Abstract
The gut microbiota (GM) is referred to as the second gene pool of the human body and a commensal, symbiotic, and pathogenic microorganism living in our intestines. The knowledge of the complex interaction between intestinal microbiota and health outcomes is a novel and rapidly expanding the field. Earlier studies have reported that the microbial communities affect the cellular responses and shape many aspects of physiology and pathophysiology within the body, including muscle and bone metabolism (formation and resorption). GM influences the skeletal homeostasis via affecting the host metabolism, immune function, hormone secretion, and the gut-brain axis. The premise of this review is to discuss the role of GM on bone homeostasis and skeletal muscle mass function. This review also opens up new perspectives for pathophysiological studies by establishing the presence of a ‘microbiota-skeletal’ axis and raising the possibility of innovative new treatments for skeletal development.
Keywords: Gut microbiota, Bone formation, Probiotics, Immune system, Osteoporosis, Skeletal muscle function
Introduction
The whole of commensal, symbiotic and pathogenic living microorganisms residing in the gastrointestinal tract, typically lining the mucosal surfaces of the host, is known as the gut microbiota (GM).1 The gut microbiome is composed of trillions of microorganisms that live in the gut and building mutually beneficial relationships with the host.2,3 The GM is acquired entirely from the mother during birth, and is subject to change under different environmental factors such as diet, age, travel, use of certain medications and disease conditions.1,4 Furthermore, this microbiota can function as multicellular organ which influences hosts in a wide variety of biological processes in various ways,2 including gut physiology,5 nutrient production and absorption,6 host growth,7 metabolic functions,8 immune system function and inflammatory processes,9 energy balancing,10 and brain-behavior.11 Additionally, alteration of gut microbiota composition has been associated with the pathogenesis of several complex diseases, such as type 1 and type 2 diabetes,12,13 irritable bowel syndrome,14 colorectal cancer15, transient ischemic attack16, and rheumatoid arthritis.17
Metabolic bone diseases like osteoporosis are characterized by increased microstructural destruction of bone tissue and low bone mass, which leads to bone fragility.18 It is reported that osteoporotic fractures are linked with increased mortality and substantial economic loss. Over the age of 50, 10.2 million Americans affected by osteoporosis and while 43.4 million Americans have low bone mass, according to the National Osteoporosis Foundation19. As per World Health Organization (WHO) recommendation, the common diagnostic criteria solely depends on the lumbar vertebrae 1~4 and femoral neck as measured by dual- energy X- ray bone densitometer.2 It is well documented that bone mass increases after birth and peaks in adulthood. This increase of bone mass occurs from mid-late adulthood, and is greatly affected by lifestyle factors as well as age and biological sex.20 Bone metabolism is balanced by the bone formation and resorption. When the bone formation is dominant, it is characterized by bone formation and anabolism, otherwise, it’s directed to resorption and catabolism.2,18 It is understood that host metabolic pathways, immune systems, and the hormonal environment can affect this bone metabolism balance, and the gut microbiota could also able to affect these pathways 21. For easy maintenance of healthy bone, the intestinal absorption of calcium and vitamin D is essential. The work of Wallace et al., (2017) suggested that probiotics can improve calcium absorption and maintain gut pH.22 Similarly, fructose, oligosaccharides, and soluble corn fibers (SCF) can improve the calcium absorption efficiency.23
Emerging evidence suggesting that the use of probiotics is beneficial to the host system in various ways.2 The majority of experimental data showed that modulation of GM by the use of probiotics can promote bone homeostasis in different pathological settings, such as sex steroid-associated bone loss in mice.2,23–25 Others have shown using yogurt that contains different probiotics (Lactobacillus species) that these probiotics can protect bone health, indicating that dairy product consumption may lead to a higher peak bone mass.26 The natural prebiotics (typically high-fiber foods) is enriched in vegetables, fruits, and grains that mainly contain high fiber. Among them, fructo- oligosaccharides and inulin- type prebiotics can improve the beneficial bacteria in the gut and promote the absorption of minerals by lowering the pH of the intestines. This led to an increase in bone formation.27
This current review summarizes the evidence on the GM may play a vital role in bone turnover and density by influencing the host metabolism, immune system, endocrine milieu, and gut-brain axis and how GM manipulation employing probiotics, prebiotics and antibiotics treatment may influence bone health. Furthermore, the review covers some aspects of knowledge on probiotics intervention that could improve skeletal muscle function.
The gut microbiome regulation on bone homeostasis by influencing host metabolism
The growing concern that recent lifestyle innovations, notably long-term dietary intake of the typical western diet (high fat/high sugar) alters the structure and metabolic activity of microbiome that resides in the human gut.28 These diets caused a change in the microbial communities that contribute to growing epidemics of chronic illness/afflictions such as obesity and inflammatory bowel disease.29,30 David et al., (2014) also suggested that the population of Bifidobacteria species was indeed increased following a high-fiber and fructo-oligosaccharides diet rather than an animal-based diet. In contrast, exogenous supplementation of probiotics can affect host metabolism and may protect the gut epithelial cell and maintain gut-barrier integrity.28 In this section, we emphasize several aspects of GM mediated host metabolism to influence bone turnover.
1. Short-Chain Fatty Acids (SCFAs)
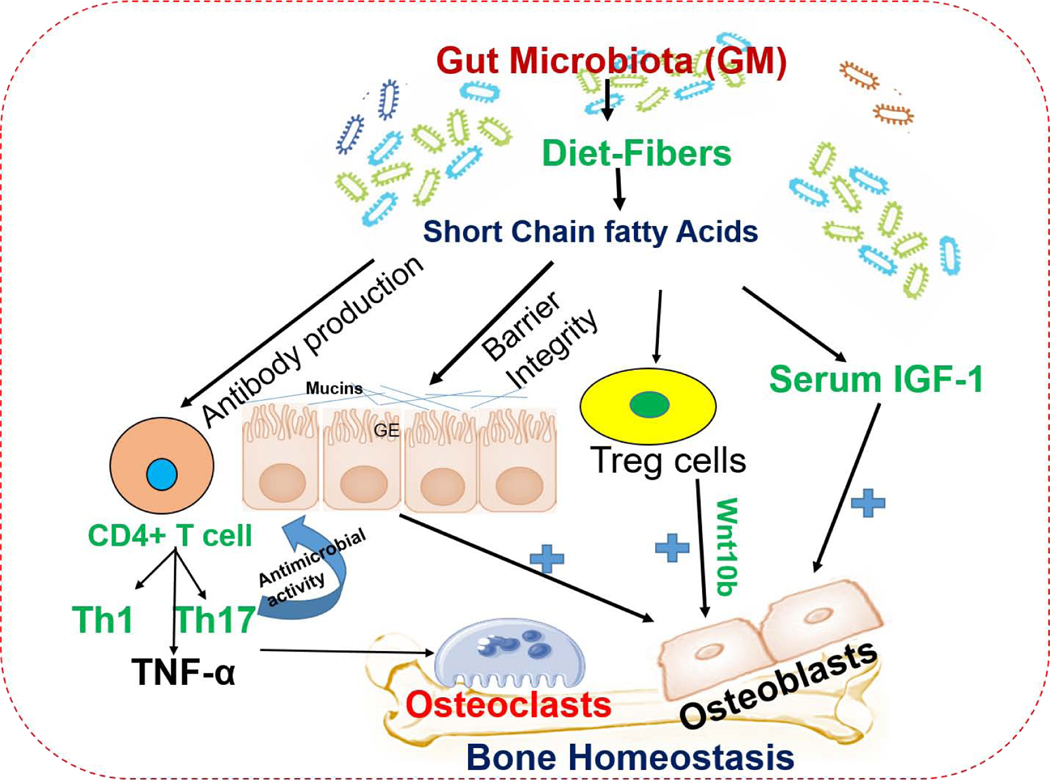
The GM produces several metabolites through fermentation of undigested prebiotic food by either modifying host products or de novo synthesis. Among these molecules, short-chain fatty acids (SCFAs, propionate, and butyrate) are the most widely investigated and have anti-inflammatory effects in the intestinal mucosa.1, 31 In contrast, intestinal bacteria (Clostridia and Peptostreptococci species) also produce SCFAs through amino acid fermentation.31 SCFAs can activate several signaling cascades such as AMP kinase and free fatty acid receptors 2/3 (FFAR2/3) or G- protein- coupled receptors 43 and 41.32 Likewise, a high oligosaccharide diet indeed changes the microbial composition and increases SCFAs production. The butyrate also activates the GPR109A/HCA2 signaling in immune cells. Both butyrate and propionate regulate gene expression by inhibiting histone deacetylases (HDAC3, HDAC4), which led to the activation of Treg function and gut protection.33, 34 The work of Yan et al., (2017) reported that SCFAs produced by microbiota induces the hormone-insulin-like growth factor 1 (IGF-1), which promotes bone growth, suggesting a mechanism by which microbiota affects bone health.3 Others have shown that SCFAs are regulators of osteoclast metabolism and bone mass in vivo. Treatment of mice with SCFA (propionate and butyrate) significantly increases bone mass and prevents post-menopausal and inflammation-induced bone loss. Mechanistically, propionate and butyrate induce metabolic reprogramming of osteoclasts resulting in the downregulation of osteoclast bone resorption activity.35 Tyagi et al., (2018) show that the administration of butyrate in the mouse model increases bone mass by activating Wnt signaling in osteoblasts.36 Therefore, SCFAs may represent new methods of intervention for the prevention of bone disease, osteoporosis (Table 1). The mechanisms of GM derived SCFAs on bone homeostasis are shown in Figure 1.
Table 1.
Modulation of gut microbiota (GM) via probiotics, prebiotics, and antibiotics treatment affects the musculoskeletal parameters
| Therapeutic agent | Animal study | Mechanism of action | Effect on the skeletal system | References |
|---|---|---|---|---|
| Prebiotics | ||||
| SCFA | C57BL/6J mice | Change in the metabolic state of pre-osteoclasts | Increased bone BMD and bone mass | 77 |
| SCFA | BALB/c mice | Increase in serum IGF-1 | Acute bone resorption and further normalize bone mass | 36 |
| GOS | C57BL/6J mice | Increased calcium retention | Increased bone BMD and bone strength | 53 |
| Inulin- type prebiotics | C57BL/6J mice | Absorption mineral contents and improve the beneficial bacteria in the gut | Bone formation increases | 28 |
| Probiotics | ||||
| Lactobacillus GG | C57BL/6J mice (OVX) | -Attenuates intestinal inflammation - Improves TJ destruction and gut epithelial permeability |
Bone formation increases | 23 |
| Lactobacillus paracasei | C57BL/6 OVX mice | Decreases inflammatory cytokines | Increases bone mass | 25 |
| Lactobacillus reuteri ATCC PTA 6475 | BALB/c mice | Decreased bone resorption | Promotes bone formation | 75 |
| Lactobacillus Plantarum WJL | BALB/c mice | Increase in serum IGF-1 | Increased femur length | 78 |
| Lactobacillus acidophilus ATCC4356 | BALB/c mice | Increased number of Treg cells and decreased number of Th17 | Promotes bone mass | 70 |
| Lactobacillus helveticus ATCC 27558 | Sprague-Dawley rat model | BMD and bone strength increases | Promotes bone mass | 89 |
| Bifidobacterium longum | OVX Sprague-Dawley rat model | BMD increases | Promotes bone mass | 26 |
| L. paracasei and L. plantarum | C57BL/6 OVX mice | Decreases inflammatory cytokines | Inhibits bone loss | 25 |
| VSL#3 | C57BL/6 OVX mice | Attenuates intestinal and BM inflammation. | completely inhibits bone loss | 23 |
| Bacillus clausii | BALB/c mice | Increased number of Treg cells and decreased number of Th17 | Inhibition of bone loss | 69 |
| Antibiotics | ||||
| penicillin, vancomycin, penicillin plus vancomycin, chlortetracycline | C57BL/6 young mice | Increase intestinal incretin, GIP and adiposity | Increases BMD | 107 |
BMD: Bone mineral density, SCFA: Short-chain fatty acid, GOS: Galacto-oligosaccharides, GIP: Glucose-dependent insulinotropic Polypeptide, TJ: Tight junction, IGF-1: Insulin-like growth factor-1.
VSL#3 contains a mixture of bacteria such as Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus bulgaricus, and Streptococcus thermophiles.
Figure 1: The influence of gut microbiota on bone metabolism through metabolite mediated barrier integrity and immune system.
SCFAs have the ability to influence Tregs development and promote bone metabolism via Wnt10 action. The production of SCFAs may be a mechanism by swhich microbial community increased the serum level of IGF- 1 which led to bone growth and homeostasis. Gut microbiota (GM) induced an increase in intestinal barrier integrity. The alternation of GM composition leads to a result in metabolic disorders. The dysfunction of the intestinal mucosal barrier may lead to an increase in serum levels of lipopolysaccharide (LPS), which could in turn increase membrane permeability, resulting in metabolic endotoxemia. Th17 cells are essential for estrogen- deficient bone loss and it produces IL-17 cytokines. The elimination of IL17 or the use of an anti- IL17 antibody may prevent bone loss from estrogen deficiency. GE: Gut epithelial cells, Treg cells: T regulatory cells, Th1: T helper-1, Th17: T helper-17 cells, SCFAs: short-chain fatty acid, IGF-1: Insulin-like growth factors.
2. Bile acid
Bile acids are small metabolic molecules that are produced in the liver. They are secreted to the small intestine to participate in the absorption of dietary lipids. Emerging evidence has suggested that the gut microbiome plays a significant role in bile acid metabolism.2, 37 In the gut, primary bile acids undergo biological transformation into secondary bile acids (primarily lithodeoxycholic acid and deoxycholic acid) via anaerobic bacteria.38 During enterohepatic recirculation, bile acids spread over in the systemic circulation and can reach every organ in the body, including the bone. A growing body of evidence suggests that bile acids regulate skeletal homeostasis through various signaling on osteoblasts and osteoclasts. In particular, in vitro, activation of FXR signaling by bile acids (chenodeoxycholic acid) or FXR agonists (Fexaramine) significantly enhanced osteoblastic mineralization through the upregulation of Runx2 and enhanced extracellular signal-regulated kinase (ERK) and β-catenin signaling.39 Upon bacterial transformation of secondary bile acids, it acts as an agonist of the membrane- bound G- protein- coupled receptor (TGR5) of intestinal cells and increases the production of glucagon- like peptide- 1 (GLP- 1). This leads to the secretion of calcitonin by thyroid cells via paracrine action, thus inhibiting bone resorption. GLP- 1 also can stimulate the proliferation and differentiation of osteoblasts.40 Monohydroxylated secondary lithocholic acid (LCA), a derivative bile acid produced by 7- dehydroxylation of intestinal bacteria, acts as vitamin D receptor (VDR) ligand,41 and affects bone metabolism.42 Excessive deposition of LCA can damage osteoblast mitochondrial activity and reduce cell viability.42 Besides, LCA reduces vitamin D effects in osteoblasts and associated with decreased osteocalcin and RANKL gene expression.43
3. Calcium absorption and intestinal barrier integrity
GM can affect the absorption of nutrients such as calcium and vitamin D that is required for skeletal development. Calcium is the dominant mineral for bone health and its absorption is facilitated by vitamin D. Calcium is absorbed by the intestinal cells via an active transcellular pathway or passive paracellular diffusion, depending on the level of calcium present in the cell, and is deposited as calcium hydroxyapatite (Ca10[PO4]6[OH]2) in bones and teeth.44 Either of calcium or vitamin D deficiency leads to severe skeletal abnormalities.45 Clinical study has revealed that high consumption of dietary calcium (47.4 mmol/day compared to the recommended 22.5 mmol/day) showed decreased bone resorption in adolescent girls. In addition to these findings, the study suggests that a low calcium diet alone is sufficient to enhance bone resorption and impaired trabecular bone formation in the rat model.46
It has been reported that fermentation of dietary prebiotics to SCFAs by the gut microbiota results in higher calcium absorption.47 Studies in adolescents found that consumption of different prebiotics diets such as galacto-oligosaccharides (GOS) and soluble corn fiber (SCF), both of which can be fermented to SCFA, led to increased calcium resorption (Table 1). This increased calcium absorption is associated with relative abundances of Parabacteroides, Bifidobacterium, Bacteroides, Butyricicoccus, Oscillibacter, and Dialister species measured in feces.48–50 Additionally, a clinical trial reported that SCF consumption in post-menopausal women showed a positive-response effect on bone calcium retention and observed a significant increase in bone-specific alkaline phosphatase activity.51 In another study, GOS feeding in the experimental rat model resulted in increased calcium absorption and higher trabecular volumetric bone mineral density (vBMD) in both the distal femur and proximal tibia with consequently greater bone strength.52 Prebiotic inulin also produced an enhancement of calcium absorption and cortical and cancellous bone density compared to oligosaccharides in the growing rat model.53 Additionally, a specific probiotic bacterium Lactobacillus salivarius stimulated calcium uptake by enterocytes in a Caco-2 cell culture model 54 and probiotics bacterial species has a beneficial function on bone mass function (Table-1).
The intestinal epithelial barrier is a one-cell-thick internal lining of different types of epithelial cells of the gut.55 Several tight junction proteins seal the paracellular pathway and conduct gate and fence functions in epithelial cells. Underneath this layer, a thin layer of connective tissue called the lamina propia is present, which cherishes the healthy communication between the microbiome and the immune system. Besides, the mucosal layer is a chemical barrier that provides the first layer of defense in the epithelium, is formed by a layer of mucus and limits the contact between the microbiome and epithelial cells. The absence of the mucus layer leads to intestinal inflammation and the onset of various metabolic diseases.55,56 Hamilton et al., (2015) demonstrated that alteration of the gut microbiota composition causes intestinal permeability and metabolic disorders.57 The dysfunction of the intestinal mucosal barrier may lead to an increase in serum levels of lipopolysaccharide (LPS), resulting in metabolic endotoxemia.58 Early studies have suggested that LPS promotes bone loss in the femur in vivo and the survival of osteoclasts in vitro.2,59 Chongwatpol P. et al., (2015) illuminated that LPS substantially decreased trabecular bone volume, lumbar vertebra bone mineral density, and the number of the vertebral bodies in the mouse model.60
The gut microbiome regulation on bone homeostasis by influencing the immune system
Recent studies have suggested that a close interplay between the immune system and bone metabolism, a term called “osteoimmunology,” which represents the role of immune cells or immune-related factors in modulating skeletal development.45 Further, GM is required for the function and maturation of the immune system and also influences host health.61, 62 Sjogren et al., (2012) discovered for the first time the relation between microbiota and bone development.63 This study also confirmed that altered immune status in germ-free mice (decreased pro-inflammatory cytokines, fewer CD4+ T cells and reduced osteoclast/precursor cells in bone marrow) may account for the higher bone mass formation than in CONV-R mice.63 Other studies have shown that intestinal filamentous bacteria were able to increase IFN-γ production and IL-17, which play an essential role in bone formation in vivo and rescues osteoporosis in mice following ovariectomy (OVX).64, 65 These studies suggest that the gut microbiota regulates bone metabolism by altering host immune status.
Th17 cells are a subset of CD4+T cells, which produces IL-17 and IL-22 which are important for innate immunity. It induces the intestinal epithelial cells to produce antimicrobial peptide against the pathogens.2 Following transplantation of segmented filamentous bacteria in GF mice increased the number of Th17 cells and maintain the antimicrobial action in the epithelium.66 CD4+FOXP3+Treg cells are associated with systemic immunity and stable at the intestinal mucosa. Also, many have shown the close relationship between intestinal microflora and Treg cells.63 Indigenous Clostridium species colonization in gnotobiotic mice resulted in increased accumulation of colonic Tregs.67 Besides, CD4+CD25+Foxp3+Treg cells are able to suppress osteoclast maturation/differentiation via cytotoxic T-lymphocyte associated protein 4 (CTLA-4) mediated pathway.68 The work of Dar et al., (2018) suggested that Bacillus clausii is known to promote bone formation via increasing Treg cells in the OVX mouse model.69 The probiotic bacteria Lactobacillus acidophilus inhibits the bone loss in OVX mice via modulating Treg-Th17 cell balance.70 Additionally, the report also revealed that the microbial population indeed affects B-cell development, which produces osteoprotegerin, an inhibitor of osteoclasts for effective bone resorption.71. Wnt signaling plays an important role in early embryonic development, organogenesis and tissue morphogenesis. Rubinson et al., (2013) and Wu et al., (2003) reported that intestinal bacteria such as Fusobacterium nucleatum and Bacteroides fragilies are known to activate the Wnt/β- catenin signaling.72, 73 Others have shown that loss of beta-catenin is associated with decreased bone formation.74 Tyagi et al., (2018) also reported that Lactobacillus rhamnosus GG (LGG) treatment regulates bone anabolism via Treg cell-mediated regulation of CD8+T cell Wnt10b production.36 The mechanisms of GM mediated bone homeostasis via immune regulation are shown in Figure 1.
The gut microbiome regulation on bone homeostasis by influencing the endocrine system
The intestinal microbiota acts as a virtual endocrine organ of the body and can engage in an interplay with the endocrine system and have a possible effect on bone homeostasis. Lack of these hormones leads to an increase in bone loss and affecting bone formation.71 Sex hormone deficiency causes intestinal permeability and osteoclastic bone resorption in a TNF and RANKL dependent manner. The work of Li et al., (2016) demonstrated that supplementation of probiotics LGG prevents sex steroid-induced bone loss by restoring intestinal permeability in the OVX mouse model.75 Other have shown that when estrogen deficiency or OVX mice were treated with Lactobacillus acidophilus, the level of bone resorption markers decreased and bone formation was improved.24 Besides, the administration of probiotic L. reuteri prevents bone loss in both estrogen deficiency (OVX) and type 1 diabetes mouse models.76, 77 The work of Yan et al., (2016) also reported that gut microbial colonization in GF mice significantly induced the serum insulin-like growth hormone 1 (IGF-1) level and promote bone formation and growth.78 Therefore, more human studies are needed to confirm that intestinal microbiota can affect bone metabolism via the activity of various hormones.
Role of the microbiome on bone health via the gut-brain axis
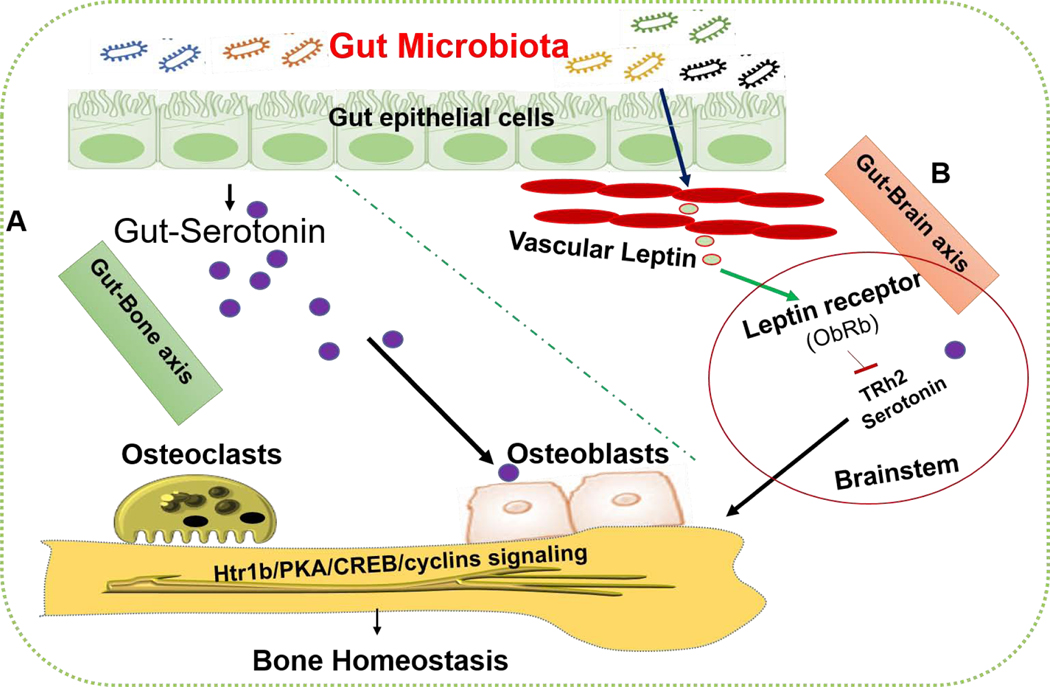
It has been reported that GM has an essential role in the nervous system to synthesize the hormone and neurotransmitters such as serotonin (5- hydroxytryptamine, 5- HT).11 Therefore, 5-HT signaling is important in the regulation of bone development and growth. Ducy and Karsenty et al., (2010) suggested that 5-HT produced in circulation has a negative action on bone metabolism. By contrast, when it is produced from the brain as a neurotransmitter, it promotes bone development.79 Recent studies have reported that intestinal microbiota has a role in regulating the blood levels of 5- HT (2). The bacteria such as Streptococcus, Corynebacterium, and E. coli have been proved to produce 5- HT in animal culture conditions.80 Additionally, Sjogren et al., (2012) have shown that decreased 5-HT levels and increased trabecular bone volume/tissue volume were observed in GF mice.63 Another study demonstrated that 5-HT levels are indeed lowered in GF mice and transplantation of intestinal microbiota can restore the levels of 5-HT in serum and colon.81 Yadav et al., (2008) demonstrated that certain spore-forming microbes can regulate gut serotonin which in turn regulates osteoblast proliferation and bone formation via Htr1b/PKA/CREB/cyclins signaling. The detailed understanding of GM-derived serotonin action on bone metabolism is described in Figure 2.
Figure 2. Effect of gut microbiota on bone homeostasis via biphasic action of serotonin.
(A) Certain microbiota such as spore-forming microbes regulates the level of serotonin in gut, serum and fecal matters. This released serotonin binds to the htr1b receptor of osteoblasts membrane and regulates its proliferation via Htr1b/PKA/CREB/cyclins signaling. (B) Another group of bacterial species (Lactococcus, Mucispirillum, Lactobacillus, and Bifidobacterium) can positively regulate peripheral/vascular leptin level which in turn regulates bone homeostasis via brain serotonin action. htr1b: 5-hydroxytryptamine receptor 1B, PKA: protein kinase A, CREB: cAMP response element-binding protein.
The adipocyte-specific hormone leptin is known to regulate the many physiological processes including bone development, energy homeostasis, etc.82 The growing body of evidence suggested that the gut microbiota regulates different physiological processes through interaction with the brain, which is called the gut-brain axis.83 Queipoortuno et al., (2013) revealed that microbiota such as Lactococcus, Mucispirilum, Lactobacillus, and Bifidobacterium positively regulate the systemic level of leptin.84 This released leptin production binds to leptin receptor (ObRb) that expressed in brainstem neurons, which led to decreased release of brain serotonin levels and may influence bone homeostasis.85, 86 Therefore, the GM affects bone metabolism via diverse potential mechanisms by influencing the host metabolism, immunity, endocrine environment, and gut-brain axis. This could potentially lead to the development of a new era for effective treatment of bone disease as well as future work to be validated in the human model using GM as potential therapeutics in promoting bone health.
The gut microbiota regulation on bone mechanical function
As we discussed that gut microbiota is indispensable for maintaining bone mass and its alternation has been associated with changes in bone mass and microstructural deterioration. Bone strength is defined as the capacity of bone to respond to mechanical demands, is ultimately determined by bone distribution, microarchitecture, material composition and quantity.44 The primary function of bone or skeleton in the body is to resist mechanical forces and impairment of the mechanical strength of bone is therefore challenged by clinical bone disease and may also lead to fragility fracture.87 Bone mineral density (BMD) typically explains bone strength and also have been preferred phenotype to study the bone and gut interaction.44 A recent study by Gus et al., (2017) suggested that alternation of gut microbiota not only changes bone mass but also impairs bone mechanical properties.88 In this study, they examined bone strength using two different mouse models such as toll-like receptor 5-deficient mouse [TLR5KO]) and WT (C57Bl/6) mice were treated selected antibiotics (ampicillin and neomycin) to deplete the microbiota (ΔMicrobiota) independently. Interestingly, the data have shown that femur bending strength was less in ΔMicrobiota mice than untreated WT mice. However, there were small differences in whole bone bending strength were observed between WT and TLR5KO mice.88 In the other study, demonstrated that probiotics strain Lactobacillus helveticus administration was reported to improve the bone strength in a three-point bending test of Ovariectomy rat model.89
Relationship between gut microbiota and dietary phosphorus metabolism
Diet is an essential environmental factor that modulates or support the digestive system and other organ function, but also shape a healthy microbial ecosystem in the GI tract.90 Among the dietary components, phosphorus (P) is an essential nutrient that helps in both microbial and host metabolism, for example in bone development, cellular signaling, energy metabolism, membrane protein synthesis and also provides a barrier against pathogens in the gut.91,92 Emerging evidence suggested that P can be stored as polyphosphates in bacterial cells and used as energy and metabolic processes.90, 93 Other have shown that P acts as a coenzyme for bacterial synthesis of fibrolytic enzymes, which is essential for dietary fibre degradation in gut.94 Komisarczuk et al. demonstrated that P supply through diet is essential for intestinal SCFA production. Further, they demonstrated that P deficiency caused a reduction in SCFA synthesis due to reduced fermentation of cellulose in the GI tract of ruminant animals in sheep.96 Others have also shown that dietary calcium phosphate (CaPi) has a positive effect on gut eubiosis (by increasing the number of ileal and fecal Lactobacillus acidophilus) and strongly protect against Salmonella enteritidis infection in a rat model.96 In a recent investigation by Mann et al., reported that feeding CaP-rich diets promoted bacterial growth and proliferation of Lactobacillus at the gastric pars non-glandularis of the stomach in pigs.97 Generally, Lactobacillus is said to produce very effective bacteriocidin and organic acid, which inhibit the growth of potential pathogens such as Escherichia coli.98–100 Therefore, CaP mediated increase of Lactobacillus in the GI tract might be essential for promoting gut microbial eubiosis and gut barrier function. However, no direct evidence is yet available demonstrating that the relationship between gut microbiota and P metabolism in bone development in mice and humans. Therefore, future research is warranted to study the role of dietary P metabolism on gut microbiota eubiosis, gut barrier integrity for better bone mass formation in osteoporotic patients.
Role of gut-microbiome on skeletal muscle mass and function
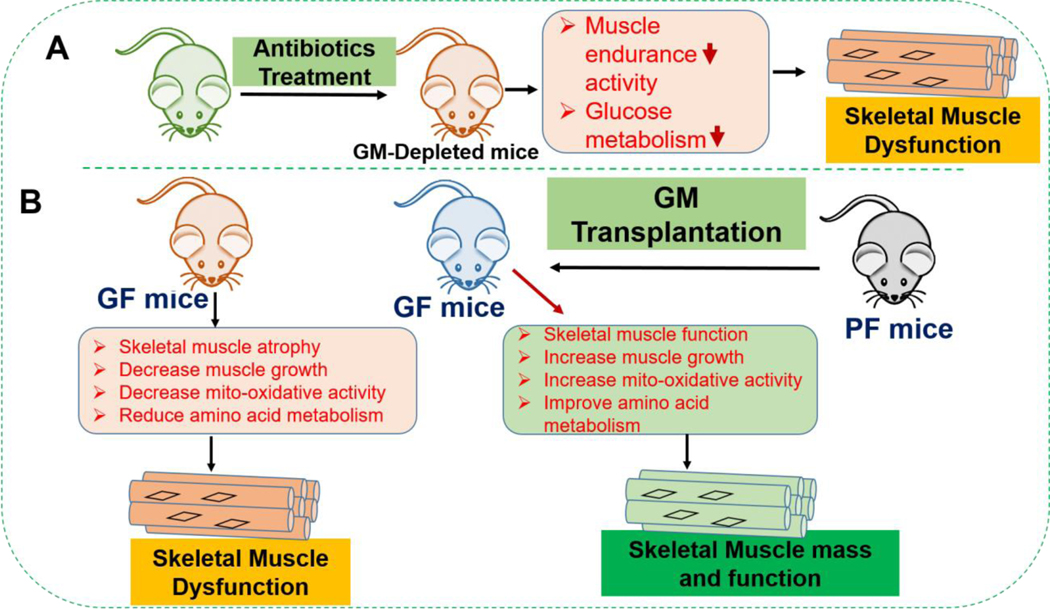
Skeletal muscle function is regulated by central nervous function via a neuro-muscular transmission.101 and it displays marked plasticity and can respond to several environmental stimuli such as exercise and nutrition. Skeletal muscle is one of the important organs involved in glucose homeostasis and fatty acid oxidation.102 Dysfunction in the skeletal muscle is associated with sarcopenia, muscle atrophy and consequently, it causes metabolic disorders.103 It has been established that the GM is involved in the onset of several pathophysiological conditions such as diabetes, cancer, obesity, and osteoporosis. However, it is still not clear how GM influences skeletal muscle function. The work of Nay et al., (2019) demonstrated that gut bacteria are indispensable for the host optimal skeletal muscle function.104 In the study, they found that GM-depleted mice following administration of broad-spectrum antibiotics, muscle endurance capacity was severely affected. Also, glucose metabolism was severely affected by GM-depletion in mice104 Others have shown that the gut microbiota is required for skeletal muscle mass and function by comparing the skeletal muscle of germ-free (GF) mice that lacked a GM to the skeletal muscle of pathogen-free (PF) mice that has a GM102 supporting the notion that gut bacteria is essential for maintaining skeletal muscle mass. In the study, they found that GF mice skeletal muscle showed atrophy, reduced expression of IGF-1, and skeletal muscle growth and mitochondrial function-related genes. GF mice also showed reduced serum choline and altered amino acid mentalism in comparison to PF mice. Transplanting the GM from PF mice into GF mice increased skeletal muscle mass and improved oxidative metabolic capacity.102 Taken together, these studies strongly support the role of GM that influences skeletal muscle mass and function in mice. One implication that might be inferred from these studies is that maintaining a healthy gut microbiota is important to the health of the muscles. The detailed study of GM’s role in skeletal muscle mass and function is shown in Figure 3.
Figure 3: The gut microbiota influences skeletal muscle mass and function in mice.
(A) The consequence of gut microbiota depletion after antibiotic treatment for 21 days, results in muscle endurance activity as well as glucose metabolism was severely affected. (B) It compares the skeletal muscle phenotype and function between GF mice and PF mice. Compared to PF mice, GF mice have increased muscle atrophy and reduced muscle growth, mitochondrial oxidative activity and amino acid metabolism. Transplantation of GM from PF mice to GF mice improved the skeletal muscle mass and oxidative metabolic function. GM: Gut microbiota, GF: Germ-free, PF: Pathogen free mice.
Translational potential and concluding remarks
The intimate association between the gut microbiota and skeletal metabolic processes suggests that the identification of important gut microbiota to be characterized and that have features for great clinical potential.105 In the current scenario, the majority of studies have focused on the preventive or protective effect of probiotics, prebiotics, and antibiotics in different diseases.106 These types of treatment have been successfully tested in the clinical conditions in many human diseases such as hypercholesterolemia, ulcerative colitis, and obesity, etc.107–110 Additionally, the antibiotic based treatment that targets microbiome has provided greater promise for effective therapy.106 However, not much focus has been directed toward understanding the potential of GM regulation as a treatment for musculoskeletal disease. However, this finding needs to be further validated in human clinical studies. In the future, the identification of therapeutic microbes could provide a promise for effective regulation of bone metabolism in musculoskeletal disease. Furthermore, pathogenic mutations that cause genetic bone diseases and its relation with intestinal microbial flora remain warranted and it needs to be further investigated.
Highlights.
✓ The gut microbiota promotes bone formation by influencing intestinal short-chain fatty acids metabolism.
✓ The gut microbiota promotes bone homeostasis by maintaining gut-barrier integrity and the immune system.
✓ Supplementation of prebiotic diet improves the microbial composition and short-chain fatty acids production leads to activation of regulatory T-cells function and gut protection.
✓ Supplementation of probiotics prevents bone loss in both sex steroid-deficiency and type-1 diabetic mouse model.
✓ The gut microbiota regulates osteoblast proliferation and bone formation via gut serotonin dependent Htr1b/PKA/CREB/cyclins signaling
✓ The gut microbiota promotes skeletal muscle mass and function through increased Insulin-like growth factor-1 expression and mitochondrial function.
Acknowledgments
This study was supported, in part, by NIH grants HL-107640 and AR-067667 to NT.
Footnotes
Disclosure of Potential Conflicts of Interest
All authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.D’Amelio P, Sassi F. Gut Microbiota, Immune System, and Bone. Calcified Tissue International. 2018;102(4);415–425. 10.1007/s00223-017-0331-y [DOI] [PubMed] [Google Scholar]
- 2.Li L, Rao S, Cheng Y, Zhuo X, Deng C, Xu N, Yang L. Microbial osteoporosis: The interplay between the gut microbiota and bones via host metabolism and immunity. Microbiology Open. 2019;8(8). 10.1002/mbo3.810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan J, Charles JF. Gut Microbiome and Bone: To Build, Destroy or Both? Curr Osteoporos Rep. 2017;15(4);376–384. 10.1007/s11914-017-0382-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Human Microbiome Project Consortium (2012) Structure, function and diversity of the healthy human microbiome. Nature 486:207–214. 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins SM. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol. 2014;11(8):497–505. 10.1038/nrgastro.2014.40. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor EM. The role of gut microbiota in nutritional status. Curr Opin Clin Nutr Metab Care. 2013;16(5):509–516. 10.1097/MCO.0b013e3283638eb3. [DOI] [PubMed] [Google Scholar]
- 7.Sommer F, Bäckhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013April;11(4):227–38. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 8.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012September;489(7415);242–249. 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 9.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014March27;157(1):121–41. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006December21;444(7122):1027–31. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2017January;112(Pt B):399–412. doi: 10.1016/j.neuropharm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 12.de Goffau MC, Fuentes S, van de Bogert B, Honanken H, de Vos WM, Welling GW, Hyöty H, Harmsen HJ. Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia. 2014August;57(8):1569–77. doi: 10.1007/s00125014-3274-0. [DOI] [PubMed] [Google Scholar]
- 13.Qin J, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012September;490:55–60. 10.1038/nature11450 [DOI] [PubMed] [Google Scholar]
- 14.Lyra A, et al. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16SrRNA gene phylotype quantification. World J Gastroenterol. 2009December21;15(47):5936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Q, et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015March11;6:6528. doi: 10.1038/ncomms7528. [DOI] [PubMed] [Google Scholar]
- 16.Yin J, et al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level inpatients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. 2015November23;4(11). pii: e002699. doi: 10.1161/JAHA.115.002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015August;21(8):895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- 18.Behera J, et al. Hydrogen Sulfide Promotes Bone Homeostasis by Balancing Inflammatory Cytokine Signaling in CBS-Deficient Mice through an Epigenetic Mechanism. Sci Rep. 2018October15;8(1):15226. doi: 10.1038/s41598-018-33149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looker AC, Borrud LG, Dawson-Hughes B Shepherd JA, & Wright N C. Osteoporosis or low bone mass at the femur neck or lumbar spine in older adults: United States, 2005–2008. NCHS Data Brief. 2012. April; (93). 1–8. [PubMed] [Google Scholar]
- 20.Weaver CM, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos Int. 2016April;27(4):1281–1386. doi: 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charles JF, Ermann J, Aliprantis. The intestinal microbiome and skeletal fitness: Connecting bugs and bones. Clin Immunol. 2015August;159(2):163–9. doi: 10.1016/j.clim.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace TC, Marzorati M, Spence L, Weaver CM, Williamson PS. New frontiers in fibers: Innovative and emerging research on the gut microbiome and bone health. J Am Coll Nutr. 2017. Mar-Apr;36(3):218–222. doi: 10.1080/07315724.2016.1257961. [DOI] [PubMed] [Google Scholar]
- 23.Li JY, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016June1;126(6):2049–63. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohlsson C, et al. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014March17;9(3):e92368. doi: 10.1371/journal.pone.0092368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parveneh K, et al. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate SPARC and BMP-2 genes in rats with bone loss resulting from Ovariectomy. Biomed Res Int. 2015;2015:897639. doi: 10.1155/2015/897639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rozneberg S, et al. Effects of dairy products consumption on health: benefits and beliefs– a commentary from the Belgian bone club and the European society for clinical and economic aspects of osteoporosis, osteoarthritis, and musculoskeletal diseases. Calcif Tissue Int. 2016January;98(1):1–17. doi: 10.1007/s00223-015-0062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Vieyra MI, Del Real A, Lopez MG. Agave fructans: Their effect on mineral absorption and bone mineral content. J Med Food. 2014November;17(11):1247–55. doi: 10.1089/jmf.2013.0137. [DOI] [PubMed] [Google Scholar]
- 28.David LA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014January23;505(7484):559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ley RE et al. Microbial ecology: human gut microbes associated with obesity. Nature.2006December21;444(7122):1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 30.Devkota S, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012July5;487(7405):104–8. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira CM, et al. The central role of the gut microbiota in chronic inflammatory diseases. J Immunol Res. 2014;2014:689492. doi: 10.1155/2014/689492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, et al. Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic lactobacillus reuteri. Endocrinology. 2015September;156(9):3169–82. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003July;133(7 Suppl):2485S–2493S. doi: 10.1093/jn/133.7.2385S. [DOI] [PubMed] [Google Scholar]
- 34.Sanford JA, et al. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci Immunol. 2016October28;1(4). pii: eaah4609. doi: 10.1126/sciimmunol.aah4609. [DOI] [PubMed] [Google Scholar]
- 35.Lucas S, et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 2018January4;9(1):55. doi: 10.1038/s41467-01702490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyagi AM, et al. The Microbial Metabolite Butyrate Stimulates Bone Formation via T Regulatory Cell-MediatedRegulation of WNT10B Expression. Immunity. 2018December18;49(6):1116–1131.e7. doi: 10.1016/j.immuni.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014May;30(3):332–8. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doerner KC, Takamine F, LaVoie CP, Mallonee DH, Hylemon PB. Assessment of fecal bacteria with bile acid 7 alpha- dehydroxylating activity for the presence of bai- like genes. Appl Environ Microbiol. 1997March; 63(3):1185–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho SW, et al. Positive regulation of osteogenesis by bile acid through FXR. J Bone Miner Res. 2013October;28(10):2109–21. doi: 10.1002/jbmr.1961. [DOI] [PubMed] [Google Scholar]
- 40.Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: Role of glucagon and GLP- 1 in health and disease. Physiological Reviews. 2015April;95(2);513–548. doi: 10.1152/physrev.00013.2014. [DOI] [PubMed] [Google Scholar]
- 41.Adachi R, et al. Selective activation of vitamin D receptor by lithocholic acid acetate, abile acid derivative. J Lipid Res. 2005January;46(1):46–57. [DOI] [PubMed] [Google Scholar]
- 42.Ceryak S, Bouscarel B, Malavolti M, Fromm H. Extrahepatic deposition and cytotoxicity of lithocholic acid: Studies in two hamster models of hepatic failure and cultured human fibroblasts. Hepatology. 1998February;27(2):546–56. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-Gaspa S, et al. Lithocholic acid downregulates vitamin D effects in human osteoblasts. Eur J Clin Invest. 2010January;40(1):25–34. doi: 10.1111/j.13652362.2009.02230.x. [DOI] [PubMed] [Google Scholar]
- 44.Bone Medina-Gomez C. and the gut microbiome: a new dimension. Jour of Lab and Prec Med. 2018. November; 3 [Google Scholar]
- 45.Xu X, et al. Intestinal microbiota: a potential target for the treatment of postmenopausal osteoporosis. Bone Res. 2017. Oct 4;5:17046. doi: 10.1038/boneres.2017.46.eCollection2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hara T, et al. Effects of ovariectomy and/or dietary calcium deficiency on bone dynamics in the rat hard palate, mandible and proximal tibia. Arch Oral Biol. 2001May;46(5):44351. [DOI] [PubMed] [Google Scholar]
- 47.Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep. 2015April;13(2):125–30. doi: 10.1007/s11914-015-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whisner CM, et al. Soluble maize fiber affects short-term calcium absorption in adolescent boys and girls: a randomized controlled trial using dual stable isotopic tracers. Br J Nutr. 2014August14;112(3):446–56. doi: 10.1017/S0007114514000981. [DOI] [PubMed] [Google Scholar]
- 49.Whisner CM, et al. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: a double-blind cross-over trial. Br J Nutr. 2013October;110(7):1292–303. doi: 10.1017/S000711451300055X. [DOI] [PubMed] [Google Scholar]
- 50.Whisner CM, et al. Soluble Corn Fiber Increases Calcium Absorption Associated with Shifts in the Gut Microbiome: A Randomized Dose-Response Trial in Free-Living Pubertal Females. J Nutr. 2016July;146(7):1298–306. doi: 10.3945/jn.115.227256. [DOI] [PubMed] [Google Scholar]
- 51.Jakeman SA, et al. Soluble corn fiber increases bone calcium retention in postmenopausal women in a dose-dependent manner: a randomized crossover trial. Am J Clin Nutr. 2016September;104(3):837–43. doi: 10.3945/ajcn.116.132761. [DOI] [PubMed] [Google Scholar]
- 52.Weaver CM, et al. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J Agric Food Chem. 2011June22;59(12):6501–10. doi: 10.1021/jf2009777. [DOI] [PubMed] [Google Scholar]
- 53.Nzeusseu A, et al. Inulin and fructo-oligosaccharides differ in their ability to enhance the density of the cancellous and cortical bone in the axial and peripheral skeleton of growing rats. Bone. 2006March;38(3):394–9. [DOI] [PubMed] [Google Scholar]
- 54.Gilman J, Cashman KD. The effect of probiotic bacteria on transepithelial calcium transport and calcium uptake in human intestinal-like Caco-2 cells. Curr Issues Intest Microbiol. 2006March;7(1):1–5. [PubMed] [Google Scholar]
- 55.Chelakkot C, Ghim J, Ho Ryu S. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp & Mol Med, 2018August; 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clemente JC, et al. The impact of the gut microbiota on human health: an integrative view. Cell. 2012March16;148(6):1258–70. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamilton MK, et al. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region-dependent. Am J Physiol Gastrointest Liver Physiol. May15;308(10):G840–51. doi: 10.1152/ajpgi.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007July;56(7):1761–72. [DOI] [PubMed] [Google Scholar]
- 59.Hou GQ, et al. Lipopolysaccharide (LPS) promotes osteoclast differentiation and activation by enhancing the MAPK pathway and COX-2 expression in RAW264.7 cells. Int J Mol Med. 2013August;32(2):503–10. doi: 10.3892/ijmm.2013.1406. [DOI] [PubMed] [Google Scholar]
- 60.Chongwatpol P, et al. Implications of compromised zinc status on bone loss associated with chronic inflammation in C57BL/6 mice. J Inflamm Res. 2015; 8: 117–128. doi: 10.2147/JIR.S82261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palm NW, de Zoete MR, Flavell RA. Immune-microbiota interactions in health and disease. Clin Immunol. 2015August;159(2):122–127. doi: 10.1016/j.clim.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peterson CT, et al. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin Exp Immunol. 2015March;179(3):363–77. doi: 10.1111/cei.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sjögren K, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012June;27(6):1357–67. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duque G, et al. Interferon-gamma plays a role in bone formation in vivo and rescues osteoporosis in ovariectomized mice. J Bone Miner Res. 2011July;26(7):1472–83. doi: 10.1002/jbmr.350. [DOI] [PubMed] [Google Scholar]
- 65.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009October30;139(3):485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goto Y, et al. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014April17;40(4):594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atarashi K, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011January21;331(6015):337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kong YY, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999November18;402(6759):304–9. [DOI] [PubMed] [Google Scholar]
- 69.Dar HY, Pal S, Shukla P, et al. Bacillus clausii inhibits bone loss by skewing Treg-Th17 cell equilibrium in postmenopausal osteoporotic mice model. Nutrition 2018;54:118–28 [DOI] [PubMed] [Google Scholar]
- 70.Dar HY, Shukla P, Mishra PK, et al. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating TregTh17 cell balance. Bone Rep 2018;8:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, et al. The impact of the intestinal microbiome on bone health. Intractable RareDis Res. 2018August; 7(3): 148–155. doi: 10.5582/irdr.2018.01055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rubinstein MR, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E- cadherin/β- catenin signaling via its FadA adhesion. Cell Host Microbe. 2013August14;14(2):195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu S, et al. Bacteroides fragilis enterotoxin induces c- Myc expression and cellular proliferation. Gastroenterology. 2003February;123(2):392–400. [DOI] [PubMed] [Google Scholar]
- 74.Holmen SL, et al. The essential role of beta- catenin in postnatal bone acquisition. J Biol Chem. 2005June3;280(22):21162–8. [DOI] [PubMed] [Google Scholar]
- 75.Li JY, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016June1;126(6):2049–63. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Britton RA, et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model.J Cell Physiol. 2014November;229(11):1822–30. doi: 10.1002/jcp.23636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J, et al. Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic Lactobacillus reuteri. Endocrinology. 2015September;156(9):3169–82. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan J, et al. Gut microbiota induces IGF-1 and promotes bone formation and growth. Proc Natl Acad Sci U S A. 2016November22;113(47):E7554–E7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ducy P, Karsenty G. The two faces of serotonin in bone biology. J Cell Biol. 2010October4;191(1):7–13. doi: 10.1083/jcb.201006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roshchina VV. New trends and perspectives in the evolution of neurotransmitters in the microbial, plant, and animal cells. Adv Exp Med Biol. 2016;874:25–77. doi: 10.1007/978-3-319-20215-0_2. [DOI] [PubMed] [Google Scholar]
- 81.Yano JM, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015April9;161(2):264–76. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Upadhyay J, Farr OM, Mantzoros CS. The role of leptin in regulating bone metabolism. Metabolism. 2015January;64(1):105–13. doi: 10.1016/j.metabol.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019October1;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 84.Queipo-Ortuño MI Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013May28;8(5):e65465. doi: 10.1371/journal.pone.0065465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scott MM, et al. Leptin targets in the mouse brain. J Comp Neurol. 2009June10;514(5):518–32. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Charnay Y, et al. Intracerebroventricular infusion of leptin decreases serotonin transporter binding sites in the frontal cortex of the rat. Neurosci Lett. 2000April7;283(2):89–92. [DOI] [PubMed] [Google Scholar]
- 87.Hernandez CJ. Bone Mechanical Function and the Gut Microbiota. Adv Exp Med Biol.2017;1033: 249–270. doi: 10.1007/978-3-319-66653-2_12 [DOI] [PubMed] [Google Scholar]
- 88.Guss JD, Horsfield MW, Fontenele FF, Sandoval TN. Alterations to the Gut Microbiome Impair Bone Strength and Tissue Material Properties. J Bone Miner Res. 2017June; 32(6):1343–1353. doi: 10.1002/jbmr.3114.Epub2017Mar27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parvaneh M, Karimi G, Jamaluddin R, et al. Lactobacillus helveticus (ATCC 27558) upregulates Runx2 and Bmp2 and modulates bone mineral density in ovariectomy-induced bone loss rats. Clin Interv Aging. 2018; August30; 13:1555–64. doi: 10.2147/CIA.S169223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heyer CM, Weiss E, Schmucker S, Rodehutscord M. The impact of phosphorus on the immune system and the intestinal microbiota with special focus on the pig. Nutr Res Rev. 2015June; 28(1):67–82. doi: 10.1017/S0954422415000049. [DOI] [PubMed] [Google Scholar]
- 91.Wood HG, Clark JE. Biological aspects of inorganic polyphosphates. Annu Rev Biochem. 1988;57:235–60. doi: 10.1146/annurev.bi.57.070188.001315 [DOI] [PubMed] [Google Scholar]
- 92.Varley PF, McCarney C, Callan JJ, O’Doherty JV. Effect of dietary mineral level and inulin inclusion on phosphorus, calcium and nitrogen utilisation, intestinal microflora and bone development. J Sci Food Agric. 2010November; 90(14):2447–54. doi: 10.1002/jsfa.4105. [DOI] [PubMed] [Google Scholar]
- 93.Anand A, Aoyagi H. Estimation of microbial phosphate-accumulation abilities. Sci Rep. 2019March19; 9(1):4879. doi: 10.1038/s41598-018-37752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Francis GL, Gawthorne JM, Storer GB. Factors affecting the activity of cellulases isolated from the rumendigesta of sheep. Appl Environ Microbiol 36, 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Komisarczuk S, Merry RJ, McAllan AB. Effect of different levels of phosphorus on rumen microbial fermen-tation and synthesis determined using a continuous culture technique. Br J Nutr. 1987March;57(2):279–90. doi: 10.1079/bjn19870033 [DOI] [PubMed] [Google Scholar]
- 96.Bovee-Oudenhoven IM, Wissink ML, Wouters JT et al. Dietary Calcium Phosphate Stimulates Intestinal Lactobacilli and Decreases the Severity of a Salmonella Infection in Rats. J Nutr. 1999March;129(3):607–12. doi: 10.1093/jn/129.3.607 [DOI] [PubMed] [Google Scholar]
- 97.Mann E, Schmitz-Esser S, Zebeli Q2, Wagner M. Mucosa-associated bacterial microbiome of the gastrointestinal tract of weaned pigs and dynamics linked to dietary calcium-phosphorus. PLoS One. 2014January23; 9(1):e86950. doi: 10.1371/journal.pone.0086950.90:1317 doi: 10.1371/journal.pone.0086950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chauvière G, Coconnier MH, Kernéis S, Fourniat J. Adhesion of human Lactobacillus acidophilus strain LB to human enter-ocyte-like Caco-2 cells. J Gen Microbiol. 1992August; 138 Pt 8:1689–96. doi: 10.1099/00221287-138-8-1689 [DOI] [PubMed] [Google Scholar]
- 99.Walter J1, Britton RA, Roos S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc Natl Acad Sci U S A. 2011March15; 108 Suppl 1:4645–52. doi: 10.1073/pnas.1000099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Vuyst L, Callewaert R, Crabbe K. Primary metabolite kinetics of bacteriocin biosynthesis by Lacto-bacillus amylovorus and evidence for stimulation of bacter-iocin production under unfavourable growth conditions. Microbiology (1996), 142, 817–827. Doi: 10.1099/00221287-142-4-817. [DOI] [PubMed] [Google Scholar]
- 101.Tintignac LA, Brenner HR, Ruegg MA. Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol Rev. 2015July;95(3):809–52. doi: 10.1152/physrev.00033.2014. [DOI] [PubMed] [Google Scholar]
- 102.Lahiri S, et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med. 2019July23;11(502). pii: eaan5662. doi: 10.1126/scitranslmed.aan5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kelley DE, et al. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999December;277(6):E1130–41. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 104.Nay K, et al. Gut bacteria are critical for optimal muscle function: a potential link with glucose homeostasis. Am J Physiol Endocrinol Metab. 2019July1;317(1):E158–E171. doi: 10.1152/ajpendo.00521.2018. [DOI] [PubMed] [Google Scholar]
- 105.Chen YC, et al. Association Between Gut Microbiota and Bone Health: Potential Mechanisms and Prospective. J Clin Endocrinol Metab. 2017October1;102(10):3635–3646. doi: 10.1210/jc.2017-00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Olle B. Medicines from microbiota. Nature Biotech. 2013April; 31; 309–315. [DOI] [PubMed] [Google Scholar]
- 107.Yoshimoto A et al. Effect of prenatal administration of low dose antibiotics on gut microbiota and body fat composition of newborn mice. J Clin Biochem Nutr 2018March;62(2):155–160. doi: 10.3164/jcbn.17-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nguyen TD, Kang JH, Lee MS. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int J Food Microbiol. 2007February15;113(3):358–61. [DOI] [PubMed] [Google Scholar]
- 109.Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora Lancet. 1989January21;1(8630):164. DOI: 10.1016/s0140-6736(89)91183-5 [DOI] [PubMed] [Google Scholar]
- 110.Kadooka Y. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010June;64(6):636–43. doi: 10.1038/ejcn.2010.19. [DOI] [PubMed] [Google Scholar]