Abstract
Background
Fatigue is the most commonly reported symptom in patients with persistent complaints following COVID-19 (ie, long COVID). Longitudinal studies examining the intensity of fatigue and differentiating between physical and mental fatigue are lacking.
Objective
The objectives of this study were to (1) assess the severity of fatigue over time in members of online long COVID peer support groups, and (2) assess whether members of these groups experienced mental fatigue, physical fatigue, or both.
Methods
A 2-wave web-based follow-up study was conducted in members of online long COVID peer support groups with a confirmed diagnosis approximately 3 and 6 months after the onset of infectious symptoms. Demographics, COVID-19 diagnosis, received health care (from medical professionals or allied health care professionals), fatigue (Checklist Individual Strength–subscale subjective fatigue [CIS-Fatigue]; 8-56 points), and physical and mental fatigue (self-constructed questions; 3-21 points) were assessed. Higher scores indicated more severe fatigue. A CIS-Fatigue score ≥36 points was used to qualify patients as having severe fatigue.
Results
A total of 239 patients with polymerase chain reaction/computed tomography–confirmed COVID-19 completed the survey 10 weeks (SD 2) and 23 weeks (SD 2) after onset of infectious symptoms, respectively (T1 and T2). Of these 239 patients, 198 (82.8%) were women; 142 (59.4%) had no self-reported pre-existing comorbidities; 208 (87%) self-reported being in good health before contracting COVID-19; and 62 (25.9%) were hospitalized during acute infection. The median age was 50 years (IQR 39-56). The vast majority of patients had severe fatigue at T1 and T2 (n=204, 85.4%, and n=188, 78.7%, respectively). No significant differences were found in the prevalence of normal, mild, and severe fatigue between T1 and T2 (P=.12). The median CIS-Fatigue score was 48 points (IQR 42-53) at T1, and it decreased from T1 to T2 (median change: –2 points, IQR –7 to 3; P<.001). At T1, a median physical fatigue score of 19 points (IQR 16-20) and a median mental fatigue score of 15 points (IQR 10-17) were reported; these scores were lower at T2 for physical but not for mental fatigue (median change for physical fatigue –1 point, IQR –3 to 0, P<.001; median change for mental fatigue 0 points, IQR –3 to 3, P=.52). At the time of completing the follow-up survey, 194/239 (81.2%) and 164/239 (68.6%) of all patients had received care from at least one medical professional and one allied health care professional, respectively.
Conclusions
Fatigue in members of online long COVID support groups with a confirmed COVID-19 diagnosis decreases from 10 to 23 weeks after onset of symptoms. Despite this, severe fatigue remains highly prevalent. Both physical and mental fatigue are present. It remains unclear whether and to what extent fatigue will resolve spontaneously in the longer term.
Trial Registration
Netherlands Trial Register NTR8705; https://www.trialregister.nl/trial/8705.
Keywords: COVID-19, SARS-CoV-2, long COVID, post-COVID-19 syndrome, post-acute sequelae of COVID-19, fatigue, post-viral fatigue, pandemic, online health, mental health, online support
Introduction
As the current COVID-19 pandemic continues to evolve, its impact becomes apparent. Clinical studies of hospitalized, laboratory-confirmed patients have shown that the acute phase of COVID-19 is characterized by a large array of respiratory and non-respiratory symptoms [1]. Over time, it has become clear that not all previously hospitalized patients fully recover from these symptoms in the months after the infection [2,3]. In addition, nonhospitalized patients can present persistent complaints months after the onset of infection-related symptoms [4]. These long-lasting symptoms after COVID-19 are referred to as long COVID [5], “a condition whereby affected individuals do not recover for several weeks or months following the onset of symptoms suggestive of COVID-19” [6], and they have a major impact on patients’ quality of life (QoL) [7,8], care dependency [9], work participation [10,11], day-to-day activities, and physical functioning [12-14].
Fatigue, defined as “a subjective, unpleasant symptom which incorporates total body feelings ranging from tiredness to exhaustion creating an unrelenting overall condition which interferes with individuals’ ability to function to their normal capacity” [15], is the most commonly reported symptom in patients with long COVID [2-4,16]. Similarly, other infections, such as severe acute respiratory syndrome (SARS) [17,18], Middle East respiratory syndrome (MERS) [19], and Q fever [20] have previously been linked to long-term fatigue, often referred to as postviral fatigue syndrome. Existing literature suggests that fatigue has several clinical presentations. A common distinction is made between physical fatigue (ie, difficulty performing physical activities) and mental fatigue (ie, difficulties concentrating and performing cognitive tasks) [21].
To date, longitudinal studies that examine fatigue intensity in patients with long COVID are lacking. Moreover, it is not known whether patients experience mostly mental or physical fatigue during and after the infection. Therefore, the objectives of this study were to (1) assess the severity of fatigue over time in members of online long COVID peer support groups; and (2) assess whether members of online long COVID peer support groups experience mental fatigue, physical fatigue, or both. We hypothesized that fatigue would be common and persistent and that both physical and mental fatigue would be present in patients with long COVID.
Methods
Study Design and Participants
This study is a prospective web-based survey of members of two Facebook peer support groups for patients with long COVID in the Netherlands (approximately 11,000 members; [22]) and Flanders (Belgium, approximately 1200 members; [23]), and a panel of approximately 1200 people who registered at a website of the Netherlands Lung Foundation (coronaplein [24]), an online platform providing additional information, advice, and peer support. Note that these totals represent the number of members of each group at the period of data collection. Between June 4 and June 11, 2020 (the time point of completing the first survey [T1]), members were invited to complete a web-based survey. Participants who completed the first survey [4,8,9] and who agreed to be contacted for a follow-up study received a second survey between August 31 and September 8, 2020 (ie, approximately three months after the first survey; the time point of completing the second survey [T2]). Ethical approval for this study was waived by the medical ethics committee of Maastricht University because the Medical Research Involving Human Subjects Act (WMO) does not apply to this study (METC2020-1978 and METC2020-2254). The medical ethics committee of Hasselt University formally judged and approved the study (MEC2020/041). Digital informed consent was obtained twice from all respondents (at the start of each survey). Exclusion criteria were intensive care unit (ICU) admission during the acute phase of infection, an onset of symptoms before January 1, 2020, being in the acute phase of COVID-19 when answering the first survey (ie, onset of infectious symptoms less than 3 weeks before filling out the first survey [25]), or an incomplete survey. Cross-sectional and follow-up data from this study on persistent symptoms, QoL, care dependency, construct-validity of the post–COVID-19 functional status scale, and information and care needs of members of online long COVID peer support groups have been published before [4,8,9,11,26,27]. This 2-wave web-based follow-up study was registered at the Netherlands Trial Registry (NTR8705). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist was used to guide reporting [28]. Of note: the current study focusses on patients with a confirmed COVID-19 diagnosis (ie, test-diagnosed cases). The results for the patients without a confirmed COVID-19 diagnosis are presented in Multimedia Appendix 1.
Assessment via Web-Based Surveys
The survey was developed in close collaboration with scientists, methodologists, health care professionals and COVID-19 patients from the long COVID peer support groups (the Netherlands and Flanders). It was digitalized by ASolutions [29] and was made available via their online platform. The survey consisted of general questions regarding demographics, clinical characteristics, and standardized questionnaires, including a fatigue questionnaire.
Demographic and Clinical Characteristics
Respondents received questions regarding demographical aspects such as gender, age, weight, height, educational level (low/medium/high; classification according to the International Standard Classification of Education 2011 [30]), and married/living with a partner (yes/no). In addition, the following clinical characteristics were assessed via self-report: pre-existing comorbidities (see Multimedia Appendix 2), health status (good/moderate/poor) during the infection and at the moment of completing the surveys, date of symptom onset, symptoms during acute phase of COVID-19 and at the moment of completing the surveys (see Multimedia Appendix 2), COVID-19–related hospitalization, and COVID-19 diagnosis (based on reverse transcription polymerase chain reaction (PCR) test or computed tomography (CT) scan of the thorax/symptom-based medical diagnosis by a physician/no formal test or diagnosis). Based upon the latter, patients were classified as either “test-diagnosed” COVID-19 (PCR or CT) or “presumed” COVID-19 (physician-diagnosed or no formal diagnosis/testing).
Received Health Care
Information regarding received health care (yes/no) by a medical professional (eg, medical specialist; general practitioner [GP]; nurse) or an allied health care professional (AHP; eg, physiotherapist [PT]; psychologist; occupational therapist [OT]; dietician; speech and language therapist) was recorded.
Standardized Fatigue Questionnaire
Fatigue, the primary outcome measure, was measured using a subscale of the Checklist Individual Strength (CIS). The Checklist Individual Strength–subscale subjective fatigue (CIS-Fatigue) is a standardized questionnaire [31,32] with high internal consistency and test-retest reliability; good discriminant, concurrent and criterion validity; and ability to detect change in subjective fatigue [33-37]. The questionnaire consists of 8 items scored on a 7-point Likert scale. Scores range from 8 to 56 points, and a higher score indicates more clinical symptoms of general fatigue (see Multimedia Appendix 3 for the CIS-Fatigue questionnaire) [31,32]. Based upon validated cutoff values, individuals can be classified as having normal (≤26 points), mild (27-35 points), and severe (≥36 points) fatigue [31-33].
Self-constructed Physical and Mental Fatigue Questions
A total of 3 self-constructed questions (all part of the CIS-Fatigue subscale) were used to evaluate physical fatigue (“Physically I feel exhausted,” “Physically I feel I am in a bad condition,” and “Physically I feel in a good shape”). In addition, to differentiate between physical and mental fatigue, 3 questions were constructed in which the word “physically” was replaced by the word “mentally” (“Mentally I feel exhausted,” “Mentally I feel I am in a bad condition,” and “Mentally I feel in a good shape,” respectively) to estimate mental fatigue. The physical and mental fatigue questions were scored on a 7-point Likert scale, with scores ranging from 3 to 21 points. A higher score indicates worse physical and mental fatigue, respectively. These self-constructed physical and mental fatigue questions and explanations of the scoring are reported in Multimedia Appendix 4.
Statistical Analyses
Data are presented as means and standard deviations, medians, and interquartile ranges or as frequencies and proportions, where appropriate. Differences over time were analyzed by a paired t-test (or Wilcoxon signed-rank test) in continuous data and a McNemar test (or McNemar-Bowker test) in categorical data. If significant, a post hoc comparison of the McNemar-Bowker test was performed, and significant Bonferroni-adjusted P values were generated as corrections for multiple comparison. Statistical analyses were conducted using SPSS 25.0 (IBM Corporation). Figures were generated via GraphPad Prism 8.3.5 (GraphPad Software) and SankeyMATIC [38]. The level of significance was set at .01 for all statistical tests (two-tailed).
Results
Participants’ Inclusion
In total, 2159 members of online long COVID peer support groups filled out the first survey, of which 220 were excluded for being in the acute phase of COVID-19 (n=14), ICU admission during the acute phase of COVID-19 (n=15), onset of symptoms before January 1, 2020 (n=8), and an incomplete first survey (n=183). From the 1939 patients who were included, 1556 consented to be approached for follow-up research, of which 1005 (64.6%) completed the second survey. Patients who did not respond to the second survey were younger and more often had a presumed COVID-19 diagnosis. Further details can be found in a previously published paper [11]. The 1005 patients completed the surveys on average 11.3 weeks (SD 2.2) and 23.5 weeks (SD 2.2) after onset of symptoms (T1 and T2, respectively). Overall, 239 test-diagnosed (hospitalized, n=62, and nonhospitalized, n=177) and 766 presumed (physician-diagnosed, n=454, and patients with no formal diagnosis/testing, n=312) patients with COVID-19 participated in this 2-wave web-based survey (see Multimedia Appendix 5 for the flowchart).
Demographical and Clinical Characteristics
Patients with confirmed COVID-19 were mostly middle-aged women (median age 50.0 years, IQR 39.0-56.0; 198/239 women, 82.8%) with a BMI indicating slight overweight (median BMI 26.0 kg/m2, IQR 23.4-30.5), and they completed the first (T1) and second (T2) survey on average 10.4 weeks (SD 2.4) and 22.6 weeks (SD 2.4) after onset of symptoms. Approximately 1 out of 4 patients (62/239, 25.9%) was hospitalized during the acute phase of COVID-19. The majority of respondents had no self-reported comorbidities (142/239, 59.4%) and good self-reported health status before the infection (208/239, 87%). Moreover, at T1 and T2, a minority of respondents self-reported good health (22/239, 9.2%, and 40/239, 16.7%, respectively [11]). Furthermore, patients retrospectively reported a median of 15 symptoms (IQR 11-18) during the acute phase of COVID-19, and 6 symptoms (IQR 4-9) and 6 symptoms (IQR 3-8) at T1 and T2, respectively. All details regarding patient characteristics can be found in Table 1.
Table 1.
Characteristics of patients with confirmed COVID-19 (n=239).
| Characteristic | Value | |
| Women, n (%) | 198 (82.8) | |
| Age (years), median (IQR) | 50.0 (39.0-56.0) | |
| BMI (kg/m2), median (IQR) | 26.0 (23.4-30.5) | |
| Time between onset of symptoms and T1a survey (weeks), mean (SD) | 10.4 (2.4) | |
| Time between onset of symptoms and T2b survey (weeks), mean (SD) | 22.6 (2.4) | |
| Married/living with partner, n (%) | 173 (72.4) | |
| Educational level, n (%) | ||
|
|
Low | 6 (2.5) |
|
|
Medium | 126 (52.7) |
|
|
High | 107 (44.8) |
| Pre-existing comorbidities, n (%) | ||
|
|
None | 142 (59.4) |
|
|
1 | 62 (25.9) |
|
|
≥2 | 35 (14.6) |
| Health status before infection, n (%) | ||
|
|
Good | 208 (87) |
|
|
Moderate | 28 (11.7) |
|
|
Poor | 3 (1.3) |
| Health status at T1, n (%) | ||
|
|
Good | 22 (9.2) |
|
|
Moderate | 156 (65.3) |
|
|
Poor | 61 (25.5) |
| Number of symptoms, median (IQR) | ||
|
|
During acute infection | 15 (11-18) |
|
|
At T1 | 6 (4-9) |
|
|
At T2 | 6 (3-8) |
| Hospitalized during acute infection, n (%) | 62 (25.9) | |
aT1: time of completing the first survey.
bT2: time of completing the second survey.
Received Health Care
During the first 10 weeks after the onset of symptoms, 2 out of 3 patients (157/239, 65.7%) received or sought care from at least one medical professional (GP: 139/239, 58.2%; medical specialist: 73/239, 30.5%; nurse: 18/239, 7.5%), whereas 1 out of 3 (90/239, 37.7%) received or sought care from at least one allied health care professional (PT: 76/239, 31.8%; psychologist: 27/239, 11.3%; OT: 7/239, 2.9%, dietician: 25/239, 10.5%; and speech and language therapist: 6/239, 2.5%). The cumulative proportion of patients who received care from a medical professional and allied health care professional at T2 respectively increased significantly to 81.2% (194/239; GP: 170/239, 71.1%; medical specialist: 131/239, 54.8%; nurse: 32/239, 13.4%; all P<.001) and 68.6% (164/239; PT: 157/239, 65.7%; psychologist: 55/239, 23%; OT: 27/239, 11.3%; dietician, 51/239, 21.3%; speech and language therapist, 21/239, 8.8%; all P<.001). Furthermore, the cumulative proportion of patients who participated in an interdisciplinary rehabilitation program (in- or outpatient) increased significantly from T1 to T2 (10/239, 4.2%, to 32/239, 13.4%, respectively; P<.001). Details regarding received health care can be found in Table 2.
Table 2.
Fatigue-related measures and received health care in patients with confirmed COVID-19 at on average 10 weeks (T1) and 23 weeks (T2) after onset of symptoms (n=239).
|
|
Value | |||||||||
|
|
|
|
|
T1a | T2b | P value | ||||
| Fatigue-related measures |
|
|||||||||
|
|
General fatigue (points on CIS-Fatiguec scale), median (IQR) | 48 (42-53) | 46 (37-50) | <.001 | ||||||
|
|
Severe fatigue, n (%) | 204 (85.4) | 188 (78.7) | .03 | ||||||
|
|
Mental fatigue (points on self-constructed questions), median (IQR) | 15 (10-17) | 14 (10-17) | .52 | ||||||
|
|
Physical fatigue (points on self-constructed questions), median (IQR) | 19 (16-20) | 18 (14-19) | <.001 | ||||||
| Received health care from a medical professional, n (%) |
|
|||||||||
|
|
Received care from ≥1 medical professionals | 157 (65.7) | 194 (81.2) | <.001 | ||||||
|
|
|
General practitioner | 139 (58.2) | 170 (71.1) | <.001 | |||||
|
|
|
Medical specialist | 73 (30.5) | 131 (54.8) | <.001 | |||||
|
|
|
Nurse | 18 (7.5) | 32 (13.4) | <.001 | |||||
| Received health care from an allied health care provider, n (%) |
|
|||||||||
|
|
Received care from ≥1 allied health care providers | 90 (37.7) | 164 (68.6) | <.001 | ||||||
|
|
|
Physiotherapist | 76 (31.8) | 157 (65.7) | <.001 | |||||
|
|
|
Psychologist | 27 (11.3) | 55 (23) | <.001 | |||||
|
|
|
Occupational therapist | 7 (2.9) | 27 (11.3) | <.001 | |||||
|
|
|
Dietician | 25 (10.5) | 51 (21.3) | <.001 | |||||
|
|
|
Speech and language therapist | 6 (2.5) | 21 (8.8) | <.001 | |||||
| Rehabilitation (in- or outpatient), n (%) | 10 (4.2) | 32 (13.4) | <.001 | |||||||
aT1: time of completing the first survey.
bT2: time of completing the second survey.
cCIS-Fatigue: Checklist Individual Strength–subscale subjective fatigue.
Standardized Fatigue Questionnaire
Patients with confirmed COVID-19 reported a median CIS-Fatigue score of 48 points (IQR 42-53) at T1. The majority (204/239, 85.4%) reported severe fatigue at approximately 3 months after the onset of COVID-19 symptoms. The median CIS-Fatigue score improved significantly between T1 and T2 (median change –2 points, IQR –7 to 3; P<.001) (Table 2), whereas no significant differences were found in the proportions of normal, mild, or severe fatigue (P=.12). An overview of the proportions of patients with normal, mild, and severe fatigue at T1 and T2, the proportional flow, and the direction of change can be found in Figure 1. In addition, Multimedia Appendix 6 shows the proportion, flow, and direction of the change of fatigue stratified for the type of diagnosis (ie, hospitalized and nonhospitalized test-diagnosed patients, physician-diagnosed patients, and patients without a formal diagnosis/test).
Figure 1.

Prevalence and change in fatigue in patients with long COVID who have confirmed COVID-19, measured using the CIS-Fatigue scale at on average 10 (T1) and 23 (T2) weeks after onset of symptoms (n=239). The width of the lines is proportional to the flow rate. No significant change in the prevalence of normal (≤26 points), mild (27-35 points), or severe (≥36 points) fatigue was found between T1 and T2 (McNemar-Bowker test, P=.12). CIS-Fatigue: Checklist Individual Strength–subscale subjective fatigue; T1: time of completing the first survey; T2: time of completing the second survey.
Self-constructed Physical and Mental Fatigue Questions
Patients with confirmed COVID-19 reported median physical and mental fatigue scores of 19 points (IQR 16-20) and 15 points (IQR 10-17) at T1. Between T1 and T2, a significant decrease was found in physical fatigue score (median change –1 point, IQR –3 to 0; P<.001), but not in mental fatigue score (median change 0 points, IQR –3 to 3; P=.52) (Table 2). Figure 2 shows the distributions of patients across the spectrum of physical and mental fatigue at T1 and T2.
Figure 2.
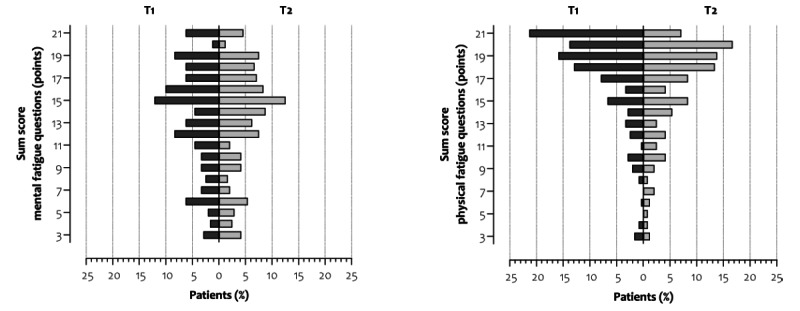
The distribution of patients with long COVID who have confirmed COVID-19 across the spectrum of self-constructed mental (left) and physical (right) fatigue at on average 10 (T1) and 23 (T2) weeks after onset of symptoms (n=239). Scores range from 3 to 21 points, and higher scores indicate higher levels of fatigue. T1: time of completing the first survey; T2: time of completing the second survey.
Discussion
Principal Findings
To the best of our knowledge, this is the first study to measure fatigue over time in members from online long COVID peer support groups with confirmed COVID-19 using a validated and standardized measurement with generic cutoff values to determine normal, mild, and severe fatigue. Our study indicates that severe fatigue is highly prevalent in patients with long COVID at approximately 3 and 6 months after the infection. Furthermore, our longitudinal follow-up data suggest that fatigue does not resolve over time in all patients, even if they receive health care. In addition, patients experience both physical and mental fatigue.
Fatigue is the most prominent symptom in patients with long COVID [2,4], irrespective of the severity of the initial infection [14]. Nevertheless, most studies are cross-sectional and use a binary question (eg, fatigued/not fatigued) to assess the prevalence of fatigue [2,16,39]. Therefore, little is known about the change in fatigue intensity over time [14,40]. Our study used a validated and standardized questionnaire to assess fatigue and was able to quantify fatigue intensity. Indeed, fatigue is highly prevalent in our sample. Moreover, fatigue was reported to be generally high. The median fatigue scores found in our sample are equal to or higher than those of other chronic diseases that are characterized by fatigue, such as chronic obstructive pulmonary disease [41], asthma [42], Q fever [20], multiple sclerosis [43], rheumatoid arthritis [44], or systemic sclerosis [45]. These findings are remarkable for such a young population with few self-reported comorbidities and good self-reported health status before the infection. Previously, other viral and nonviral infections have been linked to prolonged and debilitating fatigue [20,46-50]. For example, Lam and colleagues investigated long-term complaints in SARS survivors and found that approximately one-third of SARS survivors met the modified 1994 US Centers for Disease Control and Prevention criteria for chronic fatigue syndrome more than 3 years after having SARS [17]. Moreover, MERS survivors often experience chronic fatigue [19]. For patients with long COVID, it remains unclear whether fatigue will resolve spontaneously in the longer term. Our follow-up data show little to no improvement in the proportion of patients with severe fatigue between 3 and 6 months, despite receiving medical and allied health care. Consequently, almost two-thirds of the patients in our sample are progressing toward chronic fatigue (ie, severe fatigue that persists longer than six months [51]). The fact that some patients may experience debilitating chronic fatigue is worrisome and could have a major long-term impact upon these individuals as well as on the health care system and society as a whole [10,11,52]. Indeed, fatigue is strongly related to health-related QoL and aspects of day-to-day life [14,25,53,54], and it often involves sick leave, increased health care consumption, and more hidden costs, such as informal care by friends or family members [55-57].
Fatigue is a complex and challenging symptom, as multiple factors can play a role in the initiation and maintenance of fatigue, as seen in other chronic diseases [58]. It can present itself as mental fatigue, physical fatigue, or both [40]. Therefore, a patient-tailored treatment based upon a holistic and comprehensive assessment of systemic, physical, psychological, and behavioral factors is proposed to alleviate the fatigue symptom burden [59]. To date, it remains unknown which treatment strategies are effective to improve fatigue in patients with long COVID. Several treatment strategies for fatigue are proposed based upon knowledge from the fast-growing evidence regarding COVID-19 and other pathologies, such as multidisciplinary rehabilitation, energy conservation techniques, pacing, cognitive behavioral therapy, graded exercise therapy, or physical training [25,54,60-65]. Future research needs to provide evidence regarding underlying pathways, evaluate the effectiveness of existing treatment strategies, and identify susceptible candidates, as it is expected that not everyone will benefit from the same treatment strategy due to the multifactorial nature of fatigue. Moreover, anecdotal evidence shows that patients report having within-day and between-day variations in their daily symptoms, including fatigue [54,66,67]; these cannot be captured in detail by completing a questionnaire once or twice over a longer period of time. In this, the use of an ecological momentary assessment may be valuable, as this approach involves repeated measurements of the participant’s symptoms, behavior, and context in vivo and in real time [68]. More insights in diurnal variation in fatigue and its association with other symptoms may be useful in the development of more tailored treatment strategies for fatigue in patients with long COVID.
Methodological Considerations and Limitations
The current study has several limitations. First, the survey was only made available to members of online long COVID peer support groups. This probably caused selection bias, as it is reasonable to assume that patients with high symptom burden are more likely to become members of online long COVID peer support groups. Second, all results were collected using a web-based survey. Therefore, besides the self-reported symptoms, the patients’ height, body weight, and medical status before and during the infection were also based on self-report, which may have affected the internal validity of the current findings to some extent. Recently, the National Institute for Health and Care Excellence [69] proposed a case definition of long COVID whereby alternative diagnosis should be excluded when identifying patients with long COVID. Due to the nature and timing of this study (ie, early phase of the pandemic), this was not possible in the current study. Third, approximately 1 out of 3 participants who consented to be approached for follow-up research did not respond to the second wave of the survey. The authors have no information about the possible reasons for not responding to the second wave of the survey, although a between-group comparison was made to find possible differences [11]. Fourth, the majority of our sample were women, which limits our external validity. Nevertheless, evidence is growing that women are more prone to develop long COVID [70]. Fifth, self-constructed questions were used to quantify mental fatigue, although validated questionnaires (such as the Chalder fatigue index) to assess mental (and physical) fatigue are available [71]. Therefore, no definite conclusions on the burden of mental fatigue in online long COVID peer support groups can be drawn based on the current study. Nevertheless, the current results indicate that COVID-19 can impact both physical and mental fatigue in the long term. Furthermore, this study was conducted in adults, although evidence regarding long COVID in children and adolescents is starting to emerge [72,73].
Conclusions
Severe fatigue is highly prevalent in members of online long COVID peer support groups both at approximately 3 and 6 months after onset of symptoms. As not enough time has passed since the start of the COVID-19 pandemic, it is unclear whether this fatigue will resolve spontaneously in the longer term. Future research needs to focus on the prognosis, possible causes, and treatment strategies for physical and mental fatigue in patients with long COVID.
Acknowledgments
The research team acknowledges the valuable input from the patient representatives to develop the survey, and the technical support by ASolutions’ Martijn Briejers and Oscar Wagemakers. The scientific work of YMJG is financially supported by Lung Foundation Netherlands grant 4.1.16.085, FVCM is financially supported by ZonMW (ERACoSysMed grant 90030355), and RM is financially supported by Lung Foundation Netherlands grant 5.1.18.232.
Abbreviations
- CIS-Fatigue
Checklist Individual Strength–subscale subjective fatigue
- CT
computed tomography
- GP
general practitioner
- ICU
intensive care unit
- MERS
Middle East respiratory syndrome
- OT
occupational therapist
- PCR
polymerase chain reaction
- PT
physiotherapist
- QoL
quality of life
- SARS
severe acute respiratory syndrome
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- T1
time point of completing the first survey
- T2
time point of completing the second survey
Members of online long COVID peer support groups with presumed COVID-19 (n=766): characteristics, received health care, and fatigue-related measures.
Additional information regarding self-reported pre-existing comorbidities and symptoms during the acute phase of COVID-19 and at the moment of completing the surveys.
The Checklist Individual Strength–subscale subjective fatigue.
Self-constructed physical and mental fatigue based upon 3 items of the Checklist Individual Strength–subscale subjective fatigue.
Flowchart of participants’ inclusion.
Prevalence of normal, mild, and severe fatigue using the Checklist Individual Strength–subscale subjective fatigue at T1 and T2, the proportional flow, and the direction of change of fatigue stratified for type of diagnosis.
Footnotes
Conflicts of Interest: MAS reports grants from Netherlands Lung Foundation, AstraZeneca, Boehringer Ingelheim, and Stichting Astma Bestrijding, all outside the submitted work. FMEF reports grants and personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Chiesi, personal fees from GlaxoSmithKline, grants and personal fees from Novartis, and personal fees from TEVA, outside the submitted work. RP reports personal fees from MEDtalk and Health Investment. DJAJ has received lecture fees from Chiesi and Boehringer Ingelheim in the last 3 years, which are unrelated to this paper. All other authors declare that they have no conflicts of interest. No financial support was received for the preparation of this manuscript.
References
- 1.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Ho A, Russell CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG, ISARIC4C investigators Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020 May 22;369:m1985. doi: 10.1136/bmj.m1985. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=32444460 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020 Aug 11;324(6):603. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021 Jan 16;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. http://europepmc.org/abstract/MED/33428867 .S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goërtz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, Houben-Wilke S, Burtin C, Posthuma R, Franssen FME, van Loon N, Hajian B, Spies Y, Vijlbrief H, van 't Hul AJ, Janssen DJA, Spruit MA. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020 Oct;6(4):00542-2020. doi: 10.1183/23120541.00542-2020. http://europepmc.org/abstract/MED/33257910 .00542-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahase E. Covid-19: What do we know about "long covid"? BMJ. 2020 Jul 14;370:m2815. doi: 10.1136/bmj.m2815. [DOI] [PubMed] [Google Scholar]
- 6.Nabavi N. Long covid: How to define it and how to manage it. BMJ. 2020 Sep 07;370:m3489. doi: 10.1136/bmj.m3489. [DOI] [PubMed] [Google Scholar]
- 7.Wong AW, Shah AS, Johnston JC, Carlsten C, Ryerson CJ. Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur Respir J. 2020 Nov;56(5):2003276. doi: 10.1183/13993003.03276-2020. http://erj.ersjournals.com:4040/lookup/pmidlookup?view=long&pmid=33008936 .13993003.03276-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meys R, Delbressine JM, Goërtz YMJ, Vaes AW, Machado FVC, Van Herck M, Burtin C, Posthuma R, Spaetgens B, Franssen FME, Spies Y, Vijlbrief H, Van't Hul AJ, Janssen DJA, Spruit MA, Houben-Wilke S. Generic and respiratory-specific quality of life in non-hospitalized patients with COVID-19. J Clin Med. 2020 Dec 09;9(12):3993. doi: 10.3390/jcm9123993. https://www.mdpi.com/resolver?pii=jcm9123993 .jcm9123993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaes AW, Machado FVC, Meys R, Delbressine JM, Goertz YMJ, Van Herck M, Houben-Wilke S, Franssen FME, Vijlbrief H, Spies Y, Van 't Hul AJ, Burtin C, Janssen DJA, Spruit MA. Care dependency in non-hospitalized patients with COVID-19. J Clin Med. 2020 Sep 12;9(9):2946. doi: 10.3390/jcm9092946. https://www.mdpi.com/resolver?pii=jcm9092946 .jcm9092946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenforde MW, Kim SS, Lindsell CJ, Billig Rose E, Shapiro NI, Files DC, Gibbs KW, Erickson HL, Steingrub JS, Smithline HA, Gong MN, Aboodi MS, Exline MC, Henning DJ, Wilson JG, Khan A, Qadir N, Brown SM, Peltan ID, Rice TW, Hager DN, Ginde AA, Stubblefield WB, Patel MM, Self WH, Feldstein LR, Network Investigators IVY, CDC COVID-19 Response Team. Network Investigators IVY. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020 Jul 31;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaes AW, Goërtz YMJ, Van Herck M, Machado FVC, Meys R, Delbressine JM, Houben-Wilke S, Gaffron S, Maier D, Burtin C, Posthuma R, van Loon NPH, Franssen FME, Hajian B, Simons SO, van Boven JFM, Klok FA, Spaetgens B, Pinxt CM, Liu LY, Wesseling G, Spies Y, Vijlbrief H, van 't Hul Alex J, Janssen DJ, Spruit MA. Recovery from COVID-19: a sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Res. 2021 Apr 26;7(2):00141-2021. doi: 10.1183/23120541.00141-2021. http://europepmc.org/abstract/MED/34041295 .00141-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belli S, Balbi B, Prince I, Cattaneo D, Masocco F, Zaccaria S, Bertalli L, Cattini F, Lomazzo A, Dal Negro F, Giardini M, Franssen FME, Janssen DJA, Spruit MA. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur Respir J. 2020 Oct;56(4):2002096. doi: 10.1183/13993003.02096-2020. http://erj.ersjournals.com:4040/lookup/pmidlookup?view=long&pmid=32764112 .13993003.02096-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Writing Committee for the COMEBAC Study Group. Morin L, Savale L, Pham T, Colle R, Figueiredo S, Harrois A, Gasnier M, Lecoq A, Meyrignac O, Noel N, Baudry E, Bellin M, Beurnier A, Choucha W, Corruble E, Dortet L, Hardy-Leger I, Radiguer F, Sportouch S, Verny C, Wyplosz B, Zaidan M, Becquemont L, Montani D, Monnet X. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021 Apr 20;325(15):1525–1534. doi: 10.1001/jama.2021.3331.2777787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend L, Dyer Adam H, Jones Karen, Dunne Jean, Mooney Aoife, Gaffney Fiona, O'Connor Laura, Leavy Deirdre, O'Brien Kate, Dowds Joanne, Sugrue Jamie A, Hopkins David, Martin-Loeches Ignacio, Ni Cheallaigh Cliona, Nadarajan Parthiban, McLaughlin Anne Marie, Bourke Nollaig M, Bergin Colm, O'Farrelly Cliona, Bannan Ciaran, Conlon Niall. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11):e0240784. doi: 10.1371/journal.pone.0240784. https://dx.plos.org/10.1371/journal.pone.0240784 .PONE-D-20-22733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ream E, Richardson A. Fatigue: a concept analysis. Int J Nurs Stud. 1996 Oct;33(5):519–29. doi: 10.1016/0020-7489(96)00004-1.0020-7489(96)00004-1 [DOI] [PubMed] [Google Scholar]
- 16.Garrigues E, Janvier P, Kherabi Y, Le Bot A, Hamon A, Gouze H, Doucet L, Berkani S, Oliosi E, Mallart E, Corre F, Zarrouk V, Moyer J, Galy A, Honsel V, Fantin B, Nguyen Y. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020 Dec;81(6):e4–e6. doi: 10.1016/j.jinf.2020.08.029. http://europepmc.org/abstract/MED/32853602 .S0163-4453(20)30562-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam MH, Wing Y, Yu MW, Leung C, Ma RCW, Kong APS, So WY, Fong SY, Lam S. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009 Dec 14;169(22):2142–7. doi: 10.1001/archinternmed.2009.384.169/22/2142 [DOI] [PubMed] [Google Scholar]
- 18.Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011 Mar 24;11(1):37. doi: 10.1186/1471-2377-11-37. https://bmcneurol.biomedcentral.com/articles/10.1186/1471-2377-11-37 .1471-2377-11-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SH, Shin H, Park HY, Kim JL, Lee JJ, Lee H, Won S, Han W. Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in Middle East respiratory syndrome survivors. Psychiatry Investig. 2019 Jan;16(1):59–64. doi: 10.30773/pi.2018.10.22.3. http://psychiatryinvestigation.org/journal/view.php?doi=10.30773/pi.2018.10.22.3 .pi.2018.10.22.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bronner MB, Haagsma JA, Dontje ML, Barmentloo L, Kouwenberg RMCEJ, Olde Loohuis AGM, de Groot A, Erasmus V, Polinder S. Long-term impact of a Q-fever outbreak: an evaluation of health symptoms, health-related quality of life, participation and health care satisfaction after ten years. J Psychosom Res. 2020 Dec;139:110258. doi: 10.1016/j.jpsychores.2020.110258. http://europepmc.org/abstract/MED/33069049 .S0022-3999(20)30820-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karshikoff B, Sundelin T, Lasselin J. Role of inflammation in human fatigue: relevance of multidimensional assessments and potential neuronal mechanisms. Front Immunol. 2017;8:21. doi: 10.3389/fimmu.2017.00021. doi: 10.3389/fimmu.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corona patiënten met langdurige klachten (Nederland) Facebook. [2020-06-04]. https://www.facebook.com/groups/236723204035929.9 .
- 23.Corona patiënten (met langdurige klachten) (Vlaanderen) Facebook. [2020-06-04]. https://www.facebook.com/groups/241043323639334/
- 24.Coronaplein. Website in Dutch. Long Fonds. [2021-09-09]. https://coronaplein.nu/
- 25.Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020 Aug 11;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 26.Machado FVC, Meys R, Delbressine JM, Vaes AW, Goërtz YMJ, van Herck M, Houben-Wilke S, Boon GJAM, Barco S, Burtin C, van 't Hul A, Posthuma R, Franssen FME, Spies Y, Vijlbrief H, Pitta F, Rezek SA, Janssen DJA, Siegerink B, Klok FA, Spruit MA. Construct validity of the Post-COVID-19 Functional Status Scale in adult subjects with COVID-19. Health Qual Life Outcomes. 2021 Feb 03;19(1):40. doi: 10.1186/s12955-021-01691-2. https://hqlo.biomedcentral.com/articles/10.1186/s12955-021-01691-2 .10.1186/s12955-021-01691-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houben-Wilke S, Delbressine JM, Vaes AW, Goërtz Yvonne Mj, Meys R, Machado FV, Van Herck M, Burtin C, Posthuma R, Franssen FM, van Loon NH, Hajian B, Vijlbrief H, Spies Y, van 't Hul Alex, Janssen DJ, Spruit MA. Understanding and being understood: information and care needs of 2113 patients with confirmed or suspected COVID-19. J Patient Exp. 2021 Mar 08;8:2374373521997222. doi: 10.1177/2374373521997222. https://journals.sagepub.com/doi/10.1177/2374373521997222?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed .10.1177_2374373521997222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche Peter C, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014 Dec;12(12):1500–24. doi: 10.1016/j.ijsu.2014.07.014. https://linkinghub.elsevier.com/retrieve/pii/S1743-9191(14)00213-1 .S1743-9191(14)00213-1 [DOI] [PubMed] [Google Scholar]
- 29.ASolutions. Webpage in Dutch. [2021-09-09]. https://www.asolutions.nl/
- 30.International Standard Classification of Education ISCED 2011. UNESCO Institute for Statistics. [2021-09-09]. http://uis.unesco.org/sites/default/files/documents/international-standard-classification-of-education-isced-2011-en.pdf .
- 31.Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer J W, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res. 1994 Jul;38(5):383–92. doi: 10.1016/0022-3999(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 32.Vercoulen J, Alberts M, Bleijenberg G. De Checklist Individual Strength (CIS) Gedragstherapie. 1999;32:131–6. doi: 10.1037/t11003-000. [DOI] [Google Scholar]
- 33.Worm-Smeitink M, Gielissen M, Bloot L, van Laarhoven HWM, van Engelen BGM, van Riel P, Bleijenberg G, Nikolaus S, Knoop H. The assessment of fatigue: psychometric qualities and norms for the Checklist individual strength. J Psychosom Res. 2017 Jul;98:40–46. doi: 10.1016/j.jpsychores.2017.05.007.S0022-3999(17)30112-5 [DOI] [PubMed] [Google Scholar]
- 34.Beurskens AJ, Bültmann U, Kant I, Vercoulen JH, Bleijenberg G, Swaen GM. Fatigue among working people: validity of a questionnaire measure. Occup Environ Med. 2000 May;57(5):353–7. doi: 10.1136/oem.57.5.353. https://oem.bmj.com/lookup/pmidlookup?view=long&pmid=10769302 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panitz S, Kornhuber M, Hanisch F. The checklist individual strength (CIS20-R) in patients with amyotrophic lateral sclerosis - a longitudinal study. Acta Neurol Scand. 2015 Jun;131(6):372–80. doi: 10.1111/ane.12349. [DOI] [PubMed] [Google Scholar]
- 36.van Koulil S, Kraaimaat FW, van Lankveld W, van Riel PLCM, Evers AWM. A patient's perspective on multidisciplinary treatment gain for fibromyalgia: an indicator for pre-post treatment effects? Arthritis Rheum. 2009 Dec 15;61(12):1626–32. doi: 10.1002/art.24792. doi: 10.1002/art.24792. [DOI] [PubMed] [Google Scholar]
- 37.Evers AWM, Kraaimaat FW, van Riel Piet L C M, de Jong Alphons J L. Tailored cognitive-behavioral therapy in early rheumatoid arthritis for patients at risk: a randomized controlled trial. Pain. 2002 Nov;100(1-2):141–53. doi: 10.1016/s0304-3959(02)00274-9.S0304395902002749 [DOI] [PubMed] [Google Scholar]
- 38.SankeyMATIC: a Sankey diagram builder for everyone. SankeyMATIC. [2021-04-08]. http://sankeymatic.com/
- 39.Daher A, Balfanz P, Cornelissen C, Müller Annegret, Bergs I, Marx N, Müller-Wieland Dirk, Hartmann B, Dreher M, Müller Tobias. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174:106197. doi: 10.1016/j.rmed.2020.106197. http://europepmc.org/abstract/MED/33120193 .S0954-6111(20)30337-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Prevalence and determinants of fatigue after COVID-19 in non-hospitalized subjects: a population-based study. Int J Environ Res Public Health. 2021 Feb 19;18(4):2030. doi: 10.3390/ijerph18042030. https://www.mdpi.com/resolver?pii=ijerph18042030 .ijerph18042030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goërtz YMJ, Spruit MA, Van 't Hul AJ, Peters JB, Van Herck M, Nakken N, Djamin RS, Burtin C, Thong MSY, Coors A, Meertens-Kerris Y, Wouters EFM, Prins JB, Franssen FME, Muris JWM, Vanfleteren LEGW, Sprangers MAG, Janssen DJA, Vercoulen JH. Fatigue is highly prevalent in patients with COPD and correlates poorly with the degree of airflow limitation. Ther Adv Respir Dis. 2019;13:1753466619878128. doi: 10.1177/1753466619878128. https://journals.sagepub.com/doi/10.1177/1753466619878128?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Herck M, Spruit MA, Burtin C, Djamin R, Antons J, Goërtz YMJ, Ebadi Z, Janssen DJA, Vercoulen JH, Peters JB, Thong MSY, Otker J, Coors A, Sprangers MAG, Muris JWM, Wouters EFM, van 't Hul AJ. Fatigue is highly prevalent in patients with asthma and contributes to the burden of disease. J Clin Med. 2018 Nov 23;7(12):471. doi: 10.3390/jcm7120471. https://www.mdpi.com/resolver?pii=jcm7120471 .jcm7120471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Heugten C, Köhler S, Francke M, Bol Y. The association between executive functioning, coping styles and depressive symptoms in patients with Multiple Sclerosis. Mult Scler Relat Disord. 2019 Nov;36:101392. doi: 10.1016/j.msard.2019.101392.S2211-0348(19)30372-4 [DOI] [PubMed] [Google Scholar]
- 44.van Hoogmoed D, Fransen J, Bleijenberg G, van Riel P. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford) 2010 Jul;49(7):1294–302. doi: 10.1093/rheumatology/keq043.keq043 [DOI] [PubMed] [Google Scholar]
- 45.Kwakkenbos L, van Lankveld WGJM, Vonk MC, Becker ES, van den Hoogen FHJ, van den Ende CHM. Disease-related and psychosocial factors associated with depressive symptoms in patients with systemic sclerosis, including fear of progression and appearance self-esteem. J Psychosom Res. 2012 Mar;72(3):199–204. doi: 10.1016/j.jpsychores.2011.12.005. https://linkinghub.elsevier.com/retrieve/pii/S0022-3999(11)00311-4 .S0022-3999(11)00311-4 [DOI] [PubMed] [Google Scholar]
- 46.Sigal LH. Summary of the first 100 patients seen at a Lyme disease referral center. Am J Med. 1990 Jun;88(6):577–81. doi: 10.1016/0002-9343(90)90520-n.0002-9343(90)90520-N [DOI] [PubMed] [Google Scholar]
- 47.Straus SE, Tosato G, Armstrong G, Lawley T, Preble OT, Henle W, Davey R, Pearson G, Epstein J, Brus I. Persisting illness and fatigue in adults with evidence of Epstein-Barr virus infection. Ann Intern Med. 1985 Jan;102(1):7–16. doi: 10.7326/0003-4819-102-1-7. [DOI] [PubMed] [Google Scholar]
- 48.Voss JG. Predictors and correlates of fatigue in HIV/AIDS. J Pain Symptom Manage. 2005 Feb;29(2):173–84. doi: 10.1016/j.jpainsymman.2004.05.006. https://linkinghub.elsevier.com/retrieve/pii/S0885-3924(04)00534-2 .S0885-3924(04)00534-2 [DOI] [PubMed] [Google Scholar]
- 49.Schanke AK, Stanghelle JK. Fatigue in polio survivors. Spinal Cord. 2001 May;39(5):243–51. doi: 10.1038/sj.sc.3101147. [DOI] [PubMed] [Google Scholar]
- 50.Epstein L, Wong KK, Kallen AJ, Uyeki TM. Post-Ebola signs and symptoms in U.S. survivors. N Engl J Med. 2015 Dec 17;373(25):2484–6. doi: 10.1056/NEJMc1506576. [DOI] [PubMed] [Google Scholar]
- 51.Korenromp IHE, Meeus M, Bleijenberg G. Dutch language area definition of chronic fatigue. Article in Dutch. Ned Tijdschr Geneeskd. 2012;156(16):A4403. [PubMed] [Google Scholar]
- 52.Del Rio Carlos, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA. 2020 Nov 03;324(17):1723–1724. doi: 10.1001/jama.2020.19719.2771581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garner P. The BMJ opinion: COVID-19 and fatigue – a game of snakes and ladders. BMJ Opinion. 2020. https://blogs.bmj.com/bmj/2020/05/19/paul-garner-covid-19-and-fatigue-a-game-of-snakes-and-ladders/
- 54.Humphreys H, Kilby L, Kudiersky N, Copeland R. Long COVID and the role of physical activity: a qualitative study. BMJ Open. 2021 Mar 10;11(3):e047632. doi: 10.1136/bmjopen-2020-047632. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=33692189 .bmjopen-2020-047632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janssen N, Kant IJ, Swaen GMH, Janssen PPM, Schröer C A P. Fatigue as a predictor of sickness absence: results from the Maastricht cohort study on fatigue at work. Occup Environ Med. 2003 Jun;60(Suppl 1):i71–6. doi: 10.1136/oem.60.suppl_1.i71. https://oem.bmj.com/lookup/pmidlookup?view=long&pmid=12782750 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCrone P, Darbishire L, Ridsdale L, Seed P. The economic cost of chronic fatigue and chronic fatigue syndrome in UK primary care. Psychol Med. 2003 Feb;33(2):253–61. doi: 10.1017/s0033291702006980. [DOI] [PubMed] [Google Scholar]
- 57.Sabes-Figuera R, McCrone P, Hurley M, King M, Donaldson AN, Ridsdale L. The hidden cost of chronic fatigue to patients and their families. BMC Health Serv Res. 2010 Mar 04;10:56. doi: 10.1186/1472-6963-10-56. https://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-10-56 .1472-6963-10-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menting J, Tack CJ, Bleijenberg G, Donders R, Droogleever Fortuyn HA, Fransen J, Goedendorp MM, Kalkman JS, Strik-Albers R, van Alfen N, van der Werf SP, Voermans NC, van Engelen BG, Knoop H. Is fatigue a disease-specific or generic symptom in chronic medical conditions? Health Psychol. 2018 Jun;37(6):530–543. doi: 10.1037/hea0000598.2018-23402-001 [DOI] [PubMed] [Google Scholar]
- 59.Spruit MA, Vercoulen JH, Sprangers MAG, Wouters EFM, FAntasTIGUE consortium Fatigue in COPD: an important yet ignored symptom. Lancet Respir Med. 2017 Jul;5(7):542–544. doi: 10.1016/S2213-2600(17)30158-3.S2213-2600(17)30158-3 [DOI] [PubMed] [Google Scholar]
- 60.Ferraro F, Calafiore D, Dambruoso F, Guidarini S, de Sire A. COVID-19 related fatigue: which role for rehabilitation in post-COVID-19 patients? A case series. J Med Virol. 2021 Apr;93(4):1896–1899. doi: 10.1002/jmv.26717. [DOI] [PubMed] [Google Scholar]
- 61.Vink M, Vink-Niese A. Could cognitive behavioural therapy be an effective treatment for long COVID and post COVID-19 fatigue syndrome? Lessons from the Qure Study for Q-Fever Fatigue Syndrome. Healthcare (Basel) 2020 Dec 11;8(4):552. doi: 10.3390/healthcare8040552. https://www.mdpi.com/resolver?pii=healthcare8040552 .healthcare8040552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Herck M, Antons J, Vercoulen JH, Goërtz YMJ, Ebadi Z, Burtin C, Janssen DJA, Thong MSY, Otker J, Coors A, Sprangers MAG, Muris JWM, Prins JB, Spruit MA, Peters JB. Pulmonary rehabilitation reduces subjective fatigue in COPD: a responder analysis. J Clin Med. 2019 Aug 20;8(8):1264. doi: 10.3390/jcm8081264. https://www.mdpi.com/resolver?pii=jcm8081264 .jcm8081264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rimes KA, Chalder T. Treatments for chronic fatigue syndrome. Occup Med (Lond) 2005 Jan;55(1):32–9. doi: 10.1093/occmed/kqi015.55/1/32 [DOI] [PubMed] [Google Scholar]
- 64.Gloeckl R, Leitl D, Jarosch I, Schneeberger T, Nell C, Stenzel N, Vogelmeier CF, Kenn K, Koczulla AR. Benefits of pulmonary rehabilitation in COVID-19: a prospective observational cohort study. ERJ Open Res. 2021 Apr 11;7(2):00108-2021. doi: 10.1183/23120541.00108-2021. http://europepmc.org/abstract/MED/34095290 .00108-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daynes E, Gerlis C, Chaplin E, Gardiner N, Singh SJ. Early experiences of rehabilitation for individuals post-COVID to improve fatigue, breathlessness exercise capacity and cognition - A cohort study. Chron Respir Dis. 2021;18:14799731211015691. doi: 10.1177/14799731211015691. https://journals.sagepub.com/doi/10.1177/14799731211015691?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y, Redfield S, Austin JP, Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021 Aug;38:101019. doi: 10.1016/j.eclinm.2021.101019. https://linkinghub.elsevier.com/retrieve/pii/S2589-5370(21)00299-6 .S2589-5370(21)00299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ladds E, Rushforth A, Wieringa S, Taylor S, Rayner C, Husain L, Greenhalgh T. Persistent symptoms after Covid-19: qualitative study of 114 "long Covid" patients and draft quality principles for services. BMC Health Serv Res. 2020 Dec 20;20(1):1144. doi: 10.1186/s12913-020-06001-y. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-020-06001-y .10.1186/s12913-020-06001-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maes IHL, Delespaul PAEG, Peters ML, White MP, van Horn Y, Schruers K, Anteunis L, Joore M. Measuring health-related quality of life by experiences: the experience sampling method. Value Health. 2015 Jan;18(1):44–51. doi: 10.1016/j.jval.2014.10.003. https://linkinghub.elsevier.com/retrieve/pii/S1098-3015(14)04728-7 .S1098-3015(14)04728-7 [DOI] [PubMed] [Google Scholar]
- 69.COVID-19 rapid guideline: managing the long-term effects of COVID-19. National Institute for Health and Care Excellence. [2021-01-21]. https://www.nice.org.uk/guidance/ng188 . [PubMed]
- 70.Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, Pujol JC, Klaser K, Antonelli M, Canas LS, Molteni E, Modat M, Jorge Cardoso M, May A, Ganesh S, Davies R, Nguyen LH, Drew DA, Astley CM, Joshi AD, Merino J, Tsereteli N, Fall T, Gomez MF, Duncan EL, Menni C, Williams FMK, Franks PW, Chan AT, Wolf J, Ourselin S, Spector T, Steves CJ. Attributes and predictors of long COVID. Nat Med. 2021 Apr;27(4):626–631. doi: 10.1038/s41591-021-01292-y.10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, Wallace EP. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147–53. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 72.Hertting O. More research is needed on the long-term effects of COVID-19 on children and adolescents. Acta Paediatr. 2021 Mar;110(3):744–745. doi: 10.1111/apa.15731. http://europepmc.org/abstract/MED/33395729 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Magnusson K, Skyrud K, Suren P, Greve-Isdahl M, Størdal K, Kristoffersen D. Health care use up to 6 months after COVID-19 in 700.000 children and adolescents: a pre-post study. medRxiv. doi: 10.1101/2021.06.02.21258211. Preprint posted online on June 05, 2021. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Members of online long COVID peer support groups with presumed COVID-19 (n=766): characteristics, received health care, and fatigue-related measures.
Additional information regarding self-reported pre-existing comorbidities and symptoms during the acute phase of COVID-19 and at the moment of completing the surveys.
The Checklist Individual Strength–subscale subjective fatigue.
Self-constructed physical and mental fatigue based upon 3 items of the Checklist Individual Strength–subscale subjective fatigue.
Flowchart of participants’ inclusion.
Prevalence of normal, mild, and severe fatigue using the Checklist Individual Strength–subscale subjective fatigue at T1 and T2, the proportional flow, and the direction of change of fatigue stratified for type of diagnosis.


