Abstract
Savings have been described as the ability of healthy humans to relearn a previously acquired motor skill faster than the first time, which in the context of motor adaptation suggests that the learning rate in the brain could be adjusted when a perturbation is recognized. Alternatively, it has been argued that apparent savings were the consequence of a distinct process that instead of reflecting a change in the learning rate, revealed an explicit re-aiming strategy. Based on recent evidence that feedback adaptation may be central to both planning and control, we hypothesized that this component could genuinely accelerate relearning in human adaptation to force fields (FFs) during reaching. Consistent with our hypothesis, we observed that on re-exposure to a previously learned FF, the very first movement performed by healthy volunteers in the relearning context was better adapted to the external disturbance, and this occurred without any anticipation or cognitive strategy because the relearning session was started unexpectedly. We conclude that feedback adaptation is a medium by which the nervous system can genuinely accelerate learning across movements.
Keywords: adaptive feedback control, force field adaptation, readaptation, savings, sensorimotor control, sensorimotor learning
Significance Statement
Savings describe the ability of healthy humans to faster relearn a previously acquired motor skill. It is debated whether savings result from implicit changes in the learning rate or an explicit strategy. Given recent evidence that feedback control plays a key role in movement adaptation, we hypothesized that this component also contributed to savings in the context of force field (FF) adaptation. We confirm that relearning was faster and demonstrate that the very first relearning movement was better adapted to the external disturbance, i.e., the online corrections in the first re-exposure trial carried imprints of feedback adaptation from the previous sessions in the absence of any explicit strategy. We conclude that this non-explicit feedback component can genuinely accelerate motor learning.
Introduction
Humans adapt to force field (FF) perturbations in reaching movements within a few trials. During adaptation, a motor memory of the newly acquired mapping is created, which is fragile in the early stage of adaptation and progressively becomes stable (Krakauer and Shadmehr, 2006). When exposed to the same perturbation a second time, it was documented that relearning is faster and more complete than during the first exposure, a phenomenon called “savings” (Brashers-Krug et al., 1996; Shadmehr and Brashers-Krug, 1997; Caithness et al., 2004; Overduin et al., 2006; Focke et al., 2013; Stockinger et al., 2014; Coltman et al., 2019). There are two leading hypotheses to explain savings: the first is that the predictive, feedforward component of motor adaptation changes faster across trials because of the repeated exposure to similar perturbation (Gonzalez Castro et al., 2014); alternatively, it was also reported that savings reflect an explicit, cognitive strategy expressed following the prior learning experience (Taylor et al., 2014; McDougle et al., 2015). Currently, the question of whether savings reflect changes in learning rate or an explicit strategy remains largely open and is central to understand neural mechanisms underlying human motor adaptation.
A key question in this debate is to understand the role of feedback control. First, it is clear that the time-varying FFs recruit and adapt the feedback control system (Wagner and Smith, 2008; Yousif and Diedrichsen, 2012; Cluff and Scott, 2013; Joiner et al., 2017; Maeda et al., 2018). Additionally, recent studies highlighted the importance of online adaptive control in reaching movements and argued that rapid feedback adaptation contributed to the trial-by-trial adaptation (Crevecoeur et al., 2020a,b; Mathew et al., 2020). Moreover, changes in long-latency feedback responses to perturbations paralleled the fast learning timescale (Coltman et al., 2019; Coltman and Gribble, 2020; Smith et al., 2006), which must be involved if an increase in learning rate occurs over the course of few tens of trials. Altogether the link between feedback control and the fast timescale of motor adaptation warranted an investigation of the potential influence of feedback adaptation in savings during FF learning.
More precisely, we hypothesized that a genuine acceleration during relearning could originate from feedback adaptation, unveiling a novel component of savings to consider. The testable prediction is that if a feedback adaptation component from the first learning session facilitates relearning, it should be evident in the online corrections made in the very first readaptation trial, in the absence of any anticipation or explicit strategy. Note that the current explanation of savings (changes in the feedforward learning rate, or explicit strategies), do not predict any effect within the very-first relearning trial when the perturbation is introduced unexpectedly. Here, we demonstrate the existence of a feedback component in savings by designing a FF adaptation task (with unexpected readaptation), that rules out any contribution of an explicit strategy and allows showing an effect of faster relearning even for the first trial of readaptation, for which there is no role of the feedforward component. Our results confirm that feedback control in the first relearning trial is better tuned to the FF, and suggest that this novel feedback-mediated component contributes to savings in human reaching adaptation.
Materials and Methods
Experimental design
Sixteen self-declared right-handed healthy adults (age = 24.4 ± 3.5, four male) were recruited. The experimental paradigm was approved by the Ethics Committee of the host institution and complied with the Declaration of Helsinki. Participants were compensated for their participation. Before the experiment, all the participants provided written informed consent.
Participants were requested to sit comfortably in front of a robotic device (KINARM), with their forehead resting on a resting pad. They were instructed to perform visually guided forward reaching movements in the horizontal plane using the handle of a robotic arm, toward a virtual target on the screen in front of them.
There were two types of movement conditions. The first type corresponds to baseline trials; these were reaching movements from starting position (a filled circle with a radius of 0.6 cm) to a goal position fixed at 15 cm from starting position. The direct vision of the arm and hand was blocked, but the cursor aligned to the handle was always visible providing feedback about the current hand position. Participants had to wait at the starting position for a random delay uniformly distributed between 2s and 4s. The goal position was initially presented as an open red circle (radius 1.2 cm) and later as a filled red circle, indicating the cue to initiate the movement. For a successful trial, the goal circle became filled with green when the participants reached the target within 600–800 ms (including reaction time) and stabilized there for at least 1 s. If they moved too fast, the goal circle changed back to an open circle. If the movement was too slow, it remained red. The feedback about timing was provided to encourage consistent movement times, and despite the failure to timing criterion, all movements were included in the analyses. The second type was FF trials. In these trials, participants experienced a lateral force perturbation proportional to the forward hand velocity (Fx = ±L , L = 13 Nsm−1). Thus, FFs were either clockwise (CW) or counterclockwise (CCW). After every trial, participants needed to return to the starting point by moving the robotic handle by themselves. There was no FF during the return movement, it was not part of a trial. Participants were divided into two groups: group G1 experienced only CCW FF during adaptation and readaptation blocks and group G2 experienced only CW FF (Fig. 1C).
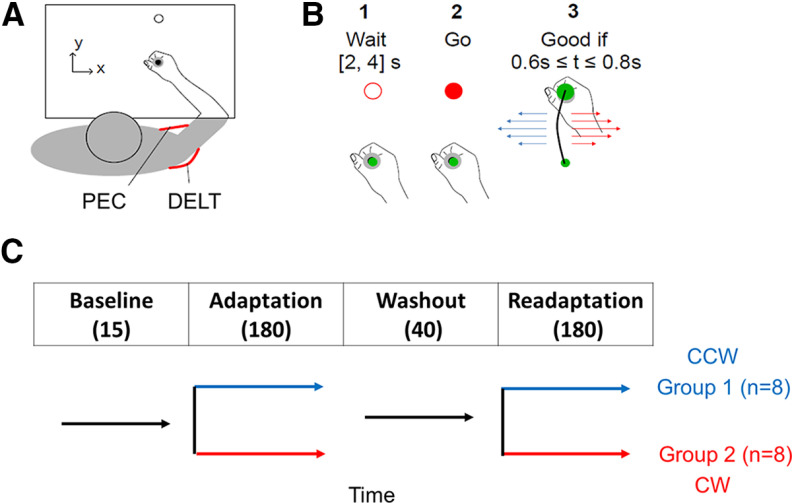
Figure 1.
A, Experimental setup, an illustration of the workspace. EMG was recorded from PEC and DELT. B, Illustration of a successful reaching trial. Participants were instructed to wait for the go signal and then to reach the goal within 0.6–0.8 s of the go signal. Visual feedback about movement timing was provided to encourage participants to adjust their movement speed, but all trials were included in the analyses. Reaching trials were with or without FF perturbation. During perturbation trials, a velocity-dependent FF was applied in either a CW (red arrows) or CCW (blue arrows) direction. C, Experimental design. Participants completed 415 trials in a session. FF was turned off during baseline and washout blocks and turned on during adaptation and readaptation blocks. The first group of participants experienced only CCW (blue timeline, n = 8) and the second group only CW FFs (red timeline, n = 8). Perturbations were introduced without prior warning.
Experimental sessions consisted of four blocks of trials. The first block was the baseline block with 15 baseline trials; the second block was the adaptation block with 180 FF trials; the third block was the washout block with 40 baseline trials, and the fourth block was the readaptation block with 180 FF trials in the same direction as during the adaptation block. Adaptation and readaptation phases were separated by 40 trials performed in the null environment to washout learning. Breaks were given two times during the adaptation block (between trial numbers 70−90 and 150–170) and two times during the readaptation block (250–270 and 350–370) randomly distributed across subjects so as to avoid any bias in a particular trial. The introduction/re-introduction of forcefield was without any explicit warning.
Data analysis and statistics
Signals from the robotic device were sampled at 1000 Hz. X and Y coordinates of the cursor/robotic handle and the forces at the interface between the participant’s hand and the handle were digitally low-pass filtered with a fourth-order, dual-pass Butterworth filter with a cutoff frequency of 50 Hz. Velocity signals were obtained from numerical differentiation of position signals (fourth order, finite difference algorithm). Electromyogram (EMG) was recorded using Bagnoli Desktop System (Delsys) at 1000-Hz sampling frequency and digitally bandpass filtered (fourth order dual-pass: [10, 400] Hz), rectified, then averaged across trials and participants. EMG electrodes were positioned over the muscle belly of the shoulder flexor, pectoralis major (PEC), and shoulder extensor, posterior deltoid (DELT), the main muscles recruited when performing lateral corrections against the FF perturbations used in this experiment, and on the right foot ankle for ground electrode.
Three events were used as timing references: (1) the Go cue to initiate the movement; (2) reach onset, which was defined as the moment when the cursor exited the home target; and (3) trial end, when the cursor stabilized at the goal position. Hand paths were averaged across the subjects. For each subject, the lateral (vx) and forward (vy) component of the peak velocity was computed for each trial. We followed standard EMG normalization procedures as follows. We performed two calibration blocks, in which participants were requested to counteract a 10N force toward left or right (three trials in each direction), activating DELT and PEC, respectively. We averaged EMG during the stationary phases of calibration trials (1-s time window) to get a reference value for each muscle. The EMG trials during the experiment were normalized to this reference value. The normalized EMG traces during the first trials of the adaptation and readaptation blocks were averaged across subjects and were aligned to the reach onset. For statistical comparison, for each subject, each EMG trace was averaged within a 100-ms window before reach onset.
The evolution of hand movement variation across the trials was assessed by means of exponential fits on parameters like path length, maximum lateral displacement, maximum lateral target overshoot, peak terminal force, initial reach angle and the correlation between commanded and measured forces. The path length and maximum lateral deviation are related, and they are minimum for straight movements. The lateral target overshoot reflects the absolute magnitude of the interaction force at the handle near to the target, i.e., the peak terminal force and they are associated with adaptation of feedback control (Crevecoeur et al., 2020a,b; Mathew et al., 2020), and the correlation between commanded and measured forces was also previously demonstrated as an indicator of adaptation. The exponential model has the following form:
| (1) |
and we regressed the three free parameters a, b, and c, which correspond to the amplitude of adaptation change, adaptation rate and asymptotic performance, respectively. The exponential models were fitted in the least square sense and the significance of the fit was determined based on whether the 99.5% confidence interval of the exponent responsible for the curvature of the fit included or not the value of zero (p < 0.005). The nonsignificant fits were associated with p > 0.05. R2 statistics were derived as follows:
| (2) |
where VRES denotes the variance of the residuals and VTOT is the total variance of the data (James et al., 2013; Crevecoeur et al., 2020b). Repeated measures ANOVA was used to document statistical differences between adaptation and readaptation blocks in CW and CCW perturbations with a significance level of p < 0.05. For ANOVAs, effect sizes are reported as partial η2 ( ; Fritz et al., 2012; Lakens, 2013).
For several analyses as specified in Results, we were interested in confirming the absence of any difference between the two datasets. The suitable approach to provide this information is to report Bayes’ factors, which compares the ratios of the likelihood of observing the two samples and can provide information about whether there is any evidence in favor of either assumption (presence or absence of difference; Faulkenberry, 2018; Rouder et al., 2009, 2012). Bayes factors (BFs) were used to characterize non-significant effects, based on the likelihood of two models (means are equal, vs means are different). When we observed non-significant effects, we calculated BF01, standing for the ratio between the likelihood of the data under H0 (there is no effect of the tested predictor), and H1 (there is an effect of the predictor). With this definition, BF01 is the evidence supporting H0, and we use the following interpretation table: BF01 between 1 and 3: only worth mentioning; BF01 between 3.2 and 10: substantial evidence; BF01 > 10: strong evidence.
FF adaptation was assessed using the correlation between the commanded force and the measured force. The commanded force in the environment was calculated offline as the forward velocity multiplied by the scaling factor that defines the FF (L = ±13 Ns/m). The measured force is the force at the interface between the hand and the robotic handle, which is measured with the force transducer. The differences between these two forces were well correlated with the lateral acceleration (for a detailed description, see Crevecoeur et al., 2020b). The initial reach angle (initial movement direction) was calculated as the angle between reach movement and the straight line connecting starting and target position, a positive value indicates an angle toward the left of this straight line.
Data availability
Data and codes used in this project is available on request.
Results
Our analyses will proceed as follows: we first confirm the presence of savings by showing that readaptation was indeed faster than adaptation. Second, we demonstrate that the very first trial in the readaptation block was already better adapted after verifying that trials at the end of the washout phase were indistinguishable from the baseline trials, and without any explicit strategy as the readaptation blocks were introduced unexpectedly. Third, we highlight the feedback control component that contributes to this better readaptation performance and savings. Finally, we present control analyses to verify the absence of anticipation at the beginning of the readaptation phase and to rule out the presence of any non-specific strategy linked to the co-contraction strategy that leads to this better performance.
Reach movements were first strongly impacted by the introduction of the perturbations and then became smoother with practice (Fig. 2A). To quantify the change in reach performance within and across blocks, we extracted few key parameters (1) the total distance traveled by the hand cursor (pathlength); (2) the maximum lateral hand deviation along the direction of the FF; (3) the lateral target overshoot near the end of the movement; (4) the peak terminal measured force; and (5) the correlation between commanded and measured forces. In all these parameters, it was visible that the initial trial in the readaptation block was different from the very first trial from the adaptation block, and their values were closer to those observed from adapted movements in the first block.
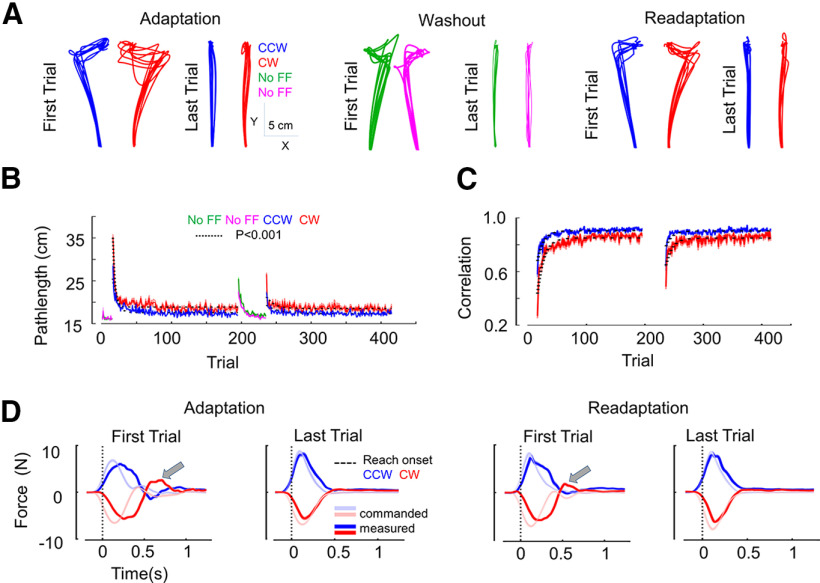
Figure 2.
A, Hand paths from all participants for the first and the last trial in adaptation, washout and readaptation blocks. CCW and CW FF trials are depicted in blue and red, respectively. No FF trials during washout block for group 1 and group 2 are depicted in green and magenta, respectively. The last trials in adaptation and readaptation blocks are well adapted to the FF, with reduced lateral deviation and target overshoot. The first trial in washout block shows after-effects. The last trial in the washout block is shown to highlight visually that the participants returned to baseline performance by the end of that block. B, Pathlength for no FF, CCW, and CW FFs across trials. The solid lines indicate strongly significant exponential fits (p < 0.001) that reveal curvature across FF trials. Shaded areas around the mean represent the SEM across participants. Adaptation (trial number: 16–195) and readaptation (trial number: 236–415) blocks are contrasted with no FF trials, i.e., baseline (trial number: 1–15) and washout (trial number:196–235). C, Correlation (R2) between commanded perturbation force and the interaction force produced at the interface between participants’ hand and the handle (measured force), across trials. The evolution of changes in force profiles across trials is evaluated using the correlation, better correlation means better compensation for the applied force. D, Commanded (light color) and measured forces (dark color) for the first and last trial of adaptation and readaptation blocks. CW in red and CCW in blue. The vertical dotted line represents the reach onset. The second peak force (highlighted with gray arrows) is nearly flattened for the last trials. Importantly, the first trial in the readaptation block shows better force control compared with the first trial in the adaptation block. The increased correlation and the better tuning of force profiles across trials are indications of better online feedback control.
For pathlength, a significant exponential decay across trials was observed in the adaptation block (p < 0.001, R2 = 0.80 for CW and R2 = 0.71 for CCW FFs; Fig. 2B) and in the readaptation block (p < 0.001, R2 = 0.30 for CW and R2 = 0.37 for CCW). We calculated the time as the number of trials to reach 90% of the asymptote of the learning curve and found that relearning was faster than learning by 75% in CCW (29 vs 7 trials to reach 90% of asymptote for adaptation and readaptation, respectively) and by 44% in CW (41 vs 23 trials). The time to reach asymptote was directly related to the rate of increase or decrease of an exponential phenomenon. We privileged this variable over individuals’ learning rates that tended to be more variable across participants.
To follow the analysis on pathlength presented above, we evaluated the correlation between commanded and interaction forces and observed a significant exponential increase in correlation across trials in the adaptation block (p < 0.001, R2 = 0.84 for CW and R2 = 0.80 for CCW; Fig. 2C), as well as in the readaptation block (p < 0.001, R2 = 0.61 for CW and R2 = 0.49 for CCW). Here, also we calculated the time to reach 90% of the asymptote of the learning curve and found that relearning was faster than learning by 80% in CCW (10 vs 2 trials to reach 90% of asymptote for adaptation and readaptation) and 36% in CW (25 vs 16 trials). Thus, this analysis confirms that relearning was indeed faster.
Since the commanded force was a function of hand forward velocity, we could interpret the increased correlation across trials as the tuning of hand force with the hand forward velocity (Fig. 2D), which is a marker of adaptation (Crevecoeur et al., 2020a,b; Mathew et al., 2020). Thus, both the reduction in pathlength and the increase in correlation reached their asymptotes faster, which highlighted the presence of savings.
In light of the tendency to reach the asymptote faster, our central question was to characterize the very first trial in learning and relearning contexts. We took the pathlength of the first trial in the two blocks and performed repeated-measures ANOVA over the learning phase (adaptation and readaptation, after pooling CW and CCW together). We found that the pathlength was significantly reduced in the first trial of readaptation block compared with that of adaptation block (G1 and G2 pooled mean: adaptation = 31.8 cm vs readaptation = 25.2 cm, 20% reduction, F(1,7) = 27.98, p = 0.001, = 0.79; Fig. 3A). Besides, we compared the correlation between commanded and interaction forces from the same very first trials from each block and found again that the correlation was significantly improved in the very first trial of readaptation block (G1 and G2 pooled mean: adaptation = 0.42 vs readaptation = 0.60, 43% improvement in readaptation, F(1,7) = 26.24, p = 0.001, = 0.79; Fig. 3B).
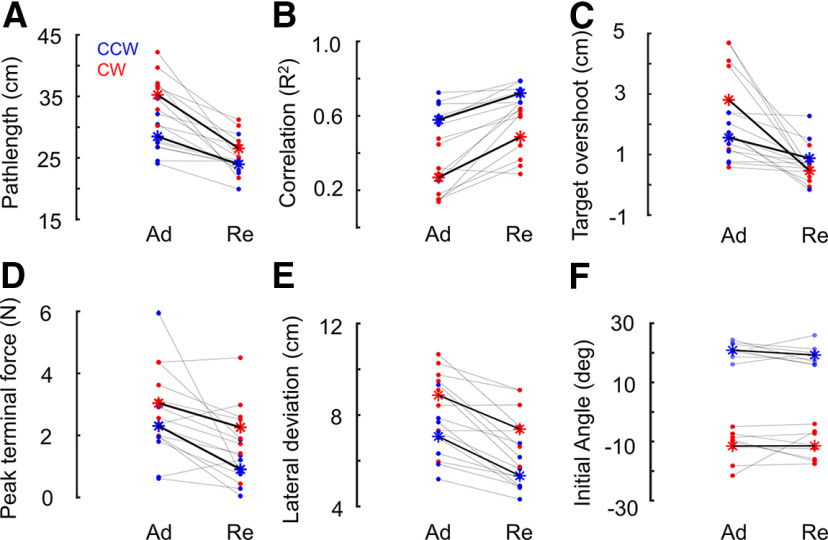
Figure 3.
A, Pathlength of the first trial in adaptation (Ad) and readaptation (Re) blocks. Each dot represents one participant, CCW and CW FF trials are depicted in blue and red, respectively. The mean of each group is represented by an asterisk (*). The gray lines represent variations for each participant and the black lines represent the mean variation between Ad and Re for each group. During the first trial in Ad and Re blocks. B, Correlation (R2) between commanded and measured force. C, Target overshoot in the lateral direction. D, Absolute peak terminal force applied on the handle. E, Maximum lateral deviation. F, Initial movement direction toward the target (initial angle made by the reaching movement with the straight line connecting starting and target position). Despite the absence of anticipation (comparable initial angle), the reduced path length, target overshoot, peak terminal force, lateral deviation, and a higher commanded-measured force correlation in the first readaptation trial highlight the recall of an efficient feedback control response during the very beginning of readaptation phase.
Similar observations (i.e., first relearn trial is better) were also made in other parameters characterizing movement kinematics like the maximum lateral hand path deviation (G1 and G2 pooled mean: adaptation = 7.9 cm vs readaptation = 6.3 cm, 20% smaller deviation for the first trial in readaptation block compared with that in adaptation block, F(1,7) = 29.33; p = 0.001, = 0.81; Fig. 3E), the lateral target overshoot near the end of the movement (G1 and G2 pooled mean: adaptation = 2.18 cm vs readaptation = 0.67 cm, 69% smaller overshoot for the first trial in readaptation block compared with that in adaptation block, F(1,7) = 19.4; p = 0.003, = 0.73; Fig. 3C), and the absolute peak terminal force (G1 and G2 pooled mean: adaptation = 2.67N vs readaptation = 1.58N, 41% smaller peak terminal force during the first readaptation trial, F(1,7) = 12.36; p = 0.009, = 0.64; Fig. 3D). Note that the reduction in terminal force (also in Fig. 2D, highlighted in gray arrows) occurred without any change in the FF, since hand forward velocities and therefore lateral forces were similar in the first and last trials of both blocks (repeated measures ANOVA, F(7,49) = 1.76; p = 0.12, = 0.20; BF = 2462, strong evidence for the null hypothesis that the means are equal).
All extracted parameters from the very first relearning trials presented the features of more adapted movements. If we can demonstrate that there was no anticipation of the FF at the beginning of the readaptation phase, that is the washout was complete, then the improvement in the first relearning trials must correspond to an adaptation of online feedback control (Crevecoeur et al., 2020a,b; Mathew et al., 2020). The foregoing analysis shows that it was indeed the case.
To further ascertain the contribution of feedback adaptation in faster relearning, it is thus necessary to verify that movements performed in the null field were similar across the baseline block and the washout block, during which the participant’s reaching movements recovered from the after-effects and returned to baseline performance. The washout block was intended to eliminate the feedforward component about the perturbation and at the end of 40 washout trials, we observed zero anticipation. We compared the last baseline trial with the last washout trial and found no significant difference in both groups in terms of pathlength (F(1,7) = 1.02, p = 0.34, = 0.13; BF =1.64, evidence for the null hypothesis: washout trials and baseline trials were equal) and initial angle (initial movement direction; F(1,7) = 1.76, p = 0.23, = 0.20; BF =1.15, evidence for the null hypothesis: washout trials and baseline trials were equal). Thus, the baseline and late washout trials were statistically indistinguishable, suggesting that deadaptation was complete. We do not reject the possibility that the feedback controller may not be completely de-adapted, although its effect was not visible on late washout trials (baseline and late washout trials were statistically indistinguishable in terms of pathlength, initial direction, and variance). Monitoring the accurate state of the feedback controller across the trials itself is challenging, it is not certain how long it will take the feedback controller to de-adapt, if it does. In such case even if we increase the number of washout trials, we may not be able to quantify the extent of de-adaptation of feedback controller.
Another way this could be verified was by looking at the initial reach angle from the first readaptation trial; indeed, if deadaptation was not complete, we would expect a difference in the initial angle, used as a proxy of feedforward strategies. Again, by comparing the first trial in adaptation and readaptation blocks, we found no significant difference in the initial angle (G1 mean = 20.9° and 19.2°, G2 mean = −11.47° and −11.5° for adaptation and readaptation blocks, respectively, F(1,7) = 0.47, p = 0.51, = 0.06; BF = 2.18, evidence for the null hypothesis: aiming angles in both blocks were equal; Fig. 3F). This confirms that the first trials in both blocks had similar (zero) anticipation, allowing us to rule out a partial anticipatory compensation, or an explicit strategy.
The previous observations confirmed that there was no residual or partial anticipation for the FF, but it remains possible that feedback control was different. To address this possibility, we analyzed the feedback control strategy by looking at the positional variance across trials over the reach time. Because perturbations may elicit changes in the control strategy independent of adaptation (Crevecoeur et al., 2019), we avoided applying disturbances to directly probe feedback gains. Instead, we observed the variance across trials as an indicator of how the natural variability of movements was regulated as a proxy of the feedback controller (Todorov and Jordan, 2002). The reduction in peak terminal force already argued against a default increase in feedback gains, since this would predict an increase in interaction forces. Besides, we computed the trial-by-trial positional variance of the whole movement in x direction, across late washout trials (15 trials) and compared it with that of baseline trials (15 trials). We concentrate on lateral variance (along x) since in our experiment we applied a lateral FF (maximum x variance: washout = 0.00203 cm, baseline = 0.00183 cm). If participants selectively increased their impedance through co-contraction or feedback gains, it should be visible in the x-axis. In the x-axis, the variance at washout is higher, which is against the idea that lateral deviations were countered by a default mechanism including biomechanical rigidity.
Finally, we investigated whether participants used or not co-contraction to modulate the limb intrinsic properties, and increased their feedback gains (Burdet et al., 2001; Franklin et al., 2008; Crevecoeur et al., 2019), potentially resulting in higher movement accuracy (Gribble et al., 2003). To evaluate the possible contribution of co-contraction to the improved control during the very first readaptation trial, we quantified EMG levels of a pair of agonist-antagonist shoulder muscles known to be strongly recruited by the perturbations that were used in this study (Fig. 4A,B; Franklin et al., 2008; Crevecoeur et al., 2019). For each participant, we computed the mean EMG activity during a 100-ms window before the reach onset for both PEC and DELT muscles (Fig. 4C). Then we compared the EMG in baseline trials with the first trial in adaptation and readaptation blocks for each participant and found no significant difference (repeated measures ANOVA for PEC: F(2,30) = 0.26; p = 0.76, = 0.01; BF = 24.31 and for DELT: F(2,30) = 0.61, p = 0.54, = 0.04; BF = 16.91, strong evidence for the null hypothesis that muscles activities are equal). This supports the argument that the better reach control in the first trial in readaptation blocks was not because of any co-contraction modulating the limb intrinsic properties, instead, it involved a feedback adaptation component that was described previously (Crevecoeur et al., 2020a; Mathew et al., 2020).
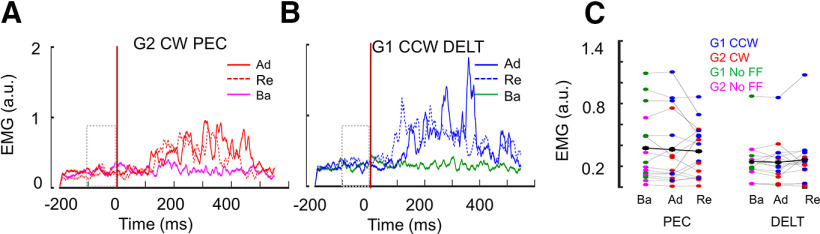
Figure 4.
A, Normalized EMG activity in pectoralis major muscle during CW perturbation (group 2). Each red EMG trace corresponds to the first trial in adaptation (Ad; dark red) and readaptation (Re; dotted red) blocks across all subjects. The mean baseline EMG activity (reach trials without FF in baseline block) is in magenta. Traces are aligned on reach onset (t = 0, vertical brown line). The dotted gray rectangular box represents 100-ms window before reach onset. B, Normalized EMG activity in DELT muscle during CCW perturbation (group 1) in Ad (dark blue) and Re (dotted blue) blocks averaged across all subjects. The mean baseline EMG activity (reach trials without FF in baseline block) is in green. Note that A, B are representative graphs to show the temporal evolution of EMG and similar graphs for G2 CW DELT and G1 CCW PEC are not shown for simplicity. C, Mean EMG activity during the 100-ms window (−100–0) before reach onset for muscles PEC and DELT during baseline (Ba), Ad, and Re blocks in both groups G1 and G2. Each dot represents the first trial in the corresponding block. The black asterisks (*) denote the mean across all participants (i.e., both CW and CCW groups). For both muscles, the mean EMG activity before reach onset is similar in all the blocks, which nullifies any possibility of coactivation strategy during the reaching movements of the readaptation block.
To summarize the Results, we first reproduced the expression of savings as faster relearning in human reach adaptation. We found evidence that the learning curves across trials converged faster to their asymptote in the readaptation blocks based on the pathlengths and the correlations between applied and measured forces. Building on these results our original contribution was to demonstrate that the very first trial in the readaptation block possessed all the characterizing features of better-adapted movements. Since the end of the washout was similar to the baseline performance, as highlighted by similar initial angles, pathlengths, positional variances, and the fact that participants were not aware that there would be a second adaptation phase, the contribution of an explicit strategy or feedforward adaptation could both be ruled out. Moreover, the readaptation phase started with similar levels of background muscle activity, and we even observed similar initial angles suggesting comparable effects of the unexpected FF. However, the kinematic and kinetics markers of better-adapted trials were present in the very first relearning trials: smaller path length, lateral deviation, target overshoot, peak terminal force and the higher correlation between commanded and applied forces. Thus, the improvement observed in the very first relearning trial can be explained by feedback adaptation, and the associated improvements contributed to the faster convergence toward the asymptote of the learning curve across trials.
Discussion
We documented the presence of a feedback adaptation component in FF reaching movements during readaptation to a FF previously encountered. Healthy participants first adapted to a unidirectional FF, followed by a washout sufficiently long to ensure that trials recovered a straight path, and then re-exposure to the same FF to quantify the nature of readaptation. First, we confirmed that readaptation was faster than adaptation based on the observation that the time to reach the asymptote of the learning curve in the readaptation block was significantly shorter than that in the adaptation block. This observation was consistent with previous findings (Smith et al., 2006; Herzfeld et al., 2014; Stockinger et al., 2014; Coltman et al., 2019) and confirmed the presence of savings (Shadmehr and Brashers-Krug, 1997; Overduin et al., 2006).
Second, in addition to the faster relearning rate characterizing trial-by-trial changes in motor performances, our main contribution was to highlight that, not only were participants relearning faster, but the very first trial of the readaptation phase was already better adapted. We observed a reduced pathlength, target overshoot, lateral deviation, terminal peak force and a higher commanded-measured force correlation in comparison with the first trials of the adaptation phase, despite the absence of anticipation in the relearning phase, and without any measurable change in average muscle activity that could have modulated limb stiffness (Burdet et al., 2001). This supports the hypothesis that an efficient feedback control response was recalled during the very first readaptation trial and improved stabilization of the ongoing movement. We believe that the link between feedback adaptation and savings is direct, considering that the state of adaptation at the end of the first relearning trial carries over to the next, which in turn enables better performance and feedback corrections for the following trials. These observations demonstrate that the neural mechanism underlying faster relearning involves a feedback adaptation component. They also provide further evidence that feedback adaptation plays a central role in the traditionally called feedforward component associated with trial-by-trial sequential adaptation scenarios (Crevecoeur et al., 2020b; Mathew et al., 2020).
In the context of adaptation to visual disturbances such as a cursor rotation, the presence of savings has been debated because of the presence of both implicit and explicit strategies (Taylor et al., 2014; McDougle et al., 2015; Morehead et al., 2015), the latter consisting in re-aiming at a remapped target. It is important to note that the possibility to handle disturbances with an explicit re-aiming strategy is potentially specific to visuomotor rotations (although subject to interference; Mazzoni and Krakauer, 2006). It is less clear that such an explicit strategy can produce apparent savings during adaptation to a FF, which involves a time-varying force that cannot be fully compensated simply by changing the aiming direction. Although, very recently, Schween et al. (2020) demonstrated explicit learning in a FF adaptation task, the apparent contribution in savings was not investigated in their study. In our experiment, washout was complete, participants did not anticipate the relearning session and we concentrated on the very first relearning trial to rule out any influence of such an explicit strategy. Note that none of the previous studies explicitly investigated the first relearning trial in detail with multiple kinematic variables (Fig. 3) to highlight the improvement in relearning (Huberdeau et al., 2015; Leow et al., 2016). As a consequence, and based on metrics specific to anticipation (for instance the initial angle), one may miss the properties of the first relearning trial that become apparent when considering the whole trace, including later within-trial corrections. We suggest that a feedback adaptation component recalling a previously acquired representation during movement produced a clear improvement during the very first relearning trial. Interestingly, previous studies documented similar adaptive control strategies when exposed to random visuomotor rotations (Braun et al., 2009); thus, there remain open questions about the possibility that feedback adaptation also influences savings in visuomotor learning.
Increasing evidence provides a compelling indication that the feedback control system plays a central role in motor adaptation. Recent studies highlighted features of the feedback adaptation component such that the feedback response was specifically and finely tuned to the ongoing FF perturbation, not only across trials with the same FF but also across different kinds of FFs (Crevecoeur et al., 2020a). The underlying mechanism associated with feedback adaptation can be preserved in memory long enough to contribute to the trial-by-trial adaptation scenarios (Mathew et al., 2020). Also, the changes in muscle activity consistent with feedback adaptation occurred within ∼250 ms following reach onset, which is proposed as an estimate of the latency of motor adaptation in the nervous system (Crevecoeur et al., 2020a,b). It was previously highlighted that modulation of long-latency feedback parallels the time course of the fast learning timescale (Coltman et al., 2019; Coltman and Gribble, 2020), which very likely is a major contributor to the speeding up of adaptation given the short time scale at which the faster relearning occurs. We add to this line of evidence by showing that feedback adaptation underlies savings in human FF learning, as it is clear that the behavioral improvement in the first relearning trial is attributable to the feedback control system, with the possibility that all feedback mechanisms are engaged (short-latency or long-latency reflexes, and visually-mediated corrections; Franklin and Wolpert, 2008; Cross et al., 2019).
We do not discard the evolution of both feedback and feedforward processes along with learning. However, most of the studies linked the trial-by-trial adaptation to feedforward process and hence the interpretation of savings was bound within feedforward concept. Our aim was to highlight the role of feedback control in adaptation (Crevecoeur, 2020a,b; Mathew, 2020) and in savings without opposing the idea of feedforward-feedback co-evolution in learning.
In conclusion, we suggest that there is a genuine acceleration of learning in a readaptation context that is supported by adaptive feedback control. By “genuinely accelerate,” we meant increase in the relearning rate by the preserved feedback component. If we analyze performance improvement trial-by-trial, the overall learning rate includes feedback and feedforward components. In this regard, we mean that feedback adaptation genuinely accelerates learning rate as assessed based on trial-by-trial behavior. The question of potential change in feedforward learning rate remains open, and our developments make clear that future work must take feedback adaptation into account in their design. Our results imply one challenging question for future work, that is to disentangle the contribution of feedback adaptation and explicit re-aiming strategies in the overall speedup of trial-by-trial learning across paradigms.
Synthesis
Reviewing Editor: Leonard Maler, University of Ottawa
Decisions are customarily a result of the Reviewing Editor and the peer reviewers coming together and discussing their recommendations until a consensus is reached. When revisions are invited, a fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision will be listed below. The following reviewer(s) agreed to reveal their identity: Alkis Hadjiosif.
Editor comments
Dear authors,
Both reviewers found this manuscript to contain interesting new data and analysis that will advance this field. Both reviewers also noted points that need addressing. I have included their comments in full to help you make the necessary revisions.
Reviewer 1
When we relearn a previously acquired motor skill, the learning is faster than the initial learning. This phenomenon is known as savings. This study proposed that the presence of feedback control adaptation can explain the savings. The authors experimentally demonstrated evidence supporting feedback control’s contribution to the savings using the force field adaptation during reaching movements. The
I think this is an interesting study demonstrating the contribution of feedback control for relearning a motor skill. I have only a few suggestions described below.
Major points
1. The pathlength and correlation measures include the contribution of the feedback controller. In contrast, savings are generally evaluated as the memory for feedforward control. Thus, I would like to see how the initial movement direction, which must directly reflect feedforward control’s contribution, changed during the adaptation and re-adaptation phase. If the savings were observed in this measure, it could be interpreted that the presence of feedback adaptation boosted the re-adaptation of the feedforward controller.
2. Do the authors predict that the increase in the number of washout trials might influence the first relearning trials’ performance? In my opinion, the results are likely to indicate the difference in the adaptation (or de-adaptation) rate between feedforward and feedback controller (de-adaptation of feedback controller seems slower). The authors should add some discussions about this possibility.
3. The dissociation between feedforward and feedback controllers implied by this study is intriguing because a lot of recent studies have indicated both types of control can co-evolve with learning (Cluff & Scott, J Neurosci 2013; Maeda et al., Curr Biol 2020). From this point of view, the value of this study is not restricted to the idea that the feedback adaptation contributes to the savings. I would like the authors to add some discussions on this issue.
Minor points
Line 413-414: A recent paper by Taylor’s group has demonstrated the contribution of explicit strategy on the force-field adaptation (Schween et al., J Neurophysiol 2020).
Reviewer 2
This paper demonstrates that feedback components of adaptation may escape washout, suggesting that this may contribute to savings. It is also shown that this feedback component is not explicitly driven. This finding is clearly interesting, and can provide valuable insights when assessing savings paradigms in general. Previous work on savings for motor adaptation tended to focus on the adaptation of feedforward components rather than feedback components (though shared mechanisms and interactions have been documented). My main issue reading the paper was whether the findings could be explained by an increase in co-contraction and feedback gains, but the latter part of the paper deals with that issue relatively convincingly (though I have a question below). I thus have only minor issues, suggestions, and general comments.
1) The potential issue with an increase in feedback gains is countered with two arguments (L321-328) that may need to be clarified a bit.
a. One argument is that the peak terminal force is already reduced, and that would be against an increase in feedback gains. But couldn’t feedback responses, with increased gains, counter the force-field so that, by the time the target is reached, deviations are lower and terminal forces along with them?
b. The other argument is about the positional variance - the authors mention that they find no evidence of differences in the positional variance between washout and baseline trials. I suggest adding the numbers there, and also explaining how the positional variance was calculated (is it the trial-to-trial variance of the maximum deviation for x and y? or is it taking the whole movement into account somehow? etc.)
2) In the abstract and intro, what is meant by “genuinely accelerate relearning”? It could be simply that feedback adaptation was not unlearned, and it accelerated resumption of performance during the re-adaptation period. This is not an acceleration of learning per se. Is “genuinely accelerate” referring to increases in the learning rate (in contrast to the re-emergence of non-unlearned components which contribute to performance, such as a better feedback response, or “hidden” feedforward components e.g. in a two-state model)? Of course, a preserved feedback component could potentially accelerate relearning, e.g. if the feedback response were training the feedforward component (e.g. Albert and Shadmehr 2016).
3) (just a general comment) While the paper clearly identifies a preserved feedback adaptation that can support savings, it’s not that clear how much of the apparent savings is to be attributed to it. The estimate of learning speed (trials to 90% of asymptote) doesn’t distinguish between feedback adaptation, latent feedforward components (40 trials may be too short a washout), or increases in the learning rate of feedforward components. This may be interesting to explore in future work.
4) Were there breaks given during the experiment? (if so, in which trials? To ensure that these did not coincide with forcefield introduction/re-introduction)
5) How did the participants return to the starting position, did the robotic arm passively move them? Or were the out and back movements separate , with forcefields on in both cases?
Author Response
Both reviewers found this manuscript to contain interesting new data and analysis that will advance this field. Both reviewers also noted points that need addressing. I have included their comments in full to help you make the necessary revisions.
We thank the editor and reviewers for their valuable feedback to improve the quality of the research work.
Reviewer 1
When we relearn a previously acquired motor skill, the learning is faster than the initial learning. This phenomenon is known as savings. This study proposed that the presence of feedback control adaptation can explain the savings. The authors experimentally demonstrated evidence supporting feedback control’s contribution to the savings using the force field adaptation during reaching movements. I think this is an interesting study demonstrating the contribution of feedback control for relearning a motor skill. I have only a few suggestions described below.
We thank the reviewer for his/her positive and detailed comments. Below we explicitly address the main concerns and express gratitude for helping us to improve our manuscript.
Major points
1. The pathlength and correlation measures include the contribution of the feedback controller. In contrast, savings are generally evaluated as the memory for feedforward control. Thus, I would like to see how the initial movement direction, which must directly reflect feedforward control’s contribution, changed during the adaptation and re-adaptation phase. If the savings were observed in this measure, it could be interpreted that the presence of feedback adaptation boosted the re-adaptation of the feedforward controller.
We thank the reviewer for the suggestion. We assessed the initial movement direction across the trials (see figure below) and observed a more complex behaviour in the learning curve as they displayed different timescales and changes in sign, possibly due to factors linked to robot dynamics.
As can be seen, this variable was noisier across the trials, we couldn’t sum up the evolution of the true nature of feedforward controller completely from this variable alone. Therefore, we would be cautious to claim that the presence of feedback adaptation impacted the re-adaptation of feedforward controller.
2. Do the authors predict that the increase in the number of washout trials might influence the first relearning trials’ performance? In my opinion, the results are likely to indicate the difference in the adaptation (or de-adaptation) rate between feedforward and feedback controller (de-adaptation of feedback controller seems slower). The authors should add some discussions about this possibility.
We predict that the improved performance in first relearning trial holds even if we increase the number of washout trials. We added the following discussion to clarify this point (line 311-317).
"We don’t reject the possibility that the feedback controller may not be completely de-adapted, even though its effect was not visible on late washout trials (baseline and late washout trials were statistically indistinguishable in terms of pathlength, initial direction and variance). Monitoring the accurate state of the feedback controller across the trials itself is challenging, it is not certain how long it will take the feedback controller to de-adapt, if it does. In such case even if we increase the number of washout trials, we may not be able to quantify the extent of de-adaptation of feedback controller.”
3. The dissociation between feedforward and feedback controllers implied by this study is intriguing because a lot of recent studies have indicated both types of control can co-evolve with learning (Cluff & Scott, J Neurosci 2013; Maeda et al., Curr Biol 2020). From this point of view, the value of this study is not restricted to the idea that the feedback adaptation contributes to the savings. I would like the authors to add some discussions on this issue.
Indeed previous works have highlighted reciprocal changes in feedforward and feedback adaptation {Cluff, Maeda}. Here our data suggest that these two models of control can be partially dissociated such that, even with complete washout, improvements based on feedback adaptation were visible. Our main message is to highlight the contribution of feedback adaptation in savings, but we don’t discard the feedforward-feedback co-evolution in learning. We added the following discussion to the manuscript (line: 463-467)
"We do not discard the evolution of both feedback and feedforward processes along with learning. However, most of the studies linked the trial-by-trial adaptation to feedforward process and hence the interpretation of savings was bound within feedforward concept. Our aim was to highlight the role of feedback control in adaptation (Crevecoeur 2020a,b, Mathew 2020) and in savings without opposing the idea of feedforward-feedback co-evolution in learning.”
Minor points
Line 413-414: A recent paper by Taylor’s group has demonstrated the contribution of explicit strategy on the force-field adaptation (Schween et al., J Neurophysiol 2020)
We thank the reviewer for pointing out this paper and we have added the following sentence to our discussion (line 431-432)
"Although very recently Schween et al (2020) demonstrated explicit learning in a force field adaptation task, the apparent contribution in savings was not investigated in their study.”
Reviewer 2
This paper demonstrates that feedback components of adaptation may escape washout, suggesting that this may contribute to savings. It is also shown that this feedback component is not explicitly driven. This finding is clearly interesting, and can provide valuable insights when assessing savings paradigms in general. Previous work on savings for motor adaptation tended to focus on the adaptation of feedforward components rather than feedback components (though shared mechanisms and interactions have been documented). My main issue reading the paper was whether the findings could be explained by an increase in co-contraction and feedback gains, but the latter part of the paper deals with that issue relatively convincingly (though I have a question below). I thus have only minor issues, suggestions, and general comments.
We thank the reviewer for his/her constructive feedback.
1) The potential issue with an increase in feedback gains is countered with two arguments (L321-328) that may need to be clarified a bit.
a. One argument is that the peak terminal force is already reduced, and that would be against an increase in feedback gains. But couldn’t feedback responses, with increased gains, counter the force-field so that, by the time the target is reached, deviations are lower and terminal forces along with them?
For every trial, participants need to wait for the “Go” cue at the starting point, upon seeing the cue make a move towards the target and stabilize there. Since this is not a shooting task, feedback responses play a good role in stabilizing the cursor at the target.
It can be demonstrated in simple settings that augmenting the feedback gains without changing the model parameters produces increases in control gains and consequently also in interaction forces, thus it is not clear to us how feedback gains can be changed selectively at the beginning of the trial, without involving a feedback adaptation component allowing to reduce them by the time the target was reached. Effectively, a precisely timed change in feedback gains with increase and decrease allowing stabilizing is indistinguishable from adaptation.
b. The other argument is about the positional variance - the authors mention that they find no evidence of differences in the positional variance between washout and baseline trials. I suggest adding the numbers there, and also explaining how the positional variance was calculated (is it the trial-to-trial variance of the maximum deviation for x and y? or is it taking the whole movement into account somehow? etc.)
We apologize for the potential confusion. We have added the following test to clarify this point (line 336-343)
"Besides, we computed the trial-by-trial positional variance of the whole movement in x direction, across late washout trials (15 trials) and compared it with that of baseline trials (15 trials). We concentrate on lateral variance (along x) since in our experiment we applied a lateral force field (Maximum X variance : Washout =0.00203 cm, Baseline = 0.00183 cm). If participants selectively increased their impedance through co-contraction or feedback gains, it should be visible in the x-axis. In the x-axis, the variance at washout is higher, which is against the idea that lateral deviations were countered by a default mechanisms including biomechanical rigidity.”
2) In the abstract and intro, what is meant by “genuinely accelerate relearning”? It could be simply that feedback adaptation was not unlearned, and it accelerated resumption of performance during the re-adaptation period. This is not an acceleration of learning per se. Is “genuinely accelerate” referring to increases in the learning rate (in contrast to the re-emergence of non-unlearned components which contribute to performance, such as a better feedback response, or “hidden” feedforward components e.g. in a two-state model)? Of course, a preserved feedback component could potentially accelerate relearning, e.g. if the feedback response were training the feedforward component (e.g. Albert and Shadmehr 2016).
The following text is added to clarify this point (line : 469-475)
"By “genuinely accelerate” we meant increase in the relearning rate by the preserved feedback component. If we analyse performance improvement trial-by-trial, the overall learning rate includes feedback and feedforward components. In this regard, we mean that feedback adaptation genuinely accelerates learning rate as assessed based on trial-by-trial behaviour. The question of potential change in feedforward learning rate remains open, and our developments make clear that future work must take feedback adaptation into account in their design.”
3) (just a general comment) While the paper clearly identifies a preserved feedback adaptation that can support savings, it’s not that clear how much of the apparent savings is to be attributed to it. The estimate of learning speed (trials to 90% of asymptote) doesn’t distinguish between feedback adaptation, latent feedforward components (40 trials may be too short a washout), or increases in the learning rate of feedforward components. This may be interesting to explore in future work.
We thank the reviewer for the fruitful suggestion for the future work. As we explained above, there can be change in feedforward learning rate and that indeed, the quantification of how each component (feedforward and feedback) accelerate learning remains open. Our work aimed at demonstrating that feedback adaptation was a player.
4) Were there breaks given during the experiment? (if so, in which trials? To ensure that these did not coincide with forcefield introduction/re-introduction)
The following text is added to the Methods section to clarify this point (line :118-121).
"Breaks were given 2 times during the adaptation block (between trial no. 70 -90 and 150-170) and 2 times during the readaptation block (250-270 and 350-370) randomly distributed across subjects so as to avoid any bias in a particular trial. The introduction/re-introduction of forcefield was without any explicit warning.”
5) How did the participants return to the starting position, did the robotic arm passively move them? Or were the out and back movements separate, with forcefields on in both cases?
The following text is added to the Methods section to clarify this point (line :108-110).
"After every trial, participants needed to return to the starting point by moving the robotic handle by themselves. There was no forcefield during the return movement, it was not part of a trial.”
References
- Brashers-Krug T, Shadmehr R, Bizzi E (1996) Consolidation in human motor memory. Nature 382:252–255. 10.1038/382252a0 [DOI] [PubMed] [Google Scholar]
- Braun DA, Aertsen A, Wolpert DM, Mehring C (2009) Learning optimal adaptation strategies in unpredictable motor tasks. J Neurosci 29:6472–6478. 10.1523/JNEUROSCI.3075-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdet E, Osu R, Franklin DW, Milner TE, Kawato M (2001) The central nervous system stabilizes unstable dynamics by learning optimal impedance. Nature 414:446–449. 10.1038/35106566 [DOI] [PubMed] [Google Scholar]
- Caithness G, Osu R, Bays P, Chase H, Klassen J, Kawato M, Wolpert DM, Flanagan JR (2004) Failure to consolidate the consolidation theory of learning for sensorimotor adaptation tasks. J Neurosci 24:8662–8671. 10.1523/JNEUROSCI.2214-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluff T, Scott SH (2013) Rapid feedback responses correlate with reach adaptation and properties of novel upper limb loads. J Neurosci 33:15903–15914. 10.1523/JNEUROSCI.0263-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltman SK, Gribble PL (2020) Time course of changes in the long latency feedback response parallels the fast process of short term motor adaptation. bioRxiv 2020.04.25.061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltman SK, Cashaback JGA, Gribble PL (2019) Both fast and slow learning processes contribute to savings following sensorimotor adaptation. J Neurophysiol 121:1575–1583. 10.1152/jn.00794.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevecoeur F, Scott SH, Cluff T (2019) Robust control in human reaching movements: a model-free strategy to compensate for unpredictable disturbances. J Neurosci 39:8135–8148. 10.1523/JNEUROSCI.0770-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevecoeur F, Mathew J, Bastin M, Lefèvre P (2020a) Feedback adaptation to unpredictable force fields in 250 ms. eNeuro 7:ENEURO.0400-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevecoeur F, Thonnard JL, Lefèvre P (2020b) A very fast time scale of human motor adaptation: within movement adjustments of internal representations during reaching. eNeuro:ENEURO.0149-19.2019. 10.1523/ENEURO.0149-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross KP, Cluff T, Takei T, Scott SH (2019) Visual feedback processing of the limb involves two distinct phases. J Neurosci 39:6751–6765. 10.1523/JNEUROSCI.3112-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkenberry TJ (2018) Computing Bayes factors to measure evidence from experiments: an extension of the BIC approximation. Biom Lett 55:31–43. 10.2478/bile-2018-0003 [DOI] [Google Scholar]
- Focke A, Stockinger C, Diepold C, Taubert M, Stein T (2013) The influence of catch trials on the consolidation of motor memory in force field adaptation tasks. Front Psychol 4:479. 10.3389/fpsyg.2013.00479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin DW, Wolpert DM (2008) Specificity of reflex adaptation for task-relevant variability. J Neurosci 28:14165–14175. 10.1523/JNEUROSCI.4406-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin DW, Burdet E, Tee KP, Osu R, Chew C-M, Milner TE, Kawato M (2008) CNS learns stable, accurate, and efficient movements using a simple algorithm. J Neurosci 28:11165–11173. 10.1523/JNEUROSCI.3099-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz CO, Morris PE, Richler JJ (2012) Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen 141:2–18. 10.1037/a0024338 [DOI] [PubMed] [Google Scholar]
- Gonzalez Castro LN, Hadjiosif AM, Hemphill MA, Smith MA (2014) Environmental consistency determines the rate of motor adaptation. Curr Biol CB 24:1050–1061. 10.1016/j.cub.2014.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble PL, Mullin LI, Cothros N, Mattar A (2003) Role of cocontraction in arm movement accuracy. J Neurophysiol 89:2396–2405. 10.1152/jn.01020.2002 [DOI] [PubMed] [Google Scholar]
- Herzfeld DJ, Vaswani PA, Marko MK, Shadmehr R (2014) A memory of errors in sensorimotor learning. Science 345:1349–1353. 10.1126/science.1253138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberdeau DM, Haith AM, Krakauer JW (2015) Formation of a long-term memory for visuomotor adaptation following only a few trials of practice. J Neurophysiol 114:969–977. 10.1152/jn.00369.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G, Witten D, Hastie T, Tibshirani R (2013) An introduction to statistical learning: with applications in R. New York: Springer. [Google Scholar]
- Joiner WM, Sing GC, Smith MA (2017) Temporal specificity of the initial adaptive response in motor adaptation. PLoS Comput Biol 13:e1005438. 10.1371/journal.pcbi.1005438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Shadmehr R (2006) Consolidation of motor memory. Trends Neurosci 29:58–64. 10.1016/j.tins.2005.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4:863. 10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow LA, de Rugy A, Marinovic W, Riek S, Carroll TJ (2016) Savings for visuomotor adaptation require prior history of error, not prior repetition of successful actions. J Neurophysiol 116:1603–1614. 10.1152/jn.01055.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda RS, Cluff T, Gribble PL, Pruszynski JA (2018) Feedforward and feedback control share an internal model of the arm’s dynamics. J Neurosci 38:10505–10514. 10.1523/JNEUROSCI.1709-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew J, Lefevre P, Crevecoeur F (2020) Rapid changes in movement representations during human reaching could be preserved in memory for at least 850 ms. eNeuro:ENEURO.0266-20.2020. 10.1523/ENEURO.0266-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW (2006) An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26:3642–3645. 10.1523/JNEUROSCI.5317-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle SD, Bond KM, Taylor JA (2015) Explicit and implicit processes constitute the fast and slow processes of sensorimotor learning. J Neurosci 35:9568–9579. 10.1523/JNEUROSCI.5061-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehead JR, Qasim SE, Crossley MJ, Ivry R (2015) Savings upon re-aiming in visuomotor adaptation. J Neurosci 35:14386–14396. 10.1523/JNEUROSCI.1046-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin SA, Richardson AG, Lane CE, Bizzi E, Press DZ (2006) Intermittent practice facilitates stable motor memories. J Neurosci 26:11888–11892. 10.1523/JNEUROSCI.1320-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G (2009) Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev 16:225–237. 10.3758/PBR.16.2.225 [DOI] [PubMed] [Google Scholar]
- Rouder JN, Morey RD, Speckman PL, Province JM (2012) Default Bayes factors for ANOVA designs. J Math Psychol 56:356–374. 10.1016/j.jmp.2012.08.001 [DOI] [Google Scholar]
- Schween R, McDougle SD, Hegele M, Taylor JA (2020) Assessing explicit strategies in force field adaptation. J Neurophysiol 123:1552–1565. 10.1152/jn.00427.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Brashers-Krug T (1997) Functional stages in the formation of human long-term motor memory. J Neurosci 17:409–419. 10.1523/JNEUROSCI.17-01-00409.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R (2006) Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol 4:e179. 10.1371/journal.pbio.0040179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger C, Focke A, Stein T (2014) Catch trials in force field learning influence adaptation and consolidation of human motor memory. Front Hum Neurosci 8:231. 10.3389/fnhum.2014.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Krakauer JW, Ivry RB (2014) Explicit and implicit contributions to learning in a sensorimotor adaptation task. J Neurosci 34:3023–3032. 10.1523/JNEUROSCI.3619-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov E, Jordan MI (2002) Optimal feedback control as a theory of motor coordination. Nat Neurosci 5:1226–1235. 10.1038/nn963 [DOI] [PubMed] [Google Scholar]
- Wagner MJ, Smith MA (2008) Shared internal models for feedforward and feedback control. J Neurosci 28:10663–10673. 10.1523/JNEUROSCI.5479-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif N, Diedrichsen J (2012) Structural learning in feedforward and feedback control. J Neurophysiol 108:2373–2382. 10.1152/jn.00315.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and codes used in this project is available on request.