Abstract
Background.
Hepatitis B vaccination is recommended for persons with current or past sexually transmitted infections (STI). Our aim is to systematically assess the association of hepatitis B virus (HBV) sero-markers for current or past infection with syphilis, chlamydia, gonorrhea, or unspecified STIs.
Methods.
We conducted a systematic review and meta-analysis. PubMed, Embase, and Web of Science from 1982 –2018 were searched using MeSH terms for HBV, STIs and epidemiology. We included studies conducted in Organization for Economic Cooperation and Development countries or Latin America that permit the calculation of prevalence ratios (PRs) for HBV and STIs and extracted PRs and counts by HBV and STI status.
Results.
Of 3,144 identified studies, 43 met inclusion requirements, yielding 72 PRs. We stratified outcomes by HBV sero-markers (surface antigen [HBsAg], core antigen [anti-HBc], combined ), STI pathogen (syphilis, gonorrhea/chlamydia, unspecified), and STI history (current, past) resulting in 18 potential outcome groups, for which results were available for 14. For the four outcome groups related to HBsAg, PR point estimates ranged from 1.65 – 6.76. For the five outcome groups related to anti-HBc, PRs ranged from 1.30 – 1.82; and for the five outcome groups related to combined HBV markers, PRs ranged from 1.15 – 1.89). The median HBsAg prevalence among people with a current or past STI was 4.17; not all studies reported HBsAg. Study settings and populations varied.
Conclusion.
This review found evidence of association between HBV infection and current or past STIs.
Keywords: systematic review, meta-analysis, hepatitis B, sexually transmitted infections, gonorrhea, chlamydia, syphilis
Summary:
Chronic hepatitis B virus (HBV) infection is a major source of morbidity and mortality in the United States and the most common source of HBV infection among adults is sexual contact. We conducted a comprehensive systematic review and meta-analysis of the association of hepatitis B prevalence among persons with current and past sexually transmitted infection and found the prevalence of hepatitis B significantly associated with sexually transmitted infection. These findings can inform potential recommendations for expanded hepatitis B testing.
Introduction
Chronic hepatitis B virus (HBV) infection is a major source of morbidity and mortality worldwide. An estimated 257 million persons worldwide are chronically infected with HBV, which results in approximately 887,000 deaths annually (1). In the United States, 847,000 persons were living with the disease in 2011–2012 (2) and 1,698 deaths were attributed to HBV in 2016 (3). About two-thirds of persons with chronic HBV infection were unaware of their infection, and an estimated 15% of treatment-eligible patients are receiving treatment (4, 5).
Chronic HBV infection is defined as the presence of hepatitis B surface antigen (HBsAg) for more than 6 months. Prevalence is highest among persons born in the Pacific Islands, Asia and Africa (2, 6). Other high-burden populations include men who have sex with men (MSM), persons who inject drugs (PWID), persons who have been incarcerated, and sexual and household contacts of persons with HBV infection (7). The Centers for Disease Control and Prevention (CDC) recommends screening for hepatitis B among person at risk; however current guidelines do not include testing persons with a history of STI (8).
Since both sexually transmitted infections (STI) and HBV infections can be transmitted through unprotected sexual contact, persons with current or past STI are at elevated risk for HBV infection. As a result, CDC recommends hepatitis B vaccination in adults seeking evaluation or treatment for STIs (9). While CDC does not recommend pre-vaccination testing for persons with current or past STI, the American Association for the Study of Liver Disease recommends hepatitis B testing for these persons (10), and the American College of Physicians recommends hepatitis B testing for persons with a history of STI as a best practice (7). Hepatitis B is commonly transmitted through sexual contact (11); however, the hepatitis B prevalence among persons with current or past STI is unknown. We report the association of hepatitis B prevalence among persons with current and past STI through a comprehensive systematic review and meta-analysis.
Methods
Our methods are based on recommendations of the Cochrane Collaboration (12), and reporting is consistent with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and checklist; details available in Appendix A (13).
Data sources and searches.
We searched PubMed, Embase and Web of Science, employing terms from Medical Subject Headings (MeSH) relevant to HBV infection, STIs and epidemiology. We reviewed the literature between 1982, when the first HBV vaccine was approved, and our search date of March 18, 2018. Search terms and strategy are shown in Appendix B.
Study selection.
To be included, studies needed to report prevalence of hepatitis B and past or current syphilis, gonorrhea/chlamydia or unspecified STIs, from any of the 36 high-income countries of the Organization for Economic Co-operation and Development (OECD) or a Latin American country. In addition, they either needed to provide measures of association such as odds ratios (ORs) or prevalence ratios (PRs) or provide data on HBV prevalence in both STI-exposed (past or current STI) and STI-unexposed (no past or current STI) sub-groups, from which PRs could be derived. Randomized controlled trials, prospective or retrospective observational cohorts, serial cross-sectional studies, and other longitudinal analyses published in any language were eligible for inclusion. Studies in peer-reviewed journals, reports from scientific conferences, and doctoral dissertations were eligible for inclusion. Studies in languages other than English were assessed by translating key passages into English using Google Translate. HBV infection could be defined as positive surface antigen (HBsAg) or as core antibody detection (anti-HBc), and we stratified results according to these two types of markers. We also included studies that combined HBV marker types without specifying which, surface antigen or core antibody, was used to define HBV infection. STI prevalence data could reflect current or past infections. Studies were excluded if they focused on persons with HIV or immigrant populations.
Data synthesis and analysis.
We used PRs as the measure of association between HBV infection and STIs. In addition to the few studies that provided PRs (or risk ratios) directly, studies were included if PRs could be constructed from the reported data. When adjusted measures were provided, typically as ORs, we used adjusted measures rather than crude measures. However, when adjusted measures were not reported, but data were available to construct unadjusted PRs from 2 × 2 data we did so, using Open-Epi’s 2 × 2 table function (14). “2 × 2 data” refers to the number of HBV negative and HBV positive cases for both STI negative and STI positive groups. We used 2 × 2 data even if unadjusted ORs were available in order to keep consistent and precise methods. We converted both adjusted ORs and unadjusted ORs when 2 × 2 data were unavailable to PRs using the formula suggested by Zhang and Yu (15),
where, P0 is the prevalence of the outcome of interest (HBV-positivity) in the unexposed group (STI-negative).
We used Comprehensive Meta Analysis™ (Version 3.3, Englewood, New Jersey) software to perform the meta-analysis. We used a random effects model because included studies were diverse in design, setting, and population (16).
Hepatitis B screening should include HBsAg, antibody to HBsAg (anti-HBs), and anti-HBc so that patients can be categorized into clinical states for appropriate management (7). Our methods divided HBV serological markers because not all the studies included results of all 3 serologic markers. Therefore, we defined 18 meta-analyses strata, a priori, according to three variables that would likely affect the association of HBV infection and STI, as well as the clinical care for persons with HBV infection or STI, as follows: HBV markers (three groups: HBsAg, anti-HBc, combined), STI type (three groups: syphilis, gonorrhea or chlamydia [treated as one group], unspecified), and STI history (two groups: current or past). We used funnel plots to visually explore the relationship between the log of the pooled PR and their associated standard errors. To portray study heterogeneity, we reported the Iota-square statistic and its p-value for each pooled outcome (17, 18).
Data extraction and quality assessment.
We imported records resulting from our search into EndNote™ (Version X8, Pittsburgh, PA). One reviewer removed duplicate records. After removing duplicate records, a reviewer performed an initial review to exclude citations that were clearly irrelevant (e.g., different disease), and then screened citation titles, abstracts and keywords of the remaining citations to identify records for full-text review. The lead author conducted quality assessment by sampling 50% of the excluded records to ensure no systematic exclusion of eligible studies. The full text of each screened-in article was obtained and then reviewed by the lead author to establish final relevance of the study according to the pre-specified inclusion criteria. Reasons for exclusion were recorded, such as no prevalence ratio reported or calculable from the available data, or the study pertained to a migrant population. The lead author extracted the measures of association as descried in the data synthesis and analysis section. Data were extracted into a database maintained in Microsoft Excel™ (Version Office 365, Redmond, Washington). A senior statistician and study co-author checked all calculations to ensure that the numbers were extracted, interpreted and calculated properly. For the risk of bias assessment, the Quality Assessment Tool for Observation Cohort and Cross-sectional Studies was used since only observational studies were identified (19). Two reviewers independently evaluated study quality and differences were resolved by a third reviewer to determine the adequacy of study population selection and recruitment, outcome and exposure measurement and assessment, and statistical analyses, relative to other observational studies, using (Appendix C).
Results
Search results.
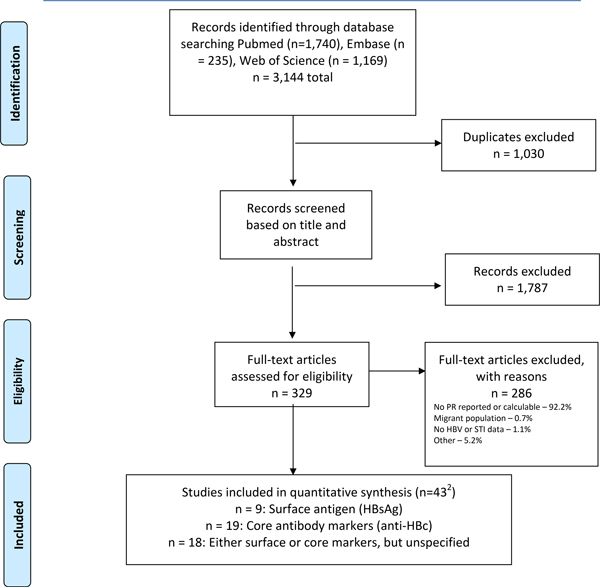
Our searches yielded 3,144 unique citations, including 1,740 from PubMed, 235 from Embase and 1,169 from Web of Science, yielding 2,114 citations after removal of duplicates. (Figure 1). Screening of titles and abstracts identified 329 citations for full text review, of which 43 met inclusion criteria and were advanced to data extraction and inclusion in the meta-analysis. Key information on the 43 included studies is shown in Table 1.
Figure 1:
Flowchart for systematic review1
1From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097
2. Three studies contributed outcomes to two HBV marker types
Table 1.
Characteristics of 43 studies included in calculation of association between HBV infections and STIs
| A. Studies using surface antigen (HbsAg) as HBV marker | ||||||||
| Hepatitis B prevalence (%) | ||||||||
| STI-negatives | STI-positives | Prevalence ratios 1 | ||||||
| Author, Pub., Year | Country | Setting | Quality rating | Population | STI measure | Point est, (Low -High 95% CI) | N, point est., (Low-High 95% CI) | |
| Carmo, Gen Hosp Psychiatry, 2014 | Brazil | 26 public mental health services | Fair | Randomly selected adults with mental illness under care | Past STIs | 2.00 | 1.92, (NR) | 2176, 0.93, (0.66–1.36) |
| Past syphilis | 4.17, (NR) | 2140, 2.36, (1.36–4.07) | ||||||
| Hart, Sex Transm Dis, 1993 | Australia | STI clinic, Adelaide | Fair | All attendees during study period - Females | Current gonorrhea | 7.10 | NR | 3998, 2.00, (0.90–4.20) |
| Hennessey, Urban Health, 2009 | USA | Jail - Chicago, Detroit, S.F. | Fair | Incoming jail inmates (M + F) | Current syphilis | 0.90 | 2.00, (0.40–3.70) | 11165, 6.02, (2.30–14.94) |
| Miranda, Sex Transm Dis., 2001 | Brazil | Antenatal clinics in Vitoria Municipality | Fair | Pregnant women | Past STIs | 0.80 | NR | 1608, 1.00, (0.13–7.15) |
| Current syphilis | 10.42 (4.09–22.61) | 1608, 12.50, (4.64–33.66) | ||||||
| Parazzini, Genitourin Med.,1992 | Italy | Two STI clinics | Fair | Patients who referred themselves to clinic for suspected STD or STD treatment | Current syphilis - TPHA | 7.80 | 15.06 (8.45–25.18) | 588, 1.55, (0.81–3.59) |
| Current syphilis - VDRL | 6.90 | 9.09 (3.91–18.78) | 588, 0.91, (0.31–2.21) | |||||
| Souto, Mem Inst Oswaldo Cruz., 2001 | Brazil | Area comprising 16 gold mine camps in Apiacas county | Fair | Malaria-exposed gold Miners | Past STIs | 2.70 | 8.92 (6.20–12.62) | 472, 3.62, (1.29–9.08) |
| van Duynhoven, Genitourin Med., 1997 | Netherlands | STI clinic at University Hospital of Rotterdam | Fair | STD clinic clients | Past STIs | 1.10 | 2.72 (1.95–3.80) | 2876, 2.46, (1.39–4.33) |
| Weinbaum, Am J Public Health, 2008 | USA | MSM-identified venues: Balt, Dallas, L.A., Miami, NYC, S.F., Seattle | Good | MSM | Current STIs | 16.00 | 33.20 (27.40–39) | 2824, 1.66, (1.46–1.96) |
| Zou, Transfusion, 2009 | USA | Blood donation sites | Fair | First time blood donors | Current syphilis | 0.05 | 0.91 (0.6–1.37) | 1203, 16.66, (11.08–24.97) |
| Repeat blood donors | 0.01 | 1.17 (0.78–1.75) | 155, 117.72, (16.57–947) | |||||
| B. Studies using core antibody (anti-Hbc) as HBV marker | ||||||||
| Author, Pub., Year | Country | Setting | Quality rating | Population | STI measure | Hepatitis B prevalence in STI-negatives |
Prevalence
ratios1 N, point est., (Low-High 95% CI) |
|
| Bratos, Sex Transm Dis., 1993 | Spain | Public health clinic | Fair | Female prostitutes | Current syphilis | 9.70 | 127, 2.54, (1.06–6.08) | |
| Carmo, Gen Hosp Psychiatry, 2014 | Brazil | 26 public mental health services | Fair | Randomly selected adults with mental illness under care | Past STIs | 15.70 | 2176, 1.20, (1.07–1.33) | |
| Past syphilis | 16.20 | 2140, 1.29, (1.05–1.58) | ||||||
| Carvalho. Cad Saude Publica, 2017 | Brazil | Public shelter in city of Goiânia |
Fair | Persons experiencing homelessness | Past STIs | 15.50 | 271, 1.13, (0.83–1.60) | |
| Corona, Epidemiol Infect., 1991 | Italy | STI clinic in Rome | Fair | Consecutive outpatients - non-PWID heterosexuals | Past STIs | 21.10 | 1136, 1.56, (1.28–1.86) | |
| Consecutive outpatients - non-PWID homosexuals | 50.00 | 196, 1.25, (0.92–1.54) | ||||||
| Corona, J Med Virol., 1996 | Italy | STI clinic in Rome | Good | Non-PWID heterosexuals | Past chlamydia / gonorrhea | 18.10 | 1497, 0.98, (0.73–1.27) | |
| Current syphilis | 13.00 | 1497, 2.01, (1.51–2.59) | ||||||
| Deininger, Klin Wochenschr., 1990 | Germany | HIV Clinic | Fair | Gay and bisexual men | Past syphilis | 52.30 | 1961, 2.00, (1.80–2.13) | |
| Fiscus, Sex Transm Dis.,1994 | USA | STI clinics; three counties in N. Carolina | Fair | STD clinic clients tested for syphilis | Current syphilis | 5.60 | 139, 1.50, (1.10–2.10) | |
| Gilson, Sex Transm Infect., 1998 | United Kingdom | Open access GUM clinic in central London | Fair | HIV-neg homosexual men | Past STIs >2 vs <2 lifetime episodes |
28.90 | 441, 5.19, (3.38–7.65) | |
| HIV-neg heterosexual men | 6.30 | 527, 0.94, (0.38–2.27) | ||||||
| HIV-neg women | 4.60 | 821, 1.00, (0.03–2.66) | ||||||
| Hakre, Sex Transm Infect., 2013 | Panama | Venues for registered and
unregistered FSWs in 8 Provinces |
Good | FSWs | Current syphilis | 8.00 | 38, 2.21, (1.10–4.46) | |
| Hawkins, J Infect Dis.,1992 | USA (see “Setting”) | Six US Navy ships on deployment to S. America, W. Africa & Mediterranean | Good | Crew members and Marine Corp personnel | Past STIs | 3.70 | 2071, 1.84, (1.10–4.21) | |
| Hennessey, J Urban Health, 2009 | USA | Jail - Chicago, Detroit, San Francisco | Fair | Incoming jail inmates (M + F) | Current syphilis | 16.00 | 11166, 1.80, (1.50–2.14) | |
| C. Studies using core antibody (anti-Hbc) as HBV marker | ||||||||
| Author, Pub., Year | Country | Setting | Quality rating | Population | STI measure | Hepatitis B prevalence in STI-negatives |
Prevalence ratios N, point est., (Low-High 95% CI) |
|
| Juarez-Figueroa, Sex Transm Infect., 1998 | Mexico | Centre for HIV/STI detection in FSWs | Fair | FSWs | Current syphilis | 5.80 | 1498, 1.81, (0.91–3.06) | |
| Mele, Eur J Epidemiol., 1988 | Italy | STI clinic | Fair | STD clinic clients, male; heterosexual & gay/bisexual | Past STIs | 46.40 | 913, 1.32, (1.15–1.58) | |
| Oliveira, Mem Inst Oswaldo Cruz, 2001 | Brazil | STI Clinic, Universidade Federal Fluminense, State of Rio de Janeiro | Fair | STD clinic clients | Current syphilis | 11.60 | 440, 1.80, (1.03–3.54) | |
| Past STIs | 12.60 | 433, 1.12, (0.68–1.85) | ||||||
| Clinic STI history | 9.50 | 440, 1.50, (0.82–2.75) | ||||||
| Ribeiro, Int J Environ Res Public Health, 2017 | Brazil | Private hemodialysis clinic & public hematology center, Fortaleza, Brazil | Fair | Male & female pts with chronic renal failure | Past STIs | 54.90 | 141, 1.07, (0.78–1.47) | |
| Male and female pts with coagulapathy | 20.00 | 50, 4.00, (2.00–8.00) | ||||||
| Tien, Clin Infect Dis., 2004 | USA | At home or work | Fair | Females at risk of HIV - PWID | Past gonorrhea Past chlamydia |
76.00 | 581, 1.06, (0.97–1.16) | |
| 581, 1.03, (0.89–1.13) | ||||||||
| Females at risk of HIV - non-PWID | 30.00 | 512, 1.46, (1.15–1.85) | ||||||
| 512, 1.67, (1.23–2.26) | ||||||||
| Females at risk of HIV - no illicit drug use | 17.00 | 850, 1.25, (0.84–1.87) | ||||||
| 850, 0.88, (0.42–1.83) | ||||||||
| Females at risk of HIV - PWID | Past syphilis | 76.00 | 581, 1.07, (0.90–1.18) | |||||
| Females at risk of HIV - non-PWID | 512, 1.18, (1.08–1.24) | |||||||
| Females at risk of HIV - no illicit drug use | 850, 1.11, (0.95–1.21) | |||||||
| Trepka, Sex Transm Dis, 2003 | USA | Large public STI clinic | Fair | Male clinic clients 18+ yo | Past STIs | 20.00 | 376, 2.32, (1.63–3.30) | |
| Female clinic clients 18+ yo | 16.00 | 242, 1.59, (1.00–2.63) | ||||||
| van Duynhoven, Genitourin Med., 1997 | Netherlands | STI clinic at University Hospital of Rotterdam | Fair | STD clinic clients | Past STIs | 12.90 | 2848, 1.33, (1.09–1.56) | |
| Weinstock, JAMA., 1993 | USA | Public STI Clinic; San Francisco | Fair | Patients with new problem and routine screening for syphilis | Past gonorrhea | 18.80 | 1159, 2.30, (1.95–2.68) | |
| Past syphilis | 24.40 | 1155, 2.72, (2.33–3.06) | ||||||
| D. Studies using either surface (HBsAg) or core (anti-HBc) HBV markers | ||||||||
| Authors | Country | Setting | Quality rating | Population | STI measure | Hepatitis B prevalence in STI-negatives |
Prevalence
ratios1 N, point est., (Low-High 95% CI) |
|
| Baddour, Sex Transm Dis, 1988 | USA | STI clinic | Fair | African American females | Past STIs | 8.60 | 136, 2.83, (1.12–7.12) | |
| Barrett, Sex Transm Dis, 1992 | USA | STI Clinic Jefferson Cty Dept. of Health; Birmingham, Ala. | Fair | Women 19 y.o.+; not pregnant and planning to use contraception for trial period | Current gon. / chlamydia 0.01 – 0.23 STIs | 14.00 | 557, 0.91, (0.54–1.48) | |
| Current gon. / chlamydia 0.24 – 1.00 STIs | 557, 1.33, (0.82–2.07) | |||||||
| El Maerrawi, International J STD AIDS, 2015 | Brazil | Sao Paulo prison for men | Fair | Sao Paulo prison for men; all inmates were included | Past STIs | 21.00 | 21, 1.81, (1.15–2.57) | |
| Hwang, Clin Infect Dis, 2000 | USA | Two drug treatment clinics in Houston & drug treatment clinic in Lubbock, Texas | Good | Indigent residents addicted to drugs and alcohol | Past STIs | 22.40 | 407, 1.41, (0.84–2.07) | |
| Lama, Am J Trop Med Hyg, 2010 | Peru | Sentinel surveillance survey in 6 HBV-endemic cities | Fair | Men 18+ y.o. and had sexual intercourse with 1+ men during previous year | Current syphilis | 25.50 | 354, 1.46, (1.21–1.74) | |
| Levine, Am J Epi, 1995 | USA | Baltimore; extensive community outreach | Fair | Former and current IV drug users who were subjects of ALIVE HIV longitudinal study | Past syphilis | 83.50 | 2193, 1.02, (0.97–1.08) | |
| Matos, Sex Transm Infect, 2008 | Brazil | Highway through Goiania City | Good | Truck drivers | Past STIs | 14.60 | 615, 1.78, (1.05–3.00) | |
| Moura, International J Inf Dis, 2015 | Brazil | Maceio, capital of State of Alagoas, Municipal Health Office | Fair | Pregnant women | Current syphilis | 0.40 | 54729, 0.81, (0.33–1.97) | |
| Oliveira, PLOS One, 2016 | Brazil | MSM in City of Goiania | Fair | MSM recruited using Respondent-Driven Sampling | Past STIs | 15.40 | 522, 3.33, (1.83–4.80) | |
| Pando, Sex Transm Dis, 2006 | Argentina | Recruitment from gay nightclubs, porn cinemas, gyms, and streets | Fair | MSM | Past STIs | 32.40 | 694, 1.47, (1.18–1.77) | |
| Pando, Am J trop Med Hyg, 2006 | Argentina | FSWs in 6 cities contacted through an NGO | Fair | FSWs | Current syphilis | 14.40 | 625, 1.80, (1.25–2.64) | |
| Pando, J Med Micro, 2008 | Argentina | Three public hospitals in Buenos Aires | Fair | TB patients > 18 years old | Past STIs | 11.6% | 181, 2.3 (1.2–4.7) | |
| Remis, Am J Pub Health, 2000 | Canada | Clinic and community settings | Fair | HIV-neg MSM | Past STIs | 40.9% | 328, 2.14 (1.57–2.39) | |
| Past gonorrhea or chlamydia | 328, 1.80 (1.38–2.11) | |||||||
| Rosenblum, J Infect Dis, 1990 | USA | Belle Glade, Florida | Fair | Adults from randomly selected households | Current syphilis | 21.0% | 723, 2.05 (2.00–4.80) | |
| Past syphilis | 29.0% | 714, 1.64 (1.16–2.32) | ||||||
| Past gonorrhea | 26.0% | 712, 1.65 (1.32–2.06) | ||||||
| Rosenblum, JAMA, 1992 | USA | Detention centers in Miami and L.A., brothels in NV, STI clinics in CO, drug Tx programs in NJ, street / social network outreach in Atlanta, NJ, and S.F. | Fair | Women 18 years old+ who had exchanged sexual services for money or drugs at least once since 1978 | Past syphilis | 70.0% | 646, 2.7 (1.9–4.0) | |
| Seage, Pub Health Rep, 1997 | USA | Student participants recruited via outreach at colleges. Other participants recruited from Fenway Community Health Center, and gay nightclubs | Fair | MSM enrolled in college in Boston area and younger than 30 or, if not students, 18 to 24 years old. | Past STIs | 11.7% | 317, 3.11 (1.11–8.76) | |
| Segura, AIDS Care, 2010 | Argentina | NGO clinic | Fair | MSM | Current syphilis | 25.0% | 811, 2.40 (1.50–3.11) | |
| Zocratto, Sub Use Misuse, 2010 | Argentina and Uruguay | Outreached clients recruited by civil associations. Hospitalized or outpatients recruited from 2 public and 1 nonpublic hospital for drug user and psychiatric diseases | Fair | Out-of-treatment and in-treatment Non-Injecting Cocaine Users from low-income neighborhoods in Montevideo and Buenos Aires. | Current syphilis | 10.8% | 89, 3.62 (2.16–5.33) | |
Classifying studies by HBV marker type, we included nine studies reporting prevalence by surface antigen (HBsAg) positivity providing 13 PRs; 19 studies reporting core antibody (anti-HBc) prevalence providing 37 PRs; and 18 studies that did not identify which HBV marker was used providing 25 PRs. Thus, a total of 75 PRs were included in the meta-analysis from a total of 43 studies. Three studies (Carmo 2014, Hennessey 2009, and van Duynhoven 1997) provided data on both HBsAg and anti-HBc (20–22). Thus, the number of studies shown in Appendix D, 72, differs from the number of PRs, 75. Nearly half (47.9%) of the PRs reported associations with syphilis, 12.9% reported associations with chlamydia or gonorrhea, and 39.2% reported associations with unspecified STI. Of the 72 PRs, 38 (52.8%) were unadjusted and 34 were adjusted, typically by basic demographics such as age and sex. By geographical classification, PRs were divided between OECD countries excluding the U.S. (24.0%), the U.S. (37.5%), and Latin America (38.5%). (See Appendix D)
Hepatitis B prevalence among persons with STIs.
Among the 13 PRs that incorporated HBsAg, we derived 11 values for hepatitis B prevalence among persons with STIs finding that the median prevalence 4.17%, and ranged from 0.91% (95% CI: 0.60, 1.37%) among first-time blood donors in the U.S. for current syphilis (23) to 33.2% (95% CI: 27.4%, 39.0%) among MSM in the U.S. for current unspecified STIs (24). See Table 1.
Meta-analysis results.
Overall, we found positive and statistically significant associations between HBV infection and STI prevalence in 12 of the 14 strata for which we were able to calculate pooled PRs. None of the pooled results showed negative associations between HBV infection and STIs. Specific results grouped by HBV marker are presented below:
Surface antigen (HBsAg) as the marker for HBV-positivity
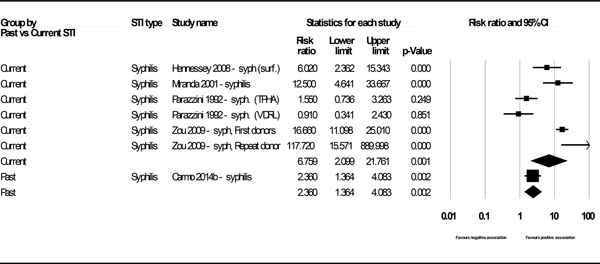
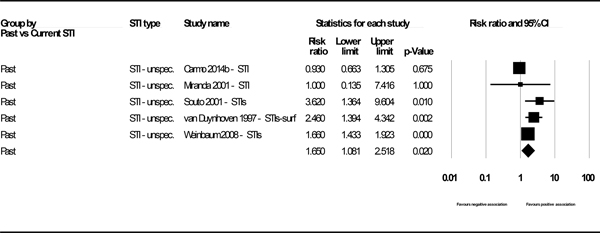
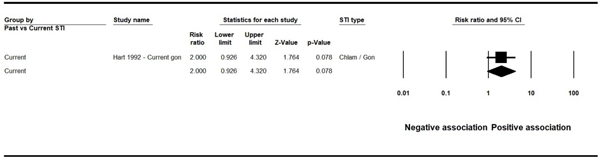
We reported four data points (two pooled and two based on a single study) for the association of STI and HBV infection for studies using HBsAg as the marker for HBV-positivity. Of these, three showed statistically significant associations: past syphilis (k=1, PR 2.36, 95% CI: 1.36, 4.08), current syphilis (k=6, PR 6.76, 95% CI: 2.10, 21.76), (Figure 2) and past unspecified STIs (k=5, PR 1.65, 95% CI: 1.08, 2.52), (See Figure 3 in web appendix). The result for current chlamydia / gonorrhea showed a positive but statistically insignificant association (k=1, PR 2.00, 95% CI: 0.93, 4.32). (See Figure 4 in web appendix). (Note that these figures do not always match the results shown in Appendix E, as the latter displays unadjusted outcomes only). We observed high heterogeneity (I2 73.6% and 91.4%) in the two analyses for which heterogeneity could be assessed.
Figure 2.
Forest plot - Association between HBsAg and past and current syphilis.
Figure 3.
Forest plot - Association between HBsAg and past unspecified STIs.
Figure 4.
Forest plot - Association between HBsAg and current gonorrhea
Core antibody (anti-HBc) as the marker for HBV-positivity
We reported five pooled data outcomes for the association of STI and HBV infection for studies using anti-HBc as the marker for HBV-positivity. All showed statistically significant associations: past syphilis (k=6, PR 1.48, 95% CI: 1.10, 1.99), current syphilis (k=7, PR 1.82, 95% CI: 1.60, 2.06), past chlamydia / gonorrhea, (k=8, PR 1.30, 95% CI: 1.02, 1.66), and past and current unspecified STIs, (k=12, PR 1.62, 95% CI: 1.31, 1.99) and (k=4, PR1.42, 95% CI: 1.23, 1.63), respectively. The result for current unspecified STIs showed moderate heterogeneity, (I2 42.4%). The other four outcomes had high heterogeneity with I2 ranging from 83.8% - 97.6%
Combined surface antigen (HBsAg) or core antibody (anti-HBc) as the marker for HBV-positivity
We reported five pooled outcomes for the association of STI and HBV infection for studies that combined HBV marker types. Three of these showed statistically significant associations: current syphilis (k=7, PR 1.69, 95% CI: 1.39, 2.07), past chlamydia / gonorrhea, (k=2, PR 1.73, 95% CI: 1.48, 2.01), and past and unspecified STIs (k=8, PR 1.89, 95% CI: 1.52, 2.37). The pooled outcomes for past syphilis and current chlamydia / gonorrhea were not statistically significant (k=3, PR 1.51, 95% CI: 0.89, 1.52) and (k=2, PR 1.15, 95% CI: 0.77, 1.62), respectively. The result showed high heterogeneity for current and past syphilis (I2 95 – 96%), moderate heterogeneity for unspecified STI (I2 45%), and low heterogeneity for current and past chlamydia / gonorrhea (I2 0% - 15%). See Table 2 below.
Table 2.
Summary of evidence for the association of HBV infection with STIs
| Outcome | Number of data points | Prevalence Ratio (95% CI); pooled if ≥2 studies | I2 (Q test p-value) | References (distinguishing characteristic for studies with >1 outcome) |
|---|---|---|---|---|
| HBV surface antigen (HBsAg ) | ||||
| Syphilis – Current | 6 | 6.76 (2.10–21.76) | 91.4% (0.00) | Hennessey 2009; Miranda 2001; Parazzini 1992 (TPHA); Parazzini 1992 (VDRL); Zou 2009 (First-time donors); Zou 2009 (Repeat donors) |
| Syphilis – Past | 1 | 2.36 (1.36–4.08) | N/A | Carmo 2014 |
| Chlamydia / gonorrhea – Current | 1 | 2.0 (0.93–4.32) | N/A | Hart 1992 |
| Unspecified STIs – Past | 5 | 1.65 (1.08–2.52) | 73.6% (0.004) | Carmo 2014; Miranda 2001; Souto 2001; van Duynhoven 1997; Weinbaum 2008 |
| HBV core antibodies (anti-HBc) | ||||
| Syphilis – Current | 7 | 1.82 (1.60– 2.06) | 84.0%; (0.00) | Bratos 1993; Corona 1993; Fiscus 1994; Hakre 2013; Hennessey 2009; Juarez-Figueroa 1998; Oliviera 2001 |
| Syphilis – Past | 6 | 1.48 (1.10–1.99) | 97.6%; (0.00) | Carmo 2014; Deininger 1990; Tien 2004 (PWID); Tien 2004 (No illicit drugs); Tien 2004 (Non- PWID); Weinstock 1993 |
| Chlamydia / gonorrhea – Past | 8 | 1.30 (1.02–1.66) | 92.3%; (0.00) | Corona 1996; Tien 2004 (No illicit drug); Tien 2004 (Chlamydia, PWID); Tien 2004 (Chlamydia, No illicit drug); Tien 2004 (Chlamydia, non-PWID); Tien 2004 (Gonorrhea, PWID); Tien 2004 (Gonorrhea, non-PWID); Weinstock 1993 |
| Unspecified STIs – Current | 4 | 1.42 (1.23–1.63) | 42.4% (0.00) | Corona 1991 (non-IDU heterosex).; Corona 1991 (non-IDU homosex.); Oliviera 2001; Oliviera 2001 clinic |
| HBsAg or anti-HBc; combined | ||||
| Syphilis – Current | 7 | 1.69 ( 1.39–2.07 | 96.1% (0.00) | Lama 2010; Moura 2015; Pando 2006; Rosenblum 1990; Rosenblum 1992; Segura 2010; Zocratto 2010 |
| Syphilis – Past | 3 | 1.51 (0.89–1.52) | 95.0% (0.00) | Levine 1995; Remis 2000; Rosenblum 1990 |
| Chlamydia / gonorrhea – Current | 2 | 1.15 (0.77–1.62) | 15.3% (0.28) | Barrett 1992 (more STIs); Barrett 1992 (fewer STIs) |
| Chlamydia / gonorrhea – Past | 2 | 1.73 (1.48–2.01) | 0.0% (0.58) | Remis 2000; Rosenblum 1990 |
| Unspecified STIs – Past | 8 | 1.89 (1.52–2.37) | 45.1% (0.08) | Baddour 1988; El Maerrawi 2005; Hwang 2000; Matos 2008; Oliveira 2015; Pando 2006; Pando 2008; Seage 1997 |
Sensitivity analysis.
Gilson 1998 used a different measure of STI prevalence than the other studies, namely <2 versus ≥2 lifetime episodes (25). If this study is removed, the pooled PR for the association between anti-HBc and past unspecified STIs is 1.43 (CI 1.23– 1.67) versus 1.62 (CI 1.31–1.99) when Gilson 1998 is included.
The majority (88%) of studies had an overall study quality rating of fair relative to other observational studies (Appendix C). No studies had an overall quality rating of poor, and thus no sensitivity analyses were conducted to assess impact of excluding studies with a poor quality rating. However, there was noted variability identified for statistical adjustment for potential confounding variables. In order to understand the effect that a mix of adjusted and unadjusted measures may have had on our results, we ran the analysis using unadjusted measures only. Of the 11 pooled PRs that were statistically significant in the full data set, two lost their statistical significance (the association between HBsAg and unspecified past STIs, and between anti-HBc and unspecified current STIs). See Appendix E.
Discussion
We undertook a systematic review and meta-analysis of the relationship between HBV infection and current or past STIs. We found a substantial body of evidence suggesting that there are positive and statistically significant associations between the prevalence of hepatitis B and STIs in the mix of countries, settings, and subpopulations for which we found usable studies. HBsAg prevalence among persons with STIs were derived from 11 of the 13 outcomes available for studies specifically reporting HBsAg prevalence with a median value of 4.17% and a range of 0.91% to 33.20% depending on the population, setting, and infection pathogen.
Among adults in the United States, the most common route of transmission for hepatitis B virus is sexual contact Among the 26% of persons with case-reports of acute HBV infection with information about risk behaviors or exposures, 22.9% reported having two or more sexual partners, 6.5% reported sexual contact with an HBV-infected person, and 12.2% of males reported having sex with another male (26). As many as 10%−40% of adults seeking treatment in STI clinics have evidence of current or past HBV infection, and 39% were screened or sought care for an STI prior to becoming infected with HBV (27). As a result, CDC recommends vaccination of adults at risk for HBV infection including persons at risk for infection by sexual exposure (28).
CDC does not currently recommend pre-vaccination serologic testing for HBV among persons with current or a history of STI (9), although the American Association for the Study of Liver Disease has recommended testing this group since 2009 (10). HBV infection and STI share common modes of transmission, and hepatitis B can be transmitted by sexual exposure. Transmission also occurs by close person-to-person contact by open cuts and sores, in hyperendemic areas (28). Therefore, persons with STI are likely at increased risk of HBV infection and should be vaccinated, and considered for pre-vaccination serologic testing (29). Screening, linkage to care, and vaccination programs for adults at risk for hepatitis B are a best practice (6). Our review suggests high hepatitis B prevalence among persons with current or past STI. As a result of this higher hepatitis B prevalence, person with a history of STI could benefit by receiving hepatitis B vaccination if they are negative or be linked to life saving care and treatment if they are found to be positive. In addition, those who are identified as positive can be counseled and is an opportunity to disrupt transmission of the virus to others.
While there was a large range of values for HBV infection and STI prevalence in the populations included in this review, the range of prevalence ratios is much more constrained. The pooled estimates vary from 1.65 to 6.76 for HBsAg, from 1.30 to 1.82 for anti-HBc and 1.15 to 1.89 unspecified HBsAg / anti-HBc. This suggests that, given a known prevalence of STIs within a potential target population, one might make reasonably precise estimates of the yield of HBV-positive persons.
We observed an overall clustering of effect sizes around the top of all three funnels reflecting the preponderance of large studies. This observation suggests that smaller studies could have been excluded. However, there is no reason to think that this exclusion was due to the magnitude or direction of their association. In addition, any potential exclusion of such studies is unlikely to affect the pooled effect sizes. For one of the three plots, we observed a few effect sizes on the right side of the plot but not on the left side at the bottom of the funnel; in addition to risk of publication bias, data heterogeneity chance, and other reasons may explain this asymmetry (17). (See Appendix F).
Among the strengths of this paper are its comprehensive look at the range of relevant HBV markers and STIs combined with a sufficiently large number of outcomes to test for statistical significance within each stratum. Thus, we were able to group ‘like with like’ and still obtain, for most pooled estimates, a fairly narrow confidence interval. The results were robust to sensitivity analyses in which a study with a different approach to portraying STI outcomes was omitted, and to confining the analyses to unadjusted outcomes only.
However, there are limitations to the methods employed in this study. We confined our literature search to PubMed, Embase and the Web of Science data bases, and did not exhaustively review the grey literature. It is thus possible that we excluded relevant information that may have affected findings. Because there are no other published systematic reviews pertaining to our research question, it is not possible to compare our results against those of other investigators who might have used different methods. As in all systematic reviews, we evaluated only studies that met inclusion criteria. Broader criteria, such as acceptance of earlier studies, or a different geographic or economic specification of included countries, might have yielded a different result, although the inclusion / exclusion criteria used are appropriate to the research question we addressed.
Given the existence of contextual heterogeneity (i.e., variation in population, study design, outcome reporting metrics) and statistical heterogeneity, we used random effects. Thus, the pooled point estimates provide an overall average effect size that may not be accurate for specific target populations and settings. Therefore, in addition to point estimates, confidence intervals should be taken into considerations for decision-making pertaining to disease screening programs or modeling. Given the cross-sectional nature of most of studies and the fact that both types of infections share common risk factors, we only assessed their association, not causal relationships. Related, during the quality assessment, we did not reduce the overall quality rating for studies that did not measure exposures prior to the outcome of interest since it would not be ethical in most instances to delay an effective vaccination in order to monitor outcomes overtime.
We derived PRs from adjusted measures of association when available and thus have a mix of adjusted and unadjusted PRs, Sensitivity analyses suggests this is not a major issue. Not all studies included HBsAg, which may have limited our estimations of the association between STI and infectious HBV. Including studies that reported on HBsAg or anti-HBc markers but did not specify which was used to determine hepatitis B status introduces an element of ambiguity in the interpretation of that sub-set of findings. However, these findings are consistent with findings pertaining to the known HBV marker types. Removing these results does not affect our overall findings. Even if we stratified data by HBV markers and STI pathogens, there is still a high degree of heterogeneity in some of the pooled analyses. This suggests that factors other than those for which we stratified may explain the heterogeneity.
Conclusion
This systematic review and meta-analysis found a substantial body of evidence suggesting robust and statistically significant associations between the prevalence of HBV infection and STIs for multiple infections and using various serologic tests. These findings may be useful in better understanding the potential benefits of pre-vaccination serologic testing for HBV among persons with or at risk for STIs and framing potential recommendations for expanded hepatitis B testing.
Supplementary Material
Funding.
This project was completed with funding from CAPE (Consortium for Assessment of Prevention Economics), of the NCHHSTP Epidemiological and Economic Modeling Agreement (Grant No: U38PS004649), with the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding source: U.S. Centers for Disease Control and Prevention
Footnotes
Compliance with Ethical Standards
Disclosure of potential conflicts of interest. The authors declare that they have no conflict of interest.
Research involving Human Participants and/or Animals. This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent. As no human subjects were involved in the research undertaken to produce this article, no informed consent was required.
References
- 1.World Health Organization. Hepatitis B [updated July 27, 2020]; cited 2021. Available from https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
- 2.Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988–2012. Hepatology 2016;63(2):388–97. doi: 10.1002/hep.28109 [published Online First: 2015/08/08] [DOI] [PubMed] [Google Scholar]
- 3.Center for Disease Control and Prevention. Surveillance for Viral Hepatitis – United States, 2016. 2016:75. [Google Scholar]
- 4.Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology 2007;46(4):1034–40. doi: 10.1002/hep.21784 [published Online First: 2007/07/27] [DOI] [PubMed] [Google Scholar]
- 5.Cohen C, Holmberg SD, McMahon BJ, et al. Is chronic hepatitis B being undertreated in the United States? Journal of viral hepatitis 2011;18(6):377–83. doi: 10.1111/j.1365-2893.2010.01401.x [published Online First: 2010/12/15] [DOI] [PubMed] [Google Scholar]
- 6.Mitchell T, Armstrong GL, Hu DJ, et al. The increasing burden of imported chronic hepatitis B--United States, 1974–2008. PloS one 2011;6(12):e27717. doi: 10.1371/journal.pone.0027717 [published Online First: 2011/12/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abara WE, Qaseem A, Schillie S, et al. Hepatitis B Vaccination, Screening, and Linkage to Care: Best Practice Advice From the American College of Physicians and the Centers for Disease Control and Prevention. Annals of internal medicine 2017;167(11):794–804. doi: 10.7326/M17-1106 [published Online First: 2017/11/22] [DOI] [PubMed] [Google Scholar]
- 8.Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep 2008;57(RR-8):1–20. [published Online First: 2008/09/20] [PubMed] [Google Scholar]
- 9.Schillie S, Vellozzi C, Reingold A, et al. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2018;67(1):1–31. doi: 10.15585/mmwr.rr6701a1 [published Online First: 2018/06/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007;45(2):507–39. doi: 10.1002/hep.21513 [published Online First: 2007/01/30] [DOI] [PubMed] [Google Scholar]
- 11.Roberts H, Jiles R, Harris AM, Gupta N, Teshale E. Incidence and Prevalence of Sexually Transmitted Hepatitis B, United States, 2013–2018. Sex Trans Dis 2021. doi: 10.1097/OLQ.0000000000001359 [published Online First: 2021/01/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 5.1.0 ed: The Cochrane Collaboration 2011. [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. British Medical Journal 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.OpenEpi: Open Source Epidemiologic Statistics for Public Health, [program]. Version 3.01 -- Released April 4 and revised April 6, 2013 version, 2013. [Google Scholar]
- 15.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA : the journal of the American Medical Association 1998;280(19):1690–1. [DOI] [PubMed] [Google Scholar]
- 16.Borenstein M. Introduction to meta-analysis. Chichester, U.K.: John Wiley & Sons; 2009. [Google Scholar]
- 17.Higgins Julian PT, Saly Green, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557 [published Online First: 2003/09/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health – National Heart, Lung, and Blood Institute. Study Quality Assessment Tools – Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies; cited 2021. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 20.van Duynhoven YT, van de Laar MJ, Schop WA, et al. Prevalence and risk factors for hepatitis B virus infections among visitors to an STD clinic. Genitourin Med 1997;73(6):488–92. [published Online First: 1998/05/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennessey KA, Kim AA, Griffin V, et al. Prevalence of infection with hepatitis B and C viruses and co-infection with HIV in three jails: a case for viral hepatitis prevention in jails in the United States. J Urban Health 2009;86(1):93–105. doi: 10.1007/s11524-008-9305-8 [published Online First: 2008/07/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmo RA, Melo AP, Dezanet LN, et al. Correlates of hepatitis B among patients with mental illness in Brazil. Gen Hosp Psychiatry 2014;36(4):398–405. doi: 10.1016/j.genhosppsych.2014.03.001 [published Online First: 2014/04/15] [DOI] [PubMed] [Google Scholar]
- 23.Zou S, Notari EP, Fang CT, et al. Current value of serologic test for syphilis as a surrogate marker for blood-borne viral infections among blood donors in the United States. Transfusion 2009;49(4):655–61. doi: 10.1111/j.1537-2995.2008.02042.x [published Online First: 2009/01/28] [DOI] [PubMed] [Google Scholar]
- 24.Weinbaum CM, Lyerla R, Mackellar DA, et al. The Young Men’s Survey phase II: hepatitis B immunization and infection among young men who have sex with men. Am J Public Health 2008;98(5):839–45. doi: 10.2105/AJPH.2006.101915 [published Online First: 2008/04/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilson RJ, de Ruiter A, Waite J, et al. Hepatitis B virus infection in patients attending a genitourinary medicine clinic: risk factors and vaccine coverage. Sex Transm Infect 1998;74(2):110–5. [published Online First: 1998/06/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC. Surveillance for Acute Viral Hepatitis — United States, 2018 Atlanta, GA. [updated July 27, 2020]. Available from: https://www.cdc.gov/hepatitis/statistics/2018surveillance/index.htm
- 27.CDC. Viral hepatitis-statistics and surveillance. 2015[updated September 26, 2017];cited 2018. Available from: https://www.cdc.gov/hepatitis/populations/stds.htm. [Google Scholar]
- 28.Mast EE, Margolis HS, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005;54(RR-16):1–31. [published Online First: 2005/12/24] [PubMed] [Google Scholar]
- 29.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50(3):661–2. doi: 10.1002/hep.23190 [published Online First: 2009/08/29] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.