Abstract
Background
Pulse pressure (PP) reflects the age-related stiffening of the central arteries, but no study addressed the management of the PP-related risk over the human lifespan.
Methods
In 4,663 young (18–49 years) and 7,185 older adults (≥50 years), brachial PP was recorded over 24 hours. Total mortality and all major cardiovascular events (MACEs) combined were coprimary endpoints. Cardiovascular death, coronary events, and stroke were secondary endpoints.
Results
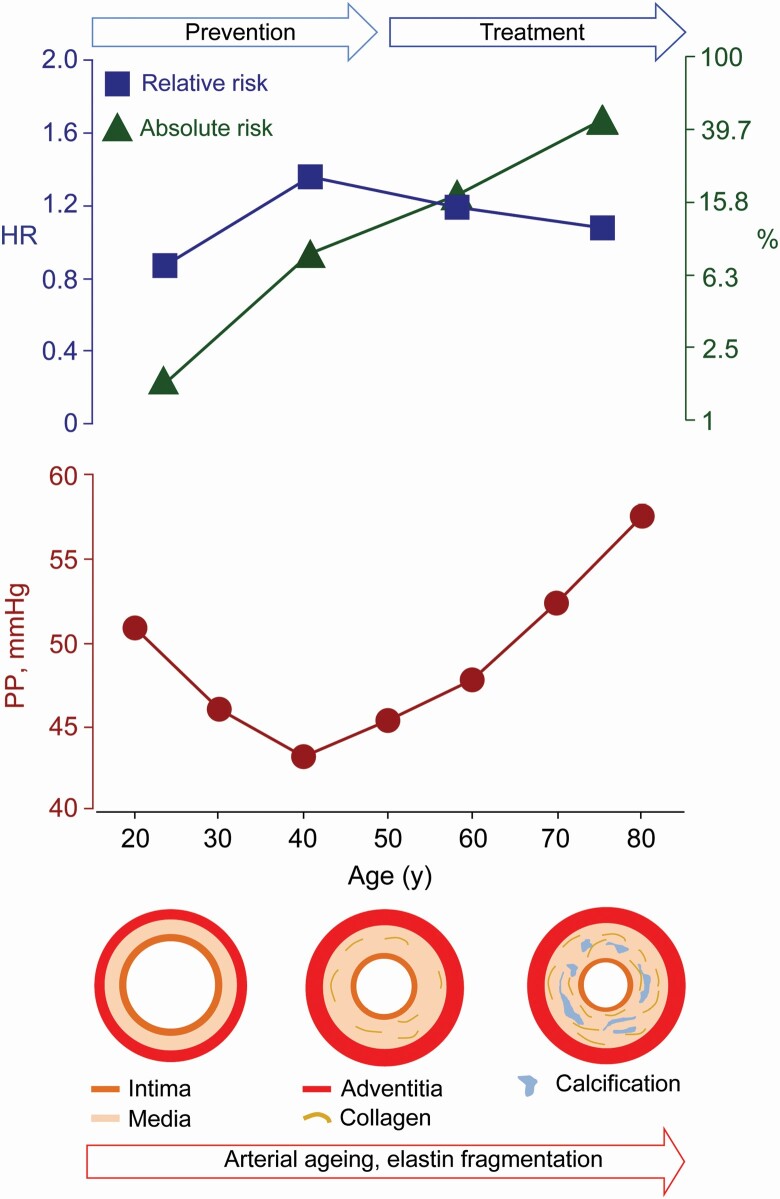
In young adults (median follow-up, 14.1 years; mean PP, 45.1 mm Hg), greater PP was not associated with absolute risk; the endpoint rates were ≤2.01 per 1,000 person-years. The adjusted hazard ratios expressed per 10-mm Hg PP increments were less than unity (P ≤ 0.027) for MACE (0.67; 95% confidence interval [CI], 0.47–0.96) and cardiovascular death (0.33; 95% CI, 0.11–0.75). In older adults (median follow-up, 13.1 years; mean PP, 52.7 mm Hg), the endpoint rates, expressing absolute risk, ranged from 22.5 to 45.4 per 1,000 person-years and the adjusted hazard ratios, reflecting relative risk, from 1.09 to 1.54 (P < 0.0001). The PP-related relative risks of death, MACE, and stroke decreased >3-fold from age 55 to 75 years, whereas absolute risk rose by a factor 3.
Conclusions
From 50 years onwards, the PP-related relative risk decreases, whereas absolute risk increases. From a lifecourse perspective, young adulthood provides a window of opportunity to manage risk factors and prevent target organ damage as forerunner of premature death and MACE. In older adults, treatment should address absolute risk, thereby extending life in years and quality.
Keywords: arterial stiffness, blood pressure, cardiovascular disease, pulse pressure, hypertension, mortality, population science
Graphical Abstract
Graphical Abstract.
Over the human lifespan, aging and age-related risk factors, such as hypertension, renal dysfunction, and type-2 diabetes, lead to stiffening of the central elastic arteries. As a consequence, the systolic load on the arterial walls is cushioned less, a phenomenon further amplified by the early return of reflected waves in early systole, while the tensile force maintaining a continuous blood flow during diastole diminishes.1 From middle age onwards, pulse pressure (PP), the difference between systolic and diastolic blood pressure (BP), widens because systolic BP continues to rise until old age, whereas diastolic BP decreases.2
Numerous studies in patient3–5 and population6,7 cohorts demonstrated that adverse health outcomes were positively associated with higher brachial PP, a commonly used marker of arterial stiffness derived from office4,7 or ambulatory3,5 BP readings. From young to old age, the risk carrying BP component gradually shifts from diastolic to systolic BP.5,6 However, to the best of our knowledge, no previous study differentiated between the absolute and relative risks associated with PP. This issue is not trivial, given that a lifecourse prevention of the cardiovascular complications associated with arterial stiffening1 and hypertension8 is of paramount importance from young adulthood onwards. In general, relative risk is high in young adults, whereas absolute risk only starts rising from middle age onwards with competing contributions of cardiovascular and noncardiovascular disease to all-cause mortality.9,10 From this perspective, we analyzed the International Database on Ambulatory Blood Pressure in Relation Cardiovascular Outcome (IDACO) database11 to evaluate the relative and absolute risk associated with PP in young and old adults and to chart the lifecourse prevention of cardiovascular disease associated with arterial stiffening.1
METHODS
Study participants
All population studies included in the IDACO database11 received ethical approval from the Institutional Review Boards in their country of origin and adhered to the Declaration of Helsinki.12 Participants provided informed written consent. The IDACO database, constructed and maintained in Leuven, did not include any data allowing identification of individuals. In line with national regulations, Review Boards either waived or provided ethical clearance for the secondary use of the IDACO data. Population studies qualified for inclusion, if the ambulatory BP and cardiovascular risk factors had been measured at baseline and if follow-up included both fatal and nonfatal outcomes. Across all studies, baseline data were collected from May 1988 until August 2010 and follow-up data from August 2007 to October 2016 (Supplementary Table S1 online). The references available in the supplementary online material describe the design of the 13 studies (Supplementary Table S2 online).
Ambulatory BP monitoring
For ambulatory BP monitoring (Supplementary Table S3 online), portable oscillometric monitors were programmed to obtain readings at 30-minute intervals throughout the whole day or at intervals of 15–30 minutes during daytime and at intervals ranging from 20 to 60 minutes during nighttime. Ambulatory recordings had to include at least 6 daytime and 3 nighttime readings.13 Mean arterial pressure was diastolic BP plus one-third of PP.
Ascertainment of endpoints
We ascertained vital status and the incidence of fatal and nonfatal endpoints (Supplementary Table S4 online) from the appropriate sources in each country. The coprimary endpoints were total mortality and a composite cardiovascular outcome consisting of cardiovascular mortality, including sudden death, nonfatal coronary events, coronary revascularization, heart failure, and stroke. Secondary endpoints included cardiovascular mortality, fatal and nonfatal coronary endpoints, and fatal and nonfatal stroke excluding transient ischemic attack. All endpoints were validated against medical records held by primary care physicians, specialists, or hospitals. In all outcome analyses, only the first event within each category was considered. Participants free of events were censored at last follow-up.
Statistical analysis
For database management and statistical analysis, SAS software, version 9.4, maintenance level 5 (Cary, NC) was used. We applied the Kolmogorov–Smirnov test for assessing the normality of distributions. For between-group comparison of means and proportions, we applied the large-sample z-test and Fisher’s exact test, respectively. After stratification for cohort and sex, we interpolated missing values of body mass index and serum cholesterol from the regression slopes on age. In participants with unknown status of smoking, drinking, diabetes, or history of cardiovascular disease, we set the variable to the cohort- and sex-specific mean of the codes (0, 1). In exploratory analyses, the incidence rates of the primary and secondary endpoints were categorized by fourths of the PP distribution. Rates were standardized by the direct method for cohort, sex and age, as appropriate, and 95% confidence intervals (CIs) were computed based on the gamma-distribution as proposed by Fay and Feuer.14
Hazard ratios associated with a 1-SD increment in PP (10 mm Hg) were estimated from multivariable-adjusted cause-specific Cox regression, accounting for cohort (using design variables), and baseline characteristics including age, body mass index, 24-hour mean arterial pressure, 24-hour heart rate, smoking and drinking status, serum cholesterol, antihypertensive drug intake, history of cardiovascular disease, and diabetes.15 To adjust for cohort, we pooled participants recruited in the framework of the European Project on Genes in Hypertension (Gdańsk, Kraków, Novosibirsk, Padova and Pilsen; Supplementary Table S1 online). We checked the proportional hazards assumption for PP by the Kolmogorov-type supremum test and by testing the interaction between BP and follow-up time. Significance of the trends in the hazard ratios associated with PP across increasing age categories in older adults (50–59, 60–69, and ≥70 years) was assessed by introducing an interaction term in the Cox models. We plotted cumulative incidence rates and estimated 5-year absolute risks from the Fine and Gray proportional subdistribution hazards model taking into account competing noncardiovascular mortality in analyses of cardiovascular death.16
RESULTS
Baseline characteristics
Of 13,728 people included in the database, we excluded 1,880, because they were adolescents younger than 18 years (n = 317), or because their ambulatory BP recording included fewer readings than the required number (n = 1,563). This left 11,848 individuals for statistical analysis (Table 1). Missing values of body mass index (n = 33), serum cholesterol (n = 822), smoking (n = 79) and drinking (n = 826) status, diabetes (n = 5), or history of cardiovascular disease (n = 1) were interpolated or set to the cohort- and sex-specific means. Table 1 lists the baseline characteristics of the participants. Mean age at enrollment was 52.9 years.
Table 1.
Baseline characteristics of participants by age group and overall
| Characteristic | Age categories | All ages | |||
|---|---|---|---|---|---|
| 18–49 y | 50–59 y | 60–69 y | ≥70 y | ||
| No. in category | 4,663 | 2,470 | 2,263 | 2,452 | 11,848 |
| No. with characteristic (%) | |||||
| Women | 2,531 (54.3) | 1,383 (56.0) | 1,192 (52.7) | 777 (31.7) | 5,883 (49.7) |
| Europeans | 3,097 (66.4) | 1,386 (56.1) | 1,060 (46.8) | 1,571 (64.1) | 7,114 (60.0) |
| Asians | 700 (15.0) | 637 (25.8) | 626 (27.7) | 452 (18.4) | 2,415 (20.4) |
| South Americans | 866 (18.6) | 447 (18.1) | 577 (25.5) | 429 (17.5) | 2,319 (19.6) |
| Current smoking | 1,451 (31.1) | 693 (28.0) | 570 (25.5) | 488 (19.9) | 3,202 (27.0) |
| Drinking alcohol | 2,111 (45.3) | 1,179 (67.7) | 1,101 (48.7) | 1,124 (45.8) | 5,515 (46.5) |
| Office hypertension | 720 (15.4) | 917 (37.1) | 1,124 (49.7) | 1,534 (62.6) | 4,295 (36.2) |
| On antihypertensive treatment | 226 (4.8) | 535 (21.7) | 644 (28.5) | 949 (38.7) | 2,354 (19.9) |
| Diabetes | 116 (2.5) | 181 (7.3) | 263 (11.6) | 306 (12.5) | 866 (7.3) |
| History of cardiovascular disease | 145 (3.1) | 251 (10.2) | 409 (18.1) | 509 (20.8) | 1,314 (11.1) |
| Mean (SD) of characteristic | |||||
| Age, y | 36.2 ± 8.6 | 54.5 ± 3.0 | 63.8 ± 3.0 | 73.2 ± 3.8 | 52.9 ± 15.9 |
| Body mass index, kg/m2 | 24.5 ± 4.2 | 26.2 ± 4.5 | 26.0 ± 4.5 | 25.6 ± 4.0 | 25.4 ± 4.4 |
| Office systolic BP, mm Hg | 120.4 ± 16.0 | 132.2 ± 21.0 | 140.9 ± 23.9 | 148.2 ± 24.4 | 132.5 ± 23.3 |
| Office diastolic BP, mm Hg | 76.4 ± 11.0 | 81.7 ± 12.2 | 82.0 ± 11.9 | 82.0 ± 11.9 | 79.7 ± 12.0 |
| Office PP, mm Hg | 43.9 ± 10.6 | 50.6 ± 14.8 | 59.0 ± 18.6 | 66.4 ± 19.1 | 52.8 ± 17.6 |
| Office MAP, mm Hg | 91.1 ± 11.9 | 98.6 ± 14.1 | 101.7 ± 14.7 | 104.4 ± 14.4 | 97.4 ± 14.5 |
| 24-Hour systolic BP, mm Hg | 117.1 ± 11.2 | 123.7 ± 11.2 | 127.6 ± 14.0 | 132.2 ± 15.1 | 123.6 ± 14.4 |
| 24-Hour diastolic BP, mm Hg | 71.9 ± 8.1 | 75.8 ± 9.1 | 75.2 ± 8.6 | 74.4 ± 3.4 | 73.9 ± 8.6 |
| 24-Hour PP, mm Hg | 45.1 ± 6.9 | 47.9 ± 8.1 | 52.4 ± 9.6 | 57.8 ± 11.2 | 49.7 ± 10.0 |
| 24-Hour MAP, mm Hg | 87.0 ± 8.7 | 91.8 ± 10.1 | 92.6 ± 9.7 | 93.6 ± 9.7 | 90.4 ± 9.8 |
| 24-Hour heart rate, bpm | 74.5 ± 9.0 | 72.5 ± 8.5 | 71.3 ± 8.8 | 69.5 ± 9.6 | 75.5 ± 9.2 |
| Serum cholesterol, mg/dl | 204.7 ± 43.3 | 224.4 ± 43.2 | 228.3 ± 47.1 | 224.1 ± 37.9 | 220.5 ± 47.2 |
Abbreviations: BP, blood pressure; MAP, mean arterial pressure derived as diastolic BP plus 1/3 of PP; PP, pulse pressure, i.e., the difference between systolic and diastolic BP. Current smoking (the daily use of smoking materials), drinking alcohol containing beverages occasionally or daily, the use of antihypertensive medications, and a history of cardiovascular disease were assessed at baseline by questionnaire. Office BP was measured using standard mercury sphygmomanometers or validated auscultatory or oscillometric devices and 2 measurements were averaged for analysis. Office hypertension was a BP of ≥140 mm Hg systolic or ≥90 mm Hg diastolic or use of antihypertensive drugs. Diabetes was a self-reported diagnosis, fasting blood glucose of ≥126 mg/dl (7.0 mmol/l), random blood glucose of ≥200 mg/dl (11.1 mmol/l), or diabetes documented in practice or hospital records. Body mass index was body weight in kilogram divided by body height in meter squared. The 24-hour BP was recorded with validated oscillometric devices (see Supplementary Table S3 online). Serum cholesterol was measured by automated methods in certified laboratories. To convert cholesterol to mmol/l, multiply by 39.67.
The median number of ambulatory readings recorded over 24 hours was 55 (5th–95th percentile interval, 34–81), ranging across cohorts (Supplementary Table S3 online) from 37 (5th–95th percentile interval, 26–42) to 80 (5th–95th percentile interval, 67–83). Among all participants, the 24-hour BP averaged 123.6 mm Hg systolic and 73.9 mm Hg diastolic and PP 49.7 mm Hg (Table 1). Analyzed by 5-year intervals (Supplementary Figure S1 online), systolic BP increased over the whole age span, while diastolic BP increased until middle age and leveled off from age 50 onwards. The association of PP with age was curvilinear with nadir at 40 years. Below 50 years of age, 24-hour PP averaged (±SD) 45.1 ± 6.9 mm Hg (Table 1 and Supplementary Figure S1 online) and 52.7 ± 9.7 mm Hg in older adults (Supplementary Figure S2 online). Mean arterial pressure increased of the whole lifespan.
Incidence of endpoints
Among 11,848 participants, median follow-up was 13.7 years (5th–95th percentile interval, 3.7–26.0 years). Across cohorts (Supplementary Table S1 online), median follow-up ranged from 4.0 years (5th–95th percentile interval, 3.5–7.6 years) to 24.5 years (5th–95th percentile interval, 8.6–27.8 years). During 162,367 person-years of follow-up (Supplementary Table S5 online), 2,846 participants died (17.5 per 1,000 person-years; 95% CI, 17.3–17.7) and 2,093 experienced a cardiovascular endpoint (14.3; CI, 14.1–14.5). The corresponding number of endpoints (rates) for the secondary endpoints (Supplementary Table S5 online) was 1,102 (6.85) for cardiovascular mortality; 932 (6.20) for coronary endpoints; and 846 (5.22) for stroke. The cohort- and sex-standardized rates of the primary and secondary endpoints increased with older age (P < 0.001; Supplementary Table S5 online). Further analyses were stratified by 50 years (<50 vs. ≥50 years).
Young adults
Incidence rates were categorized by increasing fourths of the PP distributions. Median observation time in the younger cohort was 14.1 years (5th–95th percentile interval, 4.0–26.5 years). In younger adults, absolute risk captured by the incidence of the primary and secondary endpoints (Supplementary Table S6 online) did not increase with higher PP (0.072 ≤ P ≤ 0.71). Similarly, among younger adults, the multivariable-adjusted hazard ratios relating primary or secondary (Table 3) endpoints to PP were all less than unity; the hazard ratios for the composite cardiovascular endpoint and cardiovascular mortality reached significance: 0.67 (CI, 0.47–0.96; P = 0.027) and 0.46 (CI, 0.21–0.99; P = 0.008), respectively.
Table 3.
Hazard ratios for primary and secondary endpoints by age class (starts)
| Endpoints | Young adults | Older adults | |||||
|---|---|---|---|---|---|---|---|
| 18–49 y | ≥50 y | 50–59 y | 60–69 y | ≥70 y | P trend | ||
| No. at risk | 4,663 | 7,185 | 2,470 | 2,263 | 2,452 | ||
| Primary endpoints | |||||||
| Total mortality | |||||||
| No. of deaths | 144 | 2,802 | 322 | 736 | 1,744 | ||
| Hazard ratio | 0.76 | 1.09 | 1.35 | 1.16 | 1.07 | 0.79 | 0.023 |
| 95% confidence interval | 0.55–1.06 | 1.04–1.14 | 1.13–1.61 | 1.06–1.29 | 1.01–1.12 | ||
| P value | 0.110 | <0.001 | 0.001 | 0.003 | 0.018 | ||
| All cardiovascular endpoint | |||||||
| No. of endpoints | 115 | 1,978 | 283 | 502 | 1,193 | ||
| Hazard ratio | 0.67 | 1.11 | 1.46 | 1.17 | 1.08 | 0.74 | 0.034 |
| 95% confidence interval | 0.47–0.96 | 1.06–1.17 | 1.22–1.74 | 1.05–1.30 | 1.02–1.15 | ||
| P value | 0.027 | <0.001 | <0.001 | 0.006 | 0.009 | ||
| Secondary endpoints | |||||||
| Cardiovascular mortality | |||||||
| No. of deaths | 24 | 1,078 | 104 | 235 | 739 | ||
| Hazard ratio | 0.33 | 1.14 | 1.54 | 1.36 | 1.08 | 0.70 | 0.25 |
| 95% confidence interval | 0.11–0.75 | 1.07–1.22 | 1.15–2.07 | 1.16–1.59 | 0.99–1.17 | ||
| P value | 0.008 | <0.001 | 0.004 | <0.001 | 0.050 | ||
| Coronary endpoints | |||||||
| No. of endpoints | 63 | 869 | 153 | 211 | 505 | ||
| Hazard ratio | 0.65 | 1.17 | 1.41 | 1.15 | 1.18 | 0.83 | 0.450 |
| 95% confidence interval | 0.40–1.04 | 1.09–1.26 | 1.12–1.78 | 0.97–1.36 | 1.08–1.28 | ||
| P value | 0.073 | <0.001 | 0.003 | 0.090 | <0.001 | ||
| Stroke | |||||||
| No. of endpoints | 40 | 806 | 99 | 232 | 475 | ||
| Hazard ratio | 1.06 | 1.12 | 1.43 | 1.23 | 1.08 | 0.75 | 0.014 |
| 95% confidence interval | 0.61–1.86 | 1.03–1.22 | 1.03–1.98 | 1.03–1.46 | 0.98–1.19 | ||
| P value | 0.820 | 0.006 | 0.032 | 0.020 | 0.132 |
Hazard ratios were adjusted cohort, sex, and baseline characteristics including age, body mass index, 24-hour mean arterial pressure, 24-hour heart rate, smoking and drinking, serum cholesterol, antihypertensive drug intake, history of cardiovascular disease, and diabetes. Hazard ratio expresses the relative risk associated with a 1-SD increment (10 mm Hg) in pulse pressure. Δ is e powered to the difference of the proportional hazards on the natural logarithmic scale in participants ≥70 years minus 50–59 years. Ptrend is the significance of the trend in the hazard ratios across age groups in older adults derived from an interaction term in the Cox models.
Older adults
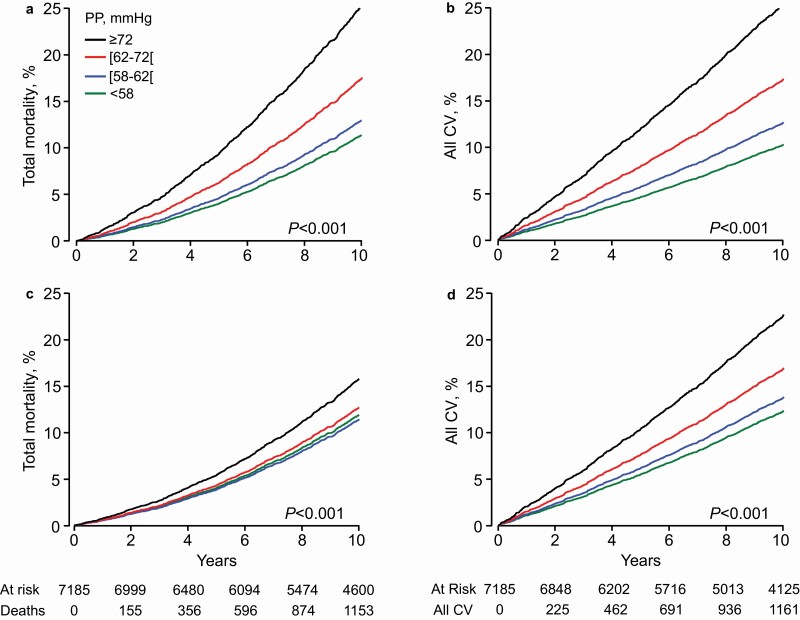
Median follow-up of the older cohort was 13.1 years (5th–95th percentile interval, 3.3–25.6 years). Among older adults, the standardized rates of all endpoints rose across increasing PP quartiles (P ≤ 0.029; Table 2). With adjustments applied for cohort and sex, the incidence of the primary (Figure 1) and secondary (Supplementary Table S3 online) endpoints augmented with higher PP category. Additional age adjustment lowered the rate ratio comparing the upper vs. the lower PP quartile from 2.41 (CI, 2.14–2.72) to 1.36 (CI, 1.20–1.53) for total mortality and from 2.95 (CI, 2.55–3.41) to 1.93 (CI, 1.67–2.24) for the composite cardiovascular endpoint (Figure 1). For cardiovascular mortality, coronary endpoints, and stroke, these rate ratios were 3.87 (CI, 3.14–4.76) vs. 2.09 (CI, 1.69–2.58), 2.63 (CI, 2.12–3.26) vs. 1.97 (CI, 1.58–2.46), and 3.78 (CI: 2.96–4.82) vs. 2.35 (CI: 1.83–3.02), respectively (Supplementary Table S3 online).
Table 2.
Rates of primary and secondary endpoints by pulse pressure quartiles in older adults
| Pulse pressure index | All participants aged ≥50 y | Pulse pressure categories | P trend | |||
|---|---|---|---|---|---|---|
| Endpoint | Low | Low-middle | High-middle | High | ||
| Quartile limits | … | <45.17 | [45.17–51.19] | [51.19–58.76] | ≥58.76 | |
| No. at risk | 7,185 | 1,796 | 1,797 | 1,796 | 1,796 | |
| Primary endpoints | ||||||
| Total mortality | ||||||
| No. of deaths | 2,802 | 424 | 582 | 769 | 1,027 | |
| Rate, per 1,000 person-years | 28.2 (27.1–29.6) | 17.8 (16.0–19.7) | 22.5 (20.6–24.5) | 31.6 (29.3–34.1) | 48.5 (45.4–51.9) | 0.008 |
| All cardiovascular endpoints | ||||||
| No. of endpoints | 1,978 | 278 | 380 | 533 | 787 | |
| Rate, per 1,000 person-years | 23.2 (22.1–24.6) | 13.1 (11.5–14.9) | 16.5 (14.8–18.4) | 25.4 (23.2–27.8) | 44.0 (41.0–47.5) | 0.016 |
| Secondary endpoints | ||||||
| Cardiovascular mortality | ||||||
| No. of deaths | 1,078 | 124 | 192 | 276 | 486 | |
| Rate, per 1,000 person-years | 11.3 (10.6–12.4) | 5.36 (4.39–6.54) | 7.56 (6.45–8.88) | 11.8 (10.4–13.5) | 23.9 (21.7–26.5) | 0.014 |
| Coronary endpoints | ||||||
| No. of endpoints | 869 | 132 | 169 | 216 | 352 | |
| Rate, per 1,000 person-years | 9.87 (9.20–10.9) | 6.13 (5.07–6.54) | 7.59 (6.45–8.88) | 11.8 (10.4–13.5) | 18.0 (16.2–20.3) | 0.029 |
| Stroke | ||||||
| No. of endpoints | 806 | 93 | 157 | 219 | 337 | |
| Rate, per 1,000 person-years | 8.46 (7.85–9.48) | 3.95 (3.12–5.01) | 6.11 (5.15–7.30) | 9.47 (8.20–11.1) | 17.0 (15.1–19.3) | 0.003 |
Rates, given with 95% confidence interval, were standardized by the direct method for cohort, sex, and median age. Ptrend denotes the significance of the rate trends across increasing pulse pressure categories.
Figure 1.
Cumulative incidence of the primary endpoints by pulse pressure quartiles in older adults. The incidence of total mortality (a, b) and the composite cardiovascular endpoint (c, b) was first adjusted for cohort and sex (a, c) and next additionally for age (b, d). Considering age significantly attenuated the gradient from the low to the high pulse pressure quartile (P < 0.0001). Tabulated data are the number of participants at risk and those experiencing an endpoint at 2-year intervals.
In older participants (Table 3), all primary and secondary endpoints were significantly (P < 0.0001) associated with PP with hazard ratios ranging from 1.09 for total mortality to 1.54 for cardiovascular death. However, across the 3 age classes (50–59, 60–69, and ≥70 years), the hazard ratios, expressing relative risk, weakened with older age; the decrease in relative risk expressed as e powered to the difference of the hazard ratio on the natural logarithmic scale in participants aged ≥70 years minus the corresponding hazard ratio in adults aged 50–59 years (i.e., the ratio of the hazard ratios) ranged from 0.70 to 0.83, reaching significance (P ≤ 0.034) for total mortality, the composite cardiovascular endpoint, and stroke (Table 3). Sensitivity analyses of the primary endpoints, only including untreated participants without history of cardiovascular disease at baseline or only 24-hour ambulatory recordings with at least 10 daytime and 5 nighttime readings, produced confirmatory results as shown for the composite cardiovascular endpoint (Supplementary Table S7 online). Similarly, replacing 24-mean arterial pressure by 24-hour diastolic BP in the multivariable-adjusted Cox models did not materially change the results.
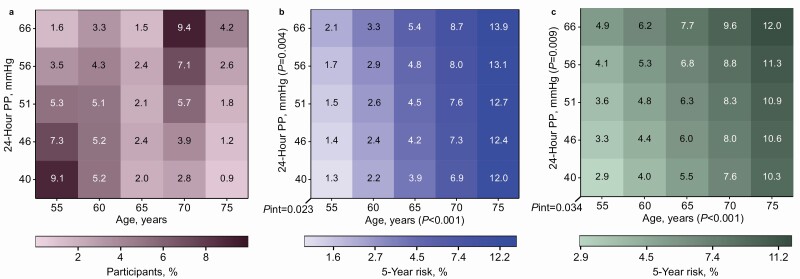
Heatmaps were constructed to show the contributions of age and PP, both analyzed as continuously distributed variables, to the 5-year risk of experiencing a primary (Figure 2) or secondary (Supplementary Table S4 online) endpoint. Both age (P < 0.0001) and PP (P ≤ 0.009) were significant drivers of the 5-year risk with the exception of PP in relation to cardiovascular mortality (P = 0.12; Supplementary Table S4 online). For total mortality, the composite cardiovascular endpoint and stroke the 5-year risk decreased with higher age (P ≤ 0.034) with similar trends for cardiovascular mortality and coronary endpoints. For total mortality (Figure 2, panel b), the 5-year age-related risk, irrespective of PP, increased 6- to 9-fold, while with PP increasing from 40 to 56 mm Hg, the 5-year risk increased by 61% (2.1/1.3%) at 55 years, but only by 16% (13.9/12.0%) at ≥75 years. For the composite cardiovascular endpoint (Figure 2, panel c) and stroke (Supplementary Figure S4, panel D online), at 55 vs. ≥75 years, the PP-related risk increment was 69% (4.9/2.9%) vs. 17% (12.0/10.3%) and 112% (1.9/0.8%) vs. 3% (3.7/3.6%).
Figure 2.
Heatmaps relating the 5-year risks of the primary endpoints to age and pulse pressure in older adults. Age was plotted along the horizontal axis and pulse pressure (PP) along the vertical axis. Numbers in the panel a grid represent the percentage of participants within each age × PP cross-classification category. Numbers in the other grids represent the 5-year risk of total mortality (b) and the composite cardiovascular endpoint (c) associated with age and PP. Estimates were generated by Cox proportional hazard regression with age and PP analyzed as continuous variables and adjusted for cohort, sex, body mass index, 24-hour mean arterial pressure, 24-hour heart rate, total cholesterol, smoking and drinking status, antihypertensive treatment at baseline, and history of cardiovascular disease and diabetes. Pint indicates the significance of the interaction term between age and PP. Models accounted for competing risks according to the Fine and Gray approach.
Discussion
Using 24-hour ambulatory monitoring, the state-of-the-art method in BP assessment,17 we demonstrated that below 50 years of age, higher PP associated with lower risk. In older adults, the incidence of death, the composite cardiovascular endpoint, and the 3 secondary endpoints showed a direct and graded positive association with PP. From 50 years onwards, absolute risk as captured by the incidence of endpoints steadily increased. Age was the predominant driver of mortality and cardiovascular complications in the older adults. Nonetheless, adjustment for age on top of cohort and sex attenuated, but did not remove, the graded rise in the rates of the primary and secondary endpoints across increasing PP quartiles. In heatmaps showing the fully adjusted risk associated with PP and age, both analyzed as continuously distributed variables, the relative risk of death, a cardiovascular endpoint or stroke in relation to PP decreased >3-fold, whereas absolute risk rose by a factor 3.
Absolute vs. relative risk
No process other than aging has greater effect on arterial stiffening.1 When it comes to absolute risk, age beats all other risk indicators by far (Supplementary Table S5 online). However, absolute risk only starts rising from middle age onwards with competing contributions of cardiovascular and noncardiovascular disease to total mortality and morbidity.10 Death rates due to noncardiovascular disease decline with advancing age with an opposite lifetime course for cardiovascular disease.18 Furthermore, population studies of cardiovascular risk factors revealed that relative risk declines with aging.9,10 In worldwide analyses of cardiovascular risk factors, including high BP, impaired fasting plasma glucose, hypercholesterolemia, and tobacco smoking, the Global Burden of Disease Investigators established that the inverse association between relative risk and age is roughly log-linear.9 The extrapolated age, at which relative risk reaches unity, was between 100 and 120 years.9
The opposing age-related trends in relative and absolute risk have great clinical relevance. Arterial stiffening occurs as a consequence of degradative processes in the wall of the large elastic and muscular arteries driven by the pulsatile BP load,1 cross-linking of elastin and collagen fibers by nonenzymatic glycation,19 calcium deposition accompanying atherosclerosis, and changes in the endothelial transcriptome and metabolic dysfunction in response to pressure overload.20 These degenerative processes, initially asymptomatic, are superimposed on the background of genetic predisposition and lifestyle influences. Assuming reversibility of risk, addressing a risk factor at young age will decrease relative risk with little effect on absolute risk, whereas at the upper end of the age distribution, relative risk will be barely affected, but absolute risk will diminish, thereby enhancing life in calendar years and in quality. In young adults, arterial stiffening as reflected by PP is a silent process (Supplementary Table S6 online and Table 3).21 However, the crux of the matter is that proper management of risk factors in young asymptomatic adults21 prevents intermediate target organ damage and the subsequent progression to major fatal and nonfatal cardiovascular complications. Thus, as highlighted by a recent review,1 addressing risk factors deteriorating the health status of arteries in young adults, moves the lifecourse trajectory of vascular disease to an optimal trajectory, whereas in the elderly such treatment extends life expectancy and reduces life-years lived with disability.
Pathophysiology underpinning the findings
Established pathophysiological concepts underpin the notion of a lifecourse management of cardiovascular risk factors causing arterial stiffening.1,22 Passive stiffness of the large elastic arteries is determined mainly by the extracellular matrix and to a much smaller extent by vascular smooth muscle cells anchored in the extracellular matrix by integrins.23,24 Elastin and collagen are the major constituents of the extracellular matrix in the media of the central elastic arteries. Elastin provides reversible extensibility during systole, while collagen generates the tensile strength of the arterial wall. As people age, the elastic fibers become fragmented and the mechanical load is transferred to collagen fibers, which are up to 1,000 times stiffer than elastin.22 This process already starts in young adulthood,1 but the deposition of elastin by vascular smooth muscle cells only occurs during fetal development and early infancy,24,25 and is switched-off thereafter. This implies that elastin fiber damage is basically irreversible. Instead, more collagen is produced, which decreases the elastin-to-collagen ratio and shifts the mechanical arterial properties towards the stiffer range of collagen fibers. The stiffness of elastin and collagen fibers can be further increased through cross-linking by advanced glycation end-products, a process accompanying normal aging, but which is accelerated in diabetic patients.19 Aberrant protein–protein cross-linking between elastin and collagen occurring along the whole length of the elastin protein chain, prevents enzymatic digestion and collagen degradation. Finally, the presence of calcium deposits in the media of large arteries is also age related with a strong correlation between the aortic calcium content and arterial stiffness in humans.19
Remarkably, in young adults the hazard ratio associating cardiovascular mortality with PP was less than unity and significant (P = 0.008; Table 3). The hazard ratios being lower than unity might be a chance finding due to the low number of cardiovascular endpoints in young adults. The absence of any trend with PP in the standardized rates of cardiovascular mortality supports this interpretation (Supplementary Table S6 online). However, hazard ratios less than unity attained significance for not only for the composite cardiovascular endpoint and cardiovascular mortality, but as well for stroke, with a directionally similar hazard ratios albeit not formally significant for total mortality and coronary endpoints (Table 3). Presumably, the most likely explanation is that a high PP in younger adults does not principally involve arterial stiffening, but rather an hyperdynamic circulation, a larger cardiac stroke volume and during daytime a higher level of physical activity, which are all factors protective from a cardiovascular point of view. Furthermore, in older adults, the age-related attenuation of the relative risk (Table 2 and Supplementary Figure S4 online) associated with PP was weaker for cardiovascular mortality and coronary endpoints than for the other endpoints, albeit directionally similar. The particular characteristics of the coronary circulation might explain these observations.26 Indeed myocardial contraction compresses the intramyocardial blood vessels and limits blood flow, and diastolic rather than systolic BP drive the coronary blood flow.
Strengths and limitations
Generalizability is among the strengths of our study. Participants were randomly recruited from populations in 12 countries and 3 continents. Endpoints were collected over a median of 13.7 years of follow-up and encompassed both fatal and nonfatal events, all adjudicated against the source documents available in each country. Ambulatory BP monitoring is the state-of-the-art method in BP assessment.17 However, our study also has limitations. Foremost, the most accurate index of arterial stiffness is aortic pulse wave velocity,27 but compared with PP is more difficult to collect in large population cohorts. Factors other than arterial stiffness influence brachial PP, such as heart rate, cardiac contractility, the venous left ventricular venous filling pressure, and the decrease in pressure amplification from central to peripheral arteries in aging individuals.27 Nevertheless, aortic pulse wave velocity is correlated with all BP indexes,1 and most closely with PP.28 Furthermore, the correlation coefficient between central and peripheral PP is 0.95, so that central PP does not generate prognostic information over and beyond its peripheral counterpart.29 Second, Asians and South Americans were underrepresented. We had no information on Blacks of African descent or Blacks born and living in Africa, who generally are more susceptible to the complications of hypertension.30 However, several studies suggest that the association of mortality and cardiovascular complications with arterial stiffness is universal without sizable differences between ethnicities, when lifestyle is accounted for.22
Adverse health outcomes were not associated with PP in young adults. In line with the lifecourse increase in arterial stiffness,1 in older adults, relative and absolute risks of fatal and nonfatal outcomes showed opposite age-related trends, relative risk decreasing, and absolute risk increasing with advancing age. Although arterial stiffening is clinically silent below 50 years of age, young adulthood provides a window of opportunity to manage the risk factors promoting arterial stiffening and, further downstream, to prevent early target organ damage caused by the pulsatile flow in the microcirculation of the brain, kidney, and heart.31 Policies shifting focus from treating to preventing disease have the added benefit of creating awareness, empowering individuals, and promoting self-management. In older adults with stiff arteries, even in the very elderly, therapeutic inertia is not an option and prevention, if possible primary prevention, is clinically mandatory, because it provides the lowest number to treat to avoid 1 fatal or nonfatal complication.32
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the clerical assistance of Vera De Leebeeck and Renilde Wolfs, Research Unit Hypertension and Cardiovascular Epidemiology, Department of Cardiovascular Sciences, Leuven, Belgium.
FUNDING
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Belgium: European Union (HEALTH-F7-305507 HOMAGE), European Research Council (Advanced Researcher Grant 2011-294713-EPLORE and Proof-of-Concept Grant 713601-uPROPHET), European Research Area Net for Cardiovascular Diseases (JTC2017-046-PROACT), and Research Foundation Flanders, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13); China: The National Natural Science Foundation of China (grants 81470533, 91639203, and 81770455), the Ministry of Science and Technology (2015AA020105-06, 2016YFC1300100, and 2018YFC1704902), Beijing, China, and by the Shanghai Commissions of Science and Technology (14ZR1436200 and 15XD1503200), and the Shanghai Bureau of Health (15GWZK0802, 2017BR025, and a Grant for Leading Academics); The Czech Republic: European Union (LSHM-CT-2006-037093 and HEALTH-F4-2007-201550) and Charles University Research Fund (project P36); Denmark: Danish Heart Foundation (01-2-9-9A-22914) and Lundbeck Fonden (R32-A2740); Ireland: the Irish Allied Bank; Italy: European Union (LSHM-CT-2006-037093 and HEALTH-F4-2007-201550); Japan: Ministry of Culture, Sports, Science and Technology (16H05243, 16H05263, 16K09472, 16K11850, 16K15359, 17H04126, 17H06533, 17K15853, 17K19930, 18K09674, 18K09904, and 18K17396), Grant-in-Aid for Young Scientists of Showa Pharmaceutical University (H28-4), Japan Atherosclerosis Prevention Fund (Comprehensive Research on Cardiovascular and Lifestyle Related Diseases), Ministry of Health, Labor and Welfare (H26-Junkankitou [Seisaku]-Ippan-001 and H29-Junkankitou-Ippan-003), Ministry of Agriculture, Forestry and Fisheries (NouEi 2-02), Academic Contributions from Pfizer Japan, and scholarship donations from Chugai Pharmaceutical Company and Daiichi Sankyo Company; Poland (Gdańsk): European Union (LSHM-CT-2006-037093 and HEALTH-F4-2007-201550); Poland (Kraków): European Union (LSHM-CT-2006-037093 and HEALTH-F4-2007-201550) and Foundation for Polish Science; The Russian Federation: European Union (LSHM-CT-2006-037093 and HEALTH-F4-2007-201550); Uruguay: Asociación Española Primera en Salud; Venezuela: The National Institute of Aging and the Fogarty International Center (1-R01AG036469 A1), the National Institutes of Health and National Institute of Aging (1 R03 AG054186-01), Fondo Nacional de Ciencia, Ternologia e innovacion, Caracas (G-97000726), and FundaConCiencia, Maracaibo (LOCTI/008-2008). The Research Institute Alliance for the Promotion of Preventive Medicine (APPREMED), Mechelen, Belgium received a nonbinding grant from OMRON Healthcare, Kyoto, Japan.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol 2019; 74:1237–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Staessen J, Amery A, Fagard R. Isolated systolic hypertension in the elderly. J Hypertens 1990; 8:393–405. [DOI] [PubMed] [Google Scholar]
- 3. Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Pede S, Porcellati C. Ambulatory pulse pressure: a potent predictor of total cardiovascular risk in hypertension. Hypertension 1998; 32:983–988. [DOI] [PubMed] [Google Scholar]
- 4. Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, Wang JG, Fagard RH, Safar ME. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med 2000; 160:1085–1089. [DOI] [PubMed] [Google Scholar]
- 5. Khattar RS, Swales JD, Dore C, Senior R, Lahiri A. Effect of aging on the prognostic significance of ambulatory systolic, diastolic, and pulse pressure in essential hypertension. Circulation 2001; 104:783–789. [DOI] [PubMed] [Google Scholar]
- 6. Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001; 103:1245–1249. [DOI] [PubMed] [Google Scholar]
- 7. Schram MT, Kostense PJ, Van Dijk RA, Dekker JM, Nijpels G, Bouter LM, Heine RJ, Stehouwer CD. Diabetes, pulse pressure and cardiovascular mortality: the Hoorn Study. J Hypertens 2002; 20:1743–1751. [DOI] [PubMed] [Google Scholar]
- 8. Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, Damasceno A, Delles C, Gimenez-Roqueplo AP, Hering D, López-Jaramillo P, Martinez F, Perkovic V, Rietzschel ER, Schillaci G, Schutte AE, Scuteri A, Sharman JE, Wachtell K, Wang JG. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet 2016; 388:2665–2712. [DOI] [PubMed] [Google Scholar]
- 9. The Global Burden of Disease Investigators. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Y, Thijs L, Zhang ZY, Asayama K, Hansen TW, Boggia J, Björklund-Bodegård K, Yang WY, Niiranen TJ, Ntineri A, Wei FF, Kikuya M, Ohkubo T, Dolan E, Hozawa A, Tsuji I, Stolarz-Skrzypek K, Huang QF, Melgarejo JD, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Aparicio L, Barochiner J, Gilis-Malinowska N, Narkiewicz K, Kawecka-Jaszcz K, Maestre GE, Jula AM, Johansson JK, Kuznetsova T, Filipovský J, Stergiou G, Wang JG, Imai Y, O’Brien E, Staessen JA; International Database on Ambulatory and Home Blood Pressure in Relation to Cardiovascular Outcome Investigators . Opposing age-related trends in absolute and relative risk of adverse health outcomes associated with out-of-office blood pressure. Hypertension 2019; 74:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang WY, Melgarejo JD, Thijs L, Zhang ZY, Boggia J, Wei FF, Hansen TW, Asayama K, Ohkubo T, Jeppesen J, Dolan E, Stolarz-Skrzypek K, Malyutina S, Casiglia E, Lind L, Filipovský J, Maestre GE, Li Y, Wang JG, Imai Y, Kawecka-Jaszcz K, Sandoya E, Narkiewicz K, O’Brien E, Verhamme P, Staessen JA; International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (IDACO) Investigators . Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA 2019; 322:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 13. Yang WY, Thijs L, Zhang ZY, Asayama K, Boggia J, Hansen TW, Ohkubo T, Jeppesen J, Stolarz-Skrzypek K, Malyutina S, Casiglia E, Nikitin Y, Li Y, Wang JG, Imai Y, Kawecka-Jaszcz K, O’Brien E, Staessen JA; International Database; on Ambulatory blood pressure in relation to Cardiovascular Outcomes (IDACO) Investigators . Evidence-based proposal for the number of ambulatory readings required for assessing blood pressure level in research settings: an analysis of the IDACO database. Blood Press 2018; 27:341–350. [DOI] [PubMed] [Google Scholar]
- 14. Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med 1997; 16:791–801. [DOI] [PubMed] [Google Scholar]
- 15. Domanski M, Norman J, Wolz M, Mitchell G, Pfeffer M. Cardiovascular risk assessment using pulse pressure in the First National Health and Nutrition Examination Survey (NHANES I). Hypertension 2001; 38:793–797. [DOI] [PubMed] [Google Scholar]
- 16. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 17. Huang QF, Yang WY, Asayama K, Zhang ZY, Thijs L, Li Y, O’Brien E, Staessen JA. Ambulatory blood pressure monitoring to diagnose and manage hypertension. Hypertension 2021; 77:254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Staessen JA, Thijs L, Yang WY, Yu CG, Wei FF, Roels HA, Nawrot TS, Zhang ZY. Interpretation of population health metrics: environmental lead exposure as exemplary case. Hypertension 2020; 75:603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 2003; 21:3–12. [DOI] [PubMed] [Google Scholar]
- 20. Trenson S, Hermans H, Craps S, Pokreisz P, de Zeeuw P, Van Wauwe J, Gillijns H, Veltman D, Wei F, Caluwé E, Gijsbers R, Baatsen P, Staessen JA, Ghesquiere B, Carmeliet P, Rega F, Meuris B, Meyns B, Oosterlinck W, Duchenne J, Goetschalckx K, Voigt JU, Herregods MC, Herijgers P, Luttun A, Janssens S. Cardiac microvascular endothelial cells in pressure overload-induced heart disease. Circ Heart Fail 2021; 14:e006979. [DOI] [PubMed] [Google Scholar]
- 21. Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 1998; 18:127–132. [DOI] [PubMed] [Google Scholar]
- 22. Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O’Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation 1983; 68:50–58. [DOI] [PubMed] [Google Scholar]
- 23. Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. J Cardiovasc Transl Res 2012; 5:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev 2009; 89:957–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Dev Biol 2004; 62:153–188. [DOI] [PubMed] [Google Scholar]
- 26. Cooper LL, Palmisano JN, Benjamin EJ, Larson MG, Vasan RS, Mitchell GF, Hamburg NM. Microvascular function contributes to the relation between aortic stiffness and cardiovascular events. Circ Cardiovasc Imaging 2016; 9:e004979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 28. Kim EJ, Park CG, Park JS, Suh SY, Choi CU, Kim JW, Kim SH, Lim HE, Rha SW, Seo HS, Oh DJ. Relationship between blood pressure parameters and pulse wave velocity in normotensive and hypertensive subjects: invasive study. J Hum Hypertens 2007; 21:141–148. [DOI] [PubMed] [Google Scholar]
- 29. Huang QF, Aparicio LS, Thijs L, Wei FF, Melgarejo JD, Cheng YB, Sheng CS, Yang WY, Gilis-Malinowska N, Boggia J, Niiranen TJ, Wojciechowska W, Stolarz-Skrzypek K, Barochiner J, Ackermann D, Tikhonoff V, Ponte B, Pruijm M, Casiglia E, Narkiewicz K, Filipovský J, Czarnecka D, Kawecka-Jaszcz K, Jula AM, Bochud M, Vanassche T, Verhamme P, Struijker-Boudier HAJ, Wang JG, Zhang ZY, Li Y, Staessen JA; IDCARS (International Database of Central Arterial Properties for Risk Stratification) Investigators . Cardiovascular end points and mortality are not closer associated with central than peripheral pulsatile blood pressure components. Hypertension 2020; 76:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Odili AN, Thijs L, Yang WY, Ogedengbe JO, Nwegbu MM, Jacobs L, Wei FF, Feng YM, Zhang ZY, Kuznetsova T, Nawrot TS, Staessen JA. Office and home blood pressures as determinants of electrocardiographic left ventricular hypertrophy among black Nigerians compared with white Flemish. Am J Hypertens 2017; 30:1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005; 46:200–204. [DOI] [PubMed] [Google Scholar]
- 32. Mortensen MB, Nordestgaard BG. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70–100 years: a contemporary primary prevention cohort. Lancet 2020; 396:1644–1652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.