), and C-3 (40.61) in GM3 ganglioside. The presence of two Neu5Ac residues is evident from the chemical shifts of the Neu5Ac H-3eq (2.98), H’−3eq (2.72), H-3ax/H’−3ax (1.74), and C-3/C’−3 (41.34 and 40.84) in GD3βSph and the Neu5Ac H-3eq (2.96), H’−3eq (2.76), H-3ax/H’−3ax (1.77–1.71), and C-3/C’−3 (41.37 and 40.77) in GD3 ganglioside.
REAGENTS AND SOLUTIONS:
Ammonium acetate (NH4OAc), 1 M
3.854 g NH4OAc (Fisher Scientific, cat. no. A637-500) to a 50-ml centrifuge tube followed by adding 50 ml dH2O. Dissolve the compound completed by inverting the tube. Store at room temperature for up to 1 year.
p-Anisaldehyde sugar stain:
Chill 850 mL of methanol on ice for 30 minutes and add 50 mL of p-anisaldehyde. Vigorously stir the solution using a stirring plate and control the speed to avoid splashing, cautiously add 100 mL of concentrate H2SO4 drop-wisely to the solution on an ice bath during a 60-min period. Store the prepared staining solution at −20 °C for up to 1 year.
CAUTION: All reagents are toxic. H2SO4 concentrate is corrosive. It can cause skin corrosion and serious eye damage upon exposure. Open the container and handle the reagent in a fume hood with care. Wear protective gloves, protective clothing, and safety goggles!
CTP (100 mM) stock solution:
Weigh 5 mg of CTP in a 1.5 mL microcentrifuge tube, add 50 μL of deionized water, adjust the pH of the solution to pH 7.0 carefully using 2 M NaOH, then add deionized water to the solution to make total volume of 103.5 μL. Cap the tube tightly. Mix by vertex to dissolve the solid completely and spin down the tube. Store the stock solution at −20 °C for up to 1 year.
GM3βSph (100 mM) stock solution:
Weigh 5 mg of GM3βSph in a 1.5 mL microcentrifuge tube, add 53.7 μL of deionized water. Cap the tube tightly. Mix by vertex to dissolve the solid completely and spin down the tube. Store the stock solution at −20 °C for up to 2 years.
HCl (2 M) aqueous solution:
Add 167 mL of hydrochloric acid (HCl) fuming 37% to 500 mL of deionized water, then add deionized water to a total volume of 1 L. Store the stock solution at room temperature for up to 1 year.
CAUTION: HCl fuming is corrosive. It can cause serious eye damage and respiratory irritation upon exposure. Open the container and handle the reagent in a fume hood with care. Wear protective gloves, protective clothing, and safety goggles! Avoid breathing fume or vapor.
LacβSph (50 mM) stock solution:
Weigh 10 mg of LacβSph in a 1.5 mL microcentrifuge tube, then add 320.8 μL of deionized water. Cap the tube tightly. Mix by vertex to dissolve the solid completely and spin down the tube. Store the stock solution at −20 °C for up to 2 years.
MgCl2 (1 M) stock solution:
Add 10.165 g of MgCl2.6H2O to a 50 mL centrifuge tube, then add 50 mL of deionized water. Cap the tube tightly and dissolve the solid completely by inverting the tube. Store the stock solution at room temperature for up to 1 year.
MgCl2 (200 mM) stock solution:
Transfer 50 μL of 1 M MgCl2 solution into a 1.5 mL centrifuge tube and add 200 μL deionized water. Cap the tube rightly. Mix by vertex and spin down. Store the stock solution at room temperature for up to 1 year.
Neu5Ac (100 mM) stock solution:
Weigh 5 mg of Neu5Ac in a 1.5 mL microcentrifuge tube and add 50 μL of deionized water. Adjust the pH of the solution to pH 7.0 carefully using a NaOH solution (2 M) and then add deionized water to a total volume of 161.7 μL. Cap the tube rightly. Mix by vertex to dissolve and spin down. Store the stock solution at −20 °C for up to 2 years.
NaHCO3 saturated solution:
At room temperature, add NaHCO3 powder to 50 mL deionized water until no more NaHCO3 will dissolve. Store the solution at room temperature for up to 1 year.
NaOH (2 M) stock solution:
Add 4 g of NaOH in a 50 mL centrifuge tube, then add 50 mL of deionized water. Cap the tube tightly and dissolve the solid completely by inverting the tube. Store the stock solution at room temperature for up to 1 year.
NaOH (4 M) stock solution:
Add 8 g of NaOH in a 50 mL centrifuge tube and then add 50 mL of deionized water. Cap the tube tightly and dissolve the solid completely by inverting the tube. Store the stock solution at room temperature for up to 1 year.
Tris-HCl buffer (1 M, pH 8.5) stock solution:
Add 121.14 g of Tris base to a 1 L bottle, then add 700 mL of deionized water. Stir the mixture using a magnetic stirrer to complete dissolve the solid. Adjust pH to 8.5 by adding concentrated HCl aqueous solution, then add deionized water to a total volume of 1 L. Store the stock solution at room temperature for up to 1 year.
COMMENTARY BACKGROUND INFORMATION:
Gangliosides play important roles in molecular recognition. Their potential applications in neurodegenerative diseases, cancer, diabetes, and others (Schnaar, 2019) have been increasingly recognized. Gangliosides are being developed as biomarkers for cancer diagnostics (Cheever et al., 2009), candidates for cancer vaccines (Zheng, Terreni, Sollogoub, & Zhang, 2019), and therapeutics for treating neurodegenerative disorders (Magistretti et al., 2019; Sipione, Monyror, Galleguillos, Steinberg, & Kadam, 2020). However, the lack of access to pure gangliosides restricts the exploration of their functions and limits their practical applications. Structurally defined gangliosides are in high demand. Although isolating gangliosides from animal brains is currently the most common approach to obtain these compounds, the types of structures that can be accessed are limited and a high purity is difficult to achieve due to the variations of ganglioside structures on both the glycan and the lipid components. Among synthetic strategies developed for gangliosides, glycosyltransferase-based chemoenzymatic methods are attractive strategies (Abhishek Santra, Yu, & Chen, 2019).
In this protocol, we describe procedures for synthesizing and purifying GM3 and GD3 gangliosides using an efficient chemoenzymatic approach. The GM3βSph is synthesized from lactosyl sphingosine (LacβSph), a water-soluble glycolipid starting substrate, using a PmST3-involved OPME sialylation system. The obtained GM3βSph is then used as the acceptor substrate for the synthesis of GD3βSph using CjCstII-dependent OPME sialylation system. The glycosphingosines formed are readily purified by a simple C18-cartridge purification process. A final chemical acylation of GM3βSph and GD3βSph with a fatty acyl chain produces target gangliosides, which again can be readily purified using a simple C18-cartridge.
CRITICAL PARAMETERS:
The success of both PmST3 and CjCstII-dependent OPME sialylation approaches relies on the activities of both NmCSS and sialyltransferases. It is necessary to carry out small-scale assays to confirm the activities of enzymes and optimize reaction conditions (amount of enzyme and reaction time) before starting preparative-scale reactions. The optimized conditions obtained from the small-scale assays regarding the amounts of enzymes used and the reaction times preferred can then be applied to preparative-scale synthesis.
TROUBLESHOOTING:
NmCSS, PmST3, and CjCstII can be lyophilized from the corresponding purified enzyme that is dialyzed against a dialysis buffer that does not contain glycerol (20 mM Tris-HCl, pH 7.5) and stored at −20 °C for up to 1 year without losing activity. The enzyme stock solutions (1 mg/mL) can be kept at 4 °C in a refrigerator for up to two days without losing significant activity. To store for up to one week, add glycerol to the enzyme stock solution to reach 10% glycerol concentration and keep the enzyme stock solutions at 4 °C. If enzymatic reactions of synthesizing GM3βSph and GD3βSph do not work or result in low yields, prepare fresh enzyme stock solutions (1 mg/mL) for test and use. Enzyme working stock solutions are always prepared right before carrying out the experiments and kept on ice before use.
Low yields may be caused by pH changes during the reaction. Check the reaction pH occasionally and adjust the pH value to the desired 8.5 if needed.
In the case of producing GD3βSph from GM3βSph, a byproduct GT3βSph can also be formed as the α2–8-sialytransferase activity of CjCstII is able to add more than one α2–8-linked sialic acid residues to GM3βSph substrate. To decrease the formation of GT3βSph, the ratio of donor (Neu5Ac) and acceptor (GM3βSph) and the reaction time can be controlled to optimized the formation of GD3βSph as the major product. The reaction process should be monitored frequently and the reaction should be stopped when the formation of GD3βSph is optimal.
UNDERSTANDING RESULTS:
Two ganglioside cancer antigens GM3 and GD3 can be successfully synthesized from LacβSph by following the protocol. Water soluble GM3βSph and GD3βSph can be synthesized using one-pot two-enzyme sialic acid activation and transfer systems and purified by a C18-cartridge. They can be readily converted to GM3 and GD3 gangliosides by a simple chemical acylation process and purified by a C18-cartridge. The reactions process can be monitored by TLC. The examples shown here produced GM3 and GD3 gangliosides from LacβSph in 90% and 57% overall yields, respectively.
TIME CONSIDERATIONS:
The small-scale enzymatic assays are usually completed in 2–12 h. For the preparative-scale synthesis of GM3βSph and GD3βSph, the process of preparing enzyme reaction mixtures needs 30 min and the reactions can be completed in 4–24 h depending on the amounts of the enzymes used. Purification of GM3βSph and GD3βSph products using a C18 cartridge takes about 30 min. The process of synthesizing GM3βCer from GM3βSph or GD3βCer from GD3βSph takes about 3 h and the purification of GM3 or GD3 ganglioside using a C18 cartridge takes 20–30 min each. An experienced chemist can anticipate the completion of the small-scale assays, preparative-scale synthesis, product purification, and characterization in two to three days.
ACKNOWLEDGEMENTS:
Research reported in this publication was supported by the United States National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under Award Number U01GM120419. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
LITERATURE CITED:
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, . . . Matrisian LM (2009). The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res, 15(17), 5323–5337. doi: 10.1158/1078-0432.Ccr-09-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Yu H, Lau K, Huang S, Chokhawala HA, Li Y, . . . Chen X. (2008). Multifunctionality of Campylobacter jejuni sialyltransferase CstII: characterization of GD3/GT3 oligosaccharide synthase, GD3 oligosaccharide sialidase, and trans-sialidase activities. Glycobiology, 18(9), 686–697. doi: 10.1093/glycob/cwn047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniotti J, Vilcaes A, Torres Demichelis V, Ruggiero F, & Rodriguez-Walker M. (2013). Glycosylation of glycolipids in cancer: basis for development of novel therapeutic approaches. Frontiers in Oncology, 3, 306. doi: 10.3389/fonc.2013.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux-Degroote S, Rodríguez-Walker M, Dewald JH, Daniotti JL, & Delannoy P. (2018). Gangliosides in cancer cell signaling. Prog Mol Biol Transl Sci, 156, 197–227. doi: 10.1016/bs.pmbts.2017.10.003 [DOI] [PubMed] [Google Scholar]
- Inokuchi JI, Inamori KI, Kabayama K, Nagafuku M, Uemura S, Go S, . . . Shishido F. (2018). Biology of GM3 ganglioside. Prog Mol Biol Transl Sci, 156, 151–195. doi: 10.1016/bs.pmbts.2017.10.004 [DOI] [PubMed] [Google Scholar]
- Itokazu Y, Wang J, & Yu RK (2018). Gangliosides in nerve cell specification. Prog Mol Biol Transl Sci, 156, 241–263. doi: 10.1016/bs.pmbts.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeller KM, & Wong CH (2000). Complex carbohydrate synthesis tools for glycobiologists: enzyme-based approach and programmable one-pot strategies. Glycobiology, 10(11), 1157–1169. doi: 10.1093/glycob/10.11.1157 [DOI] [PubMed] [Google Scholar]
- Li Y, Yu H, Cao H, Muthana S, & Chen X. (2012). Pasteurella multocida CMP-sialic acid synthetase and mutants of Neisseria meningitidis CMP-sialic acid synthetase with improved substrate promiscuity. Appl Microbiol Biotechnol, 93(6), 2411–2423. doi: 10.1007/s00253-011-3579-6 [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Geisler FH, Schneider JS, Li PA, Fiumelli H, & Sipione S. (2019). Gangliosides: Treatment avenues in neurodegenerative disease. Front Neurol, 10, 859. doi: 10.3389/fneur.2019.00859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Z, Zhu M, Han S, & Fink AL (2007). GM1 specifically interacts with alpha-synuclein and inhibits fibrillation. Biochemistry, 46(7), 1868–1877. doi: 10.1021/bi061749a [DOI] [PubMed] [Google Scholar]
- Mazorra Z, Mesa C, Fernández A, & Fernández LE (2008). Immunization with a GM3 ganglioside nanoparticulated vaccine confers an effector CD8(+) T cells-mediated protection against melanoma B16 challenge. Cancer Immunol Immunother, 57(12), 1771–1780. doi: 10.1007/s00262-008-0503-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Serna JA, Boutureira O, Diaz Y, Matheu MI, & Castillon S. (2007). Recent advances in the glycosylation of sphingosines and ceramides. Carbohydr Res, 342(12–13), 1595–1612. doi:S0008–6215(07)00167-X [pii] 10.1016/j.carres.2007.03.028 [DOI] [PubMed] [Google Scholar]
- Muthana S, Cao H, & Chen X. (2009). Recent progress in chemical and chemoenzymatic synthesis of carbohydrates. Curr Opin Chem Biol, 13(5–6), 573–581. doi: 10.1016/j.cbpa.2009.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragupathi G, Meyers M, Adluri S, Howard L, Musselli C, & Livingston PO (2000). Induction of antibodies against GD3 ganglioside in melanoma patients by vaccination with GD3-lactone-KLH conjugate plus immunological adjuvant QS-21. International Journal of Cancer, 85(5), 659–666. doi: [DOI] [PubMed] [Google Scholar]
- Santra A, Li Y, Yu H, Slack TJ, Wang PG, & Chen X. (2017). Highly efficient chemoenzymatic synthesis and facile purification of alpha-Gal pentasaccharyl ceramide Galalpha3nLc4betaCer. Chem Commun (Camb), 53(59), 8280–8283. doi: 10.1039/c7cc04090c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra A, Yu H, & Chen X. (2019). CHAPTER 10 Synthesis of Glycosphingolipids (GSLs). In Synthetic Glycomes (pp. 226–253): The Royal Society of Chemistry. [Google Scholar]
- Schnaar RL (2019). The biology of gangliosides. Adv Carbohydr Chem Biochem, 76, 113–148. doi: 10.1016/bs.accb.2018.09.002 [DOI] [PubMed] [Google Scholar]
- Schnaar RL, & Kinoshita T. (2015). Glycosphingolipids. In rd A Varki RD Cummings JD Esko P Stanley GW Hart M Aebi AG Darvill T Kinoshita NH Packer JH Prestegard RL Schnaar, & Seeberger PH (Eds.), Essentials of Glycobiology (pp. 125–135). Cold Spring Harbor; (NY: ). [PubMed] [Google Scholar]
- Sipione S, Monyror J, Galleguillos D, Steinberg N, & Kadam V. (2020). Gangliosides in the brain: Physiology, pathophysiology and therapeutic applications. Front Neurosci, 14, 572965. doi: 10.3389/fnins.2020.572965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon V, Li Y, Yu H, Lau K, & Chen X. (2012). PmST3 from Pasteurella multocida encoded by Pm1174 gene is a monofunctional alpha2–3-sialyltransferase. Appl Microbiol Biotechnol, 94(4), 977–985. doi: 10.1007/s00253-011-3676-6 [DOI] [PubMed] [Google Scholar]
- Vankar YD, & Schmidt RR (2000). Chemistry of glycosphingolipids-carbohydrate molecules of biological significance Chem. Soc. Rev, 29, 201–216 [Google Scholar]
- Yu H, & Chen X. (2016). One-pot multienzyme (OPME) systems for chemoenzymatic synthesis of carbohydrates. Org Biomol Chem, 14(10), 2809–2818. doi: 10.1039/c6ob00058d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Santra A, Li Y, McArthur JB, Ghosh T, Yang X, . . . Chen X. (2018). Streamlined chemoenzymatic total synthesis of prioritized ganglioside cancer antigens. Org Biomol Chem, 16(22), 4076–4080. doi: 10.1039/c8ob01087k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Yu H, Karpel R, & Chen X. (2004). Chemoenzymatic synthesis of CMP-sialic acid derivatives by a one-pot two-enzyme system: comparison of substrate flexibility of three microbial CMP-sialic acid synthetases. Bioorg Med Chem, 12(24), 6427–6435. doi: 10.1016/j.bmc.2004.09.030 [DOI] [PubMed] [Google Scholar]
- Zheng C, Terreni M, Sollogoub M, & Zhang Y. (2019). Ganglioside GM3 and Its Role in Cancer. Curr Med Chem, 26(16), 2933–2947. doi: 10.2174/0929867325666180129100619 [DOI] [PubMed] [Google Scholar]