Abstract
Background:
Adjuvant chemotherapy, postoperative radiation (PORT), and prophylactic cranial irradiation (PCI) have been individually examined in limited-stage small cell lung cancer (SCLC). There is a paucity of data on the effectiveness of each adjuvant treatment modality when used in combination after surgical resection of SCLC.
Methods:
Data were collected from 5 cancer centers on all patients with limited-stage SCLC who underwent surgical resection between 1986 and 2019. Univariate and multivariable models were conducted to identify predictors of long-term outcomes, focusing on freedom from recurrence and survival benefit of adjuvant chemotherapy, PORT, and PCI.
Results:
A total of 164 patients were analyzed. Multivariable Cox regression analysis did not identify any adjuvant therapies to significantly influence recurrence in this cohort. Specifically, PORT was not associated with a significant influence on locoregional recurrence and PCI was not significantly associated with intracranial outcomes. Adjuvant chemotherapy improved survival in all stage I through III disease (hazard ratio, 0.49; 95% confidence interval, 0.29–0.81; P = .005) and even in pathologically node negative patients (hazard ratio, 0.49; 95% confidence interval, 0.27–0.91; P = .024). Although PCI was found to improve survival in univariate analysis, it was not significant in a multivariable model. PORT was not found to affect survival on either univariate or multivariable analysis.
Conclusions:
This is among the largest multi-institutional studies on surgically resected limited-stage SCLC. Our results highlight survival benefit of adjuvant chemotherapy, but did not identify a statistically significant influence from mediastinal PORT or PCI in our cohort. Larger prospective studies are needed to determine the benefit of PORT or PCI in a surgically resected limited-stage SCLC population.
Keywords: small cell lung cancer, adjuvant chemotherapy, adjuvant radiation, prophylactic, cranial irradiation
Graphical Abstract
Disease-free survival of node negative patients with and without adjuvant chemotherapy.
Small cell lung cancer (SCLC) is an aggressive malignancy, with more than 180,000 cases diagnosed annually worldwide, comprising roughly 13% of thoracic malignancies.1,2 Combined modality therapy with concurrent chemoradiation has been the standard of care.3–6 Recently, there was renewed interest in surgical resection of limited-stage SCLC, which is typically defined as disease confined to 1 hemithorax with regional lymph node metastases, equivalent to stage I through III of the tumor, node, and metastases staging criteria system.7 A 2010 analysis of the Surveillance, Epidemiology, and End Results database demonstrated improved survival for patients with localized and regional disease after resection; median survival increased from 15 months to 42 months and from 12 months to 22 months, respectively.8 A more recent retrospective review also demonstrated superior results of surgery for stage I SCLC, with a 5-year survival of 62% after resection compared with 25% with nonsurgical therapy.9 Current National Comprehensive Cancer Network guidelines recommend that patients with suspected limited-stage disease undergo invasive mediastinal staging and subsequent resection if there is no involvement of mediastinal lymph nodes.10,11 Despite this recommendation, surgical therapy has been remarkably underutilized for SCLC even in potentially appropriate patients,12,13 Thus, there are relatively little data on the outcomes of surgically resected cohorts and the effect of combined modality therapies such as chemotherapy, postoperative radiation (PORT), prophylactic intracranial radiation (PCI), and their influence on outcomes.
The vast majority of clinical trials evaluating the roles of chemotherapy,14–17 chest radiation,18 and PCI18–21 have been conducted in patients without surgery as a part of the treatment modality. Guideline recommendations for adjuvant therapies in limited-stage disease are largely based on retrospective studies.8,9,22 Although adjuvant chemotherapy appears to improve survival in most studies,23,24 it is unclear what influence PORT may have for node-positive patients.24,25 PCI is also controversial because it has been shown in some studies to be beneficial in all limited-stage disease, but there may be no benefit in patients with stage I disease,24,26,27 in which case, surveillance strategy with close imaging can be a viable option given the known neurotoxic effects of PCI. Nevertheless, there is a lack of large studies that evaluate all the adjuvant treatment modalities in postresection patients.
In this study, we combined institutional data from 5 large-volume cancer centers across the United States and Canada to generate a large multi-institutional cohort in an attempt to gain insight into the utility of adjuvant therapies in resected limited-stage SCLC. We hypothesized that combined modality therapy would improve oncologic outcomes after surgical resection of SCLC, and the purpose of this study was to examine the survival and recurrence patterns following various adjuvant therapies in limited-stage SCLC after surgical resection.
MATERIALS AND METHODS
Patient Cohort
This multi-institutional retrospective cohort study received approval from the institutional review board at the University of Texas MD Anderson Cancer Center (protocol PA18–0055) and interinstitutional data user agreements were obtained from all involved institutions. Patients who are aged 18 years or older with the diagnosis of limited-stage SCLC and who underwent upfront surgical resection were retrospectively identified. In this study, limited-stage SCLC was defined as T1 2N0 2M0 disease confirmed on the final pathology. Considering the fact that resections for SCLC are rather rare, we collected all available data, regardless of the time period, to capture the largest patient cohort. Although this approach has its limitations, we were compelled to include all available data to maximize the patient cohort. The resulting aggregate multi-institutional data were then analyzed. Patients who received any induction therapy were excluded. Preoperative staging procedures varied within and between institutions over time because they represented trends in care at that time and also individual surgeon’s judgment. Surgical resections were categorized into sublobar resections (wedge or segmentectomy), lobectomy, or greater than lobectomy (bilobectomy, sleeve resections, and pneumonectomy). All pathologic stages were reported according to the seventh edition of the tumor, node, and metastases staging criteria by the American Joint Committee on Cancer, consistent with the time period of the study conception.28
Pathologic confirmation of SCLC was required. In cases of mixed histology, a predominant SCLC histology was required to meet inclusion criteria. There were 2 patients who died within 30 days of surgery (1.08%), and were excluded because they were unlikely to have been administered adjuvant therapies. Baseline clinicopathological variables were collected and analyzed, as well as treatment variables such as delivery of adjuvant chemotherapy, mediastinal PORT, and PCI.
Statistical Analysis
Continuous data were compared using Wilcoxon rank-sum test, and categorical data were compared using the χ2 or Fisher exact test, where appropriate. Median potential follow-up time duration was calculated using the reverse Kaplan-Meier method.29 Overall survival (OS) was defined as the time from surgery to death from any cause or last follow-up visit, and recurrence-free survival (RFS) was defined as the time from surgery to first recurrence, death, or last follow-up visit. Freedom from recurrence (FFR) was defined as the time from surgery to recurrence or the last follow-up. To identify the relationship between various treatment modalities and disease control, we assessed the following outcomes: the benefit of adjuvant chemotherapy by the analysis of FFR, the benefit of adjuvant PORT using the analysis of freedom from locoregional recurrence (LRR), and the benefit of PCI by assessing the freedom from brain recurrence. The Kaplan-Meier method was used to estimate time-to-event outcomes, and the log-rank test was used to compare groups. To account survival differences by time period, we compared survival by 4 decades: 1986–1989, 1990–1999, 2000–2009, and 2010–2019.
Cox proportional hazard model was used to identify clinicopathological factors associated with outcomes of interest. For the multivariable model, we included all factors with a P value<.2 in the univariate analysis. Due to its clinical importance, adjuvant chemotherapy was included in the final models for OS and FFR models a priori, whereas adjuvant PORT was included a priori for OS and freedom from LRR, and PCI was included a priori for OS and freedom from brain recurrence. All statistical analyses were performed using IBM SPSS version 24.0 (IBM-SPS Inc, Armonk, NY) and RStudio software version 1.1.463 (RStudio Inc, Boston, Mass).
RESULTS
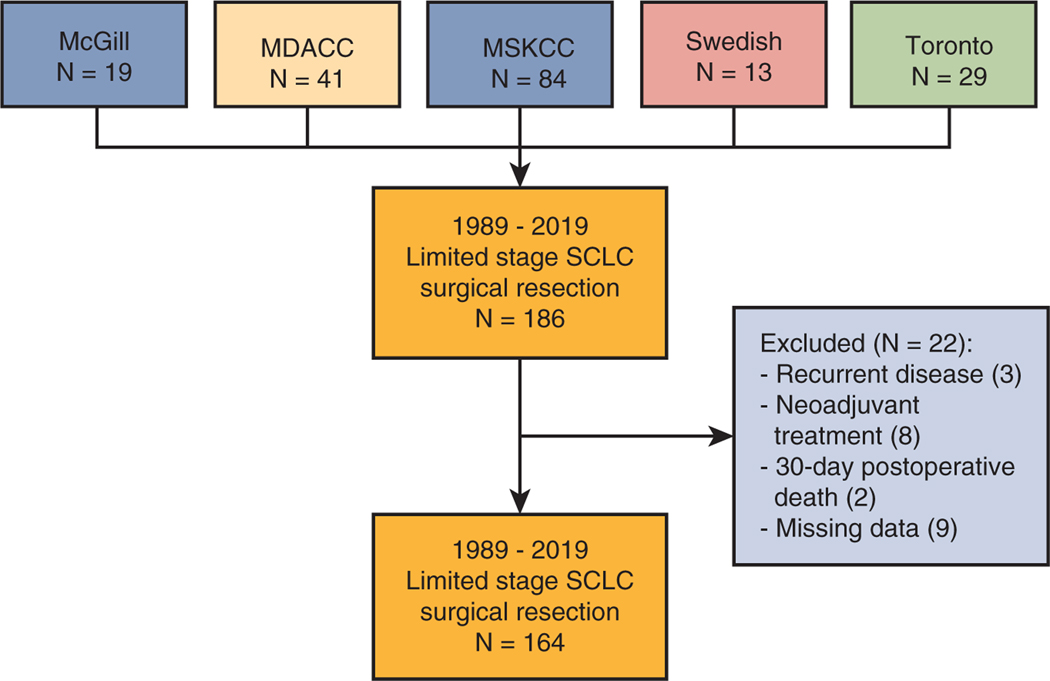
We identified 164 out of 186 patients who met inclusion criteria for analysis (Figure E1). Patient baseline, operative, and perioperative characteristics are summarized in Table 1. In general, SCLC patients were elderly (median age, 68 years), mostly smokers (88%), and underwent lobectomy (62%). There were 83% of patients who completed positron-emission tomography-computed tomography, 63% completed mediastinal staging with mediastinoscopy or endobronchial ultrasound, and 67% completed brain magnetic resonance imaging before surgery. Patients were staged as pathologic stage I, II, or III in 82 (50%), 43 (27%), and 39 (24%) instances. The 90-day mortality rate was 4% (6 out of 164). The examination of final pathology revealed that 154 (94%) of specimens were confirmed to be pure SCLC, and for 10 (6%) with mixed histology, SCLC was the predominant histology. Survival improved over time. Median OS for each decade was as follows: 26 months for 1986–1989, 37 months for 1990–1999, 60 months for 2000–2010, and 59 months for 2010–2019. The latter 2 decades (2000–2009 and 2010–2019) had significantly better OS compared with 1986–1989. Upon Cox multivariable analysis for OS, coronary artery disease (hazard ratio [HR], 3.33; 95% confidence interval [CI], 1.6–6.96; P = .001) and nodal disease (HR, 1.61; 95% CI, 1.00–2.60; P = .049) were independently associated with poor survival, whereas lobectomy (HR, 0.54; 95% CI, 0.34–0.86; P = .010) and adjuvant chemotherapy (HR, 0.49; 95% CI, 0.27–0.91; P = .024) were associated with improved survival (Table 2). Recurrence patterns were as follows: there were a total of 50 (30.5%) recurrences, 23 (14%) locoregional recurrences, 33 (20.1%) distant recurrences, and 12 (7.3%) brain recurrences.
TABLE 1.
Baseline and perioperative characteristics
| Variable | Overall (N = 164) |
|---|---|
| Age | 68 (62–73) |
| Male sex | 83 (51) |
| Smoker | 145 (88) |
| COPD | 56 (34) |
| CAD | 18 (11) |
| CHF | 3 (2) |
| DM | 20 (12) |
| Tumor size (cm) | 2.1 (1.5–3.5) |
| pT | |
| pT1 | 90 (55) |
| pT2 | 50 (31) |
| pT3 | 16 (10) |
| pT4 | 2 (1) |
| pTx | 6 (4) |
| pN | |
| pN0 | 102 (62) |
| pN1 | 31 (19) |
| pN2 | 31 (19) |
| pTNM | |
| Stage I | 82 (50) |
| Stage IIa | 14 (9) |
| Stage IIb | 29 (18) |
| Stage III | 39 (24) |
| Resection method | |
| Sublobar | 46 (28) |
| Single lobectomy | 101 (62) |
| >Lobectomy | 17 (11) |
| R0 resection | 156 (94) |
| 90-d mortality | 6 (3.7) |
| Adjuvant chemotherapy | 112 (68) |
| Adjuvant chest radiation | 42 (26) |
| PCI | 43 (26) |
Values are presented as n (%) or median (interquartile range). COPD, Chronic obstructive pulmonary disease; CAD, coronary artery disease; CHF, chronic heart failure; DM, diabetes mellitus; PCI, prophylactic cranial irradiation.
TABLE 2.
Cox proportional hazards model for mortality among the entire cohort
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
|
|
|
|||
| Variable | Hazard ratio (95% confidence interval) | P value | Hazard ratio (95% confidence interval) | P value |
| Age>65 y | 1.91 (1.23–2.98) | <.001 | 1.71 (1.00–2.94) | .052 |
| Female sex | 0.73 (0.50–1.08) | .114 | 0.87 (0.55–1.39) | .565 |
| Smoker | 0.66 (0.37–1.15) | .139 | 1.09 (0.45–2.64) | .854 |
| COPD | 1.24 (0.81–1.90) | .314 | ||
| CAD | 1.91 (1.06–3.46) | .032 | 3.33 (1.60–6.96) | .001 |
| CHF | 0.61 (0.08–4.38) | .623 | ||
| DM | 2.03 (1.16–3.53) | .013 | 1.54 (0.76–3.15) | .232 |
| Tumor size | 1.09 (0.97–1.24) | .145 | 1.15 (1.00–1.33) | .056 |
| Lobectomy (vs other) | 0.58 (0.40–0.86) | .006 | 0.54 (0.34–0.86) | .010 |
| Positive surgical margin | 2.72 (1.41–5.28) | .003 | 1.83 (0.72–4.65) | .204 |
| pN+ | 1.65 (1.31–2.09) | <.001 | 1.61 (1.00–2.60) | .049 |
| Adjuvant chemotherapy | 0.41 (0.27–0.60) | <.001 | 0.49 (0.29–0.81) | .005 |
| PORT | 0.69 (0.43–1.08) | .107 | 0.79 (0.42–1.50) | .471 |
| PCI | 0.49 (0.30–0.80) | .004 | 0.78 (0.41–1.49) | .447 |
| Treatment decade | 0.92 (0.73–1.16) | .501 | ||
COPD, Chronic obstructive pulmonary disease; CAD, coronary artery disease; CHF, chronic heart failure; DM, diabetes mellitus; pN+, pathological nodal disease; PORT, postoperative radiation therapy; PCI, prophylactic cranial irradiation.
Adjuvant Chemotherapy
Adjuvant chemotherapy was administered in 111 (68%) patients. Cisplatin or carboplatin with etoposide for 4 to 6 cycles was the predominant regimen. The rate of adjuvant chemotherapy for patients with pathological nodal status of pN0, pN1, and pN2 was 70 (68%), 27 (87%), and 14 (45%), respectively (P = .002).
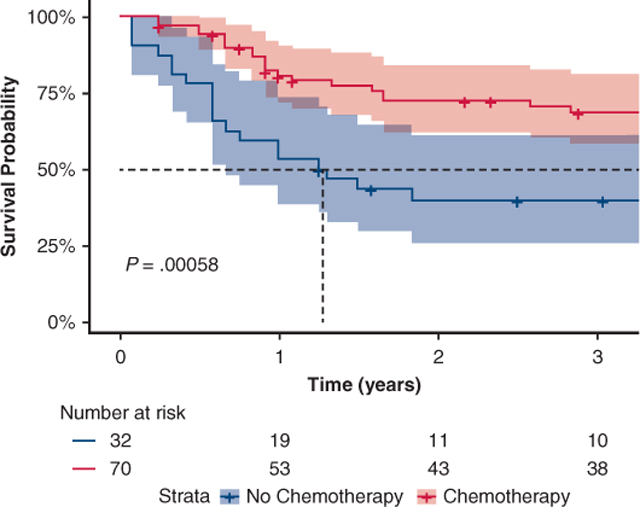
There was a survival benefit among those who received adjuvant chemotherapy, with a median survival time of 75 months (95% CI, 57–93 months) versus 17 months (95% CI, 13–20 months) (P < .001). Local recurrence (8% vs 8%), regional (9% vs 9%), or distant (20% vs 21%) recurrence were not different between those who received adjuvant chemotherapy and those without. Adjuvant chemotherapy was not a significant factor in FFR in Kaplan-Meier analysis (Figure 1), or in Cox multivariable regression analysis (HR, 0.85; 95% CI, 0.46–1.58; P = .610) for FFR (Tables E1 and E2).
FIGURE 1.
Freedom from recurrence after adjuvant chemotherapy. In patients with limited-stage small cell lung cancer after surgical resection, this graph depicts freedom from recurrence for patients who have received adjuvant chemotherapy (red), and patients who have not received adjuvant chemotherapy (blue).
Adjuvant Chest Radiation (PORT)
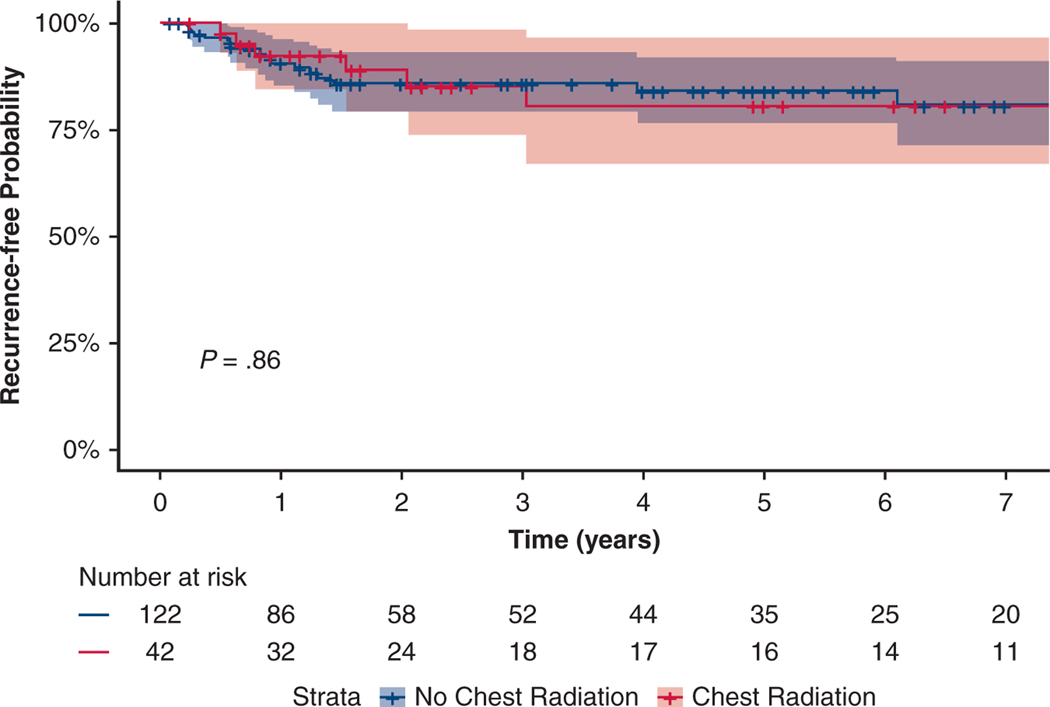
PORT was administered to 42 (26%) patients. The average time from surgery to start of radiation was 5.2 months (interquartile range [IQR], 2.4–6.7 months), with most patients receiving radiation dose of 45 Gy in 30 fractions. There were 36 (86%) patients who received PORT also received adjuvant chemotherapy. PORT was significantly more likely to be used in patients with pathologically involved lymph nodes, with 20 (20%) in pN0, 9 (29%) in pN1, and 13 (32%) in pN2 disease (P = .040).
The median survival time was 73 months (95% CI, 54–92 months) versus 48 months (95% CI, 21–75 months) among those treated with versus without PORT, respectively (P = .106). We consider this difference clinically although not statistically significant. The local (7% vs 10%), regional (9% vs 10%), and distant (20% vs 21%) recurrences were not different between those who received PORT versus those who did not. PORT was not associated with differences in freedom from LRR in Kaplan-Meier analysis (Figure 2) or Cox multivariable regression analysis (HR, 0.85; 95% CI, 0.34–2.11; P = .724).
FIGURE 2.
Freedom from locoregional recurrence after postoperative radiation therapy (PORT). In patients with limited-stage small cell lung cancer after surgical resection, this graph depicts freedom from locoregional recurrence for patients after PORT (red), and patients who have not received PORT (blue).
PCI
PCI was administered in 43 (26%) patients. The average time from surgery to the start of PCI was 6.7 months (IQR, 5.6–7.4 months), and patients most frequently received 25 Gy in 10 fractions. Thirty-seven (86%) patients who received PCI also received adjuvant chemotherapy. Administration of PCI was not different for patients based on nodal disease, the rate of treatment for patients with pN0, pN1, and pN2 was 27 (26.5%), 8 (25.8%), and 8 (25.8%), respectively (P = .996).
The median survival time for patients who received PCI was 76 months (95% CI, 45–107 months) versus 36 months (95% CI, 10–62 months) for those without PCI (P = .003). There were 3 (7%) brain recurrences among patients who received PCI, and 9 (7%) brain recurrences among patients without PCI (P = 1.000). PCI was not associated with freedom from brain recurrence in Kaplan-Meier analysis (Figure 3) or upon Cox univariate regression analysis (HR, 0.76; 95% CI, 0.21–2.83; P = .687).
FIGURE 3.
Freedom from brain recurrence after prophylactic cranial irradiation (PCI). In patients with limited-stage small cell lung cancer after surgical resection, this graph depicts freedom from brain recurrence of patients who have received adjuvant PCI (red), and patients who have not received adjuvant PCI (blue).
Subset Analysis for pN0 and pN+ Disease
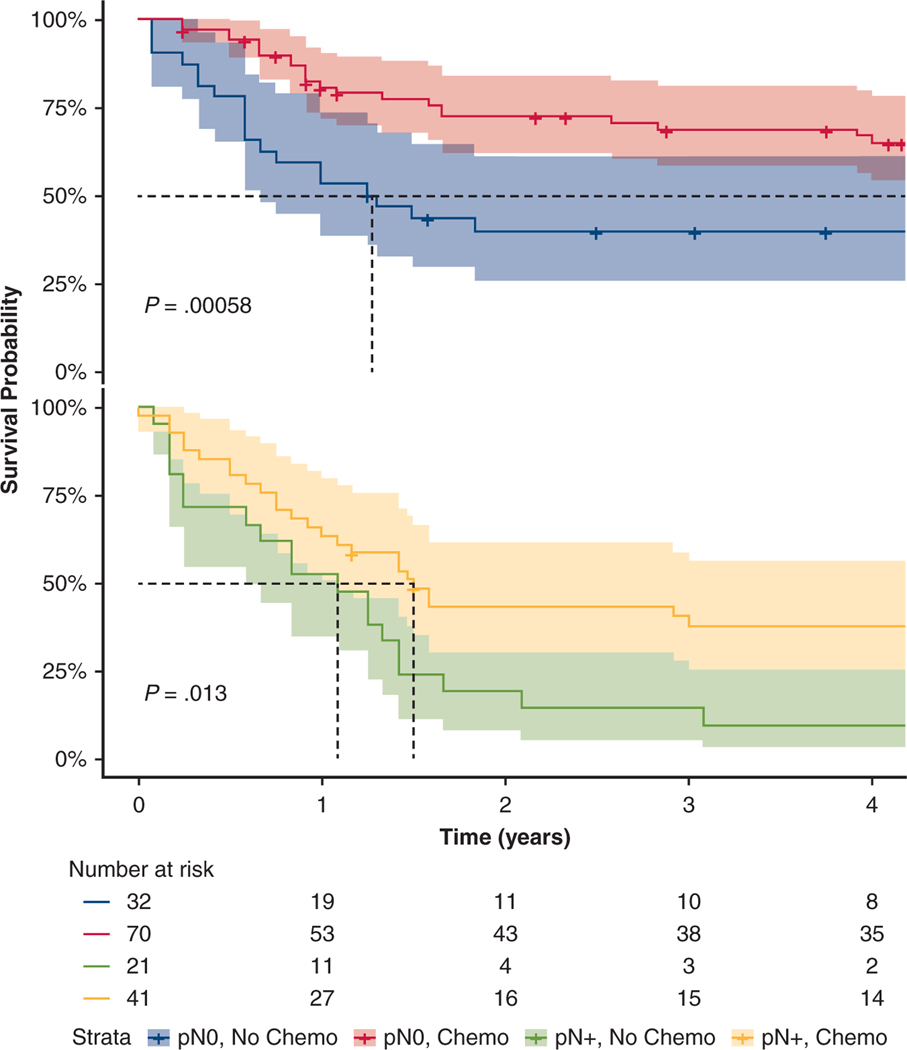
It is of clinical importance to analyze which adjuvant therapy, if any, provided survival benefit in the subset of patients with and without pathologic nodal disease. Thus, we performed subset analyses for pN0 (n = 102) and pN+ (n = 62) patients. In evaluating RFS, adjuvant chemotherapy patients had increased median RFS compared with those without adjuvant chemotherapy for both pN0 (83 vs 15 months; P <.001) and pN+ patients (18 vs 13 months; P <.001) (Figure 4). In Cox multivariable model, adjuvant chemotherapy was associated with survival benefit for both pN0 and pN+ patients, (HR, 0.49; 95% CI, 0.27–0.91; P = .024 and HR, 0.41; 95% CI, 0.18–0.94; P = .035, respectively). However, neither PORT nor PCI were associated with recurrence or survival upon multivariable analysis.
FIGURE 4.
Recurrence-free survival of patients with pathological nodal status (pN) 0 disease (top) and pN+ disease (bottom). With or without adjuvant chemotherapy. Top, Recurrence-free survival for pN0 patients without adjuvant chemotherapy (blue) and with adjuvant chemotherapy (red). Bottom, Recurrence-free survival for pN+ patients without adjuvant chemotherapy (green), and with adjuvant chemotherapy (yellow).
DISCUSSION
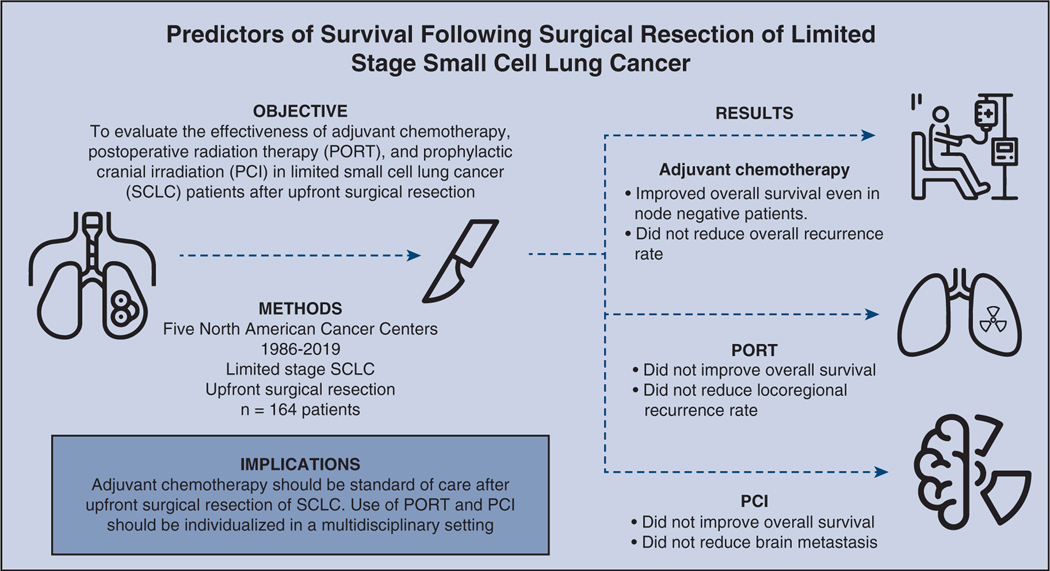
Surgical resection followed by adjuvant chemotherapy is a therapeutic option for early limited-stage SCLC.11 Early limited-stage disease treated with chemoradiation in the Concurrent Once-daily Versus twice-daily Radiotherapy trial approached 50% 5-year survival,16 and the available phase 2 trial data of surgical resection in this cohort reached similar survival rates.30–33 These findings were further corroborated in a study of National Cancer Database data by Yang and colleagues,23 with a 5-year survival rate of 52% following surgery and chemotherapy. Our analysis confirmed the benefit of adjuvant chemotherapy in limited-stage SCLC in both node-positive and node-negative patients. However, in our patient cohort, we were not able to detect the difference in the freedom from recurrence or survival benefit for PORT or PCI (Figure 5). Additionally, nodal disease appears to be an important factor for both survival and recurrence.
FIGURE 5.
In a cohort of 164 patients from 5 North American cancer centers spanning from 1986 to 2019. Resected limited-stage small cell cancer patients receive overall survival benefit from adjuvant chemotherapy. The benefit of postoperative radiation therapy (PORT) and prophylactic cranial irradiation (PCI) should be individualized.
In our study, PORT did not affect freedom from locoregional recurrence nor did it affect OS. Similarly, in the analysis of pT1 2N0 M0 patients by Yang and colleagues,23 chemotherapy with radiation to the lung (HR, 0.88; 95% CI, 0.63–1.23; P = .45), as well as lung radiation alone (HR, 0.83; 95% CI, 0.42–1.64; P = .59) were not found to be significant predictors of survival on multivariable analysis.23 In the study by Wakeam and colleagues,25 survival benefit with PORT was seen only in pN1 (HR, 0.79; 95% CI, 0.62–1.00; P = .05), and pN2 disease (HR, 0.60; 95% CI, 0.48–0.75; P <.001). Although our study did find statistically significant survival benefit with PORT after surgery for limited-stage SCLC patients, we also did not find PORT to have an effect on freedom from LRR or on OS for pN0 or pN+ limited-stage disease. We have shown that patients with higher nodal stage were more likely to receive adjuvant PORT, and it is important to note that these patients were likely highly selected for PORT. The addition of PORT may have added survival benefit, but were not statistically significant when compared with patients without PORT, who were more likely to be of lower disease stage. This draws parallel to findings seen in a meta-analysis of PORT in non–small cell lung cancer,34 where there is unclear survival benefit of PORT in stage III N2 disease, and no survival benefit, even potential detriment in stage I and II patients. However, larger patient cohort may have potentially demonstrated PORT benefit.
The role of PCI in limited stage SCLC remains controversial. There are considerable side effects of neurotoxicity and cognitive effects with whole brain radiation35; however, the justification for PCI has been the rather high rate of brain metastases in SCLC and some evidence for potentially prolonged survival. In the meta-analysis of 7 clinical trials with 987 patients, PCI was shown to have a 4.5% OS advantage in primarily limited-stage disease.21 These trials were completed in nonsurgical patients, and to date there have been no clinical trials of PCI as an adjuvant treatment after surgically resected SCLC. A retrospective study in the Chinese cohort showed that PCI in resected SCLC has a survival benefit for stage II or III, but not stage I patients.36 National Cancer Database data also demonstrated PCI with adjuvant chemotherapy to provide survival benefit in T1 2N0 M0 disease (HR, 0.52; 95% CI, 0.36–0.75; P <.01), but not as an independent treatment modality (HR, 0.83; 95% CI, 0.46–4.64; P = .52).23 In our study, patients who received PCI had improved survival in univariate analysis, but this was not significant in the multivariable model. As mentioned previously, 86% of patients who received PCI have also received chemotherapy, therefore the survival benefit may have been derived mostly from chemotherapy; likewise, patients likely to survive long-term may have been selected for PCI administration. Through our time-to-event analysis and Cox multivariable model of freedom from brain recurrence, we also did not find a difference in the rate of brain recurrence. There was a 7% brain recurrence rate for stage I through III disease, this is in contrast to the estimated 10% to 15% for stage I and 15% to 25% for stage II seen in data from China and Germany.26 Nevertheless, the 4.5% OS advantage is likely diminished further with earlier-stage disease. Given the known side effects of brain radiation,37 and modern brain imaging strategies for close follow-up, the use of PCI in limited stage SCLC, especially early SCLC should be approached with careful discussion. Furthermore, the use of low-dose computed tomography for lung cancer screening will likely result in an increased incidence of early-stage lung cancers of all types, especially in high smoking population. This may represent an increase in the diagnosis of early limited-stage SCLC, and also opportunities for future trials in this population.
Upon reviewing the data, we were surprised to find that although patients with pN1 disease were more likely to receive adjuvant chemotherapy than pN0, pN2 disease had the lowest rate of adjuvant chemotherapy. It is possible that patients with pN2 disease may have opted to receive chemotherapy outside of the institution where they received surgery, and data on adjuvant chemotherapy were not captured correctly. We were also surprised to find that no adjuvant therapies had an effect on recurrence. It is possible that recurrence rates are altered after surgical resection, another potential reason is that patients may have left the treating institution and received follow-up care near their home, leading to reporting error. Another finding of our study is that 3.7% patients died before 90 days, and although only 24% of the study cohort had pN2 disease, 4 out of 6 patients (67%) who died within 90 days after surgery had pN2 status. The pN2 patients were unlikely to have completed their chemotherapy, another contributing factor to the low rate of adjuvant chemotherapy in pN2 disease patients seen in this cohort. This alludes to the importance of optimal patient selection in terms of surgical fitness as well as likelihood of survival benefit. Current National Comprehensive Cancer Network guidelines state the need for pathological mediastinal staging before operating on clinical stage I to IIa patients, this may be difficult when the initial diagnosis is unknown, thus, mediastinal lymph node evaluation preoperatively or intraoperatively becomes important for planning adjuvant treatments. Our study further highlights the need for careful patient selection and consideration for treatment from a multidisciplinary approach.
Our study has limitations associated with its retrospective design, which include mainly small sample size because the cohort of resected limited-stage SCLC has historically been small. Our multi-institutional collaborative efforts yielded 164 patients for the analysis, with the hope that we would be able to contribute more granular information to the existing literature on surgical treatment of SCLC. However, the results of our analyses based on the number of patients and events may be subject to type II errors. There are also limitations to institutional data because each institution has different protocol and treatment decisions for patients, thus we cannot account for all the differences between institutions. Next, performance status could not be incorporated into this study, but it is a potential confounder with the administration of adjuvant therapies. Lastly, another major limitation is the time-duration of the dataset collection from 1986–2019 or 33 years. Our analysis for the past 2 decades showed significant improvement in survival compared with the first decade. However, further analysis into survival and recurrence after chemotherapy, PORT and PCI yielded no significant differences. During this time period much has changed in the treatment of lung cancer in terms of diagnosis, staging, surgical, and radiation techniques.
Although we did not detect a survival benefit with PORT and PCI in our multivariable model, we caution against concluding that those treatments do not provide benefit because we believe treatment of SCLC is a multidisciplinary endeavor. It is likely that a subset of patients may benefit from 1 or a combination of these treatments, best determined in a multidisciplinary setting. Other factors that may influence the interpretation of this study include the retrospective design that lends itself to selection bias, as well as temporal changes in practice across centers.
Our study provides a glimpse into the importance of developing multicenter collaboration in the surgical treatment of rare diseases such as limited-stage SCLC. Although we present a robust cohort of patients for this rare disease, particularly managed surgically, the importance of a well-annotated database for uncommon surgical diseases cannot be overstated. We need further data to determine optimal adjuvant therapy combination, yet there are other novel agents already being tested for the treatment of SCLC. The IMpower133 trial,38 which evaluated atezolizumab in addition to chemotherapy as the first-line treatment, was shown to prolong survival in extensive-stage SCLC. Both the IMpower13338 and CASPIAN (PMID: 31590988) trials have recently demonstrated an overall survival advantage with the addition of immune checkpoint inhibitors to frontline chemotherapy in extensive-stage SCLC. Accordingly, trials evaluating the addition of immune checkpoint inhibitors to concurrent chemoradiation are already underway for limited-stage SCLC such as NRG Oncology-LU005. Future trials evaluating surgical resection in limited-stage SCLC should anticipate these ongoing paradigm shifts and consider evaluation of induction or adjuvant chemoimmunotherapy combination, as with non–small cell lung cancer. Other novel therapeutic classes, including PARP inhibitors and lurbinectedin (PMID: 32276927), have shown promising efficacy in relapsed SCLC and warrant evaluation in less advanced disease states.39,40
The benefits of surgical approaches are not limited to the clinical outcomes described here. In SCLC, patients frequently undergo a single fine-needle aspiration at diagnosis, leaving little to no tissue for biomarker discovery or characterization of resistance mechanisms. Therapeutic strategies, including surgical resection offer the distinct advantage of providing valuable tissue for translational analyses, including after induction treatment, and could benefit patients across all stages of this devastating disease. Future trials will undoubtedly take place in limited-stage SCLC, and it is imperative that surgeons partake in these trials and take a seat at the table with regard to the multimodality treatment of SCLC, and determine how to utilize these treatments in an induction or adjuvant fashion.
CONCLUSIONS
Adjuvant chemotherapy after surgical resection is associated with improved survival in limited-stage SCLC regardless of nodal disease. Although PORT and PCI was not statistically significantly associated with a survival benefit, this cohort may have been underpowered to detect such a benefit, and we recommend that decisions regarding the use of these adjuvant modalities be discussed on individual basis in multidisciplinary settings.
Extended Data
FIGURE E1.
Consolidated Standards of Reporting Trials of patient contribution from each institution and reasons for exclusion. Patients with missing data were excluded due to missing adjuvant therapy treatment information that could not be recovered. McGill, McGill University Health Centre; MDACC, MD Anderson Cancer Center; MSKCC, Memorial Sloan Kettering Cancer Center; Swedish, Swedish Cancer Institute; Toronto, University Health Network - Toronto General Hospital.
TABLE E1.
Cox multivariable regression for disease recurrence
| Variable | Hazard ratio (95% confidence interval) | P value |
|---|---|---|
| Lobectomy | 0.77 (0.44–1.36) | .366 |
| Positive margin | 1.11 (0.42–2.91) | .837 |
| pN+ | 2.91 (1.61–5.28) | <.001 |
| Adjuvant chemotherapy | 0.85 (0.46–1.58) | .610 |
| Prophylactic cranial irradiation | 0.59 (0.30–1.18) | .136 |
pN+, Positive pathological nodal status.
TABLE E2.
Cox multivariable model for locoregional recurrence
| Variable | Hazard ratio (95% confidence interval) | P value |
|---|---|---|
| pN+ | 3.10 (1.32–7.30) | .009 |
| PORT | 0.85 (0.34–2.11) | .724 |
pN+, Positive pathological nodal status; PORT, postoperative radiation therapy.
CENTRAL MESSAGE.
For patients with surgically resected limited-stage small cell lung cancer, adjuvant chemotherapy improves survival regardless of mediastinal nodal involvement.
PERSPECTIVE.
There is a paucity of data evaluating the influence of chemotherapy, postoperative chest radiation, and prophylactic cranial irradiation used in combination after surgical resection of limited-stage small cell lung cancer. In this multi-institutional cohort, adjuvant chemotherapy was associated with a survival benefit. Larger studies may be needed to detect the influence of prophylactic cranial irradiation and postoperative chest radiation in this cohort.
Acknowledgments
Funding provided by generous philanthropic donations from the Mason Family Research Fund.
Members of the Small Cell Lung Cancer Working Group are listed in the Acknowledgments.
Abbreviations and Acronyms
- FFR
freedom from recurrence
- SCLC
small cell lung cancer
- PCI
prophylactic cranial irradiation
- PORT
postoperative radiation therapy
- OS
overall survival
- RFS
recurrence-free survival
- LRR
locoregional recurrence
Discussion
Presenter: Dr Nicolas Zhou

Dr Thomas A. D’Amico (Durham, NC). Congratulations to Dr Zhou and his colleagues for this important multi-institutional study. The role of surgery and the management of small cell lung cancer continue to evolve. Recent studies have demonstrated that surgery for early-stage small cell was associated with improved long-term survival when compared with concurrent chemoradiation and that adjuvant therapy improves survival. Despite these findings, the rate of surgery in these patients has not appeared to increase. Analysis of outcomes of the surgical treatment of small cell lung cancer are difficult to interpret because the treatment guidelines do not replicate the guidelines for non–small cell lung cancer, and the diagnosis of early-stage small cell lung cancer is often not available at the time of decision making regarding surgery. In this multi-institutional study, Dr Zhou and his colleagues analyzed the outcomes of patients after surgical resection for limited-stage small cell lung cancer and both node-negative status and the use of adjuvant chemotherapy are identified as important predictors of survival. More than half of patients had T1 tumors and without an established diagnosis of small cell lung cancer, preresectional pathologic mediastinal lymph node assessment would not be expected.
However, approximately 40% of patients were found to have node-positive disease at the time of surgery, with long-term survival of approximately only 15% and it is uncertain whether induction therapy would have improved the survival of these groups. With that in mind, I have these questions for Dr Zhou: The National Comprehensive Cancer Network guideline algorithm for patients who are clinical T1 N0 or T2 N0 small cell lung cancer is the following: Pathologic mediastinal lymph node assessment followed by surgery for patients who are not N2. In your study, 15% of patients were T3 or T4, 19% were N1, and 19% were N2.
Although some of the patients are certainly upstaged at surgery, this would not explain the majority of patients who are beyond T2 N0. Would you explain your algorithm to select patients for surgery?

Dr Nicolas Zhou (Houston, Tex). Thank you for your question. So we collected surgically resected data for small cell lung cancer from various institutions. Therefore, we cannot comment 100% accurately on why some patients had surgical resection for N2 disease or not. There may be several reasons that this happened. First, when you look back at the literature, there are several phase 2 trials in the 1990s that showed that there was survival benefit for limited stage disease patients after surgery—even in those patients who are stage IIb to IIIa. So it is within reason that some patients have surgical resection, even if they were known to have N2 disease.
Secondly, some of these patients may have been initially diagnosed as atypical carcinoid or non–small cell lung cancer with neuroendocrine features, and a lot of times, the histology of the cancer was determined intraoperatively. So for those patients, the surgeon does not have the luxury of knowing beforehand that this is small cell lung cancer.
So we do assume that some of these were resected upfront and were either upstaged or the diagnosis was changed on a fine pathology. Lastly, we also included patients who were mixed histology as long as a small cell lung cancer was the driving histology for survival. So all those factors may have contributed to why some N2 disease patients had resection.
Dr D’Amico. I’m sorry: You said as long as the small cell component was the driving feature of survival?
Dr Zhou. Yes.
Dr D’Amico. How do you know that in advance?
Dr Zhou. Just based on historical survival for each histology.
Dr D’Amico. Okay. Regarding adjuvant therapy: the National Comprehensive Cancer Network guidelines recommend adjuvant chemotherapy for all patients after surgical resection and adjuvant chemotherapy has already been demonstrated to improve survival, from studies from the National Cancer Database. Why did only 68% of your patients receive adjuvant chemotherapy?
Dr Zhou. Thank you. This came to us as a surprise as well. It is something that we will point out in the print publication. When we looked at patients who receive adjuvant chemo binodal stage, we found that those with N0 disease, 69% of them received chemo. And for those with N1 disease, 87% of them received chemo. For N2 disease, only 45% received chemo. So that is kind of puzzling.
It could be that with some of the higher referral centers, perhaps when patients get diagnosed with N2 disease, they either forego chemotherapy to receive other treatments or receive chemo back home instead of at the treating institution, so that could have contributed some missing data.
Dr D’Amico. Thank you. Regarding prophylactic cranial irradiation, how was this determined to be utilized? In your study, it was used in only 43 patients, yet nearly twice that many were beyond stage I and might have been considered.
Dr Zhou. Thank you. PCI and limited small cell lung cancer is an area of controversy. While some studies showed that the benefit was survival, some studies really highlight the side effects of PCI whole brain radiation.
There was a large meta-analysis of 7 clinical trials with nearly 1000 patients that showed that in small cell lung cancer (and these were mainly limited-stage disease), it was a 4.5% overall survival benefit. However, a recent Japanese trial in 2017 showed that the use of PCI made no difference in progression-free survival and the group that was on a surveillance arm actually trended toward a better survival.
So clinically this is controversial and our institution tends to evaluate each patient on a case-by-case basis and really weigh the benefit of survival versus the side effects. It was 1 of our study’s aims to map out the rate of PCI and its effects. As we can see, even in major institutions, decisions regarding PCI are individualized. Some patients may refuse it and elect to get brain radiation only when metastasis occurs. So unfortunately, we don’t we don’t think that we have enough statistical power to answer this question.
Dr D’Amico. Finally, it’s very important to have institutional studies in addition to the national database studies in order to develop guidelines for small cell lung cancer. It requires multi-institutional series due to the relatively small number of surgical patients at any 1 institution. So congratulations, once again to you and your coinvestigators. Can you please briefly summarize how this study may inform the development of future guidelines and improve the care of patients with early-stage small cell lung cancer.
Dr Zhou. Thank you. And we thank all of our collaborative partners in this endeavor. We think that this study strengthens the claim that adjuvant chemotherapy has survival benefit in limited-stage small cell lung cancer, regardless of whether patients have nodal disease or not. As for adjuvant chest radiation, we did not find that it was an independent predictor of survival once patients have local disease control. As for PCI, we did not find that it reduced the recurrence rate of brain metastases and it was not an independent predictor for survival. Small cell lung cancer is on those diseases that the resection rates are generally very low in any 1 institution, similar to primary chest wall tumors or thymomas. This collaborative effort or retrospective data is important, but moving forward, it should be beneficial to create a well-annotated, multi-institutional database.
The benefit of resection in small cell is evident, from multiple retrospective studies. And right now, atezolizumab has demonstrated survival benefit in extensive stage disease and other poly ADP ribose polymerase inhibitors have also shown promise. So all these novel agents are soon to be studied in limited stage small cell lung cancer, but those trials are likely to be done in patients with radiation such as stereotactic body radiation therapy and not have surgery be a part of it. So we ought to make sure that surgeons are aware of these novel agents and have a seat at the table when trials are designed for small cell lung cancer, especially limited-disease small cell lung cancer.
We believe that surgery offers a tremendous benefit in these patients and offers value not only in local and regional disease control, but also the pathology, the responses to therapy, and translational studies. So we see a lot of benefit for collaborative work in this area.
Dr D’Amico. Brief question: I think you mentioned that there was no difference noted in survival—and as you said surprisingly—between N0 and N1. Was that correct?
Dr Zhou. Yes, for overall survival.
Dr D’Amico. Indeed, and along those lines, can you comment on the median number of lymph nodes that were taken during the pathologic analysis? And do you think that was a problem that maybe just not enough nodes were taken to ensure that you were truly N0? Could you comment on that?
Dr Zhou. I appreciate the question; that is something that we want to look at. But unfortunately, not all institutions have those data, so we cannot comment completely on overall how many lymph nodes were taken for each patient.
Dr D’Amico. I see. Thank you.
Footnotes
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://aats.blob.core.windows.net/media/20AM/Presentations/Predictors%20of%20Survival%20Following%20Sur.mp4.

Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Members of the Small Cell Lung Cancer Working Group: Kyle G. Mitchell, MD, Erin M. Corsini, MD, Mara B. Antonoff, MD, Wayne L. Hofstetter, MD, Reza J. Mehran, MD, David C. Rice, MBBCh, Stephen G. Swisher, MD, Ara A. Vaporciyan, MD, and Garrett L. Walsh, MD (Department of Thoracic and Cardiovascular Surgery, University of Texas MD Anderson Cancer Center, Houston, Tex); Caroline Huynh, MD, CM, and Pierre-Olivier Fiset, MD, PhD (Division of Thoracic and Upper Gastrointestinal Surgery, McGill University Health Centre, Montreal, Québec, Canada); Stephen G. Chun, MD (Department of Radiation Oncology, University of Texas MD Anderson Cancer Center, Houston, Tex); and Carl M. Gay, MD, PhD (Department of Thoracic Head and Neck Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, Tex).
References
- 1.Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–44. [DOI] [PubMed] [Google Scholar]
- 2.van Meerbeeck JP, Fennell DA, De Ruysscher DKM. Small-cell lung cancer. Lancet. 2011;378:1741–55. [DOI] [PubMed] [Google Scholar]
- 3.Fox W, Scadding JG. Medical research council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus: ten-year follow-up. Lancet. 1973;302:63–5. [DOI] [PubMed] [Google Scholar]
- 4.Lad T, Piantadosi S, Thomas P, Payne D, Ruckdeschel J, Giaccone G. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest. 1994;106:320s–3s. [DOI] [PubMed] [Google Scholar]
- 5.Pignon JP, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327:1618–24. [DOI] [PubMed] [Google Scholar]
- 6.Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10:890–5. [DOI] [PubMed] [Google Scholar]
- 7.Stahel RA, Ginsberg R, Havemann K, Hirsch FR, Ihde DC, Jassem J, et al. Staging and prognostic factors in small cell lung cancer: a consensus report. Lung Cancer. 1989;5:119–26. [Google Scholar]
- 8.Schreiber D, Rineer J, Weedon J, Vongtama D, Wortham A, Kim A, et al. Survival outcomes with the use of surgery in limited-stage small cell lung cancer. Cancer. 2010;116:1350–7. [DOI] [PubMed] [Google Scholar]
- 9.Takenaka T, Takenoyama M, Inamasu E, Yoshida T, Toyokawa G, Nosaki K, et al. Role of surgical resection for patients with limited disease-small cell lung cancer. Lung Cancer. 2015;88:52–6. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. Small Cell Lung Cancer (version3.2020); 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/sclc_blocks.pdf.Accessed March 9, 2020.
- 11.Kalemkerian GP, Loo BW, Akerley W, Attia A, Bassetti M, Boumber Y, et al. NCCN Guidelines Insights: small cell lung cancer, version 2.2018. J Natl Comprehens Cancer Netw. 2018;16:1171–82. [DOI] [PubMed] [Google Scholar]
- 12.Wakeam E, Varghese TK Jr, Leighl NB, Giuliani M, Finlayson SRG, Darling GE. Trends, practice patterns and underuse of surgery in the treatment of early stage small cell lung cancer. Lung Cancer. 2017;109:117–23. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed Z, Kujtan L, Kennedy KF, Davis JR, Subramanian J. Disparities in the management of patients with stage I small cell lung carcinoma (SCLC): a surveillance, epidemiology and end results (SEER) analysis. Clin Lung Cancer. 2017; 18:e315–25. [DOI] [PubMed] [Google Scholar]
- 14.Turrisi AT, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999; 340:265–71. [DOI] [PubMed] [Google Scholar]
- 15.Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (convert): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salem A, Mistry H, Hatton M, Locke I, Monnet I, Blackhall F, et al. Association of chemoradiotherapy with outcomes among patients with stage I to II vs stage III small cell lung cancer: secondary analysis of a randomized clinical trial. JAMA Oncol. 2019;5:e185335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skarlos DV, Samantas E, Briassoulis E, Panoussaki E, Pavlidis N, Kalofonos HP, et al. Randomized comparison of early versus late hyperfractionated thoracic irradiation concurrently with chemotherapy in limited disease small-cell lung cancer: a randomized phase II study of the Hellenic Cooperative Oncology Group (HECOG). Ann Oncol. 2001;12:1231–8. [DOI] [PubMed] [Google Scholar]
- 18.Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan clinical oncology group study 9104. J Clin Oncol. 2002;20: 3054–60. [DOI] [PubMed] [Google Scholar]
- 19.Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–72. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi T, Yamanaka T, Seto T, Harada H, Nokihara H, Saka H, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:663–71. [DOI] [PubMed] [Google Scholar]
- 21.Auperin A, Arriagada R, Pignon JP, Le Pechoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview collaborative group. N Engl J Med. 1999;341:476–84. [DOI] [PubMed] [Google Scholar]
- 22.Combs SE, Hancock JG, Boffa DJ, Decker RH, Detterbeck FC, Kim AW. Bolstering the case for lobectomy in stages I, II, and IIIa small-cell lung cancer using the national cancer database. J Thorac Oncol. 2015;10: 316–23. [DOI] [PubMed] [Google Scholar]
- 23.Yang CF, Chan DY, Speicher PJ, Gulack BC, Wang X, Hartwig MG, et al. Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol. 2016;34:1057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bischof M, Debus J, Herfarth K, Muley T, Kappes J, Storz K, et al. Surgery and chemotherapy for small cell lung cancer in stages I-II with or without radiotherapy. Strahlenther Onkol. 2007;183:679–84. [DOI] [PubMed] [Google Scholar]
- 25.Wakeam E, Giuliani M, Leighl NB, Finlayson SRG, Varghese TK, Darling GE. Indications for adjuvant mediastinal radiotherapy in surgically resected small cell lung cancer. Ann Thorac Surg. 2017;103:1647–53. [DOI] [PubMed] [Google Scholar]
- 26.Bloom BC, Augustyn A, Sepesi B, Patel S, Shah SJ, Komaki RU, et al. Prophylactic cranial irradiation following surgical resection of early-stage small-cell lung cancer: a review of the literature. Front Oncol. 2017;7:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Zhang D, Zhou X, Bao W, Ji Y, Sheng L, et al. Prophylactic cranial irradiation in resected small cell lung cancer: a systematic review with meta-analysis. J Cancer. 2018;9:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallières E, Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, et al. The IASLC lung cancer staging project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4: 1049–59. [DOI] [PubMed] [Google Scholar]
- 29.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trial. 1996;17:343–6. [DOI] [PubMed] [Google Scholar]
- 30.Karrer K, Ulsperger E. Surgery for cure followed by chemotherapy in small cell carcinoma of the lung: for the ISC-Lung Cancer Study Group. Acta Oncolog. 1995;34:899–906. [DOI] [PubMed] [Google Scholar]
- 31.Macchiarini P, Hardin M, Basolo F, Bruno J, Chella A, Angeletti CA. Surgery plus adjuvant chemotherapy for T1–3 N0 M0 small-cell lung cancer. Rationale for current approach. Am J Clin Oncol. 1991;14:218–24. [DOI] [PubMed] [Google Scholar]
- 32.Rea F, Callegaro D, Favaretto A, Loy M, Paccagnella A, Fantoni U, et al. Long term results of surgery and chemotherapy in small cell lung cancer. Eur J Cardiothorac Surg. 1998;14:398–402. [DOI] [PubMed] [Google Scholar]
- 33.Tsuchiya R, Suzuki K, Ichinose Y, Watanabe Y, Yasumitsu T, Ishizuka N, et al. Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I-IIIa small cell lung cancer: the Japan clinical oncology lung cancer study group trial (JCOG9101). J Thorac Cardiovasc Surg. 2005;129:977–83. [DOI] [PubMed] [Google Scholar]
- 34.Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet. 1998;352:257–63. [PubMed] [Google Scholar]
- 35.Li J, Bentzen SM, Li J, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Intl J Radiat Oncol Biol Phys. 2008;71:64–70. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Yang H, Fu X, Jin B, Lou Y, Zhang Y, et al. Prophylactic cranial irradiation for patients with surgically resected small cell lung cancer. J ThoracOncol. 2017; 12:347–53. [DOI] [PubMed] [Google Scholar]
- 37.Gondi V, Paulus R, Bruner DW, Meyers CA, Gore EM, Wolfson A, et al. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of radiation therapy oncology group randomized trials 0212 and 0214. Intl J Radiat Oncol Biol Phys. 2013; 86:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–9. [DOI] [PubMed] [Google Scholar]
- 39.Pietanza MC, Waqar SN, Krug LM, Dowlati A, Hann CL, Chiappori A, et al. Randomized, double-blind, Phase II study of temozolomide in combination with either veliparib or placebo in patients with relapsed-sensitive or refractory small-cell lung cancer. J Clin Oncol. 2018;36:2386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farago AF, Yeap BY, Stanzione M, Hung YP, Heist RS, Marcoux JP, et al. Combination olaparib and temozolomide in relapsed small cell lung cancer. Cancer Discov. 2019;9:1372–87. [DOI] [PMC free article] [PubMed] [Google Scholar]